Abstract
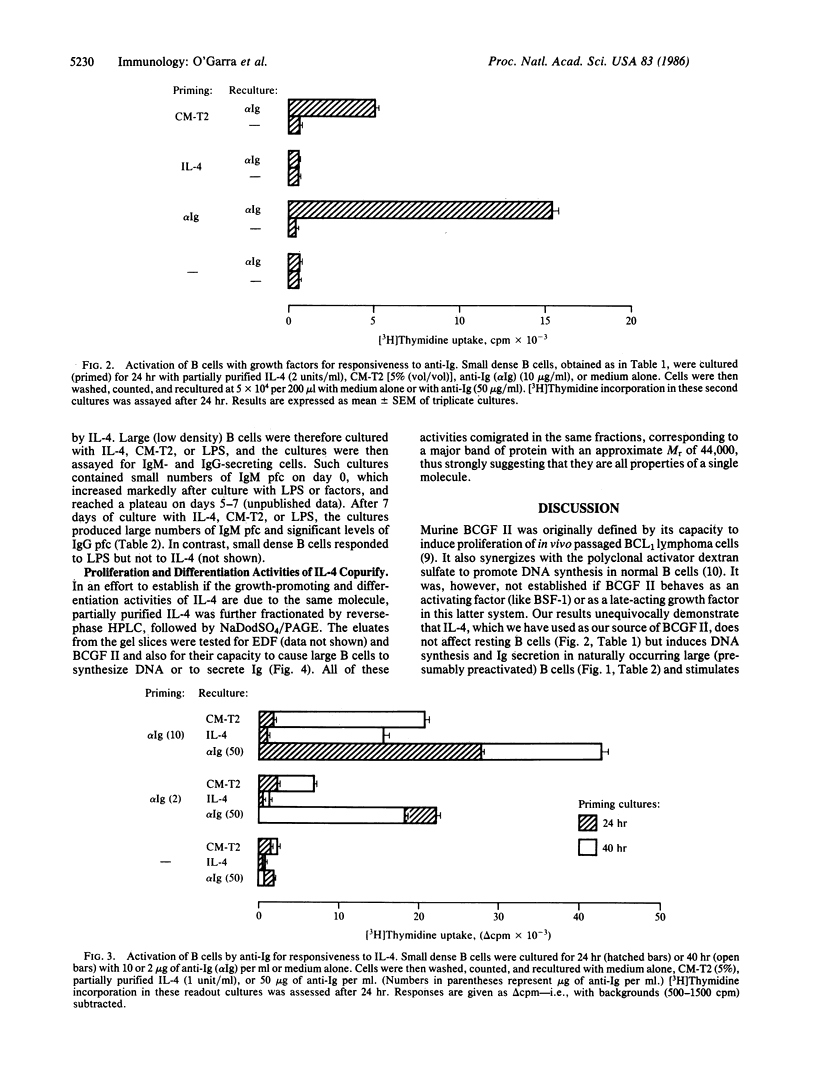
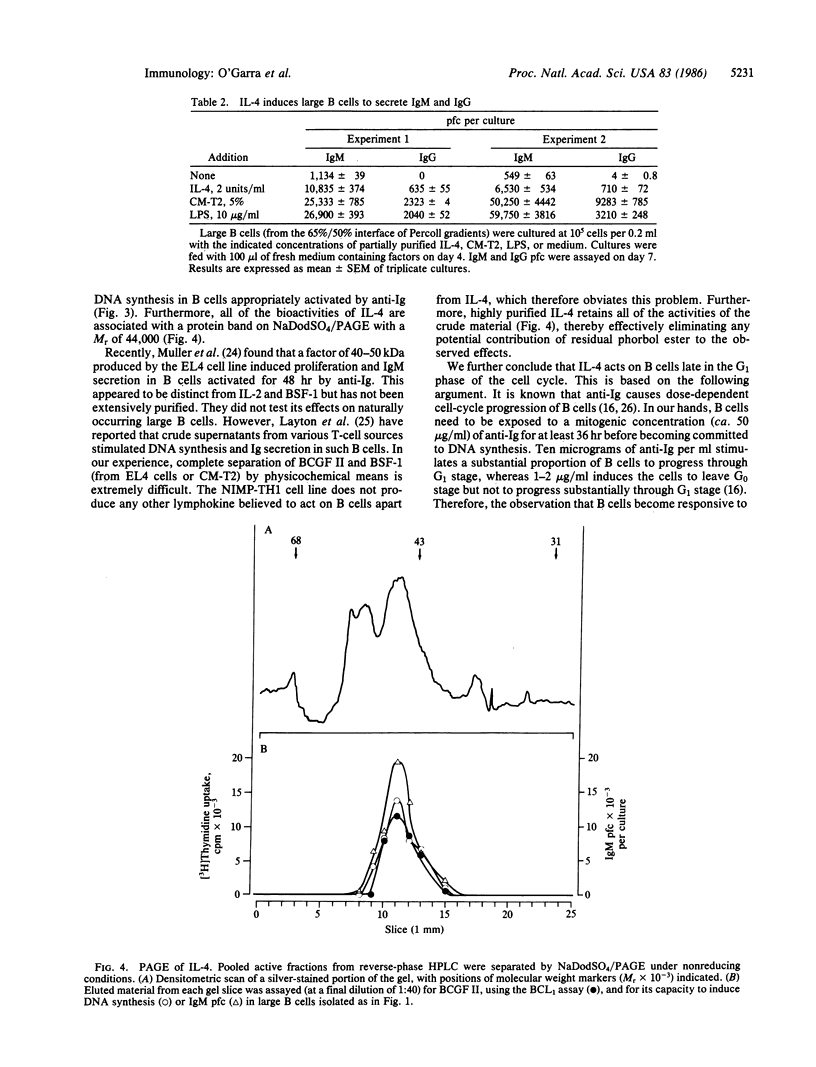
Recently we described a murine T-cell hybrid that produces activities that promote the differentiation of eosinophils (eosinophil differentiation factor) and cause proliferation of the BCL1 B-cell lymphoma (B-cell growth factor II activity). Both activities appear to be associated with the same molecule, which has therefore been termed interleukin 4. The hybrid does not produce any other known lymphokines. We now find that purified interleukin 4 has no effects on small resting B cells but induces naturally occurring large B cells (which have presumably been preactivated in vivo) to synthesize DNA and to secrete IgM and low levels of IgG. B cells activated by anti-Ig antibodies apparently only become responsive to the factor once they have reached late G1 stage. All bioactivities of interleukin 4 are associated with a protein of Mr 44,000 (by NaDodSO4/PAGE). Therefore these results demonstrate that this lymphokine alone is sufficient to induce clonal expansion and maturation of activated B cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter J., Dresser D. W. Fetal stimulation of maternal immunoglobulin production in mice. J Reprod Fertil. 1985 Jul;74(2):535–542. doi: 10.1530/jrf.0.0740535. [DOI] [PubMed] [Google Scholar]
- DeFranco A. L., Raveche E. S., Paul W. E. Separate control of B lymphocyte early activation and proliferation in response to anti-IgM antibodies. J Immunol. 1985 Jul;135(1):87–94. [PubMed] [Google Scholar]
- Dresser D. W., Popham A. M. Rheumatoid factors in mice: plaque assay for homophile and heterophile rheumatoid factors. Immunology. 1980 Nov;41(3):569–577. [PMC free article] [PubMed] [Google Scholar]
- Harada N., Kikuchi Y., Tominaga A., Takaki S., Takatsu K. BCGFII activity on activated B cells of a purified murine T cell-replacing factor (TRF) from a T cell hybridoma (B151K12). J Immunol. 1985 Jun;134(6):3944–3951. [PubMed] [Google Scholar]
- Hawrylowicz C. M., Keeler K. D., Klaus G. G. Activation and proliferation signals in mouse B cells. I. A comparison of the capacity of anti-Ig antibodies or phorbol myristic acetate to activate B cells from CBA/N or normal mice into G1. Eur J Immunol. 1984 Mar;14(3):244–250. doi: 10.1002/eji.1830140308. [DOI] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Paul W. E. Regulation of B-cell growth and differentiation by soluble factors. Annu Rev Immunol. 1983;1:307–333. doi: 10.1146/annurev.iy.01.040183.001515. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Factors affecting B-cell growth and differentiation. Annu Rev Immunol. 1985;3:133–157. doi: 10.1146/annurev.iy.03.040185.001025. [DOI] [PubMed] [Google Scholar]
