Abstract
Sandhoff’s disease is a lysosomal storage disease in which the ganglioside, GM2, accumulates in lysosomes. It has been reported that MRI cannot detect abnormalities in spin echo images in clinically pre-symptomatic Sandhoff’s disease patients. Because one of the results of GM2 accumulation is cell swelling and lysosomal distension, our goal was to determine if changes in the diffusion of water is perturbed. We utilized the MRI imaging modality, diffusion weighted imaging (DWI), to measure the apparent diffusion coefficient (ADC) in a mouse models of Sandhoff’s disease, the hexb−/− mouse, and determined if DWI could be utilized to detect early changes prior to behavioral or overt disease symptom onset. Here we report for the first time, a comprehensive behavioral characterization of the hexb−/− mouse in conjunction with the ADC measurement. Our data indicate that the ADC decreases in the hexb−/− mouse in many but not all brain regions prior to disease symptoms (< 3.5 – 4 months of age) and behavioral deficits (3-months of age). The magnitude of the decrease ranged from 4–18%.
INTRODUCTION
Ganglioside accumulation has been reported in a multitude of diseases ranging from the relatively rare lysosomal storage diseases such as Sandhoff’s disease to Down’s syndrome to Alzheimer’s disease to individuals infected with AIDS (1,2). Gangliosides are glycosphingolipids that contain a sialic acid group and are concentrated on the surfaces of neurons in the outer leaflet of the plasma membrane(3–5). Functionally, gangliosides are involved in multiple cellular processes including cell differentiation and morphogenesis, binding sites for viruses, bacteria and toxins as well as cell specific adhesion processes (4,6–8). Ganglioside synthesis begins within the endoplasmic reticulum (ER) continuing within the Golgi Apparatus (GA) and ganglioside catabolism occurs within the lysosome. Excessive ganglioside accumulation is categorized as a lipid storage disease. Individual lipid storage diseases are considered to be rare. However, given that there are 40 different types of lipid storage diseases, collectively, they affect approximately 1 in 5,000 people according to the National Tay-Sachs and Allied Diseases Association (NTSAD).
Sandhoff’s disease is a lipid storage disease in which the ganglioside, GM2, accumulates in lysosomes and is hence also referred to as a lysosomal storage disease (9–11). In Sandhoff’s disease, there is a dysfunctional beta subunit of the hexosaminidase enzyme that catabolyzes GM2 (9–11). Children with juvenile Sandhoff’s disease exhibit excessive lysosomal accumulation of GM2. By the age of 6 months, the children develop muscle weakness that progressively declines (9–11). Additionally, these children exhibit a classic cherry red spot at the back of the eye, a “doll-like” expression, an exaggerated startle reaction to sound as well as mental deterioration and cardiac hypertrophy (9–11).
A mouse model of Sandhoff’s disease has been generated that has the gene that codes for the beta subunit of the hexosaminidase enzyme, the hexb gene, knocked out (hexb−/− mice) (12,13). These mice develop progressive neurodegeneration that is physically apparent by 4 months of age (muscle stiffness, ataxia, tremors) and eventually, these mice expire at approximately 4.5 – 5 months of age(12,13).
Clinically, symptomatic Sandhoff’s disease in human patients has been reported to present as hypo-intensities in the thalamus in spin-spin (T2)-weighted spin echo images (14–18). Additionally, enhanced signal in the white matter in spin-spin (T2)-weighted spin echo images has also been reported as well as cortical atrophy in Sandhoff’s disease patients (14–17). However, it has been reported that MRI cannot detect abnormalities in spin echo images in presymptomatic Sandhoff’s disease human patients (15). Because one result of GM2 accumulation is cell swelling and lysosomal distension, our goal was to determine if changes in the diffusion of water is perturbed. We utilized the MRI modality, diffusion weighted imaging (DWI), to measure the apparent diffusion coefficient (ADC) in the hexb−/− mouse model of Sandhoff’s disease and determined if DWI could be utilized to detect early changes prior to behavioral or overt symptom onset. Here we report for the first time, a comprehensive behavioral characterization of the hexb−/− mouse in conjunction with the ADC measurements. Our data indicate that ADC changes in the hexb−/− mouse occur prior to disease symptoms and behavioral deficits.
METHODS
Mouse Models
Breeding pairs of Hexosaminidase B knockout mice (Hexbtm1Rlp referred to as Hexb−/− throughout the manuscript) were obtained from Jackson Labs (Bar Harbor, ME). These mice were donated to the Jackson Labs mouse colony by Dr. Richard Proia (13). For all behavioral, imaging and histology assays, two age points were assessed: 6–7 week old hexb−/− mice that were asymptomatic as well as 3-month old hexb−/− mice that were close to the symptomatic range of 3.5 – 4 months. It is important to note that the mice at the 3-month time point did not possess any obvious phenotypic differences that would bias our assessment. Age-matched wild-type mice from the same litters were included as controls for each assessment. The background strain for the hexb−/− was a mixed background of C57/Bl6 and 129. In this study, the 6–7 week old group included N = 5 wildtype (3 female and 2 male) and N = 6 Hexb−/− (5 female and 1 male). The 3-month old group consisted of N = 5 wildtype (2 female and 3 male) and N = 7 Hexb−/− (2 female and 5 male).
All the animals used in this study were handled in compliance with institutional and national regulations and policies. The protocols were approved by the Institutional Animal Subjects Committee at Baylor College of Medicine.
