Abstract
Chapter summary
Several groups have documented the expression of cytokines in rheumatoid arthritis synovial tissue over the past 15 years or so. These studies have indicated that most cytokines examined are expressed at the mRNA levels at least, and many other cytokines are found in abundance as proteins. Our attention has recently focused on the mechanisms that induce and regulate tumour necrosis factor and IL-10. Other workers and ourselves have found that cell–cell contact is an important signal for the induction of cytokines, and our work has demonstrated that tumour necrosis factor and IL-10 production in rheumatoid arthritis synovial joint cells cultures is dependent on T cell/macrophage interaction. In this chapter, we review recent advances in this area and also highlight areas where new therapeutic intervention opportunities arise.
Keywords: cognate, differentiation, macrophage, signalling, T cells
Historical background
It is now well accepted that the spontaneous production of proinflammatory cytokines (in particular, tumour necrosis factor [TNF] and IL-1) produced locally in the inflamed synovial joint contribute directly/indirectly to the pathogenesis of rheumatoid arthritis (RA) [1]. These observations have arisen from ex vivo studies on human synovial cultures, immunohistochemical and mRNA analysis of synovium, and in vivo studies in animal models of arthritis.
These investigations led to the development of several TNF and IL-1 inhibitors, two of which are currently licensed Remicade (chimeric anti-TNF antibody) and Enbrel (TNF-receptor fusion protein). While such therapies targeting TNF in chronic inflammatory disease are very successful [2], it is also apparent that long-term blockade of a cytokine such as TNF, which is important in innate and acquired immunity, may lead to an increase in latent and/or opportunistic infections. This is now apparent, with a small but significant increase in unusual infections, as well as the re-emergence of latent tuberculosis, particularly in Central and Eastern Europe [3].
There is thus a need to understand what mechanisms lead to the production of proinflammatory cytokines in RA synovial tissue, and further to determine how this is linked to homeostatic regulation. It has been observed that while the production of proinflammatory cytokines and enzymes is increased in RA, this is offset to some degree by the action of the endogenous anti-inflammatory cytokines and cytokine inhibitors. Of particular importance in this respect is IL-10, an important regulator of TNF-α and IL-1β spontaneously produced by macrophages in the rheumatoid joint [4,5]. Thus, if endogenous IL-10 is blocked in RA synovial cell cultures, the spontaneous production of both TNF and IL-1 increases significantly [4]. There is therefore an important need to develop therapies that block proinflammatory pathways but leave unaffected those pathways that regulate immunoregulatory cytokines such as IL-10.
Cognate-dependent interactions
Histological studies of synovium in RA have indicated that this tissue is very cellular, and that several different cell types including macrophages and T cells are in close proximity [6]. This may suggest that contact signals between macrophages and T cells could be of importance in vivo in modulating macrophage function. We have found that TNF-α production in RA synovium is T-cell dependent, as removal of CD3-positive T cells from RA synovial mononuclear cells resulted in significant reduced macrophage TNF-α production [7]. Furthermore, this signal was abrogated if physical contact between the two cell types was blocked.
