Abstract
Clinical applications of human interferon (IFN)-α have met with varying degrees of success. Nevertheless, key molecules in cell viability regulated by IFN-α have not been clearly identified. Our previous study indicated that IFN (α, β and ω) receptor (IFNAR) 1/2- and IFN regulatory factor (IRF) 9-RNA interference (RNAi) completely restored cell viability and transcription of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) following IFN-α treatment, in human ovarian adenocarcinoma OVCAR3 cells sensitive to IFN-α. In the present study, IFNAR1/2- and IRF9-RNAi inhibited the gene expression of TRAIL, but not of Fas ligand (FasL), following IFN-α treatment. In fact, TRAIL but not FasL inhibited the viability of OVCAR3 cells. IFN-α notably up-regulated the levels of TRAIL protein in the supernatant and on the membrane of OVCAR3 cells. Following TRAIL signaling, Caspase 8 inhibitor and BH3 interacting domain death agonist (BID)-RNAi significantly restored cell viability in response to IFN-α and TRAIL in OVCAR3 cells. Furthermore, BID-RNAi prevented both IFN-α and TRAIL from collapsing the mitochondrial membrane potential (ΔΨm). Finally, we provided important evidence that BID overexpression led to significant inhibition of cell viability following IFN-α or TRAIL treatments in human lung carcinoma A549 cells resistant to IFN-α. Thus, this study suggests that BID is crucial for cell viability regulated by IFN-α which can induce mitochondria-mediated apoptosis, indicating a notable potential to be a targeted therapy for IFN-α resistant tumors.
Keywords: IFN-α, cell viability, TRAIL, BID, apoptosis
INTRODUCTION
It has been shown that interferon (IFN) exhibits antiviral (AV), antiproliferative (AP) and immunomodulatory effects (1). Due to its ability to reduce tumor burden and prolong survival, IFN-α has been used in the treatment of certain malignancies (2, 3). However the clinical applications for cancer treatment are limited and varied in success (4). IFN-α elicits its AP effects primarily through the Janus kinase/Signal Transducers and Activators of Transcription (JAK-STAT) pathway (2). Its effect on apoptosis, a form of programmed cell death (PCD), is also of great interest. However, critical molecules in IFN-α-induced apoptosis have not yet been fully identified. The present study has sought to determine which factor in the IFN-α-induced apoptosis pathway is key to regulating cell viability for overcoming IFN-α resistance.
The role of the apical caspases, particularly in the context of apoptosis mediated by death ligands that are type II transmembrane proteins. They bind to specific receptors of the tumor necrosis factor (TNF) receptor superfamily have been reported (5, 6). Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a death ligand that rapidly induces apoptosis in a wide range of transformed cell lines upon binding with TRAIL-Receptor (R)1 or TRAIL-R2 (6–8). It has been reported that IFN-α treatment up-regulates the levels of TRAIL messenger RNA expression and serum-soluble TRAIL in patients with chronic myeloid leukemia (9). We have also demonstrated that TRAIL may be one of the potential mediators for inhibiting cell viability following IFN-α treatment via JAK-STAT pathway in which IFN-α can induce apoptosis mediated by IFN regulatory factor (IRF) 9 (10).
BH3 interacting domain death agonist (BID) has also been reported to have a roll in apoptosis because of its interaction with caspase 8 and its role in mitochondrial membrane permeabilization (MMP), an event considered as the “point-of-no-return” in numerous forms of PCD (11–13). The apoptotic pathway via mitochondria progresses when molecules sequestered between inner membrane (IM) and outer membrane (OM) are released to the cytosol as a result of MMP that eventually leads to cell death (11–15). BID, a pro-apoptotic member of the BCL-2 family, is activated by caspase 8, and translocates to OM followed by catalysis of MMP (16–20). This process is regulated by the BCL-2 family containing both pro- and anti-apoptotic proteins, however, the mechanisms are still controversial (11, 15, 21, 22).
It is also reported that the activation of caspase 6 can trigger caspase 8 (6, 23). Particularly in the Fas-mediated apoptosis, activation of caspase 8 (initiator caspase) directly contributes to that of caspase 3 (effector caspase) without mitochondrial involvement (6, 18, 24). In the other apoptotic process mediated by Fas, only a small amount of death-inducing signaling complex (DISC) is formed and then a small amount of caspase 8 is activated, followed by activation of the cytosolic BID which is a specific proximal substrate of caspase 8 (6, 18, 24, 25). Mitochondrial intermembrane space (IMS) proteins are then released followed by formation of the apoptosome consisting of caspase 9 and the adaptor molecule Apaf-1 (6, 18, 24, 26). In the apoptosome, caspase 9 is autoactivated and further activates the effector caspases 3 and 6 (6, 18, 24).
On the other hand, poly (ADP-ribose) polymerase (PARP), a DNA-binding enzyme, prevents excessive DNA strand breaks during DNA base excision repair in response to DNA damage (27, 28). PARP undergoes proteolytic cleavage catalyzed by caspase 3 during the apoptotic process (29). It is reported that overactivation of PARP-1, the most abundant PARP, results in cell death, and that the inhibitors of PARP-1 are successfully used in in vitro and in vivo studies of different diseases (27).
Our study provides important evidence supporting the central role of TRAIL-mediated death receptor pathway via caspase 8 and BID following IFN-α treatment. We investigated the caspases, BID, and PARP as death receptor pathways downstream of TRAIL receptors following IFN-α treatment. These results demonstrate that inhibition of BID contributes significantly to preventing mitochondrial membrane potential (ΔΨm) from collapsing due to IFN-α and TRAIL. These results are particularly significant because they suggest the importance of BID via mitochondria-mediated apoptosis induced by IFN-α. Accordingly, our current study revealed that BID overexpression significantly inhibited cell viability following IFN-α or TRAIL treatments in IFN-α resistant cells.
MATERIALS AND METHODS
Cell culture
OVCAR3 (human ovarian adenocarcinoma) cells were obtained from the National Cancer Institute (Bethesda, MD). A549 (human lung carcinoma) were purchased from the American Type Culture Collection (ATCC; Manassas, VA). OVCAR3 and A549 cells were maintained in RPMI 1640 medium (Invitrogen; Carlsbad, CA) and Kaighn’s Modification of Ham’s F-12 Medium (F-12K; ATCC), respectively. These media were adjusted to 10% FBS, 2 mM L-glutamine and antibiotics (100 μg/ml gentamicin for RPMI 1640, and 50 units/ml penicillin-G and 50 μg/ml streptomycin for F-12K, respectively).
