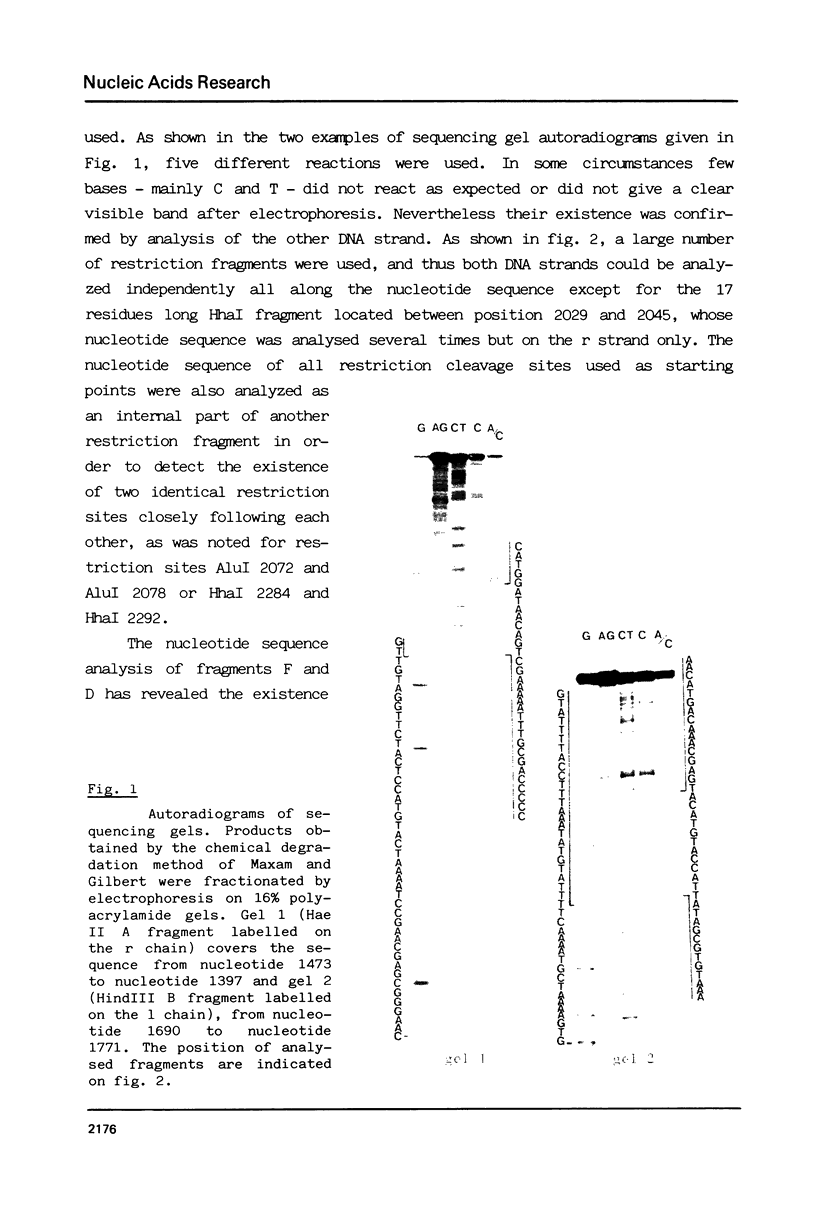
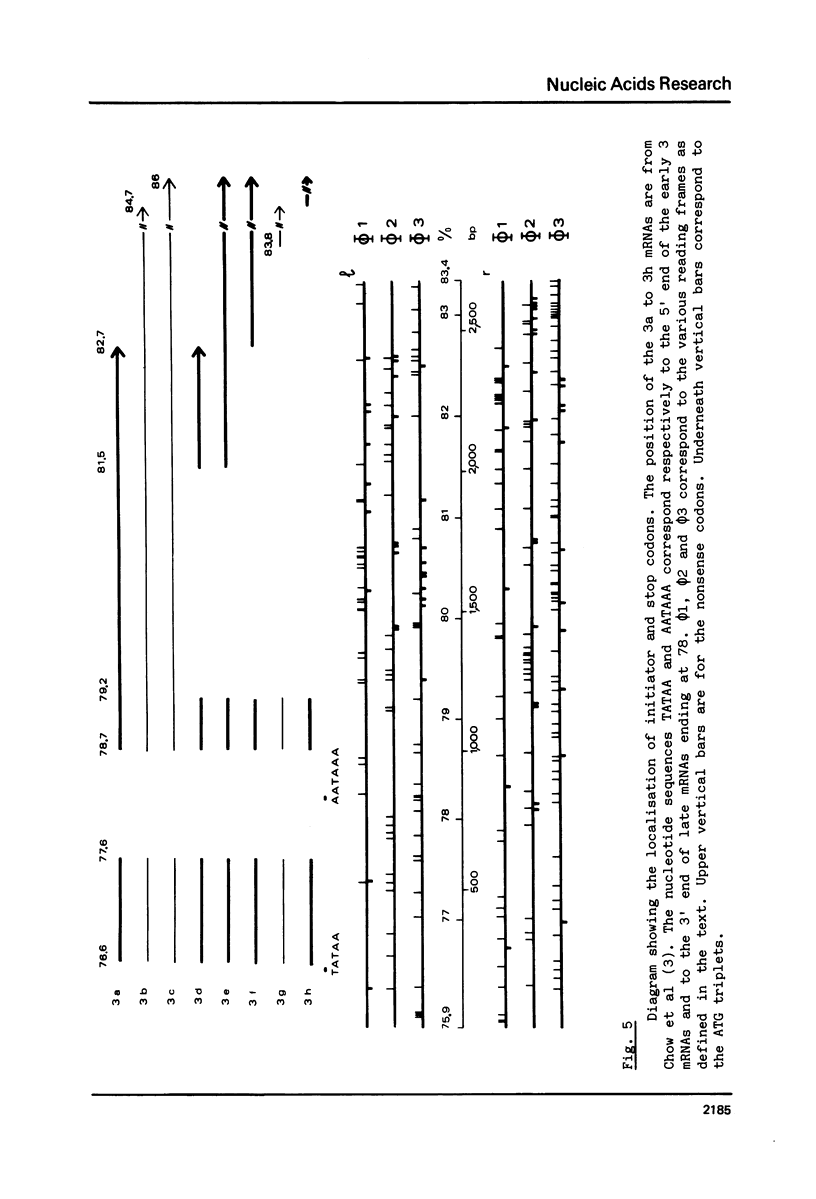
Abstract
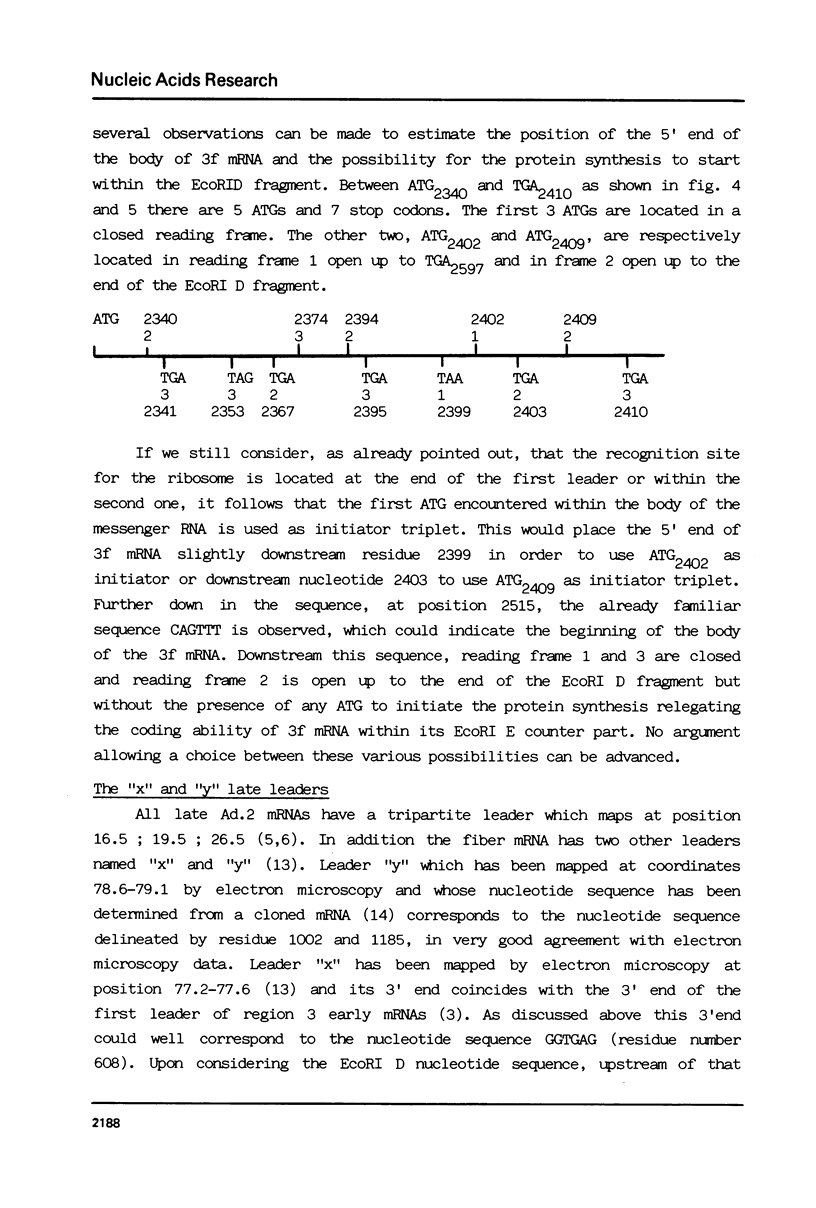
The entire nucleotide sequence of the Ad. 2 EcoRI D fragment has been determined using the Maxam and Gilbert method. This sequence of 2678 bp contains informations relative to late mRNAs ending at position 78 and for which an AATAAA sequence corresponding to their 3' ends is found at residue number 833. Position of the PVIII mRNA is determined thus allowing deduction of the probable amino acid sequence of the PVIII protein. The position and the sequence of the first leader of early 3 mRNAs is determined as well as the sequence and position of the second early leader of region 3 mRNAs, which also correspond to the "y" leader of the fiber mRNA. Following the localization of an open reading frame in which an ATG could initiate protein synthesis it can be predicted that 3a, b, c mRNAs code for the 16K early protein and the probable amino acid sequence of this protein can be deduced. The CAGTTT sequence frequently present at the 5' end of a leader or of a mRNA body as well as the GGTGAG sequence which is found at the 3' end of several leaders were used to postulate the position of various early mRNAs of region 3 and to suggest the existence of an additional splicing event during the processing of mRNAs 3a, b and c. They were also used to predict the position of the additional "x" late leaders. The imbrication of information concerning (i) the family of late mRNAs ending at position 78, (ii) the position of the "x" leader and the "y" leader and (iii) the beginning of early region 3 is also depicted.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Pettersson J. Sequence analysis of adenovirus DNA. IV. The genomic sequences encoding the common tripartite leader of late adenovirus messenger RNA. J Mol Biol. 1979 Oct 15;134(1):143–158. doi: 10.1016/0022-2836(79)90417-0. [DOI] [PubMed] [Google Scholar]
