Abstract
Silent information regulator two proteins (sirtuins or SIRTs) are a group of histone deacetylases whose activities are dependent on and regulated by nicotinamide adenine dinucleotide (NAD+). They suppress genome-wide transcription, yet upregulate a select set of proteins related to energy metabolism and pro-survival mechanisms, and therefore play a key role in the longevity effects elicited by calorie restriction. Recently, a neuroprotective effect of sirtuins has been reported for both acute and chronic neurological diseases. The focus of this review is to summarize the latest progress regarding the protective effects of sirtuins, with a focus on SIRT1. We first introduce the distribution of sirtuins in the brain and how their expression and activity are regulated. We then highlight their protective effects against common neurological disorders, such as cerebral ischemia, axonal injury, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and multiple sclerosis. Finally, we analyze the mechanisms underlying sirtuin-mediated neuroprotection, centering on their non-histone substrates such as DNA repair enzymes, protein kinases, transcription factors, and coactivators. Collectively, the information compiled here will serve as a comprehensive reference for the actions of sirtuins in the nervous system to date, and will hopefully help to design further experimental research and expand sirtuins as therapeutic targets in the future.
Keywords: SIRT1, deacetylation, cell death, cerebral ischemia, neurodegenerative disease, neuroprotection
1. Introduction
1.1 Histone deacetylases
Proteins undergo many posttranslational modifications to alter their function. One such modification is that certain proteins are acetylated on their lysine residues, a reaction mediated by acetyltransferases (Mellert and McMahon, 2009). Removal of these acetyl groups is facilitated by another family of enzymes--deacetylases (Mellert and McMahon, 2009; Yang and Seto, 2007). The prototypical proteins that exemplify the effects of acetylation are histones, as acetylated histones are unbound to DNA and allow transcription, while deacetylated histones bind tightly to DNA and restrict transcription (for a more detailed description see Section 5.1).
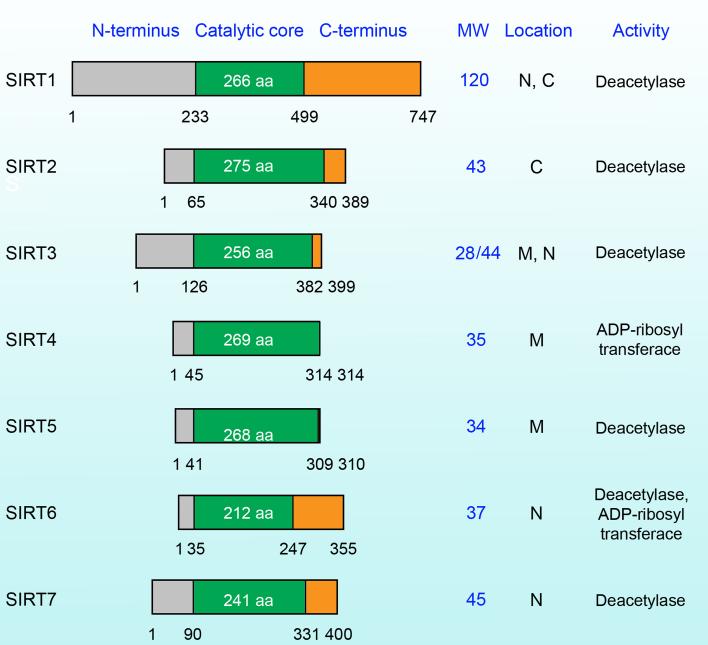
There are four classes of deacetylases in mammals; among them, class III is unique because its members require nicotinamide adenine dinucleotide (NAD+) for catalysis. Therefore, they are also known as the NAD+-dependent class III histone deacetylases (Imai et al., 2000; Mellert and McMahon, 2009; Yang and Seto, 2007). More commonly, they are referred to as silent information regulator two proteins (sirtuins or SIRTs), named after their yeast homologue, silent information regulator 2 (sir2) (Afshar and Murnane, 1999). To date, seven sirtuins have been identified, and they are known as sirtuin 1 (SIRT1) through SIRT 7 (Figure 1) (Michan and Sinclair, 2007). Structurally, they share significant sequence homology, and they all contain a conserved catalytic domain and a NAD+-binding domain (Finnin et al., 2001; Sherman et al., 1999; Yamamoto et al., 2007).
Figure 1. Molecular comparison of SIRT1 to SIRT7.
The SIRT family consists of 7 proteins that all have a conserved catalytic core domain. This diagram illustrates the protein domains in relation to the catalytic core domain along with the molecular weights, cellular localizations, and enzymatic activities of the seven human sirtuins. Molecular weights are in kDa. SIRT3 has two isoforms; the long form is 44 kDa and a short truncated form without the N-terminus is 28 kDa. ADP, adenosine diphosphate; C, cytosol; M, mitochondrial; N, nucleus.
1.2 SIRT1 mediates longevity under calorie restriction
SIRT1 is the best-characterized sirtuin among the seven. It contains 747 amino acids in human, with a predicted molecular weight of 81 kDa and a measured one of 120 kDa. In addition to histones, SIRT1 also deacetylates a number of non-histone substrates, such as p53 (Luo et al., 2001) and peroxisome proliferator-activated receptor γ (PPARγ) coactivator-1α (PGC-1α) (Nemoto et al., 2005). SIRT1 is drawing even more attention since it is considered to be one of the determining factors in lifespan elongation induced by calorie restriction, a phenomenon observed in phylogenetically diverse organisms including yeast, worm, fruit fly and mouse (Howitz et al., 2003; Kaeberlein et al., 1999; Rogina and Helfand, 2004; Tissenbaum and Guarente, 2001). Its beneficial roles are further supported by the findings that putative SIRT1-activating compounds, such as resveratrol, also promote longevity in several species, including yeast (Howitz et al., 2003), worm, (Wood et al., 2004) and mouse (Baur et al., 2006), making it an anti-aging target.
The longevity effects of SIRT1 rely on its enzymatic activity of deacetylation on histone and non-histone substrates. While the deacetylation of histones leads to their interaction with DNA and consequent gene silencing (Braunstein et al., 1993; Dali-Youcef et al., 2007; Sauve et al., 2006), the deacetylation of non-histone proteins has a wide range of biological effects, including metabolic adjustment, survival promotion, and autophagy (Brooks and Gu, 2009; Campisi, 2005; Dali-Youcef et al., 2007; Madeo et al., 2010). For example, SIRT1 inhibits p53 (Luo et al., 2001), reducing its pro-apoptotic effect. It also inhibits nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) (Yeung et al., 2004), reducing its pro-inflammatory effects. In contrast, SIRT1 activates a transcriptional coactivator, PGC-1α (Nemoto et al., 2005), leading to increased glucose levels, insulin sensitivity, and mitochondrial biogenesis. These effects, along with others, collectively contribute to the longevity effect of calorie restriction.
These metabolic changes and cytoprotective endorsements are generally considered to happen in non-neural organs, such as the liver, pancreas, muscle, and fat tissues (Brooks and Gu, 2009; Imai and Guarente, 2010). However, recent studies suggest that the hypothalamus may also contribute to the longevity effects of SIRT1 and calorie restriction via coordination of neurobehavioral and neuro-endocrinal changes including body temperature, appetite, and overall physical activity (Dietrich et al., 2010; Satoh et al., 2010). SIRT1 is abundantly expressed in several regions in the hypothalamus of mice, especially in the arcuate, paraventricular, ventro- and dorsomedial nuclei; and calorie restriction increases SIRT1 levels in the hypothalamus, which increases body temperature, food intake, and physical activity (Dietrich et al., 2010; Ramadori et al., 2008; Satoh et al., 2010). SIRT1 appears to be required for the aforementioned behavioral changes, as the changes are prevented if SIRT1 is knocked out or inhibited (Chen et al., 2005a; Satoh et al., 2010).
In addition to the hypothalamus, SIRT1 is also expressed in other regions of the brain, including the cortex, striatum and hippocampus (Ramadori et al., 2008). Shortly after this finding, a neuroprotective role of sirtuins, especially SIRT1, has been delineated (Morris et al., 2011; Tang, 2009). Hence, in this article, we intend to summarize recent progress on the neural benefits of the sirtuins. First, we briefly review how the sirtuins are regulated in terms of their expression and activity. Next, we look at their distribution in the brain and examine their neuroprotective effects against neurological disorders. Finally, we highlight the neuroprotective mechanisms of the sirtuins, with a focus on SIRT1.
2. Regulation of SIRT1 expression and activity
2.1 Transcriptional regulation of SIRT1 expression
2.1.1 Upregulation
Expression of SIRT1 is regulated at the transcriptional level. The basal level of SIRT1 is regulated by the transcription factor E2F transcription factor 1 (E2F1), through binding to the SIRT1 promoter at a consensus site (Wang et al., 2006). Calorie restriction and cellular stresses increase the transcriptional activity of E2F1 and upregulate the level of SIRT1. For instance, DNA damage and oxidative stress stabilize and activate E2F1, leading to increased SIRT1 transcription (Wang et al., 2006). Forkhead box proteins (FOXO) belong to another group of transcription factors that upregulate SIRT1. FOXO1 binds to several consensus sites within the SIRT1 promoter and enables its transcription (Xiong et al., 2011). FOXO3a is another SIRT1 regulator, and starvation in mammal cells activates FOXO3a and consequently augments SIRT1 expression, indicating an important role of these proteins in nutrient-sensing signaling (Nemoto et al., 2004).
Feedback mechanisms exist between SIRT1 and E2F1 and FOXO1. In a negative manner, SIRT1 can deacetylate E2F1 and inhibit its transcriptional activity, and therefore maintain SIRT1 protein level homeostasis (Wang et al., 2006). In contrast, SIRT1 deacetylates FOXO1 and increases its transcriptional activity, forming a positive feedback loop (Xiong et al., 2011). These feedback loops may play important roles in the fine regulation of SIRT1 expression.
Additionally, SIRT1 is upregulated by a nuclear receptor, a human homologue of the Drosophila tailless gene (TLX). It was recently reported that TLX binds the TLX-activating element on the promoter of mammalian SIRT1, and upregulates SIRT1 expression (Iwahara et al., 2009). Knockdown of TLX with siRNA reduces the level of SIRT1 (Iwahara et al., 2009).
2.1.2 Downregulation
Alternatively, SIRT1 is downregulated under certain conditions. Hypermethylated in cancer 1 (HIC1) is a transcriptional repressor, with SIRT1 as one of its targets (Chen et al., 2005c). HIC1 binds SIRT1, forming a transcriptional repression complex through its N-terminus. This complex directly binds to the SIRT1 promoter, consequently represses the transcriptional activity of the SIRT1gene, and thereby inhibits SIRT1-mediated p53 inactivation (Chen et al., 2005c). A recent study showed that PPARγ binds the promoter of SIRT1 and inhibits its expression (Han et al., 2010). There are two p53 binding sites (−178bp and −168bp) within the promoter of the SIRT1 gene, and their interaction normally represses the transcription of SIRT1 (Nemoto et al., 2004). In the absence of nutrients, FOXO3a physically binds p53 and inhibits the suppressive activity of p53 on SIRT1, resulting in increased transcription of SIRT1 (Nemoto et al., 2004; Zschoernig and Mahlknecht, 2008).
2.2 Post-transcriptional regulation of SIRT1 expression
2.2.1 MicroRNAs
In addition to transcriptional control, the post-transcriptional regulation of SIRT1mRNA is another major determinant of SIRT1 protein expression (Cheadle et al., 2005). These procedures are governed by specific RNA binding proteins or microRNAs (miRs), leading to either stabilization or degradation of the mRNA. MicroRNAs refer to a group of short RNAs with an average length of 22 nucleotides (Bartel, 2009; Lee and Kemper, 2010). They cause gene silencing by binding to complementary sequences on their target mRNAs, leading to the degradation of mRNAs (Bartel, 2009; Lee and Kemper, 2010). To date, several microRNAs have been identified that reduce SIRT1 expression, including miR-9 (Saunders et al., 2010), miR-34a (Lee and Kemper, 2010; Yamakuchi et al., 2008), miR-132 (Strum et al., 2009), miR-181 (Saunders et al., 2010) and miR-199 (Rane et al., 2009; Saunders et al., 2010), miR-217 (Menghini et al., 2009), demonstrating an additional means to regulate SIRT1 expression at the post-transcriptional level.
2.2.2 RNA-binding proteins
An example of RNA binding proteins is the Hu family of RNA-binding protein (HuR). Under physiological conditions, HuR associates with the 3′ untranslated region of the SIRT1 mRNA. This interaction leads to increased stability of SIRT1 mRNA, promoting the translation of SIRT1 (Abdelmohsen et al., 2007; Brennan and Steitz, 2001). However, the complex between HuR and SIRT1 mRNA is disrupted by DNA damage and oxidative stress, leading to the instability of SIRT1 mRNA (Brennan and Steitz, 2001). These insults also lead to the activation of a serine/threonine kinase, the cell cycle checkpoint kinase 2 (CHK2), which then phosphorylates HuR at Ser88, Ser100, and Thr118. When hyperphosphorylated, HuR loses its binding ability to SIRT1 mRNA, setting the mRNA free to be degraded (Abdelmohsen et al., 2007; Zschoernig and Mahlknecht, 2008).
2.3 The regulation of SIRT1 activity via protein-protein interactions
It is well established that protein-protein interaction, or complex formation, is a key regulatory mechanism for the activity of histone deacetylases (Table 2), including sirtuins (Sengupta and Seto, 2004). Thus far, two regulatory proteins have been found that alter SIRT1 activity by forming complexes with SIRT1 in response to cellular stresses (Sengupta and Seto, 2004; Zschoernig and Mahlknecht, 2008). Active regulator of SIRT1 (AROS) is a recently identified nuclear protein that directly interacts with SIRT1 and increases the deacetylating activity of SIRT1 on p53 following DNA damage, inhibiting p53-mediated transcription of pro-apoptotic genes (Kim et al., 2007b). In contrast, deleted in breast cancer-1 (DBC1), another nuclear protein, functions as a negative regulator of SIRT1 (Chini et al., 2010; Kim et al., 2008; Zhao et al., 2008). DBC1 directly forms a complex with SIRT1 via the interaction of a leucine zipper motif and the deacetylase domain of SIRT1. This interaction consequently inhibits the enzymatic activity of SIRT1 on p53 (Kim et al., 2008; Zhao et al., 2008). DBC-1 interacts only with SIRT1 of the sirtuin family, implying that the underlying regulatory mechanism is highly specific (Chini et al., 2010). However, the molecular mechanisms underlying SIRT1 activation by AROS and SIRT1 inhibition by DBC-1 are not completely understood.
