Abstract
Chapter summary
Contact-mediated signaling of monocytes by human stimulated T lymphocytes (TL) is a potent proinflammatory mechanism that triggers massive upregulation of the proinflammatory cytokines IL-1 and tumor necrosis factor-α. These two cytokines play an important part in chronic destructive diseases, including rheumatoid arthritis. To date this cell–cell contact appears to be a major endogenous mechanism to display such an activity in monocyte-macrophages. Since TL and monocyte-macrophages play a pivotal part in the pathogenesis of chronic inflammatory diseases, we investigated the possible ligands and counter-ligands involved in this cell–cell interaction. We also characterized an inhibitory molecule interfering in this process, apolipoprotein A-I. This review aims to summarize the state of the art and importance of contact-mediated monocyte activation by stimulated TL in cytokine production in rheumatoid arthritis and mechanisms that might control it.
Keywords: cytokines, inflammation, monocytes, rheumatoid arthritis, T lymphocytes
Introduction
Inflammation is usually the consequence of tissue damage and its purpose is to direct plasma factors and immune cells to the lesion site to eradicate infection and repair damaged tissue. In pathological conditions such as chronic inflammation, infiltration of immune cells into the target tissue precedes tissue damage; the lesion occurs after infiltration of immune cells. Based on observations in animal models it is usually thought that the first cells to infiltrate the tissue are T lymphocytes (TL), suggesting a pathogenic role for the latter cells. However, the mechanisms underlying the extravasation of T cells into the joint are still elusive [1]. T cell cytokines such as IL-4, IL-10, IL-13, and transforming growth factor (TGF)-β have predominantly anti-inflammatory effects and, in the human system, IFN-γ alone displays weak activation capacity in terms of IL-1β and tumor necrosis factor (TNF)-α induction, suggesting that soluble factors produced by T cells are not pathological mediators. Therefore, T cells might exert a pathological effect through direct cellular contact with monocyte-macrophages (Mφ). Studies carried out during the past ten years in our laboratory proved the premise that IL-1β and TNF-α are markedly increased in this interaction [2-8]. This was further confirmed by others [9-12]. This review aims to assess the importance of contact-mediated monocyte activation by stimulated TL resulting in cytokine production, and its relevance to chronic inflammatory conditions as exemplified by rheumatoid arthritis (RA). Possible modulations and controls of this mechanism are discussed.
Historical background
The history of contact-mediated activation of Mφ by stimulated TL (reviewed in [13]) began in the mid-eighties when it was observed that the expression of membrane-associated IL-1 (IL-1α) in mouse Mφ was mediated by both soluble factors and direct contact with T cells. The importance of cellular contact was confirmed by experiments showing that IL-1 was induced upon T cell-Mφ contact with both Th1 and Th2 cells in the absence of lymphokine release. We also observed that direct contact with stimulated T cells was a potent stimulus of Mφ activation. Although observations have shown that the induction of murine Mφ effector functions mediated by TL in living cell co-cultures involved signals delivered by cell–cell contact together with IFN-γ, fixed, stimulated T cells induced TNF production in Mφ in the absence of IFNγ. Furthermore, when isolated plasma membranes from different stimulated T-cell clones were used, both stimulated Th1 and Th2 cells were able to activate Mφ, establishing that direct contact with stimulated T cells was a potent mechanism inducing Mφ effector functions.
Triggering of cytokine production in chronic inflammation
Based on histology, function, animal models and clinical studies, Mφ appear to play a key role in chronic inflammation by producing large amounts of IL-1 and TNF-α under various stimuli. In chronic inflammation, infiltration of TL into the target tissue precedes tissue damage suggesting that their effect is pathogenic. Angiogenesis and proliferation of resident cells accompany this infiltration. However, Mφ are rapidly found in the lesion and interactions occur between TL and Mφ. These interactions lead to IL-1 and TNF-α production, a process that is potentiated by many other factors including IFN-γ, IL-15, and IL-18; T cell cytokines such as IL-4, IL-10, IL-13, and TGF-β are inhibitory (Fig. 1). Our previous seminal studies and further works by others strongly argue that direct cellular contact with stimulated T cells is a major pathway for the production of IL-1 and TNF-α in Mφ [13]. Indeed, contact-mediated activation of Mφ by stimulated TL is as potent as optimal doses of lipopolysaccharide (LPS) or phorbol myristate acetate in inducing IL-1β and TNF-α production in monocytes and cells of the monocytic lineage, such as THP-1 cells [13]. We therefore postulate that this mechanism is highly relevant to the pathogenesis and maintenance of chronic inflammation in diseases such as RA.
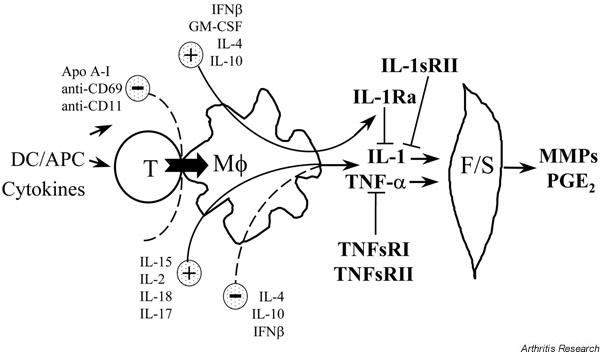
Figure 1.
Scheme of the activation cascade from T lymphocytes (TL) to monocyte-macrophages (Mφ) and fibroblasts/synoviocytes (F/S). Activated TL trigger Mφ to produce proinflammatory cytokines that in turn induce the production of matrix-destructive metalloproteinases (MMPs) and prostaglandin E2 (PGE2), the latter products being involved in cartilage destruction and bone resorption. These processes are controlled by proinflammatory factors (IL-15, IL-2, IL-18, IL-17) and anti-inflammatory factors (IL-4, IL-10, granulocyte/macrophage colony stimulating factor [GM-CSF], IFN-β). Furthermore, naturally occurring inhibitors (IL-1sRII, IL-1Ra, tumor necrosis factor [TNF]sRI, TNFsRII) inhibit the activity of IL-1 and TNF-α, the production of which is blocked by apolipoprotein (apo) A-I and decreased by exogenous antibodies to CD69 and β2-integrins (CD11b>CD11c>CD11a). APC, antigen presenting cells; DC, dendritic cells.
