Abstract
Gene therapy is reaching a stage where some clinical benefits have been demonstrated on patients involved in phase I/II clinical trials. However, in many cases, the clinical benefit is hardly measurable and progress in the improvement of gene therapy formulations is hampered by the lack of objective clinical endpoints to measure transgene delivery and to quantitate transgene expression. However, these endpoints rely almost exclusively on the analysis of biopsies by molecular and histopathological methods. These methods provide only a limited picture of the situation. Therefore, there is a need for a technology that would allow precise, spacio-temporal measurement of gene expression on a whole body scale upon administration of the gene delivery vector. In the field of gene therapy, a considerable effort is being invested in the development of noninvasive imaging of gene expression and this review presents the various strategies currently being developed.
INTRODUCTION
Gene therapy can be defined as the introduction of genetic material into cells for a therapeutic purpose. The field itself can be divided into ex vivo gene therapy, where the target cells are taken from a patient, genetically engineered and reinfused into the patient and in vivo gene therapy, where the gene medicine formulation is directly injected into the patient. There are now some reports where clinical efficacy [1, 2, 3, 4, 5] or even complete cure [6] have been demonstrated. However, these successes remain exceptional and the large majority of phase I/II trials have failed to demonstrate any objective clinical efficacy. Therefore, the challenges of the field are to understand the reasons for the inefficacy of the current formulations in order to develop new and more efficient gene delivery vectors. For this purpose, the definition of relevant clinical endpoints is crucial. Currently, these endpoints rely on analysis of biopsies. From these patient samples, the presence and expression of the transgene can be detected by PCR, RT-PCR, or histopathological methods and in some cases enzymatic activity of the transgene can be measured. However, the information that can be gathered from this type of approach is restricted to a few cubic millimeters of biopsy material and, therefore, is only a partial reflection of the real situation in vivo.
Ideally, the monitoring of transgene delivery and expression should cover the whole body, should be noninvasive and could be repeated over time in the same patient to provide information on the location, magnitude, and kinetics of gene expression. The availability of such noninvasive techniques could be pivotal in the rational development of new formulations designed to selectively target particular tissues, organs, or disease sites and, therefore, a significant effort is currently being invested by the gene therapy community to develop in vivo, noninvasive molecular imaging technologies.
METHODS TO DETECT GENE EXPRESSION IN VIVO IN PRECLINICAL MODELS
As in the case of in vitro transfection experiments, detection of gene expression in vivo requires a reporter gene and a technology capable of detecting its activity or presence in a particular tissue and in a noninvasive way. Two types of methodologies that are already used in medicine for other purposes are currently being adapted to usage in gene therapy: nuclear medical methods and magnetic resonance tomography. More recently, a technology exploiting the fact that bioluminescence can travel through tissues and be detected by very sensitive cameras has been developed.
The general principle is that upon expression of the reporter gene the biodistribution of a tracer molecule is altered, leading to its local concentration at the site of reporter gene expression. Three types of reporter genes are currently being considered and developed: enzymes, receptors, and transport proteins (Figure 1). A general overview of the different reporter genes (proven or potential) is presented in Table 1.
Figure 1.
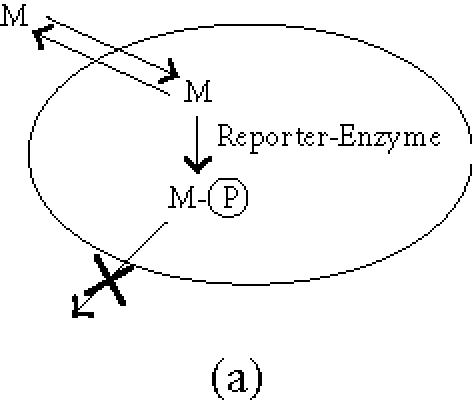
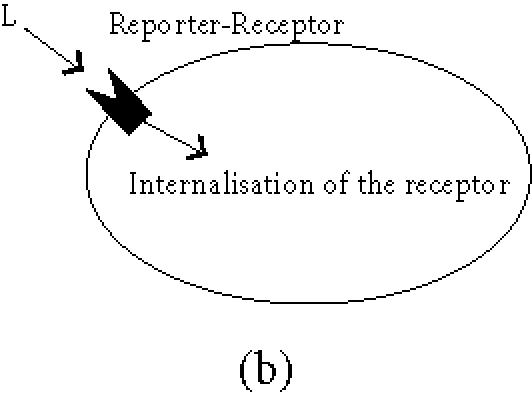
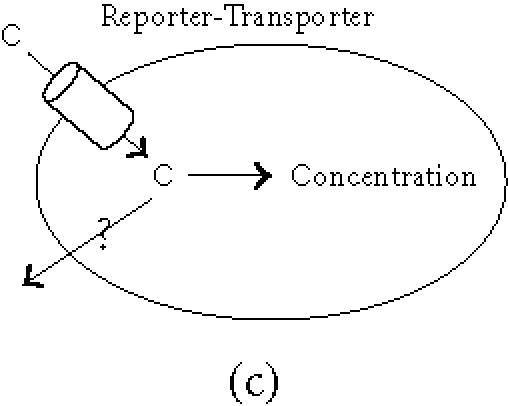
General principles of biological imaging. (a) Enzymes as reporter genes; upon expression of the reporter gene, the imaging marker (M) that can freely cross the plasmic membrane is metabolised within the cell (the most commonly used enzymatic reaction is a phosphorylation). As a result, the phosphorylated marker becomes incapable of recrossing the plasmic membrane and is trapped within the cell. (b) Receptors as reporter genes; upon expression of the reporter gene, the imaging ligand (L) binds to its receptor, resulting in the accumulation of the detectable ligand in the transduced tissue. This signal can be amplified when the receptor gets internalised. (c) Transporter as reporter genes; upon expression of the transporter, the imaging compound (C) is selectively transported into the cell where it concentrates. Depending on its nature, the imaging tracer is either trapped into the cell, or released when the extracellular concentration decreases (question mark), leading to a transient signal.
Table 1.