Table 2.
Regulators of SIRT1 activity
| Routes | Regulators | Results | Mechanisms | References |
|---|---|---|---|---|
|
Protein-protein
interaction |
AROS | ↑ | Binds and enhances deacetylating activity to p53 |
Kim, 2007b |
| DBC-1 | ↓ | Interacts with leucine zipper motif and the catalytic domain |
Kim, 2008; Zhao, 2008 |
|
|
Post-
translational modification |
Sumo | ↑ | Sumoylation of Lys734 | Yang, 2007b |
| JNK1 | ↑ | Phosphorylation of Ser27 and Ser47 |
Beausoleil, 2004 | |
| DYRK1,3 | ↑ | Phosphorylation of Thr522 | Guo, 2010 | |
| CHK1 | ↑ | Phosphorylation of Thr530 and Thr540 |
Sasaki, 2008 | |
| CK2 | ↑ | Phosphorylation of Ser659 and Ser661 |
Zschoernig, 2009 | |
| MST1 | ↓ | Phosphorylation of C-terminus | Yuan, 2011 | |
| Pharmacological | Resveratrol | ↑ | Unclear |
Howitz, 2003; Wood, 2004 |
| Quercetin | ↑ | Unclear | de Boer, 2006 | |
| Sirtinol | ↓ | Interferes with body axis formation |
Grozinger, 2001 | |
| Splitomicin | ↓ | Inhibits the access of the acetylated lysines with SIER1 |
Bedalov, 2001 | |
| Oxidative | 4-HNE, acrolein |
↓ | Reacts with Cys467 and Cys692 | Caito, 2010 |
| Metabolic | NAD+ | ↑ | Substrate of sirtuins |
Vaziri, 2001; Araki, 2004 |
| Nicotinamide | ↓ | Switches between deacetylation and base exchange |
Sauve, 2001; Jackson, 2003 |
|
| ADP-ribose | ↓ | Unclear | Zhao, 2004 |
2.4 Regulation of SIRT1 activity by post-translational modification
2.4.1 Sumoylation
As seen in other enzymes, SIRT1 enzymatic activity is also altered by post-translational modification (Table 2) (Chung et al., 2010; Tang, 2009). The most common post-translational modifications for SIRT1 are sumoylation and phosphorylation. The small ubiquitin-like modifiers (SUMO) are a group of proteins that are covalently attached to lysine residues of targeting proteins via a process called sumoylation. Distinct from the degradation function of ubiquitination, sumoylation exerts a regulatory function on its target proteins, and those regulations include subcellular translocation and altered enzymatic activity of the target proteins (Verger et al., 2003; Zschoernig and Mahlknecht, 2008). SIRT1 is one target of sumoylation (Yang et al., 2007b). Sumoylation of Lys734 significantly increases the enzymatic activity of SIRT1, and the abrogation of sumoylation by site-directed mutagenesis impairs its deacetylase activity on p53 and histones. The desumoylation of SIRT1 occurs after genotoxic stresses, leading to increased cell death (Yang et al., 2007b; Zschoernig and Mahlknecht, 2008). These results suggest that the sumoylation and desumoylation of SIRT1can function as a molecular switch to regulate SIRT1 activity in response to cellular stresses.
2.4.2 Phosphorylation
2.4.2.1 Increasing SIRT1 activity
Reversible phosphorylation of proteins is the most common post-translational modification that functions as a ‘molecular switch’ in the concerted control of biological systems (Figure 2). There are at least 13 candidate sites for phosphorylation in SIRT1 (Sasaki et al., 2008), including Ser27 and Ser47 in its N-terminus (Beausoleil et al., 2004; Beausoleil et al., 2006). Indeed, Nasrin and co-workers reported that c-jun N-terminal kinase 1 (JNK1) phosphorylates these two serine residues plus Thr530 of SIRT1 (Nasrin et al., 2009). The phosphorylation of SIRT1 occurs under oxidative stress and increases the nuclear translocation and enzymatic activity of SIRT1 specifically toward histone H3 but not p53 (Nasrin et al., 2009), suggesting this interaction may play a role in a stress-protective pathway.
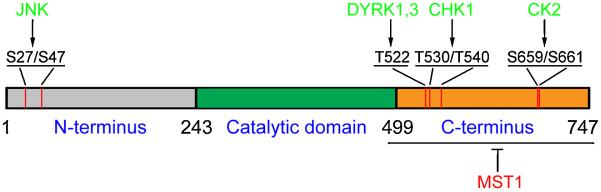
Figure 2. Regulation of SIRT1 activity via phosphorylation.
The activity of SIRT1 is regulated by several post-translational modifications, including phosphorylation. Here we provide a diagram illustrating the phosphorylation sites of SIRT1 by several protein kinases. Kinases depicted in green increase the enzymatic activity of SIRT1 upon phosphorylation. The kinase depicted in red, MST1, inhibits SIRT1 activity. CHK1, checkpoint kinase 1; CK2, casein kinase II; DYRK1/3, dual specificity tyrosine phosphorylation-regulated kinase 1/3; JNK, c-jun N-terminal kinase 1; S, serine; T, threonine.
The cell cycle checkpoint kinases (CHKs) are a group of kinases that also phosphorylate SIRT1. CHK1 is responsible for phosphorylation of Thr530 and Thr540 of SIRT1 to increase its activity; accordingly, dephosphorylation of SIRT1 results in decreased enzymatic activity (Sasaki et al., 2008).
Another family of protein kinases, the dual specificity tyrosine phosphorylation-regulated kinases (DYRKs), is also reported to phosphorylate SIRT1. DYRKs are important in the embryonic development of brain, with a special role in the pathogenesis of Down’s syndrome (Guo et al., 2010; Tejedor and Hammerle, 2011). One of their roles is to regulate apoptosis. Among its seven members, DYRK1A and DYRK3 inhibit apoptosis in various cell types, whereas DYRK2 induces apoptosis via activating p53 (Guo et al., 2010; Taira et al., 2007). Two of their members, namely the pro-survival DYRK1a and DYRK3, directly phosphorylate SIRT1 at its Thr522 and activate it, leading to increased p53 deacetylation (Guo et al., 2010).
The kinase identified most recently to increase SIRT1 activity is casein kinase II (CK2), a eukaryotic protein kinase with more than 100 substrates. CK2 is recruited to SIRT1 after cellular stresses and phosphorylates multiple conserved serine and threonine residues of SIRT1, including Ser154, 649, 651 and 683 (Kang et al., 2009), as well as Ser659 and Ser661 (Zschoernig and Mahlknecht, 2009) (Figure 2). Phosphorylation of SIRT1 by CK2 increases its substrate-binding affinity and deacetylation rate, especially in regard to p53 (Kang et al., 2009; Sasaki et al., 2008; Zschoernig and Mahlknecht, 2009).
2.4.2.2 Decreasing SIRT1 activity
Phosphorylation does not ubiquitously amplify the activity of SIRT1. Mammalian sterile 20-like kinase 1 (MST1) is a serine/threonine kinase, and the overexpression of MST1 induces apoptosis via activation of p53 (Lin et al., 2002; Yuan et al., 2011). A recent study showed that SIRT1 is phosphorylated by MST1 at its C-terminus (aa 489-747) after induced DNA damage, leading to reduced activity of SIRT1 and increased acetylation of p53, and ultimately causing cell death (Yuan et al., 2011) (Figure 2). Taken together, these results show that SIRT1 is phosphorylated at multiple sites by several protein kinases; which, together with sumoylation, play important roles in SIRT1 functional regulation.
2.5 Pharmacological regulation of SIRT1 activity
Several chemical compounds have been shown to inhibit the deacetylase activity of the sirtuin family. Inhibition of SIRT1 by splitomicin is observed in multiple systems, including budding yeast (Bedalov et al., 2001), mammalian cells (Nadtochiy et al.), and mice (Breitenstein et al., 2010). Sirtinol is another frequently used SIRT1 inhibitor that is effective both in vitro and in vivo (Grozinger et al., 2001; Ota et al., 2006; Shindler et al., 2010). Recent studies show that a series of indoles (Napper et al., 2005) are potent and selective inhibitors of SIRT1. Indeed, one of the most potent and specific inhibitors for SIRT1 is the indole EX-527 (Solomon et al., 2006),
SIRT1 is also activated by several small-molecule compounds. Most of these activators are polyphenols (Chung et al., 2010), including resveratrol (Baur et al., 2006; Howitz et al., 2003; Wood et al., 2004), quercetin, curcumin, and catechins (Chung et al., 2010; Davis et al., 2009; de Boer et al., 2006; Howitz et al., 2003). Resveratrol is the first and most studied SIRT1 activator. While its stimulatory effect is strongly supported by many studies (Alcain and Villalba, 2009; Howitz et al., 2003; Kim et al., 2007a; Pervaiz, 2003; Raval et al., 2008; Sun et al., 2010), the underlying mechanisms are not fully understood. Additionally, resveratrol may have some biological functions other than activating SIRT1 (Beher et al., 2009; Pacholec et al., 2010; Tang, 2010). A viable explanation for the diversity of biological targets of resveratrol is that a direct interaction and allosteric mechanism are involved in its mechanism of action (Dai et al., 2010). More detailed information about SIRT1 activators and inhibitors can be found in reviews dedicated to this topic (Chung et al., 2010; Neugebauer et al., 2008).
2.6 Oxidative regulation of SIRTs activity
Reactive oxygen species (ROS) causes lipid peroxidation and generates unsaturated aldehydes, such as acrolein and 4-hydroxynonenal (4-HNE), which are reactive to cysteines in proteins. It is reported that these aldehydes react with the cysteine residues of human SIRT1 between amino acids 467 and 492 (Caito et al., 2010). This modification not only decreases the enzyme activity of SIRT1 but also promotes its proteasomal degradation (Caito et al., 2010). A recent report shows that 4-HNE covalently binds Cys-280 of human mitochondrial SIRT3, resulting in allosteric inhibition of SIRT3 deacetylase activity (Fritz et al., 2011). Collectively, these findings suggest that 4-HNE directly binds some sirtuins and inhibits their activities.
2.7 Metabolic regulation of SIRT1 activity
Sirtuins catalyze the deacetylation reaction of their targets, which is an NAD+-dependent process. During this process, an acetyl group of the substrate is transferred to the ADP-ribose (ADPR) moiety of NAD+. After losing the acetyl group, the SIRT1 substrate becomes a deacetylated protein. Once NAD+ gains the acetyl group, it becomes destabilized, breaking down to one molecule of nicotinamide and one molecule of 2′-O-acetyl-ADP ribose (OAADPR) (Landry et al., 2000; Smith et al., 2000; Tanner et al., 2000; Tanny and Moazed, 2001). SIRT1 thus has two coupled enzymatic activities: deacetylating targets and breaking down NAD+, where NAD+ functions as the substrate of SIRT1 and nicotinamide and OAADPR are the products of the deacetylation reaction. Therefore, it is a reasonable prediction that NAD+ can increase the enzymatic activity of SIRT1, whereas nicotinamide and OAADPR may inhibit it (Bitterman et al., 2002; Neugebauer et al., 2008; Sauve and Schramm, 2003; Tong and Denu, 2010). Next, we review the literature showing the negative regulation of sirtuins via deacetylation byproducts.
2.7.1 Inhibition
As an end product of sirtuin deacetylation, nicotinamide is an effective inhibitor of SIRT1 activity both in vivo and in vitro (Luo et al., 2001; Sauve et al., 2001; Sauve and Schramm, 2003). At normal concentrations, nicotinamide inhibits Sir2 activity in vitro via binding to a conserved pocket on the enzyme and blocking the hydrolysis of NAD+ (Bitterman et al., 2002; Jackson et al., 2003). In yeast cells, exogenous nicotinamide suppresses Sir2 activity and shortens life span. Nicotinamide is also known to inhibit mammalian SIRT1 (Vaziri et al., 2001; Zhao et al., 2004), promoting p53-dependent apoptosis in mammalian cells (Vaziri et al., 2001). Compared with nicotinamide, OAADPR is less characterized regarding its role in sirtuin regulation (Tong and Denu, 2010), although ADP-ribose is known to be a potent SIRT1 inhibitor (Zhao et al., 2004).
2.7.2 Activation
As discussed above, SIRT1 requires NAD+ for activation, and NAD+ plays a critical role in the positive regulation of SIRT1 activity. During calorie restriction, the ratio of NAD+/NADH is increased, and the relatively high level of NAD+ enhances the activity of sirtuins (Lin et al., 2004; Vaziri et al., 2001). However, it is difficult to study the biological effects of NAD+ when it is exogenously supplied, due to its low membrane permeability and high compartmentalization within mitochondrial, nuclear, and cytosolic pools. In the case of SIRT1, the role of NAD+ is highlighted through its endogenous synthesis. There are two pathways for NAD+ synthesis, the de novo synthesis and salvage pathways (Chung et al., 2010; Sauve et al., 2006). The de novo synthesis is an eight-step process using tryptophan as the precursor (Penberthy and Tsunoda, 2009), and the salvage pathway is a two-step process using nicotinamide as the precursor. Thus, the salvage pathway turns the end product of SIRT1-mediated reactions into its substrate, therefore enhancing SIRT1 activity via two mechanisms: providing substrate and removing end product. In the salvage pathway, the two synthetic steps are catalyzed by two enzymes—nicotinamide phosphoribosyltransferase (NAMPT) and nicotinamide mononucleotide adenylyltransferase 1 (NMNAT-1) (Chung et al., 2010; Penberthy and Tsunoda, 2009). Of the two, NAMPT is the rate-limiting enzyme that catalyzes the conversion of nicotinamide to nicotinamide mononucleotide; NMNAT-1 then produces NAD+ from nicotinamide mononucleotide.
The significance of the NAD+ salvage pathway was initially supported by a yeast study demonstrating that an intact NAD+ salvage pathway is needed to elongate the lifespan by caloric restriction, as mutations of the enzymes abolish lifespan extension (Lin et al., 2000). On the other hand, overexpression of these enzymes not only extends the lifespan of yeast (Anderson et al., 2003; Lin et al., 2000) but also the lifespan of human vascular smooth muscle cells (Ho et al., 2009).