Relevance of contact-mediated activation of Mφ to chronic inflammatory diseases
T lymphocytes are likely to play a pivotal role in the pathogenesis of RA [1]. In RA, TL displaying a mature helper phenotype (i.e. CD3+CD4+ CD45RO+) are the main infiltrating cells in the pannus, at percentages ranging from 16% of total cells in 'transitional areas' to 75% in 'lymphocyte-rich areas' [14,15]. The latter are to be found in perivascular regions, around 'high endothelial venule'-like vessels, where TL extravasation occurs forming germinal center-like structures [16]. Although they are the most abundant infiltrating cells in the pannus, the importance of TL in RA pathogenesis has been mainly proven in animal models [1]. Indeed, T cells from RA patients that were transferred to SCID mice induced arthritis [17]. Although evidence suggests that TL play a pathogenic part in chronic inflammatory diseases, the mechanism by which they exert their pathogenicity has not been clearly elucidated [1], thus contact-mediated induction of proinflammatory cytokines in Mφ might represent one of these mechanisms.
T cell signaling of Mφ by direct cell–cell contact
The activation of effector cells mediated by stimulated TL has been abundantly substantiated in the T-B lymphocyte model for the induction of cell proliferation and antibody secretion, which require both direct cell–cell contact and soluble signals. Indeed, B cells can be activated in the absence of antigen by direct contact with activated T cells. A considerable amount of signaling crosstalk was observed in T and B cells, that triggered various synchronized signals resulting in the activation of effector functions in both cell types, as recently exemplified in RA [18]. Similarly, ample data exist regarding the crosstalk between dendritic cells and TL. However, much less is known about the crosstalk between TL and Mφ, other than antigen presentation.
Most T cell types, including T cell clones, freshly isolated TL, and T cell lines (such as HUT-78 cells) induce IL-1 and TNF-α in Mφ [2,3,5,13]. Various stimuli other than phytohemagglutinin/phorbol myristate acetate induce TL to activate monocytes by direct cellular contact: cross-linking of CD3 by immobilized anti-CD3 mAb with or without cross-linking of the co-stimulatory molecule CD28 [7,12,19]; antigen recognition on antigen-specific T cell clones [7]; and cytokines [9,10,20]. Furthermore, depending on T cell type and T cell stimulus, direct cell–cell contact with stimulated TL can induce different patterns of products in Mφ (Table 1). This suggests that multiple ligands and counter-ligands are involved in the contact-mediated activation of Mφ, which are differentially induced in T cells depending on the stimulus. Recent studies by Brennan et al. [12] have led to the concept that, based on their effect on Mφ, T>L can be classified as cytokine-activated TL (Tck) (which are likely to be present in RA synovium [12]) or T-cell-receptor-activated TL. In some cases, an imbalance in production of proinflammatory versus anti-inflammatory cytokines has been observed, where Th1 cell clones preferentially induce IL-1β rather than IL-1Ra production, and cytokine-stimulated TL induce TNF-α production but not that of IL-10 [7,9]. Besides, we demonstrated that upon contact with stimulated T cells, the balance between IL-1β and IL-1Ra production in monocytes is ruled by Ser/Thr phosphatase(s) [8] and that contact-activated THP-1 cells express membrane-associated protease(s), neutralizing TNF-α activity both by degrading the latter cytokine and by cleaving its receptors at the cell surface [6]. Thus triggering these intra- and extra-cellular processes by direct contact with stimulated TL may regulate the proinflammatory cytokinesand their inhibitors. The balance of their production in monocytes dictates, in part, the outcome of the inflammatory process, as depicted in Figure 1.
Table 1.
Depending on T-cell stimulus, various products are induced in monocytes upon cell–cell contact
| Stimulus | Type of T cell | Type of monocyte | Products | References |
|---|---|---|---|---|
| PHA/PMA | PB TL, HUT-78 cells, Jurkat cells, synovial and PB T cell clones: CD4+; CD8+; Th1; and Th2 | PB monocytes, THP-1 cells | TNF-α, IL-1β, IL-6, IL-8, IL-1α, IL-1Ra, TNFsRs, MMP-1 MMP-9, TIMP-1 | [2-6,8,62] |
| PdBu/ionomycine | PB TL | PB monocytes | TNF-α, IL-10 | [19] |
| anti-CD3 | PB TL, synovial T cells | PB monocytes, THP-1 cells | TNF-α, IL-10, IL-1β; MMP-1 | [4,12,19,22,62] |
| anti-CD3 and anti-CD28 or specific Ag | Th1 cell clones | THP-1 cells | IL-1β, low IL-1Ra | [7] |
| anti-CD3 and anti-CD28 or specific Ag | Th2 cell clones | THP-1 cells | IL-1Ra, low IL-1β | [7] |
| Cytokines* | PB TL, synovial T cells, Th1 and Th2 cell clones | PB monocytes | TNF-α, IL-1β | [9-12,20,63] |
| Anti-CD3 | PB TL | PMA/IFN-γ-treated U937 cell | TNF-α, IL-10, IL-12 and IL-4 | [64] |
*IL-2 or IL-15 alone or in combination with IL-6 and TNF-α. Ag, antigen; MMP, matrix metalloproteinase; PB, peripheral blood; PHA, phytohemagglutinin; PMA, phorbol myristate acetate; Th, T helper cell; TIMP, tissue inhibitor of matrix metalloproteinases; TL, T lymphocyte; TNF, tumor necrosis factor; TNFsRs, TNF soluble receptors. Modified and published with permission from European Cytokine Network[13].
