Abstract
Plants are exposed to a suite of herbivorous attackers that often arrive sequentially. Herbivory affects interactions between the host plants and subsequently attacking herbivores. Moreover, plants may respond to herbivory by emitting volatile organic compounds (VOCs) that attract carnivorous natural enemies of the herbivores. However, information borne by VOCs is ubiquitous and may attract carnivores, such as parasitoids, that differ in their effectiveness at releasing the plant from its herbivorous attackers. Furthermore, the development of parasitoids within their herbivorous hosts, attacking a given host plant, may influence the elicitation of defensive reactions in the host plant. This may, in turn, affect the behavior of subsequent herbivores attacking the host plant. Here, we show that the species identity of a parasitoid had a more significant effect on defense responses of Brassica oleracea plants than the species identity of the herbivorous hosts of the parasitoids. Consequently, B. oleracea plants that were damaged by caterpillars (Pieris spp.) parasitized by different parasitoid species varied in the degree to which diamondback moths (Plutella xylostella) selected the plants for oviposition. Attracting parasitoids in general benefitted the plants by reducing diamondback moth colonization. However, the species of parasitoid that parasitized the herbivore significantly affected the magnitude of this benefit by its species-specific effect on herbivore–plant interactions mediated by caterpillar regurgitant. Our findings show that information-mediated indirect defense may lead to unpredictable consequences for plants when considering trait-mediated effects of parasitized caterpillars on the host plant and their consequences because of community-wide responses to induced plants.
Keywords: herbivore preference, induced gene transcription, parasitoid-dependent effect
Across their whole kingdom, plants have been found to interact with carnivorous arthropods that act to control herbivorous attackers of the plants. Such interactions between plants and carnivorous arthropods may ultimately increase fitness of the plants (1–3). Plant traits involved in such interactions include those that provide a predator/parasitoid with a resource such as, for example, food or shelter, or with information on the presence and abundance of herbivorous prey/hosts (4, 5). Although resource- and information-mediated indirect defenses of plants have long been collectively viewed within the context of ecological and evolutionary theory of indirect defenses, interactions between plants and carnivorous arthropods based on resource provisioning are distinctly different from those mediated by providing information (6). Whereas resource provisioning can be obligate and is often restricted to interactions with a limited number of carnivorous species, information emitted by herbivore-infested plants is generally ubiquitous, allowing all other organisms to respond to this information (5). Release of herbivore-induced volatile organic compounds (VOCs) by plants has been shown to attract predators and parasitoids that may benefit the plants. At the same time, the VOCs may attract other herbivores, affect interactions between the plants and other mutualists such as, for example, pollinators, and affect neighboring plants (5, 7–10). Plants may benefit more from particular predators or parasitoids of their herbivorous attacker than from others. Attracting a predator that directly removes an herbivore is obviously more effective at reducing herbivore damage than attracting a parasitoid that allows the herbivore to feed on the food plant to complete its own development. Many, if not all, herbivores are attacked by a suite of parasitoid species (11) that each may affect the herbivore differently and, thus, may differ in their value in terms of indirect defense to plants.
In addition to uncontrolled attraction of specific parasitoid species, plants may experience another ecological constraint caused by a network of trait-mediated species interactions because of differences in host regulation among parasitoid species (12, 13). When a parasitoid parasitizes an herbivore, the parasitoid larva affects performance of the herbivore that, as a result, interacts differently with the host plant (13, 14). One effect that parasitoid larvae may have on herbivores is that, because of their feeding, they alter the physiology of the herbivore and, consequently, their oral secretions (13, 14). Compounds in the oral secretions or midgut regurgitant of caterpillars have been found to play a major role in the induction of herbivore-induced VOCs, which is mediated by the jasmonic acid (JA) signal-transduction pathway (15–19). Parasitoid larvae inside an herbivorous host may, thus, affect herbivore-induced plant responses through their effect on the herbivore. The resulting plant phenotype in turn affects ecological interactions with and physiological responses to other organisms associated with the plant (13). When induced responses to herbivores parasitized by different parasitoid species result in plant phenotypes that differentially affect subsequent colonization by other herbivore species, then information-mediated indirect defense may lead to unpredictable fitness consequences to the plant.