Reporter genes and corresponding probes for in vivo imaging.
| Reporter genes | Mechanism | Imaging agents | Imaging | References |
| Cytosine deaminase | Deamination | 5-[19F]fluorocytosine | MRS | [50] |
| HSV1-tk | Phosphorylation | [131I]FIAU, [131I]FIAU | SPECT, gamma camera | [51, 52] |
| [124I]FIAU | PET | [53] | ||
| [123/125I]FIAU | Gamma camera | [54] | ||
| [14C]GCV, [3H]GCV | Autoradiography | [55, 56] | ||
| [18F]GCV | PET | [57, 58] | ||
| [18F]PCV | PET | [14] | ||
| [18F]FHPG | PET | [59, 60] | ||
| [18F]FHBG | PET | [12] | ||
| HSV1-sr39tk | Phosphorylation | [18F]PCV, [18F]FHBG | PET | [10, 12, 61, 62] |
| D2R | Receptor-ligand | [18F]FESP | PET | [13] |
| Mutant D2R | Receptor-ligand | [18F]FESP | PET | [11] |
| Somatostatin receptor | Affinity binding | [111In]DTPA-D-Phe1-octreotide | Gamma camera | [63] |
| [64Cu]-TETA-octreotide | Tissue dose counting | [64] | ||
| [188Re]-somatostatin analogue, 99mTc somatostatin analogue | Gamma camera | [65, 66, 67] | ||
| Na/I symporter | Active transport | [131I], [123I] | Gamma camera | [24, 30, 68] |
| PET | [69] | |||
| Luciferase | Luciferin reaction | Bioluminescence | CCD camera | [41, 70] |
| Cathepsin D | Quenched fluorochromes | Fluorescence activation | CCD camera | [71] |
| Metalloproteinase | Quenched fluorochromes | Fluorescence activation | CCD camera | [72] |
| β-galactosidase | Hydrolysis of β-glycoside bond | EgadMe | MRI | [73] |
| Mutated transferrin receptor | Receptor-ligand | Tf-MION | MRI | [40, 74] |
| Creatine kinase | Dephosphorylation | Phosphocreatine | MRS | [75] |
| Arginine kinase | Dephosphorylation | Phosphoarginine | MRS | [76] |
Enzymes
The reporter gene can be an enzyme expressed inside the cell that alters a labelled compound. The most common approach consists of the phosphorylation of a substrate that can cross the plasma membrane of mammalian cells. The phosphorylated product becomes incapable of traversing the cell membrane and gets trapped inside the cell.
The first tracers were developed for cytosine deaminase [7, 8] but cellular uptake proved slow and this system was superseded by an evolution of tracers that have been created for the prodrug-activating enzyme herpes simplex virus-thymidine kinase (HSV-1-Tk, [9]). In the search for medications against herpes simplex virus, compounds have been found that are preferential substrates for the HSV-1-tk rather than for cellular thymidine kinase. Radiolabelled derivatives have been produced that can be used for imaging. Currently, FPCV (8-[18F]fluoropenciclovir) is the tracer compound that enables the highest sensitivity even with weak expression of HSV-1-tk. A mutated HSV-1-tk (HSV1-sr39tk) featuring a higher specificity to acycloguanosines such as FPCV provides a further increase in sensitivity [10]. Adenoviral delivery of CMV-driven HSV1-sr39tk probed with FHPG (9-[(3-[18F]fluoro-1-hydroxy-2-propoxy) methyl] guanine) induced uptake of 8.5% ID/g in the liver of nude mice if 2 × 109 plaque forming units (pfu) were injected intravenously [11]. Following intratumoural injection of a similar adenovirus at a dose of 1 × 109 pfu an FHPG concentration of 6.3% ID/g has been described [12]. Images can be obtained about one hour after tracer injection [11].
Receptor binding
Much experience exists in the imaging community with tracers that bind to surface receptors. These receptors can be expressed as transgenes and can serve as reporter genes together with their specific labelled ligand.
Dopamine receptor
The dopamine D2 receptor can be expressed as a transgene in the cell membrane to induce binding of the ligand FESP (3-(2′-[18F]fluoroethyl)spiperone) which can be imaged by positron emission tomography (PET) [13]. Because ectopic expression of the D2 receptor sensitises cells to circulating adrenergic signals, a mutated receptor (D80RA) has been reported uncouples ligand binding from intracellular signal transduction [11]. When nude mice were intravenously injected with 2 × 109 pfu, adenovirus in which CMV drives either D2R or D2R80A, binding of 17.5% ID/g liver of FESP was observed [11]. FESP requires about 3-hours binding time before obtaining the image [11]. In a direct comparison of the D2 receptor/FESP combination and the HSV-1-tk/FPCV system, similar results were obtained [14].
Somatostatin receptor type 2 (SSTR2)
Radiolabelled somatostatin analogues, such as [111In]octreotide, are routinely used in the clinic for the detection of rare neuroendocrine tumours expressing the SSTR2. Expression of the receptor in tissues by gene delivery has been shown to lead to uptake of the ligand. When subcutaneous tumours in nude mice were injected with 1 × 109 pfu adenovirus carrying the sstr2 gene driven by a CMV promoter, 8% ID/g located to the tumour if probed with [99mTc]P2045, another somatostatin analogue. The time delay between injection and imaging was 5 hours [15]. In another study, intraperitoneal injection of 1 × 109 pfu of the same virus led to uptake of 2.2% ID/g [99mTc]P2045 in an intraperitoneal nude mice model for ovarian cancer [16]. The native SSTR2 activates intracellular signalling pathways resulting in cell cycle arrest [17]. The engineering of a mutated variant has been recommended [11] although the potential growth arrest properties of the SSTR2 receptor may be beneficial for application in cancer gene therapy [18, 19].
Transporter proteins: the Na/I symporter (NIS)
Transport proteins have high specificity for certain compounds and can be expressed in the cell membrane as reporter genes. They use active transport to concentrate the labelled compound in a defined compartment such as the cell cytosol.
Figure 1 illustrates the function of NIS in the thyroid gland. Driven by the sodium gradient across the basal membrane it transports iodide into the cytoplasm and concentrates it twenty to forty fold [20]. Iodide then leaves the cytoplasm entering the thyroid follicle through the apical membrane by facilitated transport, a process which involves at least one (pendrin) [21]. NADPH oxidase on the luminal side of the apical membrane generates H2O2 which oxydizes iodide to iodine through the action of thyroperoxidase (TPO). TPO allows binding of iodide to the tyrosine residues in the thyroglobulin present in the thyroid follicle. Iodine is therefore trapped in the thyroid follicle and is organified.