- Alberty H., Raba M., Gross H. J. Isolation from rat liver and sequence of a RNA fragment containing 32 nucleotides from position 5 to 36 from the 3' end of ribosomal 18S RNA. Nucleic Acids Res. 1978 Feb;5(2):425–434. doi: 10.1093/nar/5.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. C., Herisse J., Courtois G., Galibert F., Ziff E. Messenger RNA for the Ad2 DNA binding protein: DNA sequences encoding the first leader and heterogenity at the mRNA 5' end. Cell. 1979 Oct;18(2):569–580. doi: 10.1016/0092-8674(79)90073-4. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J. R., Panasenko S. M., Lehman I. R., Davis R. W. In vitro construction of bacteriophage lambda carrying segments of the Escherichia coli chromosome: selection of hybrids containing the gene for DNA ligase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3416–3420. doi: 10.1073/pnas.72.9.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall J. F., O'Malley B. W., Robertson M. A., Staden R., Tanaka Y., Brownlee G. G. Nucleotide sequence homology at 12 intron--exon junctions in the chick ovalbumin gene. Nature. 1978 Oct 12;275(5680):510–513. doi: 10.1038/275510a0. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R. The spliced structures of adenovirus 2 fiber message and the other late mRNAs. Cell. 1978 Oct;15(2):497–510. doi: 10.1016/0092-8674(78)90019-3. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Roberts J. M., Lewis J. B., Broker T. R. A map of cytoplasmic RNA transcripts from lytic adenovirus type 2, determined by electron microscopy of RNA:DNA hybrids. Cell. 1977 Aug;11(4):819–836. doi: 10.1016/0092-8674(77)90294-x. [DOI] [PubMed] [Google Scholar]
- Cordell B., Bell G., Tischer E., DeNoto F. M., Ullrich A., Pictet R., Rutter W. J., Goodman H. M. Isolation and characterization of a cloned rat insulin gene. Cell. 1979 Oct;18(2):533–543. doi: 10.1016/0092-8674(79)90070-9. [DOI] [PubMed] [Google Scholar]
- De Wachter R. Do eukaryotic mRNA 5' noncoding sequences base-pair with the 18 S ribosomal RNA 3' terminus? Nucleic Acids Res. 1979 Dec 11;7(7):2045–2054. doi: 10.1093/nar/7.7.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maniatis T. The primary structure of rabbit beta-globin mRNA as determined from cloned DNA. Cell. 1977 Apr;10(4):571–585. doi: 10.1016/0092-8674(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Flint J. The topography and transcription of the adenovirus genome. Cell. 1977 Feb;10(2):153–166. doi: 10.1016/0092-8674(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Fraser N., Ziff E. RNA structures near poly(A) of adenovirus-2 late messenger RNAs. J Mol Biol. 1978 Sep 5;124(1):27–31. doi: 10.1016/0022-2836(78)90145-6. [DOI] [PubMed] [Google Scholar]