In The Netherlands, Pieris rapae and Pieris brassicae (Lepidoptera: Pieridae) colonize Brassica oleracea plants early in the season, and their caterpillars are attacked by several species of parasitoids (20, 21). Among the most common herbivores that arrive later in the season is the diamondback moth (Plutella xylostella; Lepidoptera: Plutellidae), which prefers to lay her eggs on herbivore-induced plants over undamaged control plants (22–25). Here, we studied whether different parasitoid species that attack Pieris caterpillars differentially affect elicitation of plant defense responses via the regurgitant of their herbivorous hosts. We also tested whether such plant defense responses result in plant phenotypes that differentially affect the oviposition behavior of late season herbivores (i.e., diamondback moths). To cover the large taxonomic diversity of parasitoids found in nature, we selected three parasitoid species in widely different evolutionary lineages: two parasitic wasps, Cotesia glomerata (Hymenoptera: Braconidae) and Hyposoter ebeninus (Hymenoptera: Ichneumonidae), and one parasitic fly, Compsilura concinnata (Diptera). To evaluate whether parasitoids affect different host herbivore species similarly, we compared the effects of parasitoid species on plant defense responses when parasitoids were developing in caterpillars from one of two closely related cabbage white butterflies, P. rapae and P. brassicae. In nature, caterpillars of these butterflies are attacked by the three parasitoid species mentioned above (20, 21). The two Pieris species themselves induce different defense responses in plants (26–28). To assess plant defense responses, we cloned nine genes from B. oleracea involved in different signal-transduction pathways (compare SI Text; Figs. S1 and S2). In the JA pathway, we cloned the JA-regulated transcription factor (BoMYC) and the JA-regulated genes coding for defensin (BoDEF) and vegetative storage protein (BoVSP), as well as the gene coding for the enzyme lipoxygenase (BoLOX), which is a key enzyme in the JA pathway. In the shikimate pathway, we cloned the phenylalanine ammonia lyase gene (BoPAL) that regulates the conversion of phenylalanine into cinnamic acid, which is an important step in salicylic acid (SA) biosynthesis and the formation of many plant secondary metabolites. Additionally, we cloned a marker gene for SA signaling (BoPR1) and a marker gene in the ethylene pathway (BoACS). We further cloned two genes involved in the regulation of biosynthesis of defensive metabolites: BoPIN that codes for a protease inhibitor, and BoMYR that codes for myrosinase, which plays an important role in the formation of brassicaceous-specific secondary metabolites through the glucosinolate–myrosinase system. Protease inhibitors as well as glucosinolates and their toxic breakdown products affect herbivore performance (29). We quantified transcript dynamics of these genes through real-time quantitative PCR (qPCR) at 2, 6, and 24 h after induction and compared plant responses to regurgitant of the two herbivore species (P. rapae, P. brassicae) when parasitized by three different parasitoids (C. glomerata, H. ebeninus, or C. concinnata) or when unparasitized (Table S1). We further ran two-choice oviposition preference experiments in which adult female diamondback moths were tested for their preferences for B. oleracea plants induced by unparasitized or parasitized caterpillars of the two Pieris species.
Results
Plant Responses to Regurgitant of Parasitized Herbivores.
To test whether parasitoid species differentially affect plant defense responses via their herbivorous hosts, we applied regurgitant of unparasitized or parasitized caterpillars to small manually punctured holes in B. oleracea leaves and quantified the expression levels of nine defense-related genes. Compared with undamaged B. oleracea plants or B. oleracea plants with mechanical damage that were treated with water as a control, B. oleracea plants that were both mechanically damaged and treated with caterpillar regurgitants had significantly higher expression levels of the BoPAL and BoPR1 genes that are associated with induction of the SA pathway (Table S2). Compared with true herbivory by neonate caterpillars, an application of regurgitants to mechanically damaged leaves resulted in similar to slightly higher expression levels of three genes related to JA signaling (BoLOX, BoMYC, and BoVSP) after 2 or 6 h. However, after 24 h, the expression levels of the JA-regulated genes in response to true herbivory were significantly higher than the expression levels of the genes in response to mechanical damage plus an application of caterpillar regurgitants. These results indicate that our method of regurgitant application mimics true herbivory up to 6 h after regurgitant was applied, but true herbivory differentiates over a longer time, possibly caused by continuous damage in natural herbivory, compared with a single damage time point in mechanical damage treatments (30). True herbivory by early-instar caterpillars of both herbivore species induced transcriptional responses in B. oleracea plants that gradually built up to the highest expression after 24 h, compared with a peak in expression after 2 h when plants were treated with regurgitant.
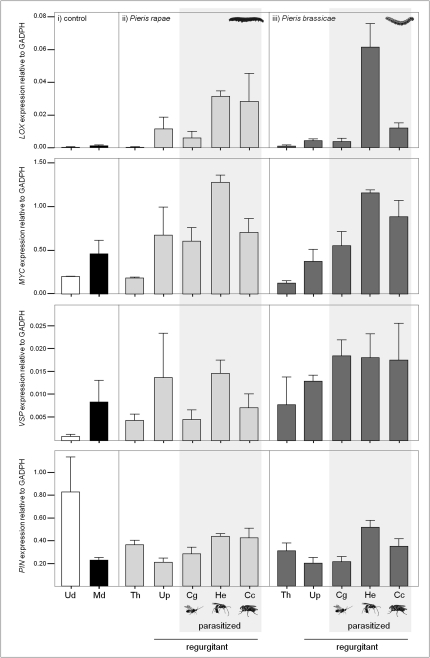
When comparing the effects of regurgitant from two different herbivores (P. rapae and P. brassicae) that were parasitized or unparasitized, we found no significant differences in expression levels of the nine defense genes for responses to regurgitant of P. rapae or P. brassicae (Table S3). Of the nine defense-related genes, only one (BoDEF) had its expression level affected by interactive effects of host herbivore and parasitoid species identity (Table S3). Hence, the parasitoid larvae affected the expression of eight other defense-related genes in plants similarly through a different host herbivore species (Fig. 1). Intriguingly, parasitoid species themselves differed widely in their effects on plant transcriptional responses through their herbivorous host (Fig. 1, Figs. S3 and S4, and Table S3). The differential effects of parasitoids on plant transcriptional responses of the nine genes were most pronounced 2 and 6 h after the application of caterpillar regurgitant to the plants. Although the expression levels of all these nine genes dropped 24 h after treatment application, four genes, all related to JA signaling (BoLOX, BoMYC, BoVSP, and BoPIN), still showed significant expression differences among the three parasitoid species interacting with the plant (Table S3). Across these four genes, responses of plants to regurgitant from unparasitized herbivores and those parasitized by the gregarious species C. glomerata were similar but generally differed from responses to regurgitant of caterpillars parasitized by the solitary parasitoids C. concinnata and H. ebeninus (Fig. 1 and Table S3). Levels of expression of each of the four genes were highest for regurgitant from caterpillars that were parasitized by the solitary wasp H. ebeninus, followed by regurgitant from caterpillars parasitized by the solitary fly C. concinnata (Fig. 1).
