Abstract
Adaptive co-evolution of mammals and bacteria has led to the establishment of complex commensal communities on mucosal surfaces. In spite of having available a wealth of immune-sensing and effector mechanisms capable of triggering inflammation in response to microbial intrusion, mucosal immune cells establish an intimate dialogue with microbes to generate a state of hyporesponsiveness against commensals and active readiness against pathogens. A key component of this homeostatic balance is IgA, a noninflammatory antibody isotype produced by mucosal B cells through class switching. This process involves activation of B cells by IgA-inducing signals originating from mucosal T cells, dendritic cells, and epithelial cells. Here, we review the mechanisms by which mucosal B cells undergo IgA diversification and production and discuss how the study of primary immunodeficiencies facilitates better understanding of mucosal IgA responses in humans.
Keywords: human, B cells, IgA, mucosa, immunodeficiency
Introduction
The intestinal mucosa is home to trillions of microbes present at densities that greatly exceed those found in other habitats, including soil.1 These commensal bacteria confer many metabolic capabilities that our mammalian genome lacks, including the ability to break down otherwise undigestible dietary carbohydrates, generate essential vitamins and iso-prenoids, and fill a niche that would otherwise be easily accessible to pathogens.2 A single layer of intestinal epithelial cells separates commensals from the sterile milieu of our body.3 By recognizing microbial molecular signatures through various families of pattern recognition receptors, such as Toll-like receptors (TLRs), intestinal epithelial cells establish a complex dialogue with innate and adaptive cells of the intestinal immune system.4 This dialogue leads to the production of a vast array of immune mediators that generate a state of hyporesponsiveness against commensals and active readiness against pathogens.1
An important component of this homeostatic balance is IgA, a noninflammatory antibody iso-type generated by follicular B cells from Peyer’s patches, mesenteric lymph nodes, and isolated lymphoid follicles.5,6 Collectively, these organized lymphoid structures form the gut-associated lymphoid tissue, which constitutes the major inductive site for intestinal IgA responses.5,6 IgA-secreting plasma cells emerging from the gut-associated lymphoid tissue migrate to the effector site of the lamina propria, where they release large amounts of IgA onto the epithelial surface.5,6 In addition to serving as a key effector site, the lamina propria has a nonorganized lymphoid tissue that includes dispersed B cells retaining some IgA-inducing function.5,6 Here, we review the cellular and signaling pathways orchestrating intestinal IgA production and discuss how the analysis of patients with specific forms of primary immunodeficiency (PID) has improved our understanding of these pathways.
Function of mucosal IgA
The intestinal mucosa has evolved several strategies to control commensals and neutralize pathogens without causing inflammatory damage to the epithelial barrier. One of these strategies involves the production of massive amounts of IgA, the most abundant antibody isotype in our body. IgA reaches the intestinal lumen by interacting with the polymeric Ig receptor on the basolateral surface of epithelial cells.5,6 After binding to polymeric Ig receptor through a joining chain, IgA dimers secreted by intestinal plasma cells translocate across epithelial cells onto the mucosal surface by undergoing transcytosis.7,8 This process involves intracellular processing of polymeric Ig receptor into a polypeptide called secretory component, which remains associated with the joining chain of the IgA dimer to form a secretory IgA complex with noninflammatory protective function.9–11 Indeed, secretory IgA can bind to bacteria without activating complement or stimulating the release of inflammatory mediators by innate immune cells.12,13
IgA neutralizes toxins, pathogenic bacteria, and inflammatory microbial molecules, such as, lipopolysaccharide.14–21 IgA also prevents commensal bacteria from adhering to the epithelial surface by generating steric hindrance, by inducing bacterial agglutination, by masking adhesion epitopes, and by interacting with mucus through the secretory component.22,23 These processes favor the growth of commensal bacteria in biofilms that prevent the outgrowth of pathogens through a mechanism involving competition for biological niches and sources of energy. Furthermore, IgA facilitates the maintenance of homeostasis by decreasing the inflammatory tone of the intestine and by favoring the maintenance of appropriate bacterial communities within specific intestinal segments.21,24,25 Finally, IgA interacts with yet poorly defined receptors to facilitate the sampling of luminal antigen by intestinal dendritic cells (DCs) and microfold (M) cells, a subset of antigen-sampling intestinal epithelial cells located in the follicular epithelium of Peyer’s patches and isolated lymphoid follicles.17,26–28
Binding modes and reactivity of mucosal IgA
Mucosal IgA antibodies emerge from B cells that follow either T cell–dependent (TD) or T cell– independent (TI) pathways (Fig. 1). Intestinal B cells generate IgA diversification and production thorugh V(D)J gene somatic hypermutation (SHM) and class switch recombination (CSR) from IgM to IgA.5,6 These processes require the DNA-editing enzyme activation–induced cytidine deaminase (AID) and predominantly occur in the germinal center of Peyer’s patches and mesenteric lymph nodes, although extrafollicular CSR and SHM have also been described.29–36
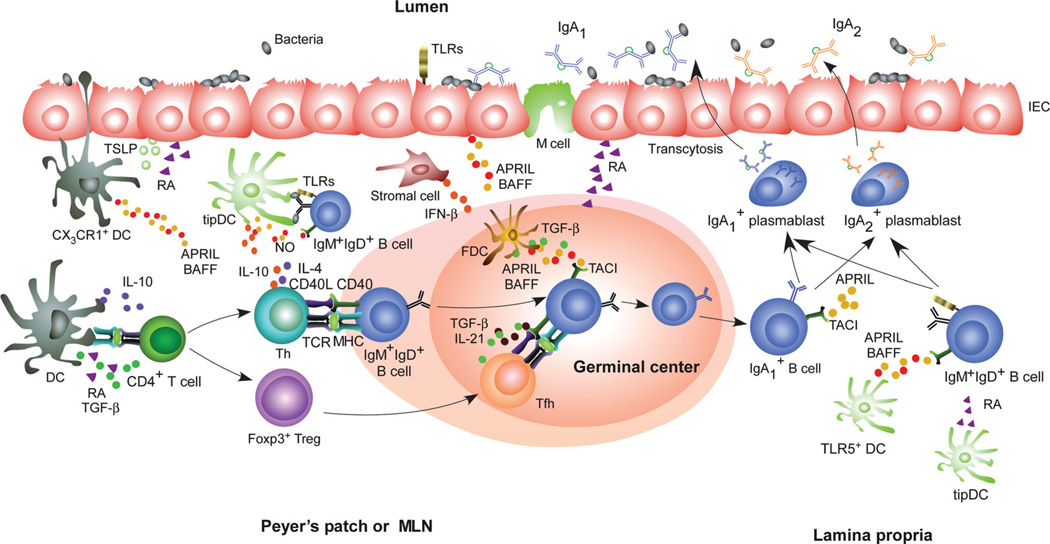
Figure 1.