- Klaus G. G., Hawrylowicz C. M., Carter C. J. Activation and proliferation signals in mouse B cells. VI. Anti-Ig antibodies induce dose-dependent cell cycle progression in B cells. Immunology. 1985 Jul;55(3):411–418. [PMC free article] [PubMed] [Google Scholar]
- Klaus G. G., Hawrylowicz C. M., Holman M., Keeler K. D. Activation and proliferation signals in mouse B cells. III. Intact (IGG) anti-immunoglobulin antibodies activate B cells but inhibit induction of DNA synthesis. Immunology. 1984 Dec;53(4):693–701. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Layton J. E., Krammer P. H., Hamaoka T., Uhr J. W., Vitetta E. S. Small and large B cells respond differently to T cell-derived B cell growth and differentiation factors. J Mol Cell Immunol. 1985;2(3):155–167. [PubMed] [Google Scholar]
- Maizel A. L., Morgan J. W., Mehta S. R., Kouttab N. M., Bator J. M., Sahasrabuddhe C. G. Long-term growth of human B cells and their use in a microassay for B-cell growth factor. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5047–5051. doi: 10.1073/pnas.80.16.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Kasahara T., Oppenheim J. J., Fauci A. S. B cell growth factor and T cell growth factor produced by mitogen-stimulated normal human peripheral blood T lymphocytes are distinct molecules. J Immunol. 1982 Dec;129(6):2486–2489. [PubMed] [Google Scholar]
- Müller W., Kühn R., Goldmann W., Tesch H., Smith F. I., Radbruch A., Rajewsky K. Signal requirements for growth and differentiation of activated murine B lymphocytes. J Immunol. 1985 Aug;135(2):1213–1219. [PubMed] [Google Scholar]
- Noelle R., Krammer P. H., Ohara J., Uhr J. W., Vitetta E. S. Increased expression of Ia antigens on resting B cells: an additional role for B-cell growth factor. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6149–6153. doi: 10.1073/pnas.81.19.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Oliver K., Noelle R. J., Uhr J. W., Krammer P. H., Vitetta E. S. B-cell growth factor (B-cell growth factor I or B-cell-stimulating factor, provisional 1) is a differentiation factor for resting B cells and may not induce cell growth. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2465–2467. doi: 10.1073/pnas.82.