Fig. 1.
Expression levels of BoLOX, BoMYC, BoVSP, and BoPIN genes 2 h after treatments were applied to B. oleracea plants. Treatments: undamaged plants (Ud, white bars); mechanical damage consisting of three needle punctures (Md, black bars); herbivore treatments with P. rapae (light gray) or P. brassicae (dark gray): true herbivory (Th), mechanical damage plus regurgitant from unparasitized caterpillars (Up) or caterpillars parasitized by Cotesia glomerata (Cg), Hyposoter ebeninus (He), or Compsilura concinnata (Cc).
Herbivore Oviposition Preference for Plants Induced by Parasitized Caterpillars.
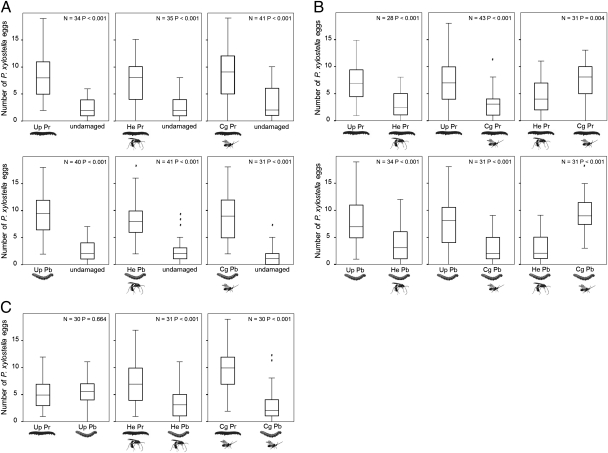
In two-choice tests, diamondback moths preferred to oviposit on plants induced by feeding of parasitized or unparasitized caterpillars of the two Pieris species over undamaged control leaves (P < 0.001 for all pair-wise combinations; Wilcoxon's matched-pairs signed-ranks test; Fig. 2A). For herbivory by P. rapae and P. brassicae, leaves damaged by unparasitized caterpillars or those parasitized by different parasitoid species differed in their attractiveness for oviposition by diamondback moths. Leaves induced by unparasitized caterpillars received larger numbers of eggs than leaves induced by parasitized caterpillars (P < 0.001; Wilcoxon's matched-pairs signed-ranks test; Fig. 2B). For both herbivore species, caterpillars parasitized by C. glomerata induced plant responses that were more attractive for diamondback moth oviposition than responses induced by H. ebeninus-parasitized caterpillars (P. rapae: P = 0.004; P. brassicae: P < 0.001; Wilcoxon's matched-pairs signed-ranks test; Fig. 2B). Diamondback moths did not differentially oviposit on plants induced by unparasitized caterpillars of either herbivore species (P = 0.664; Fig. 2C). However, when these caterpillars were parasitized by the same parasitoid species, the moths differentially oviposited on the leaves (P < 0.001; Wilcoxon's matched pairs signed-ranks test; Fig. 2C): the moths laid more eggs on plants induced by parasitized caterpillars of P. rapae than on plants induced by parasitized caterpillars of P. brassicae when either H. ebeninus or C. glomerata had parasitized the herbivores (Fig. 2C). Although parasitized and unparasitized herbivores differed in the amount of damage they inflicted to the plants (Table S4), oviposition preferences of diamondback moths were not explained by the difference in amount of feeding damage inflicted to the two leaves offered in two-choice tests (Table S5).
Fig. 2.
Oviposition preference of diamondback moth (P. xylostella) for B. oleracea leaves induced by feeding damage of parasitized or unparasitized caterpillars of P. rapae or P. brassicae. Treated leaves were offered in two-choice tests, testing (A) leaves damaged by unparasitized (Up) caterpillars, H. ebeninus-parasitized (He) caterpillars, and C. glomerata-parasitized (Cg) caterpillars of P. rapae (Pr) and P. brassicae (Pb) against undamaged leaves; (B) pairs of leaves damaged by the same herbivore species but parasitized by different parasitoids; and (C) pairs of leaves damaged by different herbivore species that were parasitized by the same parasitoid species.