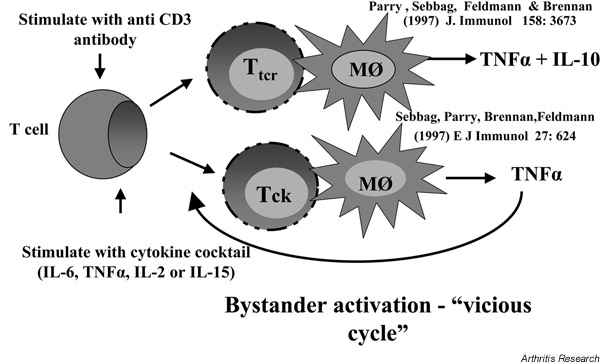
Direct-contact-mediated interactions have been studied by several groups using transformed T-cell lines and monocytic lines, and have been found to play a role in inducing the synthesis of several cytokines including IL-1β, TNF-α, IL-10 and metalloproteinases [8-12]. We have studied T cell/monocyte cognate interactions using cells isolated from the peripheral blood of normal donors. Importantly, we observed that the manner in which T cells were activated influenced the profile of cytokines induced in the monocytes. Thus, if blood T cells were activated with cross-linked anti-CD3, this induced the production of TNF-α and IL-10 in monocytes [12]. However, if the T cells were stimulated with a cocktail of cytokines (TNF-α, IL-2 and IL-6) for 8 days (bystander activation), TNF-α production followed but IL-10 production did not [11]. These observations suggested to us that cytokine-stimulated T cells (Tck) may be the actual T cells in RA synovial tissue that induce macrophage TNF-α production, because they induce an unbalanced, proinflammatory cytokine response from monocytes and they could be part of a vicious cycle (Fig. 1). Indeed, in addition to the mechanism of T-cell activation determining the cytokine profile produced by monocytic cells, the corresponding T-cell phenotype would also appear to be important, as one study [13] suggested a differential regulation of monocyte-derived cytokine production by Th1-like and Th2-like cells. This study describes CD4+ Th1 clones inducing high levels of IL-1β production by THP-1 monocytes, whereas Th2 clones induced higher levels of IL-1ra. This implies that Th1 cells are proinflammatory whereas Th2 cells are anti-inflammatory.
Figure 1.
Cytokine disequilibrium induced by cytokine-stimulated T cells (Tck). Mφ, monocytes; Ttcr, T-cell-receptor-dependent stimulated T cells.
The hypothesis that RA T cells mimic the action of Tck cells is attractive since T cells found in RA synovium have unusual characteristics. The T cells are relatively small and noncycling, but have features of activation, with over one-half expressing HLA class II, VLA antigens, CD25 and CD69 [14,15]. T-cell receptor analysis has not revealed a consistent pattern, and responses to putative autoantigens have not been easy to reproduce (reviewed in [16]). Based on these features and the low capacity of T cells to produce T-cell-derived cytokines, it has been proposed that T cells in the joint may not be involved in the later stages of the disease [17]. We have, however, proposed that they are involved in disease pathology through the activation of macrophages to induce TNF. The environment of the RA synovium is favourable to Tck cells, as it is rich in cytokines. Unutmaz et al. described bystander-activated T cells generated from normal peripheral blood mononuclear cells (PBMC) with IL-2, IL-6 and TNF-α [18]. We found that IL-15 could, by itself, mimic the IL-6/TNF-α/IL-2 cocktail used to activate Tck [11]. IL-15 is of particular interest as it is found in RA synovium [19] and can activate peripheral blood T cells to induce TNF-α synthesis in U937 cells or in adherent RA synovial cells in a contact-dependent manner [20,21]
Other cognate cell-to-cell interactions occur in the synovial joint, which contribute to the disease pathology observed in RA. These interactions include endothelial cell/T cell and fibroblast/T cell interactions. During the early stages of inflammation there is a large cellular infiltration from the peripheral circulation to the synovial joint, where interactions between T cells and vascular endothelium drive further extravasation and infiltration by the expression of cell adhesion molecules, chemokines and cytokines [22-25]. In addition, the earliest infiltrating cells, neutrophils, can be activated by contact-mediated interaction with T cells, as determined by the ability of these neutrophils to be primed for respiratory burst by formylmethionine leucine phenylalanine peptide[26].
As the pathology of RA progresses to chronic inflammation and pannus forms at the cartilage–pannus junction, the interactions between T cells and fibroblasts/macrophages predominate. The interaction between stimulated T cells and dermal fibroblasts or synoviocytes has recently been shown to induce MMP-1 (collagenase) and TIMP-1, with an imbalance in favour of the proinflammatory MMP-1 [27], also inhibiting the synthesis of type I and type III collagen by fibroblast cells [28].
Studies undertaken thus far have documented the potent stimulatory activity of T cells on monocyte cytokine production during the pathology of RA. The abundance of T cells and monocytes in the peripheral circulation, which have the potential to physically interact with each other, however, does not seem to induce cytokine production. This led to the hypothesis and subsequent characterisation of an inhibitory factor, apolipoprotein A-1, preventing monocyte activation in the plasma/serum [29]. The presence of apolipoprotein A-1 and subsequent inhibition of T-cell-mediated macrophage activation may suggest this molecule to have a useful anti-inflammatory therapeutic effect in chronic inflammatory diseases such as RA.