Cell surface molecules involved in contact-mediated monocyte activation
A critical issue arising from these observations is the identity of the molecules on the T cell surface that are involved in contact-mediated signaling of Mφ activation, as well as their counter-ligands. It has been postulated that T cell membrane-associated TNF-α was involved in Mφ activation. However, fixed, stimulated Th2 cells from a T cell line that did not express membrane-associated TNF induced both TNF and IL-1 production in Mφ [21] demonstrating that TNF-α might play a part, but not a primary one. We have shown that neither soluble TNF-α receptors nor IL-1Ra block T cell signaling of the monocytic cell line THP-1. Besides, neutralizing antibodies to TNF-α, IL-1, IL-2, IFN-γ, and granulocyte/macrophage colony stimulating factor all failed to affect monocyte activation by membranes from stimulated T cells [2-4]. Similarly, although lymphotoxin (LT)-α receptor is expressed in Mφ, it is not likely that membrane-associated LT is involved in Mφ signaling upon contact with stimulated T cells, since Th2 cells do not express LT at either protein or mRNA levels. It must be emphasized that the contact between TL and Mφ could involve different ligands and counter-ligands in the murine and the human systems.
In addition to membrane-associated cytokines, other surface molecules have been assessed for their ability to activate Mφ upon contact with stimulated T cells (e.g. lymphocyte function-associated antigen [LFA]-1/intercellular adhesion molcule-1, CD2/LFA 3, CD40/CD40L, and lymphocyte activation antigen-3. Thus CD40/CD40L interaction was shown to be involved in the contact activation of both human and mouse Mφ by TL stimulated for 6 hours [22]. However, when stimulated for 24 hours, TL isolated from both CD40L knockout and wild type mice triggered Mφ activation, although to a lesser extent [23]. In our system, where human TL were stimulated for 48 hours and expressed a high capacity to induce cytokines in Mφ, we never observed any inhibition of contact-induced cytokine production, by blocking antibodies to either CD40L or soluble CD40. Furthermore, HUT-78 cells, which efficiently induce cytokine production in Mφ, do not express CD40L mRNA in resting or activated condition [24]. Finally, THP-1 cells that respond to contact-mediated activation by membranes of stimulated T cells do not express CD40. Another study of ours shows that in co-cultures of living cells stimulated with IL-15, Th1- but not Th2-clones induce IL-1β production in monocytes [20]. In the latter system, blockade of the CD40–CD40L interaction results in inhibition of IL-1β production while IL-1Ra induction is unaffected. This differential effect indicates the selective relevance of CD40–CD40L engagement upon monocyte activation by Th1 clones. However, the levels of CD40L expression did not differ in Th1 and Th2 cell clones, implying that additional, unidentified molecule(s) preferentially expressed by Th1 cells are involved in their capacity to induce IL-1β. Therefore, CD40–CD40L might be a cofactor in contact-mediated activation of Mφ by stimulated TL. Lymphocyte activation gene (LAG)-3 might also be one of the latter factors since it is able to synergize with low amounts of CD40L in inducing TNF-α and IL-12 on monocyte-derived dendritic cells [11]. In our system LAG-3 did not induce the production of IL-1β and TNF-α (unpublished results). Others have found that soluble CD23 induces cytokine production on monocytes [25]. In monocytes, the counter-ligands of CD23 are CD11b/CD18 and CD11c/CD18 rather than CD21. LFA-1 (CD11a/CD18) and CD69 also contribute to the activation of human monocytic cells by stimulated T cells [2,26]. This was substantiated by a study showing that IL-15 induced synovial T cells from RA patients to activate the production of TNF-α by Mφ. This effect was inhibited by antibodies to CD69, LFA-1 and intracellular adhesion molecule-1 [10].
Together these studies suggest that some known surface molecules are indeed involved in T cell signaling of Mφ. However, inhibitors (e.g. antibodies) of these molecules fail to abolish monocyte activation altogether, suggesting that the factor(s) required for T cell signaling of human monocytes by direct contact remain(s) to be identified.
Intracellular pathways involved in cytokine production by monocyte-macrophages upon contact with stimulated T lymphocytes
Depending on the type of stimulus, different intracellular pathways are used in Mφ for the production of the same cytokine. Some stimuli (i.e. microbial products) can induce the production of TNF-α, IL-1β, and IL-1Ra in human Mφ. In other cases, IL-1Ra might be induced in the absence of IL-1 induction, but from what is known at present, all stimuli eliciting IL-1β production also trigger IL-1Ra production, at least in Mφ. The tight control of proinflammatory cytokine production is indeed a prerequisite to avoid a cascade of events that could lead to uncontrolled inflammation. It is also well known that the production of both IL-1β and TNF-α is tightly regulated at several levels, including the dissociation between transcription and translation [27]. For example, stimuli such as C5a, hypoxia, blood clotting, or surface contact are not sufficient to provide a signal for translation, despite a vigorous signal for transcription [28]. Consequently, some stimuli might provide signals for complete cytokine gene transcription but no translational signaling. IL-1β and TNF-α translation can be blocked by pyridinyl-imidazol compounds that bind and inactivate the mitogen-activated protein kinase p38 [28].
Lipopolysaccharide has been the most frequently used stimulus for in vitro studies aimed at identifying transduction pathways underlying cytokine production in Mφ. Components of the transduction pathways that are induced by toll-like receptor 4 [29,30] and lead to the translocation of nuclear factor (NF)-κB, AP-1, protein kinase C [31], and p44/42 (extracellular signal-regulated kinase) [32] are all likely to be also involved in cytokine gene induction by other stimuli. Other components of transcription pathways leading to cytokine synthesis have been identified after signaling by engagement of cell surface molecules. The engagement of CD45 leading to TNF-α production in monocytes adopts a unique signaling pathway (phospho-inositol-3 kinase [PI3-K]) and pathways shared with LPS (NF-κB and p38 kinase). Furthermore, different products induced by the same stimulus can depend on different signaling pathways (e.g. PI3-K pathway selectively controls IL-1Ra, and not IL-1β, in 'septic' leukocytes [33]).