Soon after the cloning of the rat NIS gene in 1996 [22], imaging of the transgene in nonthyroid cells was demonstrated by gamma camera [23]. Intratumoural injection of 2 × 109 adenovirus, in which NIS expression is controlled by the immediate-early CMV promoter control, was shown to redirect 11% ID/g of the injected radioiodine to the tumour [24].
Imaging using NIS offers several advantages. Iodide is a tracer without requirement for radiochemistry and this has significant logistic and cost advantages. Decaying isotope does not produce cold tracer but disappears from the system. Iodide has several isotopes with different nuclear physical properties that are widely used in different imaging protocols. [99mTc]pertechnetate can be used in place of iodide and is the tracer of choice for thyroid scintigraphy. [188Re]pertechnetate, a powerful beta emitter, is transported by NIS in a similar way to [99mTc]pertechnetate and has been suggested for targeted radiotherapy delivering higher tissue doses than can be achieved with 131I [25]. These two isotopes can easily be obtained from generators. However, one potential limitation of the system is that NIS alone is incapable of the organification of iodide. Therefore, the accumulation of iodide can be predicted to be a dynamic phenomenon, largely dependent on the clearance of the tracer and for which this clearance effect will vary between organs. A PET image is presented in Figure 2.
Figure 2.
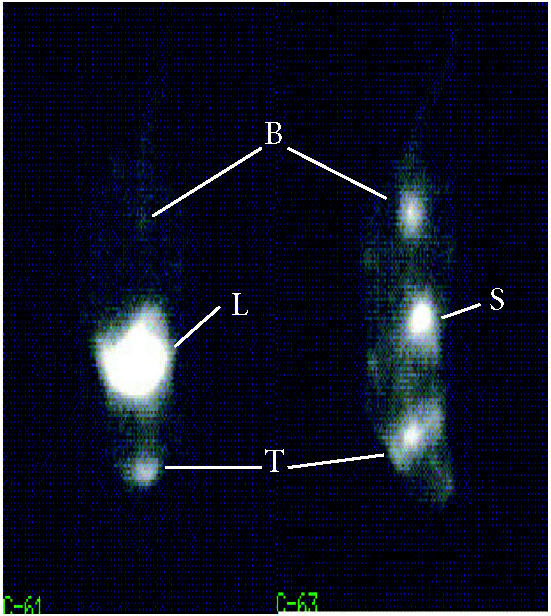
Imaging of hNIS expression by PET. Individual coronal slices from PET images of two nude mice intravenously treated with 5 × 107 GFU adenovirus in which a CMV promoter drives hNIS expression (left) and a PBS-treated control (right) followed, 72 hours later, by injection of [124I] iodide as a tracer. In both pictures, the chosen slice lies at the level of the thyroid region (T). Tracer is seen in the stomach (S) and in the bladder (B) of the control animal. Tracer uptake in the treated animal is in the upper abdomen consistent with adenoviral homing to the liver (for detailed experimental conditions, see [69]).
Transgenes with combined therapeutic and in vivo imaging potential
By definition, reporter genes should demonstrate a total lack of toxicity. However, an attractive alternative would be able to monitor the expression of a therapeutic transgene. This would combine monitoring of gene expression with monitoring of the efficacy of the treatment which has implications in reducing the cost of these experiments. Such an approach has been developed in cancer gene therapy where a large number of studies have aimed to develop tracer compounds that could be used to detect the activity of prodrug-converting enzymes (HSV-1-tk, cytosine deaminase).
In this context, the potential of the human NIS gene (hNIS) transfer for targeted radiotherapy has to be recognised. Concentration of radioiodine for the treatment of differentiated metastatic thyroid cancer has been successfully utilized since 1943 [26]. Several authors have reported the effect of high doses of radioiodine on experimental tumours following expression of NIS [27, 28]. In some cases, tumour reduction was demonstrated in permanently NIS-expressing cell lines [29]. The biological half-life of radioiodine in these studies is usually short [24, 30] and the benefit of a therapeutic dose has been shown in only one in vivo model [27]. From clinical reports it is known that failures of radioiodine therapy are linked to a short biological half-life of activity in the tumour [31] and pharmacological agents such as lithium have been described to prolong the biological half-life of iodide thyroid tissue [32]. Differentiated thyroid tissue generally expresses NIS and TPO and maintains the capability of retaining activity by organification. Coexpression of thyroid peroxidase has recently been reported in vitro but the effect on iodide retention is limited [33, 34].
Finally, the somatostatin receptor SSTR2 has been described to induce cell cycle arrest [17] and reports have demonstrated that this effect can provide therapeutic benefits in pancreatic carcinoma models [18, 19].
NON-NUCLEAR-MEDICINE METHODS
Nuclear medical methods currently offer the greatest potential to be translated into clinical applications. They are highly sensitive, provide good time resolution, and acceptable spatial resolution. But alternative methods are currently in development.
Magnetic resonance imaging (MRI)
MRI techniques have recently shown some remarkable images at very high anatomical resolution in small animals [35], during development [36, 37] and in clinical practice. However, compared to nuclear techniques, the temporal resolution remains limited and the detection of the probes by MRI is several orders of magnitude lower. In an attempt to improve the sensitivity of MR for gene therapy applications, an engineered transferrin receptor has recently been used to shuttle and accumulate supramagnetic nanoparticles into the cells [38]. The MR tracer consists of 3 nm monocristalline iron oxide nanoparticles (MION), sterically protected by a layer of dextran [39]. These MION can be covalently conjugated to the human holotransferrin (Tf-MION). The use of Tf-MION allows an amplification of the signal due to the fact that an average of 2064 Fe molecules are taken up through the transferrin receptor, as opposed to two molecules of Fe in holotransferrin. Upon binding of the Tf-MION to the transferrin receptor, the complex is internalised and MION are accumulated into the endosome. The proof of principle of noninvasive in vivo imaging of gene expression has been demonstrated [40] but it is likely that the development of complementary strategies will be necessary before MRI imaging can be used as a gene therapy tool in the clinic or even in preclinical models.