Discussion
Our results show that various parasitoid species differentially affect the interactions of their herbivorous hosts with a host plant, and that this is mediated through changes in regurgitant of the herbivores. Most notably, species identity of the parasitoids had stronger effects on induced plant defense responses than species identity of herbivorous hosts of these parasitoids. That is, each of the three parasitoid species provoked different defense responses in B. oleracea plants, and such different responses were consistent across the two herbivorous hosts of the parasitoids (Fig. 1). These results are supported by our targeted plant-gene expression approach, although this method does not identify the mechanisms underlying the species interactions studied. Parasitism affected the expression of three genes encoding early signaling in the JA pathway and was found to also affect the expression of BoPIN that codes for a protease inhibitor downstream of the JA pathway (31). The induced responses of B. oleracea plants to unparasitized or parasitized caterpillars of the two herbivore species, as expressed in the transcript levels of genes in the JA pathway, resulted in plant phenotypes that were differentiated in terms of oviposition responses by the subsequently colonizing diamondback moths. Plants damaged by unparasitized herbivores showed lower expression levels of genes related to JA signaling than plants damaged by parasitized caterpillars. Furthemore, plants damaged by unparasitized caterpillars were more attractive to diamondback moth oviposition than plants damaged by parasitized caterpillars (Fig. 2B). Diamondback moths laid fewest eggs on plants induced by H. ebeninus-parasitized caterpillars that also elicited the strongest responses in transcript levels of genes involved in plant defenses to caterpillars (Figs. 1 and 2B). Herbivory by unparasitized caterpillars of the two Pieris species did not result in differential oviposition by diamondback moths (Fig. 2C). However, parasitism of the two Pieris species caused oviposition preferences by diamondback moths, such that plants damaged by parasitized P. rapae were more prone to oviposition by the diamondback moth than plants damaged by parasitized P. brassicae (Fig. 2C). Thus, parasitism of herbivores strongly affected the interactions between the herbivores and a host plant. Specifically, attracting parasitoids, in general, benefitted host plants by reducing the pressure of colonization by diamondback moths. That the species identity of a parasitoid influenced colonization pressure of the host plant by diamondback moth, indirectly via a parasitoid species-specific effect on regurgitant of herbivorous hosts of the parasitoids, is noteworthy.
Larval development of the three parasitoids inside their caterpillar hosts had differential effects on regurgitant of the caterpillars, as indicated by the coloration patterns (Fig. S5). The gregarious hemolymph-feeding larvae of C. glomerata had less profound effects on the color of the regurgitant, as well as plant gene expression resulting from feeding by their host, than the solitary larvae of H. ebeninus that start feeding on herbivore tissue early in their development (21). In addition to their feeding pattern, compounds in the saliva or regurgitant of herbivores have a major effect on the interactions with the plant (19, 30). Plants may distinguish the type of attacking herbivore by the perception of herbivore-associated elicitors (3, 19, 32), but herbivores may also suppress plant responses by elicitors in their saliva (33, 34). The elicitors in saliva are often species-specific and diverse in structure (35, 36). They range from plant cell wall fragments (37, 38), peptides derived from ingested plant proteins (39), to fatty acid–amino acid conjugates (16, 40), sulfur-containing fatty acids (41), and enzymes (15, 42). In regurgitant of Pieris caterpillars, the enzyme β-glucosidase has been identified as playing a major role in eliciting plant responses after herbivory, including volatile emission (15). In the present study, volatiles produced by B. oleracea plants damaged by H. ebeninus-parasitized Pieris caterpillars could have made those plants less apparent or less attractive to diamondback moths. These moths have been suggested to locate their host plant on the basis of volatile compounds specific for their brassicaceous host plants (i.e., glucosinolate breakdown products), which are released in larger concentrations after herbivory by unparasitized Pieris caterpillars (43). Additionally, diamondback moths may have been selected to exploit volatile cues of plants attacked by Pieris caterpillars, because diamondback moth caterpillars are parasitized less frequently when they feed on plants with Pieris caterpillars (22). The effect of parasitoids on the composition of caterpillar regurgitant, and the nature of genetic and metabolic changes in plants after induction that are causal to a change in behavior of the diamondback moth, merit further investigation.
Salivary compounds may have evolved to allow herbivores to optimize nitrogen intake (35) or to suppress plant defense responses (33, 34). In an evolutionary arms race, plants may have evolved to recognize the type of attacker through their elicitors and respond with enhancing their resistance level to the attacker. However, when parasitism of these herbivores leads to altered composition of saliva of the herbivore, this may disrupt the potential of herbivores to modify plant responses or plants to accurately recognize the type of attacker. We show that this may lead to variation in plant phenotype when plants are colonized by different parasitoid species, as is a likely result from ubiquitous volatile information upon herbivory. These plant phenotypes influence subsequent colonization of the plant by other herbivores, and thus parasitoids may mediate community changes through their effect on herbivores, and indirectly affect plant fitness. In our study, parasitoids indirectly benefitted B. oleracea plants by making them less susceptible to diamondback moth attack. However, within insect communities, herbivore species may respond contrastingly to induced plants (23, 24), and induced responses may also affect interactions with beneficial organisms such as pollinators (9, 10, 44). The net benefits or costs of the responses to attack by parasitized caterpillars may thus heavily rely on the composition of the local insect community.
