Abstract
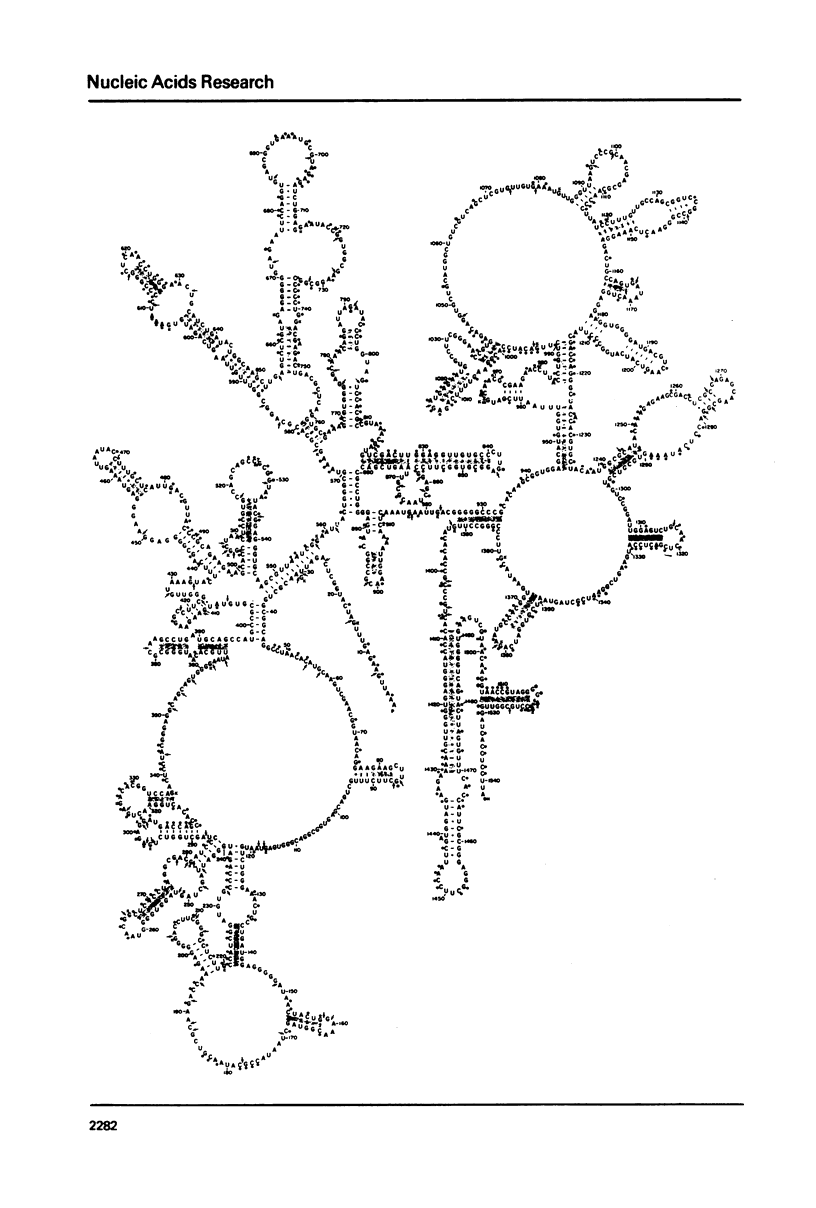
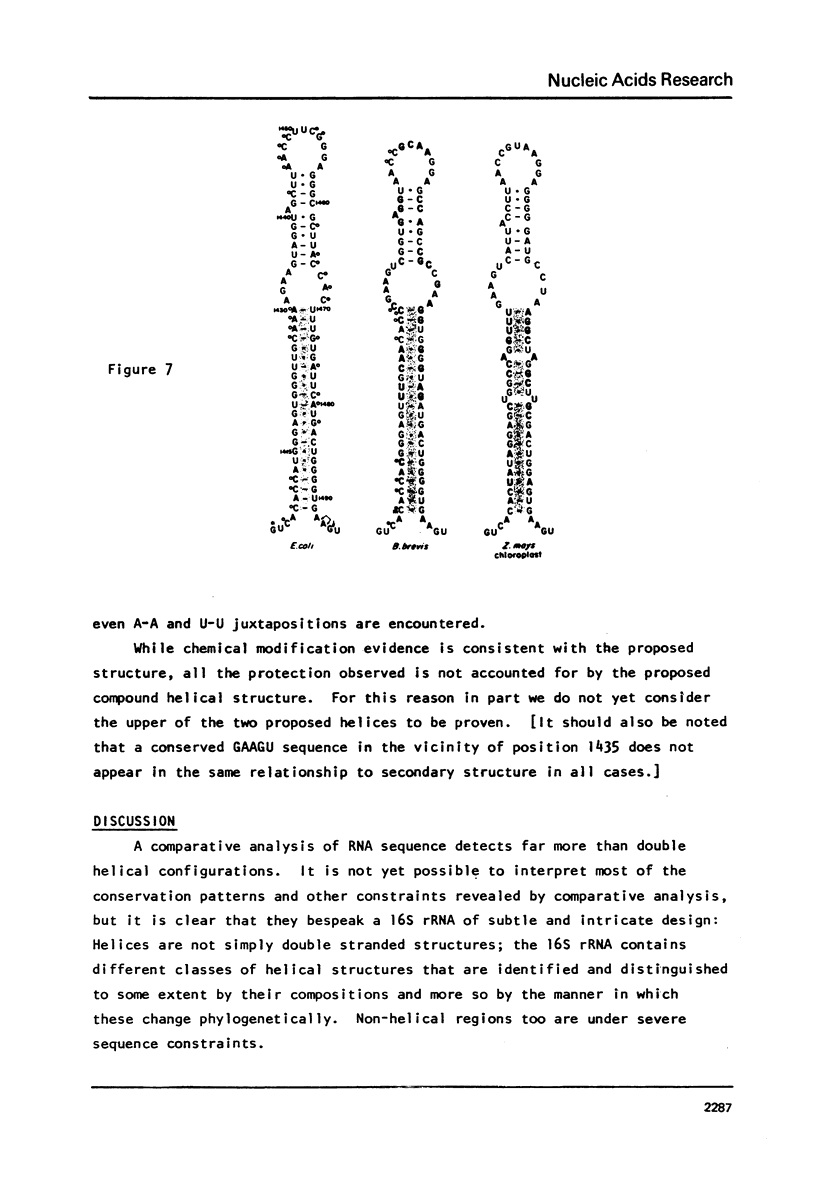
We have derived a secondary structure model for 16S ribosomal RNA on the basis of comparative sequence analysis, chemical modification studies and nuclease susceptibility data. Nucleotide sequences of the E. coli and B. brevis 16S rRNA chains, and of RNAse T1 oligomer catalogs from 16S rRNAs of over 100 species of eubacteria were used for phylogenetic comparison. Chemical modification of G by glyoxal, A by m-chloroperbenzoic acid and C by bisulfite in naked 16S rRNA, and G by kethoxal in active and inactive 30S ribosomal subunits was taken as an indication of single stranded structure. Further support for the structure was obtained from susceptibility to RNases A and T1. These three approaches are in excellent agreement. The structure contains fifty helical elements organized into four major domains, in which 46 percent of the nucleotides of 16S rRNA are involved in base pairing. Phylogenetic comparison shows that highly conserved sequences are found principally in unpaired regions of the molecule. No knots are created by the structure.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon P., Ehresmann C., Ehresmann B., Ebel J. P. The complete nucleotide sequence of the ribosomal 16-S RNA from Excherichia coli. Experimental details and cistron heterogeneities. Eur J Biochem. 1979 Oct 15;100(2):399–410. doi: 10.1111/j.1432-1033.1979.tb04183.x. [DOI] [PubMed] [Google Scholar]
- Chapman N. M., Noller H. F. Protection of specific sites in 16 S RNA from chemical modification by association of 30 S and 50 S ribosomes. J Mol Biol. 1977 Jan 5;109(1):131–149. doi: 10.1016/s0022-2836(77)80049-1. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Buzash-Pollert E., Studier F. W. Mutations of bacteriophage T7 that affect initiation of synthesis of the gene 0.3 protein. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2741–2745. doi: 10.1073/pnas.75.6.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Carbon P., Ungewickell E., Garrett R. A. The topography of the 5' end of 16-S RNA in the presence and absence of ribosomal proteins S4 and S20. Eur J Biochem. 