IFN, TRAIL, FasL and inhibitors
Human IFN-α2c was generated as previously described (30). Recombinant human TRAIL and FasL were obtained from R&D Systems (Minneapolis, MN). Caspase inhibitors as follows were purchased from BD Pharmingen (San Jose, CA); Caspase inhibitors negative control (Z-FA-FMK), Caspase 3 inhibitor (Z-DEVD-FMK), Caspase 6 inhibitor (Z-VEID-FMK), Caspase 8 inhibitor (Z-IETD-FMK) and Caspase 9 inhibitor (Z-LEHD-FMK). PARP inhibitor (3,4-dihydro-5-[4-(1-piperidinyl)butoxy]-1(2H)-isoquinolinone; DPQ) was obtained from Merck4Biosciences (Darmstadt, Germany).
RNA interference (RNAi)
Duplex RNAi, 25 base pair (bp) and 21 bp, were purchased from Invitrogen and Dharmacon (Lafayette, CO), respectively. Stealth RNAi Negative Control Low GC (Invitrogen) and ON-TARGETplus siCONTROL Non-targeting siRNA #1 (Dharmacon) were used as negative-RNAi(#1) and (#2), respectively. IFN (α, β and ω) receptor (IFNAR) 1-, IFNAR2-, and IRF9-RNAi(#1) were used as previously described (10). Sequences for BID-RNAis (#1, Stealth (Invitrogen); #2, ON-TARGETplus (Dharmacon)) are shown in Table 1. The RNAi oligos were mixed with Lipofectamine (Invitrogen) in OPTI-MEM medium (Invitrogen), and the mixture was added to the medium on cells. The cells were incubated for 4 h at 37°C then the medium was replaced with medium containing antibiotics.
TABLE 1.
Sequences of RNAi
| RNAi | Sequences | |
|---|---|---|
| BID-RNAi(#1) | sense | 5′-GGG AAG AAU AGA GGC AGA UUC UGA A-3′ |
| antisense | 5′-UUC AGA AUC UGC CUC UAU UCU UCC C-3′ | |
| BID-RNAi(#2) | sense | 5′-GGG AUG GAC UGA ACG GAC AUU-3′ |
| antisense | 5′-(P) UGU CCG UUC AGU CCA UCC CUU-3′ |
BID indicates BH3 interacting domain death agonist; RNAi, RNA interference.
Phase-contrast imaging
The imaging of cells was obtained using a phase-contrast microscope and ZoomBrowser EX software (Canon; Tokyo, Japan).
Flow cytometry analysis
JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide) Mitochondrial Membrane Potential Detection Kit (Agilent Technologies; La Jolla, CA) was used to determine ΔΨm using the FACS Calibur with CELLQuest software (BD; Franklin Lakes, NJ). The ratios (%) of ΔΨm were then analyzed by FlowJo software (Tree Star; Ashland, OR) and FACSDiva software (BD).
Cell viability assay
Cell viability assay was performed as previously described (10). Briefly, 6×103 cells/well in 96-well plates were treated as indicated followed by incubation with 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, MO) for 4 h. The stained live cells were solubilized with acidified isopropanol and the absorbance was measured at 570 nm. The absorbance corresponding to the live cells was calculated as ratios (%) compared to the absorbance of the respective control. The ratios were expressed as the concentration of IFN-α or TRAIL necessary to inhibit cell viability by 50%..
Western blot analysis
Western blot analysis was performed as previously described (10). Briefly, cells were lysed in NP40 buffer (10). Equivalent amounts of proteins from the cell lysates were separated on Tris-Glycine gels (Invitrogen) followed by transfer to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA). These proteins on the membranes were detected using primary/secondary antibodies and enhanced chemiluminescent substrate (Thermo Scientific; Rockford, IL). Blot images were developed using Fujifilm LAS-3000 with Multigauge software (FUJIFILM holdings; Tokyo, Japan). Primary antibodies of BID and β-actin were obtained from BD Transduction Laboratories (Lexington, KY) and Cell Signaling (Danvers, MA), respectively.
Quantification of western blot analysis
Images of western blotting were quantified as previously described (10). Briefly, sums of the pixel values of the target protein bands were obtained using Kodak Molecular Imaging Software (Eastman Kodak Company; Rochester, NY). Each value was divided by the value of its respective β-actin. The ratios were obtained in comparison to their controls, negative-RNAi(#1) and (#2), respectively.
Gene expression microarray
Amino allyl-oligo-dT (5′ amino-modified) primer (1μg/μl) (31) was incubated with 5 μg total RNA, purified using RNeasy Kit (Qiagen; Valencia, CA), and then reverse transcription (RT) labeling reaction mixture from FairPlay Microarray Labeling Kit (Agilent Technologies) was added to each sample. Following incubation at 42°C for 90 min, cDNA was purified using MinElute PCR Purification columns (Qiagen). After eluting and drying cDNA samples, they were resuspended in 5μl 2× Coupling Buffer. Following, 5μl of either Cy5 or Cy3 mono-reactive dye (GE Healthcare/Amersham Biosciences; Piscataway, NJ) was added to the samples and incubated in the dark at room temperature for 90 min. Using MinElute PCR Purification columns, dye coupled cDNA samples were purified and then respective samples were mixed together, heated at 95°C for 5 min followed by incubation at 42°C for 5 min. SlideHyb Buffer #1 (Ambion; Austin, TX) was added to the combined samples, and then samples were loaded onto ~21K 70mer oligonucleotide microarrays (printed by Genomics Technology Section RTB/NIAID) sealed with MAUI Mixer FL hybridization chambers (BioMicro Systems; Salt Lake City, UT) and incubated overnight at 42°C. Arrays were then washed for 5 min each in buffers; Buffer #1: 1× saline sodium citrate (SSC) with 0.05% SDS; Buffer #2: 0.1× SSC. After drying, arrays were scanned using an Axon GenePix 4200A microarray scanner with GenePix Pro 5.1 software (Molecular Devices; Sunnyvale, CA). Raw microarray data were uploaded to mAdb microarray database (http://nciarray.nci.nih.gov/) and Gene Expression Omnibus (GEO). Array data were normalized via 50th percentile normalization and spot intensity was calculated by subtracting local median background from mean foreground intensity.
