Abstract
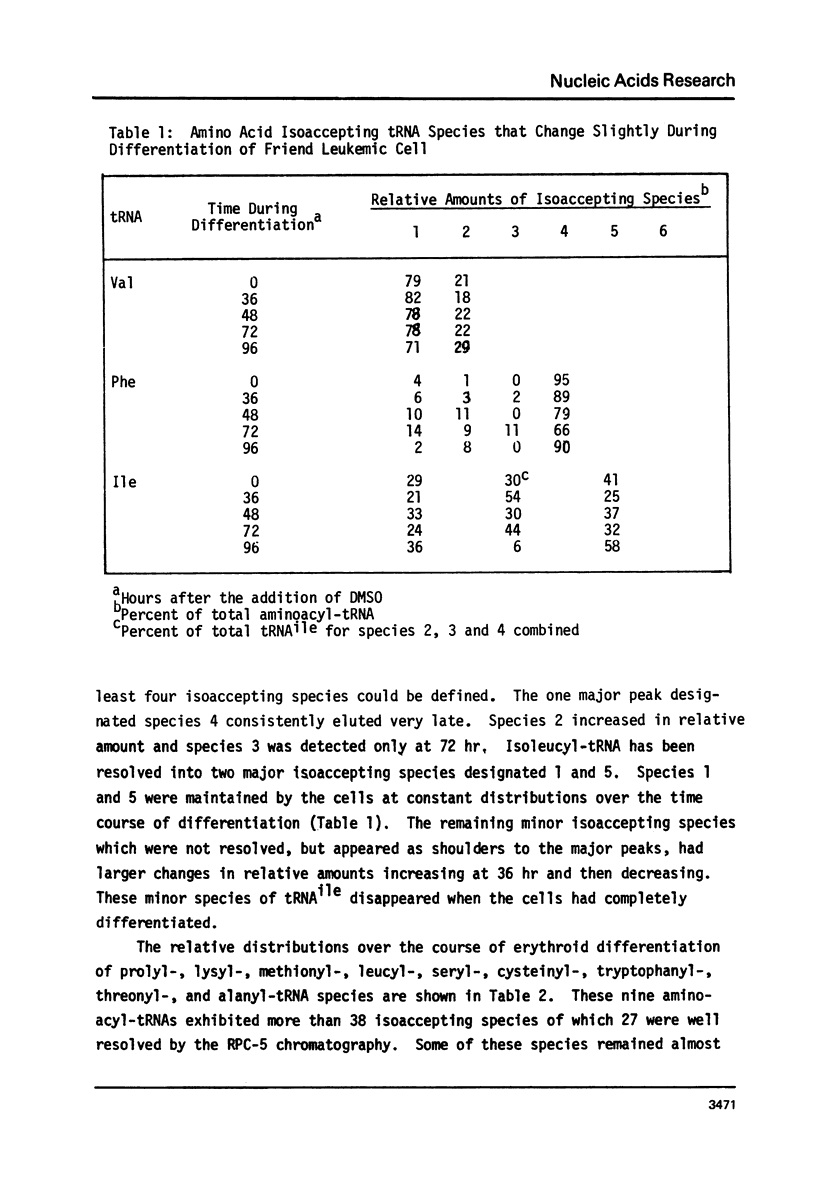
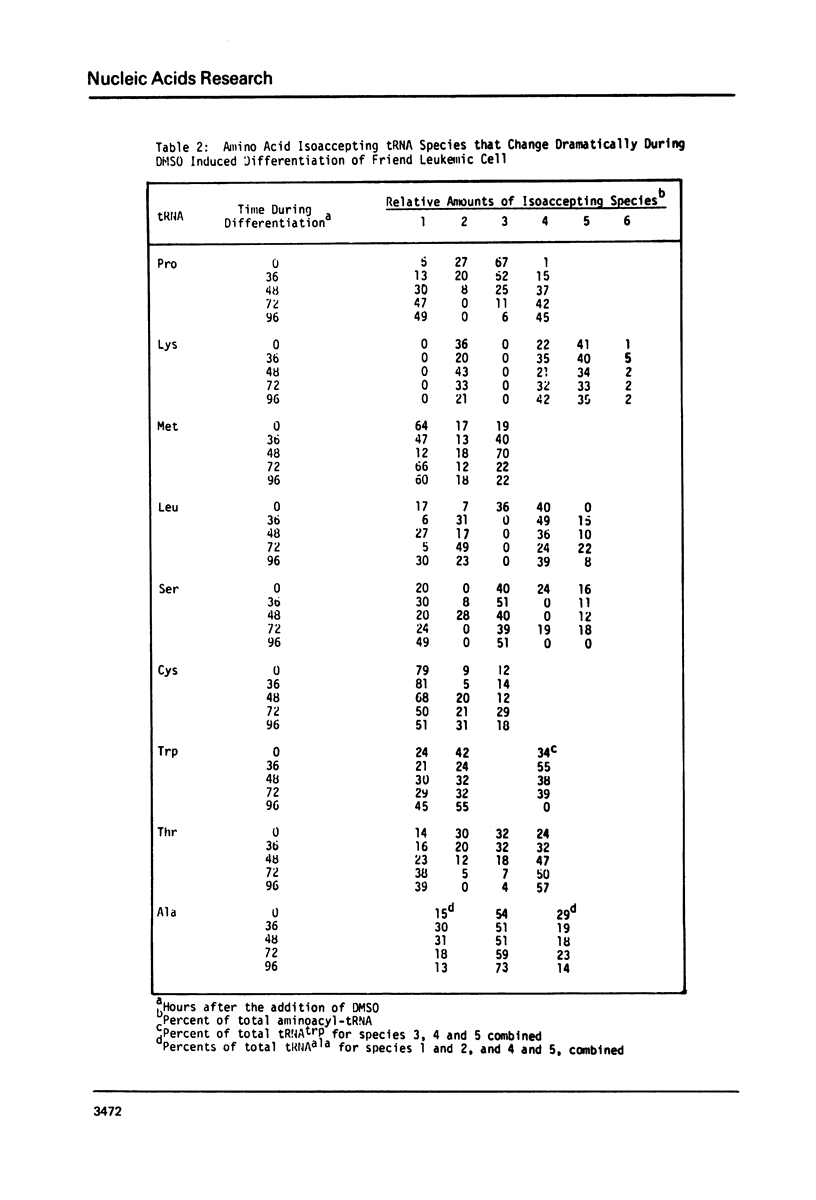
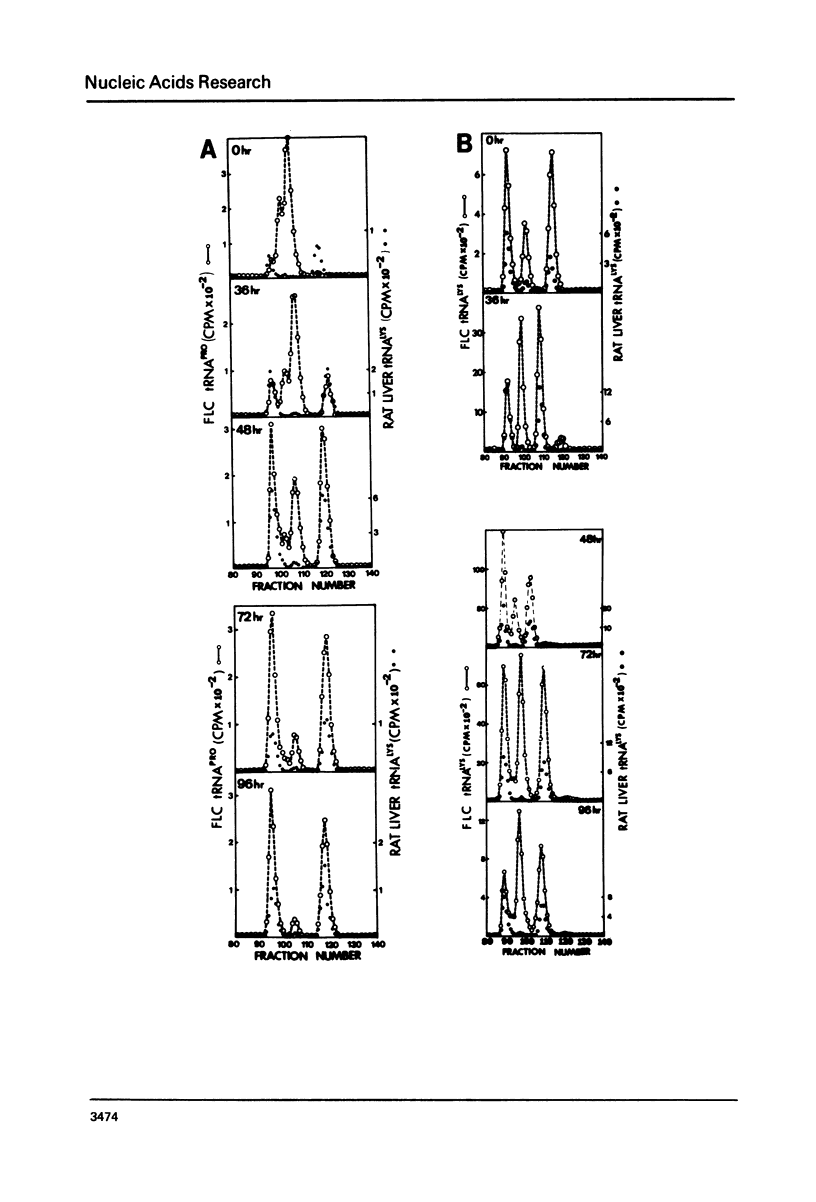
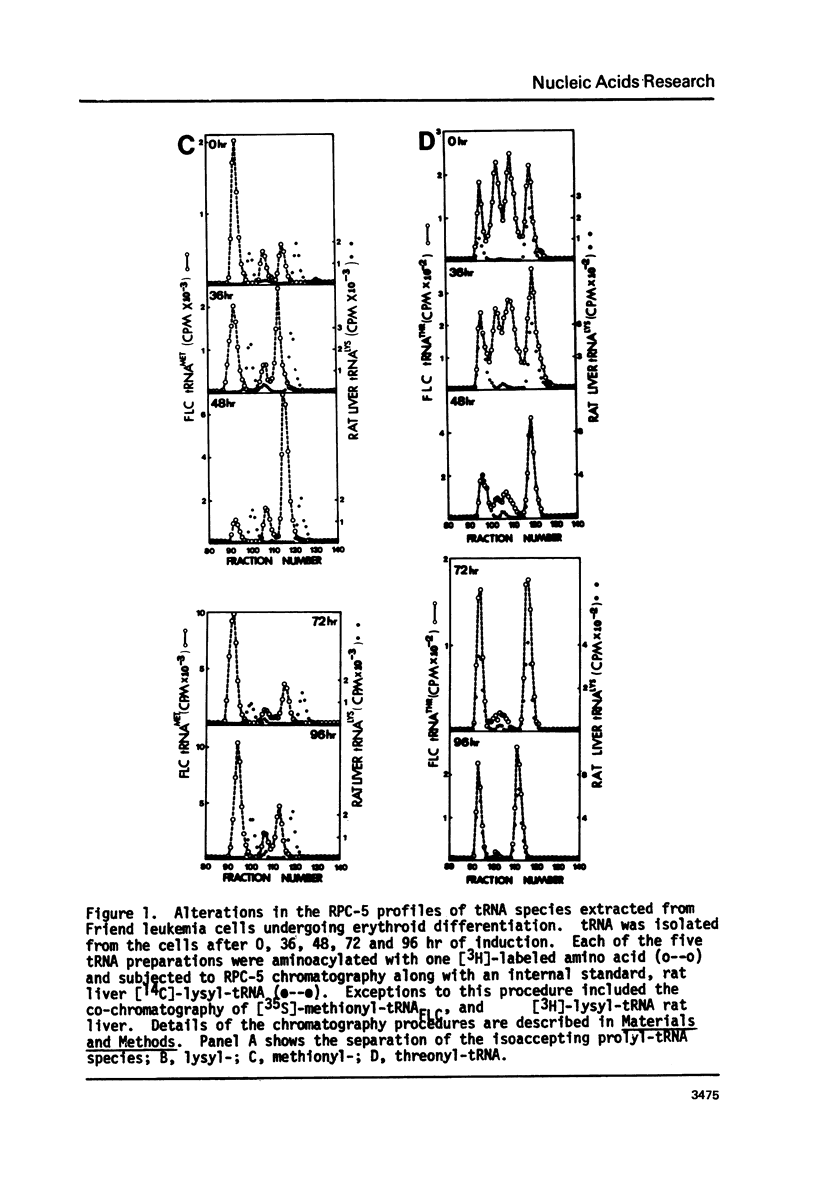
The chromatographic profiles of isoaccepting tRNAs were analyzed at five time points during the 96 hr, dimethylsulfoxide induced, erythroid-like differentiation of Friend leukemia cells. Sixty-four isoaccepting species of tRNA for 16 amino acids were resolved by RPC-5 chromatography. The relative amounts of tRNAphe, tRNAile, and tRNAval species were maintained by the cells during differentiation; whereas the relative amounts of some of the isoacceptor tRNAs for the other 13 amino acids changed significantly. Fluctuations in amounts of isoacceptors occurred between 36 and 72 hr after addition of dimethysulfoxide, corresponding to globin mRNA appearance and hemoglobin synthesis, respectively. In most cases, thepredominant tRNA isoacceptors of uninduced cells were retained throughout differentiation. Notable exceptions were tRNA species for threonine, proline, and methionine. Some of the isoacceptors occurring in relatively smaller amounts were not expressed at all times. These changes possibly reflect the cell's functional adaptation of tRNA in differentiation for hemoglobin synthesis.