Abstract
Pain remains a pervasive problem throughout medicine, transcending all specialty boundaries. Despite the extraordinary insights into pain and its mechanisms over the past few decades, few advances have been made with analgesics. Most pain remains treated by opiates, which have significant side effects that limit their utility. We now describe a potent opiate analgesic lacking the traditional side effects associated with classical opiates, including respiratory depression, significant constipation, physical dependence, and, perhaps most important, reinforcing behavior, demonstrating that it is possible to dissociate side effects from analgesia. Evidence indicates that this agent acts through a truncated, six-transmembrane variant of the G protein-coupled mu opioid receptor MOR-1. Although truncated splice variants have been reported for a number of G protein-coupled receptors, their functional relevance has been unclear. Our evidence now suggests that truncated variants can be physiologically important through heterodimerization, even when inactive alone, and can comprise new therapeutic targets, as illustrated by our unique opioid analgesics with a vastly improved pharmacological profile.
Keywords: opiate receptor, rewarding behavior, kappa3 receptor
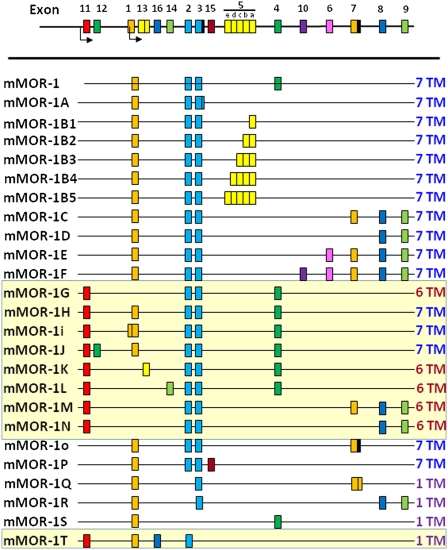
The utility of opioids in the management of pain is not disputed, but they come at a price. Along with their ability to relieve pain comes a variety of opioid receptor-mediated side effects, including respiratory depression, constipation, physical dependence, and reward behavior felt by many to contribute to their addictive potential. Most of the clinical opioids act through mu receptors, which mediate both analgesia and these side effects. Pharmacological studies have long suggested subtypes of mu receptors (1) and the possibility of dissociating analgesia from respiratory depression (2, 3), physical dependence (4), and the inhibition of gastrointestinal transit (5, 6). However, attempts to develop opiate analgesics that avoid these side effects have not been very fruitful. The isolation of a series of splice variants of the cloned mu opioid receptor from mice (Fig. 1), rats, and humans with similar splicing patterns (7, 8) reveals a complexity far exceeding the pharmacological classification of mu receptor subtypes (1). However, this complexity has yet to be exploited in generating new classes of opioid analgesics. We now report an unexpected and unusual target for potent opioid analgesic drugs that lack respiratory depression, physical dependence, reward behavior, and significant constipation.
Fig. 1.
Schematic of alternative splicing of the mouse Oprm1 gene. The mouse gene is presented in schematic form and not to scale, with splice variants shown underneath. There are two major classes of splice variants: those generated by the promoter associated with exon 1 and an additional set generated by the exon 11 promoter (designated by the yellow background). All of the exon 1-associated variants are traditional G protein-coupled receptors with 7-TM regions. The exon 11-associated variants include several full-length receptors and both 6-TM and 1-TM variants as indicated.
Results
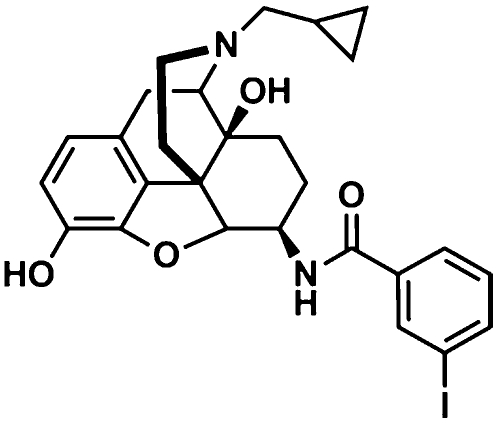
Recently, we synthesized iodobenzoylnaltrexamide (IBNtxA), a naltrexone derivative (Fig. 2) (9). In vivo, it is a very potent analgesic (ED50 = 0.48 ± 0.05 mg/kg s.c.) (Fig. 3A and Fig. S1), ∼10-fold more potent than morphine (4.6 ± 0.97 mg/kg s.c.) (10), with a mechanism of action quite distinct from traditional opiates. It was active s.c. as well as orally (Fig. S1), with a peak effect after oral administration that was delayed relative to parenteral administration. In addition to its high potency, IBNtxA also displayed full efficacy, as indicated by most mice reaching cutoff values at higher doses. We also assessed IBNtxA analgesia by using a graded response [percentage maximal possible effect (%MPE)] (Fig. S2). Its ED50 value (0.34 mg/kg; 95% confidence limits: 0.23, 0.52) was very close to that with quantal responses (Fig. 3A). Furthermore, IBNtxA analgesia was not limited to the radiant heat tail-flick assay. IBNtxA was a potent analgesic in the hot plate assay (ED50 = 0.6 mg/kg) as well (Fig. S3).
Fig. 2.
Structure of IBNtxA.
Fig. 3.
