Abstract
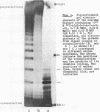
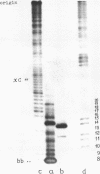
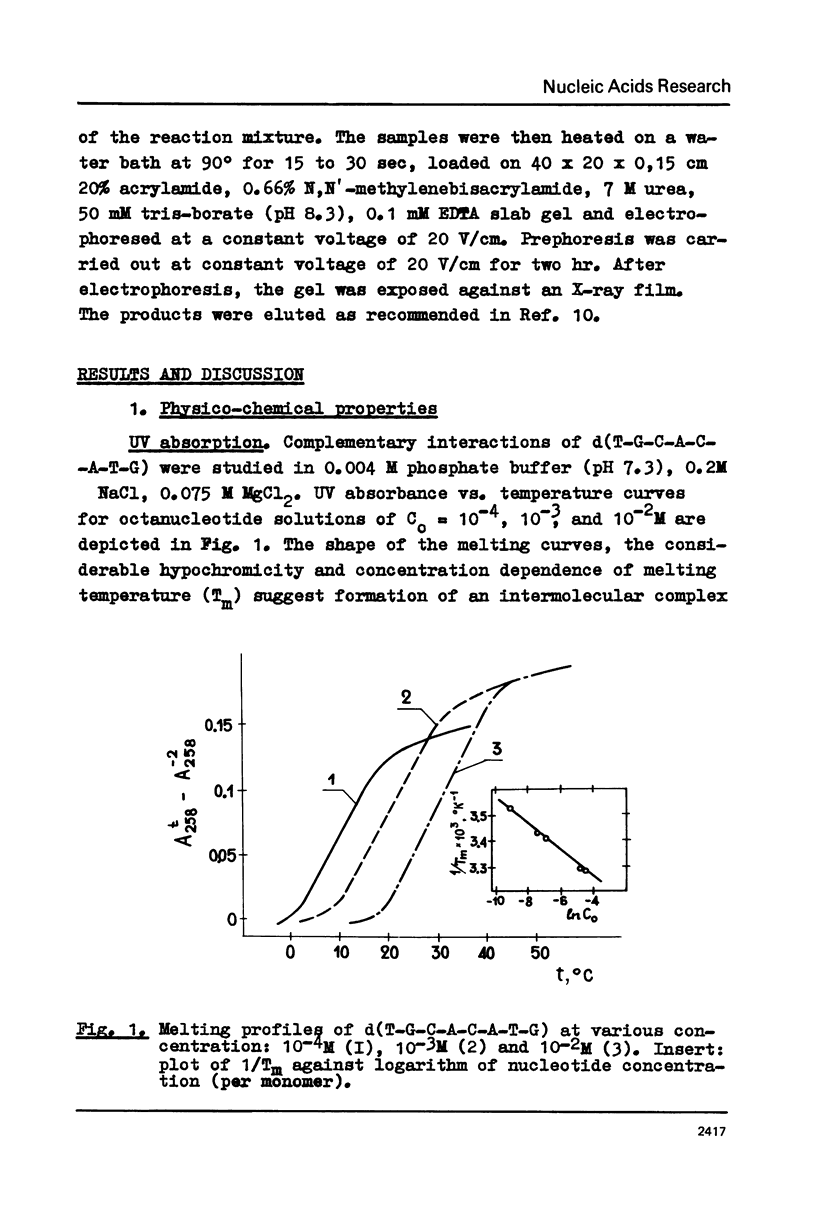
A new class of synthetic DNA duplexes containing repeating oligonucleotide sequences, double-helical concatemers, is characterized. The UV-absorption and circular dichroism of a concatemer formed in self-association of d(T-G-C-A-C-A-T-G) have been studied. The thermodynamical parameters of complex formation are the following: delta Ho1 = -9.2 +/- 0.3 kcal/mol, delta So1 = -27 +/- 1 e.u. The data obtained show that pseudopolymeric duplexes having structures that are similar to DNA-B-type helices are formed in solutions of d(T-G-C-A-C-A-T-G). Polymerization of 32P-d(pT-G-C-A-C-A-T-G) induced by water-soluble carbodiimide has been carried out under the conditions of concatemer stability. The yield of the dimer, a 16-member oligonucleotide, was 13%.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Arnott S. The sequence dependence of circular dichroism spectra of DNA duplexes. Nucleic Acids Res. 1975 Sep;2(9):1493–1502. doi: 10.1093/nar/2.9.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl C. P., Wu R., Brousseau R., Sood A. K., Hsiung H. M., Narang S. A. Chemical synthesis of versatile adaptors for molecular cloning. Biochem Biophys Res Commun. 1978 Apr 14;81(3):695–703. doi: 10.1016/0006-291x(78)91408-0. [DOI] [PubMed] [Google Scholar]
- Barth O., Maass H. Verhalten von Enzymaktivitäten, speziell der Thymidylat-Synthetase, nach Hepatektomie und deren Beeinflussbarketi durch Trenimon und Aktinomycin. Z Krebsforsch. 1970 Nov 20;75(1):45–54. [PubMed] [Google Scholar]
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Gennis R. B., Cantor C. R. Optical properties of specific complexes between complementary oligoribonucleotides. Biochemistry. 1970 Nov 24;9(24):4714–4725. doi: 10.1021/bi00826a014. [DOI] [PubMed] [Google Scholar]
- Gennis R. B., Cantor C. R. Optical studies of a conformational change in DNA before melting. J Mol Biol. 1972 Apr 14;65(3):381–399. doi: 10.1016/0022-2836(72)90196-9. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Morgan A. R., Ratliff R. L. A comparison of the circular dichroism spectra of synthetic DNA sequences of the homopurine . homopyrimidine and mixed purine- pyrimidine types. Nucleic Acids Res. 1978 Oct;5(10):3679–3695. doi: 10.1093/nar/5.10.