1980 Feb;103(3):439–446. doi: 10.1111/j.1432-1033.1980.tb05967.x. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Fellner P., Ebel J. P. The determination of the primary structure of the 16S ribosomal RNA of Escherichia coli. III. Further studies. Biochimie. 1975;57(6-7):711–748. doi: 10.1016/s0300-9084(75)80047-2. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Magrum L. J., Balch W. E., Wolfe R. S., Woese C. R. Classification of methanogenic bacteria by 16S ribosomal RNA characterization. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4537–4541. doi: 10.1073/pnas.74.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Woese C. R. 5S RNA secondary structure. Nature. 1975 Aug 7;256(5517):505–507. doi: 10.1038/256505a0. [DOI] [PubMed] [Google Scholar]
- HOLLEY R. W., APGAR J., EVERETT G. A., MADISON J. T., MARQUISEE M., MERRILL S. H., PENSWICK J. R., ZAMIR A. STRUCTURE OF A RIBONUCLEIC ACID. Science. 1965 Mar 19;147(3664):1462–1465. doi: 10.1126/science.147.3664.1462. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Mechanism of kasugamycin resistance in Escherichia coli. Nat New Biol. 1972 Jan 5;235(53):6–9. doi: 10.1038/newbio235006a0. [DOI] [PubMed] [Google Scholar]
- Herr W., Chapman N. M., Noller H. F. Mechanism of ribosomal subunit association: discrimination of specific sites in 16 S RNA essential for association activity. J Mol Biol. 1979 Jun 5;130(4):433–449. doi: 10.1016/0022-2836(79)90433-9. [DOI] [PubMed] [Google Scholar]
- Hogan J. J., Noller H. F. Altered topography of 16S RNA in the inactive form of Escherichia coli 30S ribosomal subunits. Biochemistry. 1978 Feb 21;17(4):587–593. doi: 10.1021/bi00597a005. [DOI] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Klug A. Crystallographic refinement of yeast phenylalanine transfer RNA at 2-5A resolution. J Mol Biol. 1976 Dec 25;108(4):619–649. doi: 10.1016/s0022-2836(76)80109-x. [DOI] [PubMed] [Google Scholar]