Detection of biolumunescence
Very recently, some highly sensitive devices capable of detecting and quantifying bioluminescent light have been designed. These devices are capable of detecting photons that are transmitted through mammalian tissues from internal sources [41]. This imaging of very weak visible light is rendered possible by the use of charged coupled device (CCD) cameras that include microchannel plate intensifiers and liquid nitrogen-cooled detectors. This technology aims at enhancing signal-to-noise ratio by decreasing the background (cooling) or amplifying the signal (intensifiers). These instruments are now commercially available.
The gene therapy applications of this technology use luciferase genes as reporter genes. Upon addition of luciferin, the product of the luciferase gene produces luninescence. In addition, luciferin has shown a remarkably good and rapid biodistribution that allows detection of gene expression in vivo feasible [42]. Most commonly, the firefly luciferase gene is employed but other luciferases emit light at different wavelengths. This allows the monitoring of different transgenes, simultaneously in the same animal [43]. More recently, this technology has been used successfully in preclinical models of diseases [44, 45]. However, if this technology appears to be very effective in small animals (mice, rats), there is no evidence that bioluminescence imaging will be adaptable to larger animals, where the distances between organs and the skin are greater and, therefore, the photonic signal is scattered and attenuated. In addition, the signal is bidimentional and tomographic images cannot be obtained by this method. Therefore, bioluminescence detection currently appears to be limited to the laboratory. An exception to this could be clinical gene therapy for skin or occular diseases in which the target tissue is directly accessible to the detector. However, this assumption is highly hypothetical and, to our knowledge, no clinical protocol for such human studies has been submitted to the regulatory bodies.
NUCLEAR MEDICAL DETECTION METHODS
The benefits and shortcoming of two main nuclear imaging technologies and their potential for application in the laboratory animal and in the clinic are outlined in Table 2. Nuclear medical methods of gene expression imaging offer sufficient sensitivity and currently hold the best potential to be scaled up for use in patients. The principle methods are gamma cameras, single photon emission-computed tomography (SPECT), and positron emission tomography (PET).
Table 2.
Comparison between SPECT and PET.
| Method | Advantages | Disadvantages | In vivo animal use | Clinical use | |
| SPECT | - resolution limited by technology only (submillimeter) | - 2D (planar images) and reconstructed 3D | - converted clinical cameras (pinhole collimator) | - readily available and in widespread use | |
| - low sensitivities | - semiquantitative data only | - dedicated cameras evolving | - wide range of clinically tested traces | ||
| - can differentiate between isotopes with different radiation energies | |||||
| PET | - high sensitivity | - short-lived isotopes | currently evolving: - HIDAC |
- [18F]FDG becoming routine in oncology | |
| - 3D acquisition | - isotopes produced in cyclotrons | - microPET | - special applications in neurology and cardiology | ||
| - good resolution, but with a physical limit | - expensive tracer production | ||||
| - quantification possible | - expensive equipment | ||||
| - higher tissue doses, but balanced by higher sensitivity | |||||
Single photon emission-computed tomography (SPECT)
SPECT uses arrays of detectors to identify individual photons emitted by the isotope independent of their direction. A directed view is obtained by fitting metal collimators. Acting like blinkers, they filter out all photons not travelling in a certain direction (eg, either right angle to detectors or through a pinhole) relative to the detector panel. However, the greatest problem with SPECT in respect to gene therapy applications is its lack of potential for quantitation.
Positron-emitting tomography (PET)
Because of its potential in terms of quantitation as well as its high sensitivity, PET appears to be the technique of choice to collect information on the location, magnitude, and kinetics of gene expression upon delivery of genetic material.
Detection in a PET scanner requires tracers that incorporate positron-emitting isotopes. These isotopes decay by a number of different decay mechanisms. In a certain percentage of these decays, positrons are released. The positron will travel for some distance, defined by its energy and by the surrounding matter, before being annihilated when hitting an electron. Two photons of 511 MeV are created by the annihilation event and these travel in opposite directions at an angle of almost exactly 180°. PET scanner detectors register all events in a ring of detectors around the radioactivity source but process only events that occur simultaneously within a certain time window (Figure 3c). The annihilation event must have taken place on a line between these two detectors recording the event. Reconstruction from the raw data is performed by different mathematical methods that can be either back-projections or iterative reconstruction methods. They differ in their resolution, resolution-noise ratio, contrast, and required processing time [46].
Figure 3.
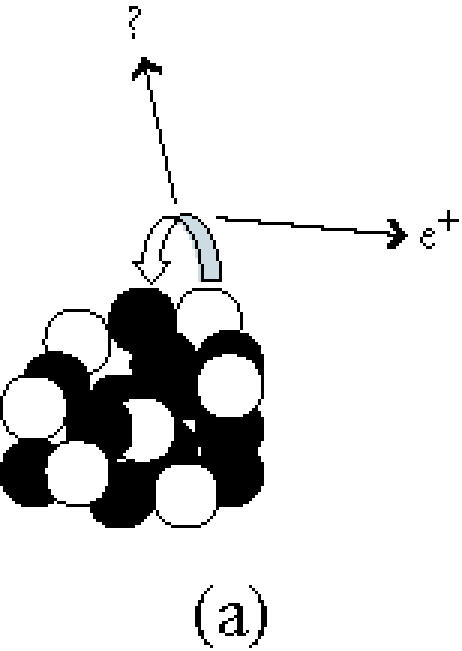
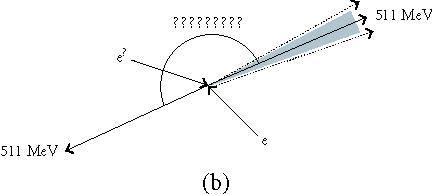
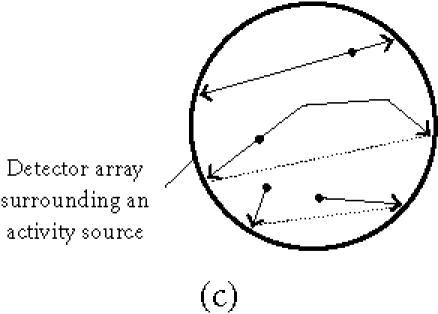
(a) A positron and a neutrino are released when a proton becomes a neutron. (b) Two annihilation photons travel away from each other at 180± 0.25°. (c) The scanner records simultaneous events within a 10–80 ns time window. These are from true coincidences, scattered coincidences, and random coincidences from independent annihilation events. Single events are not processed further (see [77]).