Cellular networks underlying mucosal IgA responses. Intestinal epithelial cells (IECs) “condition” dendritic cells (DCs) by releasing thymic stromal lymphopoietin (TSLP) and retinoic acid (RA) in response to TLR ligands from commensal bacteria. Different subsets of intestinal DCs release TGF-β, IL-10, RA, and nitric oxide (NO) that promote IgA responses in Peyer’s patches and mesenteric lymph nodes (MLNs) by inducing T regulatory (Treg) and T helper (Th) cells, including Treg-derived T follicular helper cells, which activate follicular B cells via CD40L, TGF-β, IL-4, IL-10, and IL-21. Follicular DCs further enhance IgA production by releasing B cell–activating factor of the TNF family (BAFF), a proliferation-inducing ligand (APRIL), and TGF-β upon exposure to TLR ligands and RA. Some subsets of intestinal DCs also induce T cell–independent IgA production in mesenteric lymph nodes or the lamina propria by releasing BAFF, APRIL, RA, and NO in response to TLR ligands from commensals or IFN-β from stromal cells. In humans, these T cell–independent signals would induce switching from IgM or IgA1 to IgA2. The IgA antibodies emerging from these pathways undergo transcytosis across IECs via the polymeric Ig receptor.
In mice, IgA forms both high-affinity and low-affinity binding systems that originate from different B cell types and likely serve distinct functions.18 High-affinity IgA originates from monoreactive and antigen-selected conventional B-2 cells that express mutated Ig V(D)J gene sequences and occupy the follicles of Peyer’s patches and mesenteric lymph nodes.5,6 Low-affinity IgA would derive from polyreactive and nonantigen-selected B-1 cells that express unmutated Ig V(D)J gene sequences and occupy the peritoneal cavity and to some extent the lamina propria.37–42 Additional low-affinity IgA may derive from conventional B-2 cells, such as those lodged in isolated lymphoid follicles.39,40,43,44 Mutated, antigen-selected, and monoreactive IgA plays an important role in the control of commensal bacteria and the neutralization of pathogens and microbial toxins.15,16,21,43 Unmutated, low-affinity, and polyreactive IgA would predominantly favor the exclusion of commensal bacteria from the surface of intestinal epithelial cells, but this distinction is not absolute, as there are examples of unmutated IgA antibodies that recognize commensal bacteria with high specificity.2,25,41 Conversely, polyreactivity has been detected in mutated IgA antibodies with clear traces of antigen selection, at least in humans.45
In humans, 75% of IgA antibodies from intestinal plasmablasts are extensively mutated, have high specificity for commensal and enteropathogenic antigens, and carry signs of antigen selection.45–47 Some of these antigen-specific antibodies seem to dominate the intestinal IgA repertoire in multiple individuals, which is remarkable considering the enormous diversity of the gut microbiota.45 The remaining 25% of human intestinal IgA antibodies show polyreactivity for diverse microbial and autologous antigens, but also these antibodies are highly mutated and show traces of antigen selection.45 It has been proposed that polyreactivity may be acquired through the introduction of somatic mutations and that selection of mutated variants of polyreactive germline gene-encoded antibodies may help to narrow down their antigen specificity.45,48 Consistent with this possibility, some human polyreactive IgA antibodies show high reactivity to specific commensal bacteria and intestinal tissue structures, but low reactivity to other foreign or autologous antigens.45 Another possibility is that mutation-induced polyreactivity enhances the binding affinity of human IgA for microbes by supporting heteroligation between one high-affinity combining site and a second low-affinity site on a different molecular structure of the microbe.49 Clearly, more studies are needed to further elucidate the nature and reactivity of intestinal IgA in humans and to determine whether specific members of the microbiota elicit different IgA responses.
Although SHM plays a dominant role in the control of intestinal bacteria, CSR from IgM to IgA is crucial to lower the overall inflammatory tone of the intestine in face of the continuous stimulation exerted by the local microbiota.21,25 The mechanism by which Cα confers noninflammatory-protective function to IgA remains poorly understood. The inability of Cα to effectively activate complement and the relative lack of high-affinity Cα-binding Fcα receptors on inflammatory intestinal immune cells likely play an important role.18 Additional noninflammatory properties of Cα may relate to its ability to interact with the secretory component of the polymeric Ig receptor, which delivers regulatory signals to DCs via an unknown receptor.10,11 Cα may deliver additional regulatory signals via a C-type lectin named DC-SIGN, which tunes down inflammatory signals from TLRs in antigen-sampling DCs.50,51
IgA induction in Peyer’s patches and mesenteric lymph nodes
Peyer’s patches are the major portal of entry of bacteria, and together with mesenteric lymph nodes, constitute the major IgA inductive site in the in-testine.28,52 Peyer’s patches develop during fetal life independently of gut colonization by bacteria and consist of large structures built on a stromal scaffold composed of several B cell follicles separated by areas containing T cells and DCs.5,6 In Peyer’s patches there is an ongoing germinal center reaction that continuously drives IgA diversification and production.52 This germinal center reaction is optimized by microbial signals, as mice depleted of intestinal bacteria have Peyer’s patches with fewer and smaller germinal centers.53,54 In both Peyer’s patches and mesenteric lymph nodes, germinal center B cells produce IgA through a TD pathway involving activation of CD4+ T cells by antigen-presenting DCs.44,55,56 The phenotype, cytokine expression profile, and immune functions of these and other gut DCs are a matter of intense investigation (Table 1).
Table 1.
Mucosal cell types involved in IgA class switching and production
| Mucosal cell type | Mucosal topography | Mechanism of IgA induction |
|---|---|---|
| Treg cell | PP, LP | CD40L, TGF-β, possibly IL-10 |
| TFH cell | PP, MLN | CD40L, IL-21, TGF-β |
| Th2 cells | PP | IL-4, IL-10 |
| FDC | PP, MLN | BAFF, APRIL, TGF-β |
| Stromal cell | ILF, LP | BAFF, APRIL, TGF-β |
| IEC | LP | BAFF, APRIL, RA, TSLP, IL-10, TGF-β |
| iNOS+TNF+ DC (tipDC)a | PP, LP | BAFF, APRIL, nitric oxide |
| CD11chiCD11bhi DC (TLR5+ DC)a | LP | RA, IL-6 |
| CX3CR1+ DCa | PP, LP | Unknown |
| CD103+ DCa | PP, LP, MLN | Induction of Treg and possibly TFH cells via RA |
| CD11c+ DC | PP | Induction of Th2 cells via IL-10 |
| CD11b+ DCa | PP | IL-6 |
Described mostly in mice. TFH, T follicular helper cell.