Behavioral Assays
In this study, we have included a comprehensive behavioral evaluation of the hexb−/− mice. The motor and visual assays are important to include to validate that the mice do not have any motor or visual deficits that could potentially skew the interpretation of the learning and memory tests that rely upon the mouse’s ability to mobilize and also see.
Open Field Locomotion
To assess motor function, we incorporated the use of the open field locomotion test. A VersaMax Animal Activity Monitoring System (AccuScan Instrument, Inc. Columbus, OH) was utilized for the open field locomotion test that records the spontaneous activity of a mouse in an open field. Each subject was placed in a testing room with standard lighting conditions as well as a white noise source for 30 minutes to acclimate the subject to the testing room prior to being placed in the center of the testing chamber. The standard lighting condition refers to an illuminance intensity of 700 lux (verified with a light meter). Both horizontal and vertical movements were recorded over a 30 minute time frame by computer-controlled photobeams. 15 readings were collected with a 2-minute duration for each reading. For the 6–7 week old mice, N = 15 hexb−/− and N = 15 wildtype. For the 3-month old mice, N = 18 hexb−/− and N = 17 wildtype.
Rota-rod Locomotion
The Rota-rod Locomotion test assesses motor coordination and balance, as well as motor learning. A UGO BASILE treadmill machine (Ugo Basile North America Inc. Malvern, PA) was utilized for the Rota-rod test. In this assay, the mice were placed on a rotating rod that linearly increased in speed over time. The rotation rate increased from 4 to 40 rpm (rotations per minute) during the 5-minute test. The latency for each mouse to fall off the rod was recorded. Mice were tested on 2 consecutive days with 4 trials per day. The inter-trial interval was 30–35 minutes. The same mice utilized for the open field test were utilized for the rota-rod test one day later.
Conditioned Fear
The Conditioned Fear test is extensively utilized in the behavioral phenotyping of transgenic and knockout mice as a second independent learning and memory task to complement the Morris Water Maze. A Med Associates/Actimertic System (Med Associates, St. Albans, VT) system was utilized for the Conditioned Fear test. For the training phase, a white noise conditioned stimulus (CS) would sound after the mouse was in the chamber for two minutes. 30 seconds following the CS, a footshock was applied for two seconds. Mice received two CS and unconditioned stimulus (US) pairs two minutes apart. To assess the memory for the conditioning event, 24 hours later mice were given a contextual test (CT) and a CS test.
For the CT, the mouse was placed in exactly the same environment as the training phase and observed for five minutes without any applied sound stimulus or footshock. 1–2 hours later, the CS test was administered. During the CS test, the environment was modified to be clearly different than during the training phase (e.g. no bedding in the cages used to transfer the mice to the testing room, different room lighting, different odors and shapes were placed in the actual test cabinet). After three minutes within the novel environment, the CS utilized for the conditioning was applied and maintained for three minutes.
Freezing behavior as defined by the absence of movement except that associated with breathing is indicative of a fear response was scored at 10-second intervals for by an experimenter blind to the genotype. A total of 30 knockout (6–7 week old, N = 15; 3-month old, N = 15;) and 30 wildtype (6–7 week old, N = 15; 3-month old, N = 15) mice were utilized in the Conditioned Fear behavioral assay, after they finished Open Field locomotion and Rota-rod locomotion on the previous two days.
Hidden-platform Morris Water Maze
A Noldus EthoVision (Noldus, Leesburg, VA) system was utilized for the Morris Water Maze test, which is a spatial learning and memory test. During the acquisition phase, mice are able to escape from a pool (130cm in diameter) filled with opaque water by climbing onto a platform that is hidden beneath the water surface. Visual spatial cues were positioned outside of the pool. A camera attached to the ceiling above the pool automatically recorded the mouse’s movement.
In this study, each mouse was given eight trials each day over four consecutive days. During each trial, the mouse was placed into the pool from one of the four quadrants and given the opportunity to find the platform during a 60 second duration. The escape platform was placed in the same quadrant throughout the training sessions. After the last training trial, mice were given a probe trial with the platform removed. For the training trials, the latency to find the platform (escape latency) as well as the distance traveled to reach to platform was recorded. For the probe trail the number of platform crossings and the time spent in each quadrant was recorded.
A total of 26 knockout (6–7 week old, N = 11; 3-month old, N = 15) and 26 wildtype (6–7 week old, N = 11; 3-month old, N = 15) mice were utilized for the Hidden Platform Morris Water Maze one week after they finished Conditioned Fear test.
Visible-platform Morris Water Maze
The Visible Platform Morris Water Maze test was used to validate the mouse’s vision as well as task learning. The visible platform consisted of a hidden platform on which a highly visible dark square object was mounted and extended several inches above the water. The platform was always fixed and only the starting locations of the mice were varied. Mice were trained eight times every day with a two-day experiment yielding a total of 16 latency records for each mouse. Four average values are calculated by grouping the data into four groups (1–4, 5–8, 9–12,13–16). A total of 20 knockout (6–7 week old, N = 10; 3-month old, N = 10) and 19 wildtype (3-month old, N = 9; 6–7 week old, N = 10) mice were assessed in the Visible Platform Morris Water Maze.
