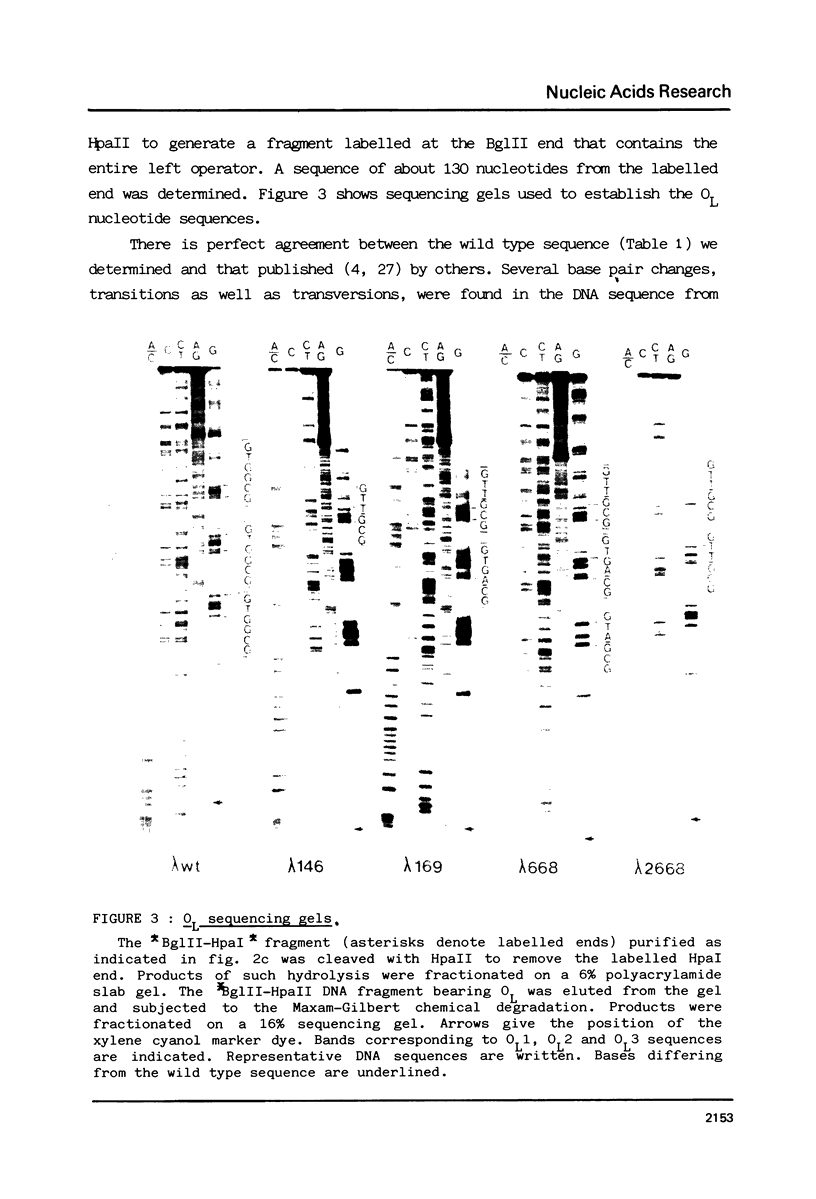
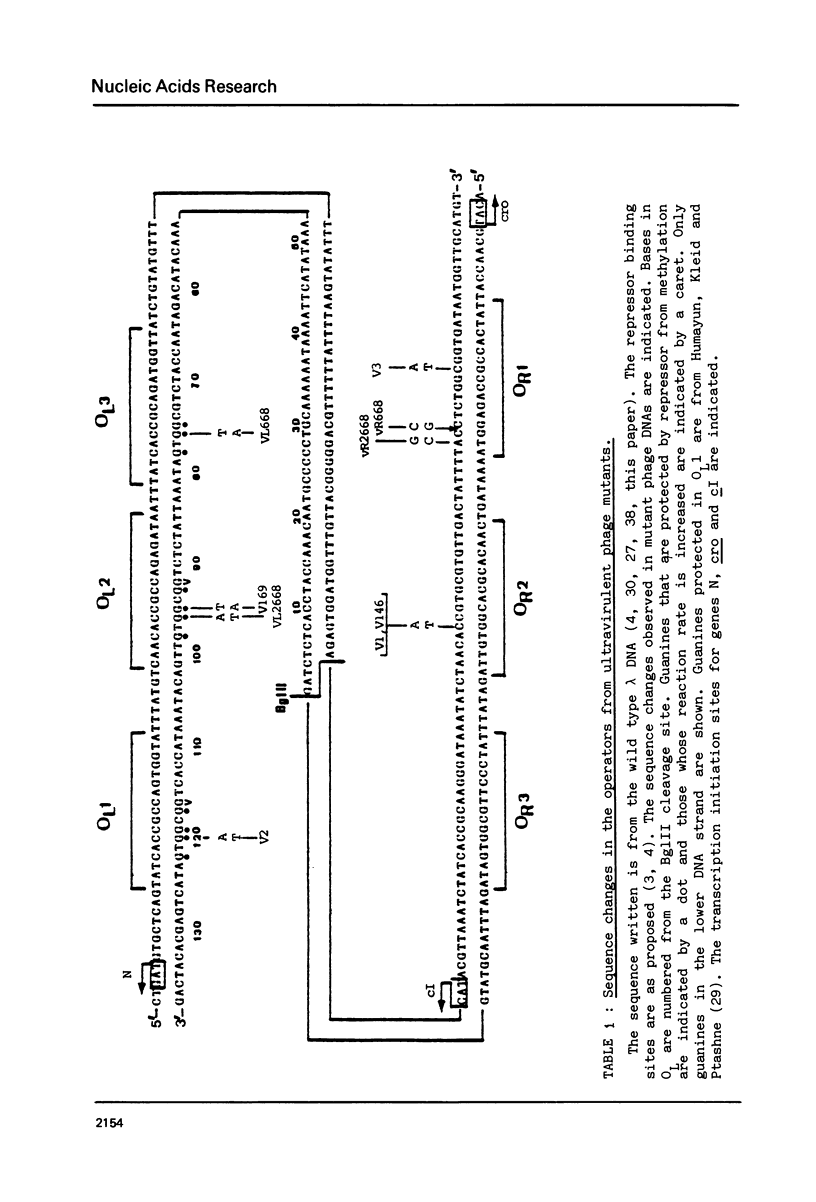
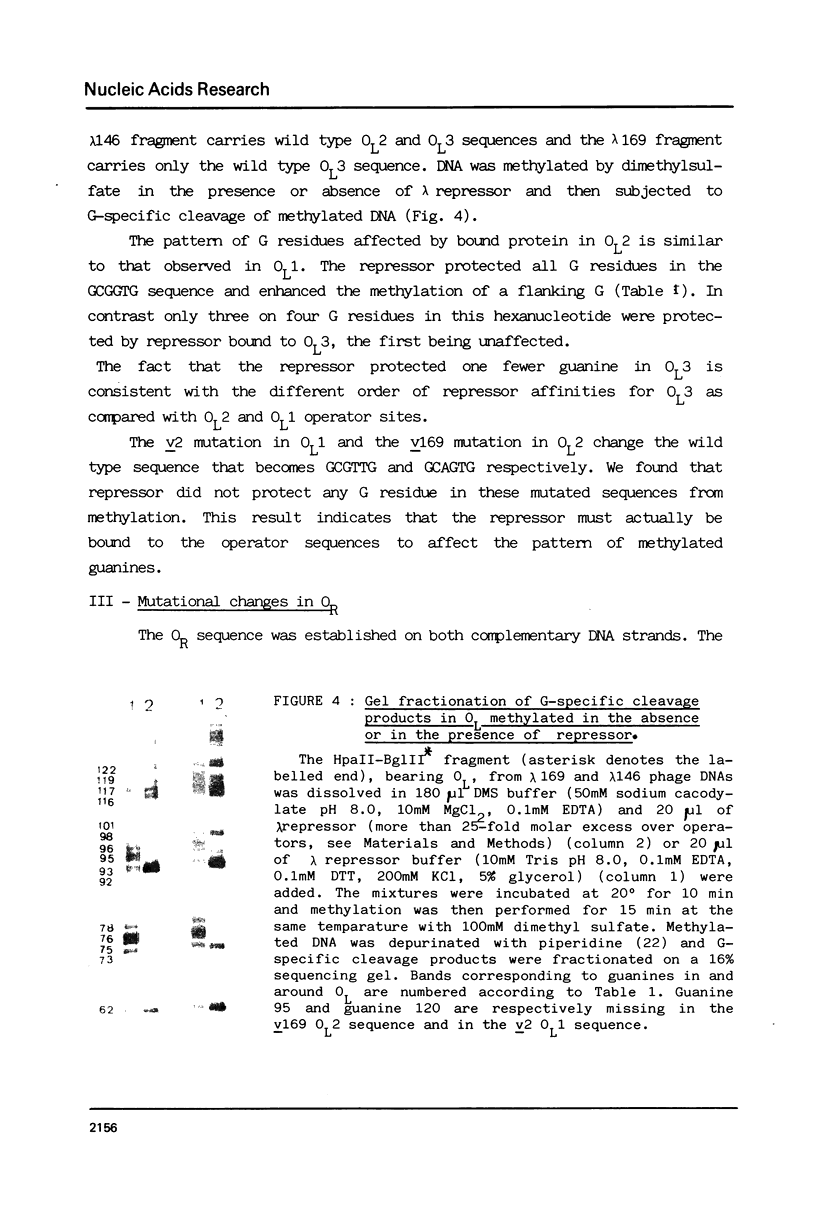
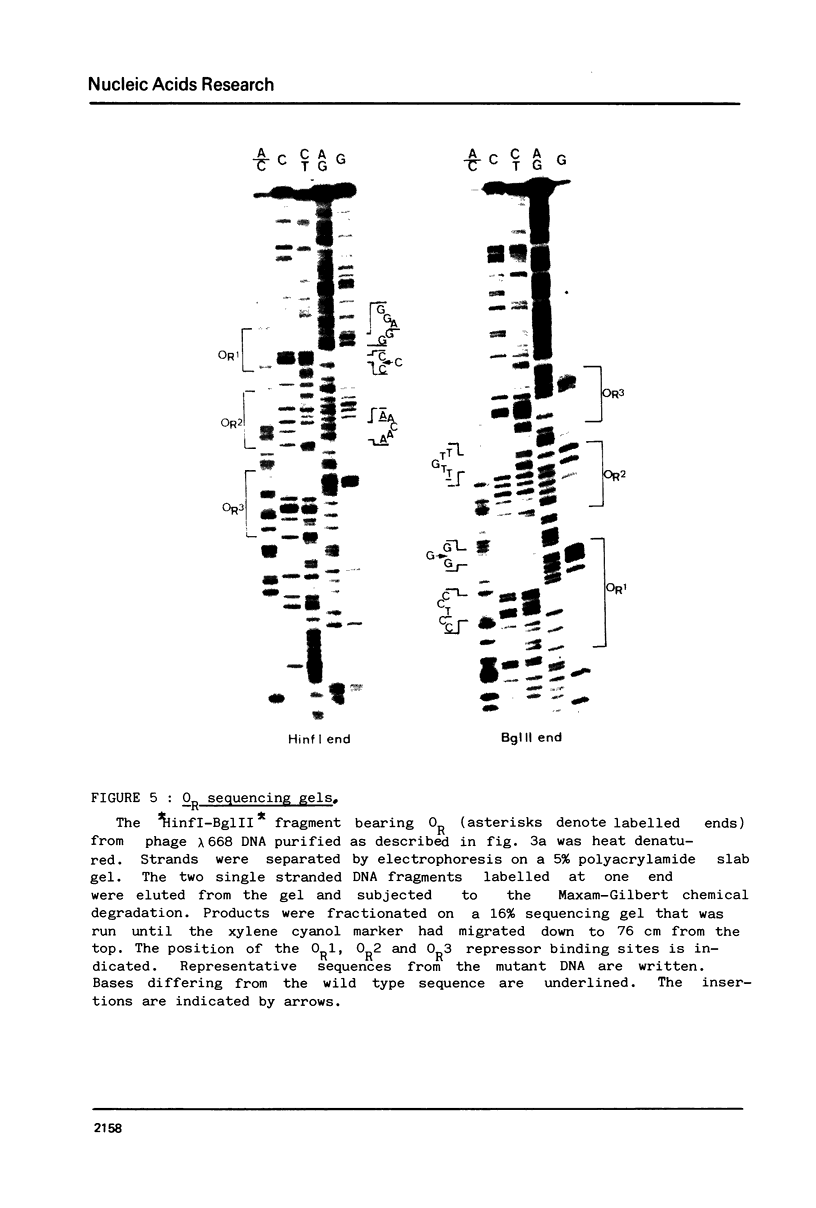
Abstract
The nucleotide sequence of the operators of ultravirulent mutants of lambda, able to grow on host cells with elevated repressor levels, was determined. It appears that ultravirulence in lambda requires multiple mutational events at the operator sequences. OL1, OL2, and OL3 operator sites are the target of mutational changes in ultravirulent phages indicating that these sites participate in vivo in repression of the PL promoter. No changes were found in the OR3 sequence, in contrast there is a mutation in OR2 and two mutations in OR1, in both lambda 668 and lambda 2668 phages. This mutated operator structure accounts for the constitutive expression of their PR promoter either in cells overproducing the lambda repressor or in cells overproducing the cro gene product. A model of the structure of the lambda operator site is proposed. The nucleotide sequence in each site can be divided into two functionally different subsets, one of which is recognized by the repressor while the other stabilizes the repressor-operator interaction.