In Peyer’s patches, DCs expressing the chemokine receptor CX3CR1 are found in close contactwith the follicle-associated epithelium and should be equivalent to CX3CR1+ DCs present in the lamina propria.57,58 In general, CX3CR1+ DCs originate from circulating monocytes, express the DC molecule CD11c together with the macrophage molecule CD11b, and extend cellular projections across interepithelial junctions to sample antigen from the intestinal lumen, including segmented filamentous bacteria.57,59–64 Although unable to migrate to the interfollicular areas of Peyer’s patches to present antigen to T cells,65 CX3CR1+ DCs might contribute to TD IgA responses by transferring antigen to migratory CD103+ DCs. In Peyer’s patches, DCs expressing the integrin CD103 have an uncertain location but seem phenotypically and functionally similar to CD103+ DCs found in the intestinal lamina propria.66 In general, CD103+ DCs originate from circulating pre-DCs, express CD11c but no or little CD11b, and migrate to the interfollicular area of Peyer’s patches and mesenteric lymph nodes to present antigen to T cells.62,63,65,67 CD103+ DCs do not seem to form interepithelial projections and, therefore, might acquire antigen from CX3CR1+ DCs or M cells.
Peyer’s patches contain additional DC subsets, including CD11c+ DCs secreting the noninflammtory cytokine IL-10 and CD11b+ DCs secreting the antibody-inducing cytokine IL-6.68,69 An additional DC subset expressing the chemokine receptor CCR6 is found in the subepithelial dome, which lies just underneath the follicle-associated epithelium of Peyer’s patches.58,70 Subepithelial CCR6+ DCs can initiate T cell immunity and may give rise to CCR7+ DCs, which are typically detected in the T cell-rich interfollicular area of mesenteric lymph nodes.71 The relationship of all these DC subsets with CX3CR1+ DCs and CD103+ DCs remains unclear.
As they sample antigen across epithelial cells, intestinal DCs receive “conditioning” signals from intestinal epithelial cells, including thymus stromal lymphopoietin (TSLP).72 These signals mitigate DC production of IL-12, a cytokine that stimulates the formation of T helper type-1 (Th1) cells producing the inflammatory cytokine IFN-γ.59,72,73 Instead, intestinal DCs, including CD103+ DCs, release IL-10 and retinoic acid (a derivative of vitamin A) to promote the development of Foxp3+ T regulatory (Treg) cells.72–75
By expressing the TNF family member CD40 ligand (CD40L) and releasing IL-10 and TGF-β1, intestinal Treg cells elicit IgA CSR and production in B cells while inhibiting the formation of inflammatory Th1 cells.56,76 Furthermore, intestinal Treg cells differentiate into T follicular helper cells, which induce germinal center B cell differentiation as well as IgA CSR and production via CD40L, IL-21, and TGF-β1.56,77,78 Yet, it is currently unclear whether intestinal T follicular helper cells arise from natural or induced Treg cells, and whether Treg and T follicular helper cells play specific roles in the generation of commensal-reactive versus pathogen-reactive, or low-affinity versus high-affinity IgA antibodies. In addition to Treg and T follicular helper cells, Th2 cells producing IL-4 may further contribute to IgA CSR and production in intestinal follicles. CD11b+ DCs appear to be particularly efficient in the induction of Th2 cells in Peyer’s patches.68,79
Together with TGF-β, CD40L is a key element in the TD pathway for IgA CSR and production.80–82 Engagement of CD40 on B cells by CD40L causes recruitment of TNF receptor–associated factor (TRAF) adaptor proteins to the cytoplasmic tail of CD40.83 This event is followed by translocation of the transcription factor NF-κB from the cytoplasm to the nucleus and by NF-κB-dependent transcription of the AID gene promoter.84 In contrast, NF-κB is not required for the activation of the Cα gene promoter, which rather involves SMA-homologue mothers against decapentaplegic (SMAD) transcription factors induced by the TGF-β receptor.84
In spite of requiring CD4+ T cells to develop a germinal center reaction, Peyer’s patches can promote IgA responses in the absence of the B cell antigen receptor (BCR or Ig receptor).54 The BCR is central to antigen-driven cognate T–B cell interactions and is required for the survival of peripheral B cells and the development of a germinal center reaction in systemic lymphoid follicles.54,85 This has led to the proposal that B cells from Peyer’s patches may undergo IgA diversification and production by utilizing a noncanonical TD pathway involving cosignals from TLRs instead of cosignals from the BCR.86 Additional BCR-independent signals may originate from TNF-inducible nitric oxide synthase (iNOS)-producing DCs, which enhance TD IgA responses by upregulating the expression of the TGF-β receptor on follicular B cells from Peyer’s patches via nitric oxide.87
Follicular B cells from Peyer’s patches can receive additional IgA-inducing signals from follicular DCs.88 These cells release CD40L-related factors known as B cell-activating factor of the TNF family (BAFF) and a proliferation-inducing ligand (APRIL) upon “priming” by mucosal signals, such as commensal TLR ligands and retinoic acid.88 Mucosal follicular DCs also release large amounts of active TGF-β1 and use their dendrites to organize commensal antigens in “periodic” arrays.88 By releasing TGF-β1, BAFF, and APRIL and stimulating BCRs and TLRs on B cells, follicular DCs would enhance the IgA-inducing function of T follicular helper cells in Peyer’s patches.56,88 A similar mechanism may enable follicular DCs to trigger IgA production in a TI manner.89,90 Follicular B cells from Peyer’s patches and mesenteric lymph nodes further undergo TI switching to IgA in response to plasmacytoid DCs.91 These cells are “primed” by type-I IFNs from intestinal stromal cells to release large amounts of BAFF and APRIL.91
