Abstract
Background
Stomatal guard cells are the regulators of gas exchange between plants and the atmosphere. Ca2+-dependent and Ca2+-independent mechanisms function in these responses. Key stomatal regulation mechanisms, including plasma membrane and vacuolar ion channels have been identified and are regulated by the free cytosolic Ca2+ concentration ([Ca2+]cyt).
Scope
Here we show that CO2-induced stomatal closing is strongly impaired under conditions that prevent intracellular Ca2+ elevations. Moreover, Ca2+ oscillation-induced stomatal closing is partially impaired in knock-out mutations in several guard cell-expressed Ca2+-dependent protein kinases (CDPKs) here, including the cpk4cpk11 double and cpk10 mutants; however, abscisic acid-regulated stomatal movements remain relatively intact in the cpk4cpk11 and cpk10 mutants. We further discuss diverse studies of Ca2+ signalling in guard cells, discuss apparent peculiarities, and pose novel open questions. The recently proposed Ca2+ sensitivity priming model could account for many of the findings in the field. Recent research shows that the stomatal closing stimuli abscisic acid and CO2 enhance the sensitivity of stomatal closing mechanisms to intracellular Ca2+, which has been termed ‘calcium sensitivity priming’. The genome of the reference plant Arabidopsis thaliana encodes for over 250 Ca2+-sensing proteins, giving rise to the question, how can specificity in Ca2+ responses be achieved? Calcium sensitivity priming could provide a key mechanism contributing to specificity in eukaryotic Ca2+ signal transduction, a topic of central interest in cell signalling research. In this article we further propose an individual stomatal tracking method for improved analyses of stimulus-regulated stomatal movements in Arabidopsis guard cells that reduces noise and increases fidelity in stimulus-regulated stomatal aperture responses ( Box 1). This method is recommended for stomatal response research, in parallel to previously adopted blind analyses, due to the relatively small and diverse sizes of stomatal apertures in the reference plant Arabidopsis thaliana.
Keywords: Stomata, ABA, guard cell, Ca2+, CDPK, calcium sensitivity priming, carbon dioxide, Arabidopsis thaliana
INTRODUCTION
Stomatal guard cells play a critical role in the regulation of plant gas exchange and water use efficiency (Kim et al., 2010). Several key physiological stimuli regulate stomatal aperture, including abscisic acid (ABA), CO2 and pathogenic elicitors (Raschke et al., 1988; Pandey et al., 2007; Joshi-Saha et al., 2010; Kim et al., 2011). One signalling component that links these diverse stimuli is the cytosolic free-calcium concentration ([Ca2+]cyt), which induces stomatal closure (DeSilva et al., 1985; Gilroy et al., 1990; McAinsh et al., 1990; Webb et al., 1996; Grabov and Blatt, 1998; Allen et al., 1999a; Staxén et al., 1999; Klüsener et al., 2002; Young et al., 2006). Several independent mechanisms have been identified that are activated by [Ca2+]cyt in guard cells, and contribute to ABA-induced stomatal closing, including [Ca2+]cyt activation of S-type anion channels (Schroeder and Hagiwara, 1989; Mori et al., 2006; Vahisalu et al., 2008; Siegel et al., 2009; Chen et al., 2010), [Ca2+]cyt activation of R-type anion channels (Hedrich et al., 1990; Meyer et al., 2010), [Ca2+]cyt down-regulation of plasma membrane proton pumps (Kinoshita et al., 1995), [Ca2+]cyt down-regulation of K+ influx channels (Schroeder and Hagiwara, 1989; Kelly et al., 1995; Grabov and Blatt, 1997; Kwak et al., 2001) and Ca2+-activation of vacuolar K+ release (VK) channels (Ward and Schroeder, 1994; Allen and Sanders, 1996; Gobert et al., 2007).
These studies and subsequent studies have identified mechanisms through which plasma membrane and tonoplast ion channels and ATPases function as key mediators in a network that controls stomatal closing and inhibits stomatal opening (reviewed in MacRobbie, 1998; Schroeder et al., 2001; Pandey et al., 2007). The importance of [Ca2+]cyt, relative to Ca2+-elevation-independent control of stomatal closure has recently been quantified in Arabidopsis showing that the Ca2+-dependent response is responsible for approx. 70 % of the ABA response (Siegel et al., 2009). This research is consistent with previous studies that demonstrated a requirement for Ca2+ in ABA-induced closure (DeSilva et al., 1985; Schwartz, 1985; McAinsh et al., 1990; Grabov and Blatt, 1998; MacRobbie, 2000). Recent ABA signalling models (Cutler et al., 2010; Hubbard et al., 2010; Melcher et al., 2010) have not yet incorporated a clear role for [Ca2+]cyt. [Ca2+]cyt elevation-independent ABA-induced stomatal closure accounted for approx. 30 % of the response (Siegel et al., 2009). Ca2+-independent signalling mechanisms function in ABA- and CO2-induced stomatal closing and have been reviewed in depth elsewhere (Levchenko et al., 2005; Israelsson et al., 2006; Sirichandra et al., 2009; Kim et al., 2010; Hubbard et al., 2010; Raghavendra et al., 2010). In this study, we examine and review studies that have identified and analysed Ca2+-dependent stomatal closure and provide an overview of the mechanisms that have been established during [Ca2+]cyt signalling in guard cells, and consider models of how specificity and plasticity in Ca2+-signalling networks may be achieved.
DYNAMICS IN ‘RESTING’ [Ca2+]cyt AND SPONTANEOUS Ca2+ TRANSIENTS
Due to the cytotoxicity of large Ca2+ concentrations inside cells and diverse Ca2+-binding proteins expressed in cells, the free cytosolic Ca2+ concentration is maintained within the 150 nm range in unstimulated cells through the action of Ca2+-ATPases and Ca2+–H+ ion exchangers at the plasma membrane and membranes of internal stores. However, a number of studies have described fluctuations in [Ca2+]cyt in guard cells, which had not yet been challenged with ABA or known stimuli (with the caveat that Ca2+ imaging experiments are performed under specific conditions which may inadvertently include physiological stimuli). For example, in Vicia faba guard cells, spontaneous increases in [Ca2+]cyt were observed before the application of ABA (Grabov and Blatt, 1998). Similarly, spontaneous transients were noted in a subset of unstimulated Commelina communis cells (Staxén et al., 1999), and spontaneous [Ca2+]cyt transients were observed in Arabidopsis guard cells expressing the non-invasive FRET reporter Yellow Cameleon with no exposure to ABA (Allen et al., 1999b; Klüsener et al., 2002). As with stimulus-induced [Ca2+]cyt increases (see below), spontaneous [Ca2+]cyt transients are not necessarily synchronized between guard cells of the same pair (Allen et al., 1999b). It is possible that [Ca2+]cyt increases happen not at the level of the cell but at the level of [Ca2+]cyt microdomains, as observed in mammalian tissue (Berridge, 2006). This may contribute to the highly stochastic nature of spontaneous [Ca2+]cyt transients. Spontaneous Ca2+ transients have also been observed in guard cells of intact leaves of Arabidopsis plants (Yang et al., 2008). These Ca2+ transients can be inhibited by loading cells with high concentrations of Ca2+ chelators including the Ca2+ chelating reporter fura-2 and its derivative BAPTA (Young et al., 2006). Interestingly, these spontaneous Ca2+ transients can be inhibited by removal of extracellular Ca2+ or by buffering the extracellular free Ca2+ concentration to 200 nm (Young et al., 2006; Siegel et al., 2009) and can be dampened or removed by more positive (depolarized) membrane potentials in guard cells (Grabov and Blatt, 1998; Staxén et al., 1999; Klüsener et al., 2002; Young et al., 2006). Spontaneous [Ca2+]cyt transients have been observed in guard cells from several species and using different [Ca2+]cyt imaging techniques indicating that spontaneous transients in [Ca2+]cyt are a conserved feature of guard cell [Ca2+]cyt dynamics that are sensitive to external Ca2+ and cytoplasmically loaded Ca2+ buffer concentrations.
While the nature and function of spontaneous Ca2+ transients is currently poorly understood, it is likely that they include a contribution from Ca2+-permeable cation channels in the plasma membrane that are activated at increasingly negative (hyperpolarized) membrane voltages (Aharon et al., 1998; Hamilton et al., 2000; Pei et al., 2000). Driving membrane voltage to more negative (hyperpolarized) membrane potentials increased the amplitude of [Ca2+]cyt transients (Grabov and Blatt, 1998) and increases the open probability of Ca2+-permeable cation channels in the plasma membrane of guard cells (Hamilton et al., 2000; Pei et al., 2000). Consistent with these findings, the proportion of guard cells with transients and the frequency of transients is dependent on the incubation buffer used. Low [K+]ext (0·1 mm) results in more negative (hyperpolarized) membrane potentials and concomitant rapid transients in the majority of cells, while high [K+]ext (100 mm) causes more positive (depolarized) membrane potentials and abolishes the Ca2+ transients (Klüsener et al., 2002). That some cells exhibit spontaneous transients while others in the same epidermal preparation have constant resting [Ca2+]cyt levels may reflect a contribution from variation in the membrane potentials of the population of cells, with only those passing a certain threshold of negative voltages exhibiting spontaneous transients.
CO2-INDUCED STOMATAL CLOSING IS STRONGLY IMPAIRED IN THE ABSENCE OF Ca2+ ELEVATIONS
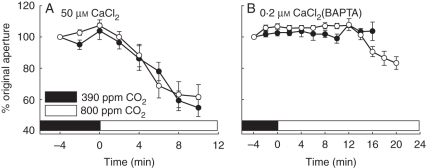
The significance of [Ca2+]cyt-dependent ABA-induced stomatal closure, relative to Ca2+-elevation-independent control of stomatal closure, has recently been quantified in Arabidopsis using the above described approaches that inhibit spontaneous and ABA-induced [Ca2+]cyt increases. Guard cells where increases in [Ca2+]cyt were prevented using the Ca2+-chelator BAPTA showed only 30 % of the ABA-induced stomatal closure response compared with guard cells where [Ca2+]cyt was allowed to increase (Siegel et al., 2009). Under the same experimental conditions that inhibit [Ca2+]cyt elevations (i.e. in the presence of BAPTA, buffering free Ca2+ concentration in the extracellular medium to 200 nm; Siegel et al., 2009), we analysed stomatal closing induced by elevation in the CO2 concentration here. Interestingly, under these conditions, CO2-induced stomatal closing was strongly inhibited (Fig. 1B), whereas addition of 50 µm Ca2+ to the bath medium was sufficient to ensure a robust CO2-induced stomatal closing response (Fig. 1A). Thus CO2-induced stomatal closing is strongly Ca2+ dependent in Arabidopsis, consistent with previous findings in Commelina guard cells (Schwartz, 1985; Webb et al., 1996). The Ca2+ requirement for CO2-induced stomatal closing (Fig. 1) is particularly interesting because recent research has revealed that the Ca2+-independent protein kinase, OST1, is required for CO2-induced stomatal closure in Arabidopsis (Xue et al., 2011). These data indicate that present models strictly separating Ca2+-dependent and Ca2+-independent stomatal closing may be oversimplified, and that these mechanisms may interact in an unknown manner and will require further research.