Full text
PDF



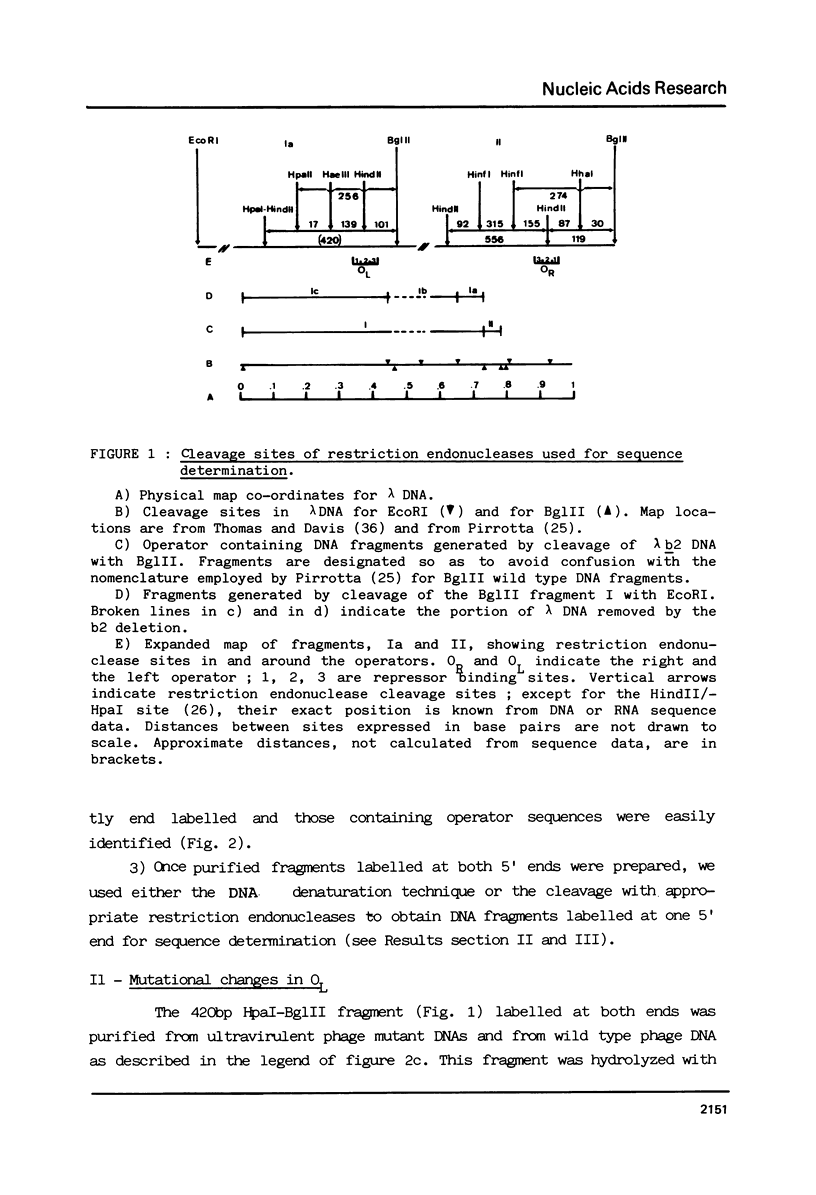



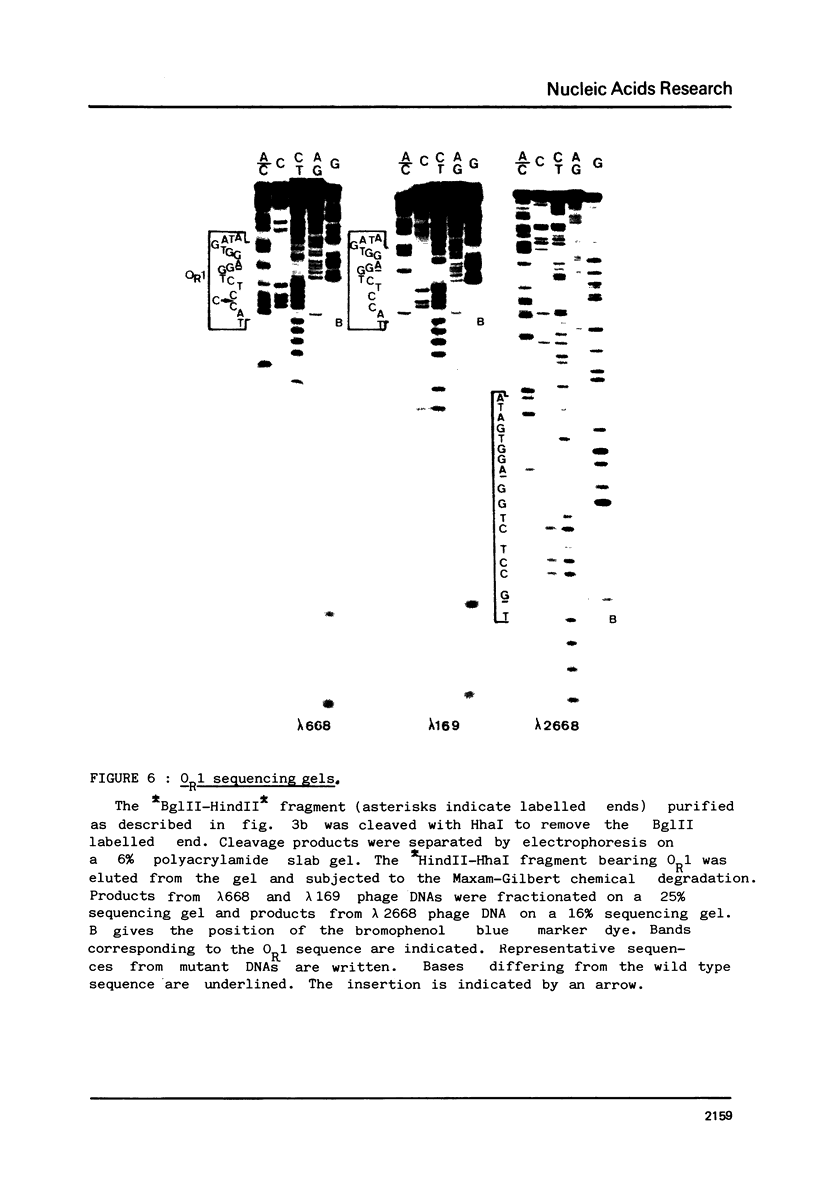

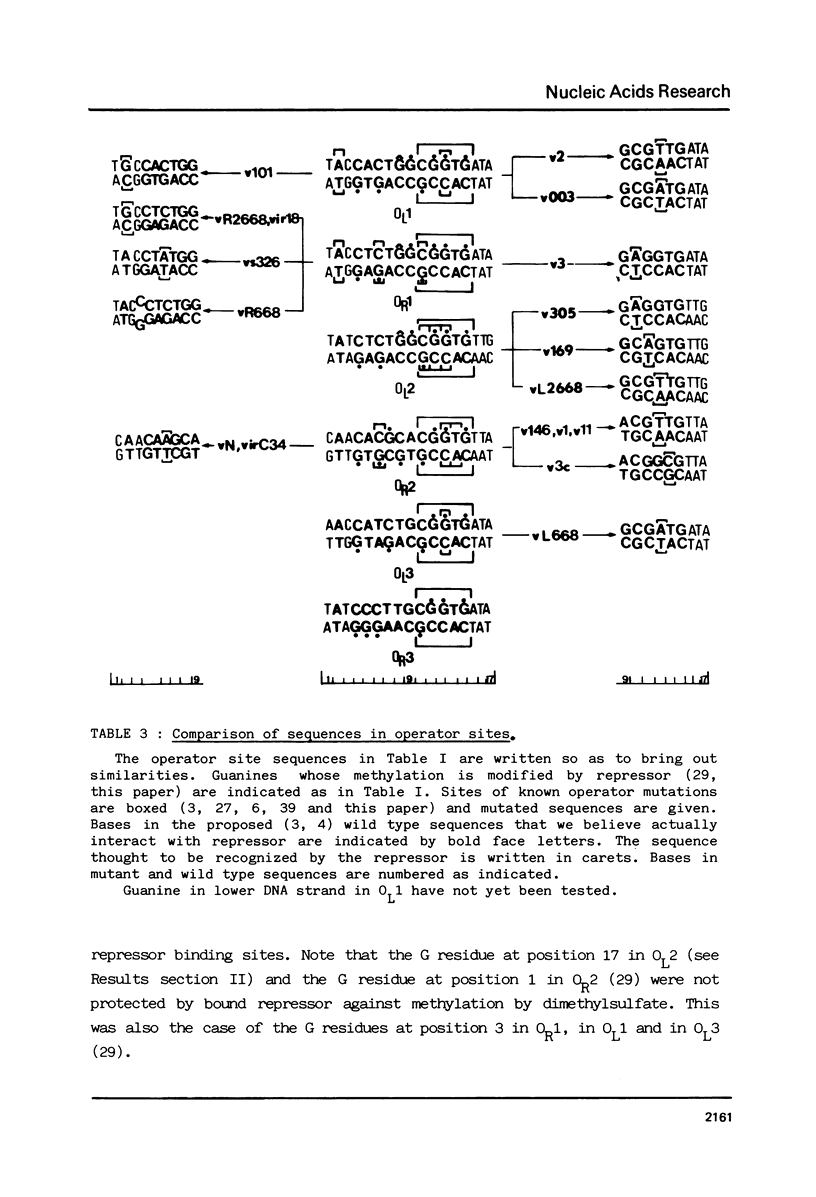



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailone A., Devoret R. Isolation of ultravirulent mutants of phage lambda. Virology. 1978 Feb;84(2):547–550. doi: 10.1016/0042-6822(78)90273-8. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E., Blattner F. R. Sequence of the promoter-operator proximal region of the major leftward RNA of bacteriophage lambda. Nucleic Acids Res. 1975 Sep;2(9):1441–1458. doi: 10.1093/nar/2.9.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H., Green L., Oppenheim A. B., Oppenheim A., Honigman A. Role of the cro gene in bacteriophage lambda development. J Mol Biol. 1973 Oct 25;80(2):203–216. doi: 10.1016/0022-2836(73)90167-8. [DOI] [PubMed] [Google Scholar]
- Flashman S. M. Mutational analysis of the operators of bacteriophage lambda. Mol Gen Genet. 1978 Oct 25;166(1):61–73. doi: 10.1007/BF00379730. [DOI] [PubMed] [Google Scholar]
- Galibert F., Hérissé J., Courtois G. Nucleotide sequence of the EcoRI-F fragment of adenovirus 2 genome. Gene. 1979 May;6(1):1–22. doi: 10.1016/0378-1119(79)90081-7. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Control of gene expression in bacteriophage lambda. Annu Rev Genet. 1973;7:289–324. doi: 10.1146/annurev.ge.07.120173.001445. [DOI] [PubMed] [Google Scholar]
- Humayun Z., Jeffrey A., Ptashne M. Completed DNA sequences and organization of repressor-binding sites in the operators of phage lambda. J Mol Biol. 1977 May 15;112(2):265–277. doi: 10.1016/s0022-2836(77)80143-5. [DOI] [PubMed] [Google Scholar]