Upon T-cell-contact-mediated activation of Mφ, different pathways are involved in the induction of IL-10 or TNF-α [34,35]. Interestingly, PI3-K is mainly involved in IL-10 induction whereas NF-κB is involved in TNF-α production, suggesting that PI3-K is preferentially involved in pathways controlling the production of anti-inflammatory factors. T cells activated by a different stimulus also induce these two pathways [12]. Indeed, T cells that had been activated for eight days using a cocktail of cytokines and designated Tck induced TNF-α production in resting monocytes in a cell-contact-dependent manner [12]. The same results were obtained with RA synovial T cells, suggesting that Tck resemble RA synovial joint T cells in terms of contact-mediated cytokine induction in monocytes. In this system, TNF-α production was abrogated by blockade of the transcription factor NF-κB but enhanced by PI3-kinase inhibitors. Production of TNF-α, induced in monocytes by peripheral blood T cells that were stimulated by crosslinked anti-CD3, was not affected by NF-κB and was inhibited in the presence of PI3-kinase inhibitors. The premise that Tck behave similarly to RA T cells further confirms the importance of T cells in inducing TNF-α in chronic inflammatory rheumatoid tissue. We recently demonstrated that PI3-kinase might represent a checkpoint signaling molecule favoring IL-1Ra synthesis over that of IL-1β [36]. Furthermore, upon contact with stimulated T cells, the balance between IL-1β and IL-1Ra production in monocytes was also regulated by Ser/Thr phosphatase(s) [8].
Modulation of contact-mediated activation of monocyte-macrophages
Since the contact-mediated activation of Mφ is a major pathway toward cytokine production, the modulation of this mechanism (i.e. the blockade of IL-1 and TNF-α production at the triggering level of contact-mediated activation) would be of therapeutic interest. We established that therapeutic agents used in RA and multiple sclerosis (i.e. leflunomide [37] and IFN-β [38,39], respectively) affect the contact-mediated activation of monocytes. Leflunomide inhibits the ability of stimulated TL to trigger IL-1β production in monocytes, resulting in an enhancement of the IL-1Ra/IL-1β molar ratio [40]. Similar results were obtained with IFN-β. Indeed, upon contact-mediated activation of monocytes, IFN-β not only inhibited IL-1β and TNF-α but it also stimulated IL-1Ra [41,42], due to the fact that IFN-β interfered with the activation of both TL and monocytes. However, surface molecules of TL that we found to be involved in contact-signaling of monocytes (i.e. TNF-α, CD25, CD69, CD18, CD11a, CD11b, CD11c, CD40L, and LAG-3) were not modulated by IFN-β or leflunomide, suggesting that other surface activators on TL are involved in the contact-mediated activation of monocytes by stimulated TL. While CD40–CD40 ligand engagement is required, it may not be sufficient for human Th1 cell induction of IL-2- or IL-15-driven, contact-dependent IL-1β production by Mφ [20]. These effects are similar to those observed in patients in vivo, suggesting the occurrence of contact-mediated activation of monocytes in vivo.
Identification of a specific inhibitor of T-cell contact-mediated activation of monocyte-macrophages
The inhibition of T cell signaling of monocytes might be important because it would maintain a low level of monocyte activation within the blood stream. We recently identified apolipoprotein (apo) A-I as being a specific inhibitor of contact-mediated activation of monocytes [43]. These results were further confirmed by using recombinant apo A-I [42]. Apo A-I is a 'negative acute-phase protein' and the main protein of high-density lipoproteins (HDL). Variations of apo A-I concentration were observed in several inflammatory diseases [44]. In RA, the levels of circulating apo A-I and HDL-cholesterol in untreated patients were lower than in normal controls [45-47]. In contrast, apo A-I was enhanced in synovial fluid of RA patients [48], although its concentrations remained 10-fold lower in synovial fluid than in plasma. The elevation of apo A-I levels in synovial fluid of RA patients was accompanied by an enhancement in cholesterol, suggesting an infiltration of HDL particles in the inflamed joint. This putative regulatory mechanism might, however, be overcome by serum amyloid A (SAA), a positive acute-phase protein, which is not only produced in the liver but also in the RA synovium [49]. Indeed SAA can displace apo A-I from HDL, and HDL-associated SAA displays proinflammatory activity [50]. Recently, it was shown that the inflammatory condition in juvenile RA was associated with hypo-high density lipoproteinemia and a significant decrease in apo A-I concentration in patient plasma [51]. Furthermore, in collaboration with B Bresnihan (Dublin, Ireland), we observed that apo A-I was present in the perivascular region of RA synovium but not in normal tissue (manuscript in preparation). In systemic lupus erythematosus, apo A-I plasma concentrations are diminished. This decrease is associated with the presence of anti-apo A-I antibodies in 32% of patients [52]. In patients with multiple sclerosis undergoing IFN-β therapy, levels of apo A-I proved to be lower in a subgroup of patients experiencing relapses and/or progression [53]. Furthermore, increasing evidence strongly supports the contention that inflammatory responses are an integral part of atherosclerosis [54]. Indeed, Mφ and TL are present at all stages of lesion development, and the earliest lesion (fatty streak) consists predominantly of Mφ and TL. In addition, transfer of CD4+ T cells aggravates atherosclerosis in immunodeficient apo E knockout mice [55]. Therefore, TL-signaling of monocytes may occur in atherosclerosis. Gene transfer of apo A-I reduced atherosclerosis in several mouse models [56]. This phenomenon has been attributed to the function of HDL-associated apo A-I in lipid metabolism and transport. However, the premise that apo A-I inhibit contact-mediated activation of Mφ by TL suggests that HDL has protective functions at several levels in atherosclerosis, including cytokine production by Mφ. Furthermore, the incidence of atherosclerotic heart disease is higher in patients with systemic lupus erythematosus and RA [57], in agreement with the inverse correlation of the concentration of HDL with the incidence of atherosclerosis.
With the exception of atherosclerosis, which had not been considered a chronic inflammatory disease until recently, and despite the scarcity of studies having dealt with the levels of HDL in 'classical' chronic inflammatory diseases, it seems that chronic inflammation is associated with low levels of HDL-associated apo A-I. We thus hypothesize that in these diseases, a vicious circle sets in, which is responsible for the maintenance of inflammation (Fig. 2).
