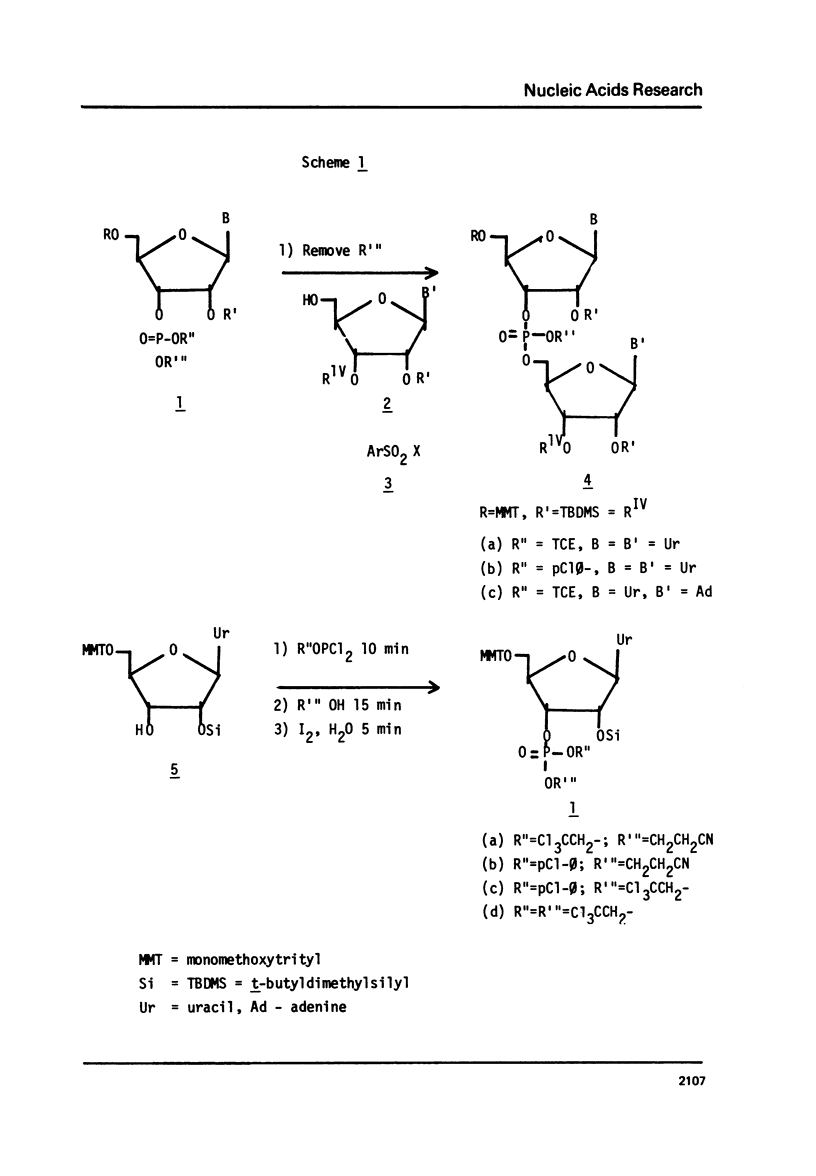
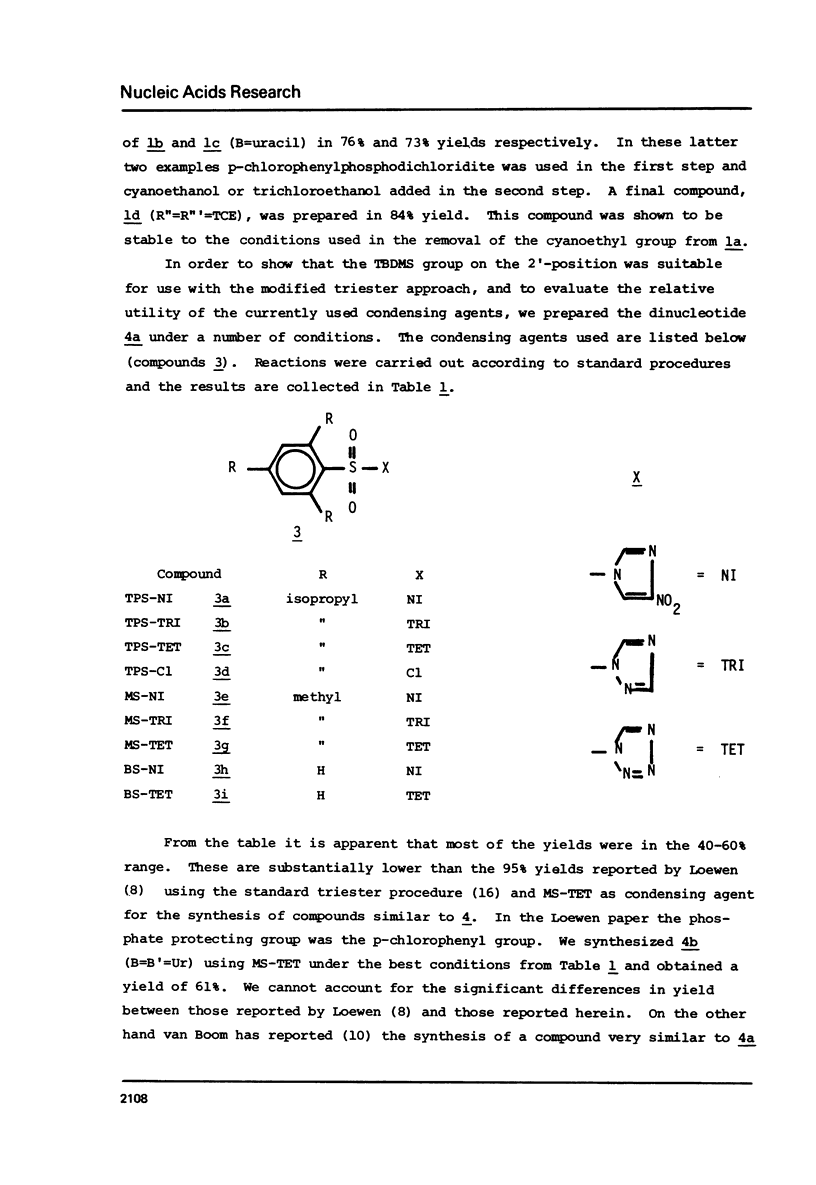
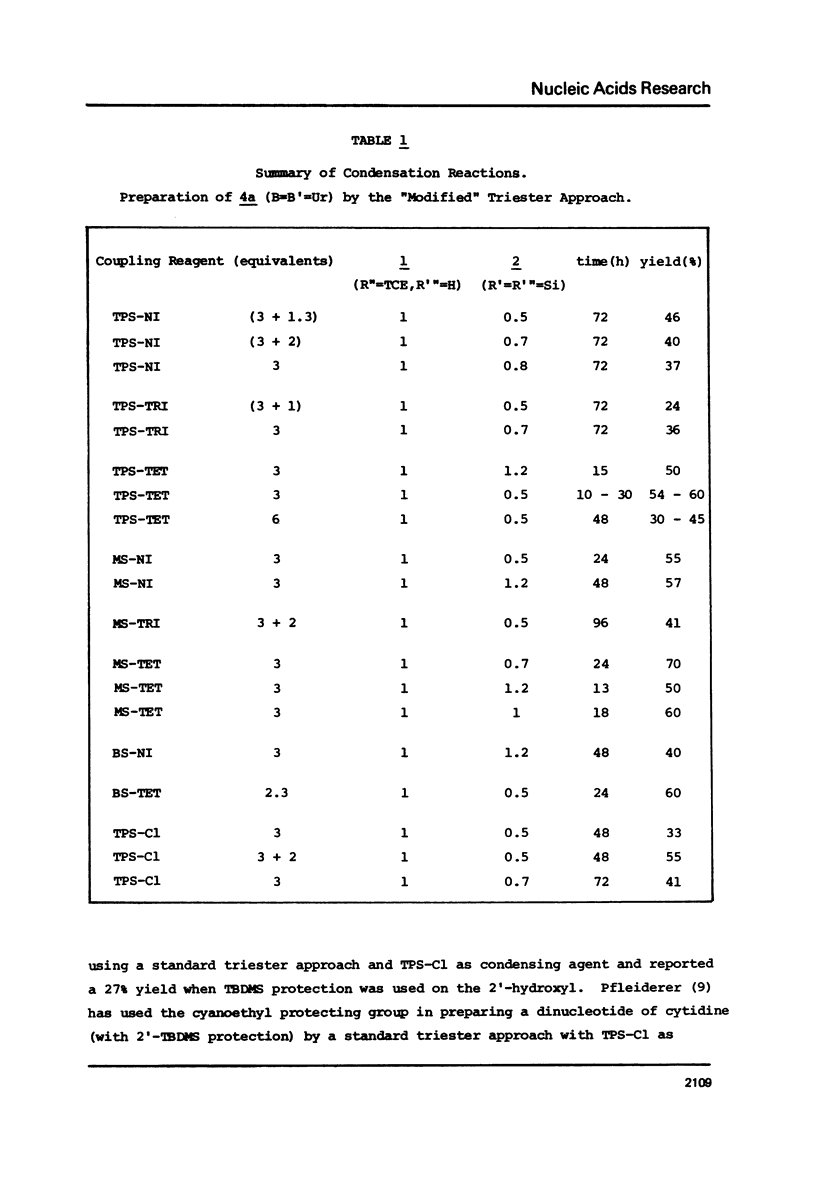
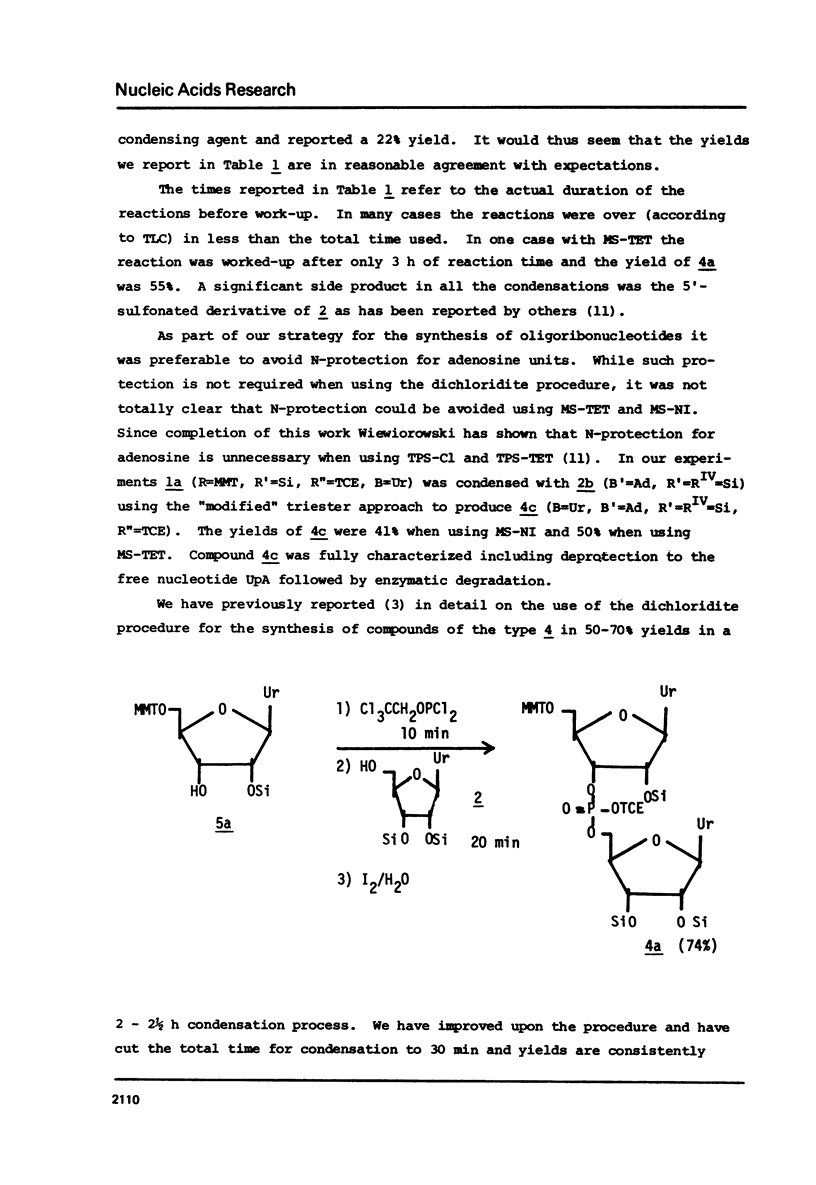
Abstract
The t-butyldimethylsilyl group is shown to be an ideal protecting group for the 2T-hydroxyl function of ribonucleosides during the synthesis of ribonucleotides using any of nine commonly used condensing agents. The phosphite coupling procedure compares favorably with all of the widely used condensing agents and provides a most convenient route to the key intermediates in the "modified" triester strategy.