IgA induction in isolated lymphoid follicles
Together with Peyer’s patches and mesenteric lymph nodes, isolated lymphoid follicles represent another important site for IgA induction.92 These lymphoid structures are scattered throughout the intestine and consist of solitary B cell clusters built on a scaffold of stromal cells with a few interspersed CD4+ T cells and more abundant perifollicular DCs expressing CD11c.56,92 Unlike Peyer’s patches, which develop in the sterile fetal microenvironment, isolated lymphoid follicles develop from smaller anlagen structures called cryptopatches after postnatal bacterial colonization of the intestine.92–94 These cryptopatches contain lymphoid tissue-inducer cells expressing the retinoic acid orphan receptor RORγt and stromal cells that recruit DCs, B, and T cells in response to bacterial signals, including TLR signals.92–94
Similar to Peyer’s patches, isolated lymphoid follicles have a follicular epithelium comprising antigen-sampling M cells as well as a subepithelial area containing CX3CR1+ DCs.92 After capturing bacteria from the intestinal lumen or M cells, CX3CR1+ DCs may present TI antigens to local follicular B cells, thereby, eliciting the activation of TLR and perhaps BCR signaling pathways. CD11c+ DCs associated with isolated lymphoid follicles also express TNF, a powerful inducer of matrix metalloproteases 9 and 13 that process active TGF-β1 from a latent precursor protein.44 In addition to forming active TGF-β1, DCs cooperate with stromal cells to release BAFF and APRIL through a pathway that is enhanced by microbial TLR signals.44 These cytokines induce IgA CSR and production through a TI pathway that does not require germinal center formation.5,44,95
IgA induction in the lamina propria
The diffuse tissue of the lamina propria can support some IgA CSR and production in the absence of follicular structures.44,96,97 Consistent with this possibility, some B cells from the lamina propria contain molecular hallmarks of ongoing IgA CSR, including AID, H2AX (a nuclear protein associated with AID-induced double-strand DNA breaks), Sα–Sµ switch circles, and Iα–Cµ switch circle transcripts.34,96,98–101 In general, lamina propria B cells are scattered and express fewer molecular byproducts of IgA CSR than Peyer’s patch B cells, which may explain why some groups failed to detect IgA CSR in the lamina propria.89,90,102 Discrepancies seem particularly evident with regard to AID expression.89,90,102 Yet, this expression has been confirmed in the lamina propria by means of a green fluorescent protein-AID reporter mouse model and through the use of in situ hybridizaton techniques.34,96
IgA CSR in the lamina propria likely involves in situ activation of B cells by DCs.34,44,87,97,103,104 In the mouse lamina propria, TNF-iNOS–producing DCs initiate TI IgA CSR and production by releasing BAFF and APRIL through a TLR-dependent mechanism involving induction of nitric oxide production by iNOS.87 Another lamina propria DC subset with IgA-licensing function is represented by TLR5+ DCs.97,105 In addition to TLR5, these DCs express CD11c, CD11b, and CD103 and induce TI IgA production by releasing retinoic acid and IL-6 upon sensing bacteria through the flagellin receptor TLR5.97,105 Also epithelial cells deliver IgA-inducing signals to lamina propria B cells by releasing BAFF and APRIL after recognizing bacteria via TLRs.34,103 Epithelial cells would further amplify IgA production by enhancing DC release of BAFF and APRIL through TSLP.34,103
In humans, APRIL is particularly effective at inducing IgA2, an IgA subclass abundant in heavily colonized mucosal districts, such as, the distal intestine.34,106,107 Consistent with studies showing the germinal center–independent origin of IgA2-producing B cells,108 APRIL triggers IgM-to-IgA2 CSR independently of CD40L.34 In addition, APRIL elicits sequential IgA1-to-IgA2 CSR, thereby, allowing hypermutated IgA1-expressing B cells from Peyer’s patches to acquire a protease-resistant IgA2 subclass in the lamina propria.34 This model could explain why IgA2 antibodies have mutated V(D)J genes in spite of emerging from a seemingly TI pathway.45
BAFF and APRIL trigger IgA CSR by engaging a CD40-related receptor known as transmembrane activator and calcium modulator and cyclophylin ligand interactor (TACI).109,110 In the presence of cosignals from cytokine receptors and TLRs (Fig. 2), TACI induces AID expression via NF-κB, followed by CSR, antibody production, and plasma cell differentiation.110,111 An important property of TACI relates to its ability to establish a close functional cooperation with B cell-intrinsic TLR signals.110 Indeed, TACI uses the adaptor protein myeloid differentiation primary response gene 88 (MyD88) and TRAF6 to activate NF-κB, as TLRs do.110 However, TLRs recruit MyD88 and downstream kinases, such as, IL-1 receptor–associated kinase 1 (IRAK-1) and IRAK4 through a cytoplasmic Toll-interleukin-1 receptor (TIR) motif, whereas TACI uses a cytoplasmic motif different from TIR.110 Given that TLR signals are also important to generate the production of TACI ligands by innate immune cells,34,103,104 these findings highlight the intimate cooperation between the innate and adaptive immune systems at both cellular and signaling levels and provide an additional mechanistic explanation for studies linking intestinal IgA responses to MyD88.54,87
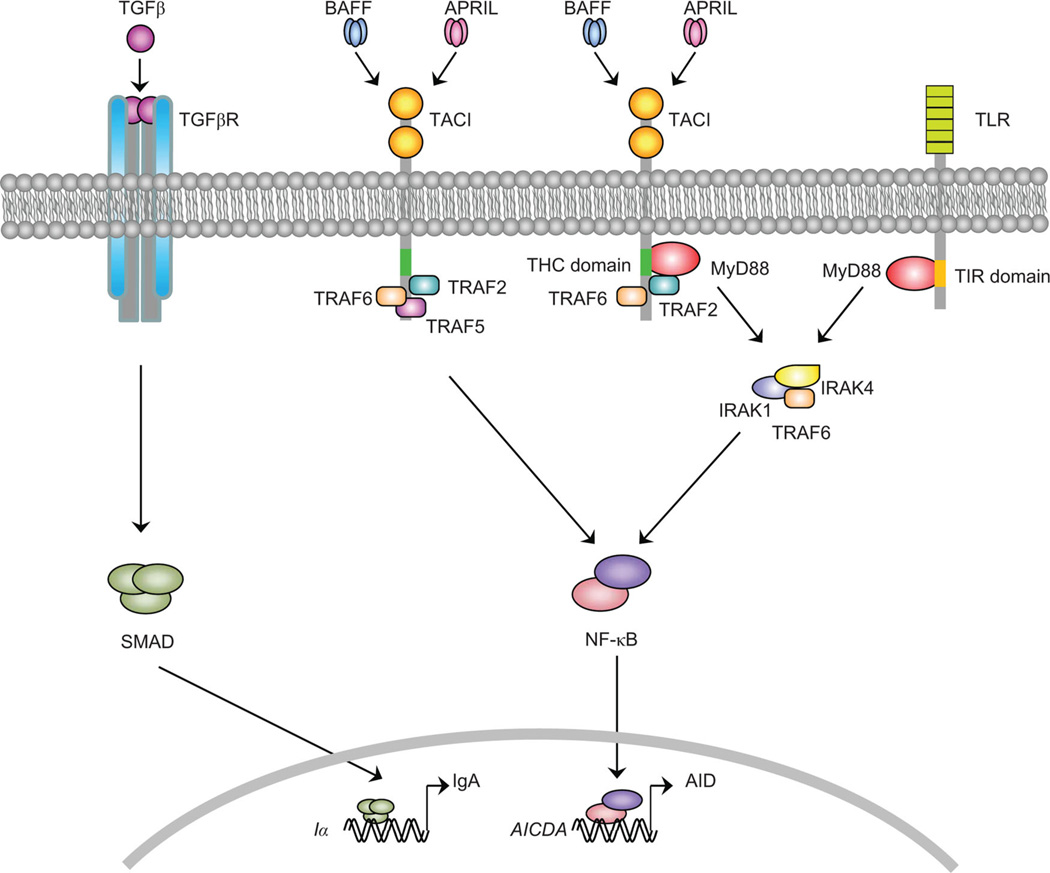
Figure 2.