Construction of BID expression vector
BID stable transfectants were generated using a pIRESpuro3 vector (Clontech; Mountain View, CA) involving multiple cloning site (mcs). Total RNA was isolated from human myeloid cell line U937 (ATCC) using TRIZOL reagent (Invitrogen). First-strand cDNA was generated by reverse transcription using a Protoscript First Strand cDNA Synthesis Kit (New England BioLabs; Ipswich, MA). The BID coding sequence was amplified by polymerase chain reaction (PCR). PCR was carried out with forward primer, 5′-ATC GAT ATC ATG TGC AGC GGT GCT GGG G-3′ and reverse primer, 5′-CTA GAA TTC TCA GTC ACT CCC ATT TCT GGC-3′, containing EcoRV and EcoRI sites, respectively (underlined). The amplified fragment was then cloned into EcoRV and EcoRI sites of the pIRESpuro3 vector involving a puromycin marker for selection of the stable transfectants. The sequence of inserted BID gene was verified by DNA sequencing. Both pIRESpuro3/mcs and pIRESpuro3/BID vectors were transformed into One Shot TOP 10 competent cells (Invitrogen). The colonies containing vectors were grown in LB medium and plasmid DNA was isolated using a QIAGEN Plasmid Purification Kit (Qiagen). These plasmids were transfected into A549 cells using FuGENE HD (Roche; Indianapolis, IN) for 24 h followed by maintenance with medium containing 2 μg/ml of puromycin (Clontech) to construct stable transfectants.
Immunoaffinity capillary electrophoresis (ICE)
ICE was performed as previously described (32, 33). Briefly, F(Ab)’2 fragments were prepared from the antibody using ImmunoPure F(Ab)’2 preparation kit (Pierce; Rockford, IL), followed by FAb construction. Fused-silica capillaries (75 μm i.d.) were obtained from Polymicro Technologies LLC (Phoenix, AR) and cut to a length of 80 cm. The first 10-cm of the capillary was silanized, and 500 nl of the FAb was introduced into the reaction end of the capillary followed by incubation overnight at 4°C. After the antibody coating, the capillary was flushed three times with 100 mM phosphate buffer, pH 7.4, and a detector window was made by removing a 2 mm section of the polyimide coating 65 cm from the reaction end. The capillary was then mounted onto a Crystal 660 capillary electrophoresis system (Prince Technologies, Amsterdam, Netherlands) equipped with a laboratory-built laser-induced fluorescence (LIF) detector. Samples were labeled with 1 ng/ml of AlexaFluor 633 laser dye (Molecular Probes; Eugene, OR) and introduced into the capillary. The sample was allowed direct contact with the immobilized antibody coating of the capillary for 10 min before purging the capillary with 200 μl of 100 mM phosphate buffer, pH 7.4, to which 0.01% of the detergent Igepal CA-630 (Sigma-Aldrich) had been added. The capillary was placed into buffer reservoirs containing 100 mM phosphate buffer-Igepal, pH 1.5, and the elution-separation performed at 100 μA constant current for 25 min at 4°C with on-line LIF detection. Analyte concentrations were calculated by comparison of the area under the peak with similar values obtained from a calibration curve run under identical conditions.
Statistical analysis
A two-tailed student’s t-test was performed. Differences were considered significant if the probability value (p-value) was less than 0.05. In the case of multiple comparisons, analysis of variance (ANOVA) was performed then differences were determined by Tukey’s multiple comparison test. False discovery rate was determined for gene expression analysis (Table 2).
TABLE 2.
IFN-α related gene expression in OVCAR3 cells
| Entrez Gene ID | Gene | Ratios of differences relative to negative-RNAi(#1) + IFN-α2c †, ‡, §, || | ||
|---|---|---|---|---|
| IFNAR1-RNAi+ IFN-α2c | IFNAR2-RNAi+ IFN-α2c | IRF9-RNAi(#1)+ IFN-α2c | ||
| 8743 | TRAIL | 0.34* | 0.27* | 0.4* |
| 356 | FASL | 1.1 | 0.9 | 1.68 |
| 2537 | IFI6 | 0.51 | 0.88 | 1.28 |
| 3429 | IFI27 | 0.4 | 0.52 | 0.87 |
| 3430 | IFI35 | 0.26* | 0.4 | 0.49 |
| 10561 | IFI44 | 0.26 | 0.65 | 1.43 |
| 10964 | IFI44L | 0.23 | 0.37 | 0.51 |
| 64135 | IFIH1 | 0.41 | 0.53 | 1.19 |
| 3434 | IFIT1 | 0.18 | 0.33 | 0.31 |
| 3437 | IFIT3 | 0.26* | 0.34 | 0.69 |
| 24138 | IFIT5 | 0.29* | 0.24* | 0.41* |
| 8519 | IFITM1 | 0.15 | 0.36 | 0.5* |
| 10581 | IFITM2 | 1.03 | 1.11 | 0.93 |
| 3659 | IRF1 | 0.31 | 0.32* | 0.59 |
| 3661 | IRF3 | 0.66 | 0.79 | 0.89 |
| 3663 | IRF5 | 0.82 | 0.67 | 1.23 |
| 3665 | IRF7 | 0.31* | 0.28 | 0.62 |
| 3394 | IRF8 | 1.02 | 1.26 | 0.95 |
| 10379 | IRF9 | 0.45 | 0.52 | 0.35* |
| 9636 | ISG15 | 0.82 | 0.92 | 0.86 |
| 3716 | JAK1 | 0.74 | 0.78 | 0.96 |
| 3717 | JAK2 | 0.78 | 0.58 | 0.78 |
| 7297 | TYK2 | 0.94 | 0.78 | 1.07 |
| 6772 | STAT1 | 0.34* | 0.4* | 0.77 |
| 6773 | STAT2 | 0.55 | 0.48 | 0.81 |
| 6774 | STAT3 | 0.66 | 0.52 | 1.01 |
| 6775 | STAT4 | 0.83 | 0.92 | 0.61 |
| 6776 | STAT5A | 0.53 | 0.52 | 0.83 |
| 6777 | STAT5B | 0.9 | 0.82 | 1.36 |
| 6778 | STAT6 | 0.58 | 0.7 | 0.79* |
OVACR3 cells were transfected with 100 nM of RNAi, then treated with or without 10 ng/ml of IFN-α2c for 24h
The data were based on the average of at least three samples, and gene expression fold values compared to untreated negative-RNAi(#1) transfected cells were determined first.
