Abstract
Chapter summary
Systemic lupus erythematosus (SLE) is the paradigm of a multisystem autoimmune disease in which genetic factors strongly influence susceptibility. Through genome scans and congenic dissection, numerous loci associated with lupus susceptibility have been defined and the complexity of the inheritance of this disease has been revealed. In this review, we provide a brief description of animal models of SLE, both spontaneous models and synthetic models, with an emphasis on the B6 congenic model derived from analyses of the NZM2410 strain. A hypothetical model of disease progression that organizes many of the identified SLE susceptibility loci in three distinct biological pathways that interact to mediate disease pathogenesis is also described. We finally discuss our recent fine mapping analysis, which revealed a cluster of loci that actually comprise the Sle1 locus.
Keywords: antinuclear autoantibodies, autoimmunity, congenic dissection, murine lupus, SLE
Introduction
SLE is a complex autoimmune disease of unknown etiology. It is estimated to affect about 1 in 2000 people, and the clinical presentation is highly variable and can sometimes be fatal, with a 10-year mortality rate of 28%. There is a strong gender bias, with a female:male ratio of about 9:1 between the ages of 15 and 50 years. Ethnicity also influences the incidence of the disease: African-Americans and Hispanics are approximately two to four times more likely to contract the disease than Caucasians [1,2].
SLE is primarily characterized by the production of auto-antibodies directed against nuclear antigens such as double-stranded DNA and chromatin. These antinuclear autoantibodies (ANAs) cause end-organ damage by a variety of mechanisms, notably via immune-complex-mediated inflammation, which can result in glomerulo-nephritis (GN), arthritis, rashes, serositis, and vasculitis. At the molecular level, defects in both immune complex clearance and in B-cell and T-cell tolerance and function have been implicated in the pathogenesis of this disease [3].
A great deal of evidence supports a genetic basis for susceptibility to SLE. Estimates of the concordance rates in monozygotic twins range between 25% and 69%, while the rate is only 1–2% in dizygotic twins. The concordance rate for the presence of ANAs in serum is as high as 92% in monozygotic twins, and 10–12% of SLE patients have first-degree or second-degree relatives with the disease [4]. Stochastic processes and environmental influences clearly also play a significant role in disease development and exacerbation. These can include stress, bacterial and viral infections, sun exposure, exogenous hormones and certain drugs [2].
SLE rarely occurs as a result of a single gene mutation. However, deficiencies in the early components of the complement cascade can cause the phenotype: 95% of C1-deficient and C4-deficient people develop lupus, as do 30% of people with C2 deficiency. In animal models, disruption of Fas and Fas ligand (FasL) also has major effects in disease susceptibility [5,6]. SLE more commonly occurs as a complex, polygenic disease, with many MHC and nonMHC genes interacting with each other and with the environment to create the varied disease phenotypes [5,6].
No single gene has been found to be necessary or sufficient to cause the disease. As with any multifactorial disease, this complicates the identification of disease genes. There is a low degree of penetrance associated with individual susceptibility loci, and different combinations of genes may be associated with disease development in different families. In addition, the lack of any strong selection against the susceptibility loci makes them fairly common in the general population. This makes their detection by linkage analysis difficult in human populations, which have a high degree of genetic variability. Nonetheless, several SLE susceptibility intervals have been identified in the human genome [7-9].
Animal models
A useful alternative strategy when dealing with genetically complex diseases like SLE is the elucidation of disease mechanisms in suitable animal models. These studies often yield valuable insights that can then be applied to human studies [10,11].
There are numerous synthetic murine models and spontaneous murine models of lupus (see Tables 1 and 2). The synthetic models include transgenics and targeted gene disruptions in which candidate disease genes that are involved in a variety of lymphocytic interactions, apoptosis, or antigen clearance are silenced or overexpressed [12-14]. As might be expected, disrupting or enhancing pathways involved in normal immune surveillance and reactivity often results in autoimmunity.
Table 1.
Synthetic murine models of lupus
| Model [reference] | Affected function |
|---|---|
| Fas [42], FasL [43], Bcl2[44] | Regulation of apoptosis |
| Sap [25], C1q [26], C4 [27], DNAse [28] | Clearance of antigen, such as apoptotic bodies and DNA |
| Ctla-4 [30], p21 [32], PD-1 [33], Lyn [38-40], Fyn [41] | Activation and regulation of T cells |
| BLyS [35-37], PD-1 [33], Lyn [38-40], Fyn [41], FcγRIIB [51] | Activation and regulation of B cells |
| FcγRIII [53], ICAM-1 [46] | Proinflammatory mechanisms |
BLyS, B-lymphocyte stimulator; FasL, Fas ligand; ICAM-1, intracellular adhesion molecule-1.
Table 2.