Fig. 1.
CO2-induced stomatal closing is strongly inhibited under conditions that prevent [Ca2+]cyt elevations in guard cells. (A) Robust CO2-induced stomatal closing was observed in response to CO2 elevation with 50 µm Ca2+ added to the bathing medium (10 mm KCl, 7·5 mm iminodeactic acid, 10 mm MES, pH 6·2). Stomatal closing of individual stomata was tracked (see Box 1 for Methods) in response to elevations in the extracellular CO2 concentration from ambient (approx. 390 ppm) to 800 ppm (added at time = 0) with 50 µm Ca2+ in the bathing medium. (B) Buffering the extracellular free Ca2+ concentration to a low level of 200 nm using the Ca2+ chelator BAPTA strongly inhibited CO2-induced stomatal closing. Extracellular bathing media and free Ca2+ buffering to 200 nm was performed using identical solutions to those reported in Siegel et al. (2009). Experiments were conducted on 4- to 5-week-old plants according to the protocols described in (Siegel et al., 2009). CO2-induced stomatal closing experiments were performed under identical Ca2+ solutions to those published for ABA-induced stomatal closing experiments with 200 nm free Ca2+ or 50 µM Ca2+ in the bath medium (Siegel et al., 2009). Two independent experiments are shown for each condition (open and closed symbols); errors are presented as ± s.e. of the mean, n = 30.
STIMULUS-INDUCED CHANGES IN [Ca2+]cyt
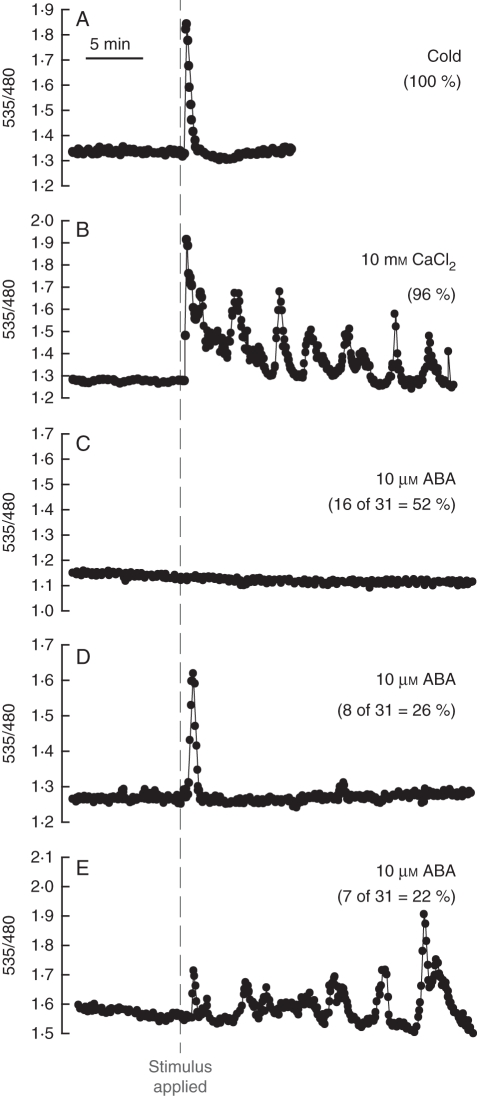
Several distinct stimuli are known to cause an increase in guard cell [Ca2+]cyt, including cold (Allen et al., 2000; Wood et al., 2000), extracellular Ca2+ ([Ca2+]ext) (McAinsh et al., 1995), ABA (McAinsh et al., 1990; Schroeder and Hagiwara, 1990; Grabov and Blatt, 1998; Allen et al., 1999a, b; Staxén et al., 1999; MacRobbie, 2000; Jung et al., 2002; Marten et al., 2007), CO2 (Webb et al., 1996; Young et al., 2006) and certain pathogenic elicitors (Klüsener et al., 2002). In cells that show constant resting Ca2+ levels, upon application of a given stimulus, [Ca2+]cyt can increase from approx. 100–150 nm to ≥350 nm. The pattern of [Ca2+]cyt increases is related to the stimulus applied and the buffer in which cells are incubated. For example, for cells incubated in a high [K+] buffer (50 mm) a cold shock results in a single large transient (Fig. 2A), whereas exposure to high [Ca2+]ext induces [Ca2+]cyt oscillations (Fig. 2B).
Fig. 2.
Stimulus-induced increases in guard cell [Ca2+]cyt. Plants transformed with the FRET-based Ca2+ sensor YC3·6, targeted to guard cells with the pGC1 guard cell promoter, were imaged (Allen et al., 1999b). Cells were initially incubated in a stomatal-opening buffer and then after 10 min of recording were challenged with (A) a cold shock (4 °C; n = 15), (B) 10 mm external CaCl2 (n = 26) or (C–E) 10 µm ABA (n = 31) as indicated. External [Ca2+] elevation induces oscillations in [Ca2+]cyt consistent with previous studies (McAinsh et al., 1995; Allen et al., 2000). The response to ABA was variable, with some cells showing no changes in [Ca2+]cyt and others exhibiting oscillations in [Ca2+]cyt in response to ABA under the imposed conditions (Allen et al., 1999b; Hugouvieux et al., 2001; Jung et al., 2002; Kwak et al., 2003), as discussed in the text. All traces are expressed as the ratio of recorded fluorescence emitted at 535 nm divided by fluorescence emitted at 480 nm after excitation at 440 nm as described in Allen et al. (1999b). Daily misting of plants with water was used to reduce endogenous ABA concentrations and increased the percentage of guard cells that showed ABA-induced [Ca2+]cyt elevation, as reported previously (Allen et al., 1999a,b). Epidermal peels were prepared from 3- to 5-week-old plants stably transformed with the cameleon construct according to the method described in Allen et al. (1999b). Samples were incubated in either a high-[K+] buffer (50 mm K+, 10 mm MES-Tris, pH 6·2) for cold and the indicated CaCl2 treatment (Fig. 2A, B) or stomatal-opening buffer (5 mm K+, 50 µm CaCl2, 10 mm MES-Tris, pH 6·2; ABA) for 3 h before imaging (C–E). Imaging was performed as described in Allen et al. (1999b). Cells were treated with either a cold shock (4 °C; n = 15), 10 mm CaCl2 (n = 26) or 10 µm ABA (dissolved in methanol). All traces were corrected for YFP bleaching through subtraction of the overall gradual decline in fluorescence in each channel from each data point (Allen et al., 1999b; Klüsener et al., 2002).
Physiological stimuli can also alter the pattern of spontaneous [Ca2+]cyt transients; for example, transfer from a low to a high [CO2] buffer reduces the rate of spontaneous Ca2+ transients in wild-type Arabidopsis (Landsberg erecta) guard cells (Young et al., 2006). This elevated CO2-induced dampening of Ca2+ transients was proposed to occur due to depolarization of the guard cell plasma membrane mediated by CO2 signalling (Young et al., 2006). Both types of responses (stimulation and dampening) have been associated with exposure of cells to ABA, with some studies showing ABA-induced [Ca2+]cyt transients (Schroeder and Hagiwara, 1990; Grabov and Blatt, 1998; Allen et al., 1999a, 2000, 2002; Staxén et al., 1999; Islam et al., 2010). However, in cells that exhibit spontaneous Ca2+ transients, prior to ABA application, ABA-dependent dampening and slowing of spontaneous [Ca2+]cyt transients was found (Grabov and Blatt, 1998; Staxén et al., 1999; Klüsener et al., 2002). A number of studies have noted a variety of ABA-induced responses ranging from repetitive oscillations to no measureable ABA-dependent change in [Ca2+]cyt (Schroeder and Hagiwara, 1990; Gilroy et al., 1991; Allen et al., 1999a; Hugouvieux et al., 2001; Jung et al., 2002; Kwak et al., 2002, 2003; Levchenko et al., 2005). These studies and data in Fig. 2 indicate that amongst a population of guard cells different microenvironments or signalling states may exist resulting in either lack of ABA-induced [Ca2+]cyt increases (Fig. 2C) or ABA-induced [Ca2+]cyt increases (Fig. 2D, E). Note that plants analysed in Fig. 2C–D were grown at high humidity, and were misted daily with water, similar to previous reports (Allen et al., 1999a; see methods in the figure captions). Daily misting of plant leaves with water may serve to reduce endogenous ABA levels in guard cells, and appears to enhance mechanisms mediating ABA-induced [Ca2+]cyt elevations.
Mechanistically, evidence suggests that [Ca2+]cyt increases induced by separate stimuli are likely generated by a different combination of mechanisms. [Ca2+]cyt oscillations recorded in response to [Ca2+]ext, cold and ABA have distinct patterns (Allen et al., 2000), indicating their generation is differently regulated. To generate a specific [Ca2+]cyt pattern, such as a repetitive oscillation, requires the co-ordinated action of both Ca2+ influx channels and active Ca2+ efflux transporters. Sorting out the specific genes encoding candidate Ca2+-permeable ion channels and efflux transporters that mediate specific [Ca2+]cyt patterns will require further research. This question is likely to be complicated by the circumstances that (a) large families of candidate Ca2+-permeable ion channels exist in plants with overlapping gene functions (Lacombe et al., 2001; Kaplan et al., 2007) and (b) more than one Ca2+ channel type is likely to contribute to any given stimulus-induced Ca2+ elevation pattern.
