Abstract
The yeast cell wall is a strong, but elastic, structure that is essential not only for the maintenance of cell shape and integrity, but also for progression through the cell cycle. During growth and morphogenesis, and in response to environmental challenges, the cell wall is remodeled in a highly regulated and polarized manner, a process that is principally under the control of the cell wall integrity (CWI) signaling pathway. This pathway transmits wall stress signals from the cell surface to the Rho1 GTPase, which mobilizes a physiologic response through a variety of effectors. Activation of CWI signaling regulates the production of various carbohydrate polymers of the cell wall, as well as their polarized delivery to the site of cell wall remodeling. This review article centers on CWI signaling in Saccharomyces cerevisiae through the cell cycle and in response to cell wall stress. The interface of this signaling pathway with other pathways that contribute to the maintenance of cell wall integrity is also discussed.
THE yeast cell wall serves four principal functions. First, it provides protection from osmotic shock. Yeast cells in the wild face the potential for exposure to rapid and extreme changes in environment, particularly with respect to osmotic potential. For example, a Saccharomyces cerevisiae cell living on the sugar-rich tissue of a grape can be exposed instantaneously to the hypo-osmotic shock of a rainfall. To survive such rapid decreases in extracellular osmolarity, the cell must limit the influx of water to avoid bursting and to maintain an intracellular water activity that is appropriate for biochemical reactions (Smits et al. 1999; Hohmann 2002). Yeasts and other fungi have solved this problem with strong, but elastic, cell walls that limit swelling. The fungal cell establishes a balance by which the force driving water across the osmotic gradient into the cell is counteracted by turgor pressure against the plasma membrane and cell wall. Second, fungal cell walls also protect against mechanical stress. The combination of strength and elasticity of the cell wall provides an effective barrier against sheer and compression forces.
Third, the yeast cell wall is required to establish and maintain cell shape (Cid et al. 1995; Klis et al. 2006), which is essential for the formation of a bud and, hence, cell division. The cell must remodel this rigid structure to accommodate cell expansion during vegetative proliferation, mating pheromone-induced morphogenesis, and starvation-driven filamentation (pseudohyphal development). Turgor pressure is critical for cell expansion because it provides the force to overcome molecular cohesion within the cell wall (Harold 2002). Because fungal cells maintain an intracellular osmolarity that exceeds that of the extracellular environment, water tends to flow into the cell, thereby providing turgor pressure. However, this pressure is equally distributed across the cell surface. Therefore, for growth to produce cell shapes other than spheres, cell wall expansion must be focused to particular regions. S. cerevisiae uses an internal actin cytoskeleton for this purpose (Drubin and Nelson 1996). During periods of polarized cell growth, the wall is loosened by digestive enzymes (e.g., glucanases and chitinases) and expanded at a single point on the cell surface, a process that must be carried out in a highly regulated manner to avoid cell lysis.
Fourth, the cell wall serves as a scaffold for cell-surface proteins. The polysaccharides that provide the mechanical strength of the cell wall also serve as the attachment matrix for a wide variety of glycoproteins (Zlotnick et al. 1984; Klis et al. 2006). These glycoproteins include sexual agglutination factors important for mating (Cappellaro et al. 1994; Zhao et al. 2001) and adhesins critical to cell–cell contact during filamentation, invasive growth, and biofilm formation (Reynolds and Fink 2001; Douglas et al. 2007). Cell-surface glycoproteins also limit the permeability of the cell wall to macromolecules, thereby protecting the glucan layer from wall-degrading enzymes (Zlotnik et al. 1984; De Nobel et al. 1990; De Nobel and Barnett 1991; Klis et al. 2006).
The focus of this review is the regulatory pathways employed by S. cerevisiae to maintain cell wall integrity during growth, morphogenesis, and in the face of environmental challenges to cell wall integrity. Although several signaling pathways contribute to the maintenance of the cell wall, the one principally responsible for orchestrating changes to the wall is known as the cell wall integrity signaling pathway, which will be abbreviated hereafter as the cell wall integrity (CWI) pathway. Recent advances in our understanding of how this pathway interfaces with the cell cycle to control spatio-temporal aspects of cell wall biogenesis will also be discussed.
Molecular Structure of the Yeast Cell Wall
Yeast cells invest considerable energy in the construction of the cell wall, which comprises some 10–25% of the cell mass depending on growth conditions (Orlean 1997; Smits et al. 1999; Aguilar-Uscanga and François 2003). The major architectural features of the S. cerevisiae cell wall are now fairly well understood. For an excellent review, see Klis et al. (2006). In brief, the cell wall is a layered structure with an electron-transparent inner layer and an electron-dense outer layer (Cappellaro et al. 1994). The inner layer is composed principally of glucan polymers and chitin (β-1,4-N-acetylglucosamine polymers). This layer is constructed mainly (80–90%) of β-1,3-glucan chains branched through β-1,6 linkages. Polymers of β-1,6-glucan chains make up most of the remainder of the inner layer (8–18%) with chitin chains representing the smallest fraction (1–2%). This layer is largely responsible for the mechanical strength and elasticity of the cell wall, owing primarily to the helical nature of β-1,3-glucan chains (Rees et al. 1982; Smits et al. 1999).
The outer cell wall layer is a lattice of glycoproteins. Two major classes of cell wall glycoproteins (CWPs) compose this layer. Members of one class, called glycosylphosphatidylinositol (GPI) proteins, are directed through the secretory pathway to the extracellular face of the plasma membrane by lipid anchors at their C termini. GPI proteins destined for the cell wall are liberated from the plasma membrane by cleavage of their anchors (Kollár et al. 1997). Lipidless GPI remnants of GPI–CWPs become linked to the external surface of the β-1,3-glucan network indirectly through β-1,6-glucan chains (Klis et al. 2006) (Figure 1). Among ∼70 GPI proteins identified in the S. cerevisiae genome (Caro et al. 1997), it is estimated that half reside in the cell wall (Smits et al. 1999).
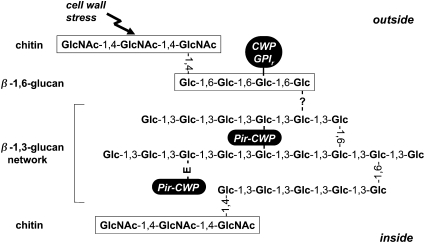
Figure 1 .
Molecular organization of the yeast cell wall (adapted from Lesage and Bussey 2006, doi: 10.1128/MMBR.00038-05; amended with permission from American Society for Microbiology). Chains of β-1,3-glucan, branched through β-1,6-linkages, form a mesh network that provides the mechanical strength of the cell wall and also serves as a scaffold for the attachment of cell wall proteins (CWPs). Pir-CWPs are attached directly to β-1,3-glucan through a Gln residue within their internal repeats that is converted to a Glu (E) residue in the linkage. These proteins have the potential to cross-link β-1,3-glucan chains through multiple repeat sequences. GPI-CWPs are attached to the network indirectly through a linkage between the lipidless GPI remnant (GPIr) and β-1,6-glucan. Chitin, a polymer of β-1,4-N-actetylglucosamine (GlcNAc), can be attached either directly to β-1,3-glucan on the inner surface or indirectly by β-1,6-glucan to the outer surface. The latter attachment is induced in response to cell wall stress. The nature of the linkage between β-1,3-glucan and β-1,6-glucan chains is still uncharacterized.
The other major class of CWPs is represented by five related polypeptides, Pir1-5 (Proteins with internal repeats) (Toh-e et al. 1993; Kapteyn et al. 1999; Mrsa and Tanner 1999; Ecker et al. 2006). Although the PIR genes are not essential, strains multiply deleted for PIR1 through PIR4 display additive defects in growth rate, morphology, and sensitivity to cell wall stress agents (Mrsa and Tanner 1999). The Pir proteins are attached directly to β-1,3-glucan chains (Figure 1) through a linkage that involves their repeat sequences, DGQΦQ [where Φ is any hydrophobic residue (Castillo et al. 2003)]. The glucan chain is linked to the protein through the γ-carboxyl group of a Glu residue evidently produced through a transglutaminase-type reaction that converts the first Gln residue in the repeat sequence to Glu (Ecker et al. 2006). Because most members of this class of proteins contain several repeat motifs, they may provide sites for cross-linking of multiple β-1,3-glucan chains. In contrast to GPI–CWPs, Pir proteins are distributed uniformly through the inner glucan network, consistent with their attachment to β-1,3-glucan (Kapteyn et al. 1999). Additionally, a subset of GPI–CWPs that includes Cwp1, Cwp2, Tir1, and Tir2 also possesses the DGQΦQ motif, suggesting the possibility that these proteins serve as bridges between β-1,3-glucan and β-1,6-glucan chains.
Although very little chitin is found in the lateral walls of cells growing under nonstress conditions, some chitin chains are attached to the internal surface of the β-1,3-glucan network in the lateral wall after cytokinesis (Kollár et al. 1995). Chitin can also be attached to β-1,6-glucan chains associated with GPI–CWPs (Cabib and Duran 2005), particularly in response to cell wall stress (Figure 1), when cell wall chitin levels can rise to as high as 20% of total wall polymer (Popolo et al. 1997; García-Rodriguez et al. 2000; Valdivieso et al. 2000; see Chitin synthase 3: the chitin emergency response). Chitin is attached to both β-1,3-glucan and β-1,6-glucan by the redundant Crh1 and Crh2 transglycosylases (Cabib et al. 2007, 2008; Cabib 2009).
Overview of CWI Signaling
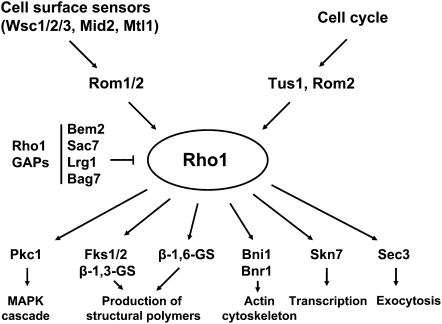
The CWI-signaling pathway exists for the purpose of detecting and responding to cell wall stress that arises during normal growth conditions or through environmental challenge. A diagrammatic representation of the core elements of this pathway is presented in Figure 2. The CWI pathway responds to cell wall stress signals through a family of cell-surface sensors coupled to a small G protein, Rho1, whose activity is also stimulated periodically through the cell cycle in a spatially defined manner. Rho1 is considered to be the master regulator of CWI signaling not only because it integrates signals from the cell surface and the cell division cycle, but also because it regulates a variety of outputs involved in cell wall biogenesis, actin organization, and polarized secretion. Moreover, it seems likely that Rho1 coordinates these functions at the cell surface.
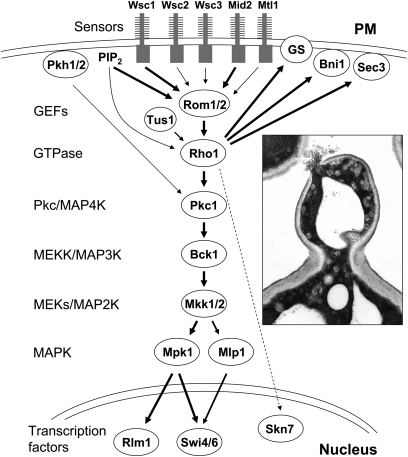
Figure 2 .
The CWI signaling pathway. Signals are initiated at the plasma membrane (PM) through the cell-surface sensors Wsc1, -2, -3, Mid2, and Mtl1. The extracellular domains of these proteins are highly O-mannosylated. Together with PIP2, which recruits the Rom1/2 GEFs to the plasma membrane, the sensors stimulate nucleotide exchange on Rho1. Relative input of each sensor is indicated by the width of the arrows. Additional regulatory inputs from the Tus1 GEF and the Pkh1/2 protein kinases are indicated. The various effectors of Rho1 include the β-1,3-glucan synthase (GS), β-1,6-glucan synthase activity (not shown), formins (Bni1), Sec3, and the Pkc1-activated MAPK cascade. Mlp1 is a pseudokinase paralog of Mpk1 that contributes to the transcriptional program through a noncatalytic mechanism. Two transcription factors, Rlm1 and SBF (Swi4/Swi6), are activated by the pathway. Skn7 (dashed line) may also contribute to the CWI transcriptional program. (Inset) Thin-section electron micrograph of a Pkc1-depleted cell that has undergone cell lysis at its bud tip. Conditions were as described in Levin et al. (1994).
Rho1 is localized to sites of polarized growth (Yamochi et al. 1994; Qadota et al. 1996) where it activates a diverse array of targets. These collectively regulate processes including β-glucan synthesis at the site of wall remodeling, gene expression related to cell wall biogenesis, organization of the actin cytoskeleton, and secretory vesicle targeting to the growth site. Both the β-1,3-glucan synthase (GS) encoded by the FKS1 and FKS2 genes and the β-1,6-glucan synthase, which has not yet been described at the molecular level, are regulated by Rho1. Actin organization is controlled by Rho1 through the actin-nucleating formin proteins Bni1 (Bud neck involved) and Bnr1 (Bni1-related). Vesicle targeting is regulated by the Rho1 control of the Sec3 exocyst protein. Finally, the transcriptional output of the CWI pathway is under the control of a MAPK cascade headed by a Rho1-activated protein kinase C (Pkc1). Disruption of signaling through the MAPK cascade compromises the integrity of the cell wall, which results in cell lysis at sites of polarized growth (Figure 2, inset). Current understanding of each of the inputs and outputs of this pathway related to the maintenance of cell wall integrity will be discussed individually. Although the CWI pathway has additionally been implicated in the responses to oxidative stress (Alic et al. 2003; Vilella et al. 2005), high and low pH stress (Claret et al. 2005; Serrano et al. 2006), and DNA damage (Queralt and Igual 2005; Dardalhon et al. 2009; Truman et al. 2009; Bandyopadhyay et al. 2010), this review article will be restricted to its role in the maintenance of cell wall integrity.
CWI Pathway Architecture
Rho GTPases and cell polarity
Members of the Rho (Ras-homologous) family of GTPases play a central role in polarized growth in animal and fungal cells (Drubin and Nelson 1996; Heasman and Ridley 2008). S. cerevisiae possesses six Rho-type GTPases: Rho1–Rho5 and Cdc42 (reviewed in Perez and Rincon 2010). They reside at the plasma membrane and serve related, but distinct, roles in the establishment and maintenance of cell polarity. Of these, only Rho1 and Cdc42 are essential—Cdc42 function is critical for bud-site assembly and for the establishment of polarized growth (Johnson and Pringle 1990; Johnson 1999)—whereas Rho1, the functional ortholog of mammalian RhoA (Qadota et al. 1994), controls CWI signaling and will be discussed in more detail below. Rho2 appears to be partially redundant with Rho1 as judged by dosage suppression results (Ozaki et al. 1996; Helliwell et al. 1998). Rho3 and Rho4 share an essential role in the establishment of polarity through actin organization (Matsui and Toh-E 1992; Imai et al. 1996; Kagami et al. 1997; Dong et al. 2003). Additionally, Rho3 serves a direct role in exocytosis that is separate from its regulation of actin organization (Adamo et al. 1999). Rho5 has been suggested to down-regulate the CWI pathway on the basis of elevated basal and induced pathway activity in a rho5Δ mutant (Schmitz et al. 2002a), but direct connections are thus far lacking. Rho proteins are tethered to the plasma membrane by prenyl groups (either farnesyl or geranylgeranyl) added to their C termini (Schafer and Rine 1992). These modifications are essential for proper localization and function of Rho proteins. Both Rho1 and Cdc42 are modified through the action of the Cdc43/Ram2 geranylgeranyl transferase (Inoue et al. 1999). Additionally, both Rho1 and Cdc42 possess a polybasic sequence near their C termini that, at least in the case of Rho1, is important for localization to specific regions of the plasma membrane (Yoshida et al. 2009).
Regulators of Rho1: Guanosine nucleotide exchange factors and GTPase-activating proteins
The Rho1 GTPase cycle is regulated by both guanosine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) (Figure 3). Three GEFs—Rom1, Rom2, and Tus1—provide overlapping functions in the activation of Rho1 (Ozaki et al. 1996; Schmelzle et al. 2002; Kono et al. 2008; Yoshida et al. 2009). Loss of either ROM2 or TUS1 function results in temperature-sensitive growth, whereas a rom1Δ rom2Δ mutation is synthetically lethal (Ozaki et al. 1996; Schmelzle et al. 2002). Like Rho1, the Rho1-GEFs reside at sites of polarized growth in a manner dependent on the actin cytoskeleton (Manning et al. 1997; Yoshida et al. 2006; Kono et al. 2008). All of the Rho-GEFs possess Dbl homology (DH) domains, which interact with GDP-bound Rho1 and catalyze the nucleotide exchange activity of these proteins (Ozaki et al. 1996; Schmelzle et al. 2002). They also possess pleckstrin homology (PH) domains that, in the case of Rom1 and Rom2, bind to phosphatidylinositol (PI)-4,5-bisphosphate (PIP2) and are responsible for their proper localization to the plasma membrane (Audhya and Emr 2002). However, the PH domain of Tus1 does not appear to bind phosphoinositides (Yu et al. 2004). Additionally, an N-terminal domain of Rom1 and Rom2 that is responsible for their association with at least the Wsc1 and Mid2 cell-surface sensors (described in the section Cell-surface sensors: Wsc1-3, Mid2, and Mtl1) (Philip and Levin 2001) is not shared with Tus1. This may be explained by the observation that Tus1 is primarily responsible for cell cycle-specific activation of Rho1 (Kono et al. 2008; see section on Rho1 activation through the cell cycle) and may therefore respond exclusively to intracellular signals.
Figure 3 .
Rho1 regulators and effectors. Rho1 localization and activity are regulated through the cell cycle and in response to cell wall stress by cell-surface sensors, a family of GEFs (Rom1, Rom2, and Tus1), and a set of GAPs (Bem2, Sac7, Lrg1, and Bag7). Six known Rho1 effectors control cell wall biogenesis through polymer synthesis, polarization of the actin cytoskeleton, directed secretion, and transcription.
S. cerevisiae possesses 11 Rho-GAPs. Of these, 4 have been shown to act on Rho1 both in vitro and in vivo: Bem2, Sac7, Bag7, and Lrg1 (Peterson et al. 1994; Schmidt et al. 1997; Cid et al. 1998; Martín et al. 2000; Roumanie et al. 2001; Watanabe et al. 2001; Schmidt et al. 2002). Interestingly, these GAPs appear to regulate Rho in a target-specific manner. Lrg1 is evidently dedicated to regulation of β-1,3-glucan synthase (Watanabe et al. 2001). By contrast, Bem2 and Sac7 collaborate to down-regulate the Pkc1-activated MAPK pathway (Martín et al. 2000; Schmidt et al. 2002), whereas Bag7 and Sac7 control the actin cytoskeleton (Schmidt et al. 1997, 2002). The apparently independent regulation of different Rho1-effector pairs by distinct GAPs indicates some compartmentalization of Rho1 functions. The differential function of Rho-GAPs is an interesting puzzle that, once solved, may yield some general principles applicable to other systems.
Cell-surface sensors: Wsc1-3, Mid2, and Mtl1
Members of a family of cell-surface sensors, which detect and transmit cell wall stress to Rho1 through a set of GEFs, are principally responsible for activation of CWI signaling (Rodicio and Heinisch 2010). These sensors include Wsc1 (Gray et al. 1997; Verna et al. 1997; Jacoby et al. 1998), Wsc2, Wsc3 (Verna et al. 1997), Mid2, and Mtl1 (Ketela et al. 1999; Rajavel et al. 1999). All five are plasma membrane proteins whose overall structures are similar in that they possess short C-terminal cytoplasmic domains, a single transmembrane domain, and a periplasmic ectodomain rich in Ser/Thr residues (Ketela et al. 1999; Lodder et al. 1999; Rajavel et al. 1999; Philip and Levin 2001). The Ser/Thr-rich regions are highly O-mannosylated, probably resulting in extension and stiffening of the polypeptide. Therefore, these proteins have been proposed to function as mechanosensors that act as rigid probes of the extracellular matrix (Rajavel et al. 1999; Philip and Levin 2001). A recent study using atomic force microscopy to probe the physical characteristics of Wsc1 supports this conclusion and suggests that this sensor behaves as a linear nanospring (Dupres et al. 2009).
Aside from the gross structural similarities between the two subfamilies of sensors, their sequences are not conserved. The Wsc proteins possess an N-terminal cysteine-rich region, termed the WSC domain, which is absent from Mid2 and Mtl1. Mutation of the conserved cysteine residues in Wsc1 destroys its function (Heinisch et al. 2010). The positions of the eight cysteine residues in this region are conserved in human polycystin 1 (PKD1), a mechanosensor whose mutation results in polycystic kidney disease (Qian et al. 2005). The same arrangement of cysteine residues is also found in a Trichoderma β-1,3-exoglucanase (Cohen-Kupiec et al. 1999), suggesting the possibility that this domain binds glucan chains, but this remains to be tested directly. On the other hand, atomic force microscopy revealed recently that cell wall stress induces clustering of Wsc1 molecules within the plasma membrane, which is dependent on the conserved cysteine residues (Heinisch et al. 2010). Therefore, the function of the WSC domain remains unresolved.
Wsc1 and Mid2 are the most important among the various sensors for response to the conditions examined to date. Deletion of WSC1 results in cell lysis at elevated growth temperature (37°–39°C), a phenotype modestly exacerbated by loss of WSC2 or WSC3 (Gray et al. 1997; Verna et al. 1997; Jacoby et al. 1998). However, a double wsc1Δ mid2Δ mutant requires osmotic support to survive (Ketela et al. 1999; Rajavel et al. 1999), revealing the complementary functions of these sensors. Functional distinctions among these proteins are revealed by the relative importance of each sensor in response to different stresses. Consistent with the importance of Wsc1 for survival of cell wall stress from growth at elevated temperature, a wsc1Δ mutant is deficient in thermal activation of Mpk1 (Gray et al. 1997; Verna et al. 1997). Similarly, stress signaling induced by caspofungin, which inhibits the GS, is mediated almost exclusively by Wsc1 (Reinoso-Martín et al. 2003; Bermejo et al. 2010), perhaps reflecting an interaction between the extracellular domain of Wsc1 and β-1,3-glucan, as noted above.
In contrast to WSC1, loss of MID2 (Mating Induced Death) results in failure to survive pheromone-induced morphogenesis. Consistent with this, Mid2 is required for Mpk1 activation in response to pheromones (Ketela et al. 1999; Rajavel et al. 1999). It should be noted that pheromone-induced activation of CWI signaling is not a direct response to pheromones, but rather a secondary response triggered by morphogenesis (Errede et al. 1995; Buehrer and Errede 1997). Mid2 also appears to be the major sensor for signaling wall stress in response to the cell wall antagonists calcofluor white (CFW) (Ketela et al. 1999) and Congo red (Bermejo et al. 2010), both of which interfere with cell wall assembly by binding to chitin (Elorza et al. 1983; Imai et al. 2005).