Signaling pathways emanating from transmembrane activator and calcium modulator and cyclophylin ligand interactor (TACI). The TGF-β receptor (TGF-βR) initiates germline Cα gene transcription by activating the Iα promoter via SMA-homologue mothers against decapentaplegic (SMAD) proteins. At the same time, engagement of TACI by BAFF and APRIL triggers the recruitment of myeloid differentiation primary response gene 88 (MyD88) to a TACI highly conserved (THC) motif located in the cytoplasmic domain of TACI receptor. TACI also recruits TNF receptor–associated factor 2 (TRAF2) to a motif located immediately downstream of THC. The THC motif is distinct from the Toll-interleukin-1 receptor (TIR) motif, which mediates the recruitment of MyD88 by Toll-like receptors (TLRs). Recruitment of IL-1 receptor–associated kinase 1 (IRAK-1), IRAK-4, and TRAF6 by TACI and TLRs leads to the activation and nuclear translocation of NF-κB, which initiates transcriptional activation of the AICDA gene encoding activation-induced cytidine deaminase (AID). Together, germline Cα gene transcription and AID induction cause class switch recombination from IgM to IgA in B cells.
Lessons from PID
In addition to neutralizing specific mucosal pathogens, IgA modulates the interaction of commensal bacteria with the mucosal immune system to mitigate the overall inflammatory tone of the intestine.25 Therefore, it is not surprising that a large proportion of patients with primary antibody disorders, such as selective IgA deficiency (SIgAD), common variable immune deficiency (CVID), and hyper-IgM (HIGM) syndrome, develop not only gastrointestinal infections, but also inflammatory bowel disease.112–116 Gastrointestinal inflammation is somewhat less prominent in patients with X-linked agammaglobulinemia (XLA), a primary antibody disorder in which deleterious substitutions of the BCR-associated enzyme Bruton’s tyrosine kinase (Btk) cause developmental arrest of B cell precursors in the bone marrow and severe depletion of mature B cells in the periphery.117,118 Perhaps, the lack of functional Btk in XLA attenuates BCR-independent inflammatory signals, such as, TLR signals in mucosal DCs, macrophages and epithelial cells.119,120 Alternatively, XLA patients may be protected from intestinal disease by the lack of heterogeneous T cell and DC abnormalities often present in patients with CVID.121–124
Patients with SIgAD or CVID also develop gut nodular lymphoid hyperplasia, a benign lymphoproliferative disorder that consists of multiple nodular lesions made up of lymphoid aggregates usually confined to the lamina propria of the small intestine.115,116,123,125 Nodular lymphoid hyperplasia is thought to originate from polyclonal activation of intestinal B cells by commensal bacteria undergoing aberrant expansion in the small intestine.126 Consistent with this interpretation, patients with SIgAD and CVID develop small bowel bacteria overgrowth syndrome, which leads to heterogeneous clinical manifestations associated with malabsorption.115,116,123 Nodular lymphoid hyperplasia and small bowel bacteria overgrowth syndrome are also present in patients with HIGM syndrome caused by deleterious AID substitutions that impair both CSR and SHM.127 The molecular basis of impaired mucosal IgA responses and gastrointestinal disorders in SIgAD and CVID remain largely unknown, but some CVID patients have deleterious TACI substitutions.128–131
Nodular lymphoid hyperplasia and bacterial overgrowth have also been observed in AID knockout mice and in mice with deleterious AID substitutions that abrogate the induction of SHM but not CSR.21,24 The small intestine of these mice shows uncontrolled expansion of segmented filamentous bacteria as well as a prominent antibiotic-sensitive hyperplasia of isolated lymphoid follicles.21,24 Together, these observations indicate that specific recognition of commensals by somatically hyper-mutated IgA plays an important role in the control of the composition and compartimentalization of the intestinal microbiota. The lack of this function would lead to increased bacterial growth in the small intestine, with subsequent polyclonal hyperactivation of local as well as systemic B cells. Over time, this process may lead to the aberrant expansion of allergen-reactive, autoreactive, and clonal B cells, which could contribute to the increased frequency of allergy, autoimmunity (celiac disease, hemolytic anemia, immune thrombocytopenic purpura), and Bcell tumors (mostly non-Hodgkin lymphoma) observed in individuals with SIgAD, CVID, or HIGM syndrome.
In cases characterized by specific gene defects, PIDs can be regarded as an “experiment of nature” that may help immunologists to better understand the regulation of mucosal IgA responses. In HIGM syndrome caused by deleterious CD40 substitutions,132 the intestinal lamina propria includes IgA-producing B cells and plasmablasts that contain AID, a hallmark of ongoing CSR.34,101,133 Consistent with the key role of CD40 in the germinal center reaction,134 AID expression and IgA CSR are virtually abolished in germinal center B cells from mucosal follicles of individuals with HIGM syndrome.104,132 In contrast, subepithelial B cells from the intestinal and respiratory mucosal surfaces of these patients show AID expression and active IgA CSR.104,132 IgA CSR is also conserved in subepithelial B cells from chronically infected HIV-infected patients with massive depletion of intestinal CD4+ T cells.100 These patients also show a profound impairment of the germinal center reaction.100 Overall, these findings suggest that human B cells can produce IgA through a germinal center–independent pathway that does not require help to B cells by CD4+ T cells expressing CD40L.
In humans, CD40-independent IgA production may mostly rely on APRIL signaling to B cells via TACI.110 Accordingly, IgA production is decreased in CVID patients with deleterious TACI substitutions, whereas patients with deleterious BAFF-R substitutions have normal IgA, at least in the circulation.128,129,135 Additional studies show that B cells from patients with MyD88 or IRAK4 deficiency, two PIDs associated with invasive bacterial infections, are less responsive to CSR signals from BAFF and APRIL, indicating a possible role of MyD88 and IRAK-4 in the generation of class-switched antibodies to bacterial TI antigens, such as, polysaccharides.110,136,137 Consistent with this possibility, MyD88-deficient mice have defective intestinal IgA production, and their B cells do not effectively undergo CSR in response to TACI engagement by BAFF or APRIL.5,54,87,95 Of note, patients lacking MyD88 or IRAK-4, a kinase downstream of MyD88, have normal levels of total IgA in the serum.136–138 However, mucosal IgA responses have never been measured in these patients.
Conclusions
IgA is the predominant antibody isotype of the gut-associated lymphoid tissue that has been selected throughout evolution to provide protection against mucosal microorganisms. IgA has long been known for its importance in the protection against pathogens, but its role in the selection and maintenance of a spatially diversified bacterial community in the gut has become clear only in recent years. A number of studies have revealed that IgA responses involve B cells from both follicular and extrafollicular districts and follow multiple TD and TI pathways. Yet, the precise cellular and signaling components of these pathways, and their relative contribution to mucosal immunity and homeostasis remain to be fully elucidated. Further studies are also needed to characterize the mechanisms by which IgA controls the inflammatory tone of the intestine and regulates the composition and compartimentalization of the intestinal microbiota. PIDs with genetic alterations affecting known B cell–regulating pathways may provide excellent models to address some of these questions in humans.