Cell surface molecules
As contact-dependent signals have been demonstrated to be of importance, much attention has focused on the probable candidate molecules on the surface of cells mediating these functions. Specific surface interaction molecules that have been reported to mediate induction of monocyte cytokine synthesis include CD69, LFA-1 [8,30], CD44 [31], CD45 [32], CD40 [33], membrane TNF [12], and signalling lymphocytic activation molecule [34]. Our studies further demonstrated that T cells activated through the T-cell receptor complex induced monocyte IL-10 synthesis, which was partially dependent on endogenous TNF-α and IL-1, and that T-cell membrane TNF-α was an important contact-mediated signal [12,35]. However, IL-10 synthesis still occurred when TNF-α and IL-1 were neutralised, suggesting that there are other TNF/IL-1-independent signals required for IL-10 synthesis.
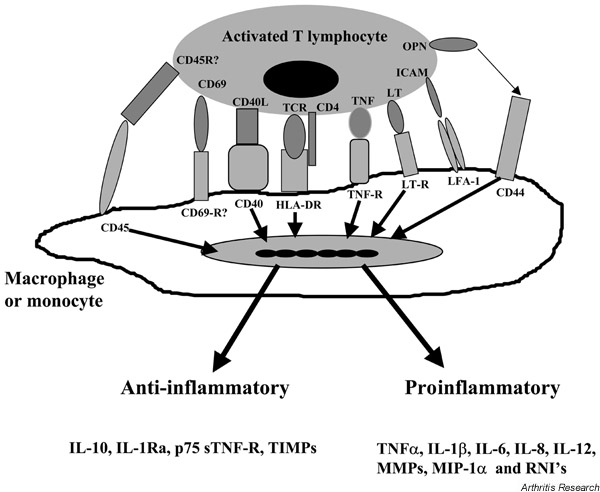
While TNF clearly plays a role in IL-10 production, there are other signals independent of TNF and IL-1 that may be involved. Of particular interest are members of the TNF/TNF-receptor family, which include CD40, CD27, CD30, OX-40 and LTβ. The ligands of these TNF-receptor molecules have been described as upregulated on T-cell activation. In addition, CD40L, 4-1BB, CD27L and CD30 have been described to be released as soluble molecules after activation [36-39]. The interaction between CD40L and CD40 has been suggested to be important for inducing both IL-1 and IL-12 synthesis following T-cell interaction with monocytes [33,40] and, more recently, to mediate IL-10 production by human microglial cells on interaction with anti-CD3-stimulated T cells [41]. In addition, we have recently shown that CD40L/CD40 interaction mediates cognate induction of macrophage IL-10 [42]. The potential involvement of these ligand/counter ligand pairs on cells interacting in synovial membrane tissue is represented in Figure 2.
Figure 2.
Potential ligand/counter ligand interactions involved in cytokine production by macrophages. HLA-DR, human leukocyte antigen – class II, subtype DR; ICAM, intracellular adhesion molecule; LT, lymphotoxin; MMP, matrix metalloproteinase; OPN, osteoprotegrin; RNI, reactive nitrogen intermediates; TCR, T-cell receptor; TNF, tumour necrosis factor.
Differentiation status
In addition to the stimulus encountered by the T cells, our data has indicated the importance of the differentiation state of the monocyte in determining cytokine profiles in response to activated T cells. We observed that CD40 signalling augmented lipopolysaccharide (LPS)-induced IL-10 production by monocytes, but also observed that CD40 ligation induced IL-10 production by differentiated monocytes (macrophages) in the absence of LPS. Indeed, the priming mechanism of the macrophage determined the cytokine profile: macrophage-colony-stimulating factor preprogrammed macrophages to produce both IL-10 and TNF-α on stimulation by CD40L or Tck, whereas IFN-γ priming resulted predominantly in TNF-α [42].
