Abstract
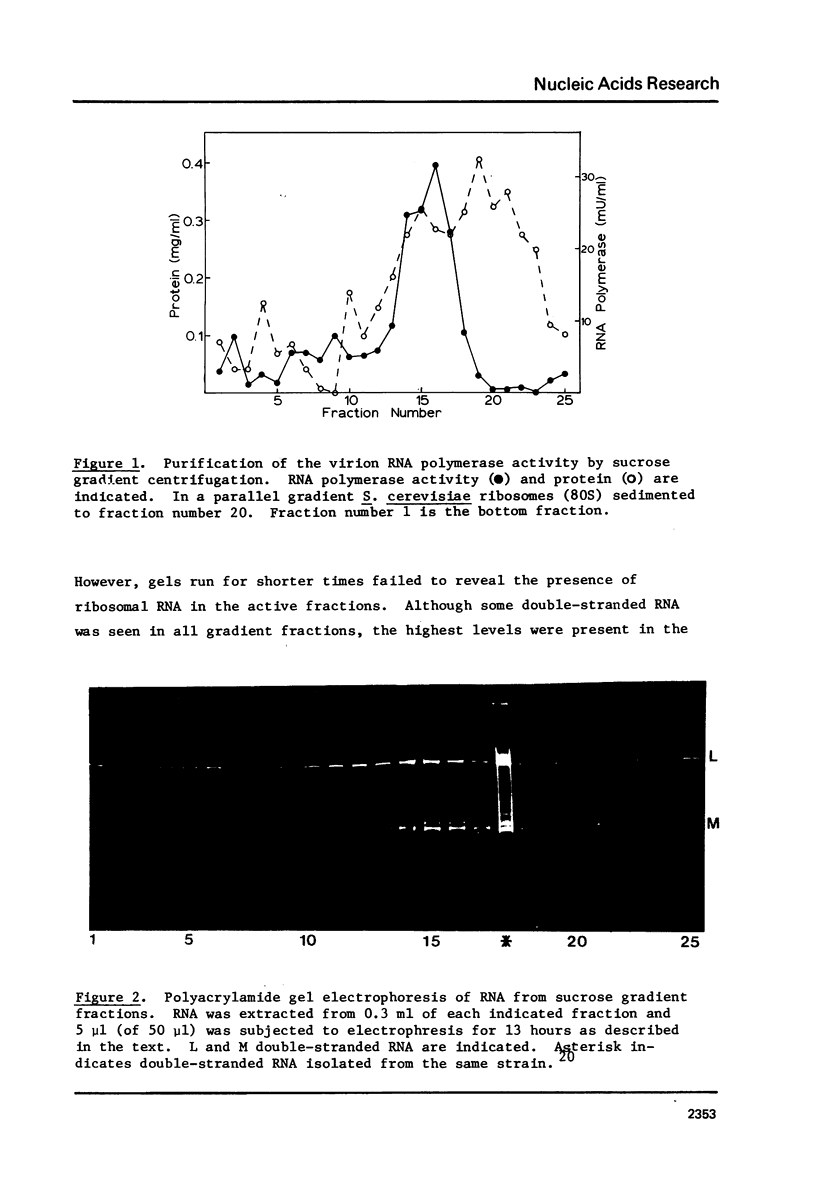
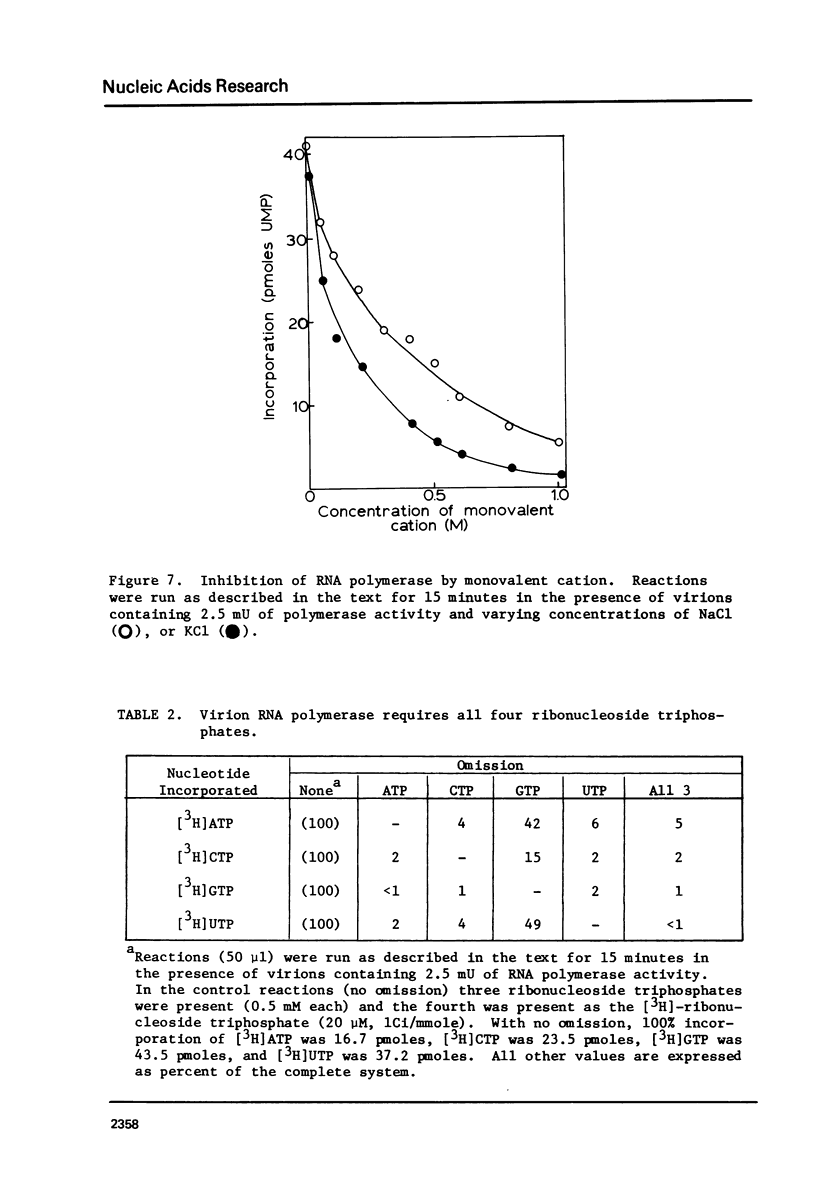
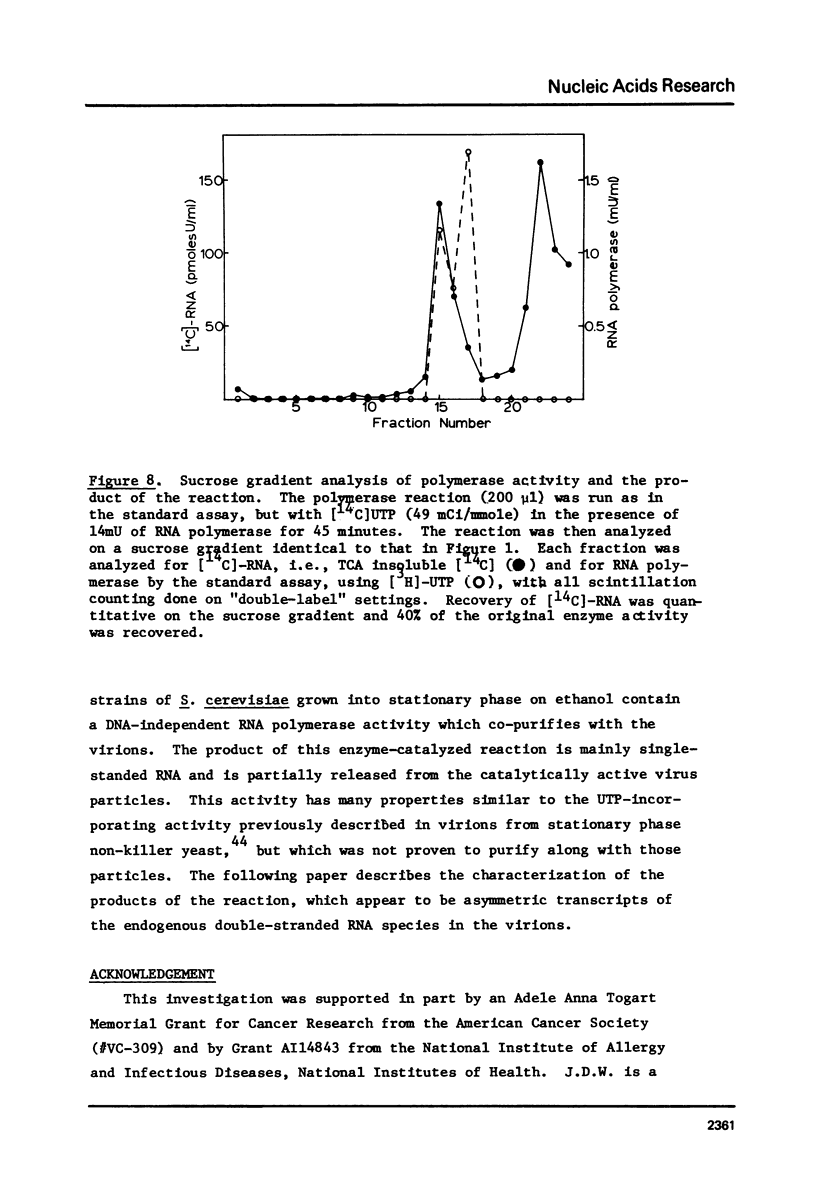
The "killer" plasmid and a larger double-stranded RNA plasmid of yeast exist in intracellular virion particles. Purification of these particles from a diploid killer strain of yeast (grown into stationary growth on ethanol) resulted in co-purification of a DNA-independent RNA polymerase activity. This activity incorporates and requires all four ribonucleoside triphosphates and will not act on deoxyribonucleoside triphosphates. The reaction requires magnesium, is inhibited by sulfhydryl-oxidizing reagents and high concentrations of monovalent cation, but is insensitive to DNase, alpha-amanitin, and actinomycin D. Pyrophosphate inhibits the reaction as does ethidium bromide. Exogenous nucleic acids have no effect on the reaction. The product is mostly single-stranded RNA, some of which is released from the enzymatically active virions.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Wood H. A., Bozarth R. F. Virus-like particles from killer, neutral, and sensitive strains of Saccharomyces cerevisiae. J Virol. 1976 Feb;17(2):472–476. doi: 10.1128/jvi.17.2.472-476.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan E. A., Herring A. J., Mitchell D. J. Preliminary characterization of two species of dsRNA in yeast and their relationship to the "killer" character. Nature. 1973 Sep 14;245(5420):81–86. doi: 10.1038/245081b0. [DOI] [PubMed] [Google Scholar]
- Black D. R., Knight C. A. Ribonucleic acid transcriptase acitvity in purified wound tumor virus. J Virol. 1970 Aug;6(2):194–198. doi: 10.1128/jvi.6.2.194-198.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsa J., Graham A. F. Reovirus: RNA polymerase activity in purified virions. Biochem Biophys Res Commun. 1968 Dec 30;33(6):895–901. doi: 10.1016/0006-291x(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Buck K. W. Replication of double-stranded RNA in particles of Penicillium stoloniferum virus S. Nucleic Acids Res. 1975 Oct;2(10):1889–1902. doi: 10.1093/nar/2.10.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck K. W. Semi-conservative replication of double-stranded RNA by a virion-associated RNA polymerase. Biochem Biophys Res Commun. 1978 Oct 16;84(3):639–645. doi: 10.1016/0006-291x(78)90753-2. [DOI] [PubMed] [Google Scholar]