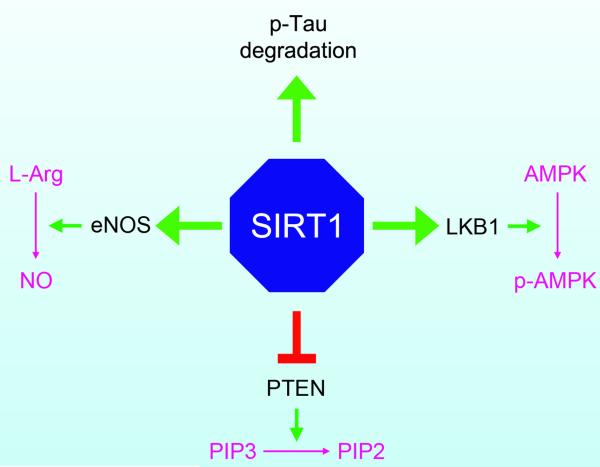
Further studies show that knockdown of either NAMPT or NMNAT-1 indeed decreases total cellular NAD+ levels (Fulco et al., 2008; Ramsey et al., 2009; Zhang et al., 2009). Individually, NAMPT directly reduces cellular nicotinamide levels (Fulco et al., 2008), increases NAD+ production, and therefore increases the ratio of NAD+/NADH in mammalian cells (Fulco et al., 2008; Nakahata et al., 2009; Ramsey et al., 2009). NMNAT-1 is especially important in the nervous system as it protects neurons against axonal injury in a SIRT1-dependent manner (Araki et al., 2004). This phenomenon remains controversial as others have shown that NAD+-dependent neuroprotection is SIRT1-independent (Wang et al., 2005b).
The notion that SIRT1 protects against axonal injury is supported by studies examining the Wallerian degeneration slow (wlds) gene, a fusion gene encoding the full-length NMNAT-1 and the N-terminal fragment of the ubiquitin assembly protein (Mack et al., 2001). In wlds transgenic mice, Wallerian degeneration, a process where the distal parts of axons undergo degeneration after injury, is slowed compared with that in wild-type mice (Araki et al., 2004; Conforti et al., 2000; Mack et al., 2001). Although not a rate-limiting enzyme, NMNAT-1 also plays an important role in Wallerian degeneration since knockdown of NMNAT-1 reduces total cellular NAD+ levels and inhibits the deacetylation activity of SIRT1 on multiple targets (Zhang and Kraus, 2009). (For more information see section 4.1.2). Taken together, these results indicate that the regulation of SIRT1 activity by NAD+, including its catabolism and synthesis, is an important link between cellular metabolism and gene transcription via protein deacetylation.
3. Distribution of sirtuins in the brain
During mouse embryogenesis, SIRT1 is highly expressed in the brain, spinal cord, and dorsal root ganglion, with the peak expression at E4.5 (Sakamoto et al., 2004). SIRT1 is also expressed in the adult brain, with high levels in the cortex, hippocampus, cerebellum, and hypothalamus, and low levels in white matter (Ramadori et al., 2008). Among the various cell types of brain, SIRT1 is predominantly, if not exclusively, expressed in neurons (Hisahara et al., 2008; Ramadori et al., 2008; Sakamoto et al., 2004). The only exception is that SIRT1 is found in microglia when co-cultured with neurons (Chen et al., 2005b). At the subcellular level, SIRT1 is viewed as a nuclear protein (Michishita et al., 2005). Yet, it is reported that SIRT1 has both nuclear import and export sequences, and that SIRT1 is present in the cytosolic fraction of mouse brain, although its cytosolic function is just beginning to be elucidated (Jin et al., 2007; Li et al., 2008; Tanno et al., 2007).
In brain, SIRT2 is a cytoplasmic protein expressed in oligodendrocytes and plays an important role in the formation of myelin sheath and in the myelin-axon interaction (Li et al., 2007c; Michishita et al., 2005; Schwer et al., 2010). The major target of SIRT2 is the Lys40 of α-tubulin (North et al., 2003). Additionally, SIRT2 is expressed in olfactory and hippocampal neurons (Suzuki and Koike, 2007).
SIRT5, a mitochondrial sirtuin, is highly expressed in the cortex of human brain, especially in layer II (Glorioso et al., 2011). Among the other sirtuins, SIRT3 and SIRT4 are also localized to the mitochondria (Ahn et al., 2008; Michishita et al., 2005), while SIRT6 and SIRT7 are nuclear proteins (Cooper and Spelbrink, 2008; Michishita et al., 2005; Schwer et al., 2010). Although they are all expressed in brain (Liszt et al., 2005; Michishita et al., 2005; Schwer et al., 2010), their cellular distributions and functions have yet to be elucidated.
4. Neuroprotective effects of sirtuins against neurological diseases
The effects of sirtuins on the outcomes of common neurological disorders are summarized in Table 3 and discussed in detail below.
Table 3.
Sirtuins and neurodegenerative diseases
| Disease | Experimental settings | Result | Mechanism | References |
|---|---|---|---|---|
| Ischemia | Res in rat global | + | Increased SIRT1 activity; decrease UCP2 |
Raval, 2006; Della-Morte, 2009 |
| Res, sirtinol and SIRT1 OE in NO exposure |
+ | Increased SIRT1 activity; | Chong, 2008 | |
| SIRT1 KO in mouse focal | + | Nampt stimulates SIRT1, SIRT1 deacetylates LKB1, LKB activates AMPK |
Wang, 2011 | |
| SIRT1 Tg in mouse focal | +/− | SIRT1 overexpression | Kakefuda, 2009 | |
| Focal in rat and mouse | + | SIRT1 mediates protection of leptin, icariin and tetrahydroxystilbene glucoside |
Wang, 2009a; Wang, 2009c; Avraham, 2010; Zhu, 2010 |
|
| WD | Axonal injury in Wlds mice | + | NMNAT increases NAD+ and activates SIRT1 |
Araki , 2004; Sasaki, 2009; Babetto, 2010 |
| Retinal injury | Res in antibody-induced apotosis |
+ | Upreguates SIRT1 expression | Anekonda, 2008 |
| SIRT1 KO mice | +? | Reduced retinal cell numbers | Cheng, 2003 | |
| AD | SIRT1 KO enhances tau acetyaltion and tauopathy |
+? | SIRT1 deacetylates tau and promotes tau degradation |
Min, 2010 |
| CR in mice increases SIRT1 and reduces Aβ neuropathy |
+ | SIRT1 inhibits ROCK1 and activates α- secrease |
Qin, 2006b | |
| SIRT1 OE suppress Aβ production in mice |
+ | SIRT1 stimulate RAR and upregulates α- secrease |
Donmez, 2010 | |
| PD | SIRT1 OE in neuron after mutant α-synuclein transfection |
+ | SIRT1 activates PGC-1 and increases mitochondrial density |
Wareski, 2009 |
| Res in mice or SK-N-BE cells | + | Res stimulates SIRT1 | Albani, 2009; Chao, 2008 | |
| Res in midbrain slice | + | SIRT1 deacetylates p53 | Okawara, 2007 | |
| MPTP-induced PD model in SIRT1 tg mice |
+/− | Kakefuda, 2009 | ||
| SIRT2 inhibition in Drosophila model of PD |
− | Outeiro, 2007 | ||
| HD | Res or SIRT1 OE in neurons after mutant huntingtin transfection |
+ | SIRT1 activates PGC-1 and increases mitochondrial density |
Parker, 2005; Wareski, 2009 |
| Res in HD mice | +/− | Ho, 2010 | ||
| Sir2 inhibition in Drosophila or striatal neuronal models of HD |
− | SIRT2 inhibition decreases sterol biosynthesis |
Pallos, 2008; Luthi-Carter, 2010 | |
| Prion disease | Res or SIRT1 OE in neurons infected with mutant prion |
+ | Bizat, 2010; Seo, 2011 | |
| ALS | Res or SIRT1 OE in a mouse model of ALS |
+ | SIRT1 deacetylates p53 | Kim, 2007; Markert, 2010 |
| MS | SIRT1 activation in a mouse model of MS |
+ | Shindler, 2007; Shindler, 2010 |
Signs abbreviation used in the table: +, protective; −, detrimental; +/−, neither protective nor detrimental; Aβ, beta amyloid; ALS, amyotrophic lateral sclerosis; CR, calorie restriction; Drosophila nicotinamidase (DN); KO, knockout; MCAO, middle cerebral artery occlusion; MS, multiple sclerosis; NO, nitric oxide; OE, overexpression; OGD, oxygen-glucose deprivation; Res, resveratrol; ROCK1, Rho-associated, coiled-coil containing protein kinase 1; Tg, transgenic; UCP2, uncoupling protein 2; WD, Wallerrian degeneration.
4.1 Acute diseases
4.1.1 Cerebral ischemia
Ischemic stroke is a common neurological disease caused by the sudden reduction or cessation of blood flow to the brain, leading to infarction. The clinical management of stroke is difficult and unsatisfactory because the only method to rescue ischemic brain tissue is to restore blood flow. For this purpose, the FDA has approved the clinical use of tissue plasminogen activator (tPA). However, this drug needs to be administered within three hours after the onset of the stroke to provide any clinical benefit, and this time window greatly limits the clinical use of tPA. Alternative and promising candidates for neuroprotective strategies include preconditioning, mild hypothermia, and the use of chemical and biological compounds targeting critical molecular mediators of neuronal death and survival. One example of these compounds is the SIRT1 activator resveratrol.
The neuroprotective effect of SIRT1 was first reported by Raval and colleagues (Raval et al., 2006; Raval et al., 2008). In their studies, they report that both ischemic preconditioning and resveratrol treatment reduce neuronal injury of hippocampal CA1 after NMDA challenge in slices and global cerebral ischemia in rats. They also show that increased SIRT1 activity is a common mechanism for the protective effects of preconditioning and resveratrol (Morris et al., 2011; Raval et al., 2006). Sirtinol, a SIRT1 activity inhibitor, abolishes the neuroprotection of preconditioning and resveratrol (Raval et al., 2006), indicating that SIRT1 plays a key role in mediating neuroprotection. The neuroprotective role of SIRT1 is further supported by two recent studies (Chong and Maiese, 2008; Della-Morte et al., 2009) showing that SIRT1 activation reduces ischemic neuronal injuries and that one possible mechanism is downregulation of the mitochondrial uncoupling protein 2 (Della-Morte et al., 2009).
Our previous study showed that, in primary neuronal culture, NAD+ pretreatment markedly reduces neuronal death induced by oxygen-glucose deprivation (OGD) (Wang et al., 2008). Our unpublished data show that SIRT1 is necessary for NAD+ neuroprotection, as NAD+ treatment upregulates SIRT1 expression and activity, and SIRT1 knockdown attenuates the protection mediated by NAD+ against excitotoxicity in neurons (Wang et al., 2009b). In addition, similar neuroprotection by NAD+ is observed in astrocytes (Ying et al., 2005), and intranasal infusion of NAD+ also decreases infarct volume in rat after focal cerebral ischemia (Ying et al., 2007). Moreover, NAMPT, the rate-limiting enzyme of the NAD+ salvage pathway, also demonstrates a protective effect against stroke. NAMPT overexpression reduces ischemic infarct whereas NAMPT inhibition aggravates ischemic injuries. The protective effect of NAMPT is SIRT1-dependent, as SIRT1 knockout blocks the protection (Wang et al., 2011).
In addition to its direct protection, SIRT1 also mediates the benefits of some other neuroprotective agents, such as leptin (Avraham et al., 2010), icariin, and tetrahydroxystilbene glucoside (Wang et al., 2009a; Wang et al., 2009c; Zhu et al., 2010). Leptin is an adipose hormone that attenuates ischemic injury (Valerio et al., 2009; Zhang and Chen, 2008; Zhang et al., 2007). One of its protective mechanisms is to upregulate SIRT1 expression (Avraham et al., 2010). In a similar way, SIRT1 upregulation is involved in the neuroprotection of icariin, a flavonol (Wang et al., 2009a; Zhu et al., 2010), and tetrahydroxystilbene glucoside, a polyphenol (Wang et al., 2009c). In the case of icariin, SIRT1 is necessary for the protection, as the knockdown of SIRT1 diminishes the flavonol’s protective effect (Zhu et al., 2010).
Despite the aforementioned evidence, controversy exists over the protective effect of SIRT1 against ischemia. In a study using SIRT1 transgenic mice, where human SIRT1 was overexpressed under the control of rat neuron-specific enolase (NSE) promoter, no neuroprotection was observed against stroke as SIRT1 and wild-type mice demonstrated almost indistinguishable infarct volumes and neurological deficiency scores (Kakefuda et al., 2009). The discrepancy between this study and the others probably derived from the sustained high level of SIRT1, because it may consume too much or even deplete NAD+, which could aggravate neuronal injury (Kakefuda et al., 2009; Liu et al., 2009; Wang et al., 2008). Therefore, it is possible that this detrimental effect of NAD+ deficiency compromises the neuroprotective effect of SIRT1. In another study, nicotinamide, a compound with a SIRT1 inhibitory action, demonstrated a neuroprotective effect against ischemic injury, seemingly implying that SIRT1 might play a detrimental role against stroke (Chong et al., 2005). However, this report might overlook other functions of nicotinamide, including that of precursor for NAD+ synthesis. In fact, the same group later reported that SIRT1 overexpression prevents neurons from apoptosis after oxidative stress (Chong and Maiese, 2008).
4.1.2 Wallerian degeneration
Wallerian degeneration refers to axonal death and degradation after focal injury, followed by myelin sheath breakdown. The neuroprotective effect of SIRT1 against Wallerian degeneration was first discovered in a study using wlds transgenic mice (Perry et al., 1990). These mice exhibited a significant delay in axonal degeneration after physical or chemical injury. The mechanistic basis for the delayed axonal damage was apparently associated with the mutant wlds chimeric protein. Wlds consists of the N-terminal 70 amino acids of the ubiquitin fusion degradation protein2a (Uf2a) and the complete sequence of NMNAT-1, a key enzyme in the NAD+ salvage pathway (Coleman, 2005). NMNAT-1 activity plays an important role in the prevention of axonal damage (Araki et al., 2004; Babetto et al., 2010; Conforti et al., 2007; Sasaki et al., 2009), exerting its protective effects through SIRT1 activation, as the neuroprotection is blocked by the SIRT1 inhibitor sirtinol and siRNA-mediated SIRT1 silencing (Araki et al., 2004; Babetto et al., 2010; Sasaki et al., 2009). It should be noted that SIRT1 cannot fully account for the neuroprotective role of the wlds gene (Conforti et al., 2007; Sasaki et al., 2009; Wang et al., 2005b). Recent studies indeed show that both the N-terminal sequence and intact NMNAT-1 activity are required for full wlds activity (Avery et al., 2009; Conforti et al., 2009), and that cytosolic translocation of NMNAT1 to axons is necessary for its neuroprotection (Babetto et al., 2010). However, the role of SIRT1 remains controversial as both SIRT1-dependent (Araki et al., 2004) and –independent mechanisms are reported (Wang et al., 2005b).