Like most other components of the CWI pathway, Wsc1 localizes to sites of polarized cell growth (Delley and Hall 1999; Huh et al. 2003; Straede and Heinisch 2007). In contrast to this, Mid2 is uniformly distributed across the plasma membrane during growth (Ketela et al. 1999; Rajavel et al. 1999; Straede and Heinisch 2007). However, consistent with the importance of Mid2 during pheromone-induced morphogenesis, this sensor becomes enriched at the tips of mating projections (Hutzler et al. 2008). The difference in localization between Wsc1 and Mid2 during vegetative growth is dictated by the presence of an endocytosis signal in the cytoplasmic C terminus of Wsc1, which is responsible for constitutive recycling of the sensor from the plasma membrane (Piao et al. 2007). A mutant form of Wsc1 that is missing its endocytosis signal is distributed evenly across the plasma membrane and results in hypersensitivity to caspofungin, revealing the importance of its focused localization to sites of polarized secretion (Piao et al. 2007).
O-mannosylation of the Mid2 and Wsc1 ectodomains requires either Pmt2 or Pmt4 (Philip and Levin 2001; Lommel et al. 2004), members of a seven-isoform family of proteins that catalyze the first step in protein O-mannosylation (Strahl-Bolsinger et al. 1999). Consistently, a double pmt2Δ pmt4Δ mutant undergoes cell lysis in the absence of osmotic support (Gentzsch and Tanner 1996). This defect is suppressed by overexpression of Pkc1, Wsc1, or Mid2 (Lommel et al. 2004), revealing that O-mannosylation of the sensors, although important, can be bypassed. Sensor mannosylation is evidently more important for stability than for function (Lommel et al. 2004), calling into question the previously proposed role of this modification in sensor rigidity. Mid2, unlike Wsc1, is additionally N-glycosylated near its N terminus (Hutzler et al. 2008). In contrast to O-mannosylation, this modification affects Mid2 signaling, rather than its stability or localization.
Rho GEFs: Signaling targets of the CWI sensors
The short cytoplasmic domains of both Wsc1 and Mid2 are essential to their functions (Lodder et al. 1999; Rajavel et al. 1999; Philip and Levin 2001; Green et al. 2003; Vay et al. 2004) and display two-hybrid interaction with the N-terminal domain of the Rho1-GEF, Rom2 (Philip and Levin 2001). This domain is different from the Rho1-interacting DH domain of Rom2, suggesting that the GEF can interact simultaneously with a sensor and with Rho1. As noted above, the sensor interaction domain of Rom2 is shared by Rom1 but not by Tus1, which appears to activate Rho1 in a cell cycle-specific manner. The sensors are not known to interact directly with Rho1. Extracts from wsc1Δ and mid2Δ cells are deficient in catalyzing GTP loading of Rho1, suggesting that the sensors recruit or activate the GEFs. In this regard, the sensors may collaborate with PIP2 (see section on Phosphoinositide metabolism: Stt4-Mss4 signaling) to recruit the GEFs to the plasma membrane as well as serve to focus their action to sites of polarized growth (Philip and Levin 2001).
The cytoplasmic domain of Wsc1 is phosphorylated (Lodder et al. 1999). Mutational analysis of this domain revealed that it possesses two short regions important for Rom2 interaction, one at the extreme C terminus and the other near the transmembrane domain (Vay et al. 2004). The phosphorylation site, a target of the Yck1/2 kinases (Levin 2005), resides between these interaction regions and serves to inhibit Wsc1 function, probably by interfering with Rom2 interaction. However, phosphorylation is not the primary means of Wsc1 regulation because a Wsc1 phosphorylation site mutant is not constitutively active, but is potentiated for activation by cell wall stress (Vay et al. 2004).
Phosphoinositide metabolism: Stt4-Mss4 signaling
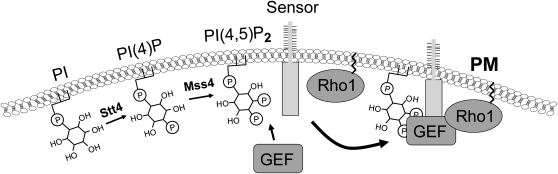
Phosphoinositides play an important role in both the activation of Rho1 and the recruitment of some of its effectors to the plasma membrane (Figure 4). STT4 encodes an essential PI 4-kinase (Cutler et al. 1997) that is responsible for the production of PI(4)P at the plasma membrane (Audhya and Emr 2002). Conditional mutants in this gene display defects in actin organization and undergo cell lysis at restrictive temperature (Yoshida et al. 1994a,b; Audhya et al. 2000).
Figure 4 .
The phosphoinositide-signaling pathway at the plasma membrane (PM). The sequential action of Stt4 and Mss4 at the cell surface generates PI(4,5)P2 (PIP2), which recruits Rho1-GEFs to the PM through their PH domains for interaction with the cytoplasmic tails of the cell-surface sensors.
The MSS4 gene encodes the only PI(4)P 5-kinase of yeast, and like STT4, it is essential for viability. Mss4 catalyzes the conversion of PI(4)P at the plasma membrane to PI(4,5)P2 (PIP2) (Desrivieres et al. 1998; Homma et al. 1998). Similar to conditional stt4 mutants, mss4 mutants display defects in actin organization and cell wall integrity at restrictive temperature (Desrivieres et al. 1998; Audhya and Emr 2003). Rom2 was identified as an effector of the Stt4-Mss4 pathway by the demonstration that a critical role of PIP2 production is to recruit this Rho-GEF (and presumably Rom1) to the plasma membrane through its PH domain (Audhya and Emr 2002). This recruitment is evidently integral to the activation of Rom2 GEF activity for Rho1.
Intracellular levels of PIP2 increase transiently in response to mild heat shock (Desrivieres et al. 1998; Audhya and Emr 2002). This stress also activates CWI signaling (Kamada et al. 1995), supporting the hypothesis that the concentration of this phosphoinositide in the plasma membrane contributes to signal activation. Moreover, although both Stt4 and Mss4 appear as discrete punctate spots distributed evenly through the plasma membrane, a recent study of PIP2 localization revealed that it is enriched at sites of polarized growth (Garrenton et al. 2010), suggesting either local activation of the PI kinases or inhibition of PI phosphatases. In either case, the concentration of PIP2 at sites of polarized growth suggests that it is a primary determinant for recruitment of Rom1, Rom2, and possibly Tus1 to sites of cell wall deposition.
Targets of Rho1
Six effectors for Rho1 have been described: the Pkc1 protein kinase, the GS, β-1,6-glucan synthase activity, the Bni1 and Bnr1 formin proteins, the Sec3 exocyst component, and the Skn7 transcription factor (Figure 3). As noted above, evidence is accumulating to suggest that each Rho1-effector pair is regulated separately by a different complement of GAPs. It is clear that spatio-temporal regulation of Rho1 by different GEFs is also important for activation of a subset of Rho1 effectors through the cell cycle (see section on Rho1 activation through the cell cycle). Together, these effectors coordinate synthesis of cell wall glucans and chitin, polarization of the actin cytoskeleton, expression of genes important for cell wall biogenesis, and polarized exocytosis.
Pkc1 and the CWI MAPK cascade
Pkc1:
The S. cerevisiae genome encodes only a single homolog of mammalian protein kinase C, designated Pkc1 (Levin et al. 1990). Although this protein kinase has several substrates, only its regulation of the Mpk1 MAPK cascade has been well studied. Deletion of PKC1 is lethal under normal growth conditions because the cells undergo cell lysis, but the growth defect of a pkc1Δ mutant can be suppressed by osmotic support (e.g., 1 M sorbitol) (Levin and Bartlett-Heubusch 1992; Paravicini et al. 1992). Electron micrographic images of pkc1Δ cells maintained in the presence of osmotic support revealed a pleiotropic set of cell wall defects (Levin et al. 1994; Roemer et al. 1994). Both the inner, glucan-containing layer and the outer, mannoprotein layer are thinner in pkc1Δ mutants. These alterations are mirrored by a reduction in both β-1,3- and β-1,6-glucans of ∼30% and a reduction in mannan of ∼20% (Roemer et al. 1994; Shimizu et al. 1994). Loss of PKC1 results in a more severe growth defect than that displayed by deletion of any of the members of the MAPK cascade under the control of Pkc1, which prompted the suggestion that Pkc1 regulates at least one additional pathway (Lee and Levin 1992). Secondary Pkc1 targets not thought to be directly involved in cell wall biosynthesis are reviewed in Levin (2005).
Pkc1 associates with and is activated by GTP-bound Rho1 (Nonaka et al. 1995; Kamada et al. 1996), which confers upon the protein kinase the ability to be stimulated by phosphatidylserine as a lone cofactor (Kamada et al. 1996). Two regions of the Pkc1 N-terminal regulatory domain, a cys-rich C1 domain and homology region 1 (HR1) domain, contribute to its ability to interact with Rho1 (Nonaka et al. 1995; Schmitz et al. 2002b). Cofactors that activate conventional PKCs, such as diacylglycerol (DAG) and Ca2+, do not activate Pkc1 even in the presence of GTP-Rho1 (Antonsson et al. 1994; Watanabe et al. 1994; Kamada et al. 1996). Consistent with this finding, a pkc1Δ mutant is complemented by human PKC-eta (Nomoto et al. 1997), a member of the so-called novel PKC subfamily, which does not respond to DAG or Ca2+. A detailed analysis of the Pkc1 domain structure as it relates to its regulation has been described elsewhere (Levin 2005).
Pkc1 is also a target of the Pkh1 and Pkh2 protein kinases (Inagaki et al. 1999; Friant et al. 2001). Pkh1 and -2 serve an essential but overlapping function in the maintenance of cell wall integrity, and their function is required for full activation of Pkc1 in response to heat shock. Regulation of Pkc1 by Pkh1/2 is exerted by phosphorylation of an activation loop residue within the catalytic domain of Pkc1 (Thr983). A mutant form of Pkc1 blocked for this phosphorylation (T983A) fails to complement a pkc1Δ mutant (Roelants et al. 2004). It is not yet clear if Pkh1/2 activity functions as a regulatory input to Pkc1 or is merely required to establish basal activity of the latter kinase. Although the sphingoid base, phytosphingosine, has been suggested to activate Pkh1/2 at the plasma membrane on the basis of weak in vitro stimulation (Friant et al. 2001), two recent studies indicate that sphingoid bases are not required for in vivo activation of either Pkh1/2 (Roelants et al. 2010) or Pkc1 (Jesch et al. 2010).
An intracellular localization study of Pkc1 revealed that it resides at sites of polarized cell growth (Andrews and Stark 2000). In G1 and S phase, Pkc1 resides at the pre-bud site and at bud tips, a pattern that is very similar to that of Rho1 (Yamochi et al. 1994; Qadota et al. 1996). Pkc1 becomes delocalized during G2 phase and finally relocalized at the mother-bud neck during mitosis, a transition that requires an intact septin ring (Denis and Cyert 2005). A molecular dissection of Pkc1 suggested that each regulatory subdomain was responsible for localizing a pool of Pkc1 to specific subcellular sites (Denis and Cyert 2005), knowledge that may contribute to our understanding of its functions beyond activation of the MAPK cascade.
CWI MAPK cascade:
Among the various Rho1 effector pathways identified, the Pkc1-activated MAPK cascade has been studied in the greatest detail. A linear protein kinase cascade is responsible for amplification of the CWI signal from Rho1 (Figure 2). MAPK cascades serve both to amplify a small signal initiated at the cell surface and to convert a graded input to a highly sensitive, switch-like response (Ferrell 1996; Huang and Ferrell 1996). The details of isolation and validation of the various components of the CWI MAPK cascade have been reviewed extensively (Gustin et al. 1998; Heinisch et al. 1999; Levin 2005). It is one of five MAPK-signaling pathways in yeast that variously regulate mating, response to high osmolarity, pseudohyphal/invasive growth, sporulation, and response to cell wall stress.
Briefly, the MAPK cascade for CWI signaling is composed of Pkc1 (Levin et al. 1990), a MEKK (Bck1) (Costigan et al. 1992; Lee and Levin 1992), a pair of redundant MEKs (Mkk1/2; Irie et al. 1993), and a MAPK (Mpk1/Slt2) (Lee et al. 1993; Martín et al. 1993). Mpk1 is a functional ortholog of human ERK5 (Truman et al. 2006), a MAPK that is activated in response to growth factors, as well as to hyperosmotic, oxidative, and fluid sheer stresses (Abe et al. 1996; Yan et al. 2001). The relative number of molecules per cell of these components (Bck1, 112 molecules per cell; Mkk1, 1040; Mkk2, 1950; and Mpk1, 3230) (Ghaemmaghami et al. 2003) reflects their hierarchical function. In addition to these protein kinases, S. cerevisiae possesses a pseudokinase paralog of Mpk1, named Mlp1 (Mpk1-like protein) (Watanabe et al. 1997), which shares with Mpk1 a specialized, noncatalytic function in transcription (Kim et al. 2008; Truman et al. 2009; Kim and Levin 2010, 2011).
Genetic and biochemical studies have established that Pkc1 activates Bck1, which activates Mkk1 and -2, which in turn activate Mpk1. Pkc1 phosphorylates Bck1 in vitro at several sites in a hinge region between its putative regulatory domain and its catalytic domain (Levin et al. 1994; Levin 2005) that is also the site of activating mutations (Lee and Levin 1992). Bck1 is presumed to phosphorylate and activate Mkk1/2 on the basis of genetic epistasis studies, two-hybrid interactions, and its requirement for activation of Mpk1 (Irie et al. 1993; Kamada et al. 1995; Paravicini and Friedli 1996; Ho et al. 2002). Mkk1 and -2 phosphorylate Mpk1 on neighboring tyrosyl and threonyl residues in a T-X-Y motif within the activation loop conserved among MAPKs. Dual phoshorylation of Mpk1 can be detected with antibodies directed against phosphorylated mammalian ERK1/2 (p42/44) (Martín et al. 2000). The Mlp1 pseudokinase possesses only the tyrosyl residue of this motif, which, nevertheless, is evidently phosphorylated by Mkk1/2 (Kim et al. 2008).
Like other MAPKs, active Mpk1 and Mlp1 associate with their targets and regulators through a canonical D motif—(Arg/Lys)1-2-(X)2-6-ΦA-x-ΦB (where Φ are hydrophobic residues Leu, Ile, or Val)—recognized by a common docking site in the MAPK (Zhang et al. 2003). Mkk1 and Mkk2 are subject to retrophosphorylation by Mpk1, which appears to be a negative feedback regulatory mechanism and requires a D motif in the MEKs (Jimenez-Sanchez et al. 2007).
Loss of function of any protein kinase below Pkc1 (or both Mkk1 and Mkk2) results in cell lysis at 37°. The growth defects of these mutants are remediated by elevated extracellular osmolarity (e.g., 1 M sorbitol), consistent with a primary defect in cell wall biogenesis. Other cell wall-related phenotypes associated with mutants in the CWI MAPK cascade include sensitivity to mating pheromone and cell wall antagonists such as CFW, Congo red, caspofungin, caffeine, and the wall lytic enzyme zymolyase (Errede et al. 1995; Kirchrath et al. 2000; Martín et al. 2000; Reinoso-Martín et al. 2003) and actin polarization defects (Mazzoni et al. 1993).
Mpk1 resides predominantly in the nucleus under nonstress conditions, but a large fraction of the nuclear protein relocates rapidly to the cytoplasm in response to cell wall stress [e.g., shift to 39° (Kamada et al. 1995)], although this translocation was not observed in another study (Hahn and Thiele 2002). Additionally, a small pool of Mpk1 localizes to sites of polarized cell growth and shuttles constitutively between these sites and the nucleus (van Drogen and Peter 2002). Similarly, during pheromone-induced morphogenesis, a minor pool of Mpk1 can be detected at the mating projection tip (Baetz et al. 2001). Polarized localization of Mpk1 during growth and morphogenesis is independent of the actin cytoskeleton, but does require Spa2 (van Drogen and Peter 2002), a component of the polarisome, a protein complex that links polarity establishment factors with actin cables (Madden and Snyder 1998; Shih et al. 2005).
Mkk1 and Mkk2 are mainly cytoplasmic proteins but, like Mpk1, can be detected at sites of polarized growth in a Spa2-dependent manner (van Drogen and Peter 2002). Moreover, Spa2 displays two-hybrid interactions with both Mpk1 and Mkk1/2 (Sheu et al. 1998), prompting the suggestion that Spa2 serves as a scaffold for these protein kinases. However, in contrast to the role of the Ste5 scaffold protein in activating the pheromone-response MAPK cascade (Elion 2000), Spa2 is not required for Mpk1 activation during vegetative growth or in response to pheromone treatment (Buehrer and Errede 1997; Sheu et al. 1998). This suggests that the function of Spa2 with regard to CWI signaling is to focus the action of the kinases to sites of polarized growth. In support of this conclusion is the additional finding that Bck1 is not recruited to sites of polarized growth (van Drogen and Peter 2002). One likely target of Mpk1 at the cell surface is the Rom2 GEF for Rho1, which is phosphorylated and delocalized from the bud tip in an Mpk1-dependent manner in response to cell wall stress (Guo et al. 2009).
β-1,3-glucan synthase
As noted above, the main structural component of the yeast cell wall is linear polymers of β-1,3-linked glucan with branches through β-1,6 linkages (Klis et al. 2006). The biochemistry of the enzyme complex that catalyzes the synthesis of β-1,3-glucan chains from UDP-glucose has been studied extensively (Inoue et al. 1996; Douglas 2001). The echinocandin antifungal agents (e.g., caspofungin), which interfere with the production of β-1,3-glucans and target the GS directly, compose the leading class of drugs directed at treating life-threatening fungal infections (Wiederhold and Lewis 2003; Perlin 2007). A pair of closely related genes, FKS1 and FKS2 (for FK506 sensitive), encode alternative catalytic subunits of the GS (Douglas et al. 1994; Mazur et al. 1995; Ram et al. 1995). S. cerevisiae Fks1 and Fks2 are large, multispan membrane proteins with a cytoplasmic central domain, either one of which is sufficient for GS activity and cell viability. Although echinocandin-resistant mutations map to the Fks1 protein (Douglas 2001), strongly suggesting that this class of agents targets the GS catalytic subunit, it is not yet clear how echinocandins inhibit GS activity. This seems a fertile area for further study.
The GS is thought to extrude glucan chains produced on the cytoplasmic face of the plasma membrane for incorporation into the wall. Although the enzyme has not been purified to homogeneity, the central domain of partially purified Neurospora crassa Fks protein was shown to cross-link to azido-UDP-glucose (Schimoler-O’Rourke et al. 2003), supporting the conclusion that this protein is the catalytic subunit. A recent functional analysis of FKS1 revealed that mutations in this central domain, which is predicted to be cytoplasmic, cause defects in GS activity (Okada et al. 2010). Unlike loss of Pkc1, loss of Fks1/2 is not suppressed by increased osmotic support. This is presumably because cell wall biosynthesis is completely shut down in an fks1Δ fks2Δ mutant.
Rho1 is an essential regulatory subunit of the GS complex, serving to stimulate GS activity in a GTP-dependent manner (Drgonova et al. 1996; Mazur and Baginsky 1996; Qadota et al. 1996). Consistent with this, Fks1 colocalizes with Rho1 in the plasma membrane at sites of cell wall remodeling (Yamochi et al. 1994; Qadota et al. 1996). A more detailed localization study revealed that GS colocalizes with cortical actin patches and moves on the cell surface in a manner dependent on actin patch mobility (Utsugi et al. 2002). The Rho1 interaction site on Fks1 and Fks2, which has not yet been identified, may give clues as to the manner by which this GTPase activates the enzyme. A third gene encoding a homolog of FKS1/2, called FKS3, is important for spore wall formation, but appears to function as a positive regulator of GS activity rather than as a catalytic subunit, possibly by stabilizing Rho1 (Ishihara et al. 2007).
An intragenic complementation analysis of conditional rho1 alleles revealed that two of its essential functions could be separated (Saka et al. 2001). Mutants in one group were defective in GS activity, and mutants in the other group were defective in activating Pkc1. Accordingly, mutants specifically deficient in Pkc1 signaling displayed cell lysis defects at restrictive temperature, whereas mutants deficient in GS activity arrested growth without cell lysis.
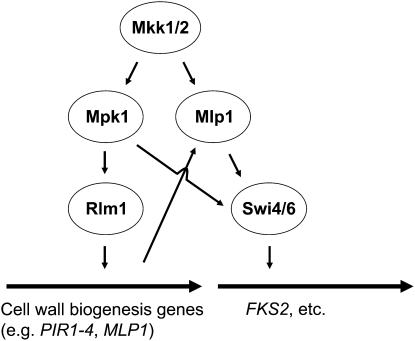
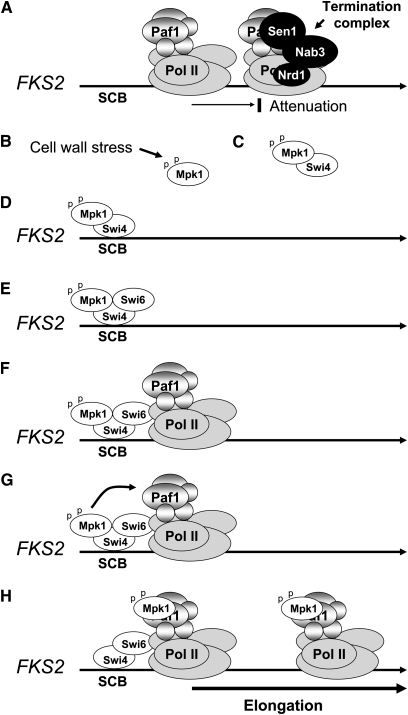
As is the case for many paralogous genes in S. cerevisiae, FKS1 and FKS2 differ primarily in the manner in which their expression is controlled. Under optimal growth conditions, FKS1 is the predominantly expressed gene, and its mRNA levels fluctuate periodically through the cell cycle, peaking in late G1 (Ram et al. 1995; Igual et al. 1996). Cell cycle-regulated expression of FKS1 is controlled primarily by the SBF transcription factor (Mazur et al. 1995; Ram et al. 1995; Igual et al. 1996; Spellman et al. 1998), which is composed of Swi4 and Swi6 (Andrews and Herskowitz 1989). Expression of FKS1 is also regulated weakly by CWI signaling (Igual et al. 1996) through the Mpk1-activated transcription factor Rlm1 (Jung and Levin 1999).
Expression of FKS2 is low under optimal growth conditions, but is induced in response to treatment with mating pheromone, cell wall stress, high extracellular Ca2+, growth on poor carbon sources, entry to stationary phase, or in the absence of FKS1 function (Mazur et al. 1995; Zhao et al. 1998). The pathway for induction of FKS2 expression by pheromone, CaCl2, or loss of FKS1 requires the Ca2+/calmodulin-dependent protein phosphatase calcineurin (Garrett-Engele et al. 1995; Mazur et al. 1995), the target of immunosuppressant FK506 action (Foor et al. 1992; Liu 1993). Because FKS1 and FKS2 provide a redundant but essential function, regulation of FKS2 expression by calcineurin explains the sensitivity of fks1 mutants to FK506 and their synthetic lethality with calcineurin mutants (Garrett-Engele et al. 1995). In response to cell wall stress, the immediate transcriptional induction of FKS2 is mediated by the calcineurin-activated transcription factor Crz1, which binds to a calcineurin-dependent response element within the FKS2 promoter (Stathopoulos and Cyert 1997; Zhao et al. 1998). Maintenance of high levels of FKS2 expression under chronic cell wall stress is driven by the CWI pathway (Zhao et al. 1998; Jung and Levin 1999), through the noncatalytic activation of the Swi4/Swi6 (SBF) transcription factor by Mpk1 and its pseudokinase paralog Mlp1 (see section on Noncatalytic transcriptional functions of Mpk1). Therefore, Rho1 controls both the activity of the GS during normal growth and the expression of its catalytic subunits under conditions of wall stress. The complex regulatory network centered on the induced expression of FKS2 is evidently a mechanism to augment Fks1-derived GS activity under emergent conditions. FKS2 also serves as the major GS for spore wall formation (Ishihara et al. 2007).