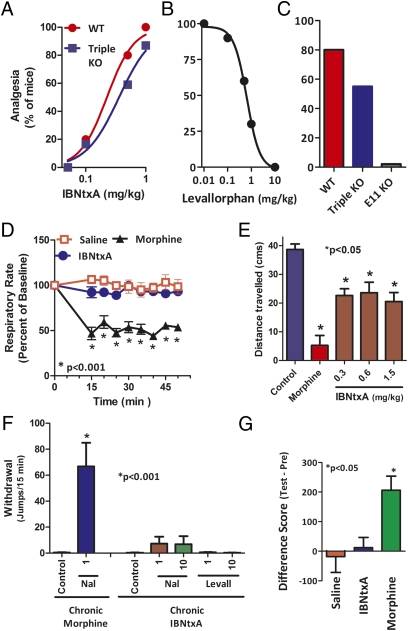
IBNtxA pharmacology. (A) IBNtxA analgesia. Groups of mice (n = 10) received IBNtxA at the indicated doses and were tested 30 min later at peak effect to generate the analgesic dose–response curve. ED50 values (and 95% confidence limits) were 0.22 mg/kg (0.13, 0.32) in WT mice and 0.39 mg/kg (0.15, 0.58) in triple-KO mice by using the radiant heat tail-flick assay. (B) Reversal of IBNtxA analgesia by levallorphan. Groups of mice (n ≥ 10) received IBNtxA (0.75 mg/kg s.c.) and the indicated dose of levallorphan, and analgesia was assessed 30 min later. (C) IBNtxA analgesia in KO mice. IBNtxA analgesia (0.5 mg/kg) was determined in groups (n = 10) of WT, triple-KO (exon 1 MOR-1/DOR-1/KOR-1), and exon 11 MOR-1 KO mice at 30 min. In the triple-KO mice, analgesia was present and not significantly different from WT mice. The responses of the exon 11 mice were significantly different from WT and triple-KO mice. Statistical significance was assessed with the Fisher exact test. (D) Respiratory rate. Animals were randomly assigned to receive saline (n = 5), IBNtxA (2.5 mg/kg, n = 5), or morphine (20 mg/kg, n = 5). Each animal's baseline average breath rate was measured every 5 min for 25 min before drug injection, and breath rates after drug injection are expressed as a percent of baseline. IBNtxA did not depress respiratory rate and was not significantly different from saline at any time point, whereas morphine decreased respiratory depression in comparison with both saline and IBNtxA (P < 0.001) as determined by repeated-measures ANOVA followed by Bonferroni multiple-comparison test. (E) Gastrointestinal transit. Groups of mice (n = 10) received saline, morphine (5 mg/kg), or IBNtxA (0.3, 0.6, and 1.5 mg/kg) before receiving an oral dose of 0.2 mL of charcoal meal (2.5% gum tragacanth in 10% activated charcoal in water) by gavage. Animals were killed 30 min later, and the distance traveled by charcoal was measured. IBNtxA lowered transit significantly compared with saline (P < 0.05) but less than morphine (P < 0.05) as determined by ANOVA followed by Tukey's multiple-comparison test. The inhibition of gastrointestinal transit seems to plateau even at doses three times higher than the ED50. (F) Physical dependence. Groups of mice (n ≥ 10) received either morphine (10 mg/kg s.c.) or IBNtxA (1 mg/kg s.c.) for 10 d. They then were challenged with the indicated antagonist. Naloxone (Nal) precipitated a profound withdrawal syndrome in the morphine-treated animals, as shown by the number of jumps per 15 min, which was significantly greater than that in the morphine or IBNtxA controls (i.e., given no antagonist) or in IBNtxA mice given the indicated antagonist. Mice chronically administered IBNtxA showed no significant difference from controls when challenged by either naloxone (Nal, 1 mg/kg s.c.) or levallorphan (Levall, 1 mg/kg s.c.). (G) Conditioned place preference. Mice randomly were assigned to receive saline (n = 10), IBNtxA (1 mg/kg, n = 10), or morphine (10 mg/kg, n = 13) in a two-compartment conditioned place preference assay. The difference score was obtained by subtracting the amount of time (seconds) spent in the drug-paired compartment on test day (Test) from the amount of time (seconds) spent in the drug-paired compartment on the preconditioning day (Pre). IBNtxA did not produce place preference and was not significantly different from saline (P > 0.05), whereas morphine showed preference in comparison with both saline and IBNtxA (P < 0.05) as determined by ANOVA followed by Tukey's multiple-comparison test.
Opioid receptor KO mice have been valuable in exploring opioid action. A number of mice targeting MOR-1 have been reported (11–14). All MOR-1 KO mice with a disruption of exon 1 are unresponsive to morphine, but one model with a disruption of exon 1 retained full sensitivity to heroin and morphine-6β-glucuronide, although with a small decrease in potency (14). Despite the loss of all of the variants containing exon 1, this mouse still expressed a series of MOR-1 variants generated from a second, upstream promoter associated with exon 11 (Fig. 1). This mouse also has been used to generate a triple-KO mouse with disruptions of the delta opioid receptor DOR-1 and the kappa1 opioid receptor KOR-1. IBNtxA displayed a full analgesic response in these triple-KO mice with a potency similar to that in WT mice (Fig. 3A), indicating that IBNtxA analgesia did not involve full-length mu receptors containing exon 1 or delta or kappa1 receptors. Its actions were opioid, as shown by its potent reversal by levallorphan (ID50 = 0.54 ± 0.05 mg/kg s.c.), a well-established opioid antagonist (Fig. 3B). Naloxone (ID50 = 10.5 ±0.6 mg/kg s.c.) also reversed its actions but at doses higher than those needed for traditional mu opiates such as morphine (ID50 = 0.01 mg/kg s.c.) (15). Its relative insensitivity to a series of selective antagonists, including β-funaltrexamine (mu), norbinaltorphimine (kappa1), and naltrindole (delta), further supported a nontraditional opioid receptor mechanism of action (Fig. S4).