4.1.3 Retinal injury
The retina is part of the nervous system, and it is susceptible to various injuries such as ultraviolet light, ischemia, inflammation and degeneration. SIRT1 is expressed in all layers of normal retina and is necessary for retinal health (Shindler et al., 2007). In SIRT1-deficient mice, however, retina have reduced cell numbers in multiple layers, and demonstrate disorganized cellular patterns, which are accompanied by p53 hyperacetylation (Cheng et al., 2003). Furthermore, a neuroprotective role of SIRT1 has been reported against retinal injury. For instance, upregulation of SIRT1 by resveratrol protects the retina from antibody-induced apoptotic cell death (Anekonda and Adamus, 2008). In retinal degeneration 10 (rd10) mice, an animal model of retinal degeneration, SIRT1 is not detected in the retina, and this absence of SIRT1 contributes to retinal degeneration (Shindler et al., 2007). Similarly, a deficiency in E2Fs, the transcription factor for SIRT1, leads to the downregulation of SIRT1, p53 hyperacetylation, and elevated apoptosis in retina (Chen et al., 2009). Collectively, these results indicate a beneficial role of SIRT1 in retina health and protection.
4.2 Neurodegenerative diseases
4.2.1 Alzheimer’s disease
Alzheimer’s disease (AD) is a terminal neurodegenerative disease, causing neuronal death and brain atrophy. The pathological hallmarks of AD are the intracellular tangles and extracellular plaques. The tangles, also known as neurofibrillary tangles, are the accumulation of insoluble tau proteins, and the plaques are deposits of β-amyloid (Aβ) peptides, typically consisting of 40-42 amino acid residues.
The protective effect of SIRT1 against AD was initially observed in caloric restriction studies, where calorie restriction reduced Aβ and plaque generation in the brains of transgenic AD mice (Patel et al., 2005; Wang et al., 2005a). Similarly, the reduction of Aβ is also noticed in the cortex of starved squirrel monkeys and is inversely correlated with SIRT1 levels (Qin et al., 2006a). These studies imply that SIRT1 is involved in the neuroprotection against AD. Indeed, recent studies demonstrate that SIRT1 activation reduces the neuronal death and brain atrophy induced by AD (Chen et al., 2005b; Donmez et al., 2010; Kim et al., 2007a; Min et al., 2010; Qin et al., 2006b). SIRT1 deficiency is associated with increased levels of phosphorylated-tau in neurons (Min et al., 2010) and the amount of neurofibrillary tangles in AD brains (Julien et al., 2009).
SIRT1 targets both tau and Aβ, two pathological hallmarks of AD. For example, degradation of phosphorylated tau improves cognitive function and reduces neuronal death in mice (Santacruz et al., 2005; Sydow et al., 2011); however, when tau is acetylated by the histone acetyltransferase, p300, the breakdown of tau is inhibited (Min et al., 2010). SIRT1 deacetylates the acetylated tau and consequently reduces its level; conversely, SIRT1 inhibition leads to the opposite effect—increasing the levels of tau and exacerbating the accumulation of pathogenic forms of phosphoryated-tau (Min et al., 2010).
Moreover, recent studies show that either resveratrol administration or SIRT1 overexpression reduces Aβ level both in vitro and in vivo (Chen et al., 2005b; Donmez et al., 2010; Qin et al., 2006b) Aβ is generated from a physiological protein, amyloid precursor protein (APP). Normally, APP is processed by α-secretase, generating soluble APP with a neurotrophic role. When sequentially processed by the β- and γ-secretases, however, APP is converted to the toxic Aβ. SIRT1 overexpression stimulates the production of α-secretase in neurons and mice via two pathways: activating the retinoic acid receptor (RAR) (Donmez et al., 2010) and inhibiting the Rho-associated, coiled-coil-containing protein kinase 1 (ROCK1) (Qin et al., 2006b). Increased level of α-secretase enhances normal precess of APP, leading to decreased generation of toxic Aβ. In addition, SIRT1 also reduces the NF-kappaB pathway in microglia and decreases Aβ level (Chen et al., 2005b). Taken together, these results show that SIRT1 is protective against AD via multiple mechanisms, including causing the degradation of tau and reducing levels of Aβ.
4.2.2 Parkinson’s disease
Parkinson’s disease (PD) is a common neurodegenerative disease caused by the death of dopaminergic neurons of the substantia nigra in the brain stem. The major symptoms of PD are rigidity, tremor, and bradykinesia. Early studies found that caloric restriction or use of 2-deoxy-D-glucose, a glucose analogue, reduces the loss of dopaminergic neurons in mice and improves motor function, implying that SIRT1 may be involved in the protection (Duan and Mattson, 1999). The levels of SIRT1 in dopaminergic neurons are sharply decreased when treated with neurotoxins, such as rotenone, 6-hydroxydopamine, α-synuclein, or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Albani et al., 2009; Alvira et al., 2007; Pallas et al., 2008), which are agents widely used to model PD. Additionally, SIRT1 overexpression (Wareski et al., 2009) or activation by resveratrol (Albani et al., 2009; Chao et al., 2008; Okawara et al., 2007) slows neuronal death as well as neurodegeneration in both in vivo and in vitro PD models, indicating a neuroprotective role of SIRT1 against PD. However, not all studies demonstrated a protective role of sirtuins. For example, no protection is noticed in a MPTP-induced PD model using SIRT1 transgenic mice (Kakefuda et al., 2009). The cytoplasmic sirtuin, SIRT2, even increases the toxicity of alpha-synuclein, as pharmaceutical and genetic inhibition of SIRT2 protects against dopaminergic cell death in a model of Parkinson’s disease (Outeiro et al., 2007b). Nevertheless, despite the controversy, most research demonstrates a protective role of SIRT1 against PD, although the mechanisms are unclear.
4.2.3 Huntington’s disease
Huntington’s disease (HD) is an autosomal dominant hereditary disease with a middle-age onset. It is caused by a trinucleotide repeat mutation in the huntingtin gene that results in an increased number of glutamine residues in the huntingtin N-terminus, which causes abnormal protein aggregation and ultimately neuronal death. Parker and colleagues showed that the upregulation of SIRT1 or resveratrol treatment rescues neurons from injury induced by mutant huntingtin in C. elegans (Parker et al., 2005). In a yeast model of HD, activation of sir2 decreases mutant polyQ aggregation (Sorolla et al., 2011). SIRT1 activation by resveratrol reduced peripheral nerve deficits in HD transgenic mice, but the benefit was not observed in the brain (Ho et al., 2010). On the other hand, SIRT2 is detrimental to HD, as inhibition of SIRT2 protects neurons from death induced by huntingtin (Luthi-Carter et al., 2010; Pallos et al., 2008). A phase 1 clinical trial is underway to treat HD with the highly specific SIRT1 inhibitor, EX-527. When released, this result will help to elucidate the role of SIRT1 in HD protection and to provide a clinically meaningful treatment for this devastating disease.
4.2.4 Prion diseases
Prion diseases, or transmissible spongiform encephalopathies, include human Creutzfeldt–Jakob disease and kuru in humans, bovine spongiform encephalopathy or mad cow disease in bovine mammals, and goat scrapie. A normal cellular prion protein (PrPC) is a membrane glycoprotein involved in several signaling pathways. The infectious form endogenous PrPC, scrapie PrP (PrPSc), has a distinct tertiary conformation from PrPC and thus is processed differently and ultimately forms aggregates. Furthermore, PrPSc is thought to be amplified following infection leading to progressive insoluble aggregate formation, ultimately causing neurotoxicity (Aguzzi et al., 2008; Bizat et al., 2010). The role of SIRT1 in prion disease was first reported in a study showing that calorie restriction reduces the level of prion protein in brain and delays the onset of prion disease (Chen et al., 2008). Further studies also demonstrate a protective effect of SIRT1 against prion diseases in C. elegans or mice models, as resveratrol and SIRT1 overexpression via adenoviral vector reduce neuronal dysfunction and death (Bizat et al., 2010; Seo et al., 2011), and SIRT1 inhibition exacerbates neuronal dysfunction (Bizat et al., 2010). In contrast, SIRT1 may have different effects on the neurotoxicity of prions, as SIRT1 knockout also delays the onset of prion disease (Chen et al., 2008). Further studies are necessary to fully elucidate the role of SIRT1 in prion-related diseases.
4.2.5 Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a chronic and fatal neurodegenerative disease, characterized pathologically by the death of motor neurons in the spinal cord and cortex, possibly induced by a deficiency in the enzyme superoxide dismutase 1 (SOD1) (Rosen, 1993). In the animal model of ALS where a mutant form of SOD1 is expressed, SIRT1 levels are upregulated in motor neurons (Kim et al., 2007a). SIRT1 overexpression protects neurons against toxicity induced by the mutant SOD1 in both cultured neurons and mouse brain (Kim et al., 2007a). This protection corresponds to the increased deacetylation of p53 (Kim et al., 2007a). Resveratrol also enhances the protective effect of SIRT1 in a mouse model of ALS (Kim et al., 2007a; Markert et al., 2010), but it seems that multiple doses are necessary to improve neurological function and increase the longevity of mice (Markert et al., 2010).
4.2.6 Multiple sclerosis
Multiple sclerosis is a myelin sheath disease with lesions typically located in the brain, spinal cord or cranial nerves, and, most commonly, in the optic nerve. The causes of multiple sclerosis are not fully identified but likely arise from an autoimmune etiology; therefore, it is traditionally treated as an inflammatory disease. Recently, however, multiple sclerosis has also been considered a neurodegenerative disease because of the co-existence of permanent axonal damage, neuronal loss, and neurological disability in patients with multiple sclerosis (Lassmann, 2010; Shindler et al., 2010). In a mouse model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE), SIRT1 activation by SRT501 or SRT1720 maintains axonal density, prevents neuronal loss and improves neuronal dysfunction (Shindler et al., 2010; Shindler et al., 2007). SIRT1 inhibition with Sirtinol attenuates the neuroprotective effects of SRT501 (Shindler et al., 2010), suggesting a protective role of SIRT1 in multiple sclerosis. However, further investigations are necessary to fully delineate the role of SIRT1 in multiple sclerosis.
5. Cellular targets and protective mechanisms of sirtuins
Sirtuins are deacetylases, having both histone and non-histone substrates. The switch between acetylated and deacetylated conditions alters the activities of their substrates. Therefore, the protective mechanisms of sirtuins are closely related to their substrates, including histones, transcription factors, transcriptional co-activators, DNA repair enzymes, protein kinases, phosphatases, and tau, a pathogenic protein of AD. We next discuss the neuroprotective targets of sirtuins in the order of the numbers of genes they influence, with the targets that influence the largest number of genes discussed first.
5.1 Nuclear targets
5.1.1 Histones
In nuclei, core histones, including H2, H3 and H4, form ball-like structures for the DNA to wrap around, keeping DNA in a resting status. These interactions occur between the basic lysines (and arginines) of histones and the acidic phosphates of DNA chains. When acetylated on the lysines, however, histones lose their positive charges and release DNA, allowing DNA unwinding and subsequent gene transcription. In contrast, deacetylation polarizes histones and promotes their binding to DNA, leading to genome-wide but non-specific transcription silencing (Figure 3). Sirtuins are histone deacetylases. For example, SIRT1 deacetylates H3 at Lys9 and Lys14, and it also deacetylates H4 at Lys16, leading to genomic silencing (Imai et al., 2000). Genomic silencing could therefore further reduce protein synthesis and energy consumption. For instance, in PC12 cells, the upregulation of SIRT1 reduces cellular oxygen consumption by about 25% (Nemoto et al., 2005).
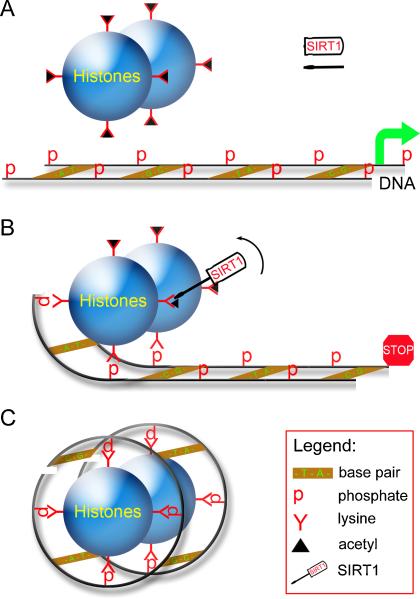
Figure 3. SIRT1 globally silences gene transcription by deacetylating histones.
(a) When SIRT1 is not active, the histones are acetylated and unable to bind DNA, thus facilitating gene transcription. (b) Once activated, SIRT1 deacetylates histones, giving them a net positive charge. Histones are then free to bind DNA at phosphate moieties and interfere with gene transcription. (c) Once histones have fully gained their positive charge upon complete deacetylation of histone lysines, DNA winds around the histones, making gene transcription unavailable and leading to genome-wide gene silencing.
Reducing metabolic and energy requirements appears to be a common strategy for cells and organisms to survive through unfavorable conditions, such as calorie restriction, hypothermia, or hibernation. Coincidentally, these measures also induce neuronal tolerance, and protect against neuronal injuries induced by ischemic stroke (Frerichs and Hallenbeck, 1998; Kitagawa et al., 1990; Maier et al., 1998; Paschen et al., 2007; Yu and Mattson, 1999), AD (Patel et al., 2005; Wang et al., 2005a), and PD (Duan and Mattson, 1999). Together, these results imply that SIRT1-mediated transcription inhibition could contribute to reducing energy requirements for neuroprotection (Morris et al., 2011).
5.1.2 Transcriptional coactivators
Sirtuins may also confer neuroprotection via a mechanism similar to ischemic tolerance (Kitagawa et al., 1990; Stenzel-Poore et al., 2003). One way for neurons to gain tolerance is to reprogram their gene expression in order to upregulate pro-survival proteins, downregulate pro-apoptotic proteins, and decrease overall energy requirements while concomitantly increasing energy metabolism efficiency (Frerichs and Hallenbeck, 1998; Paschen et al., 2007; Stenzel-Poore et al., 2003). Sirtuins are able to make such contributions via deacetylating a set of proteins, including transcriptional coactivators, transcription factors, and nuclear receptors. This is summarized in Figure 4 and discussed in the next three sections.