[Ca2+]cyt-REACTIVE MECHANISMS
There are a large number of [Ca2+]cyt-dependent mechanisms that have been described within the guard-cell signalling network, most of which function in mediation of stomatal closing, leading to the proposal that [Ca2+]cyt functions as a ‘hub’ within the stomatal signalling network (Hetherington and Woodward, 2003). The diversity of [Ca2+]cyt-dependent processes is illustrated by the large number (approx. 250) of proteins containing Ca2+-binding ‘EF-hand’ motifs encoded within the Arabidopsis thaliana genome (Day et al., 2002). This is likely to be an underestimate of the total number of Ca2+-binding proteins present, as some proteins such as the 14-3-3 protein GF14 have been described as Ca2+-binding (Lu et al., 1994; Athwal and Huber, 2002) but do not contain an EF hand (Day et al., 2002). EF-hand-containing proteins include several families of signalling components commonly referred to as Ca2+-sensors which include calmodulins (CaM) and CaM-like (CML) proteins, calcineurin-B-like (CBL) proteins and Ca2+-dependent protein kinases (CPKs); these protein families have been extensively reviewed elsewhere (Dodd et al., 2010; Kudla et al., 2010) and so will not be discussed in detail here. Moreover, additional ‘target proteins’ including ion channels contain EF-hand(s), and can be directly regulated by [Ca2+]cyt. For example, the K+- and Ca2+-permeable two-pore channel 1 (TPC1) protein which encodes the slow vacuolar (SV) channel (Peiter et al., 2005) contains two EF hands and is Ca2+ activated (Hedrich and Neher, 1987; Pei et al., 1999).
The literature might in a few cases over-interpret findings as mainly supporting a model in which only unique stimulus-specific patterns in [Ca2+]cyt elevations, rather than Ca2+ elevations above thresholds in concentrations and durations, are required in plant cells for Ca2+ to elicit an output. (We have avoided the attractive term ‘Ca2+ signature’ in our previous studies, as this can have several definitions. Nevertheless, further research in plant model cell systems is needed to elucidate the underlying cell-signalling and biochemical mechanisms, which would represent important breakthroughs in plant Ca2+ signalling.) Experimental imposition of [Ca2+]cyt transients through repetitive buffer swap experiments showed that any Ca2+ elevation above a threshold value could trigger a rapid ‘Ca2+-reactive’ stomatal closing response [see supplemental data for Allen et al. (2001)]. Thus the Ca2+-reactive stomatal closing response was shown to be largely independent of the [Ca2+]cyt elevation frequency, number or duration of transients (Allen et al., 2001). Nevertheless, the rate and the final degree of Ca2+-reactive stomatal closing depends on the number and integrated amplitude of Ca2+ elevations, which per se could also be modelled by a threshold-type mechanism. S-type anion channels in the plasma membrane of guard cells play a central role in controlling stomatal closure (Schroeder and Hagiwara, 1989; Schroeder, 1995). The rapid Ca2+-reactive phase of stomatal closing in response to imposed Ca2+ transients is reduced in the Ca2+-dependent protein kinase double mutant cpk3cpk6 (Mori et al., 2006; see Fig. 4A). Moreover, mutations that disrupt the gene encoding the S-type anion channel, SLAC1 (Negi et al., 2008; Vahisalu et al., 2008), show a dramatically impaired Ca2+-reactive stomatal closure (Vahisalu et al., 2008). These findings are consistent with the model that Ca2+-dependent protein kinases activate S-type anion channels, thus mediating stomatal closing (Mori et al., 2006; Geiger et al., 2010).
In contrast to Ca2+-reactive stomatal closing, imposed Ca2+ oscillations of particular frequencies and durations resulted in inhibition of stomatal re-opening, after Ca2+ transients had been terminated (Allen et al., 2001; Li et al., 2004). This latter Ca2+-dependent inhibition of stomatal re-opening has been coined long-term ‘Ca2+-programmed’ stomatal closure (Allen et al., 2001; Yang et al., 2003; Li et al., 2004). The gca2 mutant exhibits more ‘spiky’ Ca2+ oscillations during ABA and elevated CO2 exposures and gca2 exhibits impaired stomatal closure responses (Allen et al., 2001; Young et al., 2006). In the gca2 mutant, experimental imposition of strong Ca2+ oscillations restored 65 % of stomatal closure compared with wild-type guard cells (Allen et al., 2001). Further research suggested that the increased rate of [Ca2+]cyt transients observed in gca2 mutant guard cells (Allen et al., 2001) could result from the more negative (hyperpolarized) membrane potential of this mutant (Young et al., 2006). Indeed membrane potential hyperpolarization enhances the rate of [Ca2+]cyt transients in guard cells, as discussed earlier (Grabov and Blatt, 1998; Klüsener et al., 2002).
CALCIUM SENSITIVITY PRIMING
Given that both spontaneous transients and stimulus-specific changes in [Ca2+]cyt have been reported in guard cells, the question remains; how are guard cells able to respond in a Ca2+-dependent manner? In other words why can Ca2+ alter, for example, anion channel activity in response to the appropriate stimulus, but not in response to spontaneous Ca2+ transients? There is increasing evidence to suggest that the prior conditions that guard cells have experienced modulate the sensitivity of the signalling network to increases in [Ca2+]cyt, thus determining the final response of the cell. For example, [Ca2+]cyt-induced activation of the S-type anion channels was found not to occur in Arabidopsis guard cells under non-stimulated conditions (see fig. 3 in Allen et al., 2002). Exposure to high extracellular Ca2+ concentrations, and even after a subsequent return to more physiological extracellular Ca2+ levels, was found to alter the signalling state of guard cells, such that [Ca2+]cyt activation of anion channels was restored (Allen et al., 2002; Mori et al., 2006; Suh et al., 2007). These data along with the resolution of spontaneous Ca2+ transients in guard cells provided first evidence that the [Ca2+]cyt sensitivity of stomatal closing mechanisms can be up- and down-regulated (i.e. primed or de-primed).
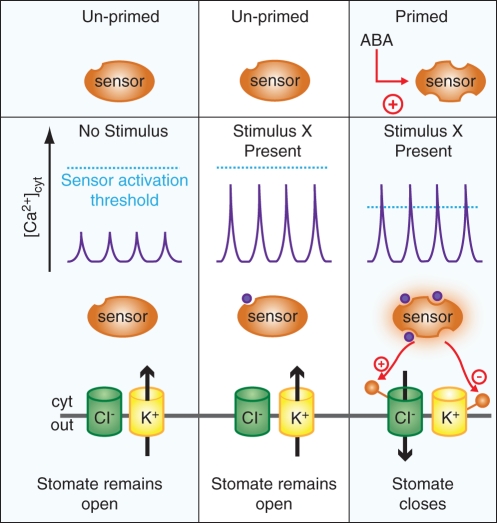
Ensuing studies showed that [Ca2+]cyt-activation of S-type anion channels is primed by pre-exposure of guard cells to ABA (Siegel et al., 2009; Chen et al., 2010). Similarly, K+-influx channels in Arabidopsis guard cells are rendered more [Ca2+]cyt sensitive by pre-exposure of guard cells to ABA as well (Siegel et al., 2009). These findings and Ca2+ imaging studies led to the proposal of the ‘Ca2+-sensitivity priming’ hypothesis (Young et al., 2006; Siegel et al., 2009), which postulates that a given physiological stimulus renders a component(s) of the signalling network more [Ca2+]cyt responsive (Fig. 3). Thus, for example, in the primed state a lower amplitude Ca2+ transient would cause a stronger activation of anion channels (Chen et al., 2010). This model resolves some of the potential paradoxes found in the literature.
Fig. 3.
Ca2+-sensitivity priming. In the Ca2+-sensitivity priming model, the response of a cell to a given stimulus depends on the conditions the cell has previously experienced. A generic model is presented, where the magnitude of Ca2+ increases and ABA-dependent priming combine to determine the eventual response (Siegel et al., 2009; Chen et al., 2010). Without a specific stimulus only spontaneous [Ca2+]cyt transients occur. In the presence of a stimulus (X) the amplitude of transients increases, but in the un-primed state this is insufficient to trigger the downstream response (e.g. ion-channel activation). A previously encountered condition (e.g. ABA) may modulate a Ca2+-response pathway, making it more Ca2+ responsive or ‘primed’. When stimulus X is perceived under the primed conditions the sensor is more Ca2+ responsive and therefore downstream responses are triggered. Note, however, that [Ca2+]cyt sensitivity enhancement could theoretically occur at any point in the network downstream of or parallel to [Ca2+]cyt. See text for details.
An important question for Ca2+ sensitivity priming remains, whether ABA or CO2 can sensitize guard cells to typical baseline resting [Ca2+]cyt concentrations of 150 nm in vivo. Some circumstantial evidence exists that this may be the case. For example, in Vicia faba and other species, guard cells have been previously shown to exhibit ABA-induced Ca2+ increases in only a minority of guard cells (Schroeder and Hagiwara, 1990; Gilroy et al., 1991; McAinsh et al., 1992). Furthermore, loading the Ca2+ reporter fura-2 into Vicia faba guard cells was shown to enable ABA-induced stomatal closing at resting [Ca2+]cyt levels in the absence of measurable [Ca2+]cyt elevations (Levchenko et al., 2005). However, in the same experiments loading a higher concentration of the fura-2-analogous Ca2+ chelator BAPTA into guard cells reduced resting Ca2+ levels to below typical baseline levels and then inhibited ABA-induced stomatal closing (Levchenko et al., 2005). Thus, ABA may enhance (prime) the sensitivity of stomatal closing mechanisms even to resting Ca2+ levels. This gives rise to the question whether Ca2+-independent signalling in vivo (Fig. 2C) is truly Ca2+ independent. More research is needed to investigate this question.
There are many potential (non-mutually exclusive) mechanisms through which Ca2+-sensitivity priming may occur, including post-translational modification of signalling proteins, sub-cellular re-localization, protein–protein interactions, coincidence detection of Ca2+ and a second parallel signal, interaction of parallel Ca2+-dependent and -independent signalling pathways and transcriptional reprogramming (for reviews and discussion of putative mechanisms, see Hubbard et al., 2010; Kim et al., 2010). These events may occur at the level of the Ca2+-sensors, but may potentially play a role at any point of the signalling network downstream of or parallel to [Ca2+]cyt responses.