Clearly, our results show that information-mediated indirect defense may lead to unpredictable consequences for plants when we consider effects of parasitized caterpillars on the host plant. Our study contributes to the awareness that evolution of information-mediated indirect defense is prone to disruptive selective forces when considering community-wide effects and trait-mediated species interactions.
Materials and Methods
Plants and Insects.
B. oleracea var. gemmifera cv. Cyrus (Brussels sprouts) were sown and grown in a greenhouse compartment (20–30 °C, 50–70% relative humidity, 16 h light/8 h dark photoperiod). Five- to six-week-old plants that had six to seven fully developed leaves were used in all reported experiments.
Caterpillars of P. rapae and P. brassicae were routinely reared on B. oleracea var. gemmifera cv. Cyrus plants in a climate room (20–24 °C, 50–70% relative humidity, 16 h light/8 h dark photoperiod). Under similar conditions, we reared three parasitoid species, each cultured on the two Pieris host species. We used two solitary parasitoids: the endoparasitoid wasp H. ebeninus and the parasitic fly C. concinnata that each oviposits a single egg per caterpillar (20, 21), and the gregarious endoparasitoid C. glomerata that lays 15–40 eggs in a single caterpillar (45).
Plant Responses to Parasitized and Unparasitized Herbivores.
To study plant responses to unparasitized and parasitized herbivores, we quantified expression of nine genes that respond to caterpillar feeding (Table S1). To rule out quantitative feeding effects by differential herbivore behavior when the herbivores are parasitized by different parasitoids, we standardized the amount of damage per treatment. We punctured three tiny holes (≈0.5 mm2) within an area of 2.5 cm in diameter (indicated in Table S1) to the youngest fully expanded leaf of each plant, using a sterile pin needle. After puncturing, 3 μL of freshly collected regurgitant from unparasitized or parasitized P. rapae or P. brassicae caterpillars was applied to the tiny holes on these mechanically damaged leaves (1 μL of regurgitant for each hole). The parasitized caterpillars were parasitized by either C. glomerata, H. ebeninus, or C. concinnata. Regurgitant of parasitized caterpillars was collected from fourth-instar caterpillars that contained full-grown parasitoid larvae. To obtain enough regurgitant for each treatment, we used a capillary to collect regurgitant from several caterpillars, each regurgitating between 1 and 5 μL (14). There were clear differences in color among regurgitants collected from unparasitized caterpillars and caterpillars parasitized by different parasitoids (Fig. S5).
As control treatments, we included plants with punctured leaves that were treated with water instead of regurgitant. Furthermore, we compared the effect of our puncturing method on gene transcription in undamaged plants and plants that were damaged by herbivory of freshly hatched first-instar larvae of P. rapae and P. brassicae. We harvested leaf disks (2.5 cm in diameter) at 2, 6, and 24 h after the treatments were applied. The disks included the damaged sites and were collected by punching as described by Zheng et al. (46). Each leaf disk from an individual plant was immediately placed into an RNase-free 2.2-mL microfuge tube as one biological replicate. These samples were immediately frozen in liquid nitrogen and stored at −80 °C for RNA isolation.
Isolation of RNA and Real-Time qPCR.
We cloned nine target genes and designed primers on the basis of their sequences (Table S6; compare SI Text). As for real-time qPCR, the primers were designed using Beacon Designer software (Premier Biosoft International) (Table S7). The RNA extraction, purification, cDNA synthesis, and the procedure of quantification of gene expression were the same as described by Zheng et al. (46, 47). The iScriptcDNA Synthesis Kit (Bio-Rad) was used for synthesis of cDNA from 1 μg of total RNA in a total volume of 20 μL. IQSYBR Green Supermix (Bio-Rad) was used for PCR. The final amount of cDNA template assayed was equivalent to 10 ng of RNA. All quantitative RT-PCRs were performed in duplicate. The following PCR program was used for all PCRs: 95 °C for 3 min for 1 cycle, followed by 40 cycles of 95 °C for 15 s and 45 s at 56 °C, with data collection at 56 °C. The PCR products for each primer set also were subjected to melt-curve analysis. The melt-curve analysis ensured that the resulting fluorescence originated from a single PCR product and did not represent primer dimer or nonspecific product formation during the PCR. A water no-template and a minus RT (10 ng of RNA) control were included to detect any spurious signals arising from amplification of any DNA contamination or primer dimer formation during the reaction. The target gene expression relative to BoGAPDH expression was quantified by comparing the threshold cycle for each PCR with their respective dilution series and dividing the resulting quantities. The BoLOX/GAPDH ratios for all samples were related to the ratio for untreated plants, which was set to 1 (46, 47). The quantification of gene expression was performed with a Rotor-gene 6000 (Corbett).
Herbivore Oviposition Preference.