Acknowledgments
This study was supported by National Institutes of Health Grants R01 AI-074378, U01 AI-095613, P01 AI-096187, and P01 AI-061093, and by Ministerio de Ciencia e Innovación Grant SAF 2008-02725 to A.C.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Sansonetti PJ. Host-bacteria homeostasis in the healthy and inflamed gut. Curr. Opin. Gastroenterol. 2008;24:435–439. doi: 10.1097/MOG.0b013e32830007f7. [DOI] [PubMed] [Google Scholar]
- 2.Macpherson AJ, Harris NL. Interactions between commensalintestinalbacteria andthe immunesystem. Nat. Rev. Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 3.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal micro-biota. Nat. Rev. Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 4.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 5.Fagarasan S, et al. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 2010;28:243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- 6.Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu. Rev. Immunol. 2010;29:273–293. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mestecky J, Zikan J, Butler WT. Immunoglobulin M and secretory immunoglobulin A: presence of a common polypeptide chain different from light chains. Science. 1971;171:1163–1165. doi: 10.1126/science.171.3976.1163. [DOI] [PubMed] [Google Scholar]
- 8.Mostov KE, Deitcher DL. Polymeric immunoglobulin receptor expressed in MDCK cells transcy-toses IgA. Cell. 1986;46:613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- 9.Mostov KE. Transepithelial transport of immunoglobulins. Annu. Rev. Immunol. 1994;12:63–84. doi: 10.1146/annurev.iy.12.040194.000431. [DOI] [PubMed] [Google Scholar]
- 10.Phalipon A, Corthesy B. Novel functions of the polymeric Ig receptor: well beyond transport of immunoglobulins. Trends Immunol. 2003;24:55–58. doi: 10.1016/s1471-4906(02)00031-5. [DOI] [PubMed] [Google Scholar]
- 11.Favre L, Spertini F, Corthesy B. Secretory IgA possesses intrinsic modulatory properties stimulating mucosal and systemic immune responses. J. Immunol. 2005;175:2793–2800. doi: 10.4049/jimmunol.175.5.2793. [DOI] [PubMed] [Google Scholar]
- 12.Monteiro RC, Van De Winkel JG. IgA Fc receptors. Annu. Rev. Immunol. 2003;21:177–204. doi: 10.1146/annurev.immunol.21.120601.141011. [DOI] [PubMed] [Google Scholar]
- 13.Pasquier B, et al. Identification of FcαRI as an inhibitory receptor that controls inflammation: dual role of FcRγ ITAM. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Hornquist CE, et al. Paradoxical IgA immunity in CD4-deficient mice. Lack of cholera toxin-specific protective immunity despite normal gut mucosal IgA differentiation. J. Immunol. 1995;155:2877–2887. [PubMed] [Google Scholar]
- 15.Fernandez MI, et al. Anti-inflammatory role for intracellular dimeric immunoglobulin a by neutralization of lipopolysaccharide in epithelial cells. Immunity. 2003;18:739–749. doi: 10.1016/s1074-7613(03)00122-5. [DOI] [PubMed] [Google Scholar]
- 16.Martinoli C, Chiavelli A, Rescigno M. Entryroute of Salmonella typhimurium directs the type of induced immune response. Immunity. 2007;27:975–984. doi: 10.1016/j.immuni.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Kadaoui KA, Corthesy B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer’s patches with restriction to mucosal compartment. J. Immunol. 2007;179:7751–7757. doi: 10.4049/jimmunol.179.11.7751. [DOI] [PubMed] [Google Scholar]
- 18.Macpherson AJ, et al. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 19.Wade TK, Wade WF. Variable gene family usage of protective and non-protective anti-Vibrio cholerae O1 LPS antibody heavy chains. Microbiol. Immunol. 2008;52:611–620. doi: 10.1111/j.1348-0421.2008.00078.x. [DOI] [PubMed] [Google Scholar]
- 20.Boullier S, et al. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruc-tion by down-regulating inflammatory circuits. J. Immunol. 2009;183:5879–5885. doi: 10.4049/jimmunol.0901838. [DOI] [PubMed] [Google Scholar]
- 21.Wei M, et al. Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nat. Immunol. 2011;12:264–270. doi: 10.1038/ni.1991. [DOI] [PubMed] [Google Scholar]
- 22.Phalipon A, et al. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 23.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 24.Fagarasan S, et al. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–1427. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 25.Peterson DA, et al. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Mantis NJ, et al. Selective adherence of IgA to murine Peyer’s patch M cells: evidence for a novel IgA receptor. J. Immunol. 2002;169:1844–1851. doi: 10.4049/jimmunol.169.4.1844. [DOI] [PubMed] [Google Scholar]
- 27.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 28.Hase K, et al. Uptake through glycoprotein2 of FimH (+) bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–230. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 29.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 30.Coker HA, Durham SR, Gould HJ. Local somatic hypermutation and class switch recombination in the nasal mucosa of allergic rhinitis patients. J. Immunol. 2003;171:5602–5610. doi: 10.4049/jimmunol.171.10.5602. [DOI] [PubMed] [Google Scholar]
- 31.Mao C, et al. T cell-independent somatic hyper-mutation in murine B cells with an immature phenotype. Immunity. 2004;20:133–144. doi: 10.1016/s1074-7613(04)00019-6. [DOI] [PubMed] [Google Scholar]
- 32.Takhar P, et al. Allergen drives class switching to IgE in the nasal mucosa in allergic rhinitis. J. Immunol. 2005;174:5024–5032. doi: 10.4049/jimmunol.174.8.5024. [DOI] [PubMed] [Google Scholar]
- 33.Han JH, et al. Class switch recombination and somatic hypermutation in early mouse B cells are mediated by B cell and toll-like receptors. Immunity. 2007;27:64–75. doi: 10.1016/j.immuni.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He B, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Herlands RA, et al. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capolunghi F, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J. Immunol. 2008;180:800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 37.Hayakawa K, Hardy RR. Normal, autoimmune, and malignant CD5+ B cells: the Ly-1 B lineage? Annu. Rev. Immunol. 1988;6:197–218. doi: 10.1146/annurev.iy.06.040188.001213. [DOI] [PubMed] [Google Scholar]
- 38.Kroese FG, et al. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int. Immunol. 1989;1:75–84. doi: 10.1093/intimm/1.1.75. [DOI] [PubMed] [Google Scholar]
- 39.Thurnheer MC, et al. B1 cells contribute to serum IgM, but not to intestinal IgA, production in gnotobiotic Ig allotype chimeric mice. J. Immunol. 2003;170:4564–4571. doi: 10.4049/jimmunol.170.9.4564. [DOI] [PubMed] [Google Scholar]
- 40.Stoel M, et al. Restricted IgA repertoire in both B-1 and B-2 cell-derived gut plasmablasts. J. Immunol. 2005;174:1046–1054. doi: 10.4049/jimmunol.174.2.1046. [DOI] [PubMed] [Google Scholar]
- 41.Rosado MM, et al. From the fetal liver to spleen and gut: the highway to natural antibody. Mucosal Immunol. 2009;2:351–361. doi: 10.1038/mi.2009.15. [DOI] [PubMed] [Google Scholar]
- 42.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 43.Harris NL, et al. Mechanisms of neonatal mucosal antibody protection. J. Immunol. 2006;177:6256–6262. doi: 10.4049/jimmunol.177.9.6256. [DOI] [PubMed] [Google Scholar]