We next addressed whether an exon 11-associated variant might be involved in IBNtxA actions because they are still expressed in the triple-KO mice. Loss of the exon 11-associated variants has no significant effect on morphine or methadone analgesia (16). In contrast, IBNtxA analgesia was lost in the exon 11 MOR-1 KO mouse (Fig. 3C), indicating that IBNtxA analgesia involves exon 11-associated MOR-1 variants. Together with the data from triple-KO mice, these data suggest that IBNtxA analgesia depends on exon 11-associated MOR-1 variants lacking exon 1.
The pharmacological profile of IBNtxA also differed from classical opioids. Respiratory depression is a major issue associated with opioids. We therefore examined the effects of IBNtxA on respiratory rate, a measure of respiratory depression. Using doses approximately fourfold greater than their respective analgesic ED50 values, we observed the expected decrease in respiratory rate in mice receiving morphine (20 mg/kg s.c.), whereas an equivalent dose of IBNtxA (2.5 mg/kg s.c.) did not change the respiratory rate compared with saline (Fig. 3D).
Inhibition of gastrointestinal transit, manifest clinically as constipation, is another major clinical concern. Morphine dramatically inhibited gastrointestinal transit (Fig. 3E). An equianalgesic dose of IBNtxA (0.6 mg/kg s.c.) lowered transit a small amount, but this effect plateaued, with far greater doses showing no further inhibition, which clearly distinguished it from the effects of morphine. However, even this small inhibition of gastrointestinal transit appears not to involve the same receptors as IBNtxA analgesia because the gastrointestinal transit inhibition persisted in the exon 11 KO mice, which displayed no IBNtxA analgesia (Fig. S5). Thus, more selective agents might lack even this small amount of inhibition of gastrointestinal transit seen with IBNtxA.
Chronic administration of traditional opioids leads to both tolerance and physical dependence. Daily administration of morphine (10 mg/kg s.c.) revealed a diminishing response with no demonstrable analgesia by day 5 (Fig. S6). These chronically morphine-treated mice were both tolerant and physically dependent, with naloxone precipitating a profound withdrawal syndrome (Fig. 3F) (4). Chronic IBNtxA dosing also produced tolerance, although it developed more slowly (Fig. S6). Unlike morphine, challenging the IBNtxA-tolerant mice with either of the antagonists naloxone or levallorphan failed to precipitate any demonstrable withdrawal symptoms, including jumping (Fig. 3F), implying that chronic IBNtxA does not produce physical dependence. We also observed no cross-tolerance between morphine and IBNtxA analgesia. Implantation of morphine pellets (75 mg of free base) in mice for 3 d led to marked analgesic tolerance and physical dependence. After 3 d, IBNtxA (1 mg/kg s.c.) displayed analgesia in 100% of the pelleted mice (n = 10), an effect comparable to that seen in naïve mice (93%, n = 15). Furthermore, IBNtxA did not elicit any signs of withdrawal in the morphine-dependent mice.
Rewarding behavior is one measure of potential abuse potential. Morphine shows rewarding behavior in the conditioned place preference assay, whereas the kappa1-selective agonist U50,488H is aversive. In our studies, IBNtxA was neutral, failing to show either rewarding or aversive behavior, whereas morphine was rewarding, as anticipated (Fig. 3G).
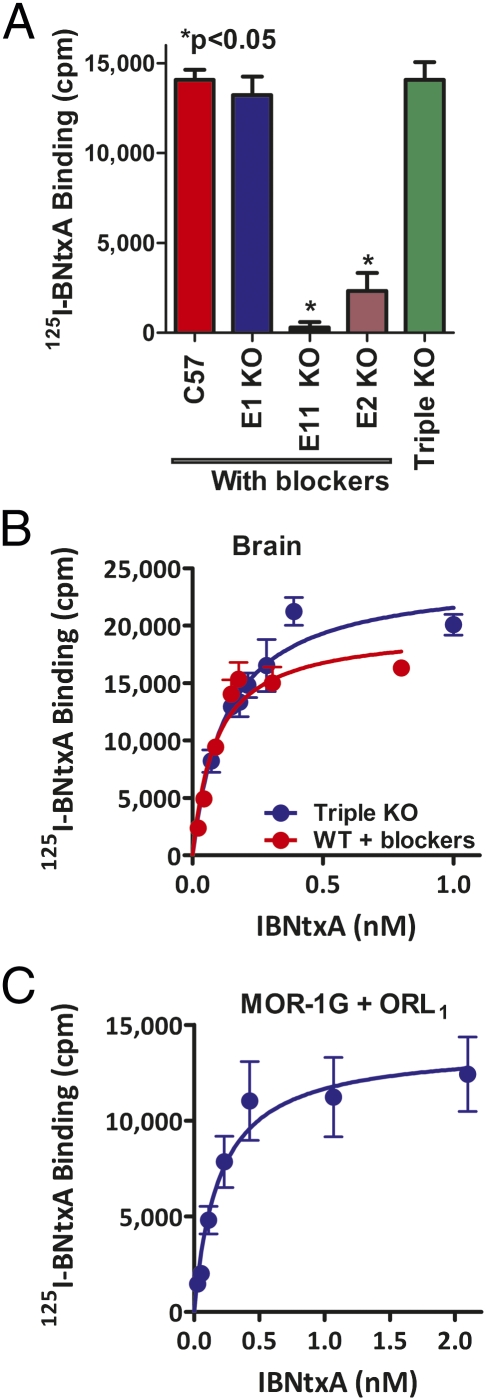
The pharmacological profile of IBNtxA suggested that it may act through a target involving exon 11-associated MOR-1 variants that lack exon 1. Although 125I-BNtxA labels traditional mu, delta, and kappa1 receptors in transfected cell lines (9), ∼20% of total specific 125I-BNtxA binding in brain tissue was insensitive to a combination of high concentrations of selective mu-, delta-, or kappa1-selective ligands (Table S1). Similar 125I-BNtxA-selective binding levels were present in triple-KO mice (Fig. 4 A and B and Table S1). Disruption of either exon 11 or exon 2 virtually eliminated the binding (Fig. 4A), mimicking in binding studies our analgesic results in the exon 11 KO model and strongly supporting a role for exon 11-associated variants in the binding target.