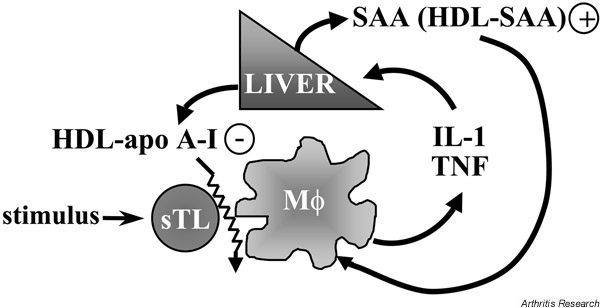
Figure 2.
Scheme of the relationship between chronic inflammation, acute-phase proteins and homeostasis of cytokines. The liver produces both apolipoprotein (apo) A-I and serum amyloid A (SAA). IL-1β and TNF-α differentially regulate the production of acute-phase proteins by increasing the production of SAA (a proinflammatory factor) and decreasing that of apo A-I (an anti-inflammatory factor). The decreased level of apo A-I results in a better activation of monocyte-macrophages (Mφ) by direct contact with stimulated T lymphocytes (sTL), enhancing the production of IL-1 and TNF. The increased levels of SAA result in the substitution of apo A-I by SAA on high-density lipoprotein (HDL), and SAA–HDL further stimulates the production of cytokines by Mφ.
The identification of HDL-associated apo A-I ligand(s) on stimulated T cells might lead to the elucidation of the mechanisms and molecules involved in T cell signaling of monocytes. HDL-associated apo A-I has been shown to bind specifically to a number of cell-surface molecules, including HDL binding protein [58], scavenger receptor B1, HDL binding protein-2, cubilin [59], ATP-binding cassette A1 transporter [60], and a 95 kDa protein at the surface of human fetal hepatocytes [61]. All these proteins display a high molecular weight (≥80 kDa) and to date have not been identified (not searched) on T cells. In conclusion, the identification of apo A-I as an important regulator of TL–Mφ interaction sheds new light on the role of HDL-associated apo A-I in innate and acquired immune response, and could be extended to other diseases.
Dissociation of IL-1β and IL-1Ra production in monocytes in contact with stimulated T lymphocytes due to the presence of HDL-associated apo A-I
Although HDL inhibited the production of TNF-α and IL-1β in both peripheral blood monocytes and THP-1 cells, this did not apply to IL-1Ra. Indeed, in peripheral blood mononuclear cells that were stimulated by either phyto-hemagglutinin or an antigen (tetanus toxoid), IL-1Ra production was not significantly inhibited, contrasting with the obvious inhibition of IL-1β and TNF-α production (Fig. 3). Furthermore, this indicates that apo A-I was able to inhibit contact-mediated Mφ activation when TL were stimulated by either nonspecific stimuli or antigens.
Figure 3.
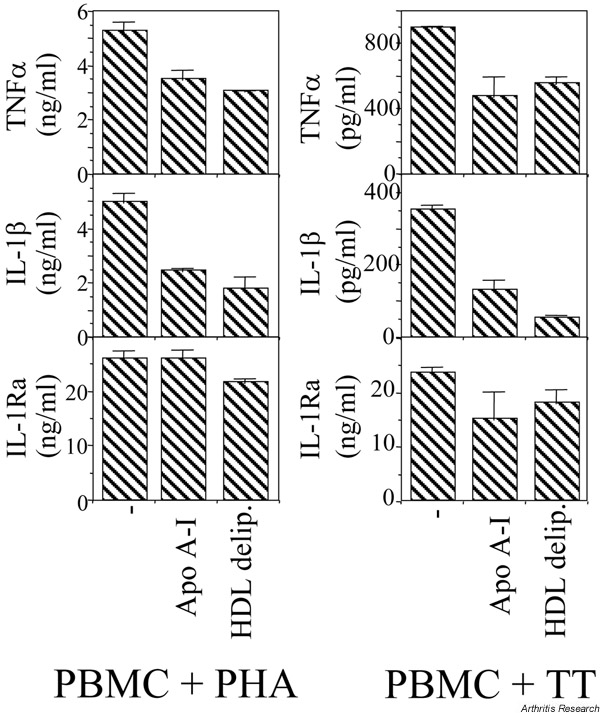
Apolipoprotein (apo) A-I does not significantly inhibit IL-1Ra production in peripheral blood mononuclear cells (PBMC) stimulated by either phytohemagglutinin (PHA) or tetanus toxoid (TT). Conditions: 0.4 × 106 cells/well/200 μl; 5 μg/ml polymyxin; 1 μg/ml PHA; 10 μg/ml TT; 48 hour incubation for PHA, 72 hours for TT.
Concluding remarks
To date, direct cell–cell contact with stimulated TL is themain pathway triggering activation of Mφ in the absence of infectious agents. The potency of this mechanism suggests that it is a major pathway by which TL exert their pathogenic effect in chronic inflammatory diseases of autoimmune etiology. Many more investigations are needed to identify the surface molecules (ligands and counter-ligands) involved in this process. However, the control of contact-mediated signaling of monocytes by apo A-I might represent the first step toward developing novel agents that interfere with the inflammatory response induced by cell–cell contact, which leads to tissue destruction in chronic inflammatory diseases.
Glossary of terms
apo = apolipoprotein; HDL = high-density lipoprotein; LAG = lymphocyte activation gene; LFA = lymphocyte-function-associated antigen; LT = lymphotoxin; PI3-K = phosiphinositol-3 kinase; PMA = phorbol myristate acetate; SAA = serum amyloid A; Tck = cytokine-activated T lymphocyte; TL = T lymphocytes.
London, UK. 24-26 June 2002
Acknowledgments
The authors gratefully acknowledge Mrs Roswitha Rehm for skilful reading of the manuscript. Unpublished results reported here were part of projects supported by grant #31-50930-97 from the Swiss National Science Foundation as well as grants from the Swiss Society for Multiple Sclerosis, and the Hans Wilsdorf Foundation.
