Abstract
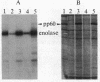
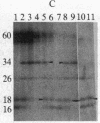
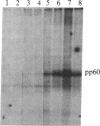
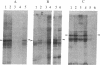
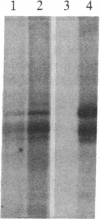
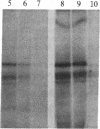
We have detected a significant increase in the levels of pp60c-src kinase activity associated with the differentiation of myeloid cell lines HL-60 and U-937. The induction of pp60c-src kinase activity becomes apparent approximately 14 hr after the addition of phorbol 12-myristate 13-acetate and increases 20-fold by 72 hr. The enhanced kinase activity can be accounted for by elevated levels of c-src protein in the differentiated cells. When nonleukemic bone marrow cells were examined, myeloid progenitor cells exhibited a low level of pp60c-src kinase activity. As these cells are allowed to differentiate in culture, the resulting adherent monocytes are as high in pp60c-src kinase activity as HL-60 cells induced to differentiate into monocytes. A strong correlation is found between the levels of pp60c-src kinase activity and the degree of monocytic differentiation of the cells from patients with acute myeloid leukemia. Our findings suggest that the activation of pp60c-src kinase activity is a normal physiological event associated with myeloid differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemà S., Casalbore P., Agostini E., Tatò F. Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature. 1985 Aug 8;316(6028):557–559. doi: 10.1038/316557a0. [DOI] [PubMed] [Google Scholar]
- Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976 Aug;33(4):451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Cotton P. C., Queral A. E., Barrett J. N., Nonner D., Keane R. W. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985 Aug 8;316(6028):554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Coussens P. M., Cooper J. A., Hunter T., Shalloway D. Restriction of the in vitro and in vivo tyrosine protein kinase activities of pp60c-src relative to pp60v-src. Mol Cell Biol. 1985 Oct;5(10):2753–2763. doi: 10.1128/mcb.5.10.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durban E. M., Boettiger D. Differential effects of transforming avian RNA tumor viruses on avian macrophages. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3600–3604. doi: 10.1073/pnas.78.6.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fults D. W., Towle A. C., Lauder J. M., Maness P. F. pp60c-src in the developing cerebellum. Mol Cell Biol. 1985 Jan;5(1):27–32. doi: 10.1128/mcb.5.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Woodgett J. R., Cooper J. A., Buss J. E., Shalloway D., Hunter T. Protein kinase C phosphorylates pp60src at a novel site. Cell. 1985 Oct;42(3):849–857. doi: 10.1016/0092-8674(85)90281-8. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Ritz J., Nadler L. M., Schlossman S. F. Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J Clin Invest. 1981 Oct;68(4):932–941. doi: 10.1172/JCI110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. D., Sabbath K. D., Herrmann F., Larcom P., Nichols K., Kornacki M., Levine H., Cannistra S. A. Differential expression of HLA-DR antigens in subsets of human CFU-GM. Blood. 1985 Oct;66(4):788–795. [PubMed] [Google Scholar]
- Harris P., Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukoc Biol. 1985 Apr;37(4):407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Iba H., Cross F. R., Garber E. A., Hanafusa H. Low level of cellular protein phosphorylation by nontransforming overproduced p60c-src. Mol Cell Biol. 1985 May;5(5):1058–1066. doi: 10.1128/mcb.5.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Raptis L., Garcea R. L., Pallas D., Roberts T. M., Cantley L. Phosphatidylinositol metabolism and polyoma-mediated transformation. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3624–3628. doi: 10.1073/pnas.83.11.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kürzinger K., Springer T. A. Purification and structural characterization of LFA-1, a lymphocyte function-associated antigen, and Mac-1, a related macrophage differentiation antigen associated with the type three complement receptor. J Biol Chem. 1982 Oct 25;257(20):12412–12418. [PubMed] [Google Scholar]
- Lipsich L., Brugge J. S., Boettiger D. Expression of the Rous sarcoma virus src gene in avian macrophages fails to elicit transformed cell phenotype. Mol Cell Biol. 1984 Jul;4(7):1420–1424. doi: 10.1128/mcb.4.7.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Mitchell R. L., Zokas L., Schreiber R. D., Verma I. M. Rapid induction of the expression of proto-oncogene fos during human monocytic differentiation. Cell. 1985 Jan;40(1):209–217. doi: 10.1016/0092-8674(85)90324-1. [DOI] [PubMed] [Google Scholar]
- Müller R., Curran T., Müller D., Guilbert L. Induction of c-fos during myelomonocytic differentiation and macrophage proliferation. Nature. 1985 Apr 11;314(6011):546–548. doi: 10.1038/314546a0. [DOI] [PubMed] [Google Scholar]
- Nienhuis A. W., Bunn H. F., Turner P. H., Gopal T. V., Nash W. G., O'Brien S. J., Sherr C. J. Expression of the human c-fms proto-oncogene in hematopoietic cells and its deletion in the 5q- syndrome. Cell. 1985 Sep;42(2):421–428. doi: 10.1016/0092-8674(85)90099-6. [DOI] [PubMed] [Google Scholar]
- Parsons S. J., McCarley D. J., Ely C. M., Benjamin D. C., Parsons J. T. Monoclonal antibodies to Rous sarcoma virus pp60src react with enzymatically active cellular pp60src of avian and mammalian origin. J Virol. 1984 Aug;51(2):272–282. doi: 10.1128/jvi.51.2.272-282.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston R., Bishop J. M. The product of the protooncogene c-src is modified during the cellular response to platelet-derived growth factor. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7845–7849. doi: 10.1073/pnas.82.23.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariban E., Mitchell T., Kufe D. Expression of the c-fms proto-oncogene during human monocytic differentiation. Nature. 1985 Jul 4;316(6023):64–66. doi: 10.1038/316064a0. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Schlossman S. F. Analysis of antigenic determinants on human monocytes and macrophages. Blood. 1982 Apr;59(4):775–786. [PubMed] [Google Scholar]
- Tushinski R. J., Oliver I. T., Guilbert L. J., Tynan P. W., Warner J. R., Stanley E. R. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982 Jan;28(1):71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- Yonemoto W., Jarvis-Morar M., Brugge J. S., Bolen J. B., Israel M. A. Tyrosine phosphorylation within the amino-terminal domain of pp60c-src molecules associated with polyoma virus middle-sized tumor antigen. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4568–4572. doi: 10.1073/pnas.82.14.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]