Light-Dark Exploration
The light-dark test is an assay that assesses anxiety-like behavior in rodents. In this assay, mice are tested in a box that contains a dark areas encompassing one-third of the box and a light area consisting of two-thirds of the box. A small opening that allowed the mice to freely move from one area to the other connected these two areas. The test commenced by placing a mouse in the lit area. The number of transitions between dark and light and the time spent in the light area are utilized as a measure for anxiety. A Psion handheld computer (Psion Teklogix, Erlanger, KY) was used to score the position of the mouse in the test chamber. The room also contained a white noise source reading approximately 69dB, and stable lighting of approximately 700 lux. 20 hexb−/− mice (6–7 week old, N = 10; 3-month old, N = 10) and 19 wildtype (6–7 week old, N = 10; 3-month old, N = 9) were utilized for the Light-Dark Exploration test.
Elevated-Plus Maze
This test is another assay routinely used for anxiety like behaviors. In this assay, mice are tested on a plus-shaped platform that is elevated approximately one meter above the floor. Two opposite arms of the maze are enclosed, whereas the other two arms are open (with a small threshold along the sides). The test begins by placing a mouse in the center part of the maze facing one of the two open arms. The number of times and the time spent within the open arm regions are utilized as a measure for anxiety. A Psion handheld computer was utilized to score the position of the mouse in the light-box as either in the enclosed arm, or in the open arm, or in the center area.
20 hexb−/− mice (6–7 week old, N = 10; 3-month old, N = 10) and 19 wildtype (6–7 week old, N = 10; 3-month old, N = 9) were utilized for the Elevated-Plus Maze test.
Behavioral Data Analysis
Behavioral data was analyzed using SPSS 14.0 statistics software (SPSS Inc., Chicago, IL). P values for Open Field, Elevated-Plus Maze and Light-Dark Exploration were obtained using univariate tests. P Values for Rota-Rod, Morris Water Maze, Morris Water Maze Probe Trial, Visible Morris Water Maze and Conditioned Fear were obtained utilizing a one-way ANOVA test. P values less than 0.05 were considered significant.
Magnetic Resonance Imaging (MRI)
Diffusion-weighted MRI experiments were performed utilizing a Bruker Avance Biospec, 9.4 T spectrometer, 21 cm bore horizontal imaging system (Bruker Biospin, Billerica, MA) with a 35 mm volume resonator. All imaging was cardiac and respiratory gated (SA Instruments, Stony Brook, NY). During the imaging the animal body temperature was maintained at 37.0°C using an animal heating system (SA Instruments, Stony Brook, NY). Three parallel axial slices with a 1.0mm thickness were selected for analysis from each mouse brain. The first slice was placed across the pituitary gland that is visible on a sagittal whole-brain reference image. The second and third slices were placed closer to the anterior side of the brain with a 1 mm gap between each of the slices. Images were acquired utilizing a 4-shot EPI protocol with the following imaging parameters: TR=1000ms, TE=32.73ms, FOV=25.6mm*25.6mm, matrix size of 128 × 128; 4 signal averages were acquired. The diffusion gradients were applied along the slice direction. The different diffusion gradient settings were as follows: δ=2.5ms, Δ=7.02ms, b=0, 300, 600 and 900 mm2/s. Saturation slices were used in non-brain regions to minimize ghosting.
Imaging for spin-lattice (T1) and spin-spin (T2) relaxation time measurements was performed for the same three slices. A FISP protocol was utilized for T1 measurements with the following imaging parameters: TR=5000ms, TE=2.019ms, 8 frames, 32 segment, FOV=30mm×30mm, matrix size of 128×128. Multi-echo spin echo sequence protocol was utilized for T2 measurements with the following imaging parameters: TR=1000ms, TE=10.287ms, 8 echoes with a echo step size of 10.287ms, FOV=30mm×30m, matrix size of 256×256,
MRI Data Analysis
The MRI image data were analyzed utilizing the Imaging Sequence Analysis Tool included in the Paravision 4.0 software (Bruker Biospin, Billerica, MA). For slices 1 and 2, 6 regions of interest (ROI) were selected at identical locations for each image set with 2 at the cortex, 2 at the thalamus and 2 at the hippocampus. For the third slice, 2 ROIs were selected at identical locations for each image set with 2 at the cortex and 2 at the striatum.
The apparent diffusion constant (ADC) values were calculated for each region based upon the equation:
where Y is the image signal intensity, A is the absolute bias, I is the multiplication constant, b is the diffusion gradient b –value and D is the ADC value. The resulting ADC values were verified with an in-house (Matlab) C language-fitting program.
The T1 values were calculated for each region based upon the equation:
where Y is the image signal intensity after t, A is the absolute bias, C is the image signal intensity at t=0s.
The T2 values were calculated for each region based upon the equation:
where Y is the image signal intensity after t, A is the absolute bias, C is the image signal intensity at t=0s.
Periodic Acid Schiff (PAS) Staining
PAS staining is a qualitative histological assay for carbohydrate rich materials such as glycogen and glycosphingolipids. After imaging, mice were perfusion fixed with 4% paraformaldehyde and brains were paraffin embedded and stained using a PAS staining kit (Sigma, St. Louis, USA) or sent to the Baylor College of Medicine Histology core.