- Galibert F., Hérissé J., Courtois G. Nucleotide sequence of the EcoRI-F fragment of adenovirus 2 genome. Gene. 1979 May;6(1):1–22. doi: 10.1016/0378-1119(79)90081-7. [DOI] [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Galibert F., Sedat J., Ziff E. Direct determination of DNA nucleotide sequences: structure of a fragment of bacteriophage phiX172 DNA. J Mol Biol. 1974 Aug 15;87(3):377–407. doi: 10.1016/0022-2836(74)90093-x. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Harter M. L., Lewis J. B. Adenovirus type 2 early proteins synthesized in vitro and in vivo: identification in infected cells of the 38,000- to 50,000- molecular-weight protein encoded by the left end of the adenovirus type 2 genome. J Virol. 1978 Jun;26(3):736–749. doi: 10.1128/jvi.26.3.736-749.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hérissé J., Courtois G., Galibert F. Mapping the DNA fragments produced by cleavage of EcoRI F fragment of adenovirus 2 with HaeIII, HpaII and AluI. Gene. 1978 Dec;4(4):279–294. doi: 10.1016/0378-1119(78)90046-x. [DOI] [PubMed] [Google Scholar]
- Klessig D. F. Two adenovirus mRNAs have a common 5' terminal leader sequence encoded at least 10 kb upstream from their main coding regions. Cell. 1977 Sep;12(1):9–21. doi: 10.1016/0092-8674(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Konkel D. A., Tilghman S. M., Leder P. The sequence of the chromosomal mouse beta-globin major gene: homologies in capping, splicing and poly(A) sites. Cell. 1978 Dec;15(4):1125–1132. doi: 10.1016/0092-8674(78)90040-5. [DOI] [PubMed] [Google Scholar]