- Hérissé J., Courtois G., Galibert F. Mapping the DNA fragments produced by cleavage of EcoRI F fragment of adenovirus 2 with HaeIII, HpaII and AluI. Gene. 1978 Dec;4(4):279–294. doi: 10.1016/0378-1119(78)90046-x. [DOI] [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. L. Etude génétique d'un bactériophage tempéré d'Escherichia coli. l. Le système genétique du bactériophage. Ann Inst Pasteur (Paris) 1954 Dec;87(6):653–673. [PubMed] [Google Scholar]
- Johnson A., Meyer B. J., Ptashne M. Mechanism of action of the cro protein of bacteriophage lambda. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1783–1787. doi: 10.1073/pnas.75.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER G., ZICHICHI M. L., WEIGLE J. A mutation affecting the DNA content of bacteriophage lambda and its lysogenizing properties. J Mol Biol. 1961 Aug;3:399–408. doi: 10.1016/s0022-2836(61)80053-3. [DOI] [PubMed] [Google Scholar]
- Kroeker W. D., Laskowski M., Sr Polynucleotide kinase: functional purification and use in the direct kinetic measurement of single- and double- strand cleavages of DNA by restriction and other endonucleases of limited action. Anal Biochem. 1977 May 1;79(1-2):63–72. doi: 10.1016/0003-2697(77)90379-7. [DOI] [PubMed] [Google Scholar]
- Levine A., Bailone A., Devoret R. Cellular levels of the prophage lambda and 434 repressors. J Mol Biol. 1979 Jul 5;131(3):655–661. doi: 10.1016/0022-2836(79)90014-7. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Ptashne M., Backman K., Kield D., Flashman S., Jeffrey A., Maurer R. Recognition sequences of repressor and polymerase in the operators of bacteriophage lambda. Cell. 1975 Jun;5(2):109–113. doi: 10.1016/0092-8674(75)90018-5. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Ptashne M., Barrell B. G., Donelson J. Sequence of a repressor-binding site in the DNA of bacteriophage lamda. Nature. 1974 Aug 2;250(465):394–397. doi: 10.1038/250394a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Ptashne M., Maurer R. Control elements in the DNA of bacteriophage lambda. Cold Spring Harb Symp Quant Biol. 1974;38:857–868. doi: 10.1101/sqb.1974.038.01.088. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Ptashne M. Multiple repressor binding at the operators in bacteriophage lambda. Proc Natl Acad Sci U S A. 1973 May;70(5):1531–1535. doi: 10.1073/pnas.70.5.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B. J., Kleid D. G., Ptashne M. Lambda repressor turns off transcription of its own gene. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4785–4789. doi: 10.1073/pnas.72.12.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W. Mutations in the right operator of bacteriophage lambda: physiological effects. J Mol Biol. 1973 Oct 5;79(4):723–729. doi: 10.1016/0022-2836(73)90074-0. [DOI] [PubMed] [Google Scholar]
- Pirrotta V. Two restriction endonucleases from Bacillus globiggi. Nucleic Acids Res. 1976 Jul;3(7):1747–1760. doi: 10.1093/nar/3.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M., Backman K., Humayun M. Z., Jeffrey A., Maurer R., Meyer B., Sauer R. T. Autoregulation and function of a repressor in bacteriophage lambda. Science. 1976 Oct 8;194(4261):156–161. doi: 10.1126/science.959843. [DOI] [PubMed] [Google Scholar]