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin E. M., Ohara J., Paul W. E. B-cell stimulatory factor 1 activates resting B cells. Proc Natl Acad Sci U S A. 1985 May;82(9):2935–2939. doi: 10.1073/pnas.82.9.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm N. W., Leibson H. J., Zlotnik A., Kappler J., Marrack P., Cambier J. C. Interleukin-induced increase in Ia expression by normal mouse B cells. J Exp Med. 1984 Sep 1;160(3):679–694. doi: 10.1084/jem.160.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., O'Garra A., Warren D. J., Klaus G. G. Eosinophil differentiation factor also has B-cell growth factor activity: proposed name interleukin 4. Proc Natl Acad Sci U S A. 1986 Jan;83(2):437–440. doi: 10.1073/pnas.83.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., Strath M., Warren D. J., O'Garra A., Kirkwood T. B. The production of lymphokines by primary alloreactive T-cell clones: a co-ordinate analysis of 233 clones in seven lymphokine assays. Immunology. 1985 Dec;56(4):575–584. [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., Warren D. J., Strath M. Identification of a lymphokine that stimulates eosinophil differentiation in vitro. Its relationship to interleukin 3, and functional properties of eosinophils produced in cultures. J Exp Med. 1985 Jul 1;162(1):60–74. doi: 10.1084/jem.162.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Dutton R. W. Production of a B cell growth-promoting activity, (DL)BCGF, from a cloned T cell line and its assay on the BCL1 B cell tumor. J Exp Med. 1982 Dec 1;156(6):1821–1834. doi: 10.1084/jem.156.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Howard M., Kappler J., Marrack P., Watson J., Booth R., Wetzel G. D., Dutton R. W. Evidence for two distinct classes of murine B cell growth factors with activities in different functional assays. J Exp Med. 1983 Sep 1;158(3):822–835. doi: 10.1084/jem.158.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren D. J., Sanderson C. J. Production of a T-cell hybrid producing a lymphokine stimulating eosinophil differentiation. Immunology. 1985 Apr;54(4):615–623. [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki K., Nakagawa T., Fukunaga K., Kaieda T., Maruyama S., Kishimoto S., Yamamura Y., Kishimoto T. Characterization of human B cell growth factor (BCGF) from cloned T cells or mitogen-stimulated T cells. J Immunol. 1983 Mar;130(3):1241–1246. [PubMed] [Google Scholar]