To study whether plants that are induced by feeding of unparasitized Pieris caterpillars or those parasitized by different parasitoid species differ in their interaction with subsequently colonizing herbivores, we offered female diamondback moths (P. xylostella) two-choice tests between Brassica leaves that differed in induction treatment (23). One week before the choice assay, B. oleracea plants were subjected to one of seven induction treatments: (i) undamaged control, unparasitized herbivore damage by (ii) P. rapae or (iii) P. brassicae caterpillars, (iv and v) damaged by caterpillars of either herbivore species parasitized by H. ebeninus, or (vi and vii) damaged by caterpillars of either herbivore species parasitized by C. glomerata. For parasitism of these caterpillars, mated females of either parasitoid species were individually presented with single early second-instar caterpillars, and each female was allowed to parasitize up to 10 caterpillars. A caterpillar was considered to have been parasitized when a female parasitoid had been observed to insert her ovipositor into the caterpillar, resulting in oviposition in 98% of the cases (13). Each plant was infested with two early second-instar caterpillars that were allowed to feed for 7 d. On the morning of the experiment, we cut the leaves of the plant, placed them in glass vials containing tap water, and matched them with a similar-sized leaf of another treatment. The pair of leaves was placed in a plastic cylinder (diameter 13 cm, height 22 cm) in which a male and female moth were released. The females were allowed to oviposit overnight, and the number of eggs on each leaf was counted the next morning. To address whether each induction treatment is more attractive to P. xylostella for oviposition than undamaged leaves, we tested each of the induction treatments against an undamaged control leaf. To study whether parasitism of herbivores by different parasitoid species affects plant responses elicited by the parasitized herbivore, each of the unparasitized and parasitized Pieris caterpillars were paired to another treatment within the herbivore species (i.e., conducted for both P. rapae or P. brassicae). Finally, to test whether herbivore species themselves induce plants differently, and whether those differences are maintained when herbivores are parasitized, we matched pairs of leaves with a similar parasitism treatment but of a different herbivore species.
Leaf Damage Assessment.
To analyze whether oviposition preference by diamondback moths correlated with the amount of leaf damage inflicted by the different plant treatments, we quantified the amount of damage on each leaf that had been used in the oviposition experiments. The leaves were taped onto a white paper sheet and scanned with a Hewlett-Packard Scanjet 3570c. The scans were analyzed for the size of damaged leaf surface by counting the number of pixels making up the damaged area using Scion Image 4.0.3 for Windows. The number of pixels was converted into mm2 by comparison with the number of pixels that make up a reference 1-cm2 square. Per oviposition choice test, we quantified the relative amount of damage on leaf A by dividing it by the total amount of damage on leaf A+B. Over the replicates of each pair-wise treatment comparison, we correlated the leaf-damage values of treatment A with the relative number of eggs on treatment A, following the same formula A/(A+B). Pearson's correlation tests were applied to each of the treatment combinations separately.
Statistical Analysis.
Oviposition preference of diamondback moths (P. xylostella) was analyzed using Wilcoxon matched-pair signed-rank tests for each of the treatment comparisons. We constructed two multiple ANOVA (MANOVA) models to analyze the relative expression of nine genes, for which the data were log-transformed to reach normality. The first model was constructed to test whether our method of manual damage is a good mimic of true herbivory. We included the data from undamaged plants, plants damaged by caterpillars, manually damaged plants treated with water, and those that were manually damaged and treated with regurgitant of unparasitized caterpillars. The MANOVA model was constructed with the fixed factors damage treatment, time (2, 6 and 24 h), and interaction between the two terms. Post hoc, differences between the four damage treatments were tested using Tukey tests.
We constructed a second MANOVA model to address whether parasitoid species differentially induce plant responses through their herbivore hosts. For this model, we included the data on manually damaged plants that were treated with regurgitant of unparasitized or parasitized caterpillars. The model was constructed with the fixed factors herbivore (P. rapae, P. brassicae), parasitoid (unparasitized, C. glomerata, H. ebeninus, C. concinnata), time (2, 6, 24 h), and full factorial interactions of the terms. We used post hoc Tukey tests to analyze the differences in gene expression for the four levels in the factor parasitoids.
Supplementary Material
Acknowledgments
We thank Colette Broekgaarden, Ayub Oduor, and two anonymous reviewers for constructive comments on an earlier version of the manuscript and Leon Westerd, Frans van Aggelen, and André Gidding for rearing the insects at the Laboratory of Entomology, Wageningen University. This work was supported by The Netherlands Organization for Scientific Research/Earth and Life Sciences, Veni Grant 863.10.012 (to E.H.P.), and Vici Grant 865.03.002 (to M.D.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110748108/-/DCSupplemental.