References
- Firestein GS, Zvaifler NJ. How important are T cells in chronic rheumatoid synovitis?: II. T cell-independent mechanisms from beginning to end. Arthritis Rheum. 2002;46:298–308. doi: 10.1002/art.502. [DOI] [PubMed] [Google Scholar]
- Vey E, Zhang JH, Dayer J-M. IFN-gamma and 1,25(OH)2D3 induce on THP-1 cells distinct patterns of cell surface antigen expression, cytokine production, and responsiveness to contact with activated T cells. J Immunol. 1992;149:2040–2046. [PubMed] [Google Scholar]
- Isler P, Vey E, Zhang JH, Dayer JM. Cell surface glycoproteins expressed on activated human T-cells induce production of interleukin-1 beta by monocytic cells: a possible role of CD69. Eur Cytokine Netw. 1993;4:15–23. [PubMed] [Google Scholar]
- Lacraz S, Isler P, Vey E, Welgus HG, Dayer JM. Direct contact between T lymphocytes and monocytes is a major pathway for induction of metalloproteinase expression. J Biol Chem. 1994;269:22027–22033. [PubMed] [Google Scholar]
- Li JM, Isler P, Dayer JM, Burger D. Contact-dependent stimulation of monocytic cells and neutrophils by stimulated human T-cell clones. Immunology. 1995;84:571–576. [PMC free article] [PubMed] [Google Scholar]
- Vey E, Burger D, Dayer JM. Expression and cleavage of tumor necrosis factor-alpha and tumor necrosis factor receptors by human monocytic cell lines upon direct contact with stimulated T cells. Eur J Immunol. 1996;26:2404–2409. doi: 10.1002/eji.1830261021. [DOI] [PubMed] [Google Scholar]
- Chizzolini C, Chicheportiche R, Burger D, Dayer JM. Human Th1 cells preferentially induce interleukin (IL)-1 beta while Th2 cells induce IL-1 receptor antagonist production upon cell/ cell contact with monocytes. Eur J Immunol. 1997;27:171–177. doi: 10.1002/eji.1830270125. [DOI] [PubMed] [Google Scholar]
- Vey E, Dayer JM, Burger D. Direct contact with stimulated T cells induces the expression of IL-1 beta and IL-1 receptor antagonist in human monocytes. Involvement of serine/threonine phosphatases in differential regulation. Cytokine. 1997;9:480–487. doi: 10.1006/cyto.1997.0191. [DOI] [PubMed] [Google Scholar]
- Sebbag M, Parry SL, Brennan FM, Feldmann M. Cytokine stimulation of T lymphocytes regulates their capacity to induce monocyte production of tumor necrosis factor-alpha, but not interleukin-10: Possible relevance to pathophysiology of rheumatoid arthritis. Eur J Immunol. 1997;27:624–632. doi: 10.1002/eji.1830270308. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- Avice MN, Sarfati M, Triebel F, Delespesse G, Demeure CE. Lymphocyte activation gene-3, a MHC class II ligand expressed on activated T cells, stimulates TNF-alpha and IL-alpha production by monocytes and dendritic cells. J Immunol. 1999;162:2748–2753. [PubMed] [Google Scholar]
- Brennan FM, Hayes AL, Ciesielski CJ, Green P, Foxwell BM, Feld-mann M. Evidence that rheumatoid arthritis synovial T cells are similar to cytokine-activated T cells: involvement of phosphatidylinositol 3-kinase and nuclear factor kappaB pathways in tumor necrosis factor alpha production in rheumatoid arthritis. Arthritis Rheum. 2002;46:31–41. doi: 10.1002/1529-0131(200201)46:1<31::AID-ART10029>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Burger D. Cell contact-mediated signaling of monocytes by stimulated T cells: a major pathway for cytokine induction. Eur Cytokine Netw. 2000;11:346–353. [PubMed] [Google Scholar]
- Tak PP, Smeets TJM, Daha MR, Kluin PM, Meijers KAE, Brand R, Meinders AE, Breedveld FC. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997;40:217–225. doi: 10.1002/art.1780400206. [DOI] [PubMed] [Google Scholar]
- Smeets TJ, Kraan MC, Galjaard S, Youssef PP, Smith MD, Tak PP. Analysis of the cell infiltrate and expression of matrix metalloproteinases and granzyme B in paired synovial biopsy specimens from the cartilage-pannus junction in patients with RA. Ann Rheum Dis. 2001;60:561–565. doi: 10.1136/ard.60.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP, Bresnihan B. The pathogenesis and prevention of joint damage in rheumatoid arthritis: advances from synovial biopsy and tissue analysis. Arthritis Rheum. 2000;43:2619–2633. doi: 10.1002/1529-0131(200012)43:12<2619::AID-ANR1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Mima T, Saeki Y, Ohshima S, Nishimoto N, Matsushita M, Shimizu M, Kobayashi Y, Nomura T, Kishimoto T. Transfer of rheumatoid arthritis into severe combined immunodeficient mice. The pathogenetic implications of T cell populations oligoclonally expanding in the rheumatoid joints. J Clin Invest. 1995;96:1746–1758. doi: 10.1172/JCI118220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T cell activation in rheumatoid synovium is B cell dependent. J Immunol. 2001;167:4710–4718. doi: 10.4049/jimmunol.167.8.4710. [DOI] [PubMed] [Google Scholar]
- Parry SL, Sebbag M, Feldmann M, Brennan FM. Contact with T cells modulates monocyte IL-10 production. Role of T cell membrane TNF-alpha. J Immunol. 1997;158:3673–3681. [PubMed] [Google Scholar]
- Ribbens C, Dayer JM, Chizzolini C. CD40-CD40 ligand (CD154) engagement is required but may not be sufficient for human T helper 1 cell induction of interleukin-2- or interleukin-15-driven, contact-dependent, interleukin-1beta production by monocytes. Immunology. 2000;99:279–286. doi: 10.1046/j.1365-2567.2000.00948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttles J, Miller RW, Tao X, Stout RD. T cells which do not express membrane tumor necrosis factor-alpha activate macrophage effector function by cell contact- dependent signaling of macrophage tumor necrosis factor-alpha production. Eur J Immunol. 1994;24:1736–1742. doi: 10.1002/eji.1830240803. [DOI] [PubMed] [Google Scholar]