Fig. 4.
125I-BNtxA binding. (A) 125I-BNtxA binding in KO mice. Brain membranes from either WT, exon 1, exon 11, or exon 2 KO mice were incubated with 125I-BNtxA (0.3 nM) in the presence of mu (CTAP), kappa1 (U50,488H), or delta (DPDPE) blockers at 1 μM. The triple-KO mice were assayed without blockers. Only specific binding is reported. The assay was replicated at least three times. ANOVA revealed no differences for the WT, exon 1, or triple-KO mice, whereas binding was lost in exon 11 KO mice and markedly reduced in exon 2 KO mice (P < 0.05). (B) Saturation studies with 125I-BNtxA. 125I-BNtxA saturation studies were carried out on mice brain membranes from either triple-KO or WT mice with blockers. Results are from a representative experiment, and only specific binding is reported. Experiments were replicated at least three times. KD and Bmax were determined by nonlinear regression analysis (Prism), and the means ± SEM of the replicates were determined. KD values were best fit with a single site and were as follows: Triple-KO mice, KD = 0.16 ± 0.04 nM, Bmax = 60.84 ± 1.62 fmol/mg; WT mice, KD = 0.12 ± 0.04 nM, Bmax = 47 ± 8.4 fmol/mg. There was no significant difference between KD or Bmax values for the two groups (t test, P > 0.05). (C) 125I-BNtxA binding in MOR-1G- and ORL1-expressing HEK cells. HEK cells were transiently transfected with MOR-1G or ORL1 alone or both together. Cell membranes from all three conditions were incubated with 125I-BNtxA (0.08–2 nM). Results represent specific binding and were best fit with a single site. The study was replicated three times, and means ± SEM of the independent replications revealed KD = 0.24 ± 0.03 nM, Bmax = 18.1 ± 2.21 fmol/mg. No specific binding was detectible in control cells expressing ORL1 or MOR-1G alone or in nontransfected HEK cells.
Competition binding studies from triple-KO and WT mouse brains with traditional mu, delta, and kappa1 blockers revealed profiles quite distinct from traditional binding sites (Table 1), although they resembled those of the kappa3 sites previously reported by our group (17–20). A wide range of traditional opioids, including the mu-selective compounds morphine and [d-Ala2,MePhe4,Gly(ol)5]enkephalin (DAMGO), the delta-selective agents [d-Pen2,d-Pen5]enkephalin (DPDPE) and SNC80, the kappa1-selective drug U50,488H, and orphanin FQ/nociceptin analogs, did not compete binding, all having Ki values greater than 1 μM. However, a number of opioids competed binding quite effectively. Martin et al. initially defined kappa receptors 35 y ago by using the benzomorphan drug ketocyclazocine (21). Ketocyclazocine competed the new site with high affinity that was comparable to or better than the traditional kappa1 receptor (Ki = 1.8 nM) (17). Other benzomorphans considered to have strong kappa actions, as defined by the criteria of Martin et al., also competed binding very potently, including the benzomorphans ethylketocyclazocine, cyclazocine, and Mr2034. Other structural scaffolds also showed high affinity. The morphinans levorphanol and butorphanol, and especially the antagonist levallorphan, were quite potent. Naloxone benzoylhydrazone (NalBzoH), which we used to define the kappa3 receptor (17, 18, 22), competed binding effectively, as did the oripavines etorphine, diprenorphine, and buprenorphine. Naloxone lowered binding (Ki = 52 nM) more than 10-fold less potently than mu binding sites (17, 23), possibly explaining its lower potency reversing IBNtxA analgesia (ID50 = 10 mg/kg s.c.) compared with reversal of morphine analgesia (ID50 = 0.01 mg/kg s.c.) (15). This finding contrasts with levallorphan, which effectively blocked IBNtxA analgesia at very low doses (ID50 = 0.54 mg/kg s.c.), consistent with its high affinity for the site (Ki = 0.34 nM).
Table 1.