- Robinson L. H., Landy A. HindII, HindIII, and HpaI restriction fragment maps of bacteriophage lambda DNA. Gene. 1977 Sep;2(1):1–31. doi: 10.1016/0378-1119(77)90019-1. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., Ptashne M. In vitro repression of RNA synthesis by purified lambda phage repressor. Nat New Biol. 1971 Mar 17;230(11):76–80. doi: 10.1038/newbio230076a0. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Folkmanis A., Echols H. Cro regulatory protein specified by bacteriophage lambda. Structure, DNA-binding, and repression of RNA synthesis. J Biol Chem. 1977 Sep 10;252(17):6177–6183. [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Walz A., Pirrotta V., Ineichen K. Lambda repressor regulates the switch between PR and Prm promoters. Nature. 1976 Aug 19;262(5570):665–669. doi: 10.1038/262665a0. [DOI] [PubMed] [Google Scholar]
- Walz A., Pirrotta V. Sequence of the PR promoter of phage lambda. Nature. 1975 Mar 13;254(5496):118–121. doi: 10.1038/254118a0. [DOI] [PubMed] [Google Scholar]
- Westmoreland B. C., Szybalski W., Ris H. Mapping of deletions and substitutions in heteroduplex DNA molecules of bacteriophage lambda by electron microscopy. Science. 1969 Mar 21;163(3873):1343–1348. doi: 10.1126/science.163.3873.1343. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Ghosh S., Echols H., Spiegelman W. G. Repression by the cI protein of phage lambda: in vitro inhibition of RNA synthesis. J Mol Biol. 1972 Jun 28;67(3):407–421. doi: 10.1016/0022-2836(72)90459-7. [DOI] [PubMed] [Google Scholar]