Spatial resolution in PET imaging has a physical limit (a) because of the distance the photon travels between the nuclear decay and the annihilation event and (b) because the angle between the two annihilation photons deviates slightly around 180° (Figure 3b). The former is influenced by the choice of isotope (Table 3), the latter is improved with smaller dimensions of the scanner. Spatial resolution is also reduced by scattering of the photons in tissue altering the angle between them (“Scattered,” in Figure 3c). The statistical quality of the image reconstruction is reduced by photons that are simultaneously registered but originate from different events (“Random,” in Figure 3c) and by multiple simultaneous registrations. Both (a) and (b) increase when the count rate approaches the saturation rate of the detection system.
Table 3.
Energy and half-life characteristics of selected positron-emitting isotopes.
| Isotope | Half-life | Maximum positron energy |
Maximum range | Spatial resolution (FWHM) |
Radiation dose with internal intake{890} |
| 18F | 109 min | 0.63 MeV | 2.6 mm | 0.22 mm | 0.049 mSv/MBq |
| 11C | 20 min | 0.96 MeV | 4.2 mm | 0.28 mm | 0.024 mSv/MBq |
| 13N | 9.9 min | 1.2 MeV | 5.4 mm | 0.35 mm | n/a |
| 15O | 122 s | 1.74 MeV | 8.4 mm | 1.22 mm | n/a |
| 82Rb | 1.3 min | 3.15 MeV | 17.1 mm | 2.6 mm | n/a |
| 124I | 4.2 d | 2.14 MeV | ∼ 1.5 mm | 13 mSv/MBq | |
| 64Cu | 12.7 h | 3.3 MeV | 0.12 mSv/MBq | ||
| 120I | 81 min | 5.6 MeV | n/a | ||
Another artifact arises from attenuation of photons when travelling through the tissue. Signals from near the body surface and in the lungs are registered with relatively higher intensity. This is of less importance for smaller animals but for patient imaging attenuation correction can be performed during image reconstruction.
Positron-emitting isotopes are generally short-lived (Table 3). They are produced in cyclotrons that should be near the laboratory or hospital where tracer synthesis, transport, and application take place. Small variations in timing have important consequences. The tissue dose from positron-emitting isotopes is relatively high because the energy of the positron is completely absorbed in the tissue. This is balanced by a short half-life but is more significant for 124I with a relatively long duration in the tissue.
HUMAN STUDY
The development of these new technologies is just reaching the clinic and, to our knowledge, only one report has been published on the use of in vivo molecular imaging in clinical gene therapy [47]. This study was preceded by the establishment of the pharmacodynamic and biodistribution parameters of the radioactive tracer [124I] FIAU (to monitor HSV-1-tk expression) in humans [48], with a particular emphasis on the head region. This radioactive tracer was shown not to be able to cross the blood-brain barrier in normal brain but showed rapid and nonspecific accumulation within recurrent glioma [48], demonstrating that [124I] FIAU was a useful tracer when the blood-brain barrier was disrupted. In a follow-up study, this tracer was used in a small clinical trial testing liposomal intratumoural delivery of the HSV-1-tk gene to patients with recurrent glioma [47]. The authors demonstrated accumulation of [124I] FIAU, indicative of HSV-1-tk expression, in the immediate periphery of the needle tract in one out of five patients. In this patient, the overall therapeutic effect was limited to a portion of the tumour. However, in the four remaining patients, histology sections of the tumour showed a significant decrease in the number of proliferating cells. This observation tends to indicate that a critical threshold of gene expression has to be reached before HSV-1-tk-associated FIAU could be detected by PET. The authors concluded that the extent of HSV-1-tk expression appeared to predict the therapeutic response. However, the overall [124I] FIAU accumulation, as well as the therapeutic response were limited [47].
CONCLUDING REMARK
There is little doubt that using whole body, noninvasive imaging technology will help to design gene therapy formulations tailored to target diseases. But the need for clinicians to monitor gene expression, and more generally to monitor biodistribution and pharmacodynamics of gene therapy formulations in a more precise and quantitative way has been sadly highlighted by the death of Jesse Gelsinger, who became the first person to die from experimental techniques of gene therapy. Following his death, one of the recommendations of the Recombinant DNA Advisory Committee (RAC) of the National Institute of Health in the USA was to develop better ways for measuring transgene expression in cells and tissues in order to improve the safety of these interventions [49].
Acknowledgments
ACKNOWLEDGMENTS
The authors would like to thank Kate and Gordon Leverton for careful reading of the manuscript and Eric O Aboagye for its help in PET scanning. Research in the authors laboratory is supported by Cancer Research UK, the Medical Research Council, and the Hammersmith Hospital Trust Research Committee.