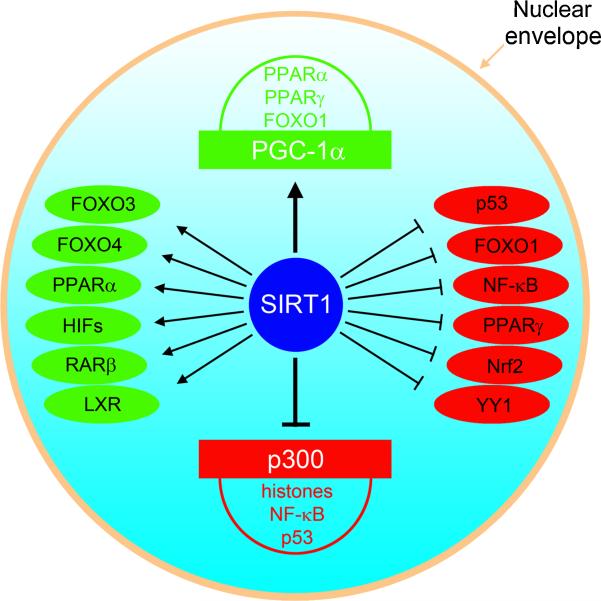
Figure 4. SIRT1 regulates gene transcriptions at a subgenomic level.
SIRT1 deacetylates two transcriptional coactivators, PGC-1α and p300, and more than a dozen transcription factors. By deacetylating and stimulating PGC-1α, SIRT1 promotes the transcriptional activity of FOXO1, PPARα, and PPARγ. By deacetylating and inhibiting p300, SIRT1 reduces the transcriptional activity of p53 and NF-κB. SIRT1 also increases the deacetylation of histones via p300. In addition, SIRT1 directly increases transcriptional activity of FOXO3, FOXO4, PPARα, HIF1, RARβ and LXR via deacetylation; and it decreases transcriptional activity of p53, NF-κB, FOXO1, YY1, Nrf2 and PPARγ. Transcriptional coactivators and transcription factors depicted in green are activated by SIRT1, while those depicted in red are inhibited by SIRT1.
5.1.2.1 Inhibition
In addition to genome-wide gene silencing, SIRT1 also suppresses gene transcription at the subgenomic level by inhibiting a transcriptional coactivator. In contrast to transcription factors, coactivators do not have DNA-binding motifs, but they can affect the transcriptional activity of several specific transcription factors. P300 is one such coactivator (Goodman and Smolik, 2000). It has built-in histone acetyltransferase activity through which p300 neutralizes histones and therefore relaxes DNA, promoting the transcription of many transcription factors, including p53 and NF-κB (Goodman and Smolik, 2000; Iyer et al., 2004). A recent study showed that SIRT1 physically interacts with p300, deacetylates it at Lys1020 and 1024, and consequently inhibits the transcriptional activity of p300 (Bouras et al., 2005). Additionally, SIRT2 also deacetylates and inhibits p300 (Black et al., 2008). Considering that knockout of p300 increases neuronal resistance to amyloid toxicity in mice (Duclot et al., 2010), the inhibitory effect of SIRT1 and SIRT2 on p300 could also be protective, although direct evidence has not been reported.
5.1.2.2 Activation
Although SIRT1 suppresses a wide range of gene transcription, it can also increase the transcription of a specific group of genes. This is achieved via activating PGC-1α, a transcriptional coactivator, as SIRT1 physically interacts with PGC-1α to increase its activity (Nemoto et al., 2005; Rodgers et al., 2005). PGC-1α was initially reported to increase the transcriptional activities of two transcription factors—PPARγ and thyroid hormone receptor (Puigserver et al., 1998)—increasing mitochondrial energy metabolism and biogenesis (Lin, 2009; Nemoto et al., 2005; Rodgers et al., 2005). Currently, its targets have extended to PPARα (Vega et al., 2000), hepatic nuclear factor-4α (HNF-4α) (Yoon et al., 2001), and FOXO1 (Puigserver et al., 2003).
Most of the aforementioned transcription factors are neuronal, such as the PPARs and FOXO1, and their activation leads to neuroprotection (Bordet et al., 2006; Luo et al., 2006; Mysiorek et al., 2009; Zhan et al., 2010). For example, PPARα reduces the expression of matrix metalloproteinase-9 (MMP-9) (Cheng et al., 2009), and protects endothelial cells against ischemic injury, thus maintaining the integrity of the blood-brain barrier (Mysiorek et al., 2009). PPARγ possesses both anti-apoptotic and anti-inflammatory functions, and reduces brain damage induced by a number of diseases, including stroke, AD, and PD (Bordet et al., 2006; Luo et al., 2006). Lastly, FOXO1 is reported to mediate ischemic tolerance against global cerebral ischemia in rats (Zhan et al., 2010).
5.1.3 Transcription factors
5.1.3.1 Inhibition
5.1.3.1.1 P53
As the first-known non-histone substrate of SIRT1, p53 plays a detrimental role in most neurological disorders because it is responsible for the upregulation of a number of pro-apoptotic molecules, especially the BH3-only members of the Bcl-2 family (Hong et al., 2010; Yi and Luo, 2010). The transcriptional activity of p53 increases when it is acetylated at its multiple lysine residues; conversely, its activity is suppressed when deacetylated. SIRT1 deacetylates p53 and reduces its transcriptional activity (Luo et al., 2001; Vaziri et al., 2001), which could be one of the mechanisms of SIRT1-mediated neuroprotection. In fact, it is reported that SIRT1 protects neurons in models of AD (Karuppagounder et al., 2009; Kim et al., 2007a), amyotrophic lateral sclerosis in mice (Kim et al., 2007a), and PD in midbrain slice (Okawara et al., 2007) via the deacetylation and inhibition of p53.
5.1.3.1.2 NF-κB
NF-κB is the major transcription factor that transcribes pro-inflammatory mediators in the nervous system, and its activation exacerbates neuronal damage after neurological insults, especially ischemic stroke (Pizzi et al., 2009; Teng and Tang, 2010; Zheng and Yenari, 2004). SIRT1 deacetylates the p65 subunit of NF-κB at Lys310 and reduces its transcriptional activity, protecting cells from apoptosis (Yeung et al., 2004). In a mixed culture of neurons and microglia, SIRT1 deacetylates NF-κB signaling in microglia, and protects neurons from Aβ toxicity (Chen et al., 2005b). In addition, the inhibition of NF-κB by SIRT1 also contributes to the neuroprotection afforded by Ginkgo extract or glucoside against AD and ischemia (Longpre et al., 2006; Wang et al., 2009c).
5.1.3.1.3 Yin Yang 1
MicroRNAs are negative post-transcriptional mediators that function in gene silencing. The miR-134 is one such short sequence. One of its targets is CREB, and the transcription of miR-134 itself is controlled by the transcription factor Yin Yang 1 (YY1) (Gao et al., 2010). The level of miR-134 is low in brain under normal conditions, because SIRT1 forms a repressor complex with YY1, which binds to the promoter of miR-143 and inhibits its transcription (Gao et al., 2010). In brain-specific SIRT1 knockout mice, however, miR-134 is upregulated in the hippocampus, and this is accompanied by decreased levels of CREB and brain-derived neurotrophic factor (BDNF), leading to impaired synaptic plasticity (Gao et al., 2010). This finding is interesting in that SIRT1 helps maintain the levels of CREB and BDNF, two molecules with established neuroprotective function, implying that this may be one of the mechanisms underlying the neuroprotection of SIRT1.
5.1.3.1.4 Nuclear factor (erythroid-derived 2)-like 2
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is a key transcription factor in the regulation of xenobiotic and oxidative stresses. The target genes of Nrf2 are collectively called phase II enzymes; these include heme oxygenase 1, NADH quinone oxidoreductase, and glutathione synthase and transferase, among others. Nrf2 activation is generally cytoprotective (Li et al., 2007a). When overactivated, however, it may be carcinogenic (Kensler and Wakabayashi, 2010). In an immortalized cell line of human leukemia, Nrf2 is acetylated at Lys591, which increases its nuclear retention and transcription activity. Conversely, SIRT1 deacetylates Nrf2 and reduces its gene transcription (Kawai et al., 2011). Considering that Nrf2 plays a protective role in brain (Li et al., 2007a), it is of interest to test whether the findings in tumor cells are translatable to neurons. This will help elucidate the role of the interaction between SIRT1 and Nrf2 in brain.
5.1.3.1.5 FOXO1
SIRT1 is reported to bind and deacetylate FOXO1, causing a reduction in its transcriptional activity (Yang et al., 2005). However, it remains unclear whether its deacetylation is neuroprotective. FOXO1 is also modified by phosphorylation, with opposite effects mediated by different kinases. For instance, when phosphorylated by Akt, FOXO1 demonstrates a neuroprotective effect against ischemic neuronal injury; when phosphorylated by mammalian sterile 20-like kinase 1 (MST1), it promotes the apoptosis of cerebellar granule neurons upon the withdrawal of growth factors (Yuan et al., 2009). Notably, these findings were determined in different model systems, and further research is necessary to fully characterize the effect of deactylated FOXO1 on neuronal viability.
5.1.3.2 Activation
5.1.3.2.1 FOXO3
SIRT1 deacetylates FOXO3 and increases the cellular resistance to oxidative stress in HEK293 cells (Brunet et al., 2004). In addition, SIRT2 also deacetylates FOXO3 and increases its transcription after calorie restriction in mice and hydrogen peroxide treatment of fat and kidney cells, reducing cellular levels of ROS (Wang et al., 2007a). Motta and colleagues also reported that SIRT1 deacetylates FOXO3; however, they show that SIRT1 inhibits the activity of FOXO3 in HeLa cells (Motta et al., 2004). In brain, overexpression of nuclear-targeted FOXO3 protects motor neurons from apoptosis induced by mutant SOD1 or polyQ-expanded androgen receptor (Mojsilovic-Petrovic et al., 2009). Knockdown of FOXO3 increases neuronal apoptosis during zebrafish development (Peng et al., 2010), indicating a neuroprotective role of FOXO3. However, this is not a universal finding. For example, it is reported that FOXO3 promotes neuronal apoptosis after overexpression (Gilley et al., 2003) or due to activation by the withdrawal of neurotrophic factors (Barthelemy et al., 2004). More studies are therefore needed to clarify the role of SIRT1 on FOXO3 activity, and to determine the overall effects of the SIRT1-FOXO3 interaction on neuronal fate.
5.1.3.2.2 FOXO4
In HEK293 cells, FOXO4 is acetylated after hydrogen peroxide treatment, leading to decreased transcriptional activity (Kobayashi et al., 2005; van der Horst et al., 2004). In contrast, its deacetylation by SIRT1 leads to increased transcriptional activity of FOXO4, increases cellular resistance against oxidative stress and promotes survival (Kobayashi et al., 2005; van der Horst et al., 2004). Although it is modestly expressed in the brain (Furuyama et al., 2000), the role of FOXO4 in neuroprotection remains unknown.
5.1.3.2.3 HIFs
Hypoxia-inducible factors (HIFs) are a family of transcription factors that also function as oxygen sensors. Once activated, HIFs upregulate proteins involved in oxygen transport, angiogenesis, cell survival, and glycolysis. Some more notable proteins in these categories include the neuroprotective proteins erythropoietin (EPO) (Sakanaka et al., 1998; Zhang et al., 2006; Zhang et al., 2010) and vascular endothelial growth factor (VEGF) (Wang et al., 2007b; Wick et al., 2002). The HIFs thus have protective roles against hypoxia and ischemia (Correia and Moreira, 2010). SIRT1 was first linked with HIF activity in hepatoma cells, where HIF2 was acetylated at its C-terminal during hypoxia, leading to decreased transcriptional activity of HIF2 (Dioum et al., 2009). SIRT1 activation reverses the acetylation of HIF2, and increases its transcription and EPO production (Dioum et al., 2009). In HEK293 cells, SIRT1 binds to HIF-1 and deacetylates it at Lys674, blocking its association with the transcriptional coactivator, p300 (Lim et al., 2010). Hypoxia suppresses SIRT1 activity due to NAD+ insufficiency, leading to the activation of HIF1 (Lim et al., 2010). Currently, no reports are available showing if or how SIRT1 can protect the brain via the HIF pathways.
5.1.4 Nuclear receptors
Unlike membrane receptors, nuclear receptors are a group of proteins that localize to the cytosol or nucleus without association with the cell membrane. Once bound by their ligands, such as some hormones and fatty acids, nuclear receptors change their conformational structures, translocate to nucleus and directly bind DNA to regulate gene expression. Therefore, nuclear factors are also considered transcription factors. The transcription efficiency of nuclear receptors is adjusted by acetylation and deacetylation. SIRT1 is one of the enzymes that can deacetylate some nuclear receptors. Below is a discussion of the known nuclear receptor targets of SIRT1.
5.1.4.1 Retinoic acid receptors
One type of nuclear receptor targeted by SIRT1 is the retinoic acid receptor (RAR). RAR has three isoforms, RAR-α, RAR-β, and RAR-γ, and all have retinoic acid as a ligand. A recent report demonstrates that SIRT1 directly binds to and deacetylates the β isoform of RAR, which is the predominant isoform in the brain (Donmez et al., 2010). The deacetylation of RAR-β increases its transcription of α-secretase, the secretase that is involved in the cleavage of APP to generate soluble APP. Simultaneously, it reduces the production of toxic Aβ (Donmez et al., 2010). Thus, it may play a role in reducing the pathogenesis of AD.
5.1.4.2 Liver X receptors
Liver X receptor (LXR) is a group of nuclear receptors with oxysterols as ligands. They are expressed in the brain, and demonstrate protective roles against cerebral ischemia, traumatic brain injury, and amyloid toxicity (Cheng et al., 2010; Fitz et al., 2010). LXR is acetylated at Lys432, blunting its response to agonists in liver (Li et al., 2007d). SIRT1 deacetylates LXR, and SIRT1 knockout increases its acetylation but reduces the expression of its target genes (Li et al., 2007d), suggesting SIRT1 may protect brain via enhancing LXR activity.