Two-sample t-test was performed between the respective log2 converted fold values of specific-RNAi (as indicated) transfected cells and those of negative-RNAi(#1) transfected cells following IFN-α2c treatment.
The ratios of differences shown were linear values and indicated how the former fold values were different from the latter ones.
Statistically significant at False Discovery Rate of 0.05 using the Benjamini-Hochberg method.
RNAi indicates RNA interference; IFN, interferon; IFNAR, IFN (α, β and ω) receptor; IRF, IFN regulatory factor; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; FASL, fas ligand; IFI6, interferon, alpha-inducible protein 6; IFI27, interferon, alpha-inducible protein 27; IFI35, interferon-induced protein 35; IFI44, interferon-induced protein 44; IFI44L, interferon-induced protein 44-like; IFIH1, interferon induced with helicase C domain 1; IFIT, interferon-induced protein with tetratricopeptide repeats; IFITM, interferon induced transmembrane protein; ISG15, ISG15 ubiquitin-like modifier; JAK, janus kinase; TYK2, tyrosine kinase 2; STAT, signal transducer and activator of transcription.
RESULTS
TRAIL, but not FasL, inhibited the viability of OVCAR3 cells sensitive to IFN-α2c
Viability of OVCAR3 and A549 cells in response to IFN-α2c was first examined using an MTT assay (Fig. 1A). OVCAR3 cell viability were inhibited by IFN-α2c (IC50 = 3.1 ng/ml). In contrast, A549 cells were essentially resistant to IFN-α2c (IC50 >1000 ng/ml). Viability of OVCAR3 cells in response to TRAIL and FasL was also examined using an MTT assay (Fig. 1B). OVCAR3 cells were inhibited by TRAIL (IC50 = 0.9 ng/ml), in contrast, essentially resistant to FasL (IC50 >100 ng/ml).
FIGURE 1.
TRAIL but not FasL inhibited the viability of OVCAR3 cells sensitive to IFN-α2c. A, Cells were treated with or without IFN-α2c (OVCAR3, day 3; A549, day 5) followed by an MTT assay. B, OVCAR3 cells were treated with TRAIL or FasL for 3 days followed by an MTT assay. These data are the mean ± standard deviation (n ≥ 3). Each arrowhead indicates the IC50. TRAIL indicates tumor necrosis factor-related apoptosis-inducing ligand; FasL, Fas ligand; IFN, interferon; MTT, 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; IC50, the concentration of IFN-α or TRAIL necessary to inhibit cell viability by 50%.
Previous investigation of the JAK-STAT pathway, demonstrated that cell viability in response to IFN-α2c was completely restored by IFNAR1-, IFNAR2-, and IRF9-RNAi in OVCAR3 cells (10). Here, we further examined how IFNAR1-, IFNAR2-, and IRF9-RNAi regulated IFN-α related gene expression including the representative death receptor ligands TRAIL and FasL in OVCAR3 cells. Table 2 shows IFN-α related gene expression microarray results. The expression of FasL and many IFN-α related genes were not significantly inhibited by, IFNAR1-, IFNAR2- and IRF9-RNAi to be inhibited. In contrast, gene expression of TRAIL and interferon-induced protein with tetratricopeptide repeats 5 (IFIT5) were significantly inhibited by IFNAR1-, IFNAR2- or IRF9-RNAi. The results of TRAIL and FasL gene expression appear to reflect the inhibition of cell viability in response to TRAIL and FasL (Fig. 1B). Supplementary Table 1 shows no significant differences between the respective IFN-α related gene expression following IFN-α2c treatments with and without negative-RNAi.
TRAIL protein levels were up-regulated in the supernatant and on the membrane of OVCAR3 cells in response to IFN-α2c
To further investigate TRAIL in response to IFN-α2c in the microenvironment of tumor cells, the amounts of TRAIL protein (in the cytosol, supernatant, and on the membrane of OVCAR3 cells) were examined using immunoaffinity capillary electrophoresis (ICE). Figure 2 shows that the amounts of TRAIL protein induced by IFN-α2c were significantly increased in the supernatant and on the membrane at the indicated times; however no significant differences between untreated and IFN-α2c-treated cells were observed in the cytosol. These findings suggest that IFN-α induces the production of TRAIL protein in the cytosol and the secretion of TRAIL protein into the supernatant and onto the membrane.
FIGURE 2.
IFN-α2c up-regulated the levels of TRAIL protein in the supernatant and on the membrane of OVCAR3 cells. OVCAR3 cells were treated with or without IFN-α2c (100 ng/ml) at the indicated time followed by ICE. The data are the mean ± standard deviation (n = 3). Differences were determined by Tukey’s multiple comparison test (*** p < 0.001). IFN indicates interferon; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; ICE, Immunoaffinity capillary electrophoresis.