Spontaneous murine models of lupus
| Model [reference] | Properties |
|---|---|
| (NZB × NZW)F1, NZM2410, (SWR × NZB)F1 | Develops antinuclear autoantibodies and glomerulonephritis that resembles human lupus; exhibits a complex inheritance |
| MRL/lpr[42], MRL/gld[43] | Contains a single-gene mutation (Fas or Fas ligand) that leads to autoimmunity when expressed in MRL background |
| BXSB/yaa | Contains the Y-linked autoimmune accelerator gene that causes a more severe disease in BXSB males |
In addition to synthetic mouse models, there are also classic spontaneous models, including the (NZB × NZW)F1 (or NZB/W) mouse and the congenic recombinant NZM2410 strain derived from this cross, the MRL/lpr mouse, and the BXSB/yaa mouse. NZB/W mice develop systemic autoimmunity with ANA production and immune-complex-mediated GN, much like that seen in humans. There is also a strong female gender bias in disease susceptibility, which is not seen in the NZM2410 strain. It is thought that this may be due to the presence of very strong susceptibility alleles in the NZM2410 strain, which cause a phenotype severe enough to mask the effects of sex hormones [15]. The lpr mutation of the Fas gene, which is involved in apoptosis, is a strongly potentiating factor for autoimmunity. In combination with a susceptible genome, it causes systemic disease in MRL/lpr mice. In the case of BXSB mice, the Y-linked autoimmune accelerator gene causes severe disease in males when expressed in the susceptible BXSB genome.
Numerous linkage analyses have been carried out in each of these strains to identify the chromosomal regions responsible for mediating susceptibility to various component phenotypes such as ANA production, splenomegaly, and GN [14]. In each study, three or more loci were linked to disease susceptibility. This holds true in the case of the (SWR × NZB)F1 strain. Mice of this strain also develop disease very similar to that seen in NZB/W mice, and linkage analysis has recently been carried out to understand the contribution of the SWR genome to disease susceptibility [16].
More than 50 loci have been found to affect susceptibility to lupus or one of its component phenotypes [13,14], pointing to the complexity and the polygenic nature of this disease. Loci on chromosomes 1, 4, 7 and 17 have been identified in multiple studies, which indicates that genes in these regions may be important in immune regulation and function, and may play a role in mediating disease in a nonstrain-specific manner (Fig. 1). Many mapped loci co-localize with regions linked to other autoimmune diseases like insulin-dependent diabetes, experimental autoimmune encephalomyelitis, and experimental induced arthritis in various murine models [17]. This makes it probable that certain loci affect autoimmune susceptibility in general by modulating processes such as immune reactivity or apoptosis, while other genes play a role in determining the specific target organ and antigens involved in disease pathogenesis.
Figure 1.
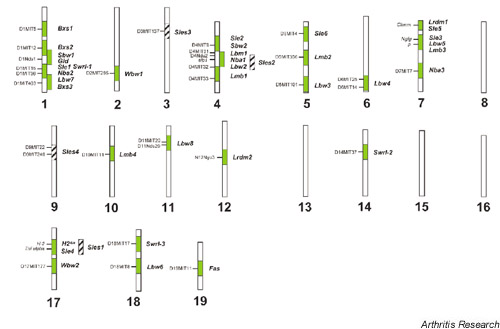
Genomic distribution of susceptibility intervals identified in linkage analysis on murine test crosses involving BSXB, MRL/lpr, PL/J, SWR, NZB, NZW, and NZM2410 strains [14,60,62,63].
Linkage analysis followed by congenic dissection is a powerful strategy that is used to narrow identified regions showing linkage to susceptibility, down to a point where physical mapping and candidate gene analysis can be meaningfully carried out. In congenic dissection, the various genomic regions are moved individually onto a resistant genome or vice versa, allowing one to see the effects of each individual locus. Using this technique, significant advances have been made towards identifying genes that may be involved in the loss of tolerance to nuclear antigens in the spontaneous models of murine lupus. Linkage analysis of susceptibility to GN and ANA production in the NZM2410 strain by Morel and Wakeland identified three prominent loci [18], and congenic strains were then made by moving each locus onto the lupus-resistant C57Bl/6J (B6) background [19].
The B6.Sle1 congenic carries the Sle1 interval, found on chromosome 1. The congenic is associated with the production of autoantibodies against H2A/H2B/DNA sub-nucleosomes and with the elevated expression of the activation markers B7.2 on B cells and CD69 on CD4+ T cells [20]. B6.Sle2 harbors the Sle2 interval on chromosome 4 and shows a reduction in the threshold for activation of B cells, leading to the production of polyclonal IgM and increased numbers of peritoneal and splenic B1 cells when present on the B6 genetic background [21]. B6.Sle3, whose congenic interval lies on chromosome 7, possesses an affected T-cell compartment, as well as the production of polyclonal IgG by B cells. These B6.Sle3 congenic mice also have an expansion of CD4+ T cells, along with a decrease in activation-induced cell death of these cells following stimulation with anti-CD3. The T cells exhibit a stronger proliferative response in vitro to T-dependent antigen stimulation, but not to T-independent antigen stimulation [22].
Significantly, neither the Sle2 nor the Sle3 interval is sufficient to cause the production of autoantibodies and kidney disease on the lupus-resistant B6 background. When combined with Sle1 as a bicongenic model, however, they cause renal lesions and proteinuria with varying penetrance [23]. This demonstrates the need for both an initiating factor and the amplification of the immune response for pathogenesis to occur. The Sle1 locus thus appears to be an initiating factor in murine lupus that causes systemic autoimmunity, resulting in GN when it is combined with either the Sle2 or Sle3 loci in bicongenic strains of mice, or with the BXSB-derived yaa gene [23].
Given the multiple loci that were identified in the NZM2410 strain, the susceptibility to lupus is highly complex. But to further complicate the issue, it was recently discovered that there existed epistatic modifiers or genes that suppress autoimmunity. Most of the Sle intervals were derived from the NZW strain, which does not have the significant autoimmune phenotype seen in the NZM2410 strain [24].