- 44.Tsuji M, et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Benckert J, et al. The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. J. Clin. Invest. 2011;121:1946–1955. doi: 10.1172/JCI44447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunn-Walters DK, Boursier L, Spencer J. Hyper-mutation, diversity and dissemination of human intestinal lamina propria plasma cells. Eur. J. Immunol. 1997;27:2959–2964. doi: 10.1002/eji.1830271131. [DOI] [PubMed] [Google Scholar]
- 47.Dunn-Walters DK, Isaacson PG, Spencer J. Sequence analysis of human IgVH genes indicates that ileal lamina propria plasma cells are derived from Peyer’s patches. Eur. J. Immunol. 1997;27:463–467. doi: 10.1002/eji.1830270217. [DOI] [PubMed] [Google Scholar]
- 48.Tiller T, et al. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mouquet H, et al. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geijtenbeek TB, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baumann J, Park CG, Mantis NJ. Recognition of secretory IgA by DC-SIGN: implications for immune surveillance in the intestine. Immunol Lett. 2010;131:59–66. doi: 10.1016/j.imlet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Craig SW, Cebra JJ. Peyer’s patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J. Exp. Med. 1971;134:188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cebra JJ, et al. Development and maintenance of the gut-associated lymphoid tissue (GALT): the roles of enteric bacteria and viruses. Dev. Immunol. 1998;6:13–18. doi: 10.1155/1998/68382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casola S, et al. B cell receptor signal strength determines B cell fate. Nat. Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 55.Weinstein PD, Cebra JJ. The preference for switching to IgA expression by Peyer’s patch germinal center B cells is likely due to the intrinsic influence of their microenvironment. J. Immunol. 1991;147:4126–4135. [PubMed] [Google Scholar]
- 56.Tsuji M, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 57.Niess JH, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 58.Salazar-Gonzalez RM, et al. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer’s patches. Immunity. 2006;24:623–632. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rescigno M, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 60.Chieppa M, et al. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bogunovic M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varol C, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 64.Ivanov II, Littman DR. Segmented filamentous bacteria take the stage. Mucosal Immunol. 2010;3:209–212. doi: 10.1038/mi.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schulz O, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J. Exp. Med. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fink LN, Frokiaer H. Dendritic cells from Peyer’s patches and mesenteric lymph nodes differ from spleen dendritic cells in their response to commensal gut bacteria. Scand. J. Immunol. 2008;68:270–279. doi: 10.1111/j.1365-3083.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 67.Jaensson E, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J. Exp. Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iwasaki A, Kelsall BL. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 1999;190:229–239. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sato A, et al. CD11b+ Peyer’s patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J. Immunol. 2003;171:3684–3690. doi: 10.4049/jimmunol.171.7.3684. [DOI] [PubMed] [Google Scholar]
- 70.Zhao X, et al. CCL9 is secreted by the follicle-associated epithelium and recruits dome region Peyer’s patch CD11b+ dendritic cells. J. Immunol. 2003;171:2797–2803. doi: 10.4049/jimmunol.171.6.2797. [DOI] [PubMed] [Google Scholar]
- 71.Iwasaki A, Kelsall BL. Localization of distinct Peyer’s patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J. Exp. Med. 2000;191:1381–1394. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rimoldi M, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 73.Rescigno M, Di Sabatino A. Dendritic cells in intestinal homeostasis and disease. J. Clin. Invest. 2009;119:2441–2450. doi: 10.1172/JCI39134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cong Y, et al. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc. Natl. Acad. Sci. U.S.A. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dullaers M, et al. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity. 2009;30:120–129. doi: 10.1016/j.immuni.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Linterman MA, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu-Amano J, et al. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J. Exp. Med. 1993;178:1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Defrance T, et al. Interleukin 10 and transforming growth factor β cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J. Exp. Med. 1992;175:671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cerutti A, et al. CD40 ligand and appropriate cytokines induce switching to IgG, IgA, and IgE and coordinated germinal center-like phenotype differentiation in a human monoclonal IgM+IgD+ B cell line. J. Immunol. 1998;160:2145–2157. [PMC free article] [PubMed] [Google Scholar]
- 82.Cazac BB, Roes J. TGF-β receptor controls B cell responsiveness and induction of IgA in vivo. Immunity. 2000;13:443–451. doi: 10.1016/s1074-7613(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 83.Bishop GA. The multifaceted roles of TRAFs in the regulation of B-cell function. Nat. Rev. Immunol. 2004;4:775–786. doi: 10.1038/nri1462. [DOI] [PubMed] [Google Scholar]
- 84.Cerutti A. The regulation of IgA class switching. Nat. Rev. Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kraus M, et al. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 86.Casola S, Rajewsky K. B cell recruitment and selection in mouse GALT germinal centers. Curr. Top. Microbiol. Immunol. 2006;308:155–171. doi: 10.1007/3-540-30657-9_7. [DOI] [PubMed] [Google Scholar]
- 87.Tezuka H, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 88.Suzuki K, et al. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 2010;33:71–83. doi: 10.1016/j.immuni.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 89.Barone F, et al. Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal Immunol. 2009;2:495–503. doi: 10.1038/mi.2009.106. [DOI] [PubMed] [Google Scholar]
- 90.Bergqvist P, et al. T cell-independent IgA class switch recombination is restricted to the GALT and occurs prior to manifest germinal center formation. J. Immunol. 2010;184:3545–3553. doi: 10.4049/jimmunol.0901895. [DOI] [PubMed] [Google Scholar]
- 91.Tezuka H, et al. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity. 2011;34:247–257. doi: 10.1016/j.immuni.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 92.Hamada H, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- 93.Lorenz RG, et al. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J. Immunol. 2003;170:5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- 94.McDonald KG, McDonough JS, Newberry RD. Adaptive immune responses are dispensable for isolated lymphoid follicle formation: antigen-naive, lymphotoxin-sufficient B lymphocytes drive the formation of mature isolated lymphoid follicles. J. Immunol. 2005;174:5720–5728. doi: 10.4049/jimmunol.174.9.5720. [DOI] [PubMed] [Google Scholar]
- 95.Suzuki K, et al. GALT: organization and dynamics leading to IgA synthesis. Adv. Immunol. 2010;107:153–185. doi: 10.1016/B978-0-12-381300-8.00006-X. [DOI] [PubMed] [Google Scholar]