Recent research has shown that elevation of the intracellular CO2 and bicarbonate concentrations in Arabidopsis guard cell protoplasts rapidly enhances the ability of [Ca2+]cyt to activate S-type anion channels, within approx. 3–5 min of whole-cell patch clamp initiation (Xue et al., 2011). These findings demonstrate that Ca2+-sensitivity priming can occur rapidly in guard cells, and may therefore not underlie a slower transcriptional regulation mechanism (Xue et al., 2011). The Ca2+-dependent protein kinase CPK1 of Mesembryanthemum crystallinum is dynamically re-localized within cells depending on growth conditions; upon transfer to low humidity the kinase moves from the plasma membrane to soluble and nuclear cell fractions, where it is presumably able to activate downstream signalling processes (Chehab et al., 2004). Further research is needed to elucidate the unknown biochemical and cell biological mechanisms by which the stomatal closing stimuli CO2 and ABA enhance the sensitivity of Ca2+-dependent stomatal closing (Young et al., 2006; Siegel et al., 2009; Chen et al., 2010; Xue et al., 2011).
MODULAR BASIS OF [Ca2+]cyt SIGNALLING
Plants contain a diverse array of proteins associated with Ca2+ signalling. For [Ca2+]cyt-signals to be generated and interpreted with specificity, a specific subset of these components must be employed at a given time and location. The concept of the ‘signalosome’ has previously been proposed for mammalian Ca2+ signalling (Berridge et al., 2003), and is equally applicable for plant systems, particularly given the large number of Ca2+-binding site-containing proteins encoded in plant genomes. The components of the signalosome may be tissue-, cell- or subcellular location-dependent, and may also vary with time, either in response to endogenous cues such as those provided by the circadian clock, or in response to particular stimuli. On a technical level, variation in growth conditions, age and physiological conditions of individual plants may influence the composition of the guard cell signalosome and therefore the sensitivity of stomata to different stimuli.
Evidence for specific control of subcellular location of Ca2+-signalling components has been elegantly demonstrated through transient expression analysis for the CBL–CIPK network, where particular CBL–CIPK pairs are restricted to defined sub-cellular locations (Waadt et al., 2008; Batistič et al., 2010). CIPK14 is located at the tonoplast when in complex with CBL2 or CBL3, but is found at the plasma membrane when co-expressed with CBL8 (Batistič et al., 2010). It would therefore be predicted for the intact plant that expression of the individual Ca2+-sensor would affect downstream signalling specificity.
Fig. 4.
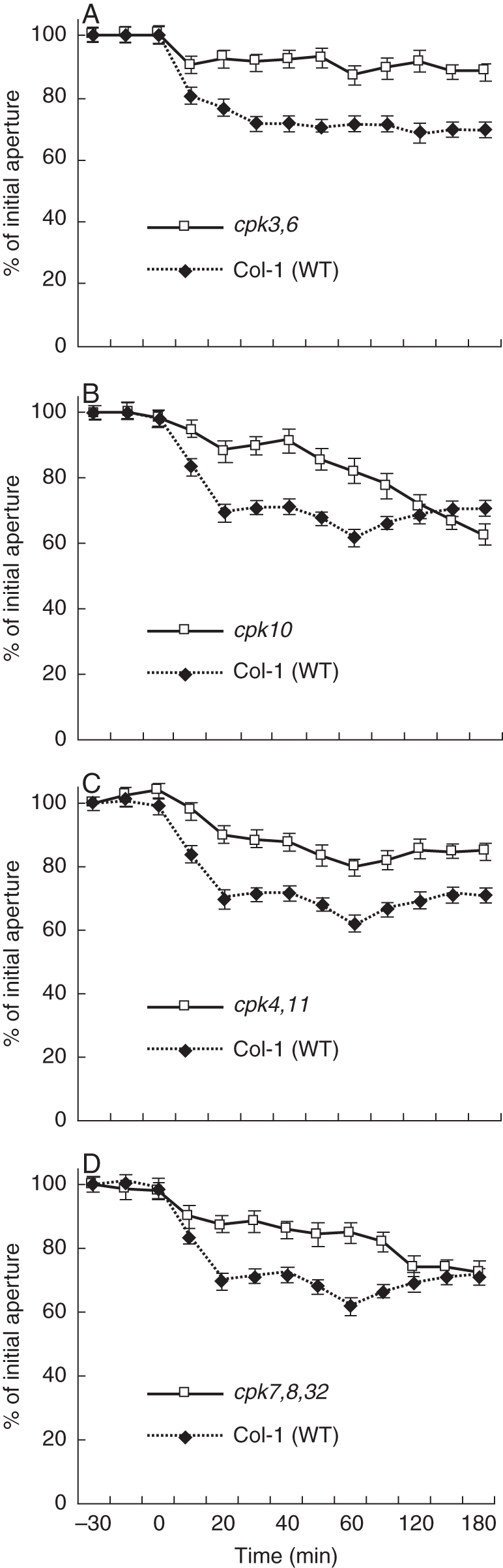
Imposed Ca2+ oscillation-induced stomatal closure is impaired in cpk3,cpk6, cpk10, cpk4,cpk11 and cpk32,cpk7,cpk8 mutants compared with wild-type stomata. Samples were pre-incubated in depolarizing stomatal-opening buffer for 3 h (50 mm KCl and 10 mm MES-Tris at pH = 5·6). Stomatal apertures of individually tracked stomata (see Box 1) were measured periodically at the indicated times. Starting at time = 0 and for the first 40 min the sample was exposed to hyperpolarizing (1 mm KCl, 1 mm CaCl2 and 10 mm MES-Tris at pH 5·6) solution four times for 5 min each (Allen et al., 2001; Mori et al., 2006). For experiments illustrated in Figs 4–6 plants were tented using transparent plastic wrap to maintain a high humidity (>95 %) to reduce endogenous ABA levels. After 2 weeks of growth, slits were cut in the plastic tent to allow enhanced air circulation. Twenty-four hours before each experiment, the old plastic was replaced with a new plastic wrap for a high humidity treatment. Stomatal movement analyses were performed following the imposed Ca2+ oscillation protocol and solutions described in Mori et al. (2006). The lower epidermis of Arabidopsis leaves was attached to glass cover slides using Hollister medical adhesive 9 to attach a leaf abaxial side down onto a coverslip. A razor blade was then used to carefully remove mesophyll layers of the leaf until only the epidermal layer remained. The coverslip was then incubated in 4 mL depolarizing buffer (50 mm KCl and 10 mm MES-Tris at pH 5·6) in a Petri dish (35 × 10 mm) for 3 h under white light (200 µE) to open stomata. The coverslip with epidermal layer attached was then sealed to a glass slide with a 2-cm hole drilled in the middle to create a 200 µL perfusion well. Depolarizing buffer was alternated with hyperpolarizing buffer (1 mm KCl, 1 mm CaCl2, and 10 mm MES-Tris at pH 5·6) using a bath perfusion system. Four 5-min extracellular Ca2+ pulses were applied in 10-min intervals in the first 40 min, as described previously (Mori et al., 2006). In between each transient and after the final transient depolarizing buffer was continuously applied. Stomatal apertures were measured using an inverted microscope at ×40. Both mutant and wild-type plants could be placed side by side on one coverslip during a single experiment. Eight individual stomata per condition were measured and tracked using Scion Image Software. Experiments were repeated at least three times for each mutant and parallel wild-type controls. Seed sources were cpk3-1 cpk6-1 (Mori et al., 2006), cpk10 (Salk_032021) and cpk32 (GABI 824E2). For information on cpk4 and cpk11 sources see Fig. 7. Experiments were performed single-blinded with the genotype identity unknown. Data are from Valerio (2007).
ANALYSIS OF Ca2+-DEPENDENT PROTEIN KINASE FUNCTIONS IN STOMATAL REGULATION
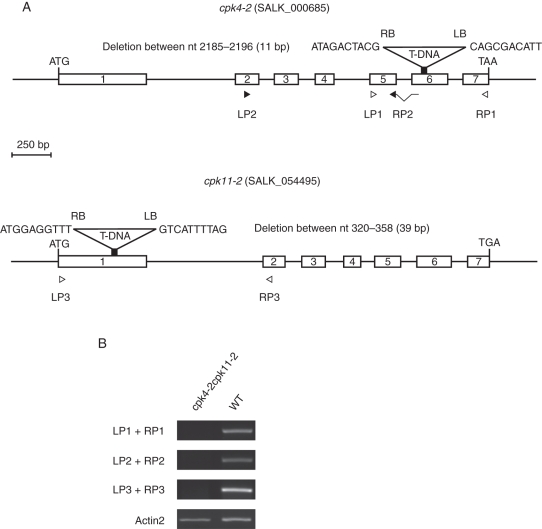
Microarray analysis provides evidence that Ca2+-signalling components are expressed in guard cells, e.g. CPK3, 6, 4, 10 and 11. Additional CPKs are expressed in guard cells, whereas CPK2, 18 and 34 (for example) are only very weakly expressed in this cell type (Leonhardt et al., 2004; Mori et al., 2006; Zhu et al., 2007; Yang et al., 2008; Zou et al., 2010). Interestingly, most of the above-mentioned Ca2+-dependent protein kinases have been reported to contribute to ABA-induced stomatal closing (Mori et al., 2006; Zhu et al., 2007; Zou et al., 2010). Here, we show that individual mutants in these same genes show partial insensitivities in the rapid Ca2+-reactive stomatal closing response, as shown for the cpk3cpk6, cpk10, cpk4cpk11 and cpk7cpk8cpk32 mutants (Fig. 4; Valerio, 2007). However, mutants in these Ca2+-dependent protein kinases (CDPKs), including cpk10, and even cpk4cpk11 double-mutant stomata and cpk7cpk8cpk32 triple-mutant stomata did not show a clear ABA-insensitive phenotype in our analyses of ABA-induced stomatal closing (Fig. 5) and ABA-inhibition of stomatal opening (Fig. 6; Valerio, 2007). These findings with a cpk4cpk11 double mutant (Figs 5 and 7; Valerio, 2007) do not correlate with a very strong ABA insensitivity reported for cpk4cpk11 (Zhu et al., 2007). Note that the cpk4 allele (cpk4-2; SALK_000685) in the double-mutant plants used for these experiments differs from the ones used in a previous study (Zhu et al., 2007; cpk4-1; SALK_081860), whereas the same cpk11-2 allele was used. The T-DNA insertion of the cpk4-2 allele analysed here was identified as lying in the 6th exon and RT-PCR analysis revealed that plants harbouring cpk4-2 and cpk11-2 alleles do not express mature transcripts (Fig. 7). These findings showing statistically functional ABA responses but slightly impaired Ca2+-reactive stomatal closing responses in the analysed cpk mutants (Valerio, 2007; Figs 4–6) point to the hypothesis that combinations of these mutants may lead to a stronger ABA insensitivity, as found for the partial ABA insensitivity of cpk3cpk6 double mutants (Mori et al., 2006). Furthermore, CPK23 is also expressed in guard cells, but functions genetically as a negative regulator in drought stress experiments as well as control of stomatal aperture (Ma and Wu, 2007). Contrary to the phenotype of cpk23 mutants, CPK23 on the other hand activates the SLAC1 anion channel in Xenopus oocytes and S-type anion currents in guard cell protoplasts of cpk23 mutants are reduced (Geiger et al., 2010). The in vivo mechanisms mediating these opposing responses will require further analysis. The closest homologue to CPK23, CPK21, can also mediate activation of the SLAH3 anion channel and to a smaller extent SLAC1 anion channels in Xenopus oocytes (Geiger et al., 2011). SLAH3 shows a relative nitrate permeability similar to S-type anion currents in Vicia faba guard cells (Schmidt and Schroeder, 1994; Geiger et al., 2011).