Linkage analysis then confirmed that there existed four suppressive loci, labeled 'Sles' (SLE suppressor), in the NZW genome. The Sles1 locus, which is a specific suppressor of Sle1, can in fact completely suppress the entire autoimmune cascade that is caused by Sle1 on a B6/NZW heterozygous background. This includes a powerful humoral autoimmune response, and a high penetrance of fatal lupus nephritis (>75%). Sles1 is located on chromosome 17, in the complement region of the murine MHC. B6 mice congenic for both the Sle1 and the Sles1 intervals are phenotypically indistinguishable from B6 mice. Heterozygosity at the H2 locus has previously been linked to lupus susceptibility in mouse models as well as human lupus [12].
Sles2 is located on a region on chromosome 4 that was previously shown to contain NZB-derived susceptibility loci, and is linked to suppression of autoantibody production. Sles3 on chromosome 3 is located near Il2, a region that has been linked to diabetes and experimental autoimmune encephalomyelitis susceptibility in murine models. It is linked with humoral autoimmunity and, more weakly, nephritis suppression. Sles4 on chromosome 9 is linked with protection from nephritis but not humoral autoimmunity, and its effect is entirely male specific [24]. These four suppressive loci, along with the Sle loci, help illustrate the complexity of SLE and the importance of epistatic interactions among susceptibility genes in leading to autoimmunity.
Biological pathways
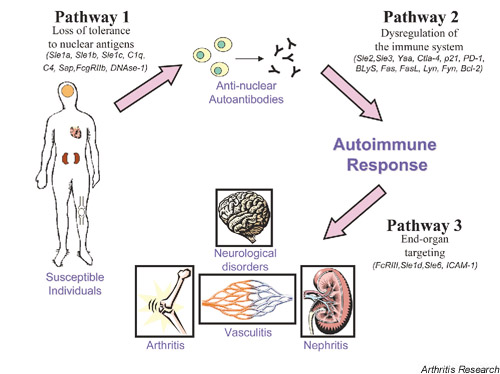
Pathway 1
Through component phenotypes detected via congenic dissection, the genes mediating lupus susceptibility in the NZM2410 model can be organized into a hypothetical pathway that provides the mechanisms for disease development (Fig. 2). The first pathway requires a breach in tolerance to nuclear antigens. As mentioned previously, the Sle1 interval is associated with the production of autoantibodies against H2A/H2B/DNA subnucleosomes [20]. Through the use of bicogenic and tricongenic strains, the combination of Sle1 with Sle2, Sle3, or yaa led to severe lupus nephritis, indicating that Sle1 is the critical factor in the pathogenesis of disease [23]. Furthermore, there are other numerous genes, particularly those involved in antigen clearance, that can potentially break tolerance to chromatin and can thus be categorized into this first pathway.
Figure 2.
Hypothetical pathways involved in the pathogenesis of SLE.
The serum amyloid P component (SAP) is known to bind the chromatin on surface blebs of apoptotic cells as well as in released nuclear debris in a calcium-dependent manner. Bickerstaff et al. have shown that the targeted disruption of SAP in mice leads to an accelerated rate of chromatin degradation when compared with SAP-sufficient mice [25]. The SAP-deficient mice spontaneously developed high titers of ANAs and severe GN. It has been suggested that SAP seems to prevent the production of autoantibodies against chromatin by binding to it and regulating its degradation and/or by sequestering it from antigen receptors [25].
Early components of complement phenotypes are also strongly associated with SLE. The first component of the complement pathway, C1q, leads to the development of autoimmunity when it is disrupted in the mouse. C1q-deficient mice show high levels of ANAs as well as increased numbers of apoptotic bodies in their glomeruli. This accumulation of apoptotic bodies suggests that there is a defect in the clearance of apoptotic debris [26]. C4 deficiency in mice has similarly been demonstrated to cause high titers of ANAs, GN and splenomegaly. In addition, these mice accumulate activated T cells and B cells, and there is impaired immune complex clearance [27].
DNase I is a major nuclease that may be involved in removing DNA from apoptotic debris. Napirei et al. constructed a knockout model, and demonstrated ANA production and GN [28]. DNase I is expressed at sites of high cellular turnover such as the skin and gastrointestinal tract. It is hypothesized that defective or decreased expression of the enzyme may cause a buildup of DNA antigen, leading to an increased risk of autoantibody formation. Treatment of NZB/W mice with DNase I results in a decrease in the level of anti-DNA IgG, supporting such a role for the molecule [29].
Pathway 2
The autoimmune response initiated by a breach in tolerance to self-antigen can be amplified by genes in the second pathway that cause a dysregulation of the immune response. Both Sle2 and Sle3, mediating the abnormal phenotypes in B cells and T cells, respectively, belong in this second pathway. The B6.Sle1 congenic mouse does not develop GN, and only when either Sle2 or Sle3 is combined with the Sle1 loci in the bicongenic strain does one see evidence of renal disease [23]. This vividly illustrates the notion that the breach in tolerance to nuclear antigen does not lead to disease unless there is an amplification of the autoimmune response, mediated by a dysregulated immune system.