References
- Khuri F R, Nemunaitis J, Ganly I, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6(8):879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- Sandmair A M, Loimas S, Puranen P, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11(16):2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J, Swisher S G, Timmons T, et al. Adenovirus-mediated p53 gene transfer in sequence with cisplatin to tumors of patients with non-small-cell lung cancer. J Clin Oncol. 2000;18(3):609–622. doi: 10.1200/JCO.2000.18.3.609. [DOI] [PubMed] [Google Scholar]
- Isner J M, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103(9):1231–1236. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M A, Manno C S, Ragni M V, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24(3):257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288(5466):669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Gambhir S S, Barrio J R, Herschman H R, Phelps M E. Assays for noninvasive imaging of reporter gene expression. Nucl Med Biol. 1999;26(5):481–490. doi: 10.1016/s0969-8051(99)00021-9. [DOI] [PubMed] [Google Scholar]
- Brix G, Bellemann M E, Haberkorn U, Gerlach L, Lorenz W J. Assessment of the biodistribution and metabolism of 5-fluorouracil as monitored by 18F PET and 19F MRI: a comparative animal study. Nucl Med Biol. 1996;23(7):897–906. doi: 10.1016/s0969-8051(96)00122-9. [DOI] [PubMed] [Google Scholar]
- Blasberg R G, Tjuvajev J G. Herpes simplex virus thymidine kinase as a marker/reporter gene for PET imaging of gene therapy. Q J Nucl Med. 1999;43(2):163–169. [PubMed] [Google Scholar]
- Gambhir S S, Bauer E, Black M E, et al. A mutant herpes simplex virus type 1 thymidine kinase reporter gene shows improved sensitivity for imaging reporter gene expression with positron emission tomography. Proc Natl Acad Sci USA. 2000;97(6):2785–2790. doi: 10.1073/pnas.97.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q, Satyamurthy N, Barrio J R, et al. Noninvasive, quantitative imaging in living animals of a mutant dopamine D2 receptor reporter gene in which ligand binding is uncoupled from signal transduction. Gene Ther. 2001;8(19):1490–1498. doi: 10.1038/sj.gt.3301542. [DOI] [PubMed] [Google Scholar]
- Yaghoubi S S, Wu L, Liang Q, et al. Direct correlation between positron emission tomographic images of two reporter genes delivered by two distinct adenoviral vectors. Gene Ther. 2001;8(14):1072–1080. doi: 10.1038/sj.gt.3301490. [DOI] [PubMed] [Google Scholar]
- MacLaren D C, Gambhir S S, Satyamurthy N, et al. Repetitive, non-invasive imaging of the dopamine D2 receptor as a reporter gene in living animals. Gene Ther. 1999;6(5):785–791. doi: 10.1038/sj.gt.3300877. [DOI] [PubMed] [Google Scholar]
- Iyer M, Barrio J R, Namavari M, et al. 8-[18F]Fluoropenciclovir: an improved reporter probe for imaging HSV1-tk reporter gene expression in vivo using PET. J Nucl Med. 2001;42(1):96–105. [PubMed] [Google Scholar]
- Zinn K R, Chaudhuri T R. The type 2 human somatostatin receptor as a platform for reporter gene imaging. Eur J Nucl Med Mol Imaging. 2002;29(3):388–399. doi: 10.1007/s00259-002-0764-y. [DOI] [PubMed] [Google Scholar]
- Chaudhuri T R, Rogers B E, Buchsbaum D J, Mountz J M, Zinn K R. A noninvasive reporter system to image adenoviral-mediated gene transfer to ovarian cancer xenografts. Gynecol Oncol. 2001;83(2):432–438. doi: 10.1006/gyno.2001.6333. [DOI] [PubMed] [Google Scholar]
- Pages P, Benali N, Saint-Laurent N, et al. sst2 somatostatin receptor mediates cell cycle arrest and induction of p27(Kip1). Evidence for the role of SHP-1. J Biol Chem. 1999;274(21):15186–15193. doi: 10.1074/jbc.274.21.15186. [DOI] [PubMed] [Google Scholar]
- Rochaix P, Delesque N, Esteve J P, et al. Gene therapy for pancreatic carcinoma: local and distant antitumor effects after somatostatin receptor sst2 gene transfer. Hum Gene Ther. 1999;10(6):995–1008. doi: 10.1089/10430349950018391. [DOI] [PubMed] [Google Scholar]
- Benali N, Cordelier P, Calise D, et al. Inhibition of growth and metastatic progression of pancreatic carcinoma in hamster after somatostatin receptor subtype 2 (sst2) gene expression and administration of cytotoxic somatostatin analog AN-238. Proc Natl Acad Sci USA. 2000;97(16):9180–9185. doi: 10.1073/pnas.130196697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filetti S, Bidart J M, Arturi F, Caillou B, Russo D, Schlumberger M. Sodium/iodide symporter: a key transport system in thyroid cancer cell metabolism. Eur J Endocrinol. 1999;141(5):443–457. doi: 10.1530/eje.0.1410443. [DOI] [PubMed] [Google Scholar]
- Royaux I E, Suzuki K, Mori A, et al. Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology. 2000;141(2):839–845. doi: 10.1210/endo.141.2.7303. [DOI] [PubMed] [Google Scholar]
- Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379(6564):458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- Shimura H, Haraguchi K, Miyazaki A, Endo T, Onaya T. Iodide uptake and experimental 131I therapy in transplanted undifferentiated thyroid cancer cells expressing the Na+/I− symporter gene. Endocrinology. 1997;138(10):4493–4496. doi: 10.1210/endo.138.10.5571. [DOI] [PubMed] [Google Scholar]
- Boland A, Ricard M, Opolon P, et al. Adenovirus-mediated transfer of the thyroid sodium/iodide symporter gene into tumors for a targeted radiotherapy. Cancer Res. 2000;60(13):3484–3492. [PubMed] [Google Scholar]
- Dadachova E, Bouzahzah B, Zuckier L S, Pestell R G. Rhenium-188 as an alternative to Iodine-131 for treatment of breast tumors expressing the sodium/iodide symporter (NIS) Nucl Med Biol. 2002;29(1):13–18. doi: 10.1016/s0969-8051(01)00279-7. [DOI] [PubMed] [Google Scholar]
- Siegel E. The beginnings of radioiodine therapy of metastatic thyroid carcinoma: a memoir of Samuel M. Seidlin, M. D. (1895–1955) and his celebrated patient. Cancer Biother Radiopharm. 1999;14(2):71–79. doi: 10.1089/cbr.1999.14.71. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, O'Connor M K, Bergert E R, Tindall D J, Young C Y, Morris J C. Treatment of prostate cancer by radioiodine therapy after tissue-specific expression of the sodium iodide symporter. Cancer Res. 2000;60(22):6526–6530. [PubMed] [Google Scholar]