Competition opioid binding assays with125I-BNtxA in triple-KO and WT mice brain membranes
| Drug |
Ki |
|
| Triple-KO, nM | WT with blockers, nM | |
| Mu | ||
| Fentanyl | 226 ± 40 | 256 ± 10.2 |
| β-Funaltrexamine | 35.6 ± 5.3 | 37.8 ± 10.6 |
| Naloxone | 51.9 ± 1.4 | 53.2 ± 7.13 |
| Naltrexone | 20.5 ± 1.8 | 32.7 ± 4.1 |
| Kappa1 | ||
| 5′-Guanidinonaltrindole (GNTI) | 22.9 ± 3.3 | 29.1 ± 0.02 |
| Norbinaltorphimine (NOR-BNI) | 3.3 ± 1.9 | 4.94 ± 0.99 |
| Delta | ||
| Naltrindole | 26.3 ± 2.3 | 28.09 ± 10.96 |
| Kappa3 | ||
| NalBzoH | 0.59 ± 0.15 | 1.0 ± 0.3 |
| Nalorphine | 3.71 ± 1.45 | 7.5 ± 0.6 |
| Levorphanol | 8.8 ± 2.5 | 5 ± 1.5 |
| Levallorphan | 0.34 ± 0.018 | 0.5 ± 0.15 |
| Ketocyclazocine | 0.04 ± 0.01 | 3.14 ± 1.08 |
| (−)-SKF10,047 | 13.5 ± 1.6 | 22.2 ± 1.9 |
| Mr2034 | 2.67 ± 0.83 | 3.99 ± 1.1 |
| (−)Ethylketocyclazocine | 0.21 ± 0.11 | 0.2 ± 0.1 |
| Cyclazocine | 1.81 ± 0.67 | 4.59 ± 0.58 |
| Nalbuphine | 3.47 ± 1.18 | 8.5 ± 5.39 |
| Butorphanol | 2.92 ± 1.55 | 2.75 ± 0.13 |
| Diprenorphine | 2.2 ± 0.71 | 3.0 ± 0.86 |
| Buprenorphine | 1.8 ± 0.93 | 2.13 ± 0.27 |
| Other | ||
| Morphine, CTAP, DAMGO, oxymorphone, oxycodone, morphine-6β-glucuronide, β-endorphin, codeine, meperidine, [d-Ala2,d-Leu5]-enkephalin (DADLE), DPDPE, SNC80, U50,488H, dynorphin A, α-neoendorphin, orphanin FQ/nociceptin (OFQ/N), OFQ/N(1\x{2013}11), J-113397, JTC801, (+)-SKF10,047, (+) pentazocine, and haloperidol | Inactive (>1,000) | |
125I-BNtxA competition studies were carried out in mice brain homogenates from triple-KO and WT mice with blockers to prevent binding to traditional mu, kappa1, and delta opioid receptors as described in Materials and Methods.
Many of the drugs active at the 125I-BNtxA binding site are potent analgesics in vivo (17–20, 24, 25). To determine whether they were acting, in part, through mechanisms similar to IBNtxA, we tested them in the exon 11 KO mouse (Table 2). The analgesic activity of ketocyclazocine, which bound this new site with very high affinity, was shifted ∼100-fold to the right, with rightward shifts for clinical analgesics levorphanol, butorphanol, and nalbuphine as well. Finally, NalBzoH lost analgesic activity in the exon 11 KO mice. Doses as high as 100 mg/kg s.c. failed to reach an ED50 comparable with an ED50 of 22 ± 5.3 mg/kg s.c. in WT mice.
Table 2.
Opioid analgesia in MOR-1 exon 11 KO mice
| ED50, mg/kg s.c. |
|||
| Drug | WT | Exon 11 KO | Shift |
| IBNtxA | 0.53 ± 0.07 | >20 | >35 |
| NalBzoH | 22 ± 5.3 | >100 | >5 |
| Ketocyclazocine | 0.18 ± 0.04 | 21.8 ± 10 | 120* |
| Nalbuphine | 46.96 ± 15.26 | >200 | >5 |
| Levorphanol | 0.1 ± 0.01 | 0.72 ± 0.13 | 7 |
| Butorphanol | 5.94 ± 3.88 | >100 | >16 |
| Morphine | 1.58 ± 0.17 | 2.58 ± 0.52 | 1.6 |
| Methadone | 1.53 ± 0.06 | 1.8 ± 0.14 | 1.2 |
Dose–response curves were performed for each ligand and replicated at least three times unless indicated otherwise. All mice were on the C57 background. Results are the means ± SEM of the independent determinations. The results for morphine and methadone are from the literature (15). A number of drugs could not be tested at very high concentrations because of solubility issues. If the ED50 could not be attained, it is stated as greater than the highest dose tested. The shifts for morphine and methadone are not significant, but the one for ketocyclazocine is (*P < 0.006).
The evidence strongly implicated a role for exon 11-associated variants in IBNtxA actions. However, attempts to demonstrate 125I-BNtxA binding after expression in cells transfected with the exon 11-associated variants lacking exon 1, such as mMOR-1G, were unsuccessful. These variants are truncated with only six-transmembrane (6-TM) domains, lacking the first TM domain of MOR-1 encoded by exon 1. We therefore examined a possible role of heterodimerization in the actions of these 6-TM variants.
Heterodimerization of full-length opioid receptors has been well described, with many showing a pharmacology distinct from either partner alone, such as the heterodimerization of DOR-1 and KOR-1 to form the kappa2 receptor (26). Earlier work from our laboratory demonstrated that the orphanin FQ receptor ORL1, which we had initially termed KOR-3 (27–33), dimerized with the full-length MOR-1 receptor to generate a binding site with a unique pharmacological profile (34). Furthermore, antisense mapping studies (27, 28) suggested an association of kappa3 analgesia with ORL1. To determine whether ORL1 could partner with a 6-TM MOR-1 variant to generate an 125I-BNtxA binding site, we cotransfected ORL1 with the 6-TM variant MOR-1G. No 125I-BNtxA binding could be detected in cells transfected with either MOR-1G or ORL1 alone. However, coexpression of MOR-1G with ORL1 produced a very high-affinity 125I-BNtxA binding site (KD = 0.24 ± 0.03 nM) (Fig. 4C), similar in affinity to that seen in brain tissue. This finding implies that the 6-TM variant alone is insufficient to generate the 125I-BNtxA site in the brain and requires a partner, which may or may not correspond to ORL1.