RESULTS
Open Field Locomotion
The total number of vertical movements (Figure 1A), central distance/total distance ratio (Figure 1B) and total distance (Figure 1C) were recorded with the behavioral software, Versamax. The hexb−/− at the age of 6–7 weeks did not significantly differ in any of the assessments compared with age-matched wildtype mice. However, at the age of 3-months, the hexb−/− exhibited a decrease in the total distance as compared to the wildtype mice (p < .002) [Figure 1C]. The number of vertical movements at the age of 3 months exhibited a trend towards being lower than wildtype but this did not reach significance (p < 0.631) [Figure 1A]. The central distance/total distance ratio also did not differ at the 3-month age point (p < 0.370) [Figure 1B].
Figure 1. Open Field data.
A. Quantification of total number of vertical movements. The number of vertical movements at the age of 3 months exhibited a trend towards being lower than wildtype but this did not reach significance.
B. Quantification of central distance/total distance ratio. The ratio did not differ at the 3-month age point.
C. Quantification of total distance. At the age of 3 months, the hexb−/− exhibited a decrease in the total distance as compared to the wildtype mice. *p<0.05.
Rota-Rod
The same mice tested in the Open Field Locomotion were assessed in the Rota-Rod test. Consistent with the work of Sango et al (13), we observed that the Hexb−/− mouse’s ability to remain on the rod was indistinguishable from wildtype at 6–7 weeks of age (p < .610) and declined at 3 months of age as compared to wildtype (p < .0001) [Figure 2]. Furthermore, the 3-month old wildtype mice improved throughout the trials whereas the hexb−/− mice exhibited a relatively flat learning curve [Figure 2].
Figure 2. Rota-Rod Data with quantification of Rota-Rod duration.
The Hexb−/− mouse’s ability to remain on the rod was indistinguishable from wildtype at 6–7 weeks of age and declined at 3 months of age as compared to wildtype. Furthermore, the 3-month old wildtype mice improved throughout the trials whereas the hexb−/− mice exhibited a relatively flat learning curve. ***p<0.0001.
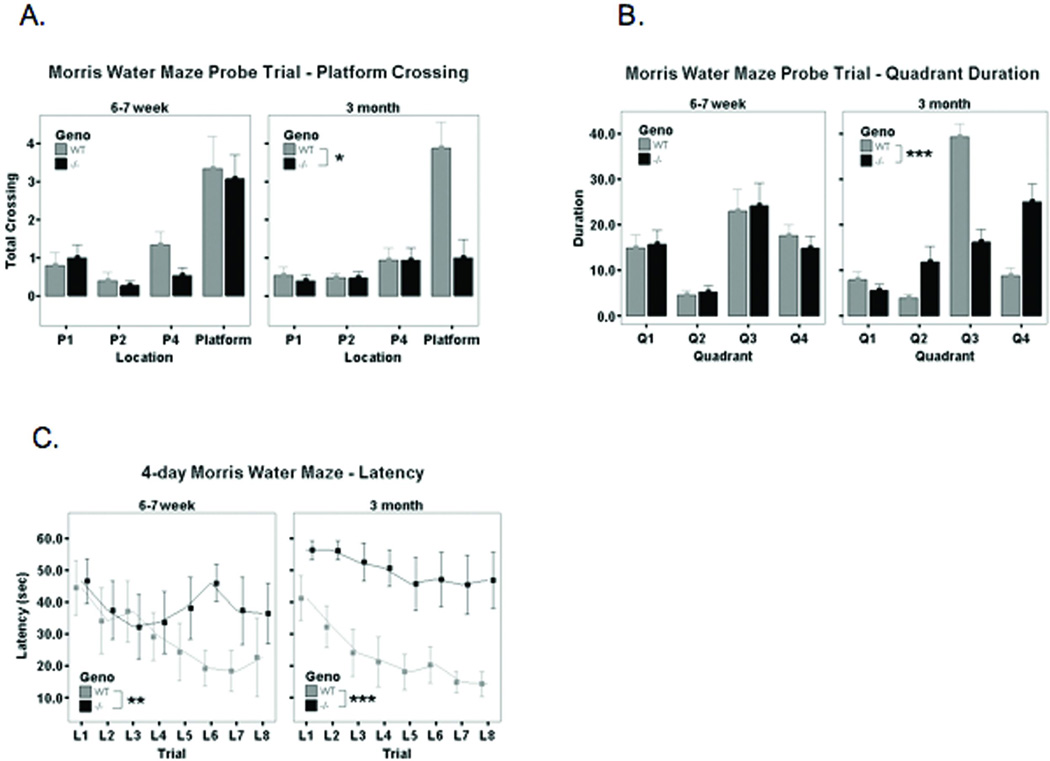
Hidden Platform Morris Water Maze
The time it took for the mouse to reach the platform 4 days after training (4 day latency) was assessed [Figure 3A]. The 6–7 week old mice took longer to reach the target quadrant (4-day latency) compared to controls (p < .003) [Figure 3A]. The total number of platform crossings were recorded [Figure 3B] as well as the quadrant duration in the actual platform location (P3) and its mirroring sites: P1, P2 and P4 in the other three quadrants [Figure 3C]. The 6–7 week old mice were indistinguishable from wildtype controls in the number of platform crossings in a probe trial as well as in the quadrant duration [Figures 3 B, C].
Figure 3. 4-day Morris Water Maze data.