- Smit J W, Schroder-van der Elst J P, Karperien M, et al. Iodide kinetics and experimental 131I therapy in a xenotransplanted human sodium-iodide symporter-transfected human follicular thyroid carcinoma cell line. J Clin Endocrinol Metab. 2002;87(3):1247–1253. doi: 10.1210/jcem.87.3.8307. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, Harrington K J, Pinke L A, Vile R G, Morris J C. Clinical review 132: The sodium iodide symporter and its potential role in cancer therapy. J Clin Endocrinol Metab. 2001;86(7):3327–3335. doi: 10.1210/jcem.86.7.7641. [DOI] [PubMed] [Google Scholar]
- La Perle K.M D, Shen D, Buckwalter T.L F, et al. In vivo expression and function of the sodium iodide symporter following gene transfer in the MATLyLu rat model of metastatic prostate cancer. Prostate. 2002;50(3):170–178. doi: 10.1002/pros.10046. [DOI] [PubMed] [Google Scholar]
- Maxon H R, Thomas S R, Hertzberg V S, et al. Relation between effective radiation dose and outcome of radioiodine therapy for thyroid cancer. N Engl J Med. 1983;309(16):937–941. doi: 10.1056/NEJM198310203091601. [DOI] [PubMed] [Google Scholar]
- Koong S S, Reynolds J C, Movius E G, et al. Lithium as a potential adjuvant to 131I therapy of metastatic, well differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1999;84(3):912–916. doi: 10.1210/jcem.84.3.5527. [DOI] [PubMed] [Google Scholar]
- Huang M, Batra R K, Kogai T, et al. Ectopic expression of the thyroperoxidase gene augments radioiodide uptake and retention mediated by the sodium iodide symporter in non-small cell lung cancer. Cancer Gene Ther. 2001;8(8):612–618. doi: 10.1038/sj.sgt.7700354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland A, Magnon C, Filetti S, et al. Transposition of the thyroid iodide uptake and organification system in nonthyroid tumor cells by adenoviral vector-mediated gene transfers. Thyroid. 2002;12(1):19–26. doi: 10.1089/105072502753451922. [DOI] [PubMed] [Google Scholar]
- Johnson G A, Benveniste H, Black R D, Hedlund L W, Maronpot R R, Smith B R. Histology by magnetic resonance microscopy. Magn Reson Q. 1993;9(1):1–30. [PubMed] [Google Scholar]
- Smith B R, Johnson G A, Groman E V, Linney E. Magnetic resonance microscopy of mouse embryos. Proc Natl Acad Sci USA. 1994;91(9):3530–3533. doi: 10.1073/pnas.91.9.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R E, Fraser S E. Magnetic resonance microscopy of embryonic cell lineages and movements. Science. 1994;263(5147):681–684. doi: 10.1126/science.7508143. [DOI] [PubMed] [Google Scholar]
- Moore A, Basilion J P, Chiocca E A, Weissleder R. Measuring transferrin receptor gene expression by NMR imaging. Biochim Biophys Acta. 1998;1402(3):239–249. doi: 10.1016/s0167-4889(98)00002-0. [DOI] [PubMed] [Google Scholar]
- Shen T, Weissleder R, Papisov M, Bogdanov A. Jr, Brady T J. Monocrystalline iron oxide nanocompounds (MION): physicochemical properties. Magn Reson Med. 1993;29(5):599–604. doi: 10.1002/mrm.1910290504. [DOI] [PubMed] [Google Scholar]
- Weissleder R, Moore A, Mahmood U, et al. In vivo magnetic resonance imaging of transgene expression. Nat Med. 2000;6(3):351–355. doi: 10.1038/73219. [DOI] [PubMed] [Google Scholar]
- Contag P R, Olomu I N, Stevenson D K, Contag C H. Bioluminescent indicators in living mammals. Nat Med. 1998;4(2):245–247. doi: 10.1038/nm0298-245. [DOI] [PubMed] [Google Scholar]
- Sweeney T J, Mailander V, Tucker A A, et al. Visualizing the kinetics of tumor-cell clearance in living animals. Proc Natl Acad Sci USA. 1999;96(21):12044–12049. doi: 10.1073/pnas.96.21.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik S, Gambhir S S. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc Natl Acad Sci USA. 2002;99(1):377–382. doi: 10.1073/pnas.012611099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Seroogy C M, Sandora M R, et al. Antigen-specific T cell-mediated gene therapy in collagen-induced arthritis. J Clin Invest. 2001;107(10):1293–1301. doi: 10.1172/JCI12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshutz G S, Gruber C A, Cao Y, Hardy J, Contag C H, Gaensler K M. In utero delivery of adeno-associated viral vectors: intraperitoneal gene transfer produces long-term expression. Mol Ther. 2001;3(3):284–292. doi: 10.1006/mthe.2001.0267. [DOI] [PubMed] [Google Scholar]
- Walledge R J, Manavaki R, Reader A J, et al. Comparison of four images reconstruction techniques for high resolution imaging. IEEE Nuclear Science Symposium and Medical Conference papers. Submitted to IEEE T Nucl Sci. 2002.
- Jacobs A, Voges J, Reszka R, et al. Positron-emission tomography of vector-mediated gene expression in gene therapy for gliomas. Lancet. 2001;358(9283):727–729. doi: 10.1016/s0140-6736(01)05904-9. [DOI] [PubMed] [Google Scholar]
- Jacobs A, Braunlich I, Graf R, et al. Quantitative kinetics of [124I]FIAU in cat and man. J Nucl Med. 2001;42(3):467–475. [PubMed] [Google Scholar]
- Hollon T. Researchers and regulators reflect on first gene therapy death. Nat Med. 2000;6(1):6. doi: 10.1038/71545. [DOI] [PubMed] [Google Scholar]
- Stegman L D, Rehemtulla A, Beattie B, et al. Noninvasive quantitation of cytosine deaminase transgene expression in human tumor xenografts with in vivo magnetic resonance spectroscopy. Proc Natl Acad Sci USA. 1999;96(17):9821–9826. doi: 10.1073/pnas.96.17.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjuvajev J G, Finn R, Watanabe K, et al. Noninvasive imaging of herpes virus thymidine kinase gene transfer and expression: a potential method for monitoring clinical gene therapy. Cancer Res. 1996;56(18):4087–4095. [PubMed] [Google Scholar]
- Tjuvajev J G, Chen S H, Joshi A, et al. Imaging adenoviral-mediated herpes virus thymidine kinase gene transfer and expression in vivo. Cancer Res. 1999;59(20):5186–5193. [PubMed] [Google Scholar]