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F. Alterations of transfer RNA during erythroid differentiation of murine virus-induced leukemia cells. Arch Biochem Biophys. 1975 Sep;170(1):114–123. doi: 10.1016/0003-9861(75)90102-2. [DOI] [PubMed] [Google Scholar]
- Agris P. F. Nucleotide composition analysis of tRNA from leukemia patient cell samples and human cell lines. Nucleic Acids Res. 1975 Jul;2(7):1083–1091. doi: 10.1093/nar/2.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpousis A., Christner P., Rosenbloom J. Preferential usage of glycyl-tRNA isoaccepting species in collagen synthesis. J Biol Chem. 1977 Apr 10;252(7):2447–2449. [PubMed] [Google Scholar]
- Chavancy G., Chevallier A., Fournier A., Garel J. P. Adaptation of iso-tRNA concentration to mRNA codon frequency in the eukaryote cell. Biochimie. 1979;61(1):71–78. doi: 10.1016/s0300-9084(79)80314-4. [DOI] [PubMed] [Google Scholar]
- Christner P. J., Rosenbloom J. A comparison of transfer RNA isoaccepting species between collagenous and noncollagenous tissues in the embryonic chick. Arch Biochem Biophys. 1976 Feb;172(2):399–409. doi: 10.1016/0003-9861(76)90091-6. [DOI] [PubMed] [Google Scholar]
- Drabkin H. J., Lukens L. N. Preferential use in collagen synthesis of the same glycyl-tRNA species that is elevated in collagen-synthesizing tissues. J Biol Chem. 1978 Sep 10;253(17):6233–6241. [PubMed] [Google Scholar]