References
- 1.Karban R, Baldwin IT. Induced Responses to Herbivory. Chicago: Chicago Univ Press; 1997. [Google Scholar]
- 2.Mumm R, Dicke M. Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can J Zool. 2010;88:628–667. [Google Scholar]
- 3.Hilker M, Meiners T. How do plants “notice” attack by herbivorous arthropods? Biol Rev Camb Philos Soc. 2010;85:267–280. doi: 10.1111/j.1469-185X.2009.00100.x. [DOI] [PubMed] [Google Scholar]
- 4.Heil M. Damaged-self recognition in plant herbivore defence. Trends Plant Sci. 2009;14:356–363. doi: 10.1016/j.tplants.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Kessler A, Heil M. The multiple faces of indirect defences and their agents of natural selection. Funct Ecol. 2011;25:348–357. [Google Scholar]
- 7.Halitschke R, Stenberg JA, Kessler D, Kessler A, Baldwin IT. Shared signals—‘alarm calls’ from plants increase apparency to herbivores and their enemies in nature. Ecol Lett. 2008;11:24–34. doi: 10.1111/j.1461-0248.2007.01123.x. [DOI] [PubMed] [Google Scholar]
- 8.Kessler A, Halitschke R. Specificity and complexity: The impact of herbivore-induced plant responses on arthropod community structure. Curr Opin Plant Biol. 2007;10:409–414. doi: 10.1016/j.pbi.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Lucas-Barbosa D, van Loon JJA, Dicke M. The effects of herbivore-induced plant volatiles on interactions between plants and flower-visiting insects. Phytochemistry. 2011;72:1647–1654. doi: 10.1016/j.phytochem.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Kessler A, Halitschke R, Poveda K. Herbivory-mediated pollinator limitation: Negative impacts of induced volatiles on plant-pollinator interactions. Ecology. 2011;92:1769–1780. doi: 10.1890/10-1945.1. [DOI] [PubMed] [Google Scholar]
- 11.Godfray HCJ. Parasitoids: Behavioural and Evolutionary Ecology. Princeton: Princeton Univ Press; 1994. [Google Scholar]
- 12.Utsumi S, Ando Y, Miki T. Linkages among trait-mediated indirect effects: A new framework for the indirect interaction web. Popul Ecol. 2010;52:485–497. [Google Scholar]
- 13.Poelman EH, et al. Indirect plant-mediated interactions among parasitoid larvae. Ecol Lett. 2011;14:670–676. doi: 10.1111/j.1461-0248.2011.01629.x. [DOI] [PubMed] [Google Scholar]
- 14.Fatouros NE, van Loon JJA, Hordijk KA, Smid HM, Dicke M. Herbivore-induced plant volatiles mediate in-flight host discrimination by parasitoids. J Chem Ecol. 2005;31:2033–2047. doi: 10.1007/s10886-005-6076-5. [DOI] [PubMed] [Google Scholar]
- 15.Mattiacci L, Dicke M, Posthumus MA. beta-Glucosidase: An elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci USA. 1995;92:2036–2040. doi: 10.1073/pnas.92.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alborn HT, et al. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949. [Google Scholar]
- 17.Schittko U, Preston CA, Baldwin IT. Eating the evidence? Manduca sexta larvae can not disrupt specific jasmonate induction in Nicotiana attenuata by rapid consumption. Planta. 2000;210:343–346. doi: 10.1007/PL00008143. [DOI] [PubMed] [Google Scholar]
- 18.Schmelz EA, Alborn HT, Tumlinson JH. The influence of intact-plant and excised-leaf bioassay designs on volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays. Planta. 2001;214:171–179. doi: 10.1007/s004250100603. [DOI] [PubMed] [Google Scholar]
- 19.Bonaventure G, VanDoorn A, Baldwin IT. Herbivore-associated elicitors: FAC signaling and metabolism. Trends Plant Sci. 2011;16:294–299. doi: 10.1016/j.tplants.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Iwao K, Ohsaki N. Inter- and intraspecific interactions among larvae of specialist and generalist parasitoids. Res Pop Ecol. 1996;38:265–273. [Google Scholar]
- 21.Harvey JA, Poelman EH, Gols R. Development and host utilization in Hyposoter ebeninus (Hymenoptera: Ichneumonidae), a solitary endoparasitoid of Pieris rapae and P. brassicae caterpillars (Lepidoptera: Pieridae) Biol Control. 2010;53:312–318. [Google Scholar]
- 22.Shiojiri K, Takabayashi J, Yano S, Takafuji A. Oviposition preferences of herbivores are affected by tritrophic interaction webs. Ecol Lett. 2002;5:186–192. [Google Scholar]
- 23.Poelman EH, Broekgaarden C, Van Loon JJA, Dicke M. Early season herbivore differentially affects plant defence responses to subsequently colonizing herbivores and their abundance in the field. Mol Ecol. 2008;17:3352–3365. doi: 10.1111/j.1365-294X.2008.03838.x. [DOI] [PubMed] [Google Scholar]
- 24.Poelman EH, van Loon JJA, van Dam NM, Vet LEM, Dicke M. Herbivore-induced plant responses in Brassica oleracea prevail over effects of constitutive resistance and result in enhanced herbivore attack. Ecol Entomol. 2010;35:240–247. [Google Scholar]