- Tjuvajev J G, Avril N, Oku T, et al. Imaging herpes virus thymidine kinase gene transfer and expression by positron emission tomography. Cancer Res. 1998;58(19):4333–4341. [PubMed] [Google Scholar]
- Haubner R, Avril N, Hantzopoulos P A, Gansbacher B, Schwaiger M. In vivo imaging of herpes simplex virus type 1 thymidine kinase gene expression: early kinetics of radiolabelled FIAU. Eur J Nucl Med. 2000;27(3):283–291. doi: 10.1007/s002590050035. [DOI] [PubMed] [Google Scholar]
- Haberkorn U, Altmann A, Morr I, et al. Monitoring gene therapy with herpes simplex virus thymidine kinase in hepatoma cells: uptake of specific substrates. J Nucl Med. 1997;38(2):287–294. [PubMed] [Google Scholar]
- Haberkorn U, Khazaie K, Morr I, Altmann A, Muller M, van Kaick G. Ganciclovir uptake in human mammary carcinoma cells expressing herpes simplex virus thymidine kinase. Nucl Med Biol. 1998;25(4):367–373. doi: 10.1016/s0969-8051(97)00210-2. [DOI] [PubMed] [Google Scholar]
- Gambhir S S, Barrio J R, Wu L, et al. Imaging of adenoviral-directed herpes simplex virus type 1 thymidine kinase reporter gene expression in mice with radiolabeled ganciclovir. J Nucl Med. 1998;39(11):2003–2011. [PubMed] [Google Scholar]
- Gambhir S S, Barrio J R, Phelps M E, et al. Imaging adenoviral-directed reporter gene expression in living animals with positron emission tomography. Proc Natl Acad Sci USA. 1999;96(5):2333–2338. doi: 10.1073/pnas.96.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alauddin M M, Conti P S, Mazza S M, Hamzeh F M, Lever J R. 9-[(3-[18F]-fluoro-1-hydroxy-2-propoxy)methyl]guanine ([18F]-FHPG): a potential imaging agent of viral infection and gene therapy using PET. Nucl Med Biol. 1996;23(6):787–792. doi: 10.1016/0969-8051(96)00075-3. [DOI] [PubMed] [Google Scholar]
- Alauddin M M, Shahinian A, Kundu R K, Gordon E M, Conti P S. Evaluation of 9-[(3-18F-fluoro-1-hydroxy-2-propoxy)methyl]guanine ([18F]-FHPG) in vitro and in vivo as a probe for PET imaging of gene incorporation and expression in tumors. Nucl Med Biol. 1999;26(4):371–376. doi: 10.1016/s0969-8051(98)00116-4. [DOI] [PubMed] [Google Scholar]
- Yu Y, Annala A J, Barrio J R, et al. Quantification of target gene expression by imaging reporter gene expression in living animals. Nat Med. 2000;6(8):933–937. doi: 10.1038/78704. [DOI] [PubMed] [Google Scholar]
- Sun X, Annala A J, Yaghoubi S S, et al. Quantitative imaging of gene induction in living animals. Gene Ther. 2001;8(20):1572–1579. doi: 10.1038/sj.gt.3301554. [DOI] [PubMed] [Google Scholar]
- Rogers B E, McLean S F, Kirkman R L, et al. In vivo localization of [(111)In]-DTPA-D-Phe1-octreotide to human ovarian tumor xenografts induced to express the somatostatin receptor subtype 2 using an adenoviral vector. Clin Cancer Res. 1999;5(2):383–393. [PubMed] [Google Scholar]
- Buchsbaum D J, Rogers B E, Khazaeli M B, et al. Targeting strategies for cancer radiotherapy. Clin Cancer Res. 1999;5(Suppl 10):3048s–3055s. [PubMed] [Google Scholar]
- Rogers B E, Zinn K R, Buchsbaum D J. Gene transfer strategies for improving radiolabeled peptide imaging and therapy. Q J Nucl Med. 2000;44(3):208–223. [PubMed] [Google Scholar]
- Zinn K R, Buchsbaum D J, Chaudhuri T R, Mountz J M, Grizzle W E, Rogers B E. Noninvasive monitoring of gene transfer using a reporter receptor imaged with a high-affinity peptide radiolabeled with 99mTc or 188Re. J Nucl Med. 2000;41(5):887–895. [PubMed] [Google Scholar]
- Hemminki A, Zinn K R, Liu B, et al. In vivo molecular chemotherapy and noninvasive imaging with an infectivity-enhanced adenovirus. J Natl Cancer Inst. 2002;94(10):741–749. doi: 10.1093/jnci/94.10.741. [DOI] [PubMed] [Google Scholar]
- Haberkorn U, Henze M, Altmann A, et al. Transfer of the human NaI symporter gene enhances iodide uptake in hepatoma cells. J Nucl Med. 2001;42(2):317–325. [PubMed] [Google Scholar]
- Groot-Wassink T, Aboagye E O, Glaser M, Lemoine N R, Vassaux G. Adenovirus biodistribution and noninvasive imaging of gene expression in vivo by positron emission tomography using the sodium/iodide symporter as a reporter gene. Hum Gene Ther. 2002;13(14):1723–1735. doi: 10.1089/104303402760293565. [DOI] [PubMed] [Google Scholar]
- Edinger M, Sweeney T J, Tucker A A, Olomu A B, Negrin R S, Contag C H. Noninvasive assessment of tumor cell proliferation in animal models. Neoplasia. 1999;1(4):303–310. doi: 10.1038/sj.neo.7900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung C H, Mahmood U, Bredow S, Weissleder R. In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer Res. 2000;60(17):4953–4958. [PubMed] [Google Scholar]
- Bremer C, Bredow S, Mahmood U, Weissleder R, Tung C H. Optical imaging of matrix metalloproteinase-2 activity in tumors: feasibility study in a mouse model. Radiology. 2001;221(2):523–529. doi: 10.1148/radiol.2212010368. [DOI] [PubMed] [Google Scholar]
- Louie A Y, Huber M M, Ahrens E T, et al. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol. 2000;18(3):321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- Moore A, Josephson L, Bhorade R M, Basilion J P, Weissleder R. Human transferrin receptor gene as a marker gene for MR imaging. Radiology. 2001;221(1):244–250. doi: 10.1148/radiol.2211001784. [DOI] [PubMed] [Google Scholar]
- Auricchio A, Zhou R, Wilson J M, Glickson J D. In vivo detection of gene expression in liver by 31P nuclear magnetic resonance spectroscopy employing creatine kinase as a marker gene. Proc Natl Acad Sci USA. 2001;98(9):5205–5210. doi: 10.1073/pnas.081508598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G, Barton E R, Sweeney H L. Noninvasive measurement of gene expression in skeletal muscle. Proc Natl Acad Sci USA. 2000;97(10):5151–5155. doi: 10.1073/pnas.97.10.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budinger T F. PET instrumentation: what are the limits? Semin Nucl Med. 1998;28(3):247–267. doi: 10.1016/s0001-2998(98)80030-5. [DOI] [PubMed] [Google Scholar]