A. Quantification of platform crossing. The 6–7 week old mice were indistinguishable from wildtype controls in the number of platform crossings in a probe trial. In 3-month age group, the hexb −/− exhibited a significant decrease in number of platform crossing as compared to wildtype mice. P*<0.05
B. Quantification of quadrant duration. The 6–7 week old mice were indistinguishable from wildtype controls in the number of platform crossings in a probe trial. In 3-month age group, the duration for staying in Quad3(where the platform was located) for Hexb −/− significantly decreased as compared to the wildtype mice. ***p<0.0001.
C. Quantification of latency. In both age groups, the Hexb −/− mice took longer to reach the target quadrant compared to the wildtype mice. **p< .001. ***p<0.0001.
The 3-month old mice exhibited deficits in the 4-day latency in the MWM test, number of platform crossings and the quadrant duration as compared to controls (p < .001, p < .05 and p < .0001 respectively) [Figure 3 A–C].
During the Morris water Maze test, we also measured the swimming velocity of the different groups and did not observe a significant difference between the 6-–7 week old hexb−/− mice and wildtype [data not shown]. However, the 3-month old mice did exhibit a decreased swimming velocity as compared to wildytpe controls (p < .004) [data not shown].
Visible Platform Morris Water Maze
A total of 20 knockout (6–7 week old, N = 10; 3-month old, N = 10) and 19 wildtype (3-month old, N = 9; 6–7 week old, N = 10) mice were assessed in the Visible Platform Morris Water Maze. The latency for each mouse to reach the platform was recorded for 2 days with 8 records for each day. These 16 records were then combined into 4 trial groups. The data indicate that the 6–7 week old hexb−/− mice perform similarly in this assay as compared to the wildtype mice [Figure 4]. However, the 3-month old hexb−/− exhibited decreased quadrant duration as compared to wildtype mice (p < .001) [Figure 4].
Figure 4. Visible-platform Morris Ware Maze data with quantification of quadrant duration.
The 6–7 week old hexb−/− mice perform similarly in this assay as compared to the wildtype mice, however, the 3-month old hexb−/− exhibited decreased quadrant duration as compared to wildtype mice. **p < .001.
Conditioned Fear
A total of 30 knockout (6–7 week old, N = 15; 3-month old, N = 15;) and 30 wildtype (6–7 week old, N = 15; 3-month old, N = 15) mice were utilized in the Conditioned Fear behavioral assay. These data indicate that the 6–7 week old hexb−/− mice performed similarly to the wildtype controls in both the contextual and cued fear conditioning assays [Figure 5 A–B]. Additionally, the 3- month old hexb−/− mice were indistinguishable from the wildtype controls in the contextual and cued fear conditioning assays [Figure 5 A–B].
Figure 5. Conditioned Fear data.
A. Quantification of freezing behavior in contextual test. In both age groups, Hexb −/− mice were indistinguishable from the wildtype mice in contextual test.
B. Quantification of freezing behavior in cued test. In both age groups, Hexb −/− mice were indistinguishable from the wildtype mice in cued test.
Elevated-Plus Maze
20 knockout mice (6–7 week old, N = 10; 3-month old, N = 10) and 19 wildtype (6–7 week old, N = 10; 3-month old, N = 9) were utilized for the elevated-Plus Maze test. The following parameters were evaluated: open arm entry frequency and total entry. The 6–7 week old hexb−/− mice did not exhibit significant changes in any of these parameters compared to wildtype [Figure 6 A–B]. However, the 3-month old hexb−/− mice exhibited deficits in the total entry (p < .010) with a trend towards a decrease in the Open Arm Entry Frequency [Figure 6 AB].
Figure 6. Elevated-Plus Maze data.
A. Quantification of open arm entry frequency. In both age groups, the hexb −/− mice did not exhibit significant changes in entry frequency as compared to wildtype mice.
B. Quantification of total entry. The 3-month old hexb −/− mice exhibited deficits in the total entry as compated to 3-month old wildtype mice. p*<0.0
Light-Dark Exploration
20 hexb−/− mice (6–7 week old, N = 10; 3-month old, N = 10) and 19 wildtype (6–7 week old, N = 10; 3-month old, N = 9) were utilized for the Light-Dark Exploration test. The number of entries into the dark and the total light duration were recorded and analyzed. The 6–7 week old hexb−/− mice were indistinguishable from the wildtype controls [Figure 7 A–B]. However, the 3-month old hexb−/− exhibited deficits in the dark frequency (p < .023) and total light total duration (p < .010) [Figure 7 A–B].
Figure 7. Light-Dark Exploration.
A. Quantification of dark frequency. The 6–7 week old hexb −/− mice were indistinguishable from the wildtype mice, however, the 3-month old hexb −/− mice exhibited deficits in dark frequency. *p<0.05.
B. Quantification of light total duration. The 6–7 week old hexb −/− mice were indistinguishable from the wildtype mice, however, the 3-month old hexb −/− mice exhibited deficits in total duration. *p<0.05.
Collectively, the behavioral data indicate that at 6–7 weeks of age, the hexb−/− mice behave similarly to wildtype mice and just prior to disease symptom onset, their performance on many of the behavioral tests deteriorates.