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Morgan D. H. Ribonucleic acid synthesis by isolated viruses of Penicillium stoloniferum. J Gen Virol. 1974 Aug;24(2):307–317. doi: 10.1099/0022-1317-24-2-307. [DOI] [PubMed] [Google Scholar]
- Cohen J. Ribonucleic acid polymerase activity associated with purified calf rotavirus. J Gen Virol. 1977 Sep;36(3):395–402. doi: 10.1099/0022-1317-36-3-395. [DOI] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H., Wickner R. B. Spermidine or spermine requirement for killer double-stranded RNA plasmid replication in yeast. J Biol Chem. 1978 Aug 10;253(15):5225–5227. [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B., TSUGITA A. Purification of viral RNA by means of bentonite. Virology. 1961 May;14:54–58. doi: 10.1016/0042-6822(61)90131-3. [DOI] [PubMed] [Google Scholar]
- Faust M., Millward S. In vitro methylation of nascent reovirus mRNA by a virion-associated methyl transferase. Nucleic Acids Res. 1974 Dec;1(12):1739–1752. doi: 10.1093/nar/1.12.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G. R., Styles C. A. Curing of a killer factor in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2846–2849. doi: 10.1073/pnas.69.10.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y. "Methylation-coupled" transcription by virus-associated transcriptase of cytoplasmic polyhedrosis virus containing double-stranded RNA. Nucleic Acids Res. 1974 Jun;1(6):809–822. doi: 10.1093/nar/1.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomatos P. J., Kuechenthal I. Reovirus-specific enzyme(s) associated with subviral particles responds in vitro to polyribocytidylate to yield double-stranded polyribocytidylate-polyriboguanylate. J Virol. 1977 Jul;23(1):80–90. doi: 10.1128/jvi.23.1.80-90.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot G. S., Poyton R. O. Oxygen control of cytochrome c oxidase synthesis in isolated mitochondria from Saccharomyces cerevisiae. Nature. 1975 May 15;255(5505):238–240. doi: 10.1038/255238a0. [DOI] [PubMed] [Google Scholar]