Caspase 8 inhibitor and BID-RNAi restored cell viability following treatment of OVCAR-3 cells with IFN-α2c or TRAIL but PARP inhibitor did not
Figure 2 showed up-regulation of TRAIL protein levels on the membrane of OVCAR3 cells in response to IFN-α2c. In addition, we previously showed that IFN-α and TRAIL predominantly signaled through TRAIL-R2 for regulating cell viability (10). In an effort to further elucidate this process, we investigated the caspases, BID, and PARP in death receptor pathways downstream of TRAIL receptors in affecting the viability of OVCAR3 cells. In Figure 3A, Caspase 8 inhibitor, but not Caspase 3/6/9 inhibitors, exhibited a significant difference from Caspase inhibitors negative control following IFN-α2c treatment. In contrast, Caspase 3/6/8/9 inhibitors demonstrated significant differences from Caspase inhibitors negative control in response to TRAIL treatment. In Figure 3B, transfection with BID-RNAi(#1) or (#2) reduced BID protein expression levels by approximately 60% in OVCAR3 cells. Figure 3C illustrates significant differences between BID-RNAi(#1) and negative-RNAi(#1) in the viability of OVCAR3 cells following whether treatment with IFN-α2c or TRAIL. BID-RNAi(#2) also demonstrated similar results with BID-RNAi(#1) on the viability of OVCAR3 cells treated with IFN-α2c (Supplementary Figure 1). DPQ, a specific PARP-1 inhibitor (27), was used to inhibit the activity downstream of caspase 3. Figure 3D shows no significant differences between DPQ and control on the viability of OVCAR3 cells following treatment of IFN-α2c or TRAIL. Taken together, these results suggest that caspase 8/BID pathway is predominantly responsible for both IFN-α and TRAIL signaling pathways in the regulation of cell viability.
FIGURE 3.
Caspase 8 inhibitor and BID-RNAi restored the viability of OVCAR3 cells in response to IFN-α2c or TRAIL. A, OVCAR3 cells were treated with Caspase inhibitors (10 μM each). Control indicates untreated cells treated with Caspase inhibitors negative control. Differences demonstrated by asterisks result from comparing all treatment groups to the control, except for the asterisks between Caspase 8 and 9 inhibitors in TRAIL treatment. B, Western blot analysis was performed 48 h after transfection of RNAi (20 nM) into OVCAR3 cells. The relative expression ratios (%) were determined by quantification of the western blot data followed by comparison to the values of the respective β-actin. C, OVCAR3 cells were transfected with 20 nM of RNAi. Control indicates untreated cells transfected with negative-RNAi. D, OVCAR3 cells were treated with 100 μM of DPQ (PARP inhibitor). Control indicates untreated cells treated with dimethyl sulfoxide (DMSO). These OVCAR3 cells (A, C, and D) were treated with IFN-α2c or TRAIL (10 ng/ml each) for 3 days followed by an MTT assay. The data are the mean ± standard deviation (n ≥ 3). Differences were determined by Tukey’s multiple comparison test (A) or a two-tailed student’s t-test (C and D) (** p < 0.01, *** p < 0.001). BID indicates BH3 interacting domain death agonist; IFN, interferon; RNAi, RNA interference; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; DPQ, 3,4-dihydro-5-[4-(1-piperidinyl)butoxy]-1(2H)-isoquinolinone; DMSO, dimethyl sulfoxide; MTT, 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide.
BID-RNAi prevented IFN-α2c and TRAIL from collapsing ΔΨm
The collapse of ΔΨm is important for the cell death contributing to MMP (11, 12) and mostly occurs in a caspase-independent manner (12, 15, 17). Here, the JC-1 fluorescence provides a sensitive assessment of ΔΨm and is relatively independent of mitochondrial mass, shape and density (12, 15, 34). Briefly, the JC-1 stains the mitochondria fluorescent red in live non-apoptotic cells. In contrast, the collapse of ΔΨm in apoptotic cells does not allow the JC-1 to accumulate within the mitochondria, resulting in having the JC-1 remain in the cytoplasm in a green fluorescent monomeric form. Consequently, apoptotic cells show green fluorescence (FL1 positive, FL2 negative), whereas live cells show both red and green fluorescence (FL1/FL2 positive). The JC-1 assay, in conjunction with flow cytometry, was used to investigate BID-RNAi transfected OVCAR3 cells treated with IFN-α2c or TRAIL. As shown in Figure 4, BID-RNAi(#1) significantly prevented IFN-α2c and TRAIL from collapsing ΔΨm.
FIGURE 4.
BID-RNAi recovered ΔΨm following IFN-α2c or TRAIL treatments. Twenty nM of RNAi transfected OVCAR3 cells were treated with IFN-α2c or TRAIL (10 ng/ml each) for 3 days followed by flow cytometry analysis. The data were analyzed by FlowJo software (Tree Star). X-axis (logarithmic scale of FL1-H) and Y-axis (logarithmic scale of FL2-H) indicate green and red fluorescence intensity, respectively. The ratios (%) for upper right and lower right quadrants in the dot plots indicate the ratios of live cells and apoptotic cells, respectively. (Live cells whose ΔΨm was intact; apoptotic cells whose ΔΨm was collapsed.) The data are the mean ± standard deviation (n = 3). Differences were determined by Tukey’s multiple comparison test (*** p < 0.001). BID indicates BH3 interacting domain death agonist; RNAi, RNA interference; ΔΨm, mitochondrial membrane potential; IFN, interferon; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Supplementary Figure 2 demonstrated that apoptotic cells were mainly populated on X-axis (FL1-H positive, FL2-H negative). FL2-H histograms also indicate decreases in apoptotic cells on X-axis (FL2-H negative) in BID-RNAi(#1) transfected OVCAR3 cells treated with IFN-α2c or TRAIL. These results suggest that BID plays an important role in the collapse of ΔΨm in response to IFN-α2c and TRAIL.
BID overexpression inhibited cell viability in response to IFN-α2c or TRAIL in IFN-α resistant A549 cells
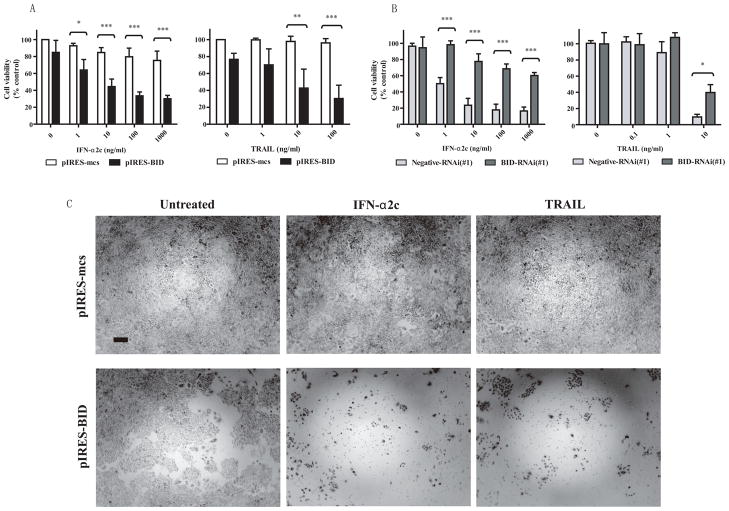
Finally, we examined whether BID overexpression could inhibit cell viability in IFN-α resistant cells treated with IFN-α2c or TRAIL. Figures 5A and 5C show notable inhibition of cell viability in BID-overexpressing A549 cells treated of IFN-α2c or TRAIL. In Figure 5B, BID-RNAi restored cell viability in BID-overexpressing A549 cells, suggesting that the pIRES-BID vector overexpressed BID gene in A549 cells. These results suggest that BID is a factor in inhibiting cell viability in response to IFN-α2c or TRAIL even in IFN-α resistant cells.