- Elder K. T., Smith A. E. Methionine transfer ribonucleic acids of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2823–2826. doi: 10.1073/pnas.70.10.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend C., Preisler H. D., Scher W. Studies on the control of differentiation of murine virus-induced erythroleukemic cells. Curr Top Dev Biol. 1974;8:81–101. doi: 10.1016/s0070-2153(08)60606-7. [DOI] [PubMed] [Google Scholar]
- Garel J. P. Functional adaptation of tRNA population. J Theor Biol. 1974 Jan;43(1):211–225. doi: 10.1016/s0022-5193(74)80054-8. [DOI] [PubMed] [Google Scholar]
- Juarez H., Juarez D., Hedgcoth C., Ortwerth B. J. Amounts of isoaccepting lysine tRNAs change with the proliferative state of cells. Nature. 1975 Mar 27;254(5498):359–360. doi: 10.1038/254359a0. [DOI] [PubMed] [Google Scholar]
- Kelmers A. D., Heatherly D. E. Columns for rapid chromatographic separation of small amounts of tracer-labeled transfer ribonucleic acids. Anal Biochem. 1971 Dec;44(2):486–495. doi: 10.1016/0003-2697(71)90236-3. [DOI] [PubMed] [Google Scholar]
- Kleiman L., Woodward-Jack J., Cedergren R. J., Dion R. Alterations in lysine transfer RNA during erythroid differentiation of the Friend cell. Nucleic Acids Res. 1978 Mar;5(3):851–859. doi: 10.1093/nar/5.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt M., Kabat D. Studies of transfer ribonucleic acids and of hemoglobin synthesis in sheep reticulocytes. J Biol Chem. 1972 Oct 25;247(20):6659–6664. [PubMed] [Google Scholar]
- Littauer U. Z., Inouye H. Regulation of tRNA. Annu Rev Biochem. 1973;42:439–470. doi: 10.1146/annurev.bi.42.070173.002255. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Harosi F. I., Leder P. Differentiation in erythroleukemic cells and their somatic hybrids. Proc Natl Acad Sci U S A. 1975 Jan;72(1):98–102. doi: 10.1073/pnas.72.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortwerth B. J., Chu-Der O. M. Studies on the specialized transfer RNA population of the lens. Exp Eye Res. 1974 Dec;19(6):521–532. doi: 10.1016/0014-4835(74)90089-x. [DOI] [PubMed] [Google Scholar]
- Ortwerth B. J., Liu L. P. Correlation between a specific isoaccepting lysyl transfer ribonucleic acid and cell division in mammalian tissues. Biochemistry. 1973 Sep 25;12(20):3978–3984. doi: 10.1021/bi00744a030. [DOI] [PubMed] [Google Scholar]
- Ortwerth B. J., Yonuschot G. R., Carlson J. V. Properties of tRNALys4 from various tissues. Biochemistry. 1973 Sep 25;12(20):3985–3991. doi: 10.1021/bi00744a031. [DOI] [PubMed] [Google Scholar]