In addition to Sle2 and Sle3, there are other molecules that belong in the second pathway and that play important roles in the activation or inhibition of lymphocytes. CTLA4 is a cell-surface molecule that, on binding to its ligand B7, inhibits the CD28 costimulation of T cells. Mice that are deficient in CTLA4 have been shown to have hyperproliferative T cells and increased titers of serum antibodies [30]. When anti-CTLA4 antibodies are administered to NZB/W mice, the production of autoantibodies is inhibited [31]. Another gene that regulates T-cell proliferation (and, potentially, tolerance to self-antigen) is p21, a cyclin-dependent kinase that is involved in the inhibition of cell-cycle progression. p21-deficient mice were generated, and they displayed T cells that had a hyperproliferative response to sustained stimulation in vitro. Furthermore, these mice developed a lupus-like syndrome with the presence of ANAs as well as GN [32].
Another gene that may play a role in amplifying an autoimmune response is PD-1, a cell-surface receptor that belongs to the immunoglobulin superfamily and contains an immunoreceptor tyrosine-based inhibitory motif. Mice deficient in PD-1 develop an autoimmune phenotype characterized by GN, which is further accelerated by the presence of the lpr mutation in the Fas gene. Although expressed on a small percentage of cells in the thymus, PD-1 becomes highly expressed on activated T cells and B cells, and has been shown to inhibit the proliferative response of primed T cells. It has been suggested that this molecule may therefore play a role in maintaining peripheral self-tolerance [33].
B-lymphocyte stimulator (BLyS), a member of the tumor necrosis factor family of ligands, also plays an important role in the immune system. It has been demonstrated to enhance B-cell responses both in vitro and in vivo[34]. When overexpressed in transgenic mice, BLyS causes an increase in the number of peripheral B cells and of autoantibodies directed against nuclear antigens, as well as in GN and lymphadenopathy [35-37]. Furthermore, BLys has been directly linked to murine lupus. Levels of circulating BLyS are elevated in the autoimmune NZB/W and MRL-lpr/lpr mice during the onset and progression of disease. When the mice were treated with an antibody directed against one of the receptors for BLyS, kidney disease was inhibited and mortality was reduced [37].
Lyn and Fyn are members of the src family protein tyrosine kinases that transduce activating signals in B cells and T cells. It has been demonstrated that mice lacking Lyn develop a lupus-like syndrome, characterized by elevated serum immunoglobulin levels and by the production of autoantibodies that lead to GN [38-40]. The absence of Fyn seems to accelerate the Lyn deficiency phenotype, with mice deficient for both molecules developing extremely severe lupus GN [41].
Apoptosis is an important process in the maintenance of homeostasis in the immune system. Fas and FasL are both cell-surface proteins that play critical roles in the regulation of cell death. Their interaction with one another results in rapid apoptosis of cells that bear Fas. The role of the apoptotic process in maintaining tolerance has been demonstrated in mice that are homozygous for the mutations lpr[42] and gld[43] in the Fas and FasL genes, respectively. These mice spontaneously develop severe lymphadenopathy and produce T-cell-dependent autoanti-bodies, indicating that Fas and FasL are important in regulating T-cell tolerance.
A member of a family of anti-apoptotic genes, Bcl2, may also be involved in autoimmunity through its role in B-cell tolerance. Strasser et al. studied mice carrying a transgene for Bcl2, and they demonstrated that the mice have a large expansion of the B-cell compartment and spontaneously produce ANAs. A large percentage of them also develop lupus-like GN. It was determined through FACS analysis that this phenotype is not caused by the increased proliferation of B cells, but by their prolonged life spans [44].
Pathway 3
A third pathway in the development of lupus nephritis is comprised of genes that potentiate end-organ damage. When the loss of tolerance to chromatin and the continued production of autoantibodies due to a dysregulated immune system occurs, genes such as Sle6 on chromosome 5 [24] and intracellular adhesion molecule-1 (ICAM-1) can target end-organ damage by the deposition of immune complexes and the subsequent recruitment of inflammatory mediators.
ICAM-1 is a member of the immunoglobulin superfamily of adhesion receptors and, through its interaction with the β2 integrin LFA-1, it is believed to be involved in the recruitment of lymphocytes to the kidney. It has been demonstrated that ICAM-1 is upregulated in the kidneys from MRL/lpr and NZB/W mice when compared with normal mice [45]. MRL/lpr mice deficient in ICAM-1 show a reduction in renal disease, as well as a remarkable decrease in mortality [46]. The ICAM-1-deficient mice exhibited less vasculitis in the kidney, lung, skin, and salivary glands when compared with wild-type MRL/lpr mice, supporting the idea that ICAM-1 may play an important role in the pathogenesis of glomerular and vascular damage in these mice.
In addition to Sle6 and ICAM-1, receptors for the IgG class of antibodies, which play a variety of complex roles in the immune system, also belong in the third pathway. There are three major classes of Fc gamma receptors present on cells of the immune system: FcγRI(CD64), FcγRII(CD32), and FcγRIII(CD16). Fc gamma receptors function in the noninflammatory clearance of immune complexes from the circulation by mononuclear phagocytes in the liver and spleen.
FcγRIIB has been found to be crucial to this process [47] and, in accordance with its role in antigen clearance, mice lacking this receptor have an increased susceptibility to collagen-induced arthritis and Goodpasture's syndrome, which are both immune complex mediated [48-50]. FcγRIIB-deficient mice spontaneously develop autoanti-bodies and GN in a strain-dependent manner [51]. Homotypic ligation of FcγRIIB on B cells sends a proapoptotic signal, and this process has been postulated to help in the maintenance of peripheral tolerance of B cells that have undergone somatic hypermutation. Follicular dendritic cells in germinal centers retain antigen in immune complexes through FcγRIIB. B cells that interact with the immune complex through both the B-cell receptor and FcγRIIB survive, whereas those that do not see the antigen through the B-cell receptor apoptose. This enables selection against those B cells that bear very low affinity, and therefore against potentially crossreactive antigen receptors, pointing to a protective regulatory role for FcγRIIB [52].