IFN-γ-primed macrophages, however, can produce an endogenous IL-10 activity that is not secreted into the supernatant on cell contact with either Tck or CD40L transfectants, as neutralisation of endogenous IL-10 resulted in a marked increase in TNF-α production. This observation may agree with the report of membrane-associated IL-10 [43] and may highlight differences in the ability of these two types of macrophage-like cells to process cytokines.
Macrophage-colony-stimulating factor can usually be readily detected in the RA joint, while IFN-γ is scarce (reviewed in [44]). This may indicate why both TNF-α and IL-10 are found in synovial membrane cell cultures. This preprogramming of macrophages would appear to be irrespective of the triggering stimulus because macrophages stimulated by either Tck, CD40 ligation or LPS result in similar cytokine profiles. It would thus appear that the route of differentiation of the monocyte is critical in the induction of IL-10 in cognate interactions between activated T cells and macrophages.
Signalling pathways
TNF-α synthesis in monocytes in response to some stimuli (LPS) but not others (zymosan or CD45 ligation) is NF-κB dependent [32,45,46]. It is thus of interest to observe that Tck-induced, but not T-cell-receptor-dependent stimulated T cell (Ttcr)-induced, TNF-α production in monocytes is NF-κB dependent. Furthermore, whereas phosphatidyl-inositol 3-kinase (PI3K) inhibitors blocked Ttcrinduction of TNF-α, they paradoxically augmented TNF-α in monocytes stimulated by Tck cells. Importantly, we then found that RA T cells behaved like Tck cells, in that the induction of TNF-α in resting peripheral blood monocytes was NF-κB dependent but superinduced if PI3K was blocked. An identical result was observed if NF-κB or PI3K was blocked in the RA synovial cell cultures.
IL-10 production in monocytes/macrophages is equally complex. In response to LPS, IL-10 production is dependent on endogenous IL-1 and TNF-α. Furthermore, there is selective mitogen-activated protein kinase (MAPK) utilisation where IL-10 production was dependent on p38 MAPK, and TNF-α production was dependent on both p38 MAPK and p42/44 MAPK [47]. The involvement of p38 MAPK activity in IL-10 production subsequently led to the characterisation of the downstream effector, hsp27, as an anti-inflammatory mediator [48].
Little is known, however, regarding the involvement of the PI3K pathway in macrophage production of IL-10. PI3K and its downstream substrate p70S6K mediate IL-10-induced proliferative responses but not anti-inflammatory effects [49]. A recent study from our laboratory has described Tck-induced macrophage IL-10 production to be dependent on PI3K and p70S6K, whereas TNF-α production is negatively regulated by PI3K and is p70S6K dependent [50]. This suggests that IL-10 and TNF-α share a common component, p70S6K, but differentially utilise PI3K activity. These results are reproduced in the spontaneous cytokine production by RA synovial mononuclear cells (RA-SMCs) and by cocultures of RA synovial T cells with macrophages (RA-T/macrophage cocultures), further suggesting the relevance of this Tck/macrophage cognate coculture system as a model for cytokine production occurring in the inflamed synovium of the rheumatoid joint.
Although many other studies have implicated other signalling cascades in the induction of IL-10 production, not much work exists on the signalling required in macrophages stimulated by cognate interactions with fixed activated T cells. PKC and cAMP signalling have been implicated in IL-10 and TNF-α production and are currently under investigation in our group. Preliminary results would suggest that both these cascades differentially regulate IL-10 and TNF-α.
Studies undertaken by other groups have reported the involvement of the cAMP/PKA pathway in the induction of IL-10 production by human PBMC. The membrane-permeable dibutyryl cAMP was capable of elevating IL-10 mRNA and of augmenting LPS-induced IL-10 production, but on its own was incapable of producing IL-10 protein [51]. This work also demonstrated a role for PKC in the induction of IL-10 by LPS using the PKC inhibitors, calphostin C and H-7. This result contradicts our data but may reflect that this study used PBMC, a heterogeneous population, as compared with purified monocyte-derived macrophages.