- Groves W. E., Davis F. C., Jr, Sells B. H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968 Feb;22(2):195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- Hafe L. A., Keller E. B. The polyadenylate polymerases from yeast. J Biol Chem. 1975 Mar 10;250(5):1838–1846. [PubMed] [Google Scholar]
- Herring A. J., Bevan E. A. Virus-like particles associated with the double-stranded RNA species found in killer and sensitive strains of the yeast Saccharomyces cerevisiae. J Gen Virol. 1974 Mar;22(3):387–394. doi: 10.1099/0022-1317-22-3-387. [DOI] [PubMed] [Google Scholar]
- Holland M. J., Hager G. L., Rutter W. J. Characterization of purified poly(adenylic acid)-containing messenger ribonucleic acid from Saccharomyces cerevisiae. Biochemistry. 1977 Jan 11;16(1):8–16. doi: 10.1021/bi00620a002. [DOI] [PubMed] [Google Scholar]
- Holm C. A., Oliver S. G., Newman A. M., Holland L. E., McLaughlin C. S., Wagner E. K., Warner R. C. The molecular weight of yeast P1 double-stranded RNA. J Biol Chem. 1978 Nov 25;253(22):8332–8336. [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapierre H., Astier-Manifacier S., Cornuet P. Activité RNA polymérase associée aux préparations purifiées de virus du Penicillium stoloniferum. C R Acad Sci Hebd Seances Acad Sci D. 1971 Sep 13;273(11):992–994. [PubMed] [Google Scholar]
- Leibowitz M. J., Wickner R. B. Pet18: a chromosomal gene required for cell growth and for the maintenance of mitochondrial DNA and the killer plasmid of yeast. Mol Gen Genet. 1978 Oct 4;165(2):115–121. doi: 10.1007/BF00269899. [DOI] [PubMed] [Google Scholar]
- Lewandowski L. J., Kalmakoff J., Tanada Y. Characterization of a Ribonucleic Acid Polymerase Activity Associated with Purified Cytoplasmic Polyhedrosis Virus of the Silkworm Bombyx mori. J Virol. 1969 Dec;4(6):857–865. doi: 10.1128/jvi.4.6.857-865.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Zweerink H. J. Isolation and characterization of two types of bluetongue virus particles. Virology. 1972 Nov;50(2):495–506. doi: 10.1016/0042-6822(72)90400-x. [DOI] [PubMed] [Google Scholar]