5.1.4.3 Peroxisome proliferator-activated receptors
Peroxisome proliferator-activated receptors (PPARs), including PPARα, PPARβ, and PPARγ, are another group of nuclear receptors whose activities are regulated by SIRT1 (Picard et al., 2004; Purushotham et al., 2009). Activation of PPARα by a two-week treatment of fenofibrate, a PPARα activator, decreases infarct volume in mice via anti-oxidant and anti-inflammatory mechanisms (Deplanque et al., 2003). In PPARα-deficient mice, however, the neuroprotection is blocked (Deplanque et al., 2003), indicating a beneficial effect of PPARα in brain. In hepatocytes, SIRT1 physically interacts with PPARα at several sites, and this increases the transcriptional activity of PPARα (Deplanque et al., 2003).
PPARγ is activated by thiazolidinedione and 15-deoxy-delta 12,14-Prostaglandin J2. When this happens, PPARγ negatively regulates the expression of some inflammatory cytokines and macrophage activation. Accordingly, PPARγ shows protective effects against neuronal injuries in a number of neurological diseases such as stroke, Alzheimer’s disease, and traumatic brain injury (Kapadia et al., 2008; Luo et al., 2006; Shie et al., 2009). In adipocytes, SIRT1 suppressed the transactivation of PPARγ by blocking its binding to DNA sequences of target genes (Picard et al., 2004). SIRT1 also directly interacts with and deacetylates PPARγ (Han et al., 2010), though the influence of deacetylation on the activity of PPARγ is not clear.
Thus far, all the mechanisms we have discussed are related to the transcriptional regulation of sirtuins. This is not an exhaustive list of the capabilities of sirtuins as they also deacetylate other proteins not involved in gene expression, including DNA repair enzymes in the nucleus, protein kinases and phosphatases in cytosol, and mitochondrial proteins.
5.1.5 DNA repair enzymes
5.1.5.1 Base excision repair
DNA damage occurs in daily life and is aggravated following metabolic and oxidative stresses. Accordingly, DNA repair is essential to maintenance of genomic integrity and cellular viability. The severity of DNA damage varies from single base damage to double-stranded DNA breaks. Base excision repair (BER) is the process of repairing single base damage that is predominantly due to oxidative DNA damage (Parsons et al., 2004; Srivastava et al., 1998). Apurinic/apyrimidinic endonuclease-1 (APE1) is one of the key enzymes in the base excision repair (BER) pathway; its function is to cleave the apurinic/apyrimidinic sites (Parsons et al., 2004; Srivastava et al., 1998). Our previous studies demonstrate that APE1 contributes to inducible DNA repair after ischemic preconditioning (Li et al., 2005) and to the neuroprotective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) (Stetler et al., 2010). We also show that NAD+ treatment protects cultured neurons against ischemic injury via enhancing the BER pathway (Wang et al., 2008).
Recent studies show that multiple lysines of APE1 are acetylated in the N-terminus of mammalian APE1, which is associated with decreased enzymatic activity (Fantini et al., 2010; Yamamori et al., 2010). Correspondingly, overexpression of SIRT1 or resveratrol treatment deacetylates APE1 by increasing its binding to the enzyme and thus increases APE enzyme activity, while knockdown of SIRT1 increases cellular abasic DNA content and sensitizes cells to death after genotoxic and oxidative stresses (Yamamori et al., 2010). These results suggest that deacetylation of APE1 to increase DNA repair could be one of the protective mechanisms of SIRT1 in the brain.
5.1.5.2 Nucleotide excision repair
Xeroderma pigmentosum (XP) is a genetic disease with seven subtypes and is characterized by causing skin cancers. The external cause of the carcinogenesis is the exposure to ultraviolet (UV) light, but the intrinsic reason is the deficiency in DNA repair, in particular, the nucleotide excision repair (NER) pathway (Taylor, 2008). The NER pathway recognizes and repairs multiple-base damages, especially thymine dimmers caused by UV exposure. Seven genes are involved in the NER pathway, named xeroderma pigmentosum A to G (XPA to XPG), and mutation in each of the XP genes causes a specific type of xeroderma pigmentosum (Taylor, 2008). Among the seven genes, XPA and XPC are more frequently mutated than others. In addition to skin cancers, neurodegeneration also occurs in patients with xeroderma pigmentosum, manifested by cognitive and neurological impairments and brain atrophy (Anttinen et al., 2008). Overall, this suggests that the NER pathway may play an important role in the well-being of the brain.
Recent studies demonstrate that SIRT1 enhances the function of the NER pathway (Fan and Luo, 2010; Ming et al., 2010). For instance, SIRT1 directly interacts with XPA and deacetylates it at Lys63 and Lys67 in HEK293 cells. Deacetylated XPA demonstrates an increased binding affinity to its partner, replication protein A32, and is necessary for optimal DNA repair activity (Fan and Luo, 2010). SIRT1 also upregulates the expression of XPC, which is required for DNA repair after UV exposure in mouse embryonic fibroblasts and keratinocytes. Lastly, loss of SIRT1 leads to DNA repair inhibition and apoptotic cell death (Ming et al., 2010). Though the NER pathway may be involved with DNA repair in the brain, its role in providing neuroprotection against neurologic diseases is currently unknown.
5.1.5.3 Single-stranded DNA breaks
SIRT1 has a role in the repair of more severe DNA damage, such as single-stranded DNA breaks. This type of repair requires several enzymes, one of which is poly(ADP-ribose) polymerase 1 (PARP-1) (Woodhouse et al., 2008). PARP-1 binds the DNA breaks and transfers ADP-ribose units from the metabolism of NAD+ to its substrates, a process known as ADP-ribosylation. When overactivated, however, PARP-1 is fatal to host cells due to the severe depletion of NAD+ and consequent release and translocation of apoptosis-inducing factor (AIF) from mitochondria to the nucleus (Moroni, 2008). In neurons, PARP-1 is highly activated following cerebral ischemia and PD, contributing to neuronal apoptosis, and inhibition of PARP-1 protects brain from neuronal death (Moroni, 2008; Outeiro et al., 2007a; Zhang et al., 2005). In cardiomyocytes, PARP-1 is acetylated after physical stress, an indicator of increased enzyme activity, and the acetylation is further enhanced in SIRT1 knockout mice (Rajamohan et al., 2009). SIRT1 directly binds and deacetylates PARP-1 in a SIRT1 catalytic site-dependent manner, which consequently reduces the enzyme activity of PARP-1 and ultimately promotes cell survival (Kolthur-Seetharam et al., 2006; Rajamohan et al., 2009). Experiments are currently underway to determine if the SIRT1-PARP-1 interaction plays a role in NAD+-mediated neuroprotection following excitotoxic insults in neurons.
5.1.5.4 Double-stranded DNA breaks
Ku70 is a 70-kDa nuclear protein involved in the repair of double-strand DNA breaks by binding to the break ends. It also has a distinct anti-apoptotic role, via binding Bax and inhibiting its mitochondrial translocation (Sawada et al., 2003). Following cerebral ischemia in mice, levels of Ku70 are decreased as early as 4 hours in the ischemic area and remain low thereafter. This is accompanied by DNA fragmentation and neuronal death (Kim et al., 2001). On the other hand, ischemic preconditioning upregulates the expression of Ku70 in the CA1 region of rat hippocampus, indicating that Ku70 plays a protective role against stroke (Sugawara et al., 2001). It is reported that SIRT1 deacetylates Ku70 on Lys539 and Lys543 of its C-terminus (Cohen et al., 2004), and increases its DNA repair activity after cellular exposure to radiation (Jeong et al., 2007).
Nijmegen breakage syndrome protein 1 (NBS1), also known as nabrin or p95, is another protein with a role in the repair of double-strand DNA breaks. It is an early sensor of DNA breaks, and its mutation results in Nijmegen breakage syndrome, a rare autosomal recessive disease in humans characterized by microcephaly, growth retardation, and radiation sensitivity (Varon et al., 1998). In HEK293 cells, NBS1 is acetylated at 10 of its 70 lysine residues, leading to decreased activity (Yuan et al., 2007). SIRT1 directly interacts with and deacetylates NBS1, increasing its activity, and this increases cell resistance to radiation challenge (Yuan et al., 2007), indicating a protective role of SIRT1. Cumulatively, current data suggest that SIRT1 enhances DNA repair activity via several mechanisms, and this may contribute to its neuroprotective effects against neurological diseases.
In addition to SIRT1, SIRT6 also plays a role in the repair of DNA double-strand breaks. Following oxidative stress in human fibroblast cell lines, SIRT6 is recruited to the DNA breaks; it then interact with PARP1 on Lys-521 and enhances PARP1 acitivity of DNA repair (Mao et al., 2011). However, it needs further investigated whether these findings apply in brain.
5.2 Cytosolic targets
5.2.1 Protein kinases and phosphatases
Protein activation and inactivation are governed by a number of post-translational modifications. Phosphorylation is a predominant means for mediating a protein’s activity status, and this is balanced by protein kinases that phosphorylate a protein and phosphatases that remove the phosphate. In addition to themselves being regulated by phosphorylation, kinases and phosphatases can also be regulated by acetylation and deacetylation.
One example is serine/threonine kinase 11 (STK11 or LKB1), the primary kinase that phosphorylates and activates AMP-activated protein kinase (AMPK) (Lan et al., 2008; Shaw et al., 2004; Zu et al., 2010). LKB1 is known to be acetylated at Lys48 in HEK293 cells, which decreases its enzymatic activity (Lan et al., 2008). Recent studies show that LKB1 physically binds to SIRT1, resulting in deacetylation (Lan et al., 2008; Wang et al., 2011; Zu et al., 2010). The effects of LKB1 deacetylation, however, are variable in different cell types. In porcine endothelial cells, deacetylation of LKB1 stimulates its proteasomal degradation and reduces phosphorylation of AMPK and endothelial senescence (Zu et al., 2010). In most cases, however, SIRT1-mediated deacetylation enhances the activity of LKB1. For instance, SIRT1 increases the activity of LKB1 in HEK293 cells, rat hepatocytes, and mouse neurons (Lan et al., 2008; Wang et al., 2011). This notion is further supported by a study showing that resveratrol stimulates AMPK activity in neurons (Dasgupta and Milbrandt, 2007). Furthermore, the overexpression of NAMPT, the rate-limiting enzyme of the NAD+ salvage pathway, increases SIRT1 activity and LKB1 deacetylation in mouse brain, which is related to a 2-3-fold amplification of AMPK activity (Wang et al., 2011). Following stroke, NAMPT transgenic mice develop a smaller infarct volume compared with wild-type mice. The protective effects disappear when SIRT1 or AMPK is knocked out (Wang et al., 2011), indicating a neuroprotective role of LKB1 deacetylation by SIRT1. It should be noted that the role of AMPK is controversial. As discussed here, AMPK is necessary for the protective effect of the MAMPT/LKB1 pathway against stroke (Wang et al., 2011); however, a previous study showed that AMPK activation was detrimental to the brain following cerebral ischemia (Li et al., 2007b).
Another example is the phosphatase and tensin homologue deleted in chromosome 10 (PTEN). This phosphatase primarily targets phosphatidylinositol (3,4,5)-trisphosphate (PIP3), therefore inhibiting the neuroprotective Akt signaling pathway. Multiple lysines of PTEN are acetylated by histone acetyltransferases, including Lys125 and Lys128 in its catalytic motif (Okumura et al., 2006). The acetylation at the catalytic motif inhibits the enzymatic activity of PTEN (Okumura et al., 2006). Acetylation also occurs at Lys402 at the PDZ domain of PTEN, enhancing its binding ability to PDZ domains of other proteins; however, it is unclear how the acetylation affects the activity of PTEN (Ikenoue et al., 2008). SIRT1 is reported to deacetylate PTEN at Lys402 and hinder the interaction of PTEN with its binding partners (Ikenoue et al., 2008). Similarly in mouse embryonic stem cells, SIRT1 deficiency leads to increased levels of acetylated PTEN, which is associated with apoptosis in response to oxidative stress (Chae and Broxmeyer, 2011). However, it has not been determined whether SIRT1 can also deacetylate PTEN in neurons, and if this action will be neuroprotective.
5.2.2 Endothelial nitric oxide synthase
Endothelial nitric oxide synthase (eNOS) plays an important role in the generation of endothelial nitric oxide (NO) and vasodilatation. In the brain, the activity of eNOS is uncoupled following subarachnoid hemorrhage (Sabri et al., 2011), and knockout or inhibition of eNOS exacerbates ischemic brain damage in mice (Atochin et al., 2007; Huang et al., 1996), indicating a protective role of eNOS. The activity of eNOS can be augmented by SIRT1 (Mattagajasingh et al., 2007). In endothelial cells, SIRT1 coprecipitates with and deacetylates eNOS at Lys496 and 506, and this stimulates eNOS activity and NO production, leading to vasodilatation (Mattagajasingh et al., 2007). It is also reported that the SIRT1 activator resveratrol reduces infarct volume in rat via an eNOS-dependent mechanism (Tsai et al., 2007). Therefore, it is likely that SIRT1 protects against ischemic and hemorrhagic strokes via eNOS-mediated blood flow improvement.
5.2.3 Tau protein
Tau is a microtubule-binding protein enriched in neurons, and it promotes the assembly of microtubules and then stabilizes them (Kar et al., 2003). These tau-related functions are compromised when tau is phosphorylated, leading to disruption of the microtubule structure. The accumulation of hyperphosphorylated tau in neurons is a hallmark of neurodegenerative tauopathies, especially in AD (Ittner and Götz, 2011; Min et al., 2010). It has been reported that tau is acetylated at multiple lysine residues and that SIRT1 is able to regulate the level of phosphorylated tau via deacetylation (Min et al., 2010). In supportive of this notion, SIRT1 directly interacts with tau and a deficiency in SIRT1 elevates both acetylated-tau and phosphorylated-tau in vivo. Moreover, inhibition of SIRT1 activity blocks tau polyubiquitination and thus the turnover of tau, resulting in accumulation of phosphorylated-tau in neurons (Min et al., 2010).
The results cited in the previous three sections indicate that, in addition to functioning in the nucleus, SIRT1 also functions in the cytosol (Figure 5), which is consistent with previous reports that SIRT1 has a cytosolic signaling sequence (Jin et al., 2007; Tanno et al., 2007).
Figure 5. Cytosolic targets of SIRT1.