FIGURE 5.
BID overexpression inhibited the viability of A549 cells in response to IFN-α2c or TRAIL. A, A549 cells transfected with pIRES vectors were treated with IFN-α2c or TRAIL for 5 days followed by an MTT assay. Control indicates untreated cells transfected with the pIRES-mcs vector. The data are the mean ± standard deviation (n = 3). Differences were determined by Tukey’s multiple comparison test (* p < 0.05, ** p < 0.01, *** p < 0.001). B, BID-overexpressing A549 cells transfected with 20 nM of BID-RNAi were treated with IFN-α2c or TRAIL for 5 days followed by an MTT assay. Control indicates untreated cells transfected with the pIRES-BID vector. The data are the mean ± standard deviation (n = 3). Differences were determined by Tukey’s multiple comparison test (* p < 0.05, *** p < 0.001). C, pIRES-mcs or pIRES-BID transfected A549 cells were treated with IFN-α2c (1000 ng/ml) or TRAIL (100 ng/ml) for 5 days, and then phase-contrast images of the cells were taken. A scale bar indicates 50 μm of length. BID indicates BH3 interacting domain death agonist; IFN, interferon; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; MTT, 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; mcs, multi-cloning site; RNAi, RNA interference.
DISCUSSION
We have reported that the JAK-STAT pathway activated by IFN-α is initially required to regulate cell viability (10). Furthermore, it appears that cell viability is also regulated by subsequent transcription of TRAIL which is a representative of death ligands (10). Here, we have investigated which death receptor pathway factor plays a key role in regulating cell viability and may be used to induce cell death and overcoming IFN-α resistance.
Death receptors are cell surface receptors belonging to the TNF receptor superfamily (6). They contain cytoplasmic death domains that can transmit apoptotic signals upon binding of the specific death ligands, such as TRAIL and FasL (6). The death receptor pathways are known as the extrinsic pathways (15). In the current study, TRAIL appears to be more effective in abrogation of cell viability in response to IFN-α2c than FasL in OVCAR3 cells (Fig. 1B). Furthermore, the gene expression microarray analysis (Table 2) suggests that TRAIL and IFIT5 are important factors in inhibiting cell viability. The role of IFIT5 requires further investigation. Collectively, these results suggest that TRAIL is responsible for regulation of cell viability rather than FasL in OVCAR3 cells treated with IFN-α2c.
Our findings also indicated that TRAIL protein was secreted into the supernatant and onto the membrane of OVCAR3 cells in response to IFN-α (Fig. 2). These results may substantiate a clinical finding that IFN-α treatment up-regulates the level of serum-soluble TRAIL in patients with chronic myeloid leukemia (9). In addition, we have also shown that TRAIL-R2 is predominantly responsible for both IFN-α and TRAIL signals in abrogating cell viability (10). Taken together, our findings suggest that TRAIL is predominantly responsible for IFN-α signaling in regulating cell viability.
Subsequently, mitochondria play a key role in the intrinsic pathway of apoptosis mediated by MMP that contributes to IM and/or OM (11–13, 15). The early mitochondrial alterations containing one or both mitochondrial membranes reflect the crucial regulation of the mitochondrial apoptosis (11–13, 15) and the collapse of ΔΨm allows for the identification of apoptotic cells (11, 12, 15, 35). In the mitochondria-mediated apoptosis, BID is a direct activator that induces pro-apoptotic effector molecules involved in the BCL-2 family, resulting in OM permeabilization and release of mitochondrial IMS proteins (11, 15, 21, 36). This mechanism appears to be independent of the effects on the IM (15). A proposed alternative mechanism is that the pro-apoptotic members of the BCL-2 family lead to formation of pores or channels, followed by collapse of ΔΨm identifying the increase in IM permeability (11–13, 15, 37). Therefore, the measurement of ΔΨm can provide an estimate of IM permeabilization (11, 15), but the mechanisms of the permeability regulation are still controversial (11, 15). These MMP mechanisms including IM and OM permeabilization may coexist (11, 13, 15).
In addition, there was no evidence of a clear linkage between cell viability and BID overexpression following IFN-α treatment. Thus, the mechanisms of mitochondria-mediated apoptosis, involving IFN-α-induced apoptosis, have not yet been fully elucidated. However, the elucidation of mitochondrial alteration pathways holds considerable therapeutic promise. Hence the estimation of the collapse of ΔΨm appears to be of particular importance in overcoming IFN-α resistance. In Figure 4, IFN-α2c and TRAIL each significantly induced the collapse of ΔΨm mediated by BID, indicating that BID, known as a downstream substrate of TRAIL death receptor signaling, may be a critical factor in regulation of cell death following IFN-α treatment. On the other hand, we previously reported that IFN-α could induce apoptosis mediated by IRF9 whose overexpression led to enhance apoptosis and inhibition of the viability of IFN-α resistant cells (10). Taken together with Figure 5A in the current study, BID overexpression appears to be more effective inhibiting the viability of A549 cells in response to IFN-α than IRF9 overexpression reported in our previous study (10). It is therefore suggested that BID in death receptor pathway may be a critical target molecule for overcoming IFN-α resistance, rather than IRF9 in the IFN-α initial pathway. It is reported that the collapse of ΔΨm occurs mostly in a caspase-independent manner (12, 15, 17). In Figure 3A, only Caspase 8 inhibitor out of the Caspase inhibitors tested restored cell viability following IFN-α treatment. Taken together with Figures 2 and 4, our results suggest that cell viability regulated by IFN-α predominantly signals through caspase 8 and BID followed by collapse of ΔΨm in the TRAIL-mediated apoptosis pathway. These results support a report which states the other mitochondrial factors mediated by MMP remain active (17).