MRI DATA
Three slices were taken throughout the brain for the MRI assessment (Figure 8A). Mice from both age groups were imaged and regions of interest (ROIs) were assigned to the cortex, hippocampus, thalamus and striatum [Figure 8B–D]. We observed a significant decrease in the ADC in the hippocampus and striatum as well as parts of the thalamus, and cortex in the 6–7 week old hexb−/− compared to controls [Figure 9A, Table 1]. In the 3-month old hexb−/− mice, we observed similar results of significant decreases in the ADC in the thalamus and hippocampus and also in parts of the cortex and striatum as compared to controls. [Figure 9B].]. However, there also appeared to be some degree of heterogeneity in the observed ADC changes. The central slice (slice 2) did not exhibit significant changes in the ADC in the cortical and thalamic regions in both groups and the cortex in slice 3 of the 3-month old groups [Figure 9B, Table 1]. Taken together, these data indicate that the ADC in the hippocampus, striatum and parts of the cortex and thalamus decreases prior to behavioral deficits and symptom onset. In the hexb−/− mice
Figure 8. Images Depicting slice and ROI placement for the T1, T2 and DWI data analysis.
A. Sagittal image indicating the placement of the three axial slices. The first slice was aligned with the pituitary gland. There was a 1mm gap between each slice.
B. Slice 1 that contained 6 ROIS: cL and cR for cortex, hL and hR for hippocampus, tL and tR for thalamus. L = Left and R = Right.
C. Slice 2 that contained 4 ROIS: cL and cR for cortex, tL and tR for thalamus.
D. Slice 3 that contained 4 ROIS: cL and cR for cortex, sL and sR for striatum.
Figure 9. ADC Values in the 6–7 week old and 3 month old Hexb−/− mice and Controls.
A. Quantification of the apparent diffusion coefficient (ADC) values in 6–7 week old Hexb−/− mice and controls. Significant decreases in the ADC in the cortex (Slice (S)1 and S3), the thalamus (S1), striatum (S3) and hippocampus (S1) in the 6–7 week old were observed. *p<0.05. **p<0.01, ***p<0.0001. N=5 for controls, N=6 for hexb−/−.
B. Quantification of the apparent diffusion coefficient (ADC) values in 3-month old Hexb−/− mice and controls. Significant decrease in the ADC in the cortex (S1), the thalamus (S1), striatum (S3) and hippocampus (S1) in the 3 month old is observed. *p<0.05. **p<0.01, ***p<0.0001. N=5 for controls, N=7 for hexb−/−.
Table 1.
In this table, the percent decreases in ADC are presented in the 6–7 week old mice and 3 month old mice for all ROIs assessed. The percent decrease was determined by subtracting the hexb−/− ADC value from the wildtype ADC value and normalizing to the wtildtype value and multiplying by 100.
| % ADC Decrease |
cS1 | tS1 | hS1 | cS2 | tS2 | cS3 | sS3 |
|---|---|---|---|---|---|---|---|
| 6–7 week | 10.8 | 18.6 | 11.4 | 6.3 | 6.0 | 10.0 | 11.2 |
| 3 month | 18.5 | 16.3 | 18.0 | 4.4 | 5.3 | 12.8 | 17.1 |
We also measured the T1 and T2s within the same ROIs. Similar to the clinical reports in human patients, we did not observe any significant differences in the 6–7 week old group [Figures 10 A–B] as well as the 3-month-old group [Figure 11 A–B] compared to controls.
Figure 10. T1 and T2 Values in 6–7 Week old Hexb−/− and Control Mice.
A. Quantification of T1 values in 6–7 week old Hexb−/− Mice and Controls. No significant difference between the two groups is observed. N=5 for WT, N=6 for the hexb−/−.
B. Quantification of T2 values in 6–7 week old Hexb−/− Mice and Contorls. No significant difference between the two groups is observed. N=5 for WT, N=6 for the hexb−/−.
Figure 11. T1 and T2 Values in 3 month old Hexb−/− and Control Mice.
A. Quantification of T1 values in 3-month old Hexb Mice−/− Mice and Controls. No significant difference between the two groups is observed. N=5 for WT, N=7 for the hexb−/−.
B. Quantification of T2 values in 3-month old Hexb−/− Mice and Controls. No significant difference between the two groups is observed. N=5 for WT, N=7 for the hexb −/−.
Histological Evaluations
Mice were perfusion fixed with 4% paraformaldehyde and brains were paraffin embedded and stained utilizing a Periodic Acid Schiff (PAS) staining kit (Sigma, St. Louis, USA). PAS staining is a qualitative stain that not only allows for the visualization of cellular morphology but also stains for structures containing high levels of carbohydrate macromolecules such as ganglioside accumulation observed in Sandhoff’s disease. The data demonstrates minor cellular swelling in the 6–7 week old hexb−/− mice as well as some PAS positive cells [Figure 12 A–C]. The 3-month old hexb−/− mice exhibit an accumulation of multiple PAS positive regions as well as many swollen cells and axons as compared to controls [Figure 12 D–F] These data indicate abnormalities on the cellular level prior to behavioral and the onset of disease symptoms.
Figure 12. Representative images of PAS staining of 6–7-week old Hexb−/− mouse and control cortex (A–C) and 3-month old Hexb−/− mouse and control cortex(D–F).
A. 6–7 week old Hexb control. B. 6–7 week old Hexb −/− demonstrates minor cellular swelling (black arrows) as well as some PAS positive regions(green arrows) as compared to control. C. Close up of B. D. 3 month old Hexb control. E. 3 month old Hexb −/− exhibit an accumulation of multiple PAS positive regions as well as many swollen cells and axons as compared to control. F. Close up of E.