β-1,6-glucan synthase activity
The site of β-1,6-glucan synthesis has been controversial for many years. β-1,6-glucan synthesis defects are caused by mutations in genes that function throughout the secretory pathway (Shahinian and Bussey 2000; Page et al. 2003), suggesting that biosynthesis of this polymer begins in the endoplasmic reticulum (ER), progresses in the Golgi, and is completed at the cell surface. Indeed, a pair of functionally redundant glucosyl hydrolases (or transglucosylases) that are critical for β-1,6-glucan synthesis, Kre6 and Skn1, reside in the Golgi (Roemer et al. 1994). However, a late secretory pathway mutant displayed only surface labeling of the polymer, indicating that a secretory block does not result in accumulation of intracellular β-1,6-glucan (Montijn et al. 1999). This suggested that β-1,6-glucan, like β-1,3-glucan, may be synthesized at the plasma membrane. An in vitro assay for β-1,6-glucan synthesis revealed requirements for UDP-glucose and GTP and, provocatively, demonstrated enhanced activity in cells overexpressing Rho1 (Vink et al. 2004). Thus, Rho1 may control the biosynthesis of both β-glucan polymers. If this is correct, it seems likely that β-1,6-glucan synthesis is carried out at sites of polarized cell growth based on the localization pattern of Rho1. It is anticipated that this assay will provide a much-needed tool for the molecular dissection of β-1,6-glucan synthesis.
Bni1 and Bnr1
The Bni1 and Bnr1 proteins are functionally redundant members of a distinct class of actin-nucleating proteins called formins that are activated by Rho-GTPases. Bni1 and Bnr1 nucleate actin filament assembly and protect actin filaments from capping protein (Fujiwara et al. 1998; Ozaki-Kuroda et al. 2001; Pruyne et al. 2002; Sagot et al. 2002a,b; Evangelista et al. 2003). Although Bni1 is a component of the polarisome and translocates between the bud tip and the bud neck, Bnr1 is primarily localized to the neck (Ozaki-Kuroda et al. 2001; Buttery et al. 2007). These proteins share functions in the assembly of actin cables, and Bni1 also has a major role in the formation of the contractile actin ring (CAR) (Tolliday et al. 2002; Yoshida et al. 2006).
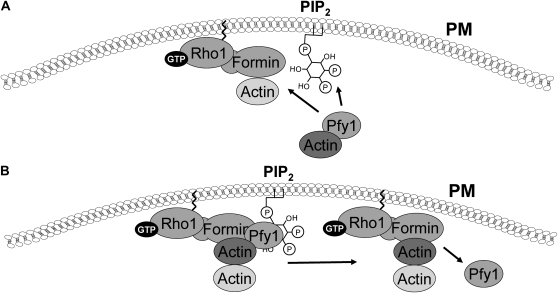
Bni1 and Bnr1 are activated by Rho GTPases through an N-terminal Rho-binding domain (RBD) (Evangelista et al. 2003). In the absence of bound Rho protein, the RBD engages in an autoinhibitory interaction with its C-terminal domain (Alberts 2001). These formins also interact physically with actin and actin-binding proteins through distinct domains (Evangelista et al. 1997; Imamura et al. 1997; Umikawa et al. 1998; Kikyo et al. 1999; Drees et al. 2001; Wen and Rubenstein 2009). Of key importance is the association of the actin-binding protein profilin (Pfy1), which enhances filament formation by delivery of actin to the formin at the plasma membrane (Sagot et al. 2002b; Pring et al. 2003; for a recent review, also see Campellone and Welch 2010). In this regard, it is interesting to note that profilin also binds PIP2, which induces release of actin from the profilin–actin complex (Sechi and Wehland 2000) (Figure 5).
Figure 5 .
The involvement of PIP2 in the delivery of actin to the Rho1–formin complex. (A) Profilin is an actin-binding protein that delivers actin to the actin-nucleating formins Bni1 and Bnr1. The profilin–actin complex is recruited to the PM by PIP2, where it is bound by the active Rho1–formin complex. At least one additional Rho1 effector, Sec3, is also recruited to the PM by PIP2 (not shown). (B) Upon delivery of actin to the formin, PIP2 is thought to stimulate the release of actin from profilin, thereby driving actin polymerization.
The GTP-bound forms of all the Rho GTPases of yeast, except Rho2, have been shown to bind Bni1 and/or Bnr1 (Kohno et al. 1996; Evangelista et al. 1997; Fujiwara et al. 1998; Robinson et al. 1999; Drees et al. 2001; Richman and Johnson 2000; Mösch et al. 2001). Expression of constitutively active versions of the formin proteins suppresses the growth defect of a rho3Δ rho4Δ mutant, prompting the suggestion that the essential function of Rho3 and Rho4 is to activate Bni1 and Bnr1 (Dong et al. 2003). However, it is not yet clear if these GTPases directly or indirectly promote formin activation because Rho3 (and likely Rho4) serves an important role in exocytosis (Adamo et al. 1999). Rho1, which is clearly critical for formin activation, is delivered to the membrane via the secretory system (Abe et al. 2003); thus, loss of Rho3 and Rho4 might reduce the level of Rho1 at the plasma membrane. Indeed, Rho1 is required for Bni1-mediated CAR assembly during cytokinesis (Tolliday et al. 2002; Yoshida et al. 2006; see section on Rho1 activation through the cell cycle).
Sec3
Cell-surface expansion in yeast is driven by polarized exocytosis, a process that involves transport of post-Golgi secretory vesicles along the actin cytoskeleton toward the cell surface. These vesicles dock with components of the exocytic machinery localized to sites of polarized growth and ultimately fuse with the plasma membrane. A multiprotein complex called the exocyst, which is involved in vesicle targeting and docking at the plasma membrane, assembles at the exocytosis site in response to the arrival of vesicles. Sec3 is a component of the exocyst with the unusual property of localizing to the site of exocytosis independently of active secretion, the actin cytoskeleton, or other components of the exocyst. Therefore, Sec3 is thought to be a spatial landmark for polarized secretion (Finger et al. 1998).
Rho1, Rho3, and Cdc42 have been proposed to control spatial regulation of the exocyst complex because Sec3 associates with these GTPases (Guo et al. 2001; Zhang et al. 2001, 2008). Sec3 also binds directly to PIP2, and both Rho and PIP2 binding are required for its polarized recruitment (Zhang et al. 2008). Moreover, Rho1 and Cdc42 compete in vitro for a direct interaction with the N-terminal domain of Sec3, and an N-terminally truncated form of Sec3 fails to localize in a polarized manner, suggesting that this region of Sec3 may receive targeting information from both Rho1 and Cdc42 (Guo et al. 2001; Zhang et al. 2001). Therefore, Rho1 and Cdc42 appear to collaborate in the process of vesicle delivery to the plasma membrane through control of both actin cytoskeleton polarization (for vesicle transport) and vesicle docking through the exocyst.
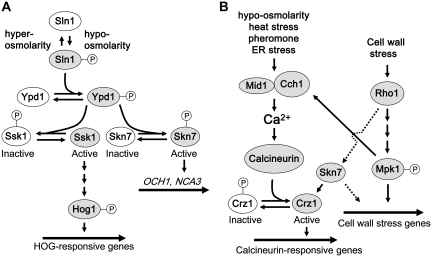
Skn7
Ssk1 and Skn7 are the only two yeast proteins related to bacterial response regulators of so-called two-component signal transduction pathways (Maeda et al. 1994; Ketela et al. 1998; Li et al. 1998). Both of these proteins are regulated by the high osmolarity glycerol (HOG) signaling pathway, which responds to changes in extracellular osmolarity (Figure 6A). Like many bacterial response regulators, Skn7 is a transcription factor. However, Ssk1 activates the MAPK cascade of the HOG pathway. In response to changes in osmotic conditions, the HOG pathway regulates Ssk1 and Skn7 in opposition through a phosphorelay switch composed of the cell-surface sensor kinase Sln1 and the histidine phosphotransfer protein Ypd1 (Ota and Varshavsky 1993; Maeda et al. 1994; Posas et al. 1996, 1998; Ketela et al. 1998; Li et al. 1998). Ypd1 transfers its phosphate to aspartyl residues within the receiver domains of both Ssk1 and Skn7, which activates Skn7 and inactivates Ssk1. Thus, under hyper-osmotic conditions, the HOG MAPK pathway is active, whereas under hypo-osmotic conditions, the Skn7 transcription factor is active.
Figure 6 .
Control of the Skn7 transcription factor. (A) The Sln1 branch of the HOG pathway. The Sln1 osmosensor controls a phosphorelay pathway, which activates Skn7 under hypo-osmotic conditions to support cell wall biosynthesis and the Hog1 MAPK cascade under hyper-osmotic conditions. Active components are shaded. (B) Coordinate activation of CWI signaling, Ca2+ signaling, and Skn7. Rho1 may independently activate the Skn7 transcription factor (dashed line), which induces stabilization of the Crz1 transcripion factor, and may have additional effects on cell wall stress-induced transcription. The Mid1-Cch1 Ca2+ channel is activated by many of the same stresses that activate CWI signaling. Additionally, activation of Mpk1 results in stimulation of the Mid1-Cch1 Ca2+ channel, at least in response to ER stress, which activates the Ca2+-dependent protein phosphatase calcineurin. The Crz1 transcription factor is activated through dephosphorylation by calcineurin, which allows its entry to the nucleus. This interplay may coordinate control of gene expression by Ca2+ signaling and cell wall stress signaling.
Although Ssk1 appears to be entirely under the control of Sln1, Skn7 activity is only partially regulated by this sensor and may also be under the control of Rho1 (Alberts et al. 1998; Ketela et al. 1999). Skn7 associates with GTP-bound Rho1 through an HR1 domain that resides between the DNA-binding domain and the response regulator domain (Alberts et al. 1998). A second observation implicating CWI signaling in Skn7 activity is that overexpression of the Mid2 CWI sensor stimulates a Skn7-LexA-dependent transcriptional reporter (Ketela et al. 1999). However, the significance of these interactions has not been tested under cell wall stress conditions.
Several lines of evidence support a role for Skn7 in cell wall biogenesis, consistent with its activation by the HOG pathway under hypo-osmotic conditions. SKN7 (Suppressor of kre nine 7) was isolated initially as a dosage suppressor of the growth defect of a kre9 mutant (Brown et al. 1993), which is deficient in β-1,6-glucan synthesis. Additionally, overexpression of SKN7 suppresses the growth defect of a pkc1Δ mutant in the absence of osmotic support, and a pkc1Δ mutation exhibits synthetic lethality with a skn7Δ mutation (Brown et al. 1994). Hypo-osmotic activation of Skn7 through the Sln1 pathway results in the transcriptional activation of at least two genes—OCH1 (Li et al. 2002), which encodes an α-1,6-mannosyltransferase involved in maturation of N-glycoproteins (Nakayama et al. 1992), and NCA3 (Shankarnarayan et al. 2008), which encodes a cell wall protein that plays a role in septation (Mouassite et al. 2000).
However, transcriptional output from the Sln1-Skn7 pathway is not activated by other cell wall stresses that stimulate CWI signaling [except zymolyase treatment (Shankarnarayan et al. 2008)], suggesting that Sln1-Skn7 signaling serves a cell wall-related function that is separate from CWI signaling.
Nevertheless, there is evidence to suggest that Skn7 makes an additional contribution to the maintenance of cell wall integrity that is independent of phosphotransfer from Sln1-Ypd1 (Figure 6B). Suppression of the pkc1Δ growth defect by increased Skn7 expression does not require aspartyl phosphorylation (Brown et al. 1994). Similarly, Skn7 binds to and stabilizes the Ca2+/calcineurin-activated transcription factor Crz1 independently of aspartyl phosphorylation (Williams and Cyert 2001). Crz1 is known to contribute to the maintenance of cell wall integrity (Garrett-Engele et al. 1995) at least through the induced expression of FKS2 and other cell wall-related genes (Stathopoulos and Cyert 1997; Zhao et al. 1998; García et al. 2004). Mutations in the Skn7 HR1 domain block its effect on Crz1, suggesting that this function is driven by Rho1 rather than by Sln1 activity (Williams and Cyert 2001). Intriguingly, Mpk1 can also activate the calcineurin-mediated signaling pathway that stimulates Crz1 activity (Bonilla and Cunningham 2003), suggesting a complex interrelationship among CWI, Skn7, and Ca2+ signaling (Figure 6B). Identification of Skn7 target genes regulated by CWI signaling should help to elucidate the CWI-specific role of this transcription factor.
Activation of CWI Signaling
CWI signaling is induced in response to a variety of cell wall stresses. Each of these cell wall stresses will be treated individually below. Additionally, CWI signaling is stimulated by oxidative stress, high and low pH, and DNA-damaging agents, as measured by Mpk1 activation. However, these stresses will not be addressed here, in part because little is understood about either the mechanisms by which these stresses activate signaling or the nature of the responses mediated by Mpk1.
Detection of CWI pathway signaling
Signaling through the CWI pathway is typically monitored by any of four approaches. Two of these approaches measure the activation state of Mpk1. The protein kinase activity of epitope-tagged Mpk1 can be measured in an immune complex using bovine myelin basic protein as a substrate (Kamada et al. 1995; Zarzov et al. 1996). Alternatively, because Mpk1 is activated by phosphorylation of neighboring threonyl and tyrosyl residues within its activation loop, residues that are analogous to Thr202/Tyr204 of mammalian p44/p42 MAPK (Erk1/2), commercially available antibodies against phospho-p42/p44 are quite effective at detecting activated Mpk1 (De Nobel et al. 2000; Martín et al. 2000). A less direct, but simpler method of measuring sustained signaling through the CWI pathway employs lacZ reporters driven by Rlm1- and Swi4/6-responsive promoters (Jung et al. 2002; Kim et al. 2008; Kim and Levin 2010). Finally, reagents designed to recognize specifically GTP-bound Rho1 can be used both to measure the activation state of the pathway and to determine subcellular sites of activity. These reagents include antibodies raised to active Rho1 (Abe et al. 2003; Yoshida et al. 2006) and a GST fusion to the Rho1-binding domain of Pkc1 (Kono et al. 2008).
Heat stress
CWI signaling is activated persistently in response to growth at elevated temperatures (e.g., 37°–39°) (Kamada et al. 1995; Zarzov et al. 1996), consistent with the finding that null mutants in many of the pathway components display cell lysis defects only when cultivated at high temperature. However, other reports indicate that the Mpk1 activation state is restored to normal after ∼2 hr at elevated temperature (Schmelzle et al. 2002; Guo et al. 2009). The reason for this discrepancy is not clear. Interestingly, Mpk1 is not activated immediately upon heat shock. Activation is detectable after ∼20 min and peaks after 30 min (Kamada et al. 1995), suggesting that the signaling pathway is not sensing temperature change directly, but is detecting some secondary effect of exposure to high temperature. One response to thermal stress is the accumulation of cytoplasmic trehalose (Neves and Francois 1992; De Virgilio et al. 1994), which reaches levels of >0.5 M for the purpose of protecting proteins from thermal denaturation and aggregation (Hottiger et al. 1994; Singer and Lindquist 1998). Such a striking increase in intracellular osmolarity would impact turgor pressure. Indeed, preventing trehalose production in response to heat stress greatly diminishes CWI signaling (Mensonides et al. 2005). The CWI sensors are important for thermal activation of Mpk1, supporting the conclusion that this stress is ultimately transmitted to the cell surface (Gray et al. 1997; Ketela et al. 1999; Rajavel et al. 1999; Martín et al. 2000). Another response to heat stress that impacts CWI signaling is the transient production of PIP2 (Desrivieres et al. 1998; Audhya and Emr 2002), which, as noted above, activates Rho1.
Hypo-osmotic shock
Hypo-osmotic shock induces a rapid, but transient, activation of CWI signaling (Davenport et al. 1995; Kamada et al. 1995). Mpk1 is activated within 15 sec of an osmotic downshift, but basal activity is restored after ∼30 min. The Sln1 cell-surface osmosensor is also stimulated by hypo-osmotic shock, which results in activation of the Skn7 transcription factor in support of cell wall biogenesis. By contrast, the Hog1 MAPK is activated in response to hyper-osmotic shift (a result of Sln1 inactivation). However, it is interesting that hyper-osmotic shock also induces a delayed and transient activation of CWI signaling (45–60 min post shock), evidently a secondary consequence of the increased intracellular glycerol generated by the HOG pathway (García-Rodríguez et al. 2005). In addition to these pathways, the Cch1/Mid1 Ca2+ channel is activated by hypo-osmotic shock (Batiza et al. 1996), which activates calcineurin in support of cell wall biosynthesis (Garrett-Engele et al. 1995).
Pheromone-induced morphogenesis
Activation of the mating pheromone response pathway induces cell cycle arrest in G1 phase followed by the formation of a mating projection toward the source of pheromone (Elion 2000). Projection formation constitutes a cell wall stress because it requires polarization of the actin cytoskeleton and the secretory pathway to mobilize remodeling of the cell surface. Consistent with this, mating pheromone stimulates CWI signaling at a time that is coincident with the onset of projection formation (Errede et al. 1995; Zarzov et al. 1996; Buehrer and Errede 1997). Indeed, mutants defective in CWI signaling undergo cell lysis during pheromone-induced morphogenesis (Errede et al. 1995), reflecting the major reorganization of the cell wall associated with projection formation. Similar to CWI signaling, calcineurin is also activated as a late response to pheromone treatment and is required for survival (Withee et al. 1997).
Both Rho1 and Pkc1 localize to projection tips of cells treated with pheromone (Ayscough and Drubin 2003; Bar et al. 2003). The Gβγ complex of the pheromone response pathway, which provides the positional clues for polarity establishment, recruits Rho1 to the site of polarized growth (Bar et al. 2003). Precedents for the association of mammalian RhoA with Gβ subunits reveal that this interaction is highly conserved (Harhammer et al. 1996; Alberts et al. 1998). As noted above, the Mid2 sensor is also recruited to mating projections (Hutzler et al. 2008). The mechanism controlling its redistribution is likely to be polarized secretion, but the requirements have not been explored.
Cell wall stress agents and cell wall biogenesis mutations
Chemical agents that induce cell wall stress—such as the chitin antagonists Calcofluor white and Congo red, enchinocandin inhibitors of GS, the cell wall lytic enzyme zymolyase, and caffeine—activate CWI signaling (Kopecka and Gabriel 1992; Ketela et al. 1999; De Nobel et al. 2000, Martín et al. 2000; Jung et al. 2002; Reinoso-Martín et al. 2003; García et al. 2004, 2009; Kuranda et al. 2006; Bermejo et al. 2008). Mutations that impair cell wall biosynthesis similarly activate CWI signaling (De Nobel et al. 2000; Terashima et al. 2000; Bulik et al. 2003; Lagorce et al. 2003). With two notable exceptions—zymolyase and caffeine—the specific nature of the cell wall stress seems to be unimportant with regard to the activation route, suggesting that any condition that interferes with maintenance of the cell wall integrity is sufficient to trigger signaling of a subset of the cell-surface sensors.
Activation of CWI signaling in response to treatment with zymolyase, an enzymatic cell wall antagonist derived from a yeast-digesting bacterium that targets both β-1,3,-glucans and cell wall proteins, is largely independent of Wsc1 and Mid2 as well as all three of the Rho1-GEFs (Bermejo et al. 2008). Instead, activation of CWI signaling requires the components of the Sho1 branch of the HOG pathway, including Hog1, suggesting sequential activation of the two pathways by zymolyase (Bermejo et al. 2008, 2010; García et al. 2009). It is conceivable that zymolyase treatment causes proteolytic destruction of the cell wall stress sensors, necessitating the evolution of an alternative activation route for CWI signaling in response to this stress. Sho1 is a multi-pass plasma membrane protein with cytoplasmic N and C termini that exposes very little sequence to the cell surface (Maeda et al. 1995; Tatebayashi et al. 2007) and may therefore be resistant to proteolytic degradation. This unusual activation route for CWI signaling requires Pkc1 and the MAPK cascade (Bermejo et al. 2008), raising the interesting question as to the point of interface between Hog1 and the CWI pathway.
Caffeine is also an unusual cell wall stress agent. The mechanism by which it induces wall stress is not understood, but genome profiling suggests that the primary target may be the TORC1 protein kinase complex (Lum et al. 2004; Kuranda et al. 2006). However, it appears likely that additional targets are involved. For example, although caffeine treatment activates Mpk1, this agent induced additional phosphorylation of the MAPK through the DNA damage checkpoint kinases, Mec1/Tel1 and Rad53 (Truman et al. 2009). The effect of these additional modifications is to prevent Mpk1 from associating with Swi4, thus blocking this part of the transcriptional program. Thus, it appears that caffeine in some way also targets DNA metabolism.
Actin cytoskeleton depolarization
When cells are subjected to heat stress, the actin cytoskeleton becomes redistributed from a polarized state to a more uniform localization around the cell periphery (Lillie and Brown 1994; Desrivieres et al. 1998). The mechanism that drives actin delocalization in response to this cell wall stress is not understood, but the process does not require CWI signaling (Levin 2005). Instead, the components of the CWI pathway also become delocalized in what has been proposed as a mechanism to repair cell wall damage that might arise at any point on the cell surface (Delley and Hall 1999; Andrews and Stark 2000). Intriguingly, although delocalization of Rho1 and Fks1 from the bud tip in response to heat stress is independent of Mpk1, delocalization of Rom2 requires the MAPK (Guo et al. 2009). This, together with the observation that Rom2 is phosphorylated in an Mpk1-dependent manner in response to cell wall stress, prompted the suggestion that Mpk1 engages in a negative feedback loop that downregulates pathway signaling by depriving Rho1 of its GEF (Guo et al. 2009).
The CWI pathway, including Mpk1, is required for repolarization of the actin cytoskeleton after cell wall stress (Delley and Hall 1999). Although the mechanism by which Mpk1 drives actin repolarization is not yet understood, one possibility involves the feedback regulation mentioned above. Support for this notion comes from the observation that the requirement for Mpk1 in actin repolarization can be overcome by artificial downregulation of Pkc1 (Guo et al. 2009), suggesting that signaling through the upper part of the pathway must be terminated to re-establish actin polarity.
Depolarization of the actin cytoskeleton by treatment with the actin antagonist latrunculin-B activates Mpk1 (Harrison et al. 2001). Similarly, rapamycin treatment, which depolarizes the actin cytoskeleton by inhibiting the shared function of the Tor1/2 protein kinases, also induces Mpk1 activation (Krause and Gray 2002; Torres et al. 2002). Although Rho1 and Pkc1 are required for Mpk1 activation in response to actin depolarization, there is disagreement as to the requirement for the cell-surface sensors. However, Mpk1 activation in response to actin depolarization was blocked by the presence of osmotic support (Harrison et al. 2001; Torres et al. 2002), suggesting that the CWI pathway senses actin depolarization as a cell wall stress. This may arise as a consequence of disrupting polarized secretion.