Fig. 5.
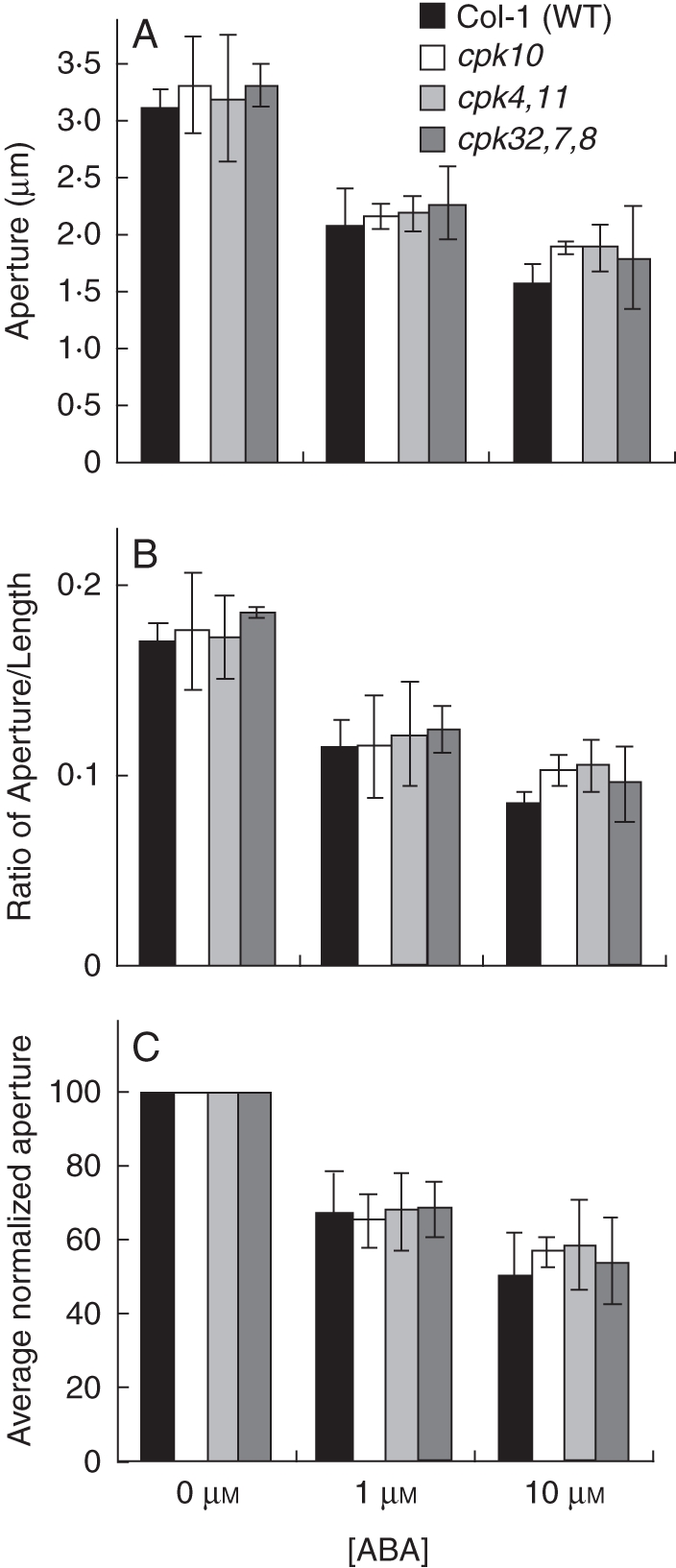
ABA-induced stomatal closure was not dramatically impaired in cpk10, cpk4cpk11 and cpk32cpk7cpk8 compared with Col-1 wild type. Whole leaves were pre-incubated in stomatal-opening buffer (10 mm KCl, 7·5 mm iminodeactic acid, 10 mm MES, pH 6·2) for 2 h and then incubated for an additional hour with 0, 1 or 10 µm ABA. Stomatal aperture (A) and ratio (width : length) (B) were then measured. In (C) the average normalized stomatal apertures from (A) are shown. Experiments were performed double blind with both genotype and ABA concentration unknown; n = 3 experiments with 30 stomata analysed per condition in each experiment (90 stomatal apertures analysed per bar in the graphs). Error bars represent ± s.e. of the mean relative to n = 3 experiments. Plants were grown at approx. 95 % humidity and were subjected to 24 h of high (>98 %) humidity prior to experimentation. ABA-dependent stomatal movement imaging analyses (Valerio, 2007) were performed following the protocol from Kwak et al. (2002). Rosette leaves from 4- to 5-week-old plants were excised and the curvature of each leaf was gently inverted using a finger so that the abaxial side did not form any air pockets when floating on solution during incubation. Using forceps the whole leaf was placed with the abaxial side down in 4 mL of stomatal-opening buffer (10 mm KCl, 7·5 mm iminodeactic acid, 10 mm MES, pH 6·2) in a Petri dish (35 × 10 mm). Leaves were incubated in stomatal-opening buffer for 2 h under white light (200 µE). Then, the stomatal-opening buffer was removed via a pipette and replaced with 4 mL stomatal-opening buffer containing 0 µm, 1 µm or 10 µm ABA and the leaves were incubated under white light for one more hour. The abaxial epidermis of each leaf was peeled using forceps and placed on a glass coverslip with approx. 200 µL of the treatment buffer. Experiments were performed double-blinded. Both genotype identity and ABA concentrations were unknown to the experimenter. Data are from Valerio (2007).
Fig. 7.
Characterization of cpk4-2 cpk11-2 mutant plants. (A) Cartoon showing genomic DNA of CPK4 and CPK11 and the T-DNA insertion sites of the corresponding mutant alleles. White boxes represent exons. Arrowheads show primer locations used for RT-PCR experiments shown in (B). The T-DNA insertion site of the cpk4-2 allele was localized in the 6th exon at nucleotide 2184 after ATG by sequencing. In the cpk4-2 mutant, 11 nucleotides (2185–2196) are deleted. The T-DNA insertion of cpk11-2 in the first exon corresponds to that published by Zhu et al. (2007) and was confirmed. (B) RT-PCR analysis of complementary DNA prepared from mutant and wild-type plant RNA revealed that in both, cpk4-2 and the cpk11-2 mutant plants no transcripts were detected (35 PCR cycles). Loading control was Actin2 (23 PCR cycles).
Fig. 6.
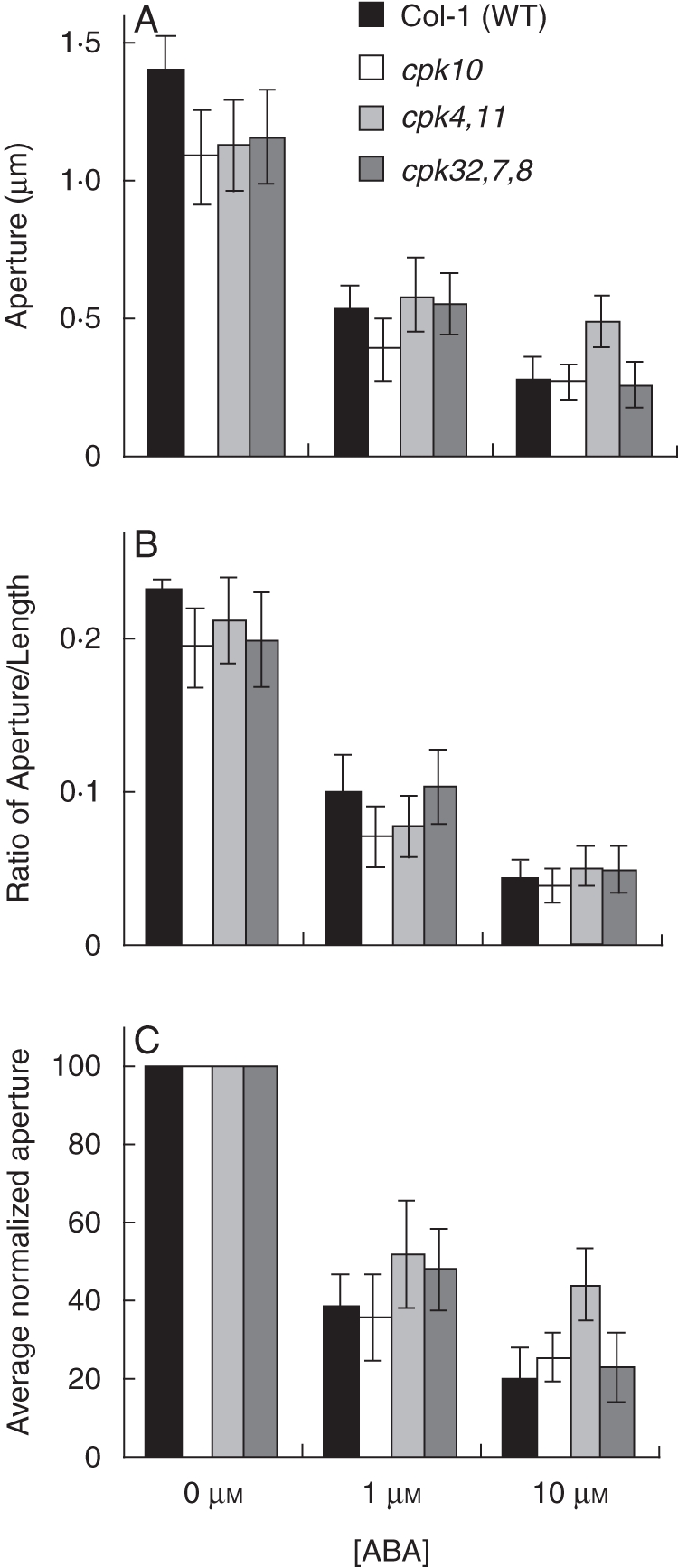
ABA inhibition of stomatal opening is not affected in cpk10, cpk4cpk11 and cpk32cpk7cpk8 compared with Col-1 wild type. Stomatal apertures (A) and ratios (width : length) (B) were measured. In (C) the averages of normalized apertures are shown. Experiments were performed double blind with both genotype and ABA concentration unknown to the experimenter; n = 3 experiments with 30 stomata per condition per experiment (90 stomatal apertures analysed per bar in graph). Error bars represent ± standard error of the mean for n = 3 experiments. For analyses of ABA inhibition of stomatal opening, plants were subjected for 24 h to darkness to reduce stomatal apertures. Whole leaves were then pre-incubated in stomatal-opening buffer with 0, 1 or 10 µm ABA concentrations for 2 h. The epidermis was then peeled off and attached onto a glass slide. Stomatal apertures were measured using a microscope at ×40. Thirty stomata per condition were measured in each epidermal peel using Scion Image Software. Data are from Valerio (2007).