- Ortwerth B. J., Yonuschot G. R., Heidlege J. F., Chu-Der O. M., Juarez D., Hedgcoth C. Induction of a new species of phenylalanine transfer RNA during lens cell differentiation. Exp Eye Res. 1975 May;20(5):417–426. doi: 10.1016/0014-4835(75)90084-6. [DOI] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Smith D. W., McNamara A. L. The distribution of transfer ribonucleic acid in rabbit reticulocytes. Levels of aminoacylation and ribosomal attachment during hemoglobin synthesis. J Biol Chem. 1974 Mar 10;249(5):1330–1334. [PubMed] [Google Scholar]
- Smith D. W., McNamara A. L. The transfer RNA content of rabbit reticulocytes: enumeration of the individual species per cell. Biochim Biophys Acta. 1972 Apr 26;269(1):67–77. doi: 10.1016/0005-2787(72)90075-5. [DOI] [PubMed] [Google Scholar]
- Vestri R., Rossi C. Correlation between the concentration of isoleucine transfer RNA and the isoleucine content of hemoglobin in rabbit and sheep reticulocytes. Ital J Biochem. 1976 Jul-Aug;25(4):327–336. [PubMed] [Google Scholar]
- van Ooyen A., van den Berg J., Mantei N., Weissmann C. Comparison of total sequence of a cloned rabbit beta-globin gene and its flanking regions with a homologous mouse sequence. Science. 1979 Oct 19;206(4416):337–344. doi: 10.1126/science.482942. [DOI] [PubMed] [Google Scholar]