ER stress
There is an intricate interrelationship between CWI signaling and ER stress. Several groups have shown that ER stress induced by tunicamycin, 2-deoxyglucose, or dithiothreitol activates CWI signaling and that Mpk1 is an important determinant of ER stress survival (Bonilla and Cunningham 2003; Chen et al. 2005; Babour et al. 2010). Genetic analyses revealed that ER stress activation of CWI signaling is independent of the unfolded protein response (UPR), the classic ER stress response pathway controlled by Ire1 and Hac1 (Chen et al. 2005). Mpk1 activation in response to tunicamycin treatment appears to be triggered principally by the Wsc1 sensor (Babour et al. 2010), but in some manner is also dependent on the Hos2/Set3 histone deacetylase complex (Cohen et al. 2008). The mechanism by which the deacetylase complex acts to control CWI pathway activation awaits elaboration. Activation of CWI signaling by ER stress drives the Rlm1-mediated transcriptional program (Cohen et al. 2008; Babour et al. 2010), indicating that response to this stress involves enhanced cell wall biogenesis.
The Cch1-Mid1 plasma membrane Ca2+ channel is also activated in response to ER stress, which causes elevation of cytosolic Ca2+ and activation of calcineurin (Bonilla et al. 2002) (Figure 6B). The proximal activator of this channel is thought to be plasma membrane stretch (Kanzaki et al. 1999), a condition that also activates CWI signaling (Kamada et al. 1995). Although activation of Cch1-Mid1 in response to ER stress is dependent on Mpk1 (Bonilla and Cunningham 2003), it is not clear if this control is direct or indirect. Nor is it known if Mpk1 activates this Cch1-Mid1 in response to all cell wall stresses. Nevertheless, it is suggestive that, in addition to activation by ER stress, Cch1-Mid1 and calcineurin are activated by pheromone treatment (Cyert and Thorner 1992; Foor et al. 1992; Moser et al. 1996), heat shock (Zhao et al. 1998), and hypo-osmotic shock (Batiza et al. 1996), all conditions that activate CWI signaling. On the other hand, calcineurin activation in response to heat shock is independent of Mpk1 (Zhao et al. 1998). Clearly, the interplay between these signaling pathways requires further dissection.
Perhaps surprisingly, activation of CWI signaling by cell wall stress also activates the UPR (Scrimale et al. 2009), revealing the existence of cross-regulation between these two systems. Krysan (2009) has suggested that this relationship might be explained in the following way. Activation of CWI signaling by cell wall stress increases the total protein flux through the ER en route to the cell surface and therefore may require increased ER capacity. Conversely, ER stress may result in the delivery of misfolded proteins to the cell surface, which consequently induces cell wall stress. Intriguingly, UPR activation by cell wall stress was shown to require Swi6, but not any of its known partners, suggesting the possibility of a nontranscriptional role for this protein. The mechanism by which the CWI signaling pathway interfaces with the Ire1 protein kinase in the ER membrane promises to be fascinating.
Turgor pressure and plasma membrane stretch
There is strong evidence that plasma membrane stretch is the principal underlying physical stress that activates CWI signaling. First, chlorpromazine, an amphipathic molecule that causes membrane stretch by asymmetric insertion into the plasma membrane, is a potent activator of Mpk1 (Kamada et al. 1995). Second, mutants that experience increased turgor pressure induced by elevated intracellular concentrations of potassium (ppz1/2Δ) or glycerol (rgc1/2Δ) display constitutively high Mpk1 activity (Merchan et al. 2004; Beese et al. 2009). Additionally, as noted above, heat stress results in elevated turgor pressure through the accumulation of trehalose. Finally, increased extracellular osmolarity, which blocks outward plasma membrane stretch by neutralizing turgor pressure, prevents activation of CWI signaling by various cell wall stressors (Kamada et al. 1995; De Nobel et al. 2000; Harrison et al. 2001; Torres et al. 2002; Mensonides et al. 2005). During periods of polarized cell growth, cell wall expansion at bud tips and mating projections may be a natural source of plasma membrane stretch. Transient weakness during cell wall remodeling may allow the plasma membrane to stretch against it.
Downregulation of signaling: MAP kinase phosphatases
Mpk1 is downregulated by four protein phosphatases: the Ptp2 and Ptp3 tyrosine-specific phosphatases and the dual-specificity (Tyr and Ser/Thr) paralogs Sdp1 and Msg5 (reviewed in Martín et al. 2005). Among these, Sdp1 is the only one to target Mpk1 specifically (Collister et al. 2002). Overexpression of SDP1 suppresses the growth defect of cells expressing constitutive MKK1 (Hahn and Thiele 2002). An sdp1Δ mutant displays a normal level of Mpk1 activity under nonstress conditions but enhanced Mpk1 activation in response to cell wall stress, suggesting that its role is to down-regulate Mpk1 after stimulation to re-establish the resting state. Expression of SDP1 is under the control of the Msn2/4 stress-activated transcription factors, but not of Rlm1. Thus, although Sdp1 may be the only protein phosphatase dedicated solely to the regulation of Mpk1, its regulation appears to be independent of Mpk1.
In contrast to Sdp1, the function of Msg5 with respect to CWI signaling appears to be to maintain a low basal activity of Mpk1 in the absence of stress (Marín et al. 2009). Like SDP1, overexpression of MSG5 suppresses the constitutive MKK1 growth defect (Watanabe et al. 1995). However, deletion of MSG5 results in increased basal phosphorylation of Mpk1 (Martín et al. 2000; Marín et al. 2009). Intriguingly, this increased Mpk1 phosphorylation is not accompanied by an increase in its protein kinase activity (Marín et al. 2009), suggesting that phosphorylation is necessary but not sufficient for Mpk1 protein kinase activity. The catalytic domain of Mpk1 associates with the N-terminal regulatory domain of Msg5 in vivo and in vitro (Andersson et al. 2004; Flandez et al. 2004). These proteins engage in reciprocal regulation in which Mpk1 phosphorylates Msg5 in response to CWI pathway activation, which results in decreased affinity between the two proteins. This appears to constitute a positive feedback loop for prolonged activation of Mpk1, which has been observed in response to chronic cell wall stress (Kamada et al. 1995; Beese et al. 2009).
The Ptp2 and Ptp3 tyrosine phosphatases, which dephosphorylate Mpk1 in vivo and in vitro, also act on the Fus3 and Hog1 MAPKs (Mattison et al. 1999). Of these, Ptp2 appears to be more effective against Mpk1 than is Ptp3. Additionally, expression of PTP2, but not of PTP3, is induced in response to heat shock in an Rlm1-dependent manner (Hahn and Thiele 2002), suggesting that activation of Mpk1 establishes a negative feedback loop for its inactivation by Ptp2. The positive regulation of PTP2 expression by Mpk1 is in contrast to the negative regulation of Msg5 activity by this MAPK. Perhaps like Sdp1, Ptp2 and Ptp3 function to re-establish the resting state of Mpk1 after stress-induced activation.
CWI Transcriptional Program
Rlm1 is a target of Mpk1
The Rlm1 (resistant to the lethality of constitutive Mkk1) transcription factor is responsible for the bulk of the CWI signaling transcriptional program. As its name suggests, RLM1 was identified in a genetic screen for mutants that could survive the growth inhibition caused by a constitutive form of Mkk1 (Watanabe et al. 1995; Yashar et al. 1995). Rlm1 possesses an N-terminal DNA-binding domain related to the MADS (MCM1, agamous deficiens, serum response factor) box family of transcriptional regulators. This factor is most closely related to mammalian MEF2, sharing the same in vitro binding specificity [CTA(T/A)4TAG] (Dodou and Treisman 1997). However, in vivo studies revealed that the binding specificity is relaxed at the terminal G/C base pairs (Jung and Levin 1999; Jung et al. 2002). Rlm1 is constitutively nuclear where Mpk1 activates it by phosphorylation at two residues within its transcriptional activation domain (Ser427 and Thr439) (Watanabe et al. 1997; Jung et al. 2002). A D motif in the Rlm1 activation domain is essential for activation by Mpk1 and is conserved in MEF2 (Jung et al. 2002).
Multiple genome-wide surveys of genes regulated through the CWI pathway have been reported. One report identified changes in gene expression in response to constitutive activation of Mkk1 (Jung and Levin 1999). This study revealed that Rlm1 regulates the expression of at least 25 genes, most of which encode cell wall proteins or have been otherwise implicated in cell wall biogenesis. All of these genes were shown to be regulated in response to cell wall stress under the control of Rlm1. A similar global gene expression study reported the use of constitutive forms of Pkc1 and Rho1 to identify an overlapping set of CWI signaling-regulated genes (Roberts et al. 2000). In this study, RLM1 was identified among the induced genes, suggesting the existence of an autoregulatory circuit for amplification of the stress response. Consistent with this, the RLM1 gene was also induced in response to cell wall stress associated with an fks1Δ mutation (Bulik et al. 2003).
A genome-wide analysis of genes induced by mutations that affect the cell wall (i.e., fks1Δ, gas1Δ, kre6Δ, mnn9Δ, and knr4Δ) identified a group of ∼80 upregulated genes common to these cell wall-stressing mutations (Lagorce et al. 2003). In silico analysis of the regulatory regions of these genes revealed that many possess sites for Rlm1, Swi4, and Crz1, as well as for Msn2/4. Similar analyses using Congo red, zymolyase, or Calcoflour white to induce cell wall damage identified an overlapping set of genes that implicated the same group of transcription factors (Boorsma et al. 2004; García et al. 2004). These results are consistent with the co-activation of CWI signaling and Ca2+ signaling as well as with general stress signaling under these conditions. Zymolyase additionally induced a set of genes under the control of the HOG pathway. This is now understood to be the consequence of sequential activation of the HOG and CWI pathways by this stress (Bermejo et al. 2008; García et al. 2009).
One intriguing transcriptional target of Rlm1 is MLP1 (Mpk1-like protein kinase) (Jung and Levin 1999), which encodes a paralog of Mpk1 (Watanabe et al. 1997). Its function remained obscure until recently due largely to an absence of phenotypic defects associated with its loss. Mlp1 is lacking several catalytic domain residues recognized to be critical for protein kinase activity (Hanks and Hunter 1995). Additionally, the Thr residue within the dual phosphorylation site of the activation loop of MAPKs (Thr-X-Tyr) is a Lys in Mlp1. Although Mlp1 protein levels increase by ∼100-fold in response to cell wall stress, efforts to detect protein kinase activity have been unsuccessful (Levin 2005). It is now clear that Mlp1 is a pseudokinase that is redundant with Mpk1 specifically for its noncatalytic transcriptional functions (see the section Noncatalytic transcriptional functions of Mpk1 below). Because the MLP1 gene is induced by Rlm1 in response to Mpk1 activity, this sets up an interesting feedback loop, which specifically augments the noncatalytic portion of the transcriptional program (Figure 7).
Figure 7 .
The CWI pathway transcriptional program. The majority of genes regulated by CWI signaling are under the control of the Rlm1 transcription factor, which is phosphorylated and activated by Mpk1. Among these genes is MLP1, which encodes a pseudokinase paralog of Mpk1. Using a mechanism that is independent of protein kinase catalytic activity, Mpk1, together with Mlp1, drive expression of a subset of cell wall stress-induced genes through the Swi4/Swi6 transcription factor (including FKS2).
Noncatalytic transcriptional functions of Mpk1
SBF (Swi4/Swi6) is an Mpk1/Mlp1 target:
A second transcription factor implicated in CWI signaling is SBF, a dimeric regulator of G1-specific transcription composed of Swi4 and Swi6 (reviewed in Breeden 2003). Swi4 is the sequence-specific DNA-binding subunit that recognizes a seven-nucleotide sequence called an SCB (CA/GCGAAA) (Taylor et al. 2000), but Swi6 is required for binding to cell cycle-regulated promoters (Andrews and Moore 1992; Sidorova and Breeden 1993; Baetz and Andrews 1999). Swi6 allows Swi4 to bind DNA by relieving an auto-inhibitory intramolecular association of the Swi4 C terminus with its DNA-binding domain. Additionally, Swi6 is the transcriptional activation component of SBF (Sedgwick et al. 1998).
Genetic and biochemical evidence suggested many years ago that SBF also participates in CWI signaling as a target of Mpk1. First, the cell lysis defect of an mpk1Δ mutant is suppressed by overexpression of Swi4 (Madden et al. 1997). Second, both swi4Δ and swi6Δ mutants are hypersensitive to Calcofluor white, supporting a role for SBF in cell wall biogenesis (Igual et al. 1996). Third, Mpk1 associates with dimeric SBF in vivo, as judged by coprecipitation experiments (Madden et al. 1997), and with Swi4 (but not Swi6) in vitro (Baetz et al. 2001). Fourth, Swi6 is phosphorylated in vivo and in vitro by Mpk1 in response to cell wall stress (Madden et al. 1997; Baetz et al. 2001). The full significance of these findings remained shrouded until recent reports established the nature of the relationship between Mpk1 and SBF.
It is now clear that SBF drives gene expression in response to cell wall stress in a manner that is independent of its role in G1-specific transcription (Kim et al. 2008; Truman et al. 2009; Kim and Levin 2010). Mpk1 and its pseudokinase paralog, Mlp1, which is also activated by the MEKs of the CWI signaling pathway, use a noncatalytic mechanism to activate SBF for transcription of a subset of cell wall stress-activated genes. This mechanism nevertheless requires Mpk1, or Mlp1, to be in the active (phosphorylated) conformation. A mutation in the ATP-binding site of Mpk1 (mpk1-K54R) did not affect induced transcription of SBF-dependent genes, but a mutation that blocked dual phosphorylation of its activation loop (mpk1-TA/YF) abolished transcription (Kim et al. 2008; Kim and Levin 2010). As noted above, Mlp1 does not possess the dual phosphorylation motif of MAPKs, but is activated by single phosphorylation of Tyr192 (Kim et al. 2008). Although not always explicitly noted in the sections below, Mlp1 can carry out all of the noncatalytic functions ascribed to Mpk1. Genes under the control of this pathway branch include FKS2, CHA1, YLR042c, and YKR013w, although this is likely not a complete list (Kim and Levin 2010).
Chromatin immunoprecipitation (ChIP) experiments revealed that activated Mpk1, or Mlp1 forms a complex with Swi4 that associates with SBF-binding sites in the promoters of cell wall stress target genes independently of Swi6 (Kim et al. 2008) (Figure 8). In this context, Mpk1 relieves the auto-inhibitory Swi4 interaction by binding to a D motif on Swi4 that is adjacent to the C-terminal Swi6-binding site (Truman et al. 2009). Although Mpk1 substitutes for Swi6 in allowing Swi4 to bind DNA at the promoters of cell wall stress-activated genes, Swi6 must be recruited to the Mpk1–Swi4 complex for transcriptional activation to ensue. Swi6 is presumed to bind to the same site in the Swi4 C terminus as it does in the control of cell cycle-regulated genes, thereby forming an Mpk1–Swi4–Swi6 trimeric complex on the promoter. This complex, and specifically Swi6, is required for recruitment of the RNA Pol II to these promoters (Kim and Levin 2011).
Figure 8 .
Model for Mpk1-driven FKS2 transcription. (A) Under non-inducing conditions, weak transcription initiation is attenuated by association of the Sen1–Nrd1–Nab3 termination complex to the elongating RNA Pol II. (B) Under inducing conditions, Mpk1 and Mlp1 (not shown) are activated in response to phosphorylation by Mkk1/2. (C) The active MAPK or pseudokinase binds to Swi4. (D) These dimers are competent to bind the FKS2 promoter. (E) Swi6 is recruited to form an Mpk1–Swi4–Swi6 complex on the FKS2 promoter. (F) RNA Pol II and the Paf1C are recruited to the promoter in a Swi6-dependent manner, completing formation of the initiation complex. (G) Mpk1 associates with Paf1, likely by hand off from Swi4. (H) Mpk1 overcomes transcriptional attenuation by blocking recruitment of the termination complex. SCB, Swi4-binding site.
Paf1 complex is an Mpk1/Mlp1 target:
Remarkably, in addition to their role in recruiting Swi4 and Swi6 to promoters, Mpk1 and Mlp1 serve a second noncatalytic function in the expression of SBF-activated cell wall stress-induced genes. ChIP experiments revealed that the MAPK and pseudokinase move from the promoter of regulated genes to the coding region, leaving Swi4 and Swi6 behind (Figure 8). This results from an apparent “hand off” of Mpk1 from Swi4 to the RNA polymerase II (Pol II)-associated complex (Paf1C) on the promoter (Kim and Levin 2011). This complex, originally identified in yeast, is composed of five subunits (Paf1, Cdc73, Rtf1, Ctr9, and Leo1) (Mueller et al. 2004). As its name implies, the Paf1C associates directly with RNA Pol II. It has been implicated in transcription start site selection (Stolinski et al. 1997), elongation (Costa and Arndt 2000; Betz et al. 2002; Rondón et al. 2004; J. Kim et al. 2010), and as a platform for the recruitment of histone methyltransferases (Krogan et al. 2003; Wood et al. 2003) and 3′-end processing factors to the elongation complex (Mueller et al. 2004; Penheiter et al. 2005; Sheldon et al. 2005).
Global gene expression analysis suggested that the Paf1C is required for the expression of <5% of yeast genes (Penheiter et al. 2005), including many involved in progression of the cell cycle (Koch et al. 1999; Porter et al. 2002) and some in cell wall biosynthesis (Chang et al. 1999). Consistent with the observation that the Paf1C is important for the expression of only a small subset of RNA Pol II-transcribed genes, the yeast Paf1C components are not essential, but their loss results in hyper-sensitivity to a variety of stresses (Betz et al. 2002), implicating this complex in the expression of stress-responsive genes. Among the conditions to which mutants in the Paf1C are hyper-sensitive is cell wall stress, prompting the suggestion by Jaehning and co-workers that the CWI pathway plays a regulatory role in Paf1C-mediated transcription (Chang et al. 1999).
A role for Mpk1 in the regulation of transcription elongation was revealed with the discovery that Mpk1 makes direct contact with the Paf1 subunit of the PafC (Kim and Levin 2011). Here, as in the case of its interaction with Swi4, the association is between the docking site of the active MAPK and a D motif in the target. A mutation in the Paf1 D motif (Paf1-4A) that specifically ablates the Mpk1 interaction blocks transcription elongation of a cell wall stress-activated target gene (FKS2), but not other Paf1C-dependent genes (e.g., CLN2). ChIP experiments demonstrated that, although the initiation complex (including Mpk1/Swi4/Swi6 and RNA Pol II/Paf1C) is assembled on the promoter in this paf1 mutant, the elongation complex does not proceed to the coding region of the gene. The phenotypic consequence of the paf1-4A mutation is specific hyper-sensitivity to cell wall stress agents, but not to other stresses to which a paf1Δ mutant is hyper-sensitive. These results revealed that the Paf1C is a physiologically important target of CWI signaling.
Mechanism of control of transcription elongation by Mpk1:
Genetic and biochemical analyses revealed that the Mpk1 interaction with Paf1 allows transcription elongation of the FKS2 gene by preventing premature termination by the Sen1–Nrd1–Nab3 complex (Kim and Levin 2011). This complex, which is recruited widely to Pol II promoters of all types (J. Kim et al. 2010), is used specifically for termination of short, nonpolyadenylated Pol II transcripts, including small nucleolar RNAs, cryptic unstable transcripts, and a few specialized sites of mRNA attenuation (Ursic et al. 1997; Rasmussen and Culbertson 1998; Steinmetz et al. 2001, 2006; Wyers et al. 2005; Arigo et al. 2006). Termination by this complex is directed by recognition of specific sequences in the nascent RNA by Nrd1 and/or Nab3 (Steinmetz et al. 2001; Morlando et al. 2002; Carroll et al. 2004). A single Nab3-binding site within the FKS2 promoter-proximal region was shown to be responsible for transcriptional attenuation under non-inducing conditions, which appears to minimize FKS2 expression in the absence of cell wall stress (Kim and Levin 2011). Attenuation must therefore be relieved under inducing conditions, and Mpk1 serves double duty in this regard. Mpk1 is responsible for recruitment of Swi4/Swi6 (and Pol II with Paf1C) to the FKS2 promoter, and the subsequent Mpk1–Paf1 interaction blocks recruitment of the termination complex to the elongating polymerase. Thus, Mpk1 and Mlp1 serve two essential functions in the induced expression of FKS2: transcription initiation and antitermination. Figure 8 outlines the various steps in this noncatalytic pathway for transcriptional control by Mpk1/Mlp1. It is interesting to note that both of these noncatalytic functions of Mpk1 and Mlp1 are complemented by the human ERK5 MAPK and that the MAPK/Paf1 interaction is conserved between ERK5 and human Paf1 (Kim et al. 2008; Kim and Levin 2011).
With regard to attenuation, if Mpk1 functions simply as a physical impediment to the recruitment of the termination complex, this would suggest a generalizable model in which other transcription factors could use the Paf1C as a platform for the same purpose. Indeed, examination of a yeast 3′ SAGE database (Neil et al. 2009) reveals that short sense transcripts are produced across the promoter regions of ∼10% of protein-coding genes (Kim and Levin 2011), suggesting that transcriptional attenuation may be a widespread phenomenon.
Control of Swi6 nucleocytoplasmic shuttling by Mpk1
Swi6 is phosphorylated in vivo and in vitro by heat stress-activated Mpk1 (Madden et al. 1997; Baetz et al. 2001). However, the results of Kim et al. (2008) made clear that whatever the function of this phosphorylation event, it was not required to drive transcription of Mpk1–Swi4–Swi6-dependent genes, which, as discussed above, is unimpaired by the absence of Mpk1 catalytic activity. In fact, FKS2 transcription induced by cell wall stress was slightly enhanced in the catalytically inactive mpk1-K54R mutant. This is explained by the observation that Mpk1 phosphorylation of Swi6 on Ser238 impairs the function of a neighboring nuclear localization signal (NLS), resulting in a net relocalization of Swi6 from the nucleus to the cytoplasm (K.-Y. Kim et al. 2010).
Interestingly, Swi6 also undergoes nucleocytoplasmic shuttling in a cell cycle-regulated manner (Sidorova et al. 1995). The Clb6/Cdc28 S-phase cell cycle kinase is responsible for phosphorylation of Swi6 on Ser160 (Geymonat et al. 2004). Swi6 resides predominantly in the cytoplasm from late G1 until late M phase, at which time it relocalizes to the nucleus in response to dephosphorylation at Ser160, where it remains for most of G1. Phosphorylation of Ser160 by Cdc28, like that of Ser238 by Mpk1, impairs the function of a neighboring NLS (Harreman et al. 2004). Thus, Swi6 possesses two distinct NLS signals, one regulated by Cdc28 periodically through the cell cycle and the other regulated by Mpk1 in response to cell wall stress. In this way, two disparate signals are integrated at a single endpoint, restricting nuclear access of Swi6 (Figure 9). The cell cycle-regulated NLS is recognized by the α/β-importin complex Srp1/Kap95 (Harreman et al. 2004), whereas the cell wall stress-regulated NLS is recognized by β-importin Kap120 (K.-Y. Kim et al. 2010).