Discussion
IBNtxA is an interesting compound from many perspectives. Its pharmacological profile in vivo distinguishes it from other opiates because of its potent analgesia without respiratory depression, physical dependence, or appreciable constipation as well as neither rewarding nor aversive behavior in conditioned place preference studies. However, it is possible to further optimize the pharmacology. Structure–activity relationships reveal compounds with increased potency and selectivity. Thus, the importance of IBNtxA comes from opening a unique area of drug development and a better understanding of receptor targets rather than its own clinical development.
The mechanism(s) of IBNtxA actions are quite unique and distinct from any of the traditional opioid receptors. Its potent reversal by the opiate antagonist levallorphan implies an opiate mechanism, but its relative insensitivity against several selective antagonists separates it from the classical drugs. The KO models give greater insights into the target. The persistence of IBNtxA analgesia and normal levels of 125I-BNtxA binding in the triple-KO mice rules out a role for delta and kappa1 receptors or any of the full-length MOR-1 splice variants, which all contain exon 1. On the other hand, the loss of both IBNtxA analgesia and receptor binding in the exon 11 KO mice clearly implicates a role for exon 11-associated variants that contain only 6-TM domains.
Heterodimerization has been well demonstrated within the opioid receptor family, with some complexes showing pharmacological profiles unlike either component examined alone (26, 34–39). However, these studies involved dimerization of full-length receptors. Our studies suggest that heterodimerization of truncated variants can generate new pharmacological targets. When examined directly, we failed to see any 125I-BNtxA binding when the 6-TM variant MOR-1G was expressed alone, leading us to consider whether the target required a partner. We tried coexpression of MOR-1G with ORL1 and observed the formation of a very high-affinity 125I-BNtxA binding site (KD = 0.24 nM). We chose to test ORL1 for a number of reasons. First, 125I-BNtxA does not label ORL1 when it is expressed alone, making the detection of a new site simpler. Second, we had already demonstrated that the full-length MOR-1 will dimerize with ORL1 (34). However, the ability of ORL1 to partner with MOR-1G to form the binding site does not necessarily mean that ORL1 is the endogenous partner. It is entirely possible that the 6-TM variants can associate with one or more other candidates. Thus, our findings further illustrate that inactivity of a truncated variant when expressed alone does not necessarily mean that a truncated receptor is inconsequential and without relevance, an observation that may prove important with other truncated G protein-coupled receptors.
Classification of the target is not straightforward. Although the KO studies clearly implicate 6-TM variants generated by the mu receptor MOR-1 gene Oprm1, its endogenous partner remains unknown. Pharmacologically, the 125I-BNtxA binding site profile in brain tissue does not correspond to traditional mu, delta, or kappa1 receptors based on its insensitivity toward highly selective mu, delta, or kappa1 agents. However, it has extremely high affinity for many of the drugs long considered to be kappa, including ketocyclazocine, which was the prototypic drug used by Martin et al. to define the receptor class (21). It is interesting that the 125I-BNtxA binding profile resembles that of the kappa3 binding site identified with [3H]NalBzoH (17, 18) and that many of the analgesics proposed to act through that site (17–20, 24, 25) lost activity in the MOR-1 exon 11 KO mouse. Regardless of the nomenclature, this target offers a major advance in the design and development of new highly potent opiates without many of the drawbacks of the drugs currently available.
Materials and Methods
Male CD-1 and C57 and exon 2 MOR-1 KO mice (25–35 g) were obtained from Charles River Breeding Laboratories. The exon 1 KO and triple-KO mice came from J.P.'s laboratory (14, 40), and the exon 11 mice were from our laboratory (16). All mice were maintained on a 12-h light/dark cycle with Purina rodent chow and water available ad libitum, housed in groups of five until testing. All animal studies were approved by the Institutional Animal Care and Use Committee of the Memorial Sloan-Kettering Cancer Center.
Opiates were a gift from the Research Technology Branch of the National Institute on Drug Abuse (Rockville, MD). IBNtxA and 125I-BNtxA were synthesized as previously described (9). Na125I was obtained from Perkin-Elmer. Miscellaneous chemicals and buffers were purchased from Sigma-Aldrich. All receptor clones are murine, unless otherwise noted.
125I-BNtxA Binding Assays.
Binding assays were carried out in whole-brain membrane homogenates prepared as previously described (17). Binding in WT, exon 1, exon 2, and exon 11 knockout mice was carried out in the presence of mu [d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP)], kappa1 (U50,488H), and delta (DPDPE) blockers at either 1 μM (saturation studies) or 200 nM (competition studies). Binding in triple-KO mice was carried out in the absence of any blockers. Nonspecific binding was defined by levallorphan (8 μM) and was subtracted from total binding to yield specific binding. Protein concentrations were determined as described in ref. 41. KD, Bmax, and Ki values were calculated by nonlinear regression analysis (GraphPad Prism).
Analgesia Assays.
Tail-flick analgesia was assessed quantally as a doubling or greater of the baseline tail-flick latency, which ranged from 2 to 3 s, in the radiant heat tail-flick technique (2, 4, 42, 43), with a maximal 10-s latency to minimize tissue damage (4, 43). Analysis of the data using graded responses with %MPE yielded similar responses. The hot plate assay was performed at 55 °C (Ugo Basile) (16). The time(s) elapsing to the first pain response (hind paw licking or jumping) was scored. A maximal latency of 30 s was used to minimize any tissue damage. Results were determined as %MPE [(latency after drug − baseline latency)/(30 − baseline latency)].