An aspect of signalosome models is that protein complexes are dynamically remodelled; and this could include a potentially [Ca2+]cyt-dependent remodelling (Berridge et al., 2003). For example, adding a continuous high extracellular Ca2+ concentration to Arabidopsis leaf epidermis causes [Ca2+]cyt elevation (McAinsh et al., 1995; Allen et al., 1999a), but under the appropriate conditions does not cause stomatal closing, in spite of the [Ca2+]cyt elevation, indicating a non-primed state (K. E. Hubbard et al., unpubl. res.). However, repetitively pulsing extracellular Ca2+ to the same leaf epidermis causes a clear Ca2+-reactive stomatal closing response (Allen et al., 2001; e.g. Fig. 4). These findings suggest that this later Ca2+ treatment of repetitive extracellular Ca2+ pulses causes a Ca2+-primed state compared with simple continuous elevation in extracellular Ca2+.
Such remodelling can establish feedback mechanisms (Berridge et al., 2003). It is entirely possible that an analogous process contributes to [Ca2+]cyt sensitivity priming in guard cell signalling. Many Arabidopsis genes are transcriptionally regulated in response to [Ca2+]cyt, which include transcripts associated with Ca2+-signalling such as CML24/TCH2 (Braam, 1992; Kaplan et al., 2006) and CPK32 (Kaplan et al., 2006). Similarly, many Ca2+-signalling components are regulated at the level of transcript abundance by ABA treatment (Hoth et al., 2002; Sánchez et al., 2004; Zeller et al., 2009). Therefore the potential exists for similar [Ca2+]cyt-dependent and conditional remodelling of the guard cell signalosome, which should be further investigated. Note, however, that CO2/HCO3-induced priming of Ca2+-dependent activation of anion channels occurs within <3 to 5 min in whole patch clamp experiments, demonstrating that relatively rapid events function in priming Ca2+sensitivity (Xue et al., 2011). Similarly the repetitive Ca2+ pulse-induced Ca2+-reactive stomatal closure is measurable within approx. 5 min of Ca2+ pulsing as resolved using tracking of individual stomatal apertures (see Box 1, overleaf).
Box 1. Improved resolution of stimulus-induced stomatal movements in guard cells by tracking of individual stomatal apertures
Arabidopsis guard cells have become a prime model system for analysing signal transduction, since early research combining genetic and ion channel analyses in this system (Ichida et al., 1997; Pei et al., 1997, 1998; Roelfsema and Prins, 1997). Arabidopsis stomata are small relative to other stomatal model systems and stomatal apertures of various plant types including Arabidopsis are known to show variability in the size of individual stomatal complexes and also variability in the opening apertures of stomata of similar size in a given leaf (Gorton et al., 1988; Mott and Buckley, 2000; Mott and Peak, 2007). Thus stomatal aperture measurements are expected to show a clear degree of statistical variation. Use of blind experiments, in which the genotype and, when possible, the stimulus being applied to guard cells is unknown to the experimenter (Murata et al., 2001) has been employed by several laboratories, has become a standard in the field and has aided in addressing the above limitations of the range of stomatal aperture sizes found under any given condition.
Research in our laboratory has shown that a major additional improvement in experiments can be made, by adding imaging of the same individual stomatal apertures over time (Allen et al., 2001; Mori et al., 2006; Vahisalu et al., 2008; Siegel et al., 2009), while performing blind experiments. In such ‘stomatal tracking’ experiments the lower side of a leaf is attached to a glass coverslip in an extracellular incubation medium (Webb et al., 2001; Young et al., 2006). The mesophyll and upper leaf epidermis are removed surgically for better optical resolution of stomatal apertures in the intact lower leaf epidermis (Young et al., 2006). For stimulus-induced stomatal closing analyses, a field of well-opened stomata is located and images are captured (e.g. using Scion Image software) for later analyses and data storage. The bottom (dry side) of coverslips can be marked with colour marker pens to label grids in the regions where apertures where imaged, for finding these same stomata subsequently if needed. Images of the same stomatal apertures are taken over time and can be stored for later analyses of individual stomatal apertures and for deposition of image files. While this approach has been used as a standard for imposed Ca2+ oscillation studies (Allen et al., 2001; Mori et al., 2006; Vahisalu et al., 2008; Fig. 4), we have found that this method also substantially improves stomatal movement response analyses to any given stimulus (Siegel et al., 2009; see Figs 1 and 4 and, Box Fig. 1). For example, while individual stomata are known to have diverse apertures (e.g. Box Fig. 1C), the relative responses of wide open stomata and smaller stomatal apertures to ABA or to CO2 were comparable (Fig. 1 and Box Fig. 1; Siegel et al., 2009). Note that this method has previously been proposed and used in Vicia faba (Gorton et al., 1988), for which stomata exhibit relatively weak ABA and CO2 responses, compared with, for example, Arabidopsis. We propose that this simple image-capturing approach, together with blind analyses, be used as a standard for stomatal response research in arabidopsis. Our research experience with this method shows that this approach will aid in greatly improving resolution and robustness and in defining the functions of individual Ca2+-independent and Ca2+-dependent components and mechanisms in stomatal response analyses.
Box Fig. 1.
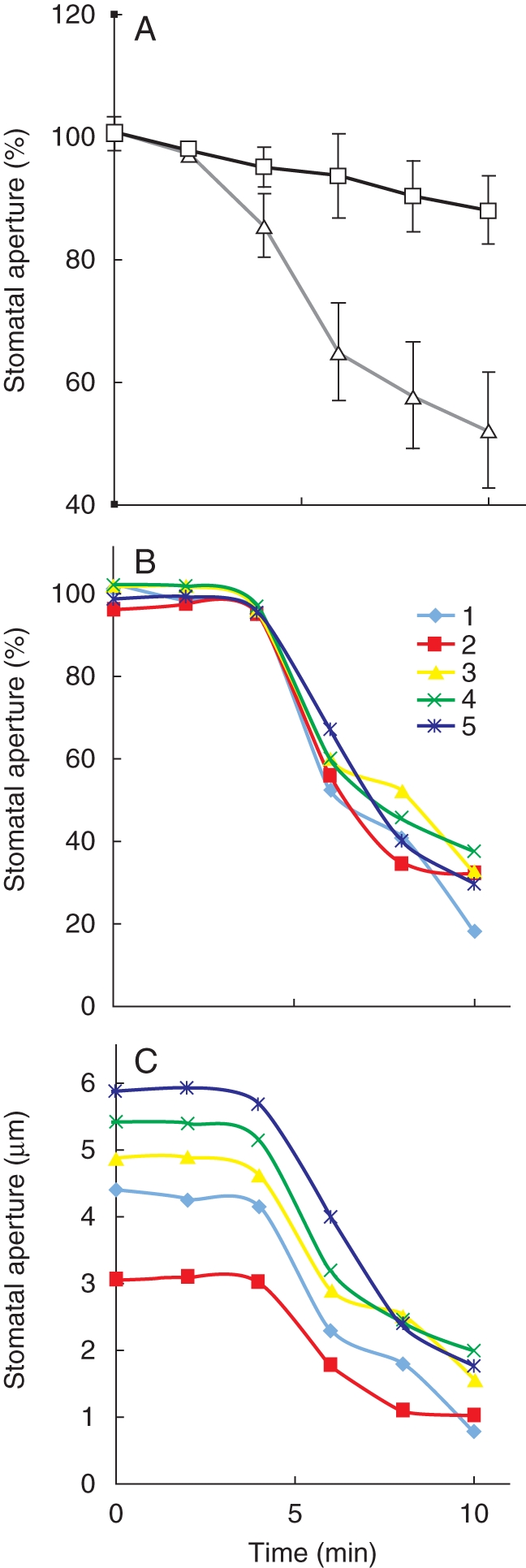
ABA-induced stomatal closing of individually tracked stomatal apertures. (A) Average individually tracked stomatal apertures in the presence of 50 µm Ca2+ (open triangles) and in the presence of 200 nm free Ca2+ (open squares) in the bath solution from three experiments are shown and were normalized to the stomatal apertures at time = 0. (B, C) ABA-induced stomatal closing in the presence of 50 µm Ca2+ in five individually tracked stomatal apertures. In (A; open triangles) normalized stomatal apertures of the same stomata depicted in (B) and (C) are shown. Methods used in these experiments tracking individual stomatal apertures are described in Siegel et al. (2009). ABA-induced stomatal closing experiments are reproduced from Siegel et al. (2009) with permission of the publisher.