- Nash C. H., Douthart R. J., Ellis L. F., Van Frank R. M., Burnett J. P., Lemke P. A. On the mycophage of Penicillium chrysogenum. Can J Microbiol. 1973 Jan;19(1):97–103. doi: 10.1139/m73-014. [DOI] [PubMed] [Google Scholar]
- Oliver S. G., McCREADY S. J., Holm C., Sutherland P. A., McLaughlin C. S., Cox B. S. Biochemical and physiological studies of the yeast virus-like particle. J Bacteriol. 1977 Jun;130(3):1303–1309. doi: 10.1128/jb.130.3.1303-1309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratti G., Buck K. W. RNA polymerase activity in double-stranded ribonucleic acid virus particles from Aspergillus foetidus. Biochem Biophys Res Commun. 1975 Sep 16;66(2):706–711. doi: 10.1016/0006-291x(75)90567-7. [DOI] [PubMed] [Google Scholar]
- Reich E., Goldberg I. H. Actinomycin and nucleic acid function. Prog Nucleic Acid Res Mol Biol. 1964;3:183–234. doi: 10.1016/s0079-6603(08)60742-4. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. RNA polymerase activity in purified reoviruses. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1462–1469. doi: 10.1073/pnas.61.4.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Christman J. K., Acs G. The reovirus replicative cycle. Annu Rev Biochem. 1976;45:375–408. doi: 10.1146/annurev.bi.45.070176.002111. [DOI] [PubMed] [Google Scholar]
- Somers J. M., Bevan E. A. The inheritance of the killer character in yeast. Genet Res. 1969 Feb;13(1):71–83. doi: 10.1017/s0016672300002743. [DOI] [PubMed] [Google Scholar]
- Stoltzfus C. M., Morgan M., Banerjee A. K., Shatkin A. J. Poly(A) polymerase activity in reovirus. J Virol. 1974 Jun;13(6):1338–1345. doi: 10.1128/jvi.13.6.1338-1345.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Vidaver A. K., Koski R. K., Semancik J. S. RNA polymerase activity associated with bacteriophage phi 6. J Virol. 1973 Sep;12(3):464–471. doi: 10.1128/jvi.12.3.464-471.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd D. W., Huismans H. Studies on the in vitro and the in vivo transcription of the bluetongue virus genome. Onderstepoort J Vet Res. 1972 Dec;39(4):185–191. [PubMed] [Google Scholar]