DISCUSSION
Previous reports have indicated that standard spin lattice, T1, and spin-spin, T2, weighted MRI imaging can detect abnormalities in symptomatic Sandhoff’s disease patients characteristic of hypointensities in the thalamus and enhanced signal in white matter regions (14–18). However, neither of these imaging modalities could readily detect differences in presymptomatic Sandhoff’s disease patients (15). Our goal was to determine if the MRI imaging modality, DWI, could be utilized to detect changes in the ADC in the pre-symptomatic mouse model of Sandhoff’s disease. The motivation for the work is one of the results of GM2 accumulation is cell swelling and lysosomal distension: two features that could potentially significantly impair water diffusion in brain tissue.
First, we performed a comprehensive behavioral characterization of the hexb−/− mouse model of Sandhoff’s disease in the pre-symptomatic age range (6–7 weeks) and also just prior to the symptomatic phase (3-months). Whereas other groups have reported rotarod and open field studies on the hexb−/− mouse, this report is the first inclusive behavioral characterization of the hexb−/− mouse model of Sandhoff’s disease. Consistent with previous reports, we observed no significant perturbations in the rotarod and open field behavioral assays at the 6–7 week time point (13). Also consistent with previous reports, at the 3-month time point, we observed significant differences in the performance in the rotatod and open field assays(13).
We observed similar responses in the Morris water maze, elevated platform maze and light dark exploration assays. The hexb−/− mice appeared indistinguishable from wildtype in most of the behavioral assays at the 6–7 week time point and exhibited significant impairments at the 3-month time point. Neither age group, however, exhibited perturbations in the fear-conditioning paradigm.
Similar to what has been reported in humans, we did not observe significant changes in T1 and T2 measurements in the hexb−/− mice at either pre-symptomatic age point (6–7 weeks and 3 months) in all of the brain regions assessed.
We also collected DWI MRI data at both of these age ranges and calculated the ADC. We observed significant decreases in the ADC in some but not all brain regions of the hexb−/− mice at 6–7 weeks of age and also at the 3-month time point compared to wildtype….
Interestingly, the ADC measured in the cortex and thalamic regions appeared to be differentially affected depending upon the location. The ADC in the cortex evaluated in slices 1 and 3 in the 6–7 week group were significantly perturbed. However, the ADC in the cortex located in slice 2 did not exhibit a significant difference. A similar case was observed with the thalamus in slices 1 and 2. Furthermore, the 3 month aged group also did not exhibit any significant differences in the ADC in the cortex and thalamus in slice 2 and in the cortex in slice 3. However, there were significant changes in the ADC in all other brain regions.
To validate the imaging data, we perfusion fixed the animals and performed PAS staining to confirm the presence of GM2 accumulation and associated pathology. We observed some GM2 accumulation and cell swelling at the 6–7 week time point that worsened at the 3-month age point in the cortex located above the hippocampus. Although we did observe PAS positive cells throughout the brain (data not shown), it has been reported that the degree of lysosomal swelling and GM2 accumulation qualitatively differs throughout the brain [12]. Such a possibility could account for the decreases in the ADC we observed in many but not all regions of the brain.
Taken together, our data indicate that DWI and the calculation of the ADC are a sensitive means to detect pre-symptomatic Sandhoff’s disease in the hexb−/− mouse However, assessments in this mouse model must be made throughout the entire brain as the degree by which the ADC decreases appears to be heterogeneous.
It remains to be determined if this strategy is also applicable to human patients afflicted with Sandhoff’s disease as at times mouse models do not perfectly mimic the corresponding human disease. For example, Tay-Sachs disease is very similar to Sandhoff’s disease with the exception that a different subunit of the same enzyme is affected (12,13,19–21). However, whereas the Sandhoff’s hexb−/− mouse model exhibits striking similarities to the human disease, the mouse model of Tay-Sachs disease (hexa−/−) is relatively unaffected due to an additional catabolic pathway present in rodent but not in humans(12,13,19–21).
In summary, we present an imaging strategy that potentially could be useful in the clinic towards assessing the severity of pre-symptomatic Sandhoff’s disease. Furthermore, further studies are warranted to assess if DWI/ADC measurements are sensitive in assessing early improvements in response to therapeutic intervention.
Acknowledgements
The authors gratefully acknowledge Ms. Taneasha Washington for her assistance with the mouse care and genotyping and Dr. Corinne Spencer for her advice on the behavioral assays. Additionally, the authors are grateful to the following funding sources for supporting this work: Alzheimer’s Association (RGP); NIH/NIA 1R01AG029977-01A1 (RGP). NIH grants NS034007 (EK) and NS047384 (EK) and Baylor College of Medicine’s IDDRC Neurobehavioral and Administrative Cores. Support was also provided by the Baylor College of Medicine Diabetes and Endocrinology Research Center (DERC), NIH/NIDDK P30DK079638 (Lawrence Chan).
