Abstract
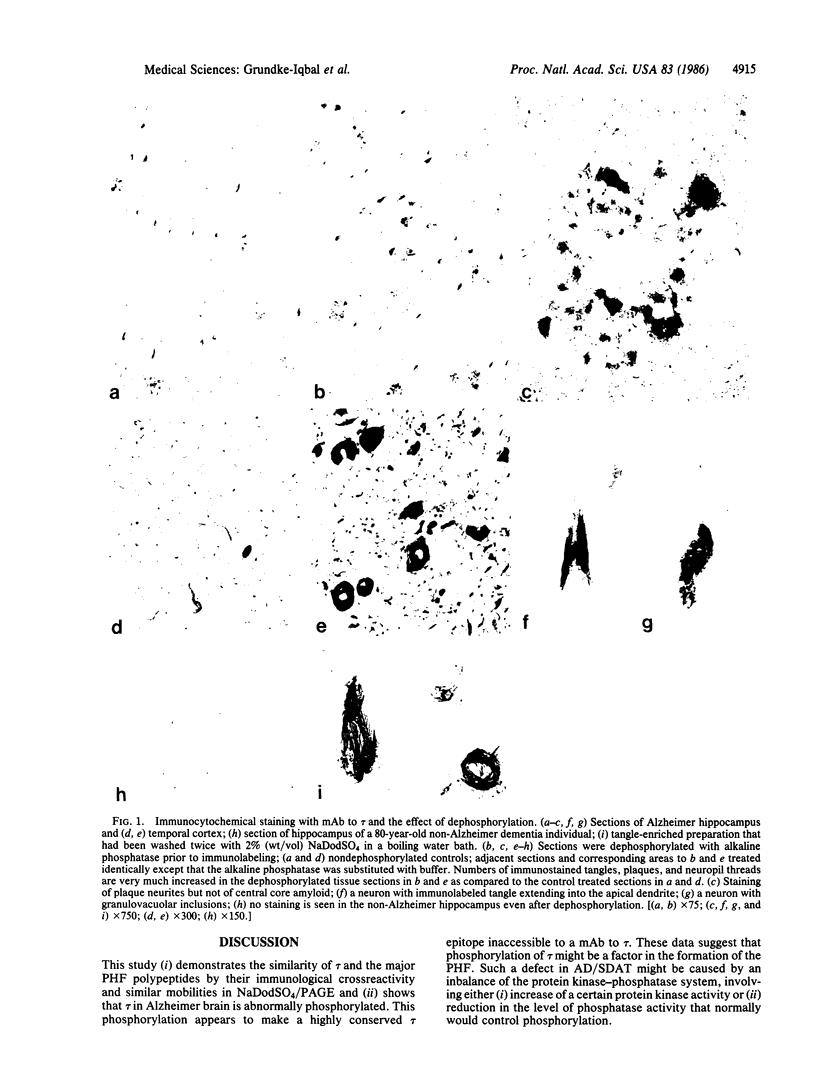
A monoclonal antibody to the microtubule-associated protein tau (tau) labeled some neurofibrillary tangles and plaque neurites, the two major locations of paired-helical filaments (PHF), in Alzheimer disease brain. The antibody also labeled isolated PHF that had been repeatedly washed with NaDodSO4. Dephosphorylation of the tissue sections with alkaline phosphatase prior to immunolabeling dramatically increased the number of tangles and plaques recognized by the antibody. The plaque core amyloid was not stained in either dephosphorylated or nondephosphorylated tissue sections. On immunoblots PHF polypeptides were labeled readily only when dephosphorylated. In contrast, a commercially available monoclonal antibody to a phosphorylated epitope of neurofilaments that labeled the tangles and the plaque neurites in tissue did not label any PHF polypeptides on immunoblots. The PHF polypeptides, labeled with the monoclonal antibody to tau, electrophoresed with those polypeptides recognized by antibodies to isolated PHF. The antibody to tau-labeled microtubules from normal human brains assembled in vitro but identically treated Alzheimer brain preparations had to be dephosphorylated to be completely recognized by this antibody. These findings suggest that tau in Alzheimer brain is an abnormally phosphorylated protein component of PHF.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986 May 5;261(13):6084–6089. [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Wang G. P., Wisniewski H. M. Alzheimer paired helical filaments: cross-reacting polypeptide/s normally present in brain. Acta Neuropathol. 1985;66(1):52–61. doi: 10.1007/BF00698295. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Wisniewski H. M. Alzheimer paired helical filaments: immunochemical identification of polypeptides. Acta Neuropathol. 1984;62(4):259–267. doi: 10.1007/BF00687607. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Johnson A. B., Wisniewski H. M., Terry R. D., Iqbal K. Evidence that Alzheimer neurofibrillary tangles originate from neurotubules. Lancet. 1979 Mar 17;1(8116):578–580. doi: 10.1016/s0140-6736(79)91006-7. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Wang G. P., Iqbal K., Tung Y. C., Wisniewski H. M. Alzheimer paired helical filaments: identification of polypeptides with monoclonal antibodies. Acta Neuropathol. 1985;68(4):279–283. doi: 10.1007/BF00690830. [DOI] [PubMed] [Google Scholar]
- Iqbal K., Zaidi T., Thompson C. H., Merz P. A., Wisniewski H. M. Alzheimer paired helical filaments: bulk isolation, solubility, and protein composition. Acta Neuropathol. 1984;62(3):167–177. doi: 10.1007/BF00691849. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindwall G., Cole R. D. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984 Apr 25;259(8):5301–5305. [PubMed] [Google Scholar]
- Selden S. C., Pollard T. D. Phosphorylation of microtubule-associated proteins regulates their interaction with actin filaments. J Biol Chem. 1983 Jun 10;258(11):7064–7071. [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. P., Grundke-Iqbal I., Kascsak R. J., Iqbal K., Wisniewski H. M. Alzheimer neurofibrillary tangles: monoclonal antibodies to inherent antigen(s). Acta Neuropathol. 1984;62(4):268–275. doi: 10.1007/BF00687608. [DOI] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen G. Y., Wisniewski H. M. Substructures of neurofilaments. Acta Neuropathol. 1984;64(4):339–343. doi: 10.1007/BF00690399. [DOI] [PubMed] [Google Scholar]
- Wisniewski H. M., Wen G. Y. Substructures of paired helical filaments from Alzheimer's disease neurofibrillary tangles. Acta Neuropathol. 1985;66(2):173–176. doi: 10.1007/BF00688696. [DOI] [PubMed] [Google Scholar]