In summary, the present study demonstrates that BID plays a crucial role in inhibiting cell viability in response to IFN-α mediated by TRAIL via caspase 8, resulting in the collapse of ΔΨm. These data appear to indicate the linkage between extrinsic (death receptor) and intrinsic (mitochondria-mediated) pathways induced by IFN-α. Our current study provides important evidence that BID overexpression led to significant inhibition of cell viability following IFN-α and TRAIL treatments in IFN-α resistant cells. These results suggest that BID overexpression has a critical potential to overcome the resistance to IFN-α, indicating that BID may become a notable target molecule for the treatment of IFN-α resistant tumors. Future studies will be necessary to continue to refine the mechanisms by how IFN-α further affect human tumors and thus provide other insight into the development of improved use of IFN-α in the treatment of cancer or identify new targets for cancer therapy.
Supplementary Material
IFN-α related gene expression in OVCAR3 cells.
Cell viability results among BID-/negative-RNAi (#1/#2) and non-RNAi transfected OVCAR3 cells treated with IFN-α2c were similar. OVCAR3 cells transfected with or without 20 nM of RNAi were treated with 10 ng/ml of IFN-α2c for 3 days followed by an MTT assay. Control indicates untreated cells transfected with negative-RNAi(#1) and (#2) for BID-RNAi(#1) and (#2), respectively. For non-RNAi, control indicates just untreated cells. The data are the mean ± standard deviation (n ≥ 3). BID indicates BH3 interacting domain death agonist; RNAi, RNA interference; IFN, interferon; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; MTT, 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; p-value, probability value.
BID-RNAi recovered ΔΨm following IFN-α2c or TRAIL treatments. A–F, Twenty nM of RNAi transfected OVCAR3 cells were treated with IFN-α2c or TRAIL (10 ng/ml each) for 3 days followed by flow cytometry analysis. The data were analyzed by FACSDiva software (BD). Figures demonstrate the measures of X-axis and Y-axis as indicated. Briefly, FL1-H and FL2-H indicate green and red fluorescence intensity as logarithmic scales, respectively. FSC-H, SSC-H, and Count indicate the number of cells as linear scales. In upper right dot plots, the ratios (%) for upper right (Q2) and lower right (Q4) quadrants indicate the ratios of live cells and apoptotic cells, respectively. (Live cells whose ΔΨm was intact; apoptotic cells whose ΔΨm was collapsed.) The X-axis indicates apoptotic cell populations that are originally seen on the X-axis in Figure 4. Blue color populations in both upper dot plots indicate apoptotic cells on X-axis (FL1-H positive, FL2-H negative). Ratios of ‘on X-axis’ are shown at the right side of the upper right dot plots. The data are the mean ± standard deviation (n = 3). BID indicates BH3 interacting domain death agonist; RNAi, RNA interference; ΔΨm, mitochondrial membrane potential; IFN, interferon; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Acknowledgments
Supported by the Intramural Research Program of the NIAID, NIH.
We thank the National Institutes of Health Fellows Editorial Board and Katherine Miller for expert assistance in editing this manuscript and Kathleen Meyer of the National Institutes of Health Center for Information Technology for her incomparable help in preparing and submitting Gene Expression Omnibus (GEO) documents. This work was supported by the Intramural Research Program of the NIAID, NIH.
Footnotes
Financial Disclosure: The authors have declared there are no conflicts of interest in regards to this work.
References
- 1.Borden EC, Sen GC, Uze G, et al. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–90. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maher SG, Romero-Weaver AL, Scarzello AJ, Gamero AM. Interferon: cellular executioner or white knight? Curr Med Chem. 2007;14:1279–89. doi: 10.2174/092986707780597907. [DOI] [PubMed] [Google Scholar]
- 3.Parmar S, Platanias LC. Interferons: mechanisms of action and clinical applications. Curr Opin Oncol. 2003;15:431–9. doi: 10.1097/00001622-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Jonasch E, Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist. 2001;6:34–55. doi: 10.1634/theoncologist.6-1-34. [DOI] [PubMed] [Google Scholar]
- 5.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 6.Petak I, Houghton JA. Shared pathways: death receptors and cytotoxic drugs in cancer therapy. Pathol Oncol Res. 2001;7:95–106. doi: 10.1007/BF03032574. [DOI] [PubMed] [Google Scholar]
- 7.Fiorucci G, Vannucchi S, Chiantore MV, Percario ZA, Affabris E, Romeo G. TNF-related apoptosis-inducing ligand (TRAIL) as a pro-apoptotic signal transducer with cancer therapeutic potential. Curr Pharm Des. 2005;11:933–44. doi: 10.2174/1381612053381729. [DOI] [PubMed] [Google Scholar]
- 8.Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–82. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka H, Ito T, Kyo T, Kimura A. Treatment with IFNalpha in vivo up-regulates serum-soluble TNF-related apoptosis inducing ligand (sTRAIL) levels and TRAIL mRNA expressions in neutrophils in chronic myelogenous leukemia patients. Eur J Haematol. 2007;78:389–98. doi: 10.1111/j.1600-0609.2007.00834.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsuno T, Mejido J, Zhao T, Schmeisser H, Morrow A, Zoon KC. IRF9 is a key factor for eliciting the antiproliferative activity of IFN-alpha. J Immunother. 2009;32:803–16. doi: 10.1097/CJI.0b013e3181ad4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galluzzi L, Zamzami N, de La Motte Rouge T, Lemaire C, Brenner C, Kroemer G. Methods for the assessment of mitochondrial membrane permeabilization in apoptosis. Apoptosis. 2007;12:803–13. doi: 10.1007/s10495-007-0720-1. [DOI] [PubMed] [Google Scholar]
- 12.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–9. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 13.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–9. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 14.Chipuk JE, Fisher JC, Dillon CP, Kriwacki RW, Kuwana T, Green DR. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proc Natl Acad Sci U S A. 2008;105:20327–32. doi: 10.1073/pnas.0808036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalvez F, Pariselli F, Dupaigne P, et al. tBid interaction with cardiolipin primarily orchestrates mitochondrial dysfunctions and subsequently activates Bax and Bak. Cell Death Differ. 2005;12:614–26. doi: 10.1038/sj.cdd.4401571. [DOI] [PubMed] [Google Scholar]