In addition, the selective inhibition of phosphodiesterase type IV by rolipram was found to augment LPS-induced IL-10 production by murine peritoneal macrophages. The mechanism of this was thought to be as a consequence of LPS inducing the anti-inflammatory mediator, prostaglandin E2, which in turn upregulates intracellular cAMP via stimulation of adenylate cyclase activity [52].
Concluding remarks
The cognate activation of macrophages by T cells has focused almost exclusively on the membrane interactions mediating the macrophage effector function, such as nitric oxide release, phagocytosis, B-cell help and, more recently, the cytokine profiles induced. The control of T-cell-induced IL-10 and TNF-α production by monocyte-derived macrophages is complex and is regulated at many levels. These levels include priming of the monocyte/macrophage, T-cell stimulation, and hence specific ligand/receptor interaction, and the resulting signal cascades and crosstalk between them. The continued study of these contact-mediated interactions, the signal transduction distal to the receptor and how they compare between the induction of IL-10 and TNF-α may discover potential therapeutic targets selectively affecting proinflammatory TNF-α production without affecting anti-inflammatory IL-10 production. Such targets will prove to be of great benefit in the treatment of such chronic inflammatory diseases as RA.
Glossary of terms
MAPK = mitogen-activated protein kinase; PI3K = phosphatidylinositol 3-kinase; Tck = cytokine-stimulated T cells; Ttcr = T-cell-receptor-dependent stimulated T cells.
London, UK. 24-26 June 2002
Acknowledgements
Andy Foey is funded by a Wellcome Trust project grant. Prof Fionula Brennan and The Kennedy Institute are supported by a core grant from the Arthritis and Rheumatism Campaign.
References
- Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Maini RN. Anti-TNF alpha therapy or rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- Katsikis PD, Chu CQ, Brennan FM, Maini RN, Feldmann M. Immunoregulatory role of interleukin 10 in rheumatoid arthritis. J Exp Med. 1994;179:1517–1527. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomarat P, Vannier E, Dechanet J, Rissoan MC, Banchereau J, Dinarello CA, Miossec P. Balance of IL-1 receptor antagonist/IL-1 beta in rheumatoid synovium and its regulation by IL-4 and IL-10. J Immunol. 1995;154:1432–1439. [PubMed] [Google Scholar]
- Duke O, Panayi GS, Janossy G, Poulter LW. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982;49:22–30. [PMC free article] [PubMed] [Google Scholar]
- Brennan FM, Hayes AL, Ciesielski CJ, Green P, Foxwell BMJ, Feldmann M. Evidence that rheumatoid arthritis synovial T cells are similar to cytokine-activated T cells: involvement of phosphatidylinositol 3-kinase and nuclear factor κB pathways in tumour necrosis factor α production in rheumatoid arthritis. Arthritis Rheum. 2002;46:31–41. doi: 10.1002/1529-0131(200201)46:1<31::AID-ART10029>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Isler P, Vey E, Zhang JH, Dayer JM. Cell surface glycoproteins expressed on activated human T cells induce production of interleukin-1 beta by monocytic cells: a possible role of CD69. Eur Cytokine Network. 1993;4:15–23. [PubMed] [Google Scholar]
- Lacraz S, Isler P, Vey E, Welgus HG, Dayer JM. Direct contact between T lymphocytes and monocytes is a major pathway for induction of metalloproteinase expression. J Biol Chem. 1994;269:22027–22033. [PubMed] [Google Scholar]
- Suttles J, Miller RW, Tao X, Stout RD. T cells which do not express membrane tumour necrosis factor-alpha activate macrophage effector function by cell contact-dependent signalling of macrophage tumour necrosis factor-alpha production. Eur J Immunol. 1994;24:1736–1742. doi: 10.1002/eji.1830240803. [DOI] [PubMed] [Google Scholar]