CONCLUSIONS
Ca2+ functions as a second messenger in guard cell signalling and stomatal movements, as was originally described over 20 years ago (DeSilva et al., 1985; Schwartz, 1985; Schroeder and Hagiwara, 1989; McAinsh et al., 1990). The recently derived Ca2+-sensitivity priming model and the concomitant signalosome model provide a mechanism for specificity in plant [Ca2+]cyt signalling. Further research at the single-cell type specific level should shed light on the underlying molecular and protein-mediated Ca2+ signalling mechanisms. Guard cells provide a potent system to pursue combined single-cell time-resolved small molecule and protein imaging, electrophysiological ion-channel regulation, cell biological, and whole-leaf and -plant response analyses which undoubtedly will spring new surprises and lead to new levels of understanding of plant-cell signalling dynamics.
ACKNOWLEDGEMENTS
We thank Drs Shintaro Munemasa and Rainer Waadt for comments on an early draft of the manuscript. This research was supported by NIH (R01GM060396) and NSF (MCB0918220) and in part by Chemical Sciences, Geosciences, and Biosciences Division of the Office of Basic Energy Sciences at the US Department of Energy (DE-FG02-03ER15449) grants (J.I.S.). Data in the Box Figure are reproduced from Siegel et al. (2009) with permission from Wiley-Blackwell.
LITERATURE CITED
- Aharon GS, Gelli A, Snedden WA, Blumwald E. Activation of a plant plasma membrane Ca2+ channel by TGalpha1, a heterotrimeric G protein alpha-subunit homologue. FEBS Letters. 1998;424:17–21. doi: 10.1016/s0014-5793(98)00129-x. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Sanders D. Control of ionic currents in guard cell vacuoles by cytosolic and luminal calcium. The Plant Journal. 1996;10:1055–1069. doi: 10.1046/j.1365-313x.1996.10061055.x. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI. Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. The Plant Cell. 1999a;11:1785–1798. doi: 10.1105/tpc.11.9.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GJ, Kwak JM, Chu SP, et al. Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. The Plant Journal. 1999b;19:735–747. doi: 10.1046/j.1365-313x.1999.00574.x. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Schumacher K, et al. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science. 2000;289:2338–2342. doi: 10.1126/science.289.5488.2338. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Harrington CL, et al. A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature. 2001;411:1053–1057. doi: 10.1038/35082575. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Murata Y, Chu SP, Nafisi M, Schroeder JI. Hypersensitivity of abscisic acid-induced cytosolic calcium increases in the Arabidopsis farnesyltransferase mutant era1-2. The Plant Cell. 2002;14:1649–1662. doi: 10.1105/tpc.010448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athwal G, Huber S. Divalent cations and polyamines bind to loop 8 of 14-3-3 proteins, modulating their interaction with phosphorylated nitrate reductase. The Plant Journal. 2002;29:119–129. doi: 10.1046/j.0960-7412.2001.01200.x. [DOI] [PubMed] [Google Scholar]
- Batistič O, Waadt R, Steinhorst L, Held K, Kudla J. CBL-mediated targeting of CIPKs facilitates the decoding of calcium signals emanating from distinct cellular stores. The Plant Journal. 2010;61:211–222. doi: 10.1111/j.1365-313X.2009.04045.x. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature Reviews Molecular Cell Biology. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Braam J. Regulated expression of the calmodulin-related TCH genes in cultured Arabidopsis cells: induction by calcium and heat shock. Proceedings of the National Academy of Sciences of the USA. 1992;89:3213–3216. doi: 10.1073/pnas.89.8.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab EW, Patharkar OR, Hegeman AD, Taybi T, Cushman JC. Autophosphorylation and subcellular localization dynamics of a salt- and water deficit-induced calcium-dependent protein kinase from ice plant. Plant Physiology. 2004;135:1430–1446. doi: 10.1104/pp.103.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z-H, Hills A, Lim CK, Blatt MR. Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. The Plant Journal. 2010;61:816–825. doi: 10.1111/j.1365-313X.2009.04108.x. [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annual Review of Plant Biology. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- Day I, Reddy V, Shad Ali G, Reddy A. Analysis of EF-hand-containing proteins in Arabidopsis. Genome Biology. 2002;3:research0056·1–research0056·24. doi: 10.1186/gb-2002-3-10-research0056. doi:10.1186/gb-2002-3-10-research0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSilva DLR, Cox RC, Hetherington AM, Mansfield TA. Synergism between calcium ions and abscisic acid in preventing stomatal opening. New Phytologist. 1985;100:473–482. [Google Scholar]
- Dodd AN, Kudla J, Sanders D. The language of calcium signalling. Annual Review of Plant Biology. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proceedings of the National Academy of Sciences of the USA. 2010;107:8023–8028. doi: 10.1073/pnas.0912030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Maierhofer T, Al-Rasheid KA, et al. Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Science Signaling. 2011;4:ra32. doi: 10.1126/scisignal.2001346. doi:10.1126/scisignal.2001346. [DOI] [PubMed] [Google Scholar]
- Gilroy S, Fricker MD, Read ND, Trewavas AJ. Role of calcium in signal transduction of commelina guard cells. The Plant Cell. 1991;3:333–344. doi: 10.1105/tpc.3.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Read ND, Trewavas AJ. Elevation of cytoplasmic calcium by caged calcium or caged inositol trisphosphate initiates stomatal closure. Nature. 1990;346:769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- Gobert A, Isayenkov S, Voelker C, Czempinski K, Maathuis FJ. The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proceedings of the National Academy of Sciences of the USA. 2007;104:10726–10731. doi: 10.1073/pnas.0702595104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorton HL, Williams WE, Binns ME. Repeated measurements of aperture for individual stomates. Plant Physiology. 1988;89:387–390. doi: 10.1104/pp.89.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Blatt MR. Parallel control of the inward-rectifier K+ channel by cytosolic free Ca2+ and pH in Vicia guard cells. Planta. 1997;201:84–95. [Google Scholar]
- Grabov A, Blatt MR. Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proceedings of the National Academy of Sciences of the USA. 1998;95:4778–4783. doi: 10.1073/pnas.95.8.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DW, Hills A, Kohler B, Blatt MR. Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proceedings of the National Academy of Sciences of the USA. 2000;97:4967–4972. doi: 10.1073/pnas.080068897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Neher E. Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature. 1987;329:833–836. [Google Scholar]
- Hedrich R, Busche H, Raschke K. Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO Journal. 1990;9:3889–3892. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- Hoth S, Morgante M, Sanchez J-P, Hanafey MK, Tingey SV, Chua N-H. Genome-wide gene expression profiling in Arabidopsis thaliana reveals new targets of abscisic acid and largely impaired gene regulation in the abi1-1 mutant. Journal of Cell Science. 2002;115:4891–4900. doi: 10.1242/jcs.00175. [DOI] [PubMed] [Google Scholar]
- Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes & Development. 2010;24:1695–1708. doi: 10.1101/gad.1953910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux V, Kwak JM, Schroeder JI. An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in arabidopsis. Cell. 2001;106:477–487. doi: 10.1016/s0092-8674(01)00460-3. [DOI] [PubMed] [Google Scholar]
- Ichida AM, Pei ZM, Baizabal-Aguirre VM, Turner KJ, Schroeder JI. Expression of a Cs+-resistant guard cell K+ channel confers Cs+-resistant, light-induced stomatal opening in transgenic arabidopsis. The Plant Cell. 1997;9:1843–1857. doi: 10.1105/tpc.9.10.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MM, Hossain MA, Jannat R, et al. Cytosolic alkalization and cytosolic calcium oscillation in Arabidopsis guard cells response to ABA and MeJA. Plant and Cell Physiology. 2010;51:1721–1730. doi: 10.1093/pcp/pcq131. [DOI] [PubMed] [Google Scholar]
- Israelsson M, Siegel RS, Young J, Hashimoto M, Iba K, Schroeder JI. Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Current Opinion in Plant Biology. 2006;9:654–663. doi: 10.1016/j.pbi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Joshi-Saha A, Valon C, Leung J. Abscisic acid signal off the STARTing block. Molecular Plant. 2011;4:562–80. doi: 10.1093/mp/ssr055. [DOI] [PubMed] [Google Scholar]
- Jung JY, Kim YW, Kwak JM, et al. Phosphatidylinositol 3- and 4-phosphate are required for normal stomatal movements. The Plant Cell. 2002;14:2399–2412. doi: 10.1105/tpc.004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan B, Davydov O, Knight H, et al. Rapid transcriptome changes induced by cytosolic Ca2+ transients reveal ABRE-related sequences as Ca2+-responsive cis elements in Arabidopsis. The Plant Cell. 2006;18:2733–2748. doi: 10.1105/tpc.106.042713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan B, Sherman T, Fromm H. Cyclic nucleotide-gated channels in plants. FEBS Letters. 2007;581:2237–2246. doi: 10.1016/j.febslet.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Kelly WB, Esser JE, Schroeder JI. Effects of cytosolic calcium and limited, possible dual, effects of G protein modulators on guard cell inward potassium channels. The Plant Journal. 1995;8:479–489. [Google Scholar]
- Kim T-H, Böhmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annual Review of Plant Biology. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Nishimura M, Shimazaki K. Cytosolic concentration of Ca2+ rregulates the plasma membrane H+-ATPase in guard cells of fava bean. The Plant Cell. 1995;7:1333–1342. doi: 10.1105/tpc.7.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüsener B, Young JJ, Murata Y, et al. Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in arabidopsis guard cells. Plant Physiology. 2002;130:2152–2163. doi: 10.1104/pp.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J, Batistic O, Hashimoto K. Calcium signals: the lead currency of plant information processing. The Plant Cell. 2010;22:541–563. doi: 10.1105/tpc.109.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Murata Y, Baizabal-Aguirre VM, et al. Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in arabidopsis. Plant Physiology. 2001;127:473–485. [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Moon J-H, Murata Y, et al. Disruption of a guard cell-expressed protein phosphatase 2A regulatory subunit, RCN1, confers abscisic acid insensitivity in arabidopsis. The Plant Cell. 2002;14:2849–2861. doi: 10.1105/tpc.003335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei Z-M, et al. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO Journal. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe B, Becker D, Hedrich R, et al. The identity of plant glutamate receptors. Science. 2001;292:1486–1487. doi: 10.1126/science.292.5521.1486b. [DOI] [PubMed] [Google Scholar]