Figure 9 .
Control of Swi6 nucleocytoplasmic shuttling. Swi6 possesses two nuclear localization (NLS) signals, NLS1 and NLS2, which are both regulated by phosphorylation. NLS1 is regulated through the cell cycle, and its function is blocked by phosphorylation on Ser160 by the S-phase CDK, Clb6/Cdc28, resulting in cytoplasmic localization of Swi6 at times other than G1. NLS2 is regulated by cell wall stress and its function is blocked by Mpk1 phosphorylation on Ser238. This feedback inhibitory event down-regulates cell wall stress-induced transcription after activation. Together, these two disparate signals converge to control Swi6 nuclear localization under different conditions. The indicated karyopherins recognize each NLS.
The apparently dual positive and negative regulation of Swi6 by Mpk1 suggests a temporal shift in which the initial stress signal mobilizes Swi4 and Swi6 for transcriptional activation. Thereafter, further transcriptional activation would be muted by the effect of Swi6 phosphorylation by Mpk1. This interpretation was supported by kinetic analysis of Swi6 phosphorylation on Ser238 and changes in its subcellular localization. Immediately upon activation of Mpk1 by heat stress, Swi6 is recruited to the nucleus (K.-Y. Kim et al. 2010). This recruitment can be mediated by either activated Mpk1 or Mlp1 and does not require catalytic activity, as described in Noncatalytic transcriptional functions of Mpk1. It is also dependent on the Mpk1–Swi4 interaction, suggesting that Swi6 is retained in the nucleus by the Mpk1 (or Mlp1)–Swi4 complex. Upon initial Mpk1 activation, very little of the Swi6 is phosphorylated on Ser238. However, as Swi6 becomes fully phosphorylated over the course of the next 40 min, it gradually returns to the cytoplasm in what appears to be a down-regulatory modulation.
An alternative interpretation is that Swi6 may have an extranuclear function during cell wall stress. This possibility is supported by the recent observation that Swi6, but not Swi4, is required for activation of the UPR at the ER in cells challenged by cell wall stress (Scrimale et al. 2009). Under this scenario, Mpk1 phosphorylation of Swi6 redirects it from its nuclear function to another function at the ER.
Chitin synthase 3: The chitin emergency response
Chitin is a linear polymer of β-1,4-N-acetylglucosamine (GlcNAc) produced from UDP-GlcNAc, which, under nonstress conditions, makes up ∼2% of the cell wall mass. In a variety of mutants that cause cell wall stress, chitin levels increase to as much as 20% of total wall polymers (Popolo et al. 1997; García-Rodriguez et al. 2000; Valdivieso et al. 2000). Additionally, the amount of chitin in the cell wall of mating projections is greatly increased (Schekman and Brawley 1979). In both cases, Chitin synthase 3 (Chs3) is responsible for the increased chitin deposition (Choi et al. 1994). This chitin is largely linked to β-1,6-glucan chains in the lateral wall, which may also be linked to GPI-CWPs (Cabib and Duran 2005) (Figure 1).
Under nonstress conditions, most of the Chs3 is maintained as an internal reservoir, called chitosomes, within the trans-Golgi network (TGN)/early endosome compartments. In response to cell wall stress, Chs3 rapidly exits the TGN and redistributes to the plasma membrane (Valdivia and Schekman 2003). Rapid mobilization of Chs3 to the cell surface was proposed to provide a mechanism for cell wall repair. Pkc1 function is required both for heat stress-induced Chs3 mobilization and for its phosphorylation in vivo (Valdivia and Schekman 2003), but it is not clear if Chs3 is a direct target of Pkc1. Moreover, the Chs3 phosphorylation level did not correlate with its stress-induced transport to the plasma membrane. Thus, the key regulatory function of Pkc1 with respect to Chs3 behavior remains unclear.
Another aspect of the chitin response to cell wall damage is the induced expression of GFA1. This gene encodes glucosamine-6-phosphate synthase, the first committed and rate-limiting step in the production of UDP-GlcNAc for biosynthesis of chitin (among other products) (Orlean 1997). Under conditions of cell wall stress (e.g., in gas1 or fks1 mutants) and in response to treatment with mating pheromone, GFA1 expression is induced severalfold (Bulik et al. 2003; Sobering et al. 2004). Ectopic overexpression of GFA1 is sufficient to drive an increase in chitin deposition in the lateral cell wall (Lagorce et al. 2002), indicating that this biosynthetic step is a critical determinant in the amount of chitin produced by Chs3. Induction of GFA1 in response to cell wall stress is under the control of the Rlm1 transcription factor (Levin 2005). Additionally, the CHS3 gene is induced by CWI signaling under the control of Rlm1 (Jung and Levin 1999). Thus, CWI signaling contributes to the chitin emergency response at least at three levels.
Cell Cycle Regulation of Cell Wall Construction
The deposition of cell wall material is tightly coordinated with cell cycle progression and is critical for proper abscission. Importantly, the pattern of wall deposition changes through the cell cycle. Young daughter cells grow isotropically, inserting new cell wall polymers into existing wall matrix. At the time of bud emergence, cell growth switches to focused (apical) growth at a single point on the cell surface directed by the polarisome. Bud emergence requires the weakening and remodeling of cell wall at the incipient bud site. As the bud enlarges, its growth switches gradually from an apical to an isotropic pattern. At the time of cytokinesis, the polarisome reassembles at the mother-bud neck to direct septum formation.
Cell cycle regulation of cell wall gene expression
Periodic expression of cell wall-related genes through the cell cycle occurs in waves that reflect specific needs for spatio-temporal insertion of wall material (Klis et al. 2006). For example, expression of some groups of genes encoding CWPs and cell wall biosynthetic proteins peaks in early G1 (Pir-CWPs, Pst1, Chs1, etc.) and others in late G1 (Crh1, Chs3, etc.), S phase (Dfg5), G2 phase (Cis3, Cwp1, Cwp2, Dcw1, Fks1, Gas1, etc.), or M phase (Gas5, Chs2). Because Mpk1 activity is also regulated periodically through the cell cycle (peaking in G1), the relative contribution of cell cycle-controlled transcription and CWI-induced transcription to this pattern remains to be teased apart.
Chitin synthesis through the cell cycle
During unstressed growth, chitin is highly concentrated at the bud neck, forms the primary septum during cytokinesis, and is present in small amounts in the lateral wall. Three chitin synthases, each with a specialized activity, are encoded by CHS1, CHS2, and CHS3 (Cabib and Duran 2005; Lesage and Bussey 2006). Chs3 is responsible for producing both the chitin that is inserted into the lateral wall during early G1 phase and the chitin ring at the incipient bud site in late G1. Chitin in the lateral wall is linked mainly through β-1,6-glucan chains. The chitin ring, which is attached directly to the β-1,3-glucan network, will ultimately define the mother/bud neck (Cabib and Duran 2005). Chs2, which is expressed specifically during mitosis, produces the chitin of the primary septum, which separates the mother and daughter cells by closing the chitin ring. Chs1, which is expressed during early G1 phase, is thought to be responsible for chitin repair of the septum after cytokinesis. The primary septum is covered on either side by a secondary septum of wall material. Dissolution of the primary septum allows separation of the bud from the mother cell.
Rho1 activation through the cell cycle
CWI signaling is regulated periodically through the cell cycle, peaking at the time of bud emergence when growth is most highly polarized (Zarzov et al. 1996). Studies from the Ohya and Pellman labs using assays for active Rho1 (Abe et al. 2003; Yoshida et al. 2006; Kono et al. 2008) revealed that this GTPase is activated at the G1/S boundary and again late in mitosis. Consistent with this are the observations that active Rho1 localizes to incipient bud sites and to the bud tip in small-budded cells (Abe et al. 2003) and to the mother-bud neck during cytokinesis (Yoshida et al. 2006). As noted above, localization of Rho1 at the bud tip is important for activation of GS and for proper actin cytoskeleton organization at least through control of Mpk1. The activation of Rho1 through the cell cycle is summarized in Figure 10.
Figure 10 .
Control of Rho1 activity through the cell cycle. Rho1 is activated at three sites through the cell cycle: the incipient bud site and bud tip during wall expansion, the mother/bud neck during mitosis, and between the septin rings during cytokinesis. Rho1’s recruitment and activation at these sites involves different regulators and is likely to result in the activation of only a subset of effectors. The secondary septum is cell wall material that is distinct from the primary septum, which is chitin produced by Chs2. GS, glucan synthase; CAR, contractile actin ring.
Rho1 activation during G1:
Rho1 is activated in G1 in response to Cln2/Cdc28 phosphorylation of several sites within the N terminus of the Rho1-GEF, Tus1 (Kono et al. 2008). Tus1 appears to act in parallel with Rom2 during G1/S because growth and actin polarization defects of tus1 mutants blocked for Cdc28 phosphorylation were exacerbated by a rom2Δ mutation. Tus1 localizes transiently to prebud sites at G1/S, dispersing immediately after bud emergence, whereas Rom2 remains polarized at the bud cortex (Manning et al. 1997; Audhya and Emr 2002; Abe et al. 2003), prompting the proposal that Tus1 augments the activity of Rom2 in Rho1 activation at an early stage of bud emergence (Kono et al. 2008).
Rho1 activation during anaphase:
Rho1 is also active at the bud neck during anaphase (Yoshida et al. 2006, 2009) and displays a peak of activity in anaphase-arrested cells (in cdc14 and cdc15 mutants) (Kono et al. 2008). Here, it functions in the assembly of the CAR, which facilitates cytokinesis (Tolliday et al. 2002; Balasubramanian et al. 2004). Rho1 contributes to CAR formation, at least in part, through the Bni1 actin nucleator. During anaphase, Rho1 is targeted to the bud neck by its GEFs. Both its recruitment to the division site and its activation during anaphase are under the control of the Polo-like kinase Cdc5 (Yoshida et al. 2006), which phosphorylates both Tus1 and Rom2. Cdc5 phosphorylation of Tus1 is responsible for its recruitment to the bud neck. Rho1 recruitment to the neck during anaphase also requires both Tus1 catalytic activity and the ability of Rho1 to undergo nucleotide exchange (Yoshida et al. 2009). It is interesting to note that Polo-box-binding motifs (Ser-Ser/Thr-Pro) are often primed by CDK phosphorylation of the residue immediately upstream of the proline. In the case of Tus1, two Cdc5-binding sites within its N terminus (at residues 7–9 and 92–94) (Yoshida et al. 2006) are situated at two of the Cln2/Cdc28 sites thought to be important for Tus1 function at the bud tip (Kono et al. 2008). These findings suggest the attractive hypothesis that Tus1 localization is controlled through sequential phosphorylation. Specifically, initial phosphorylation by Cdc28 results in its recruitment to the incipient bud site, which is followed by Cdc5 phosphorylation of Cdc28-primed Tus1 in late mitosis, resulting in its redeployment to the neck.
Rho1 activation during cytokinesis:
It is well established that Rho GTPases are required for CAR assembly in yeast as well as in animals (Yoshida et al. 2006; Takaki et al. 2008). However, in yeast, unlike animal cells, the CAR is not essential for cytokinesis because there is an alternative pathway involving the formation of a cell wall septum, thus allowing the study of cell division events in the absence of a CAR. Using live cell imaging, Yoshida et al. (2009) found that Rho1 is recruited to the bud neck after mitotic exit to perform a second, CAR-independent function in cytokinesis. Post-mitotic recruitment of Rho1 to the division site was detected in mutants lacking Rho1-GEFs and in a constitutively GTP-bound Rho1 mutant, both of which, as noted above, are blocked for recruitment of Rho1 to the neck during anaphase and CAR formation. This secondary recruitment of Rho1 to the division site contributes to cytokinesis through cell wall biosynthesis.
Post-mitotic recruitment of Rho1 to thebud neck is through a spatially and mechanistically separate pathway from its recruitment to the neck during anaphase (Yoshida et al. 2009). Rather than being dependent on the Rho1-GEFs, recruitment requires a PIP2-binding polybasic sequence within the Rho1 C terminus. Upon mitotic exit, the septin ring splits into two rings on either side of the bud neck to facilitate cytokinesis. Rho1 is recruited specifically to the membrane region between the split rings in a manner dependent on PIP2 production by Mss4 (Yoshida et al. 2009), which is enriched at the bud neck during cytokinesis (Garrenton et al. 2010). This post-mitotic recruitment pathway is essential to cytokinesis in mutants unable to form a CAR because of the requirement for septum formation. Although Tus1 is the Rho-GEF principally responsible for recruitment and activation of Rho1 to the bud neck during anaphase, Rom2 may be responsible for post-mitotic Rho1 activation on the basis of the observation that Rom2 is not recruited to the neck until after mitotic exit (Yoshida et al. 2006). This possibility is also consistent with the recruitment of Rom2 to sites of PIP2 accumulation (Audhya and Emr 2002). Thus, these studies demonstrated that Rho1 promotes cytokinesis through two separable pathways—one that drives CAR assembly during anaphase and another that drives septum formation after mitotic exit.
Role for CWI signaling in the control of mitosis: The CWI checkpoint
A growing body of evidence suggests that Pkc1 plays a role in the G2/M transition that is separate from its regulation of the MAPK cascade, perhaps as a mechanism to integrate the process of cell-surface expansion with progression of the cell cycle. First, conditional pkc1 mutants undergo cell lysis uniformly with small buds and duplicated DNA, but prior to spindle pole body (SPB) separation (Levin et al. 1990; Levin and Bartlett-Heubusch 1992). Second, under growth conditions in which cell lysis was prevented by osmotic support, a conditional pkc1 mutant was shown to linger at G2/M, a phenotype that was attributed to a delay in spindle elongation (Hosotani et al. 2001). Third, several studies have revealed genetic connections between Pkc1 and the chromatin remodeling complex called RSC (reviewed in Levin 2005), which serves a critical function in the progression from G2 to mitosis. Fourth, a pkc1 allele was isolated in a screen for mutants with elevated rates of mitotic recombination (Huang and Symington 1994). Mutants in the MAPK cascade do not share these pkc1 phenotypes. Finally, the demonstration that the N-terminal C2-like domain of Pkc1 localizes to the mitotic spindle (Denis and Cyert 2005) supports the notion that this kinase has a target at the mitotic apparatus.
Work from the Ohya laboratory revealed the existence of a checkpoint that monitors cell wall biosynthesis and links it to progression of the cell cycle across the G2/M boundary (Suzuki et al. 2004). These investigators described a conditional allele of FKS1 (in an fks2Δ background) that is temperature sensitive for growth (Sekiya-Kawasaki et al. 2002). Under restrictive conditions, this mutant arrests growth at a point in the cell cycle shortly after bud emergence, DNA replication, and SPB duplication, but prior to SPB separation (Suzuki et al. 2004; Negishi and Ohya 2010). This arrest was reminiscent of the terminal state of conditional pkc1 cells, except that it was not accompanied by cell lysis and was reversible, suggesting the existence of a checkpoint that blocks cell cycle progression in response to a block in cell wall biosynthesis. The cell cycle arrest is the consequence of inhibited CLB2 expression, which encodes a mitotic cyclin required for spindle assembly. CLB2 expression is under the control of the Fkh2–Mcm1–Ndd1 transcription factor complex.
A genetic screen for mutants that failed to survive the fks1 cell cycle arrest revealed a novel form of the Arp1 subunit of the dynactin complex that allows bipolar spindle formation and progression to M phase (Suzuki et al. 2004). The dynactin complex is an activator of dynein-mediated nuclear migration, but serves a second, mutationally separable function in the CWI checkpoint (Igarashi et al. 2005). The Nip100 and Jnm1 dynactin subunits were also required for the CWI checkpoint (Suzuki et al. 2004). The dynactin complex appears to act through blocking expression of CLB2 through the Fkh2–Mcm1–Ndd1 complex. The nature of the interaction between the cell wall stress signal initiated at the bud tip and the dynactin complex remains unknown, as does the mechanism by which dynactin controls Fkh2-mediated CLB2 transcription. This appears to be a fertile area for future investigation.
Perspectives and Future Directions
The discovery of multiple signaling pathways involved in regulating the reorganization of the yeast cell wall in response to various environmental signals and through the cell cycle has led to the recognition of cell wall biogenesis as a highly dynamic process and to a new understanding of the interrelated nature of cell wall remodeling, polarized secretion, and the actin cytoskeleton. Several intriguing questions have arisen recently as a consequence of this understanding. Other important questions have remained unanswered for many years.
One longstanding issue centers on the differential function of Rho1-GAPs and their role in signal integration and output by Rho1. The observation that different Rho1-effector pairs are regulated by distinct GAPs suggests that there exists a mechanism to compartmentalize Rho1 functions. It is not yet clear if the observed specialization represents spatial segregation of distinct pools of Rho1, effector-specific interactions of the GAPs, or some other mechanism at work. However, generalizable insights may come from a resolution of this issue.
Another important question that has remained unanswered for more than a decade concerns the identity of the molecular entities responsible for production of β-1,6-glucan. Now that an assay is available to study the production of this polymer in extracts, identification of the enzyme should be forthcoming. On a related note, despite the centrality of the β-1,3-glucan synthase to the maintenance of cell wall integrity and as the target of the echinocandin antifungal drugs, the mechanistic details of both its activity and its inhibition remain obscure. For example, although the location of the catalytic domain of Fks1 was confirmed recently, the Rho1-binding site has not yet been identified, nor has the mechanism by which Rho1 functions as a regulatory subunit been elucidated.
Although great progress has been made with regard to the transcriptional targets of CWI signaling, the branch of the transcriptional program regulated by Skn7 has remained shrouded. It is expected that the identification of genes under the control of Rho1-Skn7 should allow the dissection of this pathway. On the other hand, identification and validation of nontranscriptional targets have lagged. Of particular interest is the mechanism by which Mpk1 drives actin polarization. Recent hints suggest the involvement of feedback down-regulation of signaling through Rho1, but this idea awaits validation and mechanistic extension. Another potential target of Mpk1 in need of more detailed analysis is the Cch1/Mid1 Ca2+ channel. Whether the activity of this channel is controlled by Mpk1 directly or indirectly remains to be shown.
Recent discoveries have brought to the forefront the need to map the cross talk circuitry between CWI signaling and other pathways that contribute to the maintenance of cell wall integrity. For example, the sequential activation of the HOG pathway and the CWI pathway by zymolyase appears to bypass the upper part of the CWI pathway. The novel interface between these two stress-signaling pathways awaits elucidation. Similarly, unraveling the complex interplay between calcium signaling, ER stress signaling, and CWI signaling is an important problem for the future.
Finally, the relationship between cell wall stress and cell cycle control promises to be very interesting. This is especially true for the role of the dynactin complex as an intermediary in the CWI checkpoint. It will also be interesting to determine if the RSC chromatin remodeling complex plays a role in this novel checkpoint. Perhaps the large collection of apparently related observations connecting CWI signaling to the G2/M transition will ultimately be unified in a coherent model for the coordination of cell-surface expansion with cell cycle progression.
Acknowledgments
I am indebted to David Pellman, Satoshi Yoshida, Peter Pryciak, and two anonymous reviewers for their comments on the manuscript. Work in my laboratory on cell wall integrity signaling is supported by a grant from the National Institutes of Health (GM48533).