- Sebbag M, Parry SL, Brennan FM, Feldmann M. Cytokine stimulation of T lymphocytes regulates their capacity to induce monocyte production of tumor necrosis factor-alpha, but not interleukin-10: possible relevance to pathophysiology of rheumatoid arthritis. Eur J Immunol. 1997;27:624–632. doi: 10.1002/eji.1830270308. [DOI] [PubMed] [Google Scholar]
- Parry SL, Sebbag M, Feldmann M, Brennan FM. Contact with T cells modulates monocyte IL-10 production: role of T cell membrane TNF-alpha. J Immunol. 1997;158:3673–3681. [PubMed] [Google Scholar]
- Chizzolini C, Chicheportiche R, Burger D, Dayer JM. Human Th1 cells preferentially induce interleukin (IL)-1beta while Th2 cells induce IL-1 receptor antagonist production upon cell/cell contact with monocytes. Eur J Immunol. 1997;27:171–177. doi: 10.1002/eji.1830270125. [DOI] [PubMed] [Google Scholar]
- Salmon M, Gaston JS. The role of T-lymphocytes in rheumatoid arthritis. Br Med Bull. 1995;51:332–345. doi: 10.1093/oxfordjournals.bmb.a072964. [DOI] [PubMed] [Google Scholar]
- Nanki T, Lipsky PE. Cytokine, activation marker, and chemokine receptor expression by individual CD4+ memory T cells in rheumatoid arthritis. Arth Res. 2000;2:415–423. doi: 10.1186/ar120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Zettl A, Weyand CM. T cell receptor repertoire in rheumatoid arthritis. Int Rev Immunol. 1998;17:339–363. doi: 10.3109/08830189809054410. [DOI] [PubMed] [Google Scholar]
- Firestein GS, Zvaifler NJ. How important are T cells in chronic rheumatoid synovitis? Arthritis Rheum. 1990;33:768–773. doi: 10.1002/art.1780330602. [DOI] [PubMed] [Google Scholar]
- Unutmaz D, Pileri P, Abrignani S. Antigen-independent activation of naive and memory resting T cells by a cytokine combination. J Exp Med. 1994;180:1159–1164. doi: 10.1084/jem.180.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes IB, al-Mughales J, Field M, Leung BP, Huang FP, Dixon R, Sturrock RD, Wilkinson PC, Liew FY. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Inter-leukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Liew FY. Interleukin 15: a proinflammatory role in rheumatoid arthritis synovitis. Immunol Today. 1998;19:75–79. doi: 10.1016/S0167-5699(97)01205-X. [DOI] [PubMed] [Google Scholar]
- Lou J, Dayer J-M, Grau GE, Burger D. Direct cell/cell contact with stimulated T lymphocytes induces the expression of cell adhesion molecules and cytokines by human brain microvas-cular endothelial cells. Eur J Immunol. 1996;26:3107–3113. doi: 10.1002/eji.1830261242. [DOI] [PubMed] [Google Scholar]
- Lou J, Ythier A, Burger D, Zheng L, Juillard P, Lucas R, Dayer J-M, Grau GE. Modulation of soluble and membrane-bound TNF-induced phenotypic and functional changes of human brain microvascular endothelial cells by recombinant TNF binding protein I. J Neuroimmunol. 1997;77:107–115. doi: 10.1016/S0165-5728(97)00067-2. [DOI] [PubMed] [Google Scholar]
- Yarwood H, Mason JC, Mahiouz D, Sugars K, Haskard DO. Resting and activated T cells induce expression of E-selectin and VCAM-1 by vascular endothelial cells through a contact-dependent but CD40 ligand-independent mechanism. J Leukoc Biol. 2000;68:233–242. [PubMed] [Google Scholar]
- Monaco C, Andreakos E, Young S, Feldmann M, Paleolog E. T cell-mediated signalling to vascular endothelium: Induction of cytokines, chemokines and tissue factor. J Leukoc Biol. 2002. in press . [PubMed]
- Li JM, Isler P, Dayer J-M, Burger D. Contact-dependent stimulation of monocytic cells and neutrophils by stimulated human T-cell clones. Immunology. 1995;84:571–576. [PMC free article] [PubMed] [Google Scholar]