- Wagner DH, Stout RD, Suttles J. Role of the CD40-CD40 ligand interaction in CD4(+) T cell contact-dependent activation of monocyte interleukin-1 synthesis. Eur J Immunol. 1994;24:3148–3154. doi: 10.1002/eji.1830241235. [DOI] [PubMed] [Google Scholar]
- Stout RD, Suttles J, Xu J, Grewal IS, Flavell RA. Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J Immunol. 1996;156:8–11. [PubMed] [Google Scholar]
- Gauchat J-F, Aubry J-P, Mazzei G, Life P, Jomotte T, Elson G, Bonnefoy J-Y. Human CD40-ligand: molecular cloning, cellular distribution and regulation of expression by factors controlling IgE production. FEBS Lett. 1993;315:259–266. doi: 10.1016/0014-5793(93)81175-Y. [DOI] [PubMed] [Google Scholar]
- Hermann P, Armant M, Brown E, Rubio M, Ishihara H, Ulrich D, Caspary RG, Lindberg FP, Armitage R, Maliszewski C, Dele-spesse G, Sarfati M. The vitronectin receptor and its associated CD47 molecule mediates proinflammatory cytokine synthesis in human monocytes by interaction with soluble CD23. J Cell Biol. 1999;144:767–775. doi: 10.1083/jcb.144.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manié S, Kubar J, Limouse M, Ferrua B, Ticchioni M, Breittmayer JP, Peyron JF, Schaffar L, Rossi B. CD3-stimulated Jurkat T-cells mediate IL-1β production in monocytic THP-1 cells: role of LFA-1 molecule and participation of CD69 T-cell antigen. Eur Cytokine Netw. 1993;4:7–13. [PubMed] [Google Scholar]
- Schindler R, Gelfand JA, Dinarello CA. Recombinant C5a stimulates transcription rather than translation of interleukin-1 (IL-1) and tumor necrosis factor: translational signal provided by lipopolysaccharide or IL-1 itself. Blood. 1990;76:1631–1638. [PubMed] [Google Scholar]
- Dinarello CA. In: In Cytokine Reference. Oppenheim JJ, Feldmann M, editor. New York, London: Academic Press; 2000. IL-1beta. pp. 351–374. [Google Scholar]
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Beutler B. Toll-like receptors: how they work and what they do. Curr Opin Hematol. 2002;9:2–10. doi: 10.1097/00062752-200201000-00002. [DOI] [PubMed] [Google Scholar]
- Shapira L, Takashiba S, Champagne C, Amar S, Van Dyke TE. Involvement of protein kinase C and protein tyrosine kinase in lipopolysaccharide-induced TNF-alpha and IL-1 beta production by human monocytes. J Immunol. 1994;153:1818–1824. [PubMed] [Google Scholar]
- Liu MK, Herrera Velit P, Brownsey RW, Reiner NE. CD14-dependent activation of protein kinase C and mitogen-activated protein kinases (p42 and p44) in human monocytes treated with bacterial lipopolysaccharide. J Immunol. 1994;153:2642–2652. [PubMed] [Google Scholar]
- Learn CA, Boger MS, Li L, McCall CE. The phosphatidylinositol 3-kinase pathway selectively controls sIL-1RA not interleukin-1beta production in the septic leukocytes. J Biol Chem. 2001;276:20234–20239. doi: 10.1074/jbc.M100316200. [DOI] [PubMed] [Google Scholar]
- Foey AD, Green P, Foxwell B, Feldmann M, Brennan F. Cytokine-stimulated T cells induce macrophage IL-10 production dependent on phosphatidylinositol 3-kinase and p70S6K: implications for rheumatoid arthritis. Arthritis Res. 2002;4:64–70. doi: 10.1186/ar385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxwell B, Browne K, Bondeson J, Clarke C, de Martin R, Brennan F, Feldmann M. Efficient adenoviral infection with IkappaB alpha reveals that macrophage tumor necrosis factor alpha production in rheumatoid arthritis is NF-kappaB dependent. Proc Natl Acad Sci USA. 1998;95:8211–8215. doi: 10.1073/pnas.95.14.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyka N, Kaufmann MT, Chicheportiche R, Dayer JM, Burger D. Interferon-beta induces interleukin-1 receptor antagonist production in human monocytes through PI3-kinase-STAT1 signaling pathway [abstract]. Autoimmunity Rev. 2002;1:64. [Google Scholar]
- Tugwell P, Wells G, Strand V, Maetzel A, Bombardier C, Crawford B, Dorrier C, Thompson A. for the Leflunomide Rheumatoid Arthritis Investigators Group. Clinical improvement as reflected in measures of function and health-related quality of life following treatment with leflunomide compared with methotrexate in patients with rheumatoid arthritis: sensitivity and relative efficiency to detect a treatment effect in a twelve-month, placebo-controlled trial. Arthritis Rheum. 2000;43:506–514. doi: 10.1002/1529-0131(200003)43:3<506::AID-ANR5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Smeets TJ, Dayer JM, Kraan MC, Versendaal J, Chicheportiche R, Breedveld FC, Tak PP. The effects of interferon-beta treatment of synovial inflammation and expression of metalloproteinases in patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:270–274. doi: 10.1002/1529-0131(200002)43:2<270::AID-ANR5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Arnason BG. Treatment of multiple sclerosis with interferon beta. Biomed Pharmacother. 1999;53:344–350. doi: 10.1016/S0753-3322(99)80105-X. [DOI] [PubMed] [Google Scholar]
- Déage V, Burger D, Dayer JM. Exposure of T lymphocytes to leflunomide but not to dexamethasone favors the production by monocytic cells of interleukin-1 receptor antagonist and the tissue-inhibitor of metalloproteinases-1 over that of interleukin-1beta and metalloproteinases. Eur Cytokine Netw. 1998;9:663–668. [PubMed] [Google Scholar]