- Vodkin M., Katterman F., Fink G. R. Yeast killer mutants with altered double-stranded ribonucleic acid. J Bacteriol. 1974 Feb;117(2):681–686. doi: 10.1128/jb.117.2.681-686.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Schmid K., Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Chromosomal and nonchromosomal mutations affecting the "killer character" of Saccharomyces cerevisiae. Genetics. 1974 Mar;76(3):423–432. doi: 10.1093/genetics/76.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Deletion of mitochondrial DNA bypassing a chromosomal gene needed for maintenance of the killer plasmid of yeast. Genetics. 1977 Nov;87(3):441–452. doi: 10.1093/genetics/87.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Killer of Saccharomyces cerevisiae: a double-stranded ribonucleic acid plasmid. Bacteriol Rev. 1976 Sep;40(3):757–773. doi: 10.1128/br.40.3.757-773.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Chromosomal genes essential for replication of a double-stranded RNA plasmid of Saccharomyces cerevisiae: the killer character of yeast. J Mol Biol. 1976 Aug 15;105(3):427–443. doi: 10.1016/0022-2836(76)90102-9. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Mak mutants of yeast: mapping and characterization. J Bacteriol. 1979 Oct;140(1):154–160. doi: 10.1128/jb.140.1.154-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Two chromosomal genes required for killing expression in killer strains of Saccharomyces cerevisiae. Genetics. 1976 Mar 25;82(3):429–442. doi: 10.1093/genetics/82.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. The killer double-stranded RNA plasmids of yeast. Plasmid. 1979 Jul;2(3):303–322. doi: 10.1016/0147-619x(79)90015-5. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Twenty-six chromosomal genes needed to maintain the killer double-stranded RNA plasmid of Saccharomyces cerevisiae. Genetics. 1978 Mar;88(3):419–425. doi: 10.1093/genetics/88.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]