- Burger D, Rezzonico R, Li JM, Modoux C, Pierce RA, Welgus HG, Dayer JM. Imbalance between interstitial collagenase and tissue inhibitor of metalloproteinases 1 in synoviocytes and fibroblasts upon direct contact with stimulated T lymphocytes: involvement of membrane-associated cytokines. Arthritis Rheum. 1998;41:1748–1759. doi: 10.1002/1529-0131(199810)41:10<1748::AID-ART7>3.3.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Rezzonico R, Burger D, Dayer JM. Direct contact between T lymphocytes and human dermal fibroblasts or synoviocytes down-regulates types I and III collagen production via cell-associated cytokines. J Biol Chem. 1998;273:18720–18728. doi: 10.1074/jbc.273.30.18720. [DOI] [PubMed] [Google Scholar]
- Hyka N, Dayer J-M, Modoux C, Kohno T, Edwards CK, Roux-Lombard P, Burger D. Apolipoprotein A-I inhibits the production of interleukin-1β and tumour necrosis factor-α by blocking contact-mediated activation of monocytes by T lymphocytes. Blood. 2001;97:2381–2389. doi: 10.1182/blood.V97.8.2381. [DOI] [PubMed] [Google Scholar]
- Manie S, Kubar J, Limouse M, Ferrua B, Ticchioni M, Breittmayer J-P, Peyron J-F, Schaffar L, Rossi B. CD3-stimulated Jurkat T cells mediate IL-1β production in monocytic THP-1 cells. Role of LFA-1 molecule and participation of CD69 T cell antigen. Eur Cytokine Network. 1993;4:7–13. [PubMed] [Google Scholar]
- Zembala M, Siedlar M, Ruggiero I, Wieckiewicz J, Mytar B, Mattei M, Colizzi V. The MHC class-II and CD44 molecules are involved in the induction of tumour necrosis factor (TNF) gene expression by human monocytes stimulated with tumour cells. Int J Cancer. 1994;56:269–274. doi: 10.1002/ijc.2910560221. [DOI] [PubMed] [Google Scholar]
- Hayes AL, Smith C, Foxwell BM, Brennan FM. CD45-induced tumor necrosis factor alpha production in monocytes is phosphatidylinositol 3-kinase-dependent and nuclear factor-kappaB-independent. J Biol Chem. 1999;274:33455–33461. doi: 10.1074/jbc.274.47.33455. [DOI] [PubMed] [Google Scholar]
- Wagner DH Jr, Stout RD, Suttles J. Role of the CD40-CD40 ligand interaction in CD4+ T cell contact-dependent activation of monocyte interleukin-1 synthesis. Eur J Immunol. 1994;24:3148–3154. doi: 10.1002/eji.1830241235. [DOI] [PubMed] [Google Scholar]
- Isomaki P, Aversa G, Cocks BG, Luukkainen R, Saario R, Toivanen P, de Vries JE, Punnonen J. Increased expression of signaling lymphocytic activation molecule in patients with rheumatoid arthritis and its role in the regulation of cytokine production in rheumatoid synovium. J Immunol. 1997;159:2986–2993. [PubMed] [Google Scholar]
- Wanidworanun C, Strober W. Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol. 1993;151:6853–6861. [PubMed] [Google Scholar]
- Graf D, Muller S, Korthauer U, van Kooten C, Weise C, Kroczek RA. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Eur J Immunol. 1995;25:1749–1754. doi: 10.1002/eji.1830250639. [DOI] [PubMed] [Google Scholar]
- Michel J, Langstein J, Hofstadter F, Schwarz H. A soluble form of CD137 (ILA/4-1BB), a member of the TNF receptor family, is released by activated lymphocytes and is detectable in sera of patients with rheumatoid arthritis. Eur J Immunol. 1998;28:290–295. doi: 10.1002/(SICI)1521-4141(199801)28:01<290::AID-IMMU290>3.3.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Pizzolo G, Vinante F, Chilosi M, Dallenbach F, Josimovic-Alasevic O, Diamantstein T, Stein H. Serum levels of soluble CD30 molecule (Ki-1 antigen) in Hodgkin's disease: relationship with disease activity and clinical stage. Br J Haematol. 1990;75:282–284. doi: 10.1111/j.1365-2141.1990.tb02664.x. [DOI] [PubMed] [Google Scholar]
- Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, Kiefer MC, Barr PJ, Mountz JD. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263:1759–1762. doi: 10.1126/science.7510905. [DOI] [PubMed] [Google Scholar]
- Shu U, Kiniwa M, Wu CY, Maliszewski C, Vezzio N, Hakimi J, Gately M, Delespesse G. Activated T cells induce interleukin-12 production by monocytes via CD40–CD40 ligand interaction. Eur J Immunol. 1995;25:1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- Chabot S, Williams G, Hamilton M, Sutherland G, Yong VW. Mechanisms of IL-10 production in human microglia–T cell interaction. J Immunol. 1999;162:6819–6828. [PubMed] [Google Scholar]
- Foey AD, Feldmann M, Brennan FM. Route of monocyte differentiation determines their cytokine production profile: CD40 ligation induces interleukin 10 expression. Cytokine. 2000;12:1496–1505. doi: 10.1006/cyto.2000.0750. [DOI] [PubMed] [Google Scholar]
- Fleming SD, Campbell PA. Macrophages have cell surface IL-10 that regulates macrophage bactericidal activity. J Immunol. 1996;156:1143–1150. [PubMed] [Google Scholar]
- Brennan FM, Maini RN, Feldmann M. Cytokine expression in chronic inflammatory disease. Br Med Bull. 1995;51:368–384. doi: 10.1093/oxfordjournals.bmb.a072967. [DOI] [PubMed] [Google Scholar]
- Foxwell BMJ, Browne K, Bondeson J, Clarke C, de Martin R, Brennan FM, Feldmann M. Efficient adenoviral infection with IκBα reveals that TNFα production in rheumatoid arthritis is NF-κB dependent. Proc Natl Acad Sci USA. 1998;95:8211–8215. doi: 10.1073/pnas.95.14.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondeson J, Foxwell BMJ, Brennan FM, Feldmann M. Defining therapeutic targets by using adenovirus: blocking NF-kB inhibits both inflammatory and destructive mechanisms in rheumatoid synovium but spares anti-inflammatory mediators. Proc Natl Acad Sci USA. 1999;96:5668–5673. doi: 10.1073/pnas.96.10.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foey AD, Parry SL, Williams LM, Feldmann M, Foxwell BM, Brennan FM. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha: role of the p38 and p42/44 mitogen-activated protein kinases. J Immunol. 1998;160:920–928. [PubMed] [Google Scholar]
- De AK, Kodys KM, Yeh BS, Miller-Graziano C. Exaggerated human monocyte IL-10 concomitant to minimal TNF-alpha induction by heat-shock protein 27 (Hsp27) suggests Hsp27 is primarily an antiinflammatory stimulus. J Immunol. 2000;165:3951–3958. doi: 10.4049/jimmunol.165.7.3951. [DOI] [PubMed] [Google Scholar]
- Crawley JB, Williams LM, Mander T, Brennan FM, Foxwell BM. Interleukin-10 stimulation of phosphatidylinositol 3-kinase and p70 S6 kinase is required for the proliferative but not the antiinflammatory effects of the cytokine. J Biol Chem. 1996;271:16357–16362. doi: 10.1074/jbc.271.27.16357. [DOI] [PubMed] [Google Scholar]
- Foey AD, Green P, Foxwell BMJ, Feldmann M, Brennan F. Cytokine stimulated T cells induce IL-10 production dependent on phosphatidylinositol 3-kinase and p70S6K: implications for rheumatoid arthritis. Arthritis Res. 2002;4:64–70. doi: 10.1186/ar385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel C, Vogt K, Platzer C, Randow F, Liebenthal C, Volk HD. Differential regulation of monocytic tumor necrosis factor-alpha and interleukin-10 expression. Eur J Immunol. 1996;26:1580–1586. doi: 10.1002/eji.1830260726. [DOI] [PubMed] [Google Scholar]
- Kambayashi T, Jacob CO, Zhou D, Mazurek N, Fong M, Strassmann G. Cyclic nucleotide phosphodiesterase type IV participates in the regulation of IL-10 and in the subsequent inhibition of TNF-alpha and IL-6 release by endotoxin-stimulated macrophages. J Immunol. 1995;155:4909–4916. [PubMed] [Google Scholar]