- Möller K., Zwieb C., Brimacombe R. Identification of the oligonucleotide and oligopeptide involved in an RNA--protein crosslink induced by ultraviolet irradiation of Escherichia coli 30 S ribosomal subunits. J Mol Biol. 1978 Dec 15;126(3):489–506. doi: 10.1016/0022-2836(78)90055-4. [DOI] [PubMed] [Google Scholar]
- Müller R., Garrett R. A., Noller H. F. The structure of the RNA binding site of ribosomal proteins S8 and S15. J Biol Chem. 1979 May 25;254(10):3873–3878. [PubMed] [Google Scholar]
- Noller H. F., Chaires J. B. Functional modification of 16S ribosomal RNA by kethoxal. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3115–3118. doi: 10.1073/pnas.69.11.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F. Topography of 16S RNA in 30S ribosomal subunits. Nucleotide sequences and location of sites of reaction with kethoxal. Biochemistry. 1974 Nov 5;13(23):4694–4703. doi: 10.1021/bi00720a003. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Liou R., Kohut J., 3rd, Schwartz I., Zimmermann R. A. Covalent cross-linking of transfer ribonucleic acid to the ribosomal P site. Mechanism and site of reaction in transfer ribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4322–4332. doi: 10.1021/bi00587a010. [DOI] [PubMed] [Google Scholar]
- Pace N. R. Structure and synthesis of the ribosomal ribonucleic acid of prokaryotes. Bacteriol Rev. 1973 Dec;37(4):562–603. doi: 10.1128/br.37.4.562-603.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldermans B., Bakker H., Van Knippenberg P. H. Studies on the function of two adjacent N6,N6-dimethyladenosines near the 3' end of 16S ribosomal RNA of Escherichia coli. IV. The effect of the methylgroups on ribosomal subunit interaction. Nucleic Acids Res. 1980 Jan 11;8(1):143–151. doi: 10.1093/nar/8.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince J. B., Hixson S. S., Zimmermann R. A. Photochemical cross-linking of tRNALys and tRNA2Glu to 16 S RNA at the P site of Escherichia coli ribosomes. J Biol Chem. 1979 Jun 10;254(11):4745–4749. [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Rinke J., Yuki A., Brimacombe R. Studies on the environment of protein S7 within the 30-S subunit Escherichia coli ribosomes. Eur J Biochem. 1976 Apr 15;64(1):77–89. doi: 10.1111/j.1432-1033.1976.tb10276.x. [DOI] [PubMed] [Google Scholar]