In contrast, FcγRIII seems to be more proinflammatory in function. It can cause degranulation, phagocytosis, antibody-dependent, cell-mediated cytotoxicity, and cytokine release on engagement, and it has been observed that the deletion of FcγRIII actually has a protective effect on end-organ damage in the autoimmune NZB/W strain. Mice lacking these FcγIII receptors still produce autoantibodies, deposit immune complexes, and activate complement, but do not develop lupus nephritis [53].
Given the large number of susceptibility loci detected by genome scans across various mouse strains, these three pathways help define a mechanistic order with which the pathogenesis of lupus occurs. Furthermore, identifying how a given disease gene functions via knockout and transgenic technology provides a better understanding of how the gene contributes to lupus and provides a better approach for therapeutic intervention.
Fine mapping of Sle1 susceptibility loci
Linkage studies have provided a large collection of loci linked to susceptibility to lupus. It is also notable from Figure 1 that, although over 50 loci have been identified, there appears to be multiple loci on chromosomes 1, 4, 7, and 17 that have been identified in multiple studies. As mentioned previously, many of the loci colocalize to regions associated with other autoimmune diseases, strongly indicating that a certain locus is involved in autoimmunity. Furthermore, it is also becoming evident that an individual locus may actually be a cluster of loci that are associated with a spectrum of phenotypes, as in the case with the Sle1 locus. Fine mapping of the NZW-derived Sle1 locus revealed that it consists of at least four closely linked, functionally related genes that can each cause a break in tolerance to chromatin: Sle1a, Sle1b, Sle1c, and Sle1d. Analysis of recombinant mice carrying the first three of these loci showed that these subcongenic intervals are associated with phenotypes that are very similar to each other and to the overall Sle1 phenotype, but are subtly different from one another [54].
Sle1a is an approximately 1 cM interval that is associated with the production of ANAs specific for H2A/H2B/DNA, but is not associated with increased CD69 or B7.2 expression. Mice of this strain have reduced numbers of T cells, and a moderate increase in B220+ cells. The CD4+ T cells have a more activated phenotype than in B6 mice, and there is a higher proportion of cells with a memory phenotype. There is an increased IgG, but not IgM, response to ovalbumin immunization. Thus, when isolated from the other subcongenic loci, Sle1a was found to have phenotypes that were masked in the entire Sle1 interval.
The 0.4 cM Sle1b locus has the highest penetrance of ANA production of the three subcongenic mouse strains, with the same subnucleosomal specificity as that of the entire interval. It also has the earliest age of onset of autoantibody production. Sle1b is the only subinterval to exhibit the female gender bias seen in Sle1 and to have an increase in total IgM and IgG levels over B6. It also shows the increased expression of CD69 and B7.2 seen in Sle1, suggesting that it is the strongest contributor to the overall phenotype of the chromosome 1 interval. The combination of Sle1a with Sle1b was equivalent to the entire Sle1 interval with respect to ANA production, whereas the region between the two loci by itself did not produce an autoimmune phenotype above background levels. This would indicate that the two loci together have a synergistic effect, which is not caused by a gene that lies in the interval between them[54].
The Sle1c locus is an approximately 3 cM interval. Unlike Sle1a and Sle1b, Sle1c does not show specificity for any one component of chromatin and shows no difference from B6 in IgM response or IgG response to ovalbumin immunization. Sle1c does not have an increased level of activation markers CD69 and B7.2, but it has an increased proportion of CD4+ T cells with a memory phenotype.
It has been shown that B6 mice and NZW mice have different alleles of the Cr2 gene, which lies within the Sle1c locus. This gene encodes the complement receptors 1 and 2 as separate splice variants. Complement receptors 1 and 2 bind antigen-bound degradation products of the complement components C3 and C4. SLE patients have lower levels of these receptors on their B cells [55], as do MRL/lpr mice, prior to disease onset [56]. This indicates that lowered expression of these receptors may play a role in pathogenesis. One of the single nucleotide polymorphisms between the B6 and NZW alleles of Cr2 occurs in the ligand-binding domain and introduces a novel glycosylation site in the NZW allele. This results in lower ligand-binding affinity, probably by interfering with receptor dimerization. Complement receptor 1/complement receptor 2-mediated signaling is lower in B cells carrying this allele, and cells are also impaired in their response to T-dependent antigens [57]. It has been shown that complement receptors may play an important role in maintaining both central and peripheral B-cell tolerance [58]. Hence, the lowered functioning of these receptors may allow autoreactive B cells to escape tolerance, leading to the ANA production phenotype.
Congenic dissection has also been applied to the Nba2 locus, which maps to the same region on chromosome 1 as does Sle1. This NZB-derived region of about 8 cM is contained in the 30 cM congenic interval in the B6.Nba2 congenic strain. Females of this strain develop IgG antibodies to chromatin, to double-stranded DNA, and to total histones. When crossed with NZW, female (B6.Nba2X-NZW)F1 mice develop ANAs and proteinuria, and they die from nephritis with a high frequency. This again points to the possibility that the loss of tolerance to the chromatin pathway may be an initiating factor in overall disease development. Microarray analysis carried out on spleen cells of preautoimmune mice of the congenic strain showed that, of 11,000 genes analyzed, two interferon-responsive genes belonging to the Ifi200 gene cluster showed a highly significant difference in expression from B6 spleen cells. Both of these genes mapped within the Nba2 locus. Ifi202, which inhibits apoptosis when overexpressed and may play a role in transcriptional regulation [36], was expressed at much higher levels in B6.Nba2 spleens, while Ifi203 was expressed at lower levels. It has been postulated that either molecule could underlie the Nba2 phenotype, which may be found to correlate with additional genes within the same locus as well, as in the case of Sle1[59].