In addition to its numerous actions in the nucleus, SIRT1 also has some newly discovered cytosolic targets. SIRT1 deacetylates and activates LKB1, leading to increased AMPK activity and attenuating ischemic brain injury. SIRT1 activity can also inhibit enzyme activity. Deacetylation of PTEN by SIRT1 inhibits the phosphatase’s ability to bind and dephosphorylate PIP3, and results in unhindered activity of the neuroprotective PI3K/Akt signaling pathway. Deacetylation of eNOS stimulates the production of NO, a potent vasodilator. Putatively, this improves cerebral perfusion following ischemic stroke or subarachnoid hemorrhage. Finally, SIRT1 also deacetylates phosphorylated tau, facilitating its degradation and reducing neuronal death in models of Alzheimer’s disease.
5.3 Mitochondrial targets
Mitochondria are not only the powerhouse for ATP production but also the main sites where ROS are generated and the intrinsic apoptotic signaling pathway is initiated (Niizuma et al., 2010; Zhang et al., 2004). The functions of mitochondrial proteins are altered when they are deacetylated by NAD+-dependent mitochondrial deacetylases, including SIRT3, SIRT4, and SIRT5. All mitochondrial sirtuins are present in the mitochondrial matrix, but SIRT5 also appears in the intermembrane space (Nakamura et al., 2008; Schlicker et al., 2008). Since mitochondria contain their own DNA, transcription factors, histone-like proteins, and protein synthesis systems, mitochondrial sirtuins deacetlyate a set of targets within the mitochondria that are distinctive from nuclear proteins (Garrido et al., 2003; Katherine et al., 2010; Kutsyi et al., 2005). Although the precise mechanistic information is still lacking, evidence is emerging to suggest that mitochondrial sirtuins are protective against oxidative stress and excitotoxic injury (Fritz et al., 2011; Kim et al., 2011). The sections described below summarize the earlier studies that examined the role of mitochondrial sirtuins in energy metabolism, protection against oxidative stress or excitotoxicity, apoptosis, and mitochondrial protein synthesis.
5.3.1 Energy metabolism
5.3.1.1 Fuel switch
Glucose is the major energy source for cells. When its availability is limited, however, alternative fuels become increasingly important for cell survival. The first step of glycolysis is the conversion of glucose to glucose-6-phosphate, a reaction catalyzed by hexokinases. In tumor cells, hexokinase II is attached to the mitochondrial outer membrane, leading to increased ATP access and enzymatic activity (Mathupala et al., 2009). When glucose is replaced by galactose in culture medium, hexokinase II disassociates from the mitochondria in tumor cells, causing decreased activity toward glucose. Alternatively, the cells consume galactose (Shulga et al., 2010). It is reported that SIRT3 deacetylates cyclophilin D (Hafner et al., 2010; Shulga et al., 2010), which leads to the dissociation of hexokinase II and mitochondria, decreases glucose metabolism, and stimulates oxidative phosphorylation (Shulga et al., 2010).
At the mitochondrial level, the main energy source is pyruvate, a product of glycolysis. Alternatively, mitochondria also burn fatty acids, amino acids, and acetates when there is a deficiency in pyruvate. In the case of fatty acid catabolism, long-chain acyl coenzyme A dehydrogenase (LCAD) is a key enzyme that breaks down fatty acids and generates acetyl-CoA, stimulating β-oxidation. In SIRT3 knockout mice, LCAD is hyperacetylated at Lys42, leading to decreases in enzymatic activity, β-oxidation, and ATP level (Hirschey et al., 2010). Interestingly, these mice do not tolerate cold exposure during fasting (Hirschey et al., 2010). SIRT3 directly deacetylates LACD at Lys42 and increases LACD activity (Hirschey et al., 2010). In addition, SIRT3 may promote β-oxidation via multiple mechanisms, such as by deacetylating other β-oxidation enzymes, including the short-chain L-3-hydroxyacyl-CoA dehydrogenase and the very-long-chain acyl coenzyme A dehydrogenase (Hallows et al., 2011), facilitating mitochondrial adaptation to fuel changes.
Amino acids can also be used as fuels. Glutamate dehydrogenase (GDH) is a key enzyme for amino acid catabolism as it converts glutamate to α-ketoglutarate, and α-ketoglutarate can directly enter the Krebs cycle. SIRT3 interacts with and deacetylates GDH in mice, and might increase its activity (Cimen et al., 2009; Lombard et al., 2007; Schlicker et al., 2008). On the other hand, SIRT4 suppresses GDH via ADP-ribosylation in pancreatic β-cells (Haigis et al., 2006). A by-product of amino acid breakdown is ammonia, which is toxic to mammals as an elevated ammonia level causes neurologic disorders due to encephalopathy and coma. Therefore, ammonia is largely converted to urea through the five-step urea cycle. Carbamoyl phosphate synthetase 1 (CPS1) catalyzes the rate-limiting and first step of the urea cycle. In SIRT5 knockout mice, blood ammonia level is increased due to the decreased activity of carbamoyl phosphate synthetase 1 (Nakagawa et al., 2009). SIRT5 deacetylates this enzyme and increases its activity during calorie restriction (Nakagawa et al., 2009; Ogura et al., 2010). Ornithine transcarbamylase (OTC) catalyzes the second reaction of the urea cycle. A recent study showed that Sirt3 directly deacetylates this enzyme and stimulates its activity (Hallows et al., 2011). Collectively, these results suggest that SIRT3 and SIRT5 may play a protective role by promoting amino acid catabolism and converting ammonia to non-toxic urea.
Derived from acetic acid and alcohol, acetate is also used as a mitochondrial fuel, though this only occurs in extreme circumstances of nutrient depletion. The initial step is for conversion of acetate to acetyl-CoA catalyzed by acetyl-CoA synthetases. Acetyl-CoA synthetase 2 is the mitochondrial form of the enzyme. Its enzymatic activity is inhibited when it is acetylated at Lys661 in mouse or Lys642 in human (Hallows et al., 2006; Schwer et al., 2006). SIRT3 deacetylates acetyl-CoA synthetase 2 and enhances its activity, leading to increased production of acetyl-CoA (Hallows et al., 2006; Schwer et al., 2006).
5.3.1.2 Krebs cycle
As discussed above, the acetyl-CoA from fatty acids and acetates can enter the Krebs cycle, and α-ketoglutarate from amino acids can also promote the Krebs cycle. These two reactions are enhanced by SIRT3 (Hallows et al., 2006; Lombard et al., 2007; Schlicker et al., 2008; Schwer et al., 2006).
Additionally, SIRT3 directly stimulates the Krebs cycle. The third step of the cycle is the conversion of 6-carbon isocitrate to 5-carbon α-ketoglutarate, a process catalyzed by isocitrate dehydrogenase 2 (IDH2). A recent study shows that SIRT3 directly deacetylates this dehydrogenase to increase its activity (Someya et al., 2010).
5.3.1.3. Electron transport and ATP synthesis
NADH dehydrogenase 1 alpha subcomplex subunit 9 (NDUFA9) is an enzyme of mitochondrial complex I which is acetylated at Lys370 (Kim et al., 2006). SIRT3 physically interacts with NDUFA9 and deacetylates it. SIRT3 knockout enhances its acetylation and reduces the activity of complex I (Ahn et al., 2008), indicating that SIRT3 is a positive regulator of complex I.
Complex II, also known as succinate dehydrogenase, is composed of four subunits, including the flavoprotein succinate dehydrogenase subunit A (SdhA). In SIRT3 knockout mice, SdhA is hyperacetylated at several lysine residues, and shows decreased activity of complex II (Cimen et al., 2009). SIRT3 overexpression reverses the acetylation of SdhA and increases complex II activity (Cimen et al., 2009), indicating that SdhA is a SIRT3 substrate, and that SIRT3 is also a positive regulator of complex II.
It is not clear whether mitochondrial sirtuins are able to affect the activities of complex III and complex IV, though it is reported that SIRT5 deacetylates cytochrome c (Yang et al., 2007a), a protein involved in electron-transfer in both complex III and IV. SIRT3 is also reported to bind the alpha subunit 1 of the F1 particle of ATP synthase (Law et al., 2009), but the function is unclear. Taken together, these results suggest that SIRT3 promotes ATP generation through enhancing several enzymes in energy metabolism. In further support of the role of SIRT3 in energy metabolism, SIRT3-knockout mice demonstrate substantial acetylation of mitochondrial proteins, and have reductions in ATP levels at baseline and during cellular stress (Ahn et al., 2008). Taken together, those findings suggest that the mitochondrial sirtuins play a role in adjusting energy metabolism when energy sources are changed.
5.3.2 Anti-oxidative proteins
Mitochondria are the major sites for the generation of the reactive oxygen species superoxide. It is also the site where the superoxide is dismuted by mitochondrial SOD, also known as SOD II or MnSOD. Recent reports show that SIRT3 deacetylates MnSOD at Lys122 and increases its activity, reducing oxidative and radiation stress in mice (Qiu et al., 2010; Tao et al., 2010). SIRT3 is also protective against cardiac hypertrophy via an anti-oxidative mechanism as overexpression of Sirt3 increases MnSOD level, reduces cellular ROS, and blocks cardiac hypertrophy (Sundaresan et al., 2009). In the citric acid cycle, the third step is the conversion of the 6-carbon isocitrate to 5-carbon α-ketoglutarate, with simultaneous reduction of NAD+ to NADH, and this process is catalyzed by isocitrate dehydrogenase 2. A recent study demonstrates that SIRT3 directly deacetylates this dehydrogenase and increases its activity (Someya et al., 2010). In addition to stimulation of ATP production, the deacetylation of this enzyme also increases the levels of reduced glutathione, and overexpression of SIRT3 protects HEK293 from oxidative stress and prevents age-related cochlear cell death in mice (Someya et al., 2010). Overall, this suggests anti-oxidative and neuroprotective roles of SIRT3.
5.3.3 Pro-apoptotic proteins
The mitochondrial apoptotic pathway, or intrinsic cell death pathway, plays a key role in neuronal death after cerebral ischemia and neurodegenerative disease (Azarashvili et al., 2010; Zhang et al., 2004). The cascade is initiated by the release of pro-apoptotic proteins from mitochondria (Azarashvili et al., 2010; Kinnally and Antonsson, 2007), with cytochrome c release playing a central role in the mitochondrial apoptotic pathway. SIRT5 is found in the intermembrane space (Nakamura et al., 2008; Schlicker et al., 2008), where it co-localizes with cytochrome c (Schlicker et al., 2008). An in vitro experiment demonstrates that SIRT5 also deacetylates cytochrome c (Schlicker et al., 2008). However, it is not clear how this reaction affects the release and pro-apoptotic activity of cytochrome c.
The mitochondrial permeability transition pore (mPTP) is composed of several proteins, including cyclophilin D and adenine nucleotide translocase (ANT). When open, the mPTP causes the leak of mitochondrial components, which is a key mechanism underlying neuronal death after excitotoxicity and stroke (Kinnally and Antonsson, 2007; Lemasters et al., 2009). Both cyclophilin D and ANT are acetylated and deacetylated. For example, cyclophilin D is acetylated in HeLa cells (Shulga et al., 2010), in HEK293 cells, and in mouse cardiomyocytes (Hafner et al., 2010). In the heart, Lys166 of cyclophilin D is acetylated, and SIRT3 deacetylates it to increase cell survival after cardiac stress (Hafner et al., 2010). ANT, another component of mPTP, co-immunoprecipitates with SIRT4 in pancreatic beta-cells (Ahuja et al., 2007), but its role in apoptotic regulation is not clear. Another study demonstrates that SIRT3 also deacetylates Ku70 outside the mitochondria of cardiomyocytes, which increases the binding ability of Ku70 to Bax, thus inhibiting Bax translocation to mitochondria (Sundaresan et al., 2008).
Under certain conditions, SIRT3 can be pro-apoptotic. In human epithelial tumor cells, SIRT3 is required to induce apoptosis by Bcl-2 silencing (Allison and Milner, 2007); however, SIRT3 is not necessary for cell death of normal human epithelial cells, nor is it needed for stress-induced apoptosis (Allison and Milner, 2007).
5.3.4 Protein synthesis
The mitochondrial genome encodes 13 proteins involved in the electron transport chain and ATP synthesis, and these proteins are synthesized by mitochondrial ribosomes (Jacobs and Turnbull, 2005). Mitochondrial ribosomal protein L10 (MRPL10) is a component of mitochondrial ribosomes (Yang et al., 2010). A recent study reports that MRPL10 is acetylated at several lysine residues, including Lys162 and Lys196, and is accompanied by increased protein synthesis. The increase in protein synthesis is further enhanced in SIRT3 knockout mice (Yang et al., 2010). Accordingly, SIRT3 deacetylates MRPL10, and further inhibits ribosomal function and mitochondrial protein synthesis (Yang et al., 2010).
Mitochondrial sirtuins are cytoprotective in non-neural cells; in contrast, their role in brain is less clear. However, emerging evidence suggests that SIRT3 may be cytoprotective in neural cells as well (Kim et al., 2011). In the study by Kim et al (2011), it was demonstrated that overexpression of SIRT3 protects against NMDA-induced neuronal death in mouse cortical neuron cultures whereas knockdown of SIRT3 expression exacerbates neuronal death. Nonetheless, further investigations are needed to determine the functional role of various mitochondrial sirtuins in the central nervous system
6. Conclusions and future perspectives
Over the last decade, our understanding of the biology of sirtuins has undergone vast expansion from its original description as a NAD+-dependent class III histone deacetylase that can control the life span of yeast. Of particular interest is the discovery that SIRT1 deacetylates not only histones, but also some well-known transcriptional regulators, thereby modulating a wide array of biological processes. An exciting aspect is that SIRT1 mediates neuroprotection against both acute and chronic neurological diseases. Importantly, SIRT1 activity is enhanced by small-molecule compounds; therefore, development of small-molecule activators could lead to novel therapies against neurological diseases. One of the broad protective mechanisms of sirtuins is to suppress genome-wide gene transcription via histone deacetylation. Furthermore, SIRT1 selectively suppresses genes involved in fat storage, apoptosis, and inflammation. Adding to the complexity of SIRT1-mediated cell survival, SIRT1 specifically promotes the transcription of a set of genes related to cell survival, energy metabolism, and mitochondrial biogenesis. Sirtuins thus have multifaceted mechanisms with the end goal to increase cell viability.