- Ross A., Brimacombe R. Experimental determination of interacting sequences in ribosomal RNA. Nature. 1979 Sep 27;281(5729):271–276. doi: 10.1038/281271a0. [DOI] [PubMed] [Google Scholar]
- Rubtsov P. M., Musakhanov M. M., Batchikova N. V., Skriabin K. S., Baev A. A. Opredelenie pervichnoi struktury fragmentov ribosomnogo operona pekarskikh drozhzhei, kodiruiushchikh 18 S rRNK. Dokl Akad Nauk SSSR. 1979;248(3):760–762. [PubMed] [Google Scholar]
- Santer M., Shane S. Area of 16S ribonucleic acid at or near the interface between 30S and 50S ribosomes of Escherichia coli. J Bacteriol. 1977 May;130(2):900–910. doi: 10.1128/jb.130.2.900-910.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M., Craven G. R. Chemical inactivation of Escherichia coli 30-S ribosomes by iodination. Identification of proteins involved in tRNA binding. Eur J Biochem. 1976 Jan 2;61(1):307–315. doi: 10.1111/j.1432-1033.1976.tb10023.x. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duin J., Kurland C. G., Dondon J., Grunberg-Mangago M., Branlant C., Ebel J. P. New aspects of the IF3-ribosome interaction. FEBS Lett. 1976 Feb 15;62(2):111–114. doi: 10.1016/0014-5793(76)80030-0. [DOI] [PubMed] [Google Scholar]
- Vasilenko S. K., Ryte V. C. [Isolation of highly purified ribonuclease from cobra (Naja oxiana) venom]. Biokhimiia. 1975 May-Jun;40(3):578–583. [PubMed] [Google Scholar]
- Yuki A., Brimacombe R. Nucleotide sequences of Escherichia coli 16-S RNA associated with ribosomal proteins S7, S9, S10, S14 and S19. Eur J Biochem. 1975 Aug 1;56(1):23–34. doi: 10.1111/j.1432-1033.1975.tb02203.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann R. A., Singh-Bergmann K. Binding sites for ribosomal proteins S8 and S15 in the 16 S RNA of Escherichia coli. Biochim Biophys Acta. 1979 Jul 26;563(2):422–431. doi: 10.1016/0005-2787(79)90061-3. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]