The region of mouse chromosome 1 that contains Sle1 and Nba2 has consistently been implicated in studies of murine models of systemic autoimmunity [12,60]. Other loci that map here include NZB-derived Lbw7 and SWR-derived Swrl-1. Significantly, the human region syntenic to the telomeric region of chromosome 1 where this cluster of murine susceptibility loci lies (1q22–1q44) has also been linked to lupus susceptibility in genome wide scans using a variety of ethnic groups [7-9,61]. Advances made in identifying the susceptibility genes in this region could yield valuable insights into the checks and balances that usually operate in the immune system, which when they fail can result in a breach in tolerance to self-antigens, initiating an autoimmune cascade.
Concluding remarks
The inheritance of lupus susceptibility is undoubtedly complex and, although numerous susceptibility loci have so far been identified, piecing them together into a meaningful pathway that illustrates the pathogenesis of the disease is very difficult. It is becoming evident through congenic dissection that there is a strong genetic interaction among multiple loci that leads to the expression of disease. A hypothetical model, containing three biologically distinct pathways, has been constructed that outlines these genetic interactions and illustrates the progression of lupus. The mechanisms within these pathways are still poorly understood, however, and further work remains to fully elucidate the pathogenesis of SLE.
Glossary of terms
ANA = antinuclear autoantibody; BLyS = B-lymphocyte stimulator; FasL = Fas ligand; GN = glomerulonephritis; SAP = serum amyloid P component; SLE = systemic lupus erythematosus; Sles = SLE suppressor.
Funds for research
Organizations that provide funding for research in this field include the Alliance for Lupus Research, the SLE Foundation, the Arthritis Foundation, and the National Institutes of Health.
London, UK. 24-26 June 2002
References
- Blatt NB, Glick GD. Anti-DNA autoantibodies and systemic lupus erythematosus. Pharmacol Ther. 1999;33:125–139. doi: 10.1016/S0163-7258(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/S0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- Sullivan KE. Genetics of systemic lupus erythematosus. Clinical implications. Rheum Dis Clin North Am. 2000;26:229. doi: 10.1016/s0889-857x(05)70137-x. [DOI] [PubMed] [Google Scholar]
- Kotzin BL, O'Dell JR. In: In Samter's Immunologic Diseases. Edited by Frank MM, Austen KF, Claman HN, Unanue ER, editor. Boston, MA: Little, Brown & Co;; 1995. Systemic lupus erythematosus. pp. 667–697. [Google Scholar]
- Johnson GC, Todd JA. Strategies in complex disease mapping. Curr Opin Genet Dev. 2000;10:330–334. doi: 10.1016/S0959-437X(00)00075-7. [DOI] [PubMed] [Google Scholar]
- Lindqvist AK, Alarcon-Riquelme ME. The genetics of systemic lupus erythematosus. Scand J Immunol. 1999;50:562–571. doi: 10.1046/j.1365-3083.1999.00664.x. [DOI] [PubMed] [Google Scholar]
- Gaffney PM, Kearns GM, Shark KB, Ortmann WA, Selby SA, Malmgren ML, Rohlf KE, Ockenden TC, Messner RP, Rich S, Behrens TW. A genome-wide search for susceptibility genes in human systemic lupus erythematosus sib-pair families. Proc Natl Acad Sci USA. 1998;95:14875–14879. doi: 10.1073/pnas.95.25.14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser KL, Neas BR, Salmon JE, Yu H, Gray-McGuire C, Asundi N, Bruner GR, Fox J, Kelly J, Henshall S, Bacino D, Dietz M, Hogue R, Koelsch G, Nightingale L, Shayer T, Abdou NI, Albert A, Carson C, Petri M, Treadwell EL, James JA, Harley JB. Genome scan of human systemic lupus erythematosus: evidence for linkage on chromosome 1q in African-American pedigrees. Proc Natl Acad Sci USA. 1998;95:14869–14874. doi: 10.1073/pnas.95.25.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai R, Quismorio F Jr, Li L, Kwon O-J, Morrison J, Wallace D, Neuwelt C, Brautbar C, Gauderman W, Jacob CO. Genome-wide screen for systemic lupus erythematosus susceptibility genes in multiplex families. Hum Mol Genet. 1999;8:639–644. doi: 10.1093/hmg/8.4.639. [DOI] [PubMed] [Google Scholar]
- Vyse TJ, Rozzo SJ, Drake CG, Izui S, Kotzin BL. Control of multiple autoantibodies linked with a lupus nephritis susceptibility locus in New Zealand black mice. J Immunol. 1997;158:5566–5574. [PubMed] [Google Scholar]
- Risch N. Searching for genes in complex diseases: lessons from systemic lupus erythematosus [comment]. J Clin Invest. 2000;105:1503–1506. doi: 10.1172/JCI10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyse TJ, Kotzin BL. Genetic susceptibility to systemic lupus erythematosus. Annu Rev Immunol. 1998;16:261–292. doi: 10.1146/annurev.immunol.16.1.261. [DOI] [PubMed] [Google Scholar]