- 96.Crouch EE, et al. Regulation of AID expression in the immune response. J. Exp. Med. 2007;204:1145–1156. doi: 10.1084/jem.20061952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uematsu S, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 98.Fagarasan S, et al. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639–643. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- 99.Shang L, et al. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529–538. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu W, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat. Immunol. 2009;10:1008–1017. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He B, Xu W, Cerutti A. Comment on “gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination”. Mucosal Immunol. 2010;3:92–94. doi: 10.1038/mi.2009.125. author reply 94–95. [DOI] [PubMed] [Google Scholar]
- 102.Bergqvist P, et al. Gut IgA class switch recombination in the absenceof CD40 does not occur in the lamina propria and is independent of germinal centers. J. Immunol. 2006;177:7772–7783. doi: 10.4049/jimmunol.177.11.7772. [DOI] [PubMed] [Google Scholar]
- 103.Xu W, et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat. Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- 104.Xu W, et al. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J. Immunol. 2008;181:276–287. doi: 10.4049/jimmunol.181.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uematsu S, Akira S. Immune responses of TLR5(+) lamina propria dendritic cells in enterobacterial infection. J. Gastroenterol. 2009;44:803–811. doi: 10.1007/s00535-009-0094-y. [DOI] [PubMed] [Google Scholar]
- 106.Kett K, et al. Different subclass distribution of IgA-producing cells in human lymphoid organs and various secretory tissues. J. Immunol. 1986;136:3631–3635. [PubMed] [Google Scholar]
- 107.Kett K, et al. Intestinal B-cell isotype response in relation to local bacterial load: evidence for immunoglobulin A subclass adaptation. Gastroenterology. 1995;109:819–825. doi: 10.1016/0016-5085(95)90389-5. [DOI] [PubMed] [Google Scholar]
- 108.Berkowska MA, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118:2150–2158. doi: 10.1182/blood-2011-04-345579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Litinskiy MB, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.He B, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat. Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Castigli E, et al. TACI and BAFF-R mediate isotype switching in B cells. J. Exp. Med. 2005;201:35–39. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Washington K, et al. Gastrointestinal pathology in patients with common variable immunodeficiency and X-linked agammaglobulinemia. Am. J. Surg. Pathol. 1996;20:1240–1252. doi: 10.1097/00000478-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 113.Knight AK, Cunningham-Rundles C. Inflammatory and autoimmune complications of common variable immune deficiency. Autoimmun. Rev. 2006;5:156–159. doi: 10.1016/j.autrev.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 114.Jesus AA, Duarte AJ, Oliveira JB. Autoimmunity in hyper-IgM syndrome. J. Clin. Immunol. 2008;28 Suppl 1:S62–S66. doi: 10.1007/s10875-008-9171-x. [DOI] [PubMed] [Google Scholar]
- 115.Malamut G, et al. The enteropathy associated with common variable immunodeficiency: the delineated frontiers with celiac disease. Am. J. Gastroenterol. 2010;105:2262–2275. doi: 10.1038/ajg.2010.214. [DOI] [PubMed] [Google Scholar]
- 116.Agarwal S, et al. Characterization of immunologic defects in patients with common variable immunodeficiency (CVID) with intestinal disease. Inflamm. Bowel Dis. 2011;17:251–259. doi: 10.1002/ibd.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.de Villartay JP, Fischer A, Durandy A. The mechanisms of immune diversification and their disorders. Nat. Rev. Immunol. 2003;3:962–972. doi: 10.1038/nri1247. [DOI] [PubMed] [Google Scholar]
- 118.Cunningham-Rundles C, Ponda PP. Molecular defects in T- and B-cell primary immunodeficiency diseases. Nat. Rev. Immunol. 2005;5:880–892. doi: 10.1038/nri1713. [DOI] [PubMed] [Google Scholar]
- 119.Sochorova K, et al. Impaired Toll-like receptor 8-mediated IL-6 and TNF-alpha production in antigen-presenting cells from patients with X-linked agammaglob-ulinemia. Blood. 2007;109:2553–2556. doi: 10.1182/blood-2006-07-037960. [DOI] [PubMed] [Google Scholar]
- 120.Horwood NJ, et al. Bruton’s tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. J. Immunol. 2006;176:3635–3641. doi: 10.4049/jimmunol.176.6.3635. [DOI] [PubMed] [Google Scholar]
- 121.Bayry J, et al. Commonvariable immunodeficiency is associated with defective functions of dendritic cells. Blood. 2004;104:2441–2443. doi: 10.1182/blood-2004-04-1325. [DOI] [PubMed] [Google Scholar]
- 122.Cunningham-Rundles C, Radigan L. Deficient IL-12 and dendritic cell function in common variable immune deficiency. Clin. Immunol. 2005;115:147–153. doi: 10.1016/j.clim.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 123.Cunningham-Rundles C, et al. TLR9 activation is defective in common variable immune deficiency. J. Immunol. 2006;176:1978–1987. doi: 10.4049/jimmunol.176.3.1978. [DOI] [PubMed] [Google Scholar]
- 124.Ochtrop ML, et al. T and B lymphocyte abnormalities in bone marrow biopsies of common variable immunodeficiency. Blood. 2011;118:309–318. doi: 10.1182/blood-2010-11-321695. [DOI] [PubMed] [Google Scholar]
- 125.Bastlein C, et al. Common variable immunodeficiency syndrome and nodular lymphoid hyperplasia in the small intestine. Endoscopy. 1988;20:272–275. doi: 10.1055/s-2007-1018192. [DOI] [PubMed] [Google Scholar]
- 126.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat. Rev. Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 127.Revy P, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 128.Salzer U, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat. Genet. 2005;37:820–828. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 129.Castigli E, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat. Genet. 2005;37:829–834. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 130.Cunningham-Rundles C, Knight AK. Common variable immune deficiency: reviews, continued puzzles, and a new registry. Immunol. Res. 2007;38:78–86. doi: 10.1007/s12026-007-0024-0. [DOI] [PubMed] [Google Scholar]
- 131.Castigli E, et al. Reexamining the role of TACI coding variants in common variable immunodeficiency and selective IgA deficiency. Nat. Genet. 2007;39:430–431. doi: 10.1038/ng0407-430. [DOI] [PubMed] [Google Scholar]
- 132.Ferrari S, et al. Mutations of CD40 gene cause an autosomal recessive form of immunodeficiency with hyper IgM. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12614–12619. doi: 10.1073/pnas.221456898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cerutti A. Location, location, location: B-cell differentiation in the gut lamina propria. Mucosal Immunol. 2008;1:8–10. doi: 10.1038/mi.2007.8. [DOI] [PubMed] [Google Scholar]
- 134.MacLennan IC. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 135.Warnatz K, et al. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13945–13950. doi: 10.1073/pnas.0903543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Picard C, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 137.von Bernuth H, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ku CL, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J. Exp. Med. 2007;204:2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]