Gastrointestinal Motility Assay.
Gastrointestinal transit was determined as described in ref. 5. Animals received the indicated drug followed by a charcoal meal by gavage. Animals were killed 30 min later, and the distance traveled by charcoal was measured.
Conditional Place Preference/Aversion.
The testing apparatus consisted of two compartments of equal size separated by a wall with a guillotine-style door (ENV-512 insert; MedAssociates). One compartment was surrounded by white walls and had a rod floor, and the other had black walls and a grid floor. Infrared photobeams lining the floor tracked the location of the mouse at all times.
Animals were habituated to the environment for 3 h for each of 2 d before testing and for 1 h on each conditioning session. Baseline preferences were determined on the preconditioning test day by letting animals explore both sides freely for 20 min, and the side in which they initially spent more time in was assigned to saline in the place preference study. Animals were injected on alternating days for 8 d with either drug or saline and restricted to one compartment for 20 min. On the postconditioning testing day, animals were placed in the side paired with saline and allowed to freely explore both compartments for 20 min. The time spent in each compartment postconditioning was calculated and subtracted from the amount of time spent in each compartment preconditioning to determine the change in each animal's preference attributable to conditioning.
Tolerance and Dependence Studies.
Tolerance was induced by twice-daily injections with either morphine (6 mg/kg s.c.) or IBNtxA (1 mg/kg s.c.) or through the implantation of free-base morphine pellets (75 mg). Dependence was determined on day 3 after pellet implantation with either IBNtxA (1 mg/kg s.c.) or naloxone (1 mg/kg s.c.) to precipitate withdrawal, and animals were evaluated for signs of diarrhea and jumping (4, 15).
Respiratory Depression Assessment.
Respiratory rate was assessed in awake, freely moving, adult male CD1 mice with the MouseOx pulse oximeter system (Starr Life Sciences). Each animal was habituated to the device for 30 min and then tested. A 5-s average breath rate was assessed at 5-min intervals. A baseline for each animal was obtained over a 25-min period before drug injection, and testing began at 15 min postinjection and continued for a period of 35 min. Groups of mice (n = 5) were treated s.c. with either morphine (20 mg/kg) or IBNtxA (2.5 mg/kg) at doses approximately four times their analgesic ED50. Groups were compared with repeated-measures ANOVA followed by Bonferroni multiple-comparison test.
Supplementary Material
Acknowledgments
This work was supported in part by National Institute on Drug Abuse Grants DA02615, DA06241, DA07242, and DA00220 (to G.W.P.); DA013997 and DA029244 (to Y.-X.P.); and DA018257 (to J.P.); as well as the Technology Development Fund of Memorial Sloan-Kettering Cancer Center (G.W.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115231108/-/DCSupplemental.
References
- 1.Wolozin BL, Pasternak GW. Classification of multiple morphine and enkephalin binding sites in the central nervous system. Proc Natl Acad Sci USA. 1981;78:6181–6185. doi: 10.1073/pnas.78.10.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ling GSF, Spiegel K, Nishimura SL, Pasternak GW. Dissociation of morphine's analgesic and respiratory depressant actions. Eur J Pharmacol. 1983;86:487–488. doi: 10.1016/0014-2999(83)90203-0. [DOI] [PubMed] [Google Scholar]
- 3.Ling GSF, Spiegel K, Lockhart SH, Pasternak GW. Separation of opioid analgesia from respiratory depression: Evidence for different receptor mechanisms. J Pharmacol Exp Ther. 1985;232:149–155. [PubMed] [Google Scholar]
- 4.Ling GSF, MacLeod JM, Lee S, Lockhart SH, Pasternak GW. Separation of morphine analgesia from physical dependence. Science. 1984;226:462–464. doi: 10.1126/science.6541807. [DOI] [PubMed] [Google Scholar]
- 5.Paul D, Pasternak GW. Differential blockade by naloxonazine of two μ opiate actions: Analgesia and inhibition of gastrointestinal transit. Eur J Pharmacol. 1988;149:403–404. doi: 10.1016/0014-2999(88)90680-2. [DOI] [PubMed] [Google Scholar]
- 6.Heyman JS, Williams CL, Burks TF, Mosberg HI, Porreca F. Dissociation of opioid antinociception and central gastrointestinal propulsion in the mouse: Studies with naloxonazine. J Pharmacol Exp Ther. 1988;245:238–243. [PubMed] [Google Scholar]
- 7.Pan Y-X, Pasternak GW. In: The Opiate Receptors. Pasternak GW, editor. New York: Springer; 2011. pp. 121–160. [Google Scholar]
- 8.Pasternak GW. Molecular insights into μ opioid pharmacology: From the clinic to the bench. Clin J Pain. 2010;26(Suppl 10):S3–S9. doi: 10.1097/AJP.0b013e3181c49d2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majumdar S, et al. Generation of novel radiolabeled opiates through site-selective iodination. Bioorg Med Chem Lett. 2011;21:4001–4004. doi: 10.1016/j.bmcl.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolesnikov YA, Pick CG, Ciszewska G, Pasternak GW. Blockade of tolerance to morphine but not to κ opioids by a nitric oxide synthase inhibitor. Proc Natl Acad Sci USA. 1993;90:5162–5166. doi: 10.1073/pnas.90.11.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthes HWD, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 12.Sora I, et al. Opiate receptor knockout mice define μ receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci USA. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loh HH, et al. μ Opioid receptor knockout in mice: Effects on ligand-induced analgesia and morphine lethality. Brain Res Mol Brain Res. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- 14.Schuller AG, et al. Retention of heroin and morphine-6β-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci. 1999;2:151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- 15.Gistrak MA, Paul D, Hahn EF, Pasternak GW. Pharmacological actions of a novel mixed opiate agonist/antagonist: Naloxone benzoylhydrazone. J Pharmacol Exp Ther. 1989;251:469–476. [PubMed] [Google Scholar]