- Leonhardt N, Kwak JM, Robert N, Waner D, Leonhardt G, Schroeder JI. Microarray expression analyses of arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. The Plant Cell. 2004;16:596–615. doi: 10.1105/tpc.019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenko V, Konrad KR, Dietrich P, Roelfsema MRG, Hedrich R. Cytosolic abscisic acid activates guard cell anion channels without preceding Ca2+ signals. Proceedings of the National Academy of Sciences of the USA. 2005;102:4203–4208. doi: 10.1073/pnas.0500146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang G-X, Xin M, Yang H-M, Wu X-J, Li T. The parameters of guard cell calcium oscillation encode inhibition of stomatal opening in Vicia faba. Plant Science. 2004;166:415–421. [Google Scholar]
- Lu G, Sehnke PC, Ferl RJ. Phosphorylation and calcium binding properties of an arabidopsis GF14 brain protein homolog. The Plant Cell. 1994;6:501–510. doi: 10.1105/tpc.6.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. Abscisic acid-induced elevation of guard-cell cytosolic Ca2+ precedes stomatal closure. Nature. 1990;343:186–188. [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. Visualizing changes in cytosolic-free Ca2+ during the response of stomatal guard cells to abscisic acid. The Plant Cell. 1992;4:1113–1122. doi: 10.1105/tpc.4.9.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Webb AAR, Staxen I, Taylor JE, Hetherington AM. Stimulus-induced oscillations in guard-cell cytosolic-free Ca2+ Plant Physiology. 1995;108:100–100. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SY, Wu WH. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Molecular Biology. 2007;65:511–518. doi: 10.1007/s11103-007-9187-2. [DOI] [PubMed] [Google Scholar]
- MacRobbie EA. Signal transduction and ion channels in guard cells. Philosophical Transactions of the Royal Society of London Series B – Biological Sciences. 1998;353:1475–1488. doi: 10.1098/rstb.1998.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie EA. ABA activates multiple Ca(2+) fluxes in stomatal guard cells, triggering vacuolar K(+)(Rb(+)) release. Proceedings of the National Academy of Sciences of the USA. 2000;97:12361–12368. doi: 10.1073/pnas.220417197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten H, Konrad KR, Dietrich P, Roelfsema MR, Hedrich R. Ca2+-dependent and -independent abscisic acid activation of plasma membrane anion channels in guard cells of Nicotiana tabacum. Plant Physiology. 2007;143:28–37. doi: 10.1104/pp.106.092643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher K, Zhou XE, Xu HE. Thirsty plants and beyond: structural mechanisms of abscisic acid perception and signaling. Current Opinion in Structural Biology. 2010;20:722–729. doi: 10.1016/j.sbi.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Mumm P, Imes D, et al. AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. The Plant Journal. 2010;63:1054–1062. doi: 10.1111/j.1365-313X.2010.04302.x. [DOI] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang Y, et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biology. 2006;4:e327. doi: 10.1371/journal.pbio.0040327. doi:10.1371/journal.pbio.0040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott KA, Buckley TN. Patchy stomatal conductance: emergent collective behaviour of stomata. Trends in Plant Science. 2000;5:258–262. doi: 10.1016/s1360-1385(00)01648-4. [DOI] [PubMed] [Google Scholar]
- Mott KA, Peak D. Stomatal patchiness and task-performing networks. Annals of Botany. 2007;99:219–226. doi: 10.1093/aob/mcl234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Pei Z-M, Mori IC, Schroeder J. Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. The Plant Cell. 2001;13:2513–2523. doi: 10.1105/tpc.010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, et al. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature. 2008;452:483–486. doi: 10.1038/nature06720. [DOI] [PubMed] [Google Scholar]
- Pandey S, Zhang W, Assmann SM. Roles of ion channels and transporters in guard cell signal transduction. FEBS Letters. 2007;581:2325–2336. doi: 10.1016/j.febslet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. Differential abscisic acid regulation of guard cell slow anion channels in arabidopsis wild-type and abi1 and abi2 mutants. The Plant Cell. 1997;9:409–423. doi: 10.1105/tpc.9.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schroeder JI. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science. 1998;282:287–290. doi: 10.1126/science.282.5387.287. [DOI] [PubMed] [Google Scholar]
- Pei ZM, Ward JM, Schroeder JI. Magnesium sensitizes slow vacuolar channels to physiological cytosolic calcium and inhibits fast vacuolar channels in fava bean guard cell vacuoles. Plant Physiology. 1999;121:977–986. doi: 10.1104/pp.121.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, et al. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Peiter E, Maathuis FJM, Mills LN, et al. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434:404–408. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Gonugunta VK, Christmann A, Grill E. ABA perception and signalling. Trends in Plant Science. 2010;15:395–401. doi: 10.1016/j.tplants.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Raschke K, Hedrich R, Reckmann U, Schroder JI. Exploring biophysical and biochemical components of the osmotic motor that drives stomatal movement. Botanica Acta. 1988;101:283–294. [Google Scholar]
- Roelfsema MRG, Prins HBA. Ion channels in guard cells of Arabidopsis thaliana. Planta. 1997;202:18–27. doi: 10.1007/s004250050098. [DOI] [PubMed] [Google Scholar]
- Sánchez J-P, Duque P, Chua N-H. ABA activates ADPR cyclase and cADPR induces a subset of ABA-responsive genes in Arabidopsis. The Plant Journal. 2004;38:381–395. doi: 10.1111/j.1365-313X.2004.02055.x. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Schroeder JI. Anion selectivity of slow anion channels in the plasma membrane of guard cells (large nitrate permeability) Plant Physiol. 1994;106:383–391. doi: 10.1104/pp.106.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI. Anion channels as central mechanisms for signal transduction in guard cells and putative functions in roots for plant–soil interactions. Plant Molecular Biology. 1995;28:353–361. doi: 10.1007/BF00020385. [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature. 1989;338:427–430. [Google Scholar]
- Schroeder JI, Hagiwara S. Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels. Proceedings of the National Academy of Sciences of the USA. 1990;87:9305–9309. doi: 10.1073/pnas.87.23.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Kwak JM, Allen GJ. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature. 2001;410:327–330. doi: 10.1038/35066500. [DOI] [PubMed] [Google Scholar]
- Schwartz A. Role of Ca and EGTA on stomatal movements in Commelina communis L. Plant Physiology. 1985;79:1003–1005. doi: 10.1104/pp.79.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RS, Xue S, Murata Y, et al. Calcium elevation-dependent and attenuated resting calcium-dependent abscisic acid induction of stomatal closure and abscisic acid-induced enhancement of calcium sensitivities of S-type anion and inward-rectifying K channels in Arabidopsis guard cells. The Plant Journal. 2009;59:207–220. doi: 10.1111/j.1365-313X.2009.03872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichandra C, Wasilewska A, Vlad F, Valon C, Leung J. The guard cell as a single-cell model towards understanding drought tolerance and abscisic acid action. Journal of Experimental Botany. 2009;60:1439–1463. doi: 10.1093/jxb/ern340. [DOI] [PubMed] [Google Scholar]
- Staxén I, Pical C, Montgomery LT, Gray JE, Hetherington AM, McAinsh MR. Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proceedings of the National Academy of Sciences of the USA. 1999;96:1779–1784. doi: 10.1073/pnas.96.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SJ, Wang Y-F, Frelet A, et al. The ATP binding cassette transporter AtMRP5 modulates anion and calcium channel activities in arabidopsis guard cells. Journal of Biological Chemistry. 2007;282:1916–1924. doi: 10.1074/jbc.M607926200. [DOI] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature. 2008;452:487–491. doi: 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio GS. 2007 The roles of Ca2+in the guard cell ABA signaling pathway and exploration of a possible ion channel gene in Arabidopsis thaliana. MSc Thesis, University of California, San Diego. [Google Scholar]
- Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. The Plant Journal. 2008;56:505–516. doi: 10.1111/j.1365-313X.2008.03612.x. [DOI] [PubMed] [Google Scholar]
- Ward JM, Schroeder JI. Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. The Plant Cell. 1994;6:669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AAR, McAinsh MR, Mansfield TA, Hetherington AM. Carbon dioxide induces increases in guard cell cytosolic free calcium. The Plant Journal. 1996;9:297–304. [Google Scholar]
- Webb AAR, Larman MG, Montgomery LT, Taylor JE, Hetherington AM. The role of calcium in ABA-induced gene expression and stomatal movements. The Plant Journal. 2001;26:351–362. doi: 10.1046/j.1365-313x.2001.01032.x. [DOI] [PubMed] [Google Scholar]
- Wood NT, Allan AC, Haley A, Viry-Moussaïd M, Trewavas AJ. The characterization of differential calcium signalling in tobacco guard cells. The Plant Journal. 2000;24:335–344. doi: 10.1046/j.1365-313x.2000.00881.x. [DOI] [PubMed] [Google Scholar]
- Xue S, Hu H, Ries A, Merilo E, Kollist H, Schroeder JI. Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO Journal. 2011;30:1645–1658. doi: 10.1038/emboj.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HM, Zhang XY, Wang GX, Li Y, Wei XP. Cytosolic calcium oscillations induce stomatal closure in Vicia faba. Plant Science. 2003;165:1117–1122. [Google Scholar]
- Yang Y, Costa A, Leonhardt N, Siegel R, Schroeder J. Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods. 2008;4:6. doi: 10.1186/1746-4811-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JJ, Mehta S, Israelsson M, Godoski J, Grill E, Schroeder JI. CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proceedings of the National Academy of Sciences of the USA. 2006;103:7506–7511. doi: 10.1073/pnas.0602225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller G, Henz SR, Widmer CK, et al. Stress-induced changes in the Arabidopsis thaliana transcriptome analyzed using whole-genome tiling arrays. The Plant Journal. 2009;58:1068–1082. doi: 10.1111/j.1365-313X.2009.03835.x. [DOI] [PubMed] [Google Scholar]
- Zhu S-Y, Yu X-C, Wang X-J, et al. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in arabidopsis. The Plant Cell. 2007;19:3019–3036. doi: 10.1105/tpc.107.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J-J, Wei F-J, Wang C, et al. Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiology. 2010;154:1232–1243. doi: 10.1104/pp.110.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]