Literature Cited
- Abe J., Kusuhara M., Ulevitch R. J., Berk B. C., Lee J. D., 1996. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J. Biol. Chem. 271: 16586–16590 [DOI] [PubMed] [Google Scholar]
- Abe M., Qadota H., Hirata A., Ohya Y., 2003. Lack of GTP-bound Rho1p in secretory vesicles of Saccharomyces cerevisiae. J. Cell Biol. 162: 85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo J. E., Rossi G., Brennwald P., 1999. The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol. Biol. Cell 10: 4121–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Uscanga B., François J. M., 2003. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 37: 268–274 [DOI] [PubMed] [Google Scholar]
- Alberts A. S., 2001. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J. Biol. Chem. 276: 2824–2830 [DOI] [PubMed] [Google Scholar]
- Alberts A. S., Bouquin N., Johnston L. H., Treisman R., 1998. Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. J. Biol. Chem. 273: 8616–8622 [DOI] [PubMed] [Google Scholar]
- Alic N., Higgins V. J., Pichova A., Breitenbach M., Dawes I. W., 2003. Lipid hydroperoxides activate the mitogen-activated protein kinase Mpk1p in Saccharomyces cerevisiae. J. Biol. Chem. 278: 41849–41855 [DOI] [PubMed] [Google Scholar]
- Andersson J., Simpson D. M., Qi M., Wang Y., Elion E. A., 2004. Differential input by Ste5 scaffold and Msg5 phosphatase route a MAPK cascade to multiple outcomes. EMBO J. 23: 2564–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews B. J., Herskowitz I., 1989. Identification of a DNA binding factor involved in cell cycle-control of the yeast HO gene. Cell 57: 21–29 [DOI] [PubMed] [Google Scholar]
- Andrews B. J., Moore L. A., 1992. Interaction of the yeast Swi4 and Swi6 cell cycle regulatory proteins in vitro. Proc. Natl. Acad. Sci. USA 89: 11852–11856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. D., Stark M. J., 2000. Dynamic, Rho1p-dependent localization of Pkc1p to sites of polarized growth. J. Cell Sci. 113: 2685–2693 [DOI] [PubMed] [Google Scholar]
- Antonsson B., Montessuit S., Friedli L., Payton M. A., Paravicini G., 1994. Protein kinase C in yeast. Characteristics of the Saccharomyces cerevisiae PKC1 gene product. J. Biol. Chem. 269: 16821–16828 [PubMed] [Google Scholar]
- Arigo J. T., Carroll K. L., Ames J. M., Corden J. L., 2006. Regulation of yeast NRD1 expression by premature transcription termination. Mol. Cell 21: 641–651 [DOI] [PubMed] [Google Scholar]
- Audhya A., Emr S. D., 2002. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell 2: 593–605 [DOI] [PubMed] [Google Scholar]
- Audhya A., Emr S. D., 2003. Regulation of PI4,5P2 synthesis by nuclear-cytoplasmic shuttling of the Mss4 lipid kinase. EMBO J. 22: 4223–4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya A., Foti M., Emr S. D., 2000. Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol. Biol. Cell 11: 2673–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough K. R., Drubin D. G., 2003. A role for the yeast actin cytoskeleton in pheromone receptor clustering and signaling. Curr. Biol. 8: 927–930 [DOI] [PubMed] [Google Scholar]
- Babour A., Bicknell A. A., Tourtellotte J., Niwa M., 2010. A surveillance pathway monitors the fitness of the endoplasmic reticulum to control its inheritance. Cell 142: 256–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetz K., Andrews B., 1999. Regulation of the cell cycle transcription factor Swi4 through auto-inhibition of DNA binding. Mol. Cell. Biol. 19: 6729–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetz K., Moffat J., Haynes J., Chang M., Andrews B., 2001. Transcriptional coregulation by the cell integrity mitogen-activated protein kinase Slt2 and the cell cycle regulator Swi4. Mol. Cell. Biol. 21: 6515–6528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian M. K., Bi E., Glotzer M., 2004. Comparative analysis of cytokinesis in budding yeast, fission yeast and animal cells. Curr. Biol. 14: R806–R818 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S., Mehta M., Kuo D., Sung M. K., Chuang R., et al. , 2010. Rewiring of genetic networks in response to DNA damage. Science 330: 1385–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar E. E., Ellicott A. T., Stone D. E., 2003. Gβγ recruits Rho1 to the site of polarized growth during mating in budding yeast. J. Biol. Chem. 278: 21798–21804 [DOI] [PubMed] [Google Scholar]
- Batiza A. F., Schulz T., Masson P. H., 1996. Yeast respond to hypotonic shock with a calcium pulse. J. Biol. Chem. 271: 23357–23362 [DOI] [PubMed] [Google Scholar]
- Beese S. E. T. N., Levin D. E., 2009. Identification of positive regulators of the yeast fps1 glycerol channel. PLoS Genet. 5: e1000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo C., Rodríguez E., García R., Rodríguez-Peña J. M., Rodríguez de la Concepción M. L., et al. , 2008. The sequential activation of the yeast HOG and SLT2 pathways is required for cell survival to cell wall stress. Mol. Biol. Cell 19: 1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo C., García R., Straede A., Rodríguez-Peña J. M., Nombela C., et al. , 2010. Characterization of sensor-specific stress response by transcriptional profiling of wsc1 and mid2 deletion strains and chimeric sensors in Saccharomyces cerevisiae. OMICS 14: 679–688 [DOI] [PubMed] [Google Scholar]
- Betz J. L., Chang M., Washburn T. M., Porter S. E., Mueller C. L., et al. , 2002. Phenotypic analysis of Paf1/RNA polymerase II complex mutations reveals connections to cell cycle regulation, protein synthesis, and lipid and nucleic acid metabolism. Mol. Genet. Genomics 268: 272–285 [DOI] [PubMed] [Google Scholar]
- Bonilla M., Cunningham K. W., 2003. Mitogen-activated protein kinase stimulation of Ca2+ signaling is required for survival of endoplasmic reticulum stress in yeast. Mol. Biol. Cell 14: 4296–4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla M., Nastase K. K., Cunningham K. W., 2002. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 21: 2343–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorsma A., de Nobel H., ter Riet B., Bargmann B., Brul S., et al. , 2004. Characterization of the transcriptional response to cell wall stress in Saccharomyces cerevisiae. Yeast 21: 413–427 [DOI] [PubMed] [Google Scholar]
- Breeden L. L., 2003. Periodic transcription: a cycle within a cycle. Curr. Biol. 13: R31–R38 [DOI] [PubMed] [Google Scholar]
- Brown J. L., North S., Bussey H., 1993. SKN7, a yeast multicopy suppressor of a mutation affecting cell wall beta-glucan assembly, encodes a product with domains homologous to prokaryotic two-component regulators and to heat shock transcription factors. J. Bacteriol. 175: 6908–6915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. L., Bussey H., Stewart R. C., 1994. Yeast Skn7p functions in a eukaryotic two-component regulatory pathway. EMBO J. 13: 5186–5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehrer B. M., Errede B., 1997. Coordination of the mating and cell integrity mitogen-activated protein kinase pathways in Saccharomyces cerevisiae. Mol. Cell. Biol. 17: 6517–6525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik D. A., Olczak M., Lucero H. A., Osmond B. C., Robbins P. W., et al. , 2003. Chitin synthesis in Saccharomyces cerevisiae in response to supplementation of growth medium with glucosamine and cell wall stress. Eukaryot. Cell 2: 886–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery S. M., Yoshida S., Pellman D., 2007. Yeast formins Bni1 and Bnr1 utilize different modes of cortical interaction during the assembly of actin cables. Mol. Biol. Cell 18: 1826–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., 2009. Two novel techniques for determination of polysaccharide cross-links show that Crh1p and Crh2p attach chitin to both β(1–6)- and β(1–3)glucan in the Saccharomyces cerevisiae cell wall. Eukaryot. Cell 8: 1626–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Duran A., 2005. Synthase III-dependent chitin is bound to different acceptors depending on location on the cell wall of budding yeast. J. Biol. Chem. 280: 9170–9179 [DOI] [PubMed] [Google Scholar]
- Cabib E., Blanco N., Grau C., Rodriguez-Peña J. M., Arroyo J., 2007. Crh1p and Crh2p are required for the cross-linking of chitin to β(1–6)glucan in the Saccharomyces cerevisiae cell wall. Mol. Microbiol. 63: 921–935 [DOI] [PubMed] [Google Scholar]
- Cabib E., Farkas V., Kosik O., Blanko N., Arroyo J., et al. , 2008. Assembly of the yeast cell wall. Crh1p and Crh2p act as transglycosylases in vivo and in vitro. J. Biol. Chem. 283: 29859–29872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone K. G., Welch M. D., 2010. A nucleator arms race: cellular control of actin assembly. Nat. Rev. Mol. Cell Biol. 11: 237–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellaro C., Baldermann C., Rachel R., Tanner W., 1994. Mating type-specific cell-cell recognition of Saccharomyces cerevisiae: cell wall attachment and active sites of a- and alpha-agglutinin. EMBO J. 13: 4737–4744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro L. H., Tettelin H., Vossen J. H., Ram A. F., Van den Ende H., et al. , 1997. In silicio identification glycosyl-phosphatidylinositol-anchored plasma membrane and cell wall proteins of Saccharomyces cerevisiae. Yeast 13: 1477–1489 [DOI] [PubMed] [Google Scholar]
- Carroll K. L., Pradhan D. A., Granek J. A., Clarke N. D., Corden J. L., 2004. Identification of cis elements directing termination of yeast nonpolyadenylated snoRNA transcripts. Mol. Cell. Biol. 24: 6241–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo L., Martinez A. I., Garcera A., Elorza M. V., Valentin E., et al. , 2003. Functional analysis of the cysteine residues and the repetitive sequence of Saccharomyces cerevisiae Pir4/Cis3: the repetitive sequence is needed for binding to the cell wall beta-1,3-glucan. Yeast 20: 973–983 [DOI] [PubMed] [Google Scholar]
- Chang M., French-Cornay D., Fan H. Y., Klein H., Denis C. L., et al. , 1999. A complex containing RNA polymerase II, Paf1p, Cdc73p, Hpr1p, and Ccr4p plays a role in protein kinase C signaling. Mol. Cell. Biol. 19: 1056–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Feldman D. E., Deng C., Brown J. A., De Giacomo A. F., et al. , 2005. Identification of mitogen-activated protein kinase signaling pathways that confer resistance to endoplasmic reticulum stress in Saccharomyces cerevisiae. Mol. Cancer Res. 3: 669–677 [DOI] [PubMed] [Google Scholar]
- Choi W. J., Santos B., Durán A., Cabib E., 1994. Are yeast chitin synthases regulated at the transcriptional or the posttranslational level? Mol. Cell. Biol. 14: 7685–7694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid V. J., Duran A., Rey F., Snyder M. P., Nombela C., et al. , 1995. Molecular basis of cell integrity and morphogenesis in Saccharomyces cerevisiae. Microbiol. Rev. 59: 345–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid V. J., Cenamor R., Sanchez M., Nombela C., 1998. A mutation in the Rho1-GAP-encoding gene BEM2 of Saccharomyces cerevisiae affects morphogenesis and cell wall functionality. Microbiology 144: 25–36 [DOI] [PubMed] [Google Scholar]
- Claret S., Gatti X., Doignon X., Thoraval D., Crouzet M., 2005. The Rgd1p Rho GTPase-activating protein and the Mid2p cell wall sensor are required at low pH for protein kinase C pathway activation and cell survival in Saccharomyces cerevisiae. Eukaryot. Cell 4: 1375–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen T. J., Mallory M. J., Strich R., Yao T. P., 2008. Hos2p/Set3p deacetylase complex signals secretory stress through the Mpk1p cell integrity pathway. Eukaryot. Cell 7: 1191–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kupiec R., Broglie K. E., Friesem D., Broglie R. M., Chet I., 1999. Molecular characterization of a novel β-1,3-exoglucanase related to mycoparasitism of Trichoderma harzianum. Gene 226: 147–154 [DOI] [PubMed] [Google Scholar]
- Collister M., Didmon M. P., MacIsaac F., Stark M. J., MacDonald N. Q., et al. , 2002. YIL113w encodes a functional dual-specificity protein phosphatase which specifically interacts with and inactivates the Slt2/Mpk1p MAP kinase in S. cerevisiae. FEBS Lett. 527: 186–192 [DOI] [PubMed] [Google Scholar]
- Costa P. J., Arndt K. M., 2000. Synthetic lethal interactions suggest a role for the Saccharomyces cerevisiae Rtf1 protein in transcription elongation. Genetics 156: 535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan C., Gehrung S., Snyder M., 1992. A synthetic lethal screen identifies SLK1, a novel protein kinase homolog implicated in yeast cell morphogenesis and cell growth. Mol. Cell. Biol. 12: 1162–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler N. S., Heitman J., Cardenas M. E., 1997. Stt4 is an essential phosphatidylinositol 4-kinase that is a target of wartmannin in Saccharomyces cerevisiae. J. Biol. Chem. 272: 27671–27677 [DOI] [PubMed] [Google Scholar]
- Cyert M. S., Thorner J., 1992. Regulatory subunit (CNB1 gene product) of yeast Ca2+/calmodulin-dependent phosphoprotein phosphatases is required for adaptation to pheromone. Mol. Cell. Biol. 12: 3460–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon M., Agoutin B., Watzinger M., Averbeck D., 2009. Slt2 (Mpk1) MAP kinase is involved in the response of Saccharomyces cerevisiae to 8-methoxypsoralen plus UVA. J. Photochem. Photobiol. B 95: 148–155 [DOI] [PubMed] [Google Scholar]
- Davenport K. R., Sohaskey M., Kamada Y., Levin D. E., Gustin M. C., 1995. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J. Biol. Chem. 270: 30157–30161 [DOI] [PubMed] [Google Scholar]
- Delley P. A., Hall M. N., 1999. Cell wall stress depolarizes cell growth via hyperactivation of Rho1. J. Cell Biol. 147: 163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis V., Cyert M. S., 2005. Molecular analysis reveals localization of Saccharomyces cerevisiae protein kinase C to sites of polarized growth and Pkc1p targeting to the nucleus and mitotic spindle. Eukaryot. Cell 4: 36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nobel J. G., Barnett J. A., 1991. Passage of molecules through yeast cell walls: a brief essay-review. Yeast 7: 313–323 [DOI] [PubMed] [Google Scholar]
- de Nobel J. G., Klis F. M., Priem J., Munnik T., van den Ende H., 1990. The glucanase-soluble mannoproteins limit cell wall porosity in Saccharomyces cerevisiae. Yeast 6: 491–499 [DOI] [PubMed] [Google Scholar]
- de Nobel H., Ruiz C., Martin H., Morris W., Brul S., et al. , 2000. Cell wall perturbation in yeast results in dual phosphorylation of the Slt2/Mpk1 MAP kinase and in an Slt2-mediated increase in FKS2-lacZ expression, glucanase resistance and thermotolerance. Microbiology 146: 2121–2132 [DOI] [PubMed] [Google Scholar]
- Desrivieres S., Cooke F. T., Parker P. J., Hall M. N., 1998. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J. Biol. Chem. 273: 15787–15793 [DOI] [PubMed] [Google Scholar]
- De Virgilio C., Hottiger T., Dominguez J., Boller T., Wiemken A., 1994. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. I. Genetic evidence that trehalose is a thermoprotectant. Eur. J. Biochem. 219: 179–186 [DOI] [PubMed] [Google Scholar]
- Dodou E., Treisman R., 1997. The Saccharomyces cerevisiae MADS-box transcription factor Rlm1 is a target for the Mpk1 mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17: 1848–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Pruyne D., Bretscher A., 2003. Formin-dependent actin assembly is regulated by distinct modes of Rho signaling in yeast. J. Cell Biol. 161: 1081–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. M., 2001. Fungal β-1,3-D-glucan synthesis. Med. Mycol. 39: 55–66 [DOI] [PubMed] [Google Scholar]
- Douglas C. M., Foor F., Marrinan J. A., Morin N., Nielsen J. B., et al. , 1994. The Saccaharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-β-D-glucan synthase. Proc. Natl. Acad. Sci. USA 91: 12907–12911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas L. M., Li L., Yang Y., Dranginis A. M., 2007. Expression and characterization of the flocculin Flo11/Muc1, a Saccharomyces cerevisiae mannoprotein with homotypic properties of adhesion. Eukaryot. Cell 6: 2214–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees B. L., Sundin B., Brazeau E., Caviston J. P., Chen G. C., et al. , 2001. A protein interaction map for cell polarity development. J. Cell Biol. 154: 549–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drgonova J., Drgon T., Tanaka K., Kollár R., Chen G. C., et al. , 1996. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science 272: 277–279 [DOI] [PubMed] [Google Scholar]
- Drubin D. G., Nelson W. J., 1996. Origins of cell polarity. Cell 84: 335–344 [DOI] [PubMed] [Google Scholar]
- Dupres V., Alsteens D., Wilk S., Hansen B., Heinisch J. J., et al. , 2009. The yeast Wsc1 cell surface sensor behaves like a nanospring in vivo. Nat. Chem. Biol. 5: 857–862 [DOI] [PubMed] [Google Scholar]
- Ecker M., Deutzmann R., Lehle L., Mrsa V., Tanner W., 2006. Pir proteins of Saccharomyces cerevisiae are attached to β-1,3-glucan by a new protein-carbohydrate linkage. J. Biol. Chem. 281: 11523–11529 [DOI] [PubMed] [Google Scholar]
- Elion E. A., 2000. Pheromone response, mating and cell biology. Curr. Opin. Microbiol. 3: 573–581 [DOI] [PubMed] [Google Scholar]
- Elorza M. V., Rico H., Sentandreu R., 1983. Calcofluor white alters the assembly of chitin fibrils in Saccharomyces cerevisiae and Candida albicans cells. J. Gen. Microbiol. 129: 1577–1582 [DOI] [PubMed] [Google Scholar]
- Errede B., Cade R. M., Yashar B. M., Kamada Y., Levin D. E., et al. , 1995. Dynamics and organization of MAP kinase signal pathways. Mol. Reprod. Dev. 42: 477–485 [DOI] [PubMed] [Google Scholar]
- Evangelista M., Blundell K., Longtine M. S., Chow C. J., Adames N., et al. , 1997. Bni1p, a yeast formin linking Cdc42p and the actin cytoskeleton during polarized morphogenesis. Science 276: 118–122 [DOI] [PubMed] [Google Scholar]
- Evangelista M., Zigmond S., Boone C., 2003. Formins: signaling effectors for assembly and polarization of actin filaments. J. Cell Sci. 116: 2603–2611 [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr 1996. Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem. Sci. 21: 460–466 [DOI] [PubMed] [Google Scholar]
- Finger F. P., Hughes T. E., Novick P., 1998. Sec3 is a spatial landmark for polarized secretion in budding yeast. Cell 92: 559–571 [DOI] [PubMed] [Google Scholar]
- Flandez M., Cosano I. C., Nombela C., Martin H., Molina M., 2004. Reciprocal regulation between Slt2 MAPK and isoforms of Msg5 dual-specificity protein phosphatase modulates the yeast cell integrity pathway. J. Biol. Chem. 279: 11027–11034 [DOI] [PubMed] [Google Scholar]
- Foor F., Parent S. A., Morin N., Dahl A. M., Ramadan N., et al. , 1992. Calcineurin mediates inhibition by FK506 and cyclosporin of recovery from alpha-factor arrest in yeast. Nature 360: 682–684 [DOI] [PubMed] [Google Scholar]
- Friant S., Lombardi R., Schmelzle T., Hall M. N., Riezman H., 2001. Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. EMBO J. 20: 6783–6792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Tanaka K., Mino A., Kikyo M., Takahashi K., et al. , 1998. Rho1p-Bni1p-Spa2p interactions: implication in localization of Bni1p at the bud site and regulation of the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Biol. Cell 9: 1221–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García R., Bermejo C., Grau C., Perez R., Rodríguez-Peña J. M., et al. , 2004. The global transcriptional response to transient cell wall damage in Saccharomyces cerevisiae and its regulation by the cell integrity signaling pathway. J. Biol. Chem. 279: 15183–15195 [DOI] [PubMed] [Google Scholar]
- García R., Rodríguez-Peña J. M., Bermejo C., Nombela C., Arroyo J., 2009. The high osmotic response and cell wall integrity pathways cooperate to regulate transcriptional responses to zymolyase-induced cell wall stress in Saccharomyces cerevisiae. J. Biol. Chem. 284: 10901–10911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rodriguez L. J., Durán A., Roncero C., 2000. Calcofluor antifungal action depends on chitin and a functional high-osmolarity glycerol response (HOG) pathway: evidence for a physiological role of the Saccharomyces cerevisiae HOG pathway under noninducing conditions. J. Bacteriol. 182: 2428–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rodríguez L. J., Valle R., Durán A., Roncero C., 2005. Cell integrity signaling activation in response to hyperosmotic shock in yeast. FEBS Lett. 579: 6186–6190 [DOI] [PubMed] [Google Scholar]
- Garrenton L. S., Stefan C. J., McMurray M. A., Emr S. D., Thorner J., 2010. Pheromone-induced anisotropy in yeast plasma membrane phosphatidylinositol-4,5-bisphosphate distribution is required for MAPK signaling. Proc. Natl. Acad. Sci. USA 107: 11805–11810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett-Engele P., Moilanen B., Cyert M. S., 1995. Calcineurin, the Ca2+/calmodulin-dependent protein phosphatase, is essential in yeast mutants with cell integrity defects and in mutants that lack a functional vacuolar H+-ATPase. Mol. Cell. Biol. 15: 4103–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzsch M., Tanner W., 1996. The PMT gene family: protein O-glycosylation in Saccharomyces cerevisiae is vital. EMBO J. 15: 5752–5759 [PMC free article] [PubMed] [Google Scholar]
- Geymonat M., Spanos A., Wells G. P., Smerdon S. J., Sedgwick S. G., 2004. Clb6/Cdc28 and Cdc14 regulate phosphorylation status and cellular localization of Swi6. Mol. Cell. Biol. 24: 2277–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami S., Huh W. K., Bower K., Howson R. W., Belle A., et al. , 2003. Global analysis of protein expression in yeast. Nature 425: 737–741 [DOI] [PubMed] [Google Scholar]
- Gray J. V., Ogas J. P., Kamada Y., Stone M., Levin D. E., et al. , 1997. A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO J. 16: 4924–4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R., Lesage G., Sdicu A.-M., Menard P., Bussey H., 2003. A synthetic analysis of the Saccharomyces cerevisiae stress sensor Mid2p, and indentification of a Mid2p-interacting protein, Zeo1, that modulates the PKC1-MPK1 cell integrity pathway. Microbiology 149: 2487–2499 [DOI] [PubMed] [Google Scholar]
- Guo S., Shen X., Yan G., Ma D., Bai X., et al. , 2009. A MAP kinase dependent feedback mechanism controls Rho1 GTPase and actin distribution in yeast. PLoS ONE 4: e6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Tamanoi F., Novick P., 2001. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 3: 353–360 [DOI] [PubMed] [Google Scholar]
- Gustin M. C., Albertyn J., Alexander M., Davenport K., 1998. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62: 1264–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J.-S., Thiele D. J., 2002. Regulation of the Saccharomyces cerevisiae Slt2 kinase pathway by the stress-inducible Sdp1 dual specificity phosphatase. J. Biol. Chem. 277: 21278–21284 [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Hunter T., 1995. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9: 576–596 [PubMed] [Google Scholar]
- Harhammer R., Gohla A., Schultz G., 1996. Interaction of G protein Gβγ dimers with small GTP-binding proteins of the Rho family. FEBS Lett. 399: 211–214 [DOI] [PubMed] [Google Scholar]
- Harold F. M., 2002. Force and compliance: rethinking morphogenesis in walled cells. Fungal Genet. Biol. 37: 271–282 [DOI] [PubMed] [Google Scholar]
- Harreman M. T., Kline T. M., Milford H. G., Harben M. B., Hodel A. E., et al. , 2004. Regulation of nuclear import by phosphorylation adjacent to nuclear localization signals. J. Biol. Chem. 279: 20613–20621 [DOI] [PubMed] [Google Scholar]
- Harrison J. C., Bardes E. S., Ohya Y., Lew D. J., 2001. A role for the Pkc1p/Mpk1p kinase cascade in the morphogenesis checkpoint. Nat. Cell Biol. 3: 417–420 [DOI] [PubMed] [Google Scholar]