- 16.Pan YX, et al. Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc Natl Acad Sci USA. 2009;106:4917–4922. doi: 10.1073/pnas.0811586106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark JA, et al. Kappa opiate receptor multiplicity: Evidence for two U50,488-sensitive kappa 1 subtypes and a novel kappa 3 subtype. J Pharmacol Exp Ther. 1989;251:461–468. [PubMed] [Google Scholar]
- 18.Paul D, et al. Naloxone benzoylhydrazone (NalBzoH) analgesia. J Pharmacol Exp Ther. 1990;255:769–774. [PubMed] [Google Scholar]
- 19.Paul D, Pick CG, Tive LA, Pasternak GW. Pharmacological characterization of nalorphine, a kappa 3 analgesic. J Pharmacol Exp Ther. 1991;257:1–7. [PubMed] [Google Scholar]
- 20.Pick CG, Paul D, Pasternak GW. Nalbuphine, a mixed kappa 1 and kappa 3 analgesic in mice. J Pharmacol Exp Ther. 1992;262:1044–1050. [PubMed] [Google Scholar]
- 21.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 22.Price M, Gistrak MA, Itzhak Y, Hahn EF, Pasternak GW. Receptor binding of [3H]naloxone benzoylhydrazone: A reversible kappa and slowly dissociable mu opiate. Mol Pharmacol. 1989;35:67–74. [PubMed] [Google Scholar]
- 23.Pan L, et al. Identification and characterization of six new alternatively spliced variants of the human μ opioid receptor gene, Oprm. Neuroscience. 2005;133:209–220. doi: 10.1016/j.neuroscience.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 24.Moulin DE, Ling GSF, Pasternak GW. Unidirectional analgesic cross-tolerance between morphine and levorphanol in the rat. Pain. 1988;33:233–239. doi: 10.1016/0304-3959(88)90095-4. [DOI] [PubMed] [Google Scholar]
- 25.Tive LA, Ginsberg K, Pick CG, Pasternak GW. Kappa 3 receptors and levorphanol-induced analgesia. Neuropharmacology. 1992;31:851–856. doi: 10.1016/0028-3908(92)90121-5. [DOI] [PubMed] [Google Scholar]
- 26.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan Y-X, Cheng J, Xu J, Pasternak GW. Cloning, expression and classification of a kappa3-related opioid receptor using antisense oligodeoxynucleotides. Regul Pept. 1994;54:217–218. [Google Scholar]
- 28.Pan Y-X, et al. Cloning and functional characterization through antisense mapping of a kappa 3-related opioid receptor. Mol Pharmacol. 1995;47:1180–1188. [PubMed] [Google Scholar]
- 29.Uhl GR, Childers S, Pasternak GW. An opiate-receptor gene family reunion. Trends Neurosci. 1994;17:89–93. doi: 10.1016/0166-2236(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, et al. Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett. 1994;347:279–283. doi: 10.1016/0014-5793(94)00560-5. [DOI] [PubMed] [Google Scholar]
- 31.Bunzow JR, et al. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a μ, δ or κ opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- 32.Wang JB, et al. cDNA cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett. 1994;348:75–79. doi: 10.1016/0014-5793(94)00557-5. [DOI] [PubMed] [Google Scholar]
- 33.Keith D, Jr, Maung T, Anton B, Evans C. Isolation of cDNA clones homologous to opioid receptors. Regul Pept. 1994;54:143–144. [Google Scholar]
- 34.Pan Y-X, Bolan E, Pasternak GW. Dimerization of morphine and orphanin FQ/nociceptin receptors: Generation of a novel opioid receptor subtype. Biochem Biophys Res Commun. 2002;297:659–663. doi: 10.1016/s0006-291x(02)02258-1. [DOI] [PubMed] [Google Scholar]
- 35.George SR, et al. Oligomerization of μ- and δ-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 36.Gomes I, et al. Heterodimerization of μ and δ opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan BA, Trapaidze N, Gomes I, Nivarthi R, Devi LA. Oligomerization of opioid receptors with β2-adrenergic receptors: A role in trafficking and mitogen-activated protein kinase activation. Proc Natl Acad Sci USA. 2001;98:343–348. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfeiffer M, et al. Heterodimerization of substance P and μ-opioid receptors regulates receptor trafficking and resensitization. J Biol Chem. 2003;278:51630–51637. doi: 10.1074/jbc.M307095200. [DOI] [PubMed] [Google Scholar]
- 39.Roumy M, et al. Physical association between neuropeptide FF and micro-opioid receptors as a possible molecular basis for anti-opioid activity. J Biol Chem. 2007;282:8332–8342. doi: 10.1074/jbc.M606946200. [DOI] [PubMed] [Google Scholar]
- 40.Zhu YX, et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in δ opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 41.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 42.D'Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- 43.Ling GSF, Pasternak GW. Spinal and supraspinal opioid analgesia in the mouse: The role of subpopulations of opioid binding sites. Brain Res. 1983;271:152–156. doi: 10.1016/0006-8993(83)91376-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.