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marck C., Guschlbauer W. A simple method for the computation of first neighbour frequencies of DNAs from CD spectra. Nucleic Acids Res. 1978 Jun;5(6):2013–2031. doi: 10.1093/nar/5.6.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. R., Kramer F. R. Structure-independent nucleotide sequence analysis. Proc Natl Acad Sci U S A. 1979 May;76(5):2232–2235. doi: 10.1073/pnas.76.5.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor R., Gilham P. T. Studies on some interactions and reactions of oligonucleotides in aqueous solution. Biochemistry. 1966 Aug;5(8):2722–2728. doi: 10.1021/bi00872a032. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Tonelli A. E. Assignment of the proton Nmr chemical shifts of the T-N3H and G-N1H proton resonances in isolated AT and GC Watson-Crick base pairs in double-stranded deoxy oligonucleotides in aqueous solution. Biopolymers. 1974;13(10):1943–1964. doi: 10.1002/bip.1974.360131003. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Thermodynamics of the helix-coil transition of (dG-dC) oligomers. Eur J Biochem. 1974 Mar 1;42(2):495–504. doi: 10.1111/j.1432-1033.1974.tb03364.x. [DOI] [PubMed] [Google Scholar]
- Pörschke D., Eggers F. Thermodynamics and kinetics of base-stacking interactions. Eur J Biochem. 1972 Apr 24;26(4):490–498. doi: 10.1111/j.1432-1033.1972.tb01791.x. [DOI] [PubMed] [Google Scholar]
- Shabarova Z. A., Prokofiev M. A. A model of enzymatic synthesis of the internucleotide bond between oligodeoxynucleotides. FEBS Lett. 1970 Dec 11;11(4):237–240. doi: 10.1016/0014-5793(70)80537-3. [DOI] [PubMed] [Google Scholar]
- Uesugi S., Ts'o P. O. Chemical polymerization of oligonucleotides directed by a complementary polynucleotide. Preparation and polymerization of oligo(2'-O-methylinosine 3'-phosphate). Biochemistry. 1974 Jul 16;13(15):3142–3152. doi: 10.1021/bi00712a022. [DOI] [PubMed] [Google Scholar]
- Usatyi A. F., Shlyakhtenko L. S. Temperature dependence of CD spectra of DNA from various sources. Biopolymers. 1973;12(1):45–51. doi: 10.1002/bip.1973.360120105. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Luck G. Conformation and reactivity of DNA. VI. Circular dichroism studies of salt-induced conformational changes of DNAs of different base composition. Biochim Biophys Acta. 1974 Aug 15;361(1):11–32. [PubMed] [Google Scholar]