- Wakeland EK, Liu K, Graham RR, Behrens TW. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15:397–408. doi: 10.1016/S1074-7613(01)00201-1. [DOI] [PubMed] [Google Scholar]
- Mohan C. Murine lupus genetics: lessons learned. Curr Opin Rheumatol. 2001;13:352–360. doi: 10.1097/00002281-200109000-00003. [DOI] [PubMed] [Google Scholar]
- Morel L, Wakeland EK. Susceptibility to lupus nephritis in the NZB/W model system. Curr Opin Immunol. 1998;10:718–725. doi: 10.1016/S0952-7915(98)80094-0. [DOI] [PubMed] [Google Scholar]
- Xie S, Chang SH, Yang P, Jacob C, Kaliyaperumal A, Datta SK, Mohan C. Genetic contributions of nonautoimmune SWR mice to lupus nephritis. J Immunol. 2001;167:7141–7149. doi: 10.4049/jimmunol.167.12.7141. [DOI] [PubMed] [Google Scholar]
- Marrack P, Kappler J, Kotzin BL. Autoimmune disease: why and where it occurs. Nat Med. 2001;7:899–905. doi: 10.1038/90935. [DOI] [PubMed] [Google Scholar]
- Morel L, Wakeland EK. Lessons from the NZM2410 model and related strains. Int Rev Immunol. 2000;19:423–446. doi: 10.3109/08830180009055506. [DOI] [PubMed] [Google Scholar]
- Morel L, Mohan C, Croker BP, Tian X-H, Wakeland EK. Functional dissection of systemic lupus erythematosus using congenic mouse strains. J Immunol. 1997;158:6019–6028. [PubMed] [Google Scholar]
- Mohan C, Alas E, Morel L, Yang P, Wakeland EK. Genetic dissection of SLE pathogenesis: Sle1 on murine chromosome 1 leads to a selective loss of tolerance to H2A/H2B/DNA sub-nucelosomes. J Clin Invest. 1998;101:1362–1372. doi: 10.1172/JCI728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan C, Morel L, Yang P, Wakeland EK. Genetic dissection of SLE pathogenesis: Sle2 on murine chromosome 4 leads to B-cell hyperactivity. J Immunol. 1997;159:454–465. [PubMed] [Google Scholar]
- Mohan C, Yu Y, Morel L, Yang P, Wakeland EK. Genetic dissection of SLE pathogenicity: Sle3 on murine chromosome 7 impacts T cell activation, differentiation, and cell death. J Immunol. 1999;162:6492–6502. [PubMed] [Google Scholar]
- Morel L, Croker BP, Blenman KR, Mohan C, Huang G, Gilkeson G, Wakeland EK. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci USA. 2000;97:6670–6675. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel L, Tian X-H, Croker BP, Wakeland EK. Epistatic modifiers of autoimmunity in a murine model of lupus nephritis. Immunity. 1999;11:131–139. doi: 10.1016/s1074-7613(00)80088-6. [DOI] [PubMed] [Google Scholar]
- Bickerstaff MC, Botto M, Hutchinson WL, Herbert J, Tennent GA, Bybee A, Mitchell DA, Cook HT, Butler PJ, Walport MJ, Pepys MB. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat Med. 1999;5:694–697. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- Botto M, Dell'Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi P, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- Chen Z, Koralov SB, Kelsoe G. Complement C4 inhibits systemic autoimmunity through a mechanism independent of complement receptors CR1 and CR2. J Exp Med. 2000;192:1339–1351. doi: 10.1084/jem.192.9.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG, Moroy T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet. 2000;25:177–181. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- Macanovic M, Sinicropi D, Shak S, Baughman S, Thiru S, Lach-mann PJ. The treatment of systemic lupus erythematosus (SLE) in NZB/W F1 hybrid mice; studies with recombinant murine DNase and with dexamethasone. Clin Exp Immunol. 1996;106:243–252. doi: 10.1046/j.1365-2249.1996.d01-839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 2002;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265:1225–1227. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- Balomenos D, Martin-Caballero J, Garcia MI, Prieto I, Flores JM, Serrano M, Martinez A. The cell cycle inhibitor p21 controls T-cell proliferation and sex-linked lupus development. Nat Med. 2000;6:171–176. doi: 10.1038/72272. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- Do RK, Chen-Kiang S. Mechanism of BLyS action in B cell immunity. Cytokin Growth Factor Rev. 2002;1:19–25. doi: 10.1016/S1359-6101(01)00025-9. [DOI] [PubMed] [Google Scholar]
- Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J ExpMed. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare SD, Sarosi I, Xia XZ, McCabe S, Miner K, Solovyev I, Hawkins N, Kelley M, Chang D, Van G, Ross L, Delaney J, Wang L, Lacey D, Boyle WJ, Hsu H. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci USA. 2000;97:3370–3375. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, Xu W, Parrish-Novak J, Foster D, Lofton-Day C, Moore M, Littau A, Grossman A, Haugen H, Foley K, Blumberg H, Harrison K, Kindsvogel W, Clegg CH. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-X. [DOI] [PubMed] [Google Scholar]
- Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Yu CC, Yen TS, Lowell CA, DeFranco AL. Lupus-like kidney disease in mice deficient in the Src family tyrosine kinases Lyn and Fyn. Curr Biol. 2001;11:34–38. doi: 10.1016/S0960-9822(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, Harris AW. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci. 1991;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich RP, Jevnikar AM, Takei F, Glimcher LH, Kelley VE. Inter-cellular adhesion molecule-1 (ICAM-1) expression is upregulated in autoimmune murine lupus nephritis. Am J Pathol. 1990;136:441–450. [PMC free article] [PubMed] [Google Scholar]
- Bullard DC, King PD, Hicks MJ, Dupont B, Beaudet AL, Elkon KB. Intercellular adhesion molecule-1 deficiency protects MRL/MpJ-Fas(lpr) mice from early lethality. J Immunol. 1997;159:2058–2067. [PubMed] [Google Scholar]
- Dijstelbloem HM, van de Winkel J, Kallenberg CG. Inflammation in autoimmunity: receptors for IgG revisited. Trends Immunol. 2001;22:510–516. doi: 10.1016/S1471-4906(01)02014-2. [DOI] [PubMed] [Google Scholar]
- Yuasa T, Kubo S, Yoshino T, Ujike A, Matsumura K, Ono M, Ravetch JV, Takai T. Deletion of fcgamma receptor IIB renders H-2(b) mice susceptible to collagen-induced arthritis. J Exp Med. 1999;189:187–194. doi: 10.1084/jem.189.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinau S, Martinsson P, Heyman B. Induction and suppression of collagen-induced arthritis is dependent on distinct fcgamma receptors. J Exp Med. 2000;191:1611–1616. doi: 10.1084/jem.191.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu WM, Miyazaki T, Terada M, Lu LM, Nishihara M, Yamada A, Mori S, Nakamura Y, Ogasawara H, Yazawa C, Nakatsuru S, Nose M. Genetic dissection of vasculitis in MRL/lpr lupus mice: a novel susceptibility locus involving the CD72c allele. Eur J Immunol. 2000;30:2027–2037. doi: 10.1002/1521-4141(200007)30:7<2027::AID-IMMU2027>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/S1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- Morel L, Blenman KR, Croker BP, Wakeland EK. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc Natl Acad Sci USA. 2001;98:1787–1792. doi: 10.1073/pnas.031336098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JG, Ratnoff WD, Schur PH, Fearon DT. Decreased expression of the C3b/C4b receptor (CR1) and the C3d receptor (CR2) on B lymphocytes and of CR1 on neutrophils of patients with systemic lupus erythematosus. Arthritis Rheum. 1986;29:739–747. doi: 10.1002/art.1780290606. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kozono Y, Waldschmidt TJ, Quigg RJ, Baron A, Holers VM. Mouse complement receptors type 1 (CR1;CD35) and type 2 (CR2;CD21): expression on normal B cell subpopulations and decreased levels during the development of autoimmunity in MRL/lpr mice. J Immunol. 1997;159:1557–1569. [PubMed] [Google Scholar]
- Boackle SA, Holers VM, Chen X, Szakonyi G, Karp D, Wakeland EK, Morel L. Cr2, a candidate gene in the murine Sle1c lupus susceptibility locus, encodes a dysfunctional protein. Immunity. 2002;15:785. doi: 10.1016/s1074-7613(01)00228-x. [DOI] [PubMed] [Google Scholar]
- Prodeus AP, Goerg S, Shen LM, Pozdnyakova OO, Chu L, Alicot EM, Goodnow CC, Carroll MC. A critical role for complement in maintenance of self-tolerance. Immunity. 1998;9:721–731. doi: 10.1016/s1074-7613(00)80669-x. [DOI] [PubMed] [Google Scholar]
- Rozzo SJ, Allard JD, Choubey D, Vyse TJ, Izui S, Peltz G, Kotzin BL. Evidence for an interferon-inducible gene, Ifi202, in the susceptibility to systemic lupus. Immunity. 2001;15:435–443. doi: 10.1016/S1074-7613(01)00196-0. [DOI] [PubMed] [Google Scholar]
- Wakeland EK, Wandstrat AE, Liu K, Morel L. Genetic dissection of systemic lupus erythematosus. Curr Opin Immunol. 1999;11:701–707. doi: 10.1016/S0952-7915(99)00039-4. [DOI] [PubMed] [Google Scholar]
- Tsao BP, Cantor RM, Kalunian C, Chen C-J, Badsha H, Singh R, Wallace DJ, Kitridou RC, Chen S, Shen N, Song YW, Isenberg DA, Yu C-L, Hahn BH, Rotter JI. Evidence for linkage of a candidate chromosome 1 region to human systemic lupus erythematosus. J ClinInvest. 1997;99:725–731. doi: 10.1172/JCI119217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman ZS, Tin SK, Buenaventura PN, Ho CH, Yap EP, Yong RY, Koh DR. A novel susceptibility locus on chromosome 2 in the (New Zealand Black × New Zealand White)F1 hybrid mouse model of systemic lupus erythematosus. J Immunol. 2002;168:3042–3049. doi: 10.4049/jimmunol.168.6.3042. [DOI] [PubMed] [Google Scholar]
- Xie S, Chang S, Yang P, Jacob C, Kaliyaperumal A, Datta SK, Mohan C. Genetic contributions of nonautoimmune SWR mice toward lupus nephritis. J Immunol. 2001;167:7141–7149. doi: 10.4049/jimmunol.167.12.7141. [DOI] [PubMed] [Google Scholar]