Full text
PDF


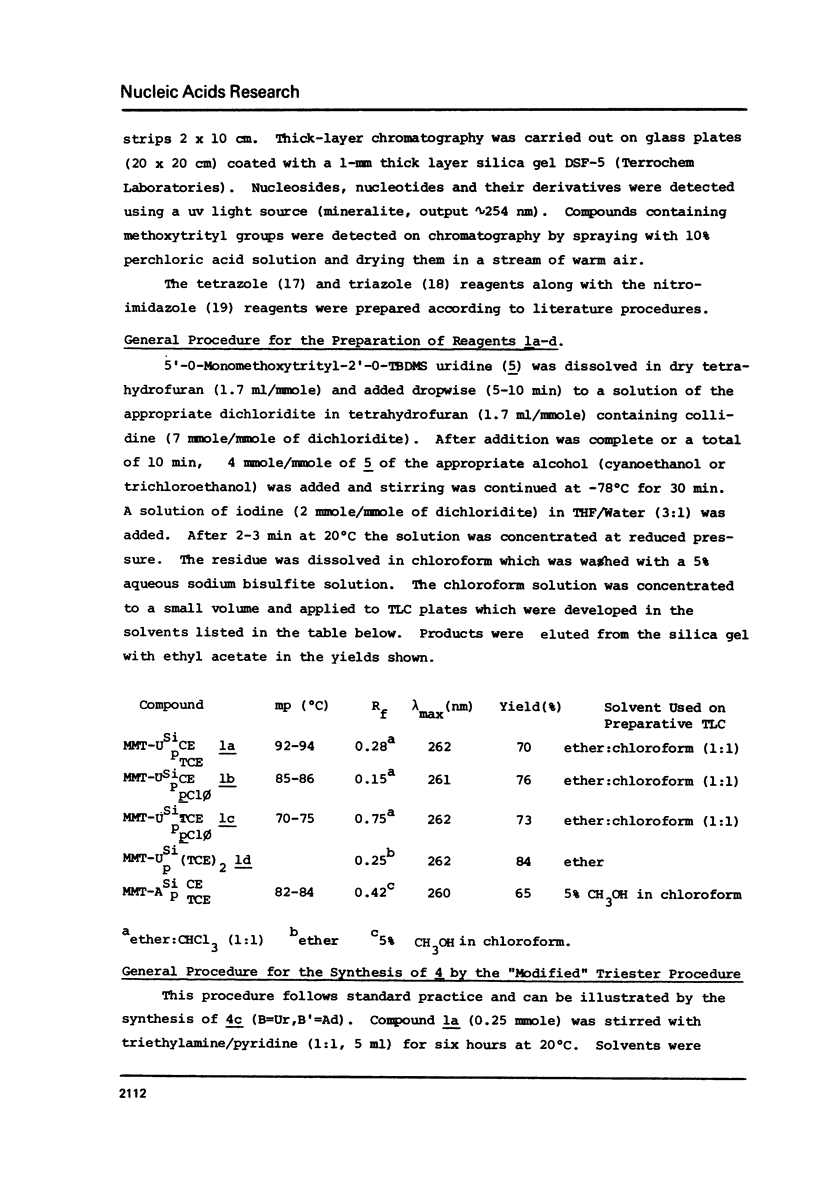
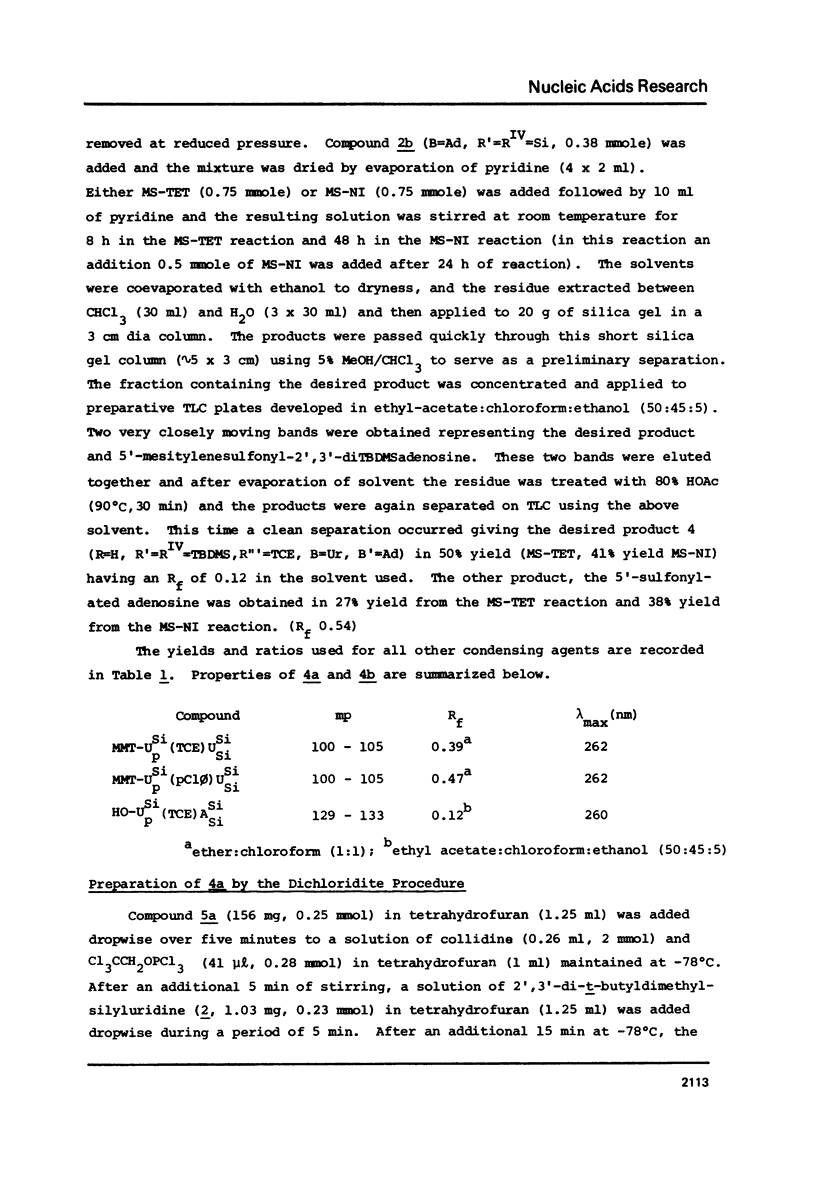


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamiak R. W., Biała E., Grześkowiak K., Kierzek R., Kraszewski A., Markiewicz W. T., Okupniak J., Stawiński J., Wiewiórowski M. The chemical synthesis of the anticodon loop of an eukaryotic initiator tRNA containing the hypermodified nucleoside N6-/N-threonylcarbonyl/-adenosine/t6A/1. Nucleic Acids Res. 1978 Jun;5(6):1889–1905. doi: 10.1093/nar/5.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin J. C., Cramer F. Deoxy oligonucleotide synthesis via the triester method. J Org Chem. 1973 Jan 26;38(2):245–250. doi: 10.1021/jo00942a011. [DOI] [PubMed] [Google Scholar]
- Katagiri N., Itakura K., Narang S. A. The use of arylsulfonyltriazoles for the synthesis of oligonucleotides by the triester approach. J Am Chem Soc. 1975 Dec 10;97(25):7332–7337. doi: 10.1021/ja00858a021. [DOI] [PubMed] [Google Scholar]
- Letsinger R. L., Lunsford W. B. Synthesis of thymidine oligonucleotides by phosphite triester intermediates. J Am Chem Soc. 1976 Jun 9;98(12):3655–3661. doi: 10.1021/ja00428a045. [DOI] [PubMed] [Google Scholar]
- Ogilvie K. K., Theriault N., Sadana K. L. Synthesis of oligoribonucleotides. J Am Chem Soc. 1977 Nov 9;99(23):7741–7743. doi: 10.1021/ja00465a073. [DOI] [PubMed] [Google Scholar]
- de Rooij J. F., Wille-Hazeleger G., Burgers P. M., van Boom J. H. Neighbouring group participation in the unblocking of phosphotriesters of nucleic acids. Nucleic Acids Res. 1979;6(6):2237–2259. doi: 10.1093/nar/6.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boom J. H., Burgers P. M., van der Marel G., Verdegaal C. H., Wille G. Synthesis of oligonucleotides with sequences identical with or analogous to the 3'-end of 16S ribosomal RNA of Escherichia coli: preparation of A-C-C-U-C-C via the modified phosphotriester method. Nucleic Acids Res. 1977 Apr;4(4):1047–1063. doi: 10.1093/nar/4.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]