- Coclet-Ninin J, Dayer JM, Burger D. Interferon-beta not only inhibits interleukin-1 beta and tumor necrosis factor-alpha but stimulates interleukin-1 receptor antagonist production in human peripheral blood mononuclear cells. Eur Cytokine Netw. 1997;8:345–349. [PubMed] [Google Scholar]
- Franceschini G. Apolipoprotein function in health and disease: insights from natural mutations. Eur J Clin Invest. 1996;26:733–746. doi: 10.1046/j.1365-2362.1996.2120536.x. [DOI] [PubMed] [Google Scholar]
- Hyka N, Dayer JM, Modoux C, Kohno T, Edwards CK III, Roux-Lombard P, Burger D. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood. 2001;97:2381–2389. doi: 10.1182/blood.V97.8.2381. [DOI] [PubMed] [Google Scholar]
- Burger D, Dayer JM. High-density lipoprotein-associated apolipoprotein A-I: the missing link between infection and chronic inflammation? Autoimmunity Rev. 2002;1:111–117. doi: 10.1016/S1568-9972(01)00018-0. [DOI] [PubMed] [Google Scholar]
- Park YB, Lee SK, Lee WK, Suh CH, Lee CW, Lee CH, Song CH, Lee J. Lipid profiles in untreated patients with rheumatoid arthritis. J Rheumatol. 1999;26:1701–1704. [PubMed] [Google Scholar]
- Doherty NS, Littman BH, Reilly K, Swindell AC, Buss JM, Ander-son NL. Analysis of changes in acute-phase plasma proteins in an acute inflammatory response and in rheumatoid arthritis using two-dimensional gel electrophoresis. Electrophoresis. 1998;19:355–363. doi: 10.1002/elps.1150190234. [DOI] [PubMed] [Google Scholar]
- Lakatos J, Harsagyi A. Serum total, HDL, LDL cholesterol, and triglyceride levels in patients with rheumatoid arthritis. Clin Biochem. 1988;21:93–96. doi: 10.1016/s0009-9120(88)80094-8. [DOI] [PubMed] [Google Scholar]
- Ananth L, Prete PE, Kashyap ML. Apolipoproteins A-I and B and cholesterol in synovial fluid of patients with rheumatoid arthritis. Metabolism. 1993;42:803–806. doi: 10.1016/0026-0495(93)90050-x. [DOI] [PubMed] [Google Scholar]
- O'Hara R, Murphy EP, Whitehead AS, Fitzgerald O, Bresnihan B. Acute-phase serum amyloid A production by rheumatoid arthritis synovial tissue. Arthritis Res. 2000;2:142–144. doi: 10.1186/ar78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H, Fellowes R, Coade S, Woo P. Human serum amyloid A has cytokine-like properties. Scand J Immunol. 1998;48:410–418. doi: 10.1046/j.1365-3083.1998.00394.x. [DOI] [PubMed] [Google Scholar]
- Tselepis AD, Elisaf M, Besis S, Karabina SA, Chapman MJ, Siamopoulou A. Association of the inflammatory state in active juvenile rheumatoid arthritis with hypo-high-density lipoproteinemia and reduced lipoprotein-associated platelet-activating factor acetylhydrolase activity. Arthritis Rheum. 1999;42:373–383. doi: 10.1002/1529-0131(199902)42:2<373::AID-ANR21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Dinu AR, Merrill JT, Shen C, Antonov IV, Myones BL, Lahita RG. Frequency of antibodies to the cholesterol transport protein apolipoprotein A1 in patients with SLE. Lupus. 1998;7:355–360. doi: 10.1191/096120398678920262. [DOI] [PubMed] [Google Scholar]
- Sena A, Pedrosa R, Ferret-Sena V, Almeida R, Andrade ML, Morais MG, Couderc R. Interferon beta-1a therapy changes lipoprotein metabolism in patients with multiple sclerosis. Clin Chem Lab Med. 2000;38:209–213. doi: 10.1515/CCLM.2000.030. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- Manzi S, Wasko MC. Inflammation-mediated rheumatic diseases and atherosclerosis. Ann Rheum Dis. 2000;59:321–325. doi: 10.1136/ard.59.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidge NH. High-density lipoprotein receptors, binding proteins, and ligands. J Lipid Res. 1999;40:187–201. [PubMed] [Google Scholar]
- Kozyraki R, Fyfe J, Kristiansen M, Gerdes C, Jacobsen C, Cui S, Christensen EI, Aminoff M, de la Chapelle A, Krahe R, Verroust PJ, Moestrup SK. The intrinsic factor-vitamin B12 receptor, cubilin, is a high-affinity apolipoprotein A-I receptor facilitating endo-cytosis of high-density lipoprotein. Nat Med. 1999;5:656–661. doi: 10.1038/9504. [DOI] [PubMed] [Google Scholar]
- Chambenoit O, Hamon Y, Marguet D, Rigneault H, Rosseneu M, Chimini G. Specific docking of apolipoprotein A-I at the cell surface requires a functional ABCA1 transporter. J Biol Chem. 2001;276:9955–9960. doi: 10.1074/jbc.M010265200. [DOI] [PubMed] [Google Scholar]
- Bocharov AV, Vishnyakova TG, Baranova IN, Patterson AP, Eggerman TL. Characterization of a 95 kDa high affinity human high density lipoprotein-binding protein. Biochemistry. 2001;40:4407–4416. doi: 10.1021/bi001503k. [DOI] [PubMed] [Google Scholar]
- Miltenburg AMM, Lacraz S, Welgus HG, Dayer JM. Immobilized anti-CD3 antibody activates T cell clones to induce the production of interstitial collagenase, but not tissue inhibitor of metalloproteinases, in monocytic THP-1 cells and dermal fibroblasts. J Immunol. 1995;154:2655–2667. [PubMed] [Google Scholar]
- Avice MN, Demeure CE, Delespesse G, Rubio M, Armant M, Sarfati M. IL-15 promotes IL-12 production by human monocytes via T cell-dependent contact and may contribute to IL-12-mediated IFN-gamma secretion by CD4+ T cells in the absence of TCR ligation. J Immunol. 1998;161:3408–3415. [PubMed] [Google Scholar]
- Chabot S, Charlet D, Wilson TL, Yong VW. Cytokine production consequent to T cell–microglia interaction: the PMA/IFN gamma-treated U937 cells display similarities to human microglia. J Neurosci Methods. 2001;105:111–120. doi: 10.1016/S0165-0270(00)00346-0. [DOI] [PubMed] [Google Scholar]