- Heasman S. J., Ridley A. J., 2008. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 9: 690–701 [DOI] [PubMed] [Google Scholar]
- Heinisch J. J., Lorberg A., Schmitz H. P., Jacoby J. J., 1999. The protein kinase C-mediated MAP kinase pathway involved in the maintenance of cellular integrity in Saccharomyces cerevisiae. Mol. Microbiol. 32: 671–680 [DOI] [PubMed] [Google Scholar]
- Heinisch J. J., Dupres V., Wilk S., Jendretzki A., Dufrêne Y. F., 2010. Single-molecule atomic force microscopy reveals clustering of the yeast plasma-membrane sensor Wsc1. PLoS ONE 5: e11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell S. B., Howald I., Barbet N., Hall M. N., 1998. TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics 148: 99–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y., Gruhler A., Heilbut A., Bader G. D., Moore L., et al. , 2002. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415: 180–183 [DOI] [PubMed] [Google Scholar]
- Hohmann S., 2002. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 66: 300–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma K., Terui S., Minemura M., Qadota H., Anraku Y., et al. , 1998. Phosphatidylinositol-4-phosphate 5-kinase localized on the plasma membrane is essential for yeast cell morphogenesis. J. Biol. Chem. 273: 15779–15786 [DOI] [PubMed] [Google Scholar]
- Hosotani T., Koyama H., Uchino M., Miyakawa T., Tsuchiya E., 2001. PKC1, a protein kinase C homologue of Saccharomyces cerevisiae, participates in microtubule function through the yeast EB1 homologue, BIM1. Genes Cells 6: 775–788 [DOI] [PubMed] [Google Scholar]
- Hottiger T., de Virgilio C., Hall M. N., Boller T., Wiemken A., 1994. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. II. Physiological concentrations of trehalose increase the thermal stability of proteins in vitro. Eur. J. Biochem. 219: 187–193 [DOI] [PubMed] [Google Scholar]
- Huang C.-Y., Ferrell J. E., Jr, 1996. Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 93: 10078–10083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. N., Symington L. S., 1994. Mutation of the gene encoding protein kinase C 1 stimulates mitotic recombination in Saccharomyces cerevisiae. Mol. Cell. Biol. 14: 6039–6045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh W. K., Falvo J. V., Gerk L. C., Carroll A. S., Howson R. W., et al. , 2003. Global analysis of protein localization in budding yeast. Nature 425: 686–691 [DOI] [PubMed] [Google Scholar]
- Hutzler F., Gerstl R., Lommel M., Strahl S., 2008. Protein N-glycosylation determines functionality of the Saccharomyces cerevisiae cell wall integrity sensor Mid2p. Mol. Microbiol. 68: 1438–1449 [DOI] [PubMed] [Google Scholar]
- Igarashi R., Suzuki M., Nogami S., Ohya Y., 2005. Molecular dissection of ARP1 regions required for nuclear migration and cell wall integrity checkpoint functions in Saccharomyces cerevisiae. Cell Struct. Funct. 30: 57–67 [DOI] [PubMed] [Google Scholar]
- Igual J. C., Johnson A. L., Johnston L. H., 1996. Coordinated regulation of gene expression by the cell cycle transcription factor SWI4 and the protein kinase C MAP kinase pathway for yeast cell integrity. EMBO J. 15: 5001–5013 [PMC free article] [PubMed] [Google Scholar]
- Imai J., Toh-e A., Matsui Y., 1996. Genetic analysis of the Saccharomyces cerevisiae RHO3 gene, encoding a Rho-type small GTPase, provides evidence for a role in bud formation. Genetics 142: 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Noda Y., Adachi H., Yoda K., 2005. A novel endoplasmic reticulum membrane protein Rcr1 regulates chitin deposition in the cell wall of Saccharomyces cerevisiae. J. Biol. Chem. 280: 8275–8284 [DOI] [PubMed] [Google Scholar]
- Imamura H., Tanaka K., Hihara T., Umikawa M., Kamei T., et al. , 1997. Bni1p and Bnr1p: downstream targets of the rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 16: 2745–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M., Schmelzle T., Yamaguchi K., Irie K., Hall M. N., et al. , 1999. PDK1 homologs activate the Pkc1-mitogen-activated protein kinase pathway in yeast. Mol. Cell. Biol. 19: 8344–8352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S. B., Qadota H., Arisawa T., Anraku Y., Watanabe T., et al. , 1996. Signaling toward 1,3-β-glucan synthesis. Cell Struct. Funct. 21: 395–402 [DOI] [PubMed] [Google Scholar]
- Inoue S. B., Qadota H., Arisawa M., Watanabe T., Ohya Y., 1999. Prenylation of Rho1p is required for activation of yeast 1,3-beta-glucan synthase. J. Biol. Chem. 274: 38119–38124 [DOI] [PubMed] [Google Scholar]
- Irie K., Takase M., Lee K. S., Levin D. E., Araki H., et al. , 1993. MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase kinase homologs, function in the pathway mediated by protein kinase C. Mol. Cell. Biol. 13: 3076–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara S., Hirata A., Nogami S., Beauvais A., Latge J. P., et al. , 2007. Homologous subunits of 1,3-beta-glucan synthase are important for spore wall assembly in Saccharomyces cerevisiae. Eukaryot. Cell 6: 143–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby J. J., Nilius S. M., Heinisch J. J., 1998. A screen for upstream components of the yeast protein kinase C signal transduction pathway identifies the product of the SLG1 gene. Mol. Gen. Genet. 258: 148–155 [DOI] [PubMed] [Google Scholar]
- Jesch S. A., Gaspar M. L., Stefan C. J., Aregullin M. A., Henry S. A., 2010. Interruption of inositol sphingolipid synthesis triggers Stt4p-dependent protein kinase C signaling. J. Biol. Chem. 285: 41947–41960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Sanchez M., Cid V. J., Molina M., 2007. Retrophosphorylation of Mkk1 and Mkk2 MAPKKs by the Slt2 MAPK in the yeast cell integrity pathway. J. Biol. Chem. 282: 31174–31185 [DOI] [PubMed] [Google Scholar]
- Johnson D. I., 1999. Cdc42: an essential rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63: 54–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. I., Pringle J., 1990. Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J. Cell Biol. 111: 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung U. S., Levin D. E., 1999. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 34: 1049–1057 [DOI] [PubMed] [Google Scholar]
- Jung U. S., Sobering A. K., Romeo M. J., Levin D. E., 2002. Regulation of the yest Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol. Microbiol. 46: 781–789 [DOI] [PubMed] [Google Scholar]
- Kagami M., Toh-e A., Matsui Y., 1997. SRO9, a multicopy suppressor of the bud growth defect in the Saccharomyces cerevisiae rho3-deficient cells, shows strong genetic interactions with tropomyosin genes, suggesting its role in organization of the actin cytoskeleton. Genetics 147: 1003–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y., Jung U. S., Piotrowski J., Levin D. E., 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9: 1559–1571 [DOI] [PubMed] [Google Scholar]
- Kamada Y., Qadota H., Python C. P., Anraku Y., Ohya Y., et al. , 1996. Activation of yeast protein kinase C by Rho1 GTPase. J. Biol. Chem. 271: 9193–9196 [DOI] [PubMed] [Google Scholar]
- Kanzaki M., Nagasawa M., Kojima I., Sato C., Naruse K., et al. , 1999. Molecular identification of a eukaryotic, stretch-activated nonselective cation channel. Science 285: 882–886 [DOI] [PubMed] [Google Scholar]
- Kapteyn J. C., van Egmond P., Sievi E., van den Ende H., Makarow M., et al. , 1999. The contribution of the O-glycosylated protein Pir2/Hsp150 to the construction of the yeast cell wall in wild type cells and β1,6-glucan-deficient mutants. Mol. Microbiol. 31: 1835–1844 [DOI] [PubMed] [Google Scholar]
- Ketela T., Brown J. L., Stewart R. C., Bussey H., 1998. Yeast Skn7p activity is modulated by the Sln1p-Ypd1p osmosensor and contributes to regulation of the HOG pathway. Mol. Gen. Genet. 259: 372–378 [DOI] [PubMed] [Google Scholar]
- Ketela T., Green R., Bussey H., 1999. Saccharomyces cerevisiae Mid2p is a potential cell wall stress sensor and upstream activator of the PKC1–MPK1 cell integrity pathway. J. Bacteriol. 181: 3330–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikyo M., Tanaka K., Kamei T., Ozaki K., Fujiwara T., et al. , 1999. An FH domain-containing Bnr1p is a multifunctional protein interacting with a variety of cytoskeletal proteins in Saccharomyces cerevisiae. Oncogene 18: 7046–7054 [DOI] [PubMed] [Google Scholar]
- Kim J., Guermah M., Roeder R. G., 2010. The human PAF1 complex acts in chromatin transcription elongation both independently and cooperatively with SII/TFIIS. Cell 140: 491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.-Y., Levin D. E., 2010. Transcriptional reporters for genes activated by cell wall stress through a non-catalytic mechanism involving Mpk1 and SBF. Yeast 27: 541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.-Y., Levin D. E., 2011. Mpk1 MAPK association with the Paf1 complex blocks Sen1-mediated premature transcription termination. Cell 144: 745–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.-Y., Truman A. W., Levin D. E., 2008. Yeast Mpk1 mitogen-activated protein kinase activates transcription through Swi4/Swi6 by a noncatalytic mechanism that requires upstream signal. Mol. Cell. Biol. 28: 2579–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.-Y., Truman A. W., Caesar S., Schlenstedt G., Levin D. E., 2010. Yeast Mpk1 cell wall integrity mitogen-activated protein kinase regulates nucleocytoplasmic shuttling of the Swi6 transcriptional regulator. Mol. Biol. Cell 21: 1609–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchrath L., Lorberg A., Schmitz H. P., Gengenbacher U., Heinisch J. J., 2000. Comparative genetic and physiological studies of the MAP kinase Mpk1p from Kluyveromyces lactis and Saccharomyces cerevisiae. J. Mol. Biol. 300: 743–758 [DOI] [PubMed] [Google Scholar]
- Klis F. M., Boorsma A., De Groot P. W. J., 2006. Cell wall construction in S. cerevisiae. Yeast 23: 185–202 [DOI] [PubMed] [Google Scholar]
- Koch C., Wollmann P., Dahl M., Lottspeich F., 1999. A role for Ctr9p and Paf1p in the regulation G1 cyclin expression in yeast. Nucleic Acids Res. 27: 2126–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno H., Tanaka K., Mino A., Umikawa M., Imamura H., et al. , 1996. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15: 6060–6068 [PMC free article] [PubMed] [Google Scholar]
- Kollár R., Petráková E., Ashwell G., Robbins P. W., Cabib E., 1995. Architecture of the yeast cell wall. The linkage between chitin and β-1,3-glucan. J. Biol. Chem. 270: 1070–1078 [DOI] [PubMed] [Google Scholar]
- Kollár R., Reinhold B. B., Petrakova E., Yeh H. J., Ashwell G., et al. , 1997. Architecture of the yeast cell wall. β-1,6-glucan interconnects mannoprotein, β-1,3-glucan, and chitin. J. Biol. Chem. 272: 17762–17775 [DOI] [PubMed] [Google Scholar]
- Kono K., Nogami S., Abe M., Nishizawa M., Morishita S., et al. , 2008. G1/S cyclin-dependent kinase regulates small GTPase Rho1p through phosphorylation of RhoGEF Tus1p in Saccharomyces cerevisiae. Mol. Biol. Cell 19: 1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecka M., Gabriel M., 1992. The influence of Congo red on the cell wall and 1,3-β-D-glucan microfibril biogenesis in Saccharomyces cerevisiae. Arch. Microbiol. 158: 115–126 [DOI] [PubMed] [Google Scholar]
- Krause S. A., Gray J. V., 2002. The protein kinase C pathway is required for viability in quiescence in Saccharomyces cerevisiae. Curr. Biol. 12: 588–593 [DOI] [PubMed] [Google Scholar]
- Krogan N. J., Dover J., Wood A., Schneider J., Heidt J., et al. , 2003. The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol. Cell 11: 721–729 [DOI] [PubMed] [Google Scholar]
- Krysan D. J., 2009. The cell wall and endoplasmic reticulum stress responses are coordinately regulated in Saccharomyces cerevisiae. Commun. Integr. Biol. 2: 233–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuranda K., Leberre V., Sokol S., Palamarczyk G., François J., 2006. Investigating the caffeine effects in the yeast Saccharomyces cerevisiae brings new insights into the connection between TOR, PKC and Ras/cAMP signalling pathways. Mol. Microbiol. 61: 1147–1166 [DOI] [PubMed] [Google Scholar]
- Lagorce A., le Berre-Anton V., Aguilar-Uscanga B., Martin-Yken H., Dagkessamanskaia A., et al. , 2002. Involvement of GFA1, which encodes glutamine-fructose-6-phosphate amidotransferase, in the activation of chitin synthesis pathway in response to cell-wall defects in Saccharomyces cerevisiae. Eur. J. Biochem. 269: 1697–1707 [DOI] [PubMed] [Google Scholar]
- Lagorce A., Hauser N. C., Labourdette D., Rodriquez C., Martin-Yken H., et al. , 2003. Genome-wide analysis of the response to cell wall mutations in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 278: 20345–20357 [DOI] [PubMed] [Google Scholar]
- Lee K. S., Levin D. E., 1992. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol. Cell. Biol. 12: 172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Irie K., Gotoh Y., Watanabe Y., Araki H., et al. , 1993. A yeast mitogen-activated protein kinase homolog (Mpk1p) mediates signaling by protein kinase C. Mol. Cell. Biol. 13: 3067–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage G., Bussey H., 2006. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70: 317–343; doi: 10.1128/MMBR.00038-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69: 262–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., Bartlett-Heubusch E., 1992. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J. Cell Biol. 116: 1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., Fields F. O., Kunisawa R., Bishop J. M., Thorner J., 1990. A candidate protein kinase C gene, PKC1, is required for the S. cerevisiae cell cycle. Cell 62: 213–224 [DOI] [PubMed] [Google Scholar]
- Levin D. E., Bowers B., Chen C. Y., Kamada Y., Watanabe M., 1994. Dissecting the protein kinase C/MAP kinase signalling pathway of Saccharomyces cerevisiae. Cell. Mol. Biol. Res. 40: 229–239 [PubMed] [Google Scholar]
- Li S., Ault A., Malone C. L., Raitt D., Dean S., et al. , 1998. The yeast histidine protein kinase, Sln1p, mediates phosphotransfer to two response regulators, Ssk1p and Skn7p. EMBO J. 17: 6952–6962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Dean S., Li Z., Horecka J., Deschenes R. J., et al. , 2002. The eukaryotic two-component histidine kinase Sln1p regulates OCH1 via the transcription factor, Skn7p. Mol. Biol. Cell 13: 412–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie S. H., Brown S. S., 1994. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J. Cell Biol. 125: 825–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., 1993. FK506 and cyclosporine, molecular probes for studying intracellular signal transduction. Immunol. Today 14: 290–295 [DOI] [PubMed] [Google Scholar]
- Lodder A. L., Lee T. K., Ballester R., 1999. Characterization of the Wsc1 protein, a putative receptor in the stress response of Saccharomyces cerevisiae. Genetics 152: 1487–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommel M., Bagnat M., Strahl S., 2004. Aberrant processing of the WSC family and Mid2p cell surface sensors results in death of Saccharomyces cerevisiae O-mannosylation mutants. Mol. Cell. Biol. 24: 46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum P. Y., Armour C. D., Stepaniants S. B., Cavet G., Wolf M. K., et al. , 2004. Discovering modes of action for therapeutic compounds using a genome-wide screen of yeast heterozygotes. Cell 116: 121–137 [DOI] [PubMed] [Google Scholar]
- Madden K., Snyder M., 1998. Cell polarity and morphogenesis in budding yeast. Annu. Rev. Microbiol. 52: 687–744 [DOI] [PubMed] [Google Scholar]
- Madden K., Sheu Y. J., Baetz K., Andrews B., Snyder M., 1997. SBF cell cycle regulator as a target of the yeast PKC-MAP kinase pathway. Science 275: 1781–1784 [DOI] [PubMed] [Google Scholar]
- Maeda T., Wurgler-Murphy S. M., Saito H., 1994. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature 369: 242–245 [DOI] [PubMed] [Google Scholar]
- Maeda T., Takekawa M., Saito H., 1995. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science 269: 554–558 [DOI] [PubMed] [Google Scholar]
- Manning B. D., Padmanabha R., Snyder M., 1997. The rho-GEF Rom2p localizes to sites of polarized cell growth and participates in cytoskeletal functions in Saccharomyces cerevisiae. Mol. Biol. Cell 8: 1829–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín M. J., Flández M., Bermejo C., Arroyo J., Martín H., et al. , 2009. Different modulation of the outputs of yeast MAPK-mediated pathways by distinct stimuli and isoforms of the dual-specificity phosphatase Msg5. Mol. Genet. Genomics 281: 345–359 [DOI] [PubMed] [Google Scholar]
- Martín H., Arroyo J., Sanchez M., Molina M., Nombela C., 1993. Activity of the yeast MAP kinase homologue Slt2 is critically required for cell integrity at 37 degrees C. Mol. Gen. Genet. 241: 177–184 [DOI] [PubMed] [Google Scholar]
- Martín H., Rodriguez-Pachon J. M., Ruiz C., Nombela C., Molina M., 2000. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 275: 1511–1519 [DOI] [PubMed] [Google Scholar]
- Martín H., Flández M., Nombela C., Molina M., 2005. Protein phosphatases in MAPK signaling: we keep learning from yeast. Mol. Microbiol. 58: 6–16 [DOI] [PubMed] [Google Scholar]
- Matsui Y., Toh-e A., 1992. Yeast RHO3 and RHO4 ras superfamily genes are necessary for bud growth, and their defect is suppressed by a high dose of bud formation genes CDC42 and BEM1. Mol. Cell. Biol. 12: 5690–5699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison C. P., Spencer S. S., Kresge K. A., Lee J., Ota I. M., 1999. Differential regulation of the cell wall integrity mitogen-activated protein kinase pathway in budding yeast by the protein tyrosine phosphatases Ptp2 and Ptp3. Mol. Cell. Biol. 19: 7651–7660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P., Baginsky W., 1996. In vitro activity of 1,3-β-D-glucan synthase requires the GTP-binding protein Rho1. J. Biol. Chem. 271: 14604–14609 [DOI] [PubMed] [Google Scholar]
- Mazur P., Morin N., Baginsky W., el-Sherbeini M., Clemas J. A., et al. , 1995. Differential expression and function of two homologous subunits of yeast 1,3-β-D-glucan synthase. Mol. Cell. Biol. 15: 5671–5681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni C., Zarov P., Rambourg A., Mann C., 1993. The SLT2 (MPK1) MAP kinase homolog is involved in polarized cell growth in Saccharomyces cerevisiae. J. Cell Biol. 123: 1821–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensonides F. I., Brul S., Klis F. M., Hellingwerf K. J., Teixeira de Mattos M. J., 2005. Activation of the protein kinase C1 pathway upon continuous heat stress in Saccharomyces cerevisiae is triggered by an intracellular increase in osmolarity due to trehalose accumulation. Appl. Environ. Microbiol. 71: 4531–4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchan S., Bernal D., Serrano R., Yenush L., 2004. Response of the Saccharomyces cerevisiae Mpk1 mitogen-activated protein kinase pathway to increases in internal turgor pressure caused by loss of Ppz protein phosphatases. Eukaryot. Cell 3: 100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montijn R. C., Vink E., Muller W. H., Verkleij A. J., Van Den Ende H., et al. , 1999. Localization of synthesis of β-1,6-glucan in Saccharomyces cerevisiae. J. Bacteriol. 181: 7414–7420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlando M., Greco P., Dichtl B., Fatica A., Keller W., et al. , 2002. Functional analysis of yeast snoRNA and snRNA 3′-end formation mediated by uncoupling of cleavage and polyadenylation. Mol. Cell. Biol. 22: 1379–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mösch H. U., Köhler T., Braus G. H., 2001. Different domains of the essential GTPase Cdc42p required for growth and development of Saccharomyces cerevisiae. Mol. Cell. Biol. 21: 235–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M. J., Geiser J. R., Davis T. N., 1996. Ca2+-calmodulin promotes survival of pheromone-induced growth arrest by activation of calcineurin and Ca2+-calmodulin-dependent protein kinase. Mol. Cell. Biol. 16: 4824–4831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouassite M., Camougrand N., Schwob E., Demaison G., Laclau M., et al. , 2000. The ‘SUN’ family: yeast SUN4/SCW3 is involved in cell septation. Yeast 16: 905–919 [DOI] [PubMed] [Google Scholar]
- Mrsa V., Tanner W., 1999. Role of NaOH-extractable cell wall proteins Ccw5p, Ccw6p, Ccw7p and Ccw8p (members of the Pir protein family) in stability of the Saccharomyces cerevisiae cell wall. Yeast 15: 813–820 [DOI] [PubMed] [Google Scholar]
- Mueller C. L., Porter S. E., Hoffman M. G., Jaehning J. A., 2004. The Paf1 complex has functions independent of actively transcribing RNA polymerase II. Mol. Cell 14: 447–456 [DOI] [PubMed] [Google Scholar]
- Nakayama K., Nagasu T., Shimma Y., Kuromitsu J., Jigami Y., 1992. OCH1 encodes a novel membrane bound mannosyltransferase: outer chain elongation of asparagine-linked oligosaccharides. EMBO J. 11: 2511–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi T., Ohya Y., 2010. The cell wall integrity checkpoint: coordination between cell wall synthesis and the cell cycle. Yeast 27: 513–519 [DOI] [PubMed] [Google Scholar]
- Neil H., Malabat C., d’Aubenton-Carafa Y., Xu X., Steinmetz L. M., et al. , 2009. Widespread bidirectional promoters are the major source of cryptic transcripts in yeast. Nature 457: 1038–1042 [DOI] [PubMed] [Google Scholar]
- Neves M. J., François J., 1992. On the mechanism by which a heat shock induces trehalose accumulation in Saccharomyces cerevisiae. Biochem. J. 288: 859–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto S., Watanabe Y., Ninomiya-Tsuji J., Yang L. X., Nagai Y., et al. , 1997. Functional analyses of mammalian protein kinase C isozymes in budding yeast and mammalian fibroblasts. Genes Cells 2: 601–614 [DOI] [PubMed] [Google Scholar]
- Nonaka H., Tanaka K., Hirano H., Fujiwara T., Kohno H., et al. , 1995. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 14: 5931–5938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H., Abe M., Asakawa-Minemura M., Hirata A., Qadota H., et al. , 2010. Multiple functional domains of the yeast l,3-β-glucan synthase subunit Fks1p revealed by quantitative phenotypic analysis of temperature-sensitive mutants. Genetics 184: 1013–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlean P., 1997. Biogenesis of yeast wall and surface components, pp. 229–362 The Molecular Biology of the Yeast Saccharomyces, Vol. 3, edited by Pringle J. R., Broach J. R., Jones E. W. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Ota I. M., Varshavsky A., 1993. A yeast protein similar to bacterial two-component regulators. Science 262: 566–569 [DOI] [PubMed] [Google Scholar]
- Ozaki K., Tanaka K., Imamura H., Hihara T., Kameyama T., et al. , 1996. Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15: 2196–2207 [PMC free article] [PubMed] [Google Scholar]
- Ozaki-Kuroda K., Yamamoto Y., Nohara H., Kinoshita M., Fujiwara T., et al. , 2001. Dynamic localization and function of Bni1p at the sites of directed growth in Saccharomyces cerevisiae. Mol. Cell. Biol. 21: 827–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page N., Gerard-Vincent M., Menard P., Beaulieu M., Azuma M., et al. , 2003. A Saccharomyces cerevisiae genome-wide mutant screen for altered sensitivity to K1 killer toxin. Genetics 163: 875–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paravicini G., Friedli L., 1996. Protein-protein interactions in the yeast PKC1 pathway: Pkc1p interacts with a component of the MAP kinase cascade. Mol. Gen. Genet. 251: 682–691 [DOI] [PubMed] [Google Scholar]
- Paravicini G., Cooper M., Friedli L., Smith D. J., Carpentier J. L., et al. , 1992. The osmotic integrity of the yeast cell requires a functional PKC1 gene product. Mol. Cell. Biol. 12: 4896–4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penheiter K. L., Washburn T. M., Porter S. E., Hoffman M. G., Jaehning J. A., 2005. A posttranscriptional role for the yeast Paf1-RNA polymerase II complex is revealed by identification of primary targets. Mol. Cell 20: 213–223 [DOI] [PubMed] [Google Scholar]