- 17.Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005;11:725–30. doi: 10.1038/nm1263. [DOI] [PubMed] [Google Scholar]
- 18.Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–87. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamzami N, El Hamel C, Maisse C, et al. Bid acts on the permeability transition pore complex to induce apoptosis. Oncogene. 2000;19:6342–50. doi: 10.1038/sj.onc.1204030. [DOI] [PubMed] [Google Scholar]
- 20.Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–5. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- 21.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–64. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rostovtseva TK, Antonsson B, Suzuki M, Youle RJ, Colombini M, Bezrukov SM. Bid, but not Bax, regulates VDAC channels. J Biol Chem. 2004;279:13575–83. doi: 10.1074/jbc.M310593200. [DOI] [PubMed] [Google Scholar]
- 23.Cowling V, Downward J. Caspase-6 is the direct activator of caspase-8 in the cytochrome c-induced apoptosis pathway: absolute requirement for removal of caspase-6 prodomain. Cell Death Differ. 2002;9:1046–56. doi: 10.1038/sj.cdd.4401065. [DOI] [PubMed] [Google Scholar]
- 24.Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem. 1999;274:5053–60. doi: 10.1074/jbc.274.8.5053. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 26.Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 27.Czapski GA, Cakala M, Kopczuk D, Strosznajder JB. Effect of poly(ADP-ribose) polymerase inhibitors on oxidative stress evoked hydroxyl radical level and macromolecules oxidation in cell free system of rat brain cortex. Neurosci Lett. 2004;356:45–8. doi: 10.1016/j.neulet.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Parsons JL, Dianova, Allinson SL, Dianov GL. Poly(ADP-ribose) polymerase-1 protects excessive DNA strand breaks from deterioration during repair in human cell extracts. FEBS J. 2005;272:2012–21. doi: 10.1111/j.1742-4658.2005.04628.x. [DOI] [PubMed] [Google Scholar]
- 29.Smulson ME, Pang D, Jung M, et al. Irreversible binding of poly(ADP)ribose polymerase cleavage product to DNA ends revealed by atomic force microscopy: possible role in apoptosis. Cancer Res. 1998;58:3495–8. [PubMed] [Google Scholar]
- 30.Hu R, Bekisz J, Hayes M, et al. Divergence of binding, signaling, and biological responses to recombinant human hybrid IFN. J Immunol. 1999;163:854–60. [PubMed] [Google Scholar]
- 31.Han J, Lee H, Nguyen NY, Beaucage SL, Puri RK. Novel multiple 5′-amino-modified primer for DNA microarrays. Genomics. 2005;86:252–8. doi: 10.1016/j.ygeno.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Phillips TM. Analysis of single-cell cultures by immunoaffinity capillary electrophoresis with laser-induced fluorescence detection. Luminescence. 2001;16:145–52. doi: 10.1002/bio.645. [DOI] [PubMed] [Google Scholar]
- 33.Phillips TM, Dickens BF. Analysis of recombinant cytokines in human body fluids by immunoaffinity capillary electrophoresis. Electrophoresis. 1998;19:2991–6. doi: 10.1002/elps.1150191632. [DOI] [PubMed] [Google Scholar]
- 34.Smiley ST, Reers M, Mottola-Hartshorn C, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991;88:3671–5. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shelton SN, Shawgo ME, Robertson JD. Cleavage of Bid by executioner caspases mediates feed forward amplification of mitochondrial outer membrane permeabilization during genotoxic stress-induced apoptosis in Jurkat cells. J Biol Chem. 2009;284:11247–55. doi: 10.1074/jbc.M809392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banerjee J, Ghosh S. Bax increases the pore size of rat brain mitochondrial voltage-dependent anion channel in the presence of tBid. Biochem Biophys Res Commun. 2004;323:310–4. doi: 10.1016/j.bbrc.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–7. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IFN-α related gene expression in OVCAR3 cells.
Cell viability results among BID-/negative-RNAi (#1/#2) and non-RNAi transfected OVCAR3 cells treated with IFN-α2c were similar. OVCAR3 cells transfected with or without 20 nM of RNAi were treated with 10 ng/ml of IFN-α2c for 3 days followed by an MTT assay. Control indicates untreated cells transfected with negative-RNAi(#1) and (#2) for BID-RNAi(#1) and (#2), respectively. For non-RNAi, control indicates just untreated cells. The data are the mean ± standard deviation (n ≥ 3). BID indicates BH3 interacting domain death agonist; RNAi, RNA interference; IFN, interferon; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; MTT, 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; p-value, probability value.
BID-RNAi recovered ΔΨm following IFN-α2c or TRAIL treatments. A–F, Twenty nM of RNAi transfected OVCAR3 cells were treated with IFN-α2c or TRAIL (10 ng/ml each) for 3 days followed by flow cytometry analysis. The data were analyzed by FACSDiva software (BD). Figures demonstrate the measures of X-axis and Y-axis as indicated. Briefly, FL1-H and FL2-H indicate green and red fluorescence intensity as logarithmic scales, respectively. FSC-H, SSC-H, and Count indicate the number of cells as linear scales. In upper right dot plots, the ratios (%) for upper right (Q2) and lower right (Q4) quadrants indicate the ratios of live cells and apoptotic cells, respectively. (Live cells whose ΔΨm was intact; apoptotic cells whose ΔΨm was collapsed.) The X-axis indicates apoptotic cell populations that are originally seen on the X-axis in Figure 4. Blue color populations in both upper dot plots indicate apoptotic cells on X-axis (FL1-H positive, FL2-H negative). Ratios of ‘on X-axis’ are shown at the right side of the upper right dot plots. The data are the mean ± standard deviation (n = 3). BID indicates BH3 interacting domain death agonist; RNAi, RNA interference; ΔΨm, mitochondrial membrane potential; IFN, interferon; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.