- Kroeker W. D., Laskowski M., Sr Polynucleotide kinase: functional purification and use in the direct kinetic measurement of single- and double- strand cleavages of DNA by restriction and other endonucleases of limited action. Anal Biochem. 1977 May 1;79(1-2):63–72. doi: 10.1016/0003-2697(77)90379-7. [DOI] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Anderson C. W., Atkins J. F. Further mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Cell. 1977 Sep;12(1):37–44. doi: 10.1016/0092-8674(77)90183-0. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Anderson C. W., Atkins J. F., Gesteland R. F. The origin and destiny of adenovirus proteins. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):581–590. doi: 10.1101/sqb.1974.039.01.072. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McParland R. H., Engelking H. M., Pearson G. D. Cleavage of type 2 adenovirus DNA by HaeIII endonuclease. I. Catalog of HaeIII fragments. Biochim Biophys Acta. 1978 May 23;518(3):413–423. doi: 10.1016/0005-2787(78)90160-0. [DOI] [PubMed] [Google Scholar]
- McReynolds L., O'Malley B. W., Nisbet A. D., Fothergill J. E., Givol D., Fields S., Robertson M., Brownlee G. G. Sequence of chicken ovalbumin mRNA. Nature. 1978 Jun 29;273(5665):723–728. doi: 10.1038/273723a0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E. Groups of adenovirus type 2 mRNA's derived from a large primary transcript: probable nuclear origin and possible common 3' ends. J Virol. 1978 Mar;25(3):811–823. doi: 10.1128/jvi.25.3.811-823.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J. Complete 3' noncoding region sequences of rabbit and human beta-globin messenger RNAs. Cell. 1977 Apr;10(4):559–570. doi: 10.1016/0092-8674(77)90089-7. [DOI] [PubMed] [Google Scholar]
- Ross S., Levine A. J. The genomic map position of the adenovirus type 2 glycoprotein. Virology. 1979 Dec;99(2):427–430. doi: 10.1016/0042-6822(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Lennarz W. J., Brew K. Primary structural requirements for the enzymatic formation of the N-glycosidic bond in glycoproteins. Studies with alpha-lactalbumin. J Biol Chem. 1978 Aug 25;253(16):5786–5794. [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]
- Ziff E. B., Evans R. M. Coincidence of the promoter and capped 5' terminus of RNA from the adenovirus 2 major late transcription unit. Cell. 1978 Dec;15(4):1463–1475. doi: 10.1016/0092-8674(78)90070-3. [DOI] [PubMed] [Google Scholar]