- Perez P., Rincón S. A., 2010. Rho GTPases: regulation of cell polarity and growth in yeasts. Biochem. J. 426: 243–253 [DOI] [PubMed] [Google Scholar]
- Perlin D. S., 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10: 121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J., Zheng Y., Bender L., Myers A., Cerione R., et al. , 1994. Interactions between the bud emergence proteins Bem1p and Bem2p and rho-type GTPases in yeast. J. Cell Biol. 127: 1395–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip B., Levin D. E., 2001. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol. Cell. Biol. 21: 271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao H. L., Machado I. M., Payne G. S., 2007. NPFXD-mediated endocytosis is required for polarity and function of a yeast cell wall stress sensor. Mol. Biol. Cell 18: 57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popolo L., Gilardelli D., Bonfante P., Vai M., 1997. Increase in chitin as an essential response to defects in assembly of cell wall polymers in the ggp1 mutant of Saccharomyces cerevisiae. J. Bacteriol. 179: 463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter S. E., Washburn T. M., Chang M., Jaehning J. A., 2002. The yeast Pafl-RNA polymerase II complex is required for full expression of a subset of cell cycle-regulated genes. Eukaryot. Cell 1: 830–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F., Wurgler-Murphy S. M., Maeda T., Witten E. A., Thai T. C., et al. , 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1–YPD1-SSK1 “two-component” osmosensor. Cell 86: 865–875 [DOI] [PubMed] [Google Scholar]
- Posas F., Takekawa M., Saito H., 1998. Signal transduction by MAP kinase cascades in budding yeast. Curr. Opin. Microbiol. 1: 175–182 [DOI] [PubMed] [Google Scholar]
- Pring M., Evangelista M., Boone C., Yang C., Zigmond S. H., 2003. Mechanism of forming-induced nucleation of actin filaments. Biochemistry 42: 486–496 [DOI] [PubMed] [Google Scholar]
- Pruyne D., Evangelista M., Yang C., Bi E., Zigmond S., et al. , 2002. Role of formins in actin assembly: nucleation and barbed-end association. Science 297: 612–615 [DOI] [PubMed] [Google Scholar]
- Qadota H., Anraku Y., Botstein D., Ohya Y., 1994. Conditional lethality of a yeast strain expressing human RHOA in place of RHO1. Proc. Natl. Acad. Sci. USA 91: 9317–9321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadota H., Python C. P., Inoue S. B., Arisawa M., Anraku Y., et al. , 1996. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-β-glucan synthase. Science 272: 279–281 [DOI] [PubMed] [Google Scholar]
- Qian F., Wei W., Germino G., Oberhauser A., 2005. The nanomechanics of polycystin-1 extracellular region. J. Biol. Chem. 280: 40723–40730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queralt E., Igual J. C., 2005. Functional connection between the Clb5 cyclin, the protein kinase C pathway and the Swi4 transcription factor in Saccharomyces cerevisiae. Genetics 17: 1485–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavel M., Philip B., Buehrer B. M., Errede B., Levin D. E., 1999. Mid2 is a putative sensor for cell integrity signaling in Saccharomyces cerevisiae. Mol. Cell. Biol. 19: 3969–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram A. F., Brekelmans S. S. C., Oehlen L. J. W. M., Klis F. M., 1995. Identification of two cell cycle regulated genes affecting the β-1,3-glucan content of cell wall in Saccharomyces cerevisiae. FEBS Lett. 358: 165–170 [DOI] [PubMed] [Google Scholar]
- Rasmussen T. P., Culbertson M. R., 1998. The putative nucleic acid helicase Sen1p is required for formation and stability of termini and for maximal rates of synthesis and levels of accumulation of small nucleolar RNAs in S. cerevisiae. Mol. Cell. Biol. 18: 6885–6896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. A., Morris E. R., Thom D., Madden J. K., 1982. Shapes and interactions of carbohydrate chains, pp. 196–290 The Polysaccharides, Vol. 1, edited by Aspinall G. O. Academic Press, New York [Google Scholar]
- Reinoso-Martín C., Schüller C., Schuetzer-Muehlbauer M., Kuchler K., 2003. The yeast protein kinase C cell integrity pathway mediates tolerance to the antifungal drug caspofungin through activation of Slt2p mitogen-activated protein kinase signaling. Eukaryot. Cell 2: 1200–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds T. B., Fink G. R., 2001. Bakers’ yeast, a model for fungal biofilm formation. Science 291: 878–881 [DOI] [PubMed] [Google Scholar]
- Richman T. J., Johnson D. I., 2000. Saccharomyces cerevisiae cdc42p GTPase is involved in preventing the recurrence of bud emergence during the cell cycle. Mol. Cell. Biol. 20: 8548–8559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J., Nelson B., Marton M. J., Stoughton R., Meyer M. R., et al. , 2000. Signaling and circuitry of multiple MAP kinase pathways revealed by a matrix of global gene expression profiles. Science 287: 873–880 [DOI] [PubMed] [Google Scholar]
- Robinson N. G., Guo L., Imai J., Tohe A., Matsui Y., et al. , 1999. Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol. Cell. Biol. 19: 3580–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodicio R., Heinisch J. J., 2010. Together we are strong: cell wall integrity sensors in yeast. Yeast 27: 531–540 [DOI] [PubMed] [Google Scholar]
- Roelants F. M., Torrance P. D., Thorner J., 2004. Differential roles of PDK1- and PDK2-phosphorylation sites in the yeast AGC kinases Ypk1, Pkc1 and Sch9. Microbiology 150: 3289–3304 [DOI] [PubMed] [Google Scholar]
- Roelants F. M., Baltz A. G., Trott A. E., Fereres S., Thorner J., 2010. A protein kinase network regulates the function of aminophospholipid flippases. Proc. Natl. Acad. Sci. USA 107: 34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer T., Paravicini G., Payton M. A., Bussey H., 1994. Characterization of the yeast (1–6)-β-glucan biosynthetic components, Kre6p and Skn1p, and genetic interactions between the PKC1 pathway and extracellular matrix assembly. J. Cell Biol. 127: 567–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondón A. G., Gallardo M., García-Rubio M., Aguilera A., 2004. Molecular evidence indicating that the yeast PAF complex is required for transcription elongation. EMBO Rep. 5: 47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumanie O., Weinachter C., Larrieu I., Crouzet M., Doignon F., 2001. Functional characterization of the Bag7, Lrg1 and Rgd2 RhoGAP proteins from Saccharomyces cerevisiae. FEBS Lett. 506: 149–156 [DOI] [PubMed] [Google Scholar]
- Sagot I., Klee S. K., Pellman D., 2002a Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 4: 42–50 [DOI] [PubMed] [Google Scholar]
- Sagot I., Rodal A. A., Moseley J., Goode B. L., Pellman D., 2002b An actin nucleation mechanism mediated by Bni1 and profilin. Nat. Cell Biol. 4: 626–631 [DOI] [PubMed] [Google Scholar]
- Saka A., Abe M., Okano H., Minemura M., Qadota H., et al. , 2001. Complementing yeast rho1 mutation groups with distinct functional defects. J. Biol. Chem. 276: 46165–46171 [DOI] [PubMed] [Google Scholar]
- Schafer R. W., Rine J., 1992. Protein prenylation: genes, enzymes, targets, and functions. Annu. Rev. Genet. 26: 209–237 [DOI] [PubMed] [Google Scholar]
- Schekman R., Brawley V., 1979. Localized deposition of chitin on the yeast cell surface in response to mating pheromone. Proc. Natl. Acad. Sci. USA 76: 645–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimoler-O’Rourke R., Renault S., Mo W., Selitrennikoff C. P., 2003. Neurospora crassa FKS protein binds to the (1,3)β-glucan synthase substrate, UDP-glucose. Curr. Microbiol. 63: 408–412 [DOI] [PubMed] [Google Scholar]
- Schmelzle T., S. B. Helliwell, Hall M. N., 2002. Yeast protein kinases and the RHO1 exchange factor TUS1 are novel components of the cell integrity pathway in yeast. Mol. Cell. Biol. 22: 1329–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Bickle M., Beck T., Hall M. N., 1997. The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell 88: 531–542 [DOI] [PubMed] [Google Scholar]
- Schmidt A., Schmelzle T., Hall M., 2002. The RHO1-GAPs SAC7, BEM2, and BAG7 control distinct RHO1 functions in Saccharomyces cerevisiae. Mol. Microbiol. 45: 1433–1441 [DOI] [PubMed] [Google Scholar]
- Schmitz H. P., Huppert S., Lorberg A., Heinisch J. J., 2002a Rho5p downregulates the yeast cell integrity pathway. J. Cell Sci. 115: 3139–3148 [DOI] [PubMed] [Google Scholar]
- Schmitz H. P., Lorberg A., Heinisch J. J., 2002b Regulation of yeast protein kinase C activity by interaction with the small GTPase Rho1p through its amino-terminal HR1 domain. Mol. Microbiol. 44: 829–840 [DOI] [PubMed] [Google Scholar]
- Scrimale T., Didone L., de Mesy Bentley K. L., Krysan D. J., 2009. The unfolded protein response is induced by the cell wall integrity mitogen-activated protein kinase signaling cascade and is required for cell wall integrity in Saccharomyces cerevisiae. Mol. Biol. Cell 20: 164–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechi A. S., Wehland J., 2000. The actin cytoskeleton and plasma membrane connection: PtdIns(4,5)P(2) influences cytoskeletal protein activity at the plasma membrane. J. Cell Sci. 113: 3685–3695 [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G., Taylor I. A., Adam A. C., Spanos A., Howell S., et al. , 1998. Structural and functional architecture of the yeast cell-cycle transcription factor Swi6. J. Mol. Biol. 281: 763–775 [DOI] [PubMed] [Google Scholar]
- Sekiya-Kawasaki M., Abe M., Saka A., Watanabe D., Kono K., et al. , 2002. Dissection of upstream regulatory components of the Rho1 effector, 1,3-β-glucan synthase, in Saccharomyces cerevisiae. Genetics 162: 663–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R., Martín H., Casamayor A., Ariño J., 2006. Signaling alkaline pH stress in the yeast Saccharomyces cerevisiae through the Wsc1 cell surface sensor and the Slt2 MAPK pathway. J. Biol. Chem. 281: 39785–39795 [DOI] [PubMed] [Google Scholar]
- Shahinian S., Bussey H., 2000. β-1,6-Glucan synthesis in Saccharomyces cerevisiae. Mol. Microbiol. 35: 477–489 [DOI] [PubMed] [Google Scholar]
- Shankarnarayan S., Malone C. L., Deschenes R. J., Fassler J. S., 2008. Modulation of yeast Sln1 kinase activity by the Ccw12 cell wall protein. J. Biol. Chem. 283: 1962–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon K. E., Mauger D. M., Arndt K. M., 2005. A requirement for the S. cerevisiae Paf1 complex in snoRNA 3′ end formation. Mol. Cell 20: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu Y. J., Santos B., Fortin N., Costigan C., Snyder M., 1998. Spa2p interacts with cell polarity proteins and signaling components involved in yeast cell morphogenesis. Mol. Cell. Biol. 18: 4053–4069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J. L., Reck-Peterson S. L., Newitt R., Mooseker M. S., Aebersold R., et al. , 2005. Cell polarity protein Spa2P associates with proteins involved in actin function in Saccharomyces cerevisiae. Mol. Biol. Cell 16: 4595–4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu J., Yoda K., Yamasaki M., 1994. The hypo-osmolarity-sensitive phenotype of the Saccharomyces cerevisiae hpo2 mutant is due to a mutation in PKC1, which regulates expression of β-glucanase. Mol. Gen. Genet. 242: 641–648 [DOI] [PubMed] [Google Scholar]
- Sidorova J. M., Breeden L. L., 1993. Analysis of the SWI4/SWI6 protein complex, which directs G1/S-specific transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 13: 1069–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorova J. M., Mikesell G. E., Breeden L. L., 1995. Cell cycle-regulated phosphorylation of Swi6 controls its nuclear localization. Mol. Biol. Cell 6: 1641–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. A., Lindquist S., 1998. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol. Cell 1: 639–648 [DOI] [PubMed] [Google Scholar]
- Smits G. J., Kapteyn J. C., van den Ende H., Klis F. M., 1999. Cell wall dynamics in yeast. Curr. Opin. Microbiol. 2: 348–352 [DOI] [PubMed] [Google Scholar]
- Sobering A. K., Watanabe R., Romeo M. J., Yan B. C., Specht C. A., et al. , 2004. Yeast Ras regulates the complex that catalyzes the first step in GPI-anchor biosynthesis at the ER. Cell 117: 637–648 [DOI] [PubMed] [Google Scholar]
- Spellman P. T., Sherlock G., Zhang M. Q., Iyer V. R., Anders K., et al. , 1998. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol. Biol. Cell 9: 3273–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos A. M., Cyert M. S., 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11: 3432–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz E. J., Conrad N. K., Brow D. A., Corden J. L., 2001. RNA-binding protein Nrd1 directs poly(A)-independent 3′-end formation of RNA polymerase II transcripts. Nature 413: 327–331 [DOI] [PubMed] [Google Scholar]
- Steinmetz E. J., Warren C. L., Kuehner J. N., Panbehi B., Ansari A. Z., et al. , 2006. Genome-wide distribution of yeast RNA polymerase II and its control by Sen1 helicase. Mol. Cell 24: 735–746 [DOI] [PubMed] [Google Scholar]
- Stolinski L. A., Eisenmann D. M., Arndt K. M., 1997. Identification of RTF1, a novel gene important for TATA site selection by TATA box-binding protein in S. cerevisiae. Mol. Cell. Biol. 17: 4490–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straede A., Heinisch J. J., 2007. Functional analyses of the extra- and intracellular domains of the yeast cell wall integrity sensors Mid2 and Wsc1. FEBS Lett. 581: 4495–4500 [DOI] [PubMed] [Google Scholar]
- Strahl-Bolsinger S., Gentzsch M., Tanner W., 1999. Protein O-mannosylation. Biochim. Biophys. Acta 1426: 297–307 [DOI] [PubMed] [Google Scholar]
- Suzuki M., Igarashi R., Sekiya M., Utsugi T., Morishita S., et al. , 2004. Dynactin is involved in a checkpoint to monitor cell wall synthesis in Saccharomyces cerevisiae. Nat. Cell Biol. 6: 861–871 [DOI] [PubMed] [Google Scholar]
- Takaki T., Trenz K., Costanzo V., Petronczki M., 2008. Polo-like kinase 1 reaches beyond mitosis: cytokinesis, DNA damage response, and development. Curr. Opin. Cell Biol. 20: 650–660 [DOI] [PubMed] [Google Scholar]
- Tatebayashi K., Tanaka K., Yang H. Y., Yamamoto K., Matsushita Y., et al. , 2007. Transmembrane mucins Hkr1 and Msb2 are putative osmosensors in the SHO1 branch of yeast HOG pathway. EMBO J. 26: 3521–3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I. A., McIntosh P. B., Pala P., Treiber M. K., Howell S., et al. , 2000. Characterization of the DNA-binding domains from the yeast cell-cycle transcription factors Mbp1 and Swi4. Biochemistry 39: 3943–3954 [DOI] [PubMed] [Google Scholar]
- Terashima H., Yabuki N., Arisawa M., Hamada K., Kitada K., 2000. Up-regulation of genes encoding glycosylphosphatidylinositol (GPI)-attached proteins in response to cell wall damage caused by disruption of FKS1 in Saccharomyces cerevisiae. Mol. Gen. Genet. 264: 64–74 [DOI] [PubMed] [Google Scholar]
- Toh-e A., Yasunaga S., Nisogi H., Tanaka K., Oguchi T., et al. , 1993. Three yeast genes, PIR1, PIR2 and PIR3, containing internal tandem repeats, are related to each other, and PIR1 and PIR2 are required for tolerance to heat shock. Yeast 9: 481–494 [DOI] [PubMed] [Google Scholar]
- Tolliday N., VerPlank L., Li R., 2002. Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Curr. Biol. 12: 1864–1870 [DOI] [PubMed] [Google Scholar]
- Torres J., di Como C. J., Herrero E., Angeles de la Torre-Ruiz M., 2002. Regulation of the cell integrity pathway by rapamycin-sensitive TOR function in budding yeast. J. Biol. Chem. 277: 43495–43504 [DOI] [PubMed] [Google Scholar]
- Truman A. W., Millson S. H., Nuttall J. M., King V., Mollapour M., et al. , 2006. Expressed in the yeast Saccharomyces cerevisiae, human ERK5 is a client of the Hsp90 chaperone that complements loss of the Slt2p (Mpk1p) cell integrity stress-activated protein kinase. Eukaryot. Cell 5: 1914–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman A. W., Kim K.-Y., Levin D. E., 2009. Mechanism of Mpk1 mitogen-activated protein kinase binding to the Swi4 transcription factor and its regulation by a novel caffeine-induced phosphorylation. Mol. Cell. Biol. 29: 6449–6461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umikawa M., Tanaka K., Kamei T., Shimizu K., Imamura H., et al. , 1998. Interaction of Rho1p target Bni1p with F-actin-binding elongation factor 1α: implication in Rho1p-regulated reorganization of the actin cytoskeleton in Saccharomyces cerevisiae. Oncogene 16: 2011–2016 [DOI] [PubMed] [Google Scholar]
- Ursic D., Himmel K. L., Gurley K. A., Webb F., Culbertson M. R., 1997. The yeast SEN1 gene is required for the processing of diverse RNA classes. Nucleic Acids Res. 25: 4778–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsugi T., Minemura M., Hirato A., Abe M., Watanabe D., et al. , 2002. Movement of yeast 1,3-β-glucan synthase is essential for uniform cell wall biosynthesis. Genes Cells 7: 1–9 [DOI] [PubMed] [Google Scholar]
- Valdivia R. H., Schekman R., 2003. The yeasts Rho1p and Pkc1p regulate the transport of chitin synthase III (Chs3p) from internal stores to the plasma membrane. Proc. Natl. Acad. Sci. USA 100: 10287–10292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivieso M. H., Ferrario L., Vai M., Duran A., Popolo L., 2000. Chitin synthesis in a gas1 mutant of Saccharomyces cerevisiae. J. Bacteriol. 182: 4752–4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drogen F., Peter M., 2002. Spa2p functions as a scaffold-like protein to recruit the Mpk1p MAP kinase module to sites of polarized growth. Curr. Biol. 12: 1698–1703 [DOI] [PubMed] [Google Scholar]
- Vay H. A., Philip B., Levin D. E., 2004. Mutational analysis of the cytoplasmic domain of the Wsc1 cell wall stress sensor. Mol. Microbiol. 150: 3281–3288 [DOI] [PubMed] [Google Scholar]
- Verna J., Lodder A., Lee K., Vagts A., Ballester R., 1997. A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94: 13804–13809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilella F., Herrero E., Torres J., de la Torre-Ruiz M. A., 2005. Pkc1 and the upstream elements of the cell integrity pathway in Saccharomyces cerevisiae, Rom2 and Mtl1, are required for cellular responses to oxidative stress. J. Biol. Chem. 280: 9149–9159 [DOI] [PubMed] [Google Scholar]
- Vink E., Rodriguez-Suarez R. J., Gérard-Vincent M., Ribas J. C., de Nobel H., et al. , 2004. An in vitro assay for (1,6)-β-D-glucan synthesis in Saccharomyces cerevisiae. Yeast 21: 1121–1131 [DOI] [PubMed] [Google Scholar]
- Watanabe D., Abe M., Ohya Y., 2001. Yeast Lrg1p acts as a specialized RhoGAP regulating 1,3-β-Glucan synthesis. Yeast 18: 943–951 [DOI] [PubMed] [Google Scholar]
- Watanabe M., Chen C.-Y., Levin D. E., 1994. Saccharomyces cerevisiae PKC1 encodes a protein kinase C (PKC) homolog with a substrate specificity similar to that of mammalian PKC. J. Biol. Chem. 269: 16829–16836 [PubMed] [Google Scholar]
- Watanabe Y., Irie K., Matsumoto K., 1995. Yeast RLM1 encodes a serum response factor-like protein that may function downstream of the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 15: 5740–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Takaesu G., Hagiwara M., Irie K., Matsumoto K., 1997. Characterization of a serum response factor-like protein in Saccharomyces cerevisiae, Rlm1, which has transcriptional activity regulated by the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17: 2615–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen K. K., Rubenstein P. A., 2009. Differential regulation of actin polymerization and structure by yeast formin isoforms. J. Biol. Chem. 284: 16776–16783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold N. P., Lewis R. E., 2003. The echinocandin antifungals: an overview of the pharmacology, spectrum and clinical efficacy. Expert Opin. Investig. Drugs 12: 1313–1333 [DOI] [PubMed] [Google Scholar]
- Williams K. E., Cyert M. S., 2001. The eukaryotic response regulator Skn7p regulates calcineurin signaling through stabilization of Crz1p. EMBO J. 20: 3473–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withee J. L., Mulholland J., Jeng R., Cyert M. S., 1997. An essential role of the yeast pheromone-induced Ca2+ signal is to activate calcineurin. Mol. Biol. Cell 8: 263–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A., Schneider J., Dover J., Johnston M., Shilatifard A., 2003. The Paf1 complex is essential for histone monoubiquitination by the Rad6-Bre1 complex, which signals for histone methylation by COMPASS and Dot1p. J. Biol. Chem. 278: 34739–34742 [DOI] [PubMed] [Google Scholar]
- Wyers F., Rougemaille M., Badis G., Rousselle J. C., Dufour M. E., et al. , 2005. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121: 725–737 [DOI] [PubMed] [Google Scholar]
- Yamochi W., Tanaka K., Nonaka H., Maeda A., Musha T., et al. , 1994. Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J. Cell Biol. 125: 1077–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Luo H., Lee J. D., Abe J., Berk B. C., 2001. Molecular cloning of mouse ERK5/BMK1 splice variants and characterization of ERK5 functional domains. J. Biol. Chem. 276: 10870–10878 [DOI] [PubMed] [Google Scholar]
- Yashar B., Irie K., Printen J. A., Stevenson B. J., Sprague G. F., Jr, et al. , 1995. Yeast MEK-dependent signal transduction: response thresholds and parameters affecting fidelity. Mol. Cell. Biol. 15: 6545–6553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Ohya Y., Goebl M., Nakano A., Anraku Y., 1994a A novel gene, STT4, encodes a phosphatidylinositol 4-kinase in the PKC1 protein kinase pathway of Saccharomyces cerevisiae. J. Biol. Chem. 269: 1166–1172 [PubMed] [Google Scholar]
- Yoshida S., Ohya Y., Nakano A., Anraku Y., 1994b Genetic interactions among genes involved in the STT4–PKC1 pathway of Saccharomyces cerevisiae. Mol. Gen. Genet. 242: 631–640 [DOI] [PubMed] [Google Scholar]
- Yoshida S., Kono K., Lowery D. M., Bartolini S., Yaffe M. B., et al. , 2006. Polo-like kinase Cdc5 controls the local activation of Rho1 to promote cytokinesis. Science 313: 108–111 [DOI] [PubMed] [Google Scholar]
- Yoshida S., Bartolini S., Pellman D., 2009. Mechanisms for concentrating Rho1 during cytokinesis. Genes Dev. 23: 810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. W., Mendrola J. M., Audhya A., Singh S., Keleti D., et al. , 2004. Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol. Cell 13: 677–688 [DOI] [PubMed] [Google Scholar]
- Zarzov P., Mazzoni C., Mann C., 1996. The Slt2 (Mpk1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO J. 15: 83–91 [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhou B., Zheng C.-F., Zhang Z.-Y., 2003. A bipartite mechanism for ERK2 recognition by its cognate regulators and substrates. J. Biol. Chem. 278: 29901–29912 [DOI] [PubMed] [Google Scholar]
- Zhang X., Bi E., Novick P., Du L., Kozminski K. G., et al. , 2001. Cdc42 interacts with the exocyst and regulates polarized exocytosis. J. Biol. Chem. 276: 46745–46750 [DOI] [PubMed] [Google Scholar]
- Zhang X., Orlando K., He B., Xi F., Zhang J., et al. , 2008. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J. Cell Biol. 180: 145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Jung U. S., Garrett-Engele P., Roe T., Cyert M. S., et al. , 1998. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol. 18: 1013–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Shen Z.-M., Kahn P. C., Lipke P. N., 2001. Interaction of alpha-agglutinin and a-agglutinin, Saccharomyces cerevisiae sexual cell adhesion molecules. J. Bacteriol. 183: 2874–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik H., Fernandez M. P., Bowers B., Cabib E., 1984. Saccharomyces cerevisiae mannoproteins form an external cell wall layer that determines wall porosity. J. Bacteriol. 181: 1018–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]