REFERENCES
- 1.Brooksbank BW, McGovern J. Gangliosides in the brain in adult Down's syndrome and Alzheimer's disease. Mol Chem Neuropathol. 1989;11(3):143–156. doi: 10.1007/BF03160048. [DOI] [PubMed] [Google Scholar]
- 2.Ariga T, Kobayashi K, Hasegawa A, Kiso M, Ishida H, Miyatake T. Characterization of high-affinity binding between gangliosides and amyloid beta-protein. Arch Biochem Biophys. 2001;388(2):225–230. doi: 10.1006/abbi.2001.2304. [DOI] [PubMed] [Google Scholar]
- 3.Leden RaY RK. New strategies for detection and resolution of minor gangliosides as applied to brain fuco-gangliosides. Methods Enzymol. 1982;83:139–189. [PubMed] [Google Scholar]
- 4.Svennerholm L. Biological Significance of Gangliosides” Cellular and Pathological Aspects of Glycoconjugate Metabolism. INSERM. 1984;126:21–44. [Google Scholar]
- 5.van Echten G, Sandhoff K. Modulation of ganglioside biosynthesis in primary cultured neurons. J Neurochem. 1989;52(1):207–214. doi: 10.1111/j.1471-4159.1989.tb10918.x. [DOI] [PubMed] [Google Scholar]
- 6.Kojima N, Hakomori S. Cell adhesion, spreading, and motility of GM3-expressing cells based on glycolipid-glycolipid interaction. J Biol Chem. 1991;266(26):17552–17558. [PubMed] [Google Scholar]
- 7.Karlsson KA. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- 8.Phillips ML, Nudelman E, Gaeta FC, Perez M, Singhal AK, Hakomori S, Paulson JC. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- 9.Jeyakumar M, Butters TD, Dwek RA, Platt FM. Glycosphingolipid lysosomal storage diseases: therapy and pathogenesis. Neuropathol Appl Neurobiol. 2002;28(5):343–357. doi: 10.1046/j.1365-2990.2002.00422.x. [DOI] [PubMed] [Google Scholar]
- 10.Jeyakumar M, Dwek RA, Butters TD, Platt FM. Storage solutions: treating lysosomal disorders of the brain. Nat Rev Neurosci. 2005;6(9):713–725. doi: 10.1038/nrn1725. [DOI] [PubMed] [Google Scholar]
- 11.Neufeld EF. Natural history and inherited disorders of a lysosomal enzyme, beta-hexosaminidase. J Biol Chem. 1989;264(19):10927–10930. [PubMed] [Google Scholar]
- 12.Phaneuf D, Wakamatsu N, Huang JQ, Borowski A, Peterson AC, Fortunato SR, Ritter G, Igdoura SA, Morales CR, Benoit G, Akerman BR, Leclerc D, Hanai N, Marth JD, Trasler JM, Gravel RA. Dramatically different phenotypes in mouse models of human Tay-Sachs and Sandhoff diseases. Hum Mol Genet. 1996;5(1):1–14. doi: 10.1093/hmg/5.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Sango K, Yamanaka S, Hoffmann A, Okuda Y, Grinberg A, Westphal H, McDonald MP, Crawley JN, Sandhoff K, Suzuki K, Proia RL. Mouse models of Tay-Sachs and Sandhoff diseases differ in neurologic phenotype and ganglioside metabolism. Nat Genet. 1995;11(2):170–176. doi: 10.1038/ng1095-170. [DOI] [PubMed] [Google Scholar]
- 14.Hittmair K, Wimberger D, Bernert G, Mallek R, Schindler EG. MRI in a case of Sandhoff's disease. Neuroradiology. 1996;38 Suppl 1:S178–S180. doi: 10.1007/BF02278152. [DOI] [PubMed] [Google Scholar]
- 15.Koelfen W, Freund M, Jaschke W, Koenig S, Schultze C. GM-2 gangliosidosis (Sandhoff's disease): two year follow-up by MRI. Neuroradiology. 1994;36(2):152–154. doi: 10.1007/BF00588086. [DOI] [PubMed] [Google Scholar]
- 16.Yun YM, Lee SN. A case refort of Sandhoff disease. Korean J Ophthalmol. 2005;19(1):68–72. doi: 10.3341/kjo.2005.19.1.68. [DOI] [PubMed] [Google Scholar]
- 17.Yuksel A, Yalcinkaya C, Islak C, Gunduz E, Seven M. Neuroimaging findings of four patients with Sandhoff disease. Pediatr Neurol. 1999;21(2):562–565. doi: 10.1016/s0887-8994(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 18.Caliskan M, Ozmen M, Beck M, Apak S. Thalamic hyperdensity--is it a diagnostic marker for Sandhoff disease? Brain Dev. 1993;15(5):387–388. doi: 10.1016/0387-7604(93)90128-u. [DOI] [PubMed] [Google Scholar]
- 19.Taniike M, Yamanaka S, Proia RL, Langaman C, Bone-Turrentine T, Suzuki K. Neuropathology of mice with targeted disruption of Hexa gene, a model of Tay-Sachs disease. Acta Neuropathol. 1995;89(4):296–304. doi: 10.1007/BF00309622. [DOI] [PubMed] [Google Scholar]
- 20.Yamanaka S, Johnson MD, Grinberg A, Westphal H, Crawley JN, Taniike M, Suzuki K, Proia RL. Targeted disruption of the Hexa gene results in mice with biochemical and pathologic features of Tay-Sachs disease. Proc Natl Acad Sci U S A. 1994;91(21):9975–9979. doi: 10.1073/pnas.91.21.9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamanaka S, Johnson ON, Norflus F, Boles DJ, Proia RL. Structure and expression of the mouse beta-hexosaminidase genes, Hexa and Hexb. Genomics. 1994;21(3):588–596. doi: 10.1006/geno.1994.1318. [DOI] [PubMed] [Google Scholar]