- 25.Bruinsma M, et al. Inhibition of lipoxygenase affects induction of both direct and indirect plant defences against herbivorous insects. Oecologia. 2010;162:393–404. doi: 10.1007/s00442-009-1459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaakmeer A, et al. Comparative headspace analysis of cabbage plants damaged by two species of Pieris caterpillars: Consequences for in-flight host location by Cotesia parasitoids. Entomol Exp Appl. 1994;73:175–183. [Google Scholar]
- 27.Geervliet JBF, Posthumus MA, Vet LEM, Dicke M. Comparative analysis of headspace volatiles from different caterpillar-infested or uninfested food plants of Pieris species. J Chem Ecol. 1997;23:2935–2954. [Google Scholar]
- 28.Geervliet JBF, Vreugdenhil AI, Dicke M, Vet LEM. Learning to discriminate between infochemicals from different plant-host complexes by the parasitoids Cotesia glomerata and C. rubecula. Entomol Exp Appl. 1998;86:241–252. [Google Scholar]
- 29.Hopkins RJ, van Dam NM, van Loon JJA. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu Rev Entomol. 2009;54:57–83. doi: 10.1146/annurev.ento.54.110807.090623. [DOI] [PubMed] [Google Scholar]
- 30.Mithöfer A, Wanner G, Boland W. Effects of feeding Spodoptera littoralis on lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiol. 2005;137:1160–1168. doi: 10.1104/pp.104.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Vos M, et al. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact. 2005;18:923–937. doi: 10.1094/MPMI-18-0923. [DOI] [PubMed] [Google Scholar]
- 32.Heil M. Indirect defence via tritrophic interactions. New Phytol. 2008;178:41–61. doi: 10.1111/j.1469-8137.2007.02330.x. [DOI] [PubMed] [Google Scholar]
- 33.Musser RO, et al. Herbivory: Caterpillar saliva beats plant defences. Nature. 2002;416:599–600. doi: 10.1038/416599a. [DOI] [PubMed] [Google Scholar]
- 34.Diezel C, von Dahl CC, Gaquerel E, Baldwin IT. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol. 2009;150:1576–1586. doi: 10.1104/pp.109.139550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshinaga N, et al. Fatty acid-amino acid conjugates diversification in lepidopteran caterpillars. J Chem Ecol. 2010;36:319–325. doi: 10.1007/s10886-010-9764-8. [DOI] [PubMed] [Google Scholar]
- 36.Mori N, Yoshinaga N. Function and evolutionary diversity of fatty acid amino acid conjugates in insects. J Plant Interact. 2011;6:103–107. [Google Scholar]
- 37.Doares SH, Syrovets T, Weiler EW, Ryan CA. Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proc Natl Acad Sci USA. 1995;92:4095–4098. doi: 10.1073/pnas.92.10.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergey DR, Orozco-Cardenas M, de Moura DS, Ryan CA. A wound- and systemin-inducible polygalacturonase in tomato leaves. Proc Natl Acad Sci USA. 1999;96:1756–1760. doi: 10.1073/pnas.96.4.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmelz EA, et al. Fragments of ATP synthase mediate plant perception of insect attack. Proc Natl Acad Sci USA. 2006;103:8894–8899. doi: 10.1073/pnas.0602328103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allmann S, Baldwin IT. Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science. 2010;329:1075–1078. doi: 10.1126/science.1191634. [DOI] [PubMed] [Google Scholar]
- 41.Alborn HT, et al. Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc Natl Acad Sci USA. 2007;104:12976–12981. doi: 10.1073/pnas.0705947104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eichenseer H, Mathews MC, Bi JL, Murphy JB, Felton GW. Salivary glucose oxidase: Multifunctional roles for helicoverpa zea? Arch Insect Biochem Physiol. 1999;42:99–109. doi: 10.1002/(SICI)1520-6327(199909)42:1<99::AID-ARCH10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 43.Gols R, Van Dam NM, Raaijmakers CE, Dicke M, Harvey JA. Are population differences in plant quality reflected in the preference and performance of two endoparasitoid wasps? Oikos. 2009;118:733–743. [Google Scholar]
- 44.Kessler A, Halitschke R. Testing the potential for conflicting selection on floral chemical traits by pollinators and herbivores: Predictions and case study. Funct Ecol. 2009;23:901–912. [Google Scholar]
- 45.Brodeur J, Geervliet JBF, Vet LEM. The role of host species, age and defensive behaviour on ovipositional decisions in a solitary specialist and gregarious generalist parasitoid (Cotesia species) Entomol Exp Appl. 1996;81:125–132. [Google Scholar]
- 46.Zheng SJ, van Dijk JP, Bruinsma M, Dicke M. Sensitivity and speed of induced defense of cabbage (Brassica oleracea L.): Dynamics of BoLOX expression patterns during insect and pathogen attack. Mol Plant Microbe Interact. 2007;20:1332–1345. doi: 10.1094/MPMI-20-11-1332. [DOI] [PubMed] [Google Scholar]
- 47.Zheng SJ, Zhang PJ, van Loon JJA, Dicke M. Silencing defense pathways in Arabidopsis by heterologous gene sequences from Brassica oleracea enhances the performance of a specialist and a generalist herbivorous insect. J Chem Ecol. 2011;37:818–829. doi: 10.1007/s10886-011-9984-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.