Although extensively studied, the biological functions of SIRT1 and other sirtuins remain only partially characterized. The new frontiers of sirtuin study will include their role in autophagy (Kume et al., 2010; Madeo et al., 2010), neurogenesis (Ichi et al., 2011; Prozorovski et al., 2008), and angiogenesis (Lim et al., 2010; Zhao et al., 2010). In terms of mechanism, there are several substantial unknowns. For example, it is not known how SIRT1 specifically increases transcription of beneficial genes while it simultaneously suppresses universal transcription. It is known that when protein synthesis is inhibited globally, select chaperone proteins such as heat shock proteins are translated either via the use of internal ribosome entry sequences (IRESs) (Jackson, 2005) or by a shunting mechanism (Yueh and Schneider, 2000). It is worthy of investigation to determine whether similar mechanisms exist for genes upregulated by SIRT1-mediated activation of transcription. Another issue is the paradoxical effects of SIRT1 on PPARγ. For example, SIRT1 directly suppresses PPARγ transcriptional activity (Picard et al., 2004), but SIRT1 also activates PGC-1α (Nemoto et al., 2005; Rodgers et al., 2005), which could increase the transcriptional activities of PPARγ (Puigserver et al., 1998).
Our current knowledge is even more deficient with regard to the other sirtuins. Like SIRT1, SIRT6 and SIRT7 are nuclear sirtuins, but their molecular targets, biological functions, and possible roles in neuroprotection are largely unknown. In regard to mitochondrial sirtuins, little is known about SIRT4 and SIRT5. Further investigation into the targets and functions of these sirtuins will help develop new strategies for protection against and recovery from common neurological diseases.
Article highlights.
Sirtuins are NAD+-dependent histone deacetylases
SIRT1 is the best characterized sirtuin expressed in neurons
SIRT1 is protective against acute and chronic neurological diseases
Deacetylation on histone and non-histone targets contributes to the protective effects of SIRT1
The activity of SIRT1 is adjustable, making it a neuroprotective target.
Table 1.
Regulation of SIRT1 levels via transcription and post-transcriptional modification
| Levels | Regulators | Roles | Results | References |
|---|---|---|---|---|
| Transcription | E2F2 | TF | ↑ | Wang, 2006 |
| FOXO1 | TF | ↑ | Xiong, 2011 | |
| FOXO3 | TF | ↑ | Nemoto, 2004 | |
| TLX | NR | ↑ | Iwahara, 2009 | |
| HIC1 | TF | ↓ | Chen, 2005c | |
| p53 | TF | ↓ | Nemoto, 2004 | |
| PPARγ | NR | ↓ | Han, 2010 | |
|
Post-
transcription |
HuR | RNA-binding proteins |
↑ |
Brennan, 2001; Abdelmohsen, 2007 |
| miR-9 | MicroRNA | ↓ | Saunders, 2010 | |
| miR-34a | MicroRNA | ↓ | Yamakuchi, 2008; Lee, 2010 | |
| miR-132 | MicroRNA | ↓ | Strum, 2009 | |
| miR-199a | MicroRNA | ↓ | Rane, 2009 | |
| miR-217 | MicroRNA | ↓ | Menghini, 2009 |
Abbreviations: NR, nuclear receptor; TF, transcription factor.
Acknowledgements
This work was supported by Special Research Funds from Chinese Ministry of Science & Technology to State Key laboratories (Y. G., M. Z., and J.C.), grants from the National Institutes of Health (NS36736, NS43802, NS45048 and NS62157 to J.C.), and the American Heart Association (10SDG2560122 to F.Z.). We thank Pat Strickler for secretarial support and Carol Culver for editorial assistance.
Abbreviations
- 4-HNE
4-Hydroxynonenal
- Aβ
beta-amyloid
- AD
Alzheimer’s disease
- ADPR
ADP-ribose
- ALS
amyotrophic lateral sclerosis
- AMPK
AMP-activated protein kinase
- APE1
apurinic/apyrimidinic endonuclease-1
- APP
amyloid precursor protein
- AROS
active regulator of SIRT1
- Bax
Bcl-2 associated X protein
- BDNF
brain-derived neurotrophic factor
- CREB
cAMP response binding protein
- DBC1
deleted in breast cancer 1
- CHK1
cycle checkpoint kinase 1
- CK2
casein kinase II
- DYRK
dual specificity tyrosine phosphorylation-regulated kinase
- E2F1
E2F transcription factor 1
- eNOS
endothelial nitric oxide synthase
- FOXO
forkhead box protein O
- HD
Huntington’s disease
- HIC1
hypermethylated in cancer 1
- HuR
Hu family of RNA-binding proteins
- IRES
internal ribosomal entry sequence
- JNK1
c-jun N-terminal kinase 1
- LXR
liver X receptor
- miR
microRNA
- MST1
mammalian sterile 20-like kinase 1
- MRPL10
mitochondrial ribosomal protein L10
- NAD+
nicotinamide adenine dinucleotide
- NADH
NAD+ reduced
- NDUFA9
NADH dehydrogenase 1 alpha subcomplex subunit 9
- NAMPT
nicotinamide phosphoribosyltransferase
- NBS1
Nijmegen breakage syndrome protein 1
- NF-κB
nuclear factor κ-light-chain-enhancer of activated B cells
- NMNAT-1
nicotinamide mononucleotide adenylyltransferase 1
- NO
nitric oxide
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- OA-ADPR
2′-O-acetyl-ADP ribose
- PARP-1
poly(ADP-ribose)polymerase 1
- PD
Parkinson’s disease
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator-1α
- PPARs
peroxisome proliferator-activated receptors
- PTEN
phosphatase and tensin homologue deleted in chromosome 10
- RAR
retinoic acid receptor
- ROS
reactive oxygen species
- SdhA
succinate dehydrogenase subunit A
- sir2
silent information regulator 2
- SIRT
sirtuin
- sirtuins
silent information regulator 2 proteins
- SODs
superoxide dismutases
- SUMO
small ubiquitin-like modifier
- Wlds
Wallerian degeneration slow
- XP
xeroderma pigmentosum
References
- Abdelmohsen K, Pullmann R, Jr., Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA, Furneaux H, Gorospe M. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell. 2007;25:543–557. doi: 10.1016/j.molcel.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshar G, Murnane JP. Characterization of a human gene with sequence homology to Saccharomyces cerevisiae SIR2. Gene. 1999;234:161–168. doi: 10.1016/s0378-1119(99)00162-6. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol. 2008;3:11–40. doi: 10.1146/annurev.pathmechdis.3.121806.154326. [DOI] [PubMed] [Google Scholar]
- Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, Verdin E. Regulation of Insulin Secretion by SIRT4, a Mitochondrial ADP-ribosyltransferase. Journal of Biological Chemistry. 2007;282:33583–33592. doi: 10.1074/jbc.M705488200. [DOI] [PubMed] [Google Scholar]
- Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, Colombo L, Manzoni C, Salmona M, Caccia S, Negro A, Forloni G. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by α-synuclein or amyloid-β (1-42) peptide. Journal of Neurochemistry. 2009;110:1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- Alcain FJ, Villalba JM. Sirtuin activators. Expert Opin Ther Pat. 2009;19:403–414. doi: 10.1517/13543770902762893. [DOI] [PubMed] [Google Scholar]
- Allison SJ, Milner J. SIRT3 is pro-apoptotic and participates in distinct basal apoptotic pathways. Cell Cycle. 2007;6:2669–2677. doi: 10.4161/cc.6.21.4866. [DOI] [PubMed] [Google Scholar]
- Alvira D, Yeste-Velasco M, Folch J, Verdaguer E, Canudas AM, Pallas M, Camins A. Comparative analysis of the effects of resveratrol in two apoptotic models: inhibition of complex I and potassium deprivation in cerebellar neurons. Neuroscience. 2007;147:746–756. doi: 10.1016/j.neuroscience.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Latorre-Esteves M, Neves AR, Lavu S, Medvedik O, Taylor C, Howitz KT, Santos H, Sinclair DA. Yeast life-span extension by calorie restriction is independent of NAD fluctuation. Science. 2003;302:2124–2126. doi: 10.1126/science.1088697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anekonda TS, Adamus G. Resveratrol prevents antibody-induced apoptotic death of retinal cells through upregulation of Sirt1 and Ku70. BMC Res Notes. 2008;1:122. doi: 10.1186/1756-0500-1-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttinen A, Koulu L, Nikoskelainen E, Portin R, Kurki T, Erkinjuntti M, Jaspers NGJ, Raams A, Green MHL, Lehmann AR, Wing JF, Arlett CF, Marttila RJ. Neurological symptoms and natural course of xeroderma pigmentosum. Brain. 2008;131:1979–1989. doi: 10.1093/brain/awn126. [DOI] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Atochin DN, Wang A, Liu VWT, Critchlow JD, Dantas APV, Looft-Wilson R, Murata T, Salomone S, Shin HK, Ayata C, Moskowitz MA, Michel T, Sessa WC, Huang PL. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. The Journal of Clinical Investigation. 2007;117:1961–1967. doi: 10.1172/JCI29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery MA, Sheehan AE, Kerr KS, Wang J, Freeman MR. Wld S requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration. J Cell Biol. 2009;184:501–513. doi: 10.1083/jcb.200808042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham Y, Davidi N, Porat M, Chernoguz D, Magen I, Vorobeiv L, Berry EM, Leker RR. Leptin reduces infarct size in association with enhanced expression of CB2, TRPV1, SIRT-1 and leptin receptor. Curr Neurovasc Res. 2010;7:136–143. doi: 10.2174/156720210791184943. [DOI] [PubMed] [Google Scholar]
- Azarashvili T, Stricker R, Reiser G. The mitochondria permeability transition pore complex in the brain with interacting proteins – promising targets for protection in neurodegenerative diseases. Biological Chemistry. 2010;391:619–629. doi: 10.1515/BC.2010.070. [DOI] [PubMed] [Google Scholar]
- Babetto E, Beirowski B, Janeckova L, Brown R, Gilley J, Thomson D, Ribchester RR, Coleman MP. Targeting NMNAT1 to Axons and Synapses Transforms Its Neuroprotective Potency In Vivo. J. Neurosci. 2010;30:13291–13304. doi: 10.1523/JNEUROSCI.1189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelemy C, Henderson CE, Pettmann B. Foxo3a induces motoneuron death through the Fas pathway in cooperation with JNK. BMC Neurosci. 2004;5:48. doi: 10.1186/1471-2202-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villen J, Li J, Cohn MA, Cantley LC, Gygi SP. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci U S A. 2004;101:12130–12135. doi: 10.1073/pnas.0404720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol. 2006;24:1285–1292. doi: 10.1038/nbt1240. [DOI] [PubMed] [Google Scholar]
- Bedalov A, Gatbonton T, Irvine WP, Gottschling DE, Simon JA. Identification of a small molecule inhibitor of Sir2p. Proc Natl Acad Sci U S A. 2001;98:15113–15118. doi: 10.1073/pnas.261574398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, Kim KW, Lu S-C, Atangan L, Wang M. Resveratrol is Not a Direct Activator of SIRT1 Enzyme Activity. Chemical Biology & Drug Design. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- Bizat N, Peyrin J-M, Haik S, Cochois V, Beaudry P, Laplanche J-L, Neri C. Neuron Dysfunction Is Induced by Prion Protein with an Insertional Mutation via a Fyn Kinase and Reversed by Sirtuin Activation in Caenorhabditis elegans. J. Neurosci. 2010;30:5394–5403. doi: 10.1523/JNEUROSCI.5831-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JC, Mosley A, Kitada T, Washburn M, Carey M. The SIRT2 Deacetylase Regulates Autoacetylation of p300. Molecular Cell. 2008;32:449–455. doi: 10.1016/j.molcel.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet R, Ouk T, Petrault O, Gele P, Gautier S, Laprais M, Deplanque D, Duriez P, Staels B, Fruchart JC, Bastide M. PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem Soc Trans. 2006;34:1341–1346. doi: 10.1042/BST0341341. [DOI] [PubMed] [Google Scholar]
- Bouras T, Fu M, Sauve AA, Wang F, Quong AA, Perkins ND, Hay RT, Gu W, Pestell RG. SIRT1 Deacetylation and Repression of p300 Involves Lysine Residues 1020/1024 within the Cell Cycle Regulatory Domain 1. Journal of Biological Chemistry. 2005;280:10264–10276. doi: 10.1074/jbc.M408748200. [DOI] [PubMed] [Google Scholar]
- Braunstein M, Rose AB, Holmes SG, Allis CD, Broach JR. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 1993;7:592–604. doi: 10.1101/gad.7.4.592. [DOI] [PubMed] [Google Scholar]
- Breitenstein A, Stein S, Holy EW, Camici GG, Lohmann C, Akhmedov A, Spescha R, Elliott PJ, Westphal CH, Matter CM, Luscher TF, Tanner FC. Sirt1 inhibition promotes in vivo arterial thrombosis and tissue factor expression in stimulated cells. Cardiovasc Res. 2010 doi: 10.1093/cvr/cvq339. [DOI] [PubMed] [Google Scholar]
- Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci. 2001;58:266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CL, Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer. 2009;9:123–128. doi: 10.1038/nrc2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Caito S, Rajendrasozhan S, Cook S, Chung S, Yao H, Friedman AE, Brookes PS, Rahman I. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J. 2010;24:3145–3159. doi: 10.1096/fj.09-151308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. Suppressing cancer: the importance of being senescent. Science. 2005;309:886–887. doi: 10.1126/science.1116801. [DOI] [PubMed] [Google Scholar]
- Chae HD, Broxmeyer HE. SIRT1 Deficiency Downregulates PTEN/JNK/FOXO1 Pathway to Block ROS-Induced Apoptosis in Mouse Embryonic Stem Cells. Stem Cells Dev. 2011 doi: 10.1089/scd.2010.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J, Yu MS, Ho YS, Wang M, Chang RC. Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity. Free Radic Biol Med. 2008;45:1019–1026. doi: 10.1016/j.freeradbiomed.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Cheadle C, Fan J, Cho-Chung YS, Werner T, Ray J, Do L, Gorospe M, Becker KG. Stability regulation of mRNA and the control of gene expression. Ann N Y Acad Sci. 2005;1058:196–204. doi: 10.1196/annals.1359.026. [DOI] [PubMed] [Google Scholar]
- Chen D, Pacal M, Wenzel P, Knoepfler PS, Leone G, Bremner R. Division and apoptosis of E2f-deficient retinal progenitors. Nature. 2009;462:925–929. doi: 10.1038/nature08544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Steele AD, Hutter G, Bruno J, Govindarajan A, Easlon E, Lin SJ, Aguzzi A, Lindquist S, Guarente L. The role of calorie restriction and SIRT1 in prion-mediated neurodegeneration. Exp Gerontol. 2008;43:1086–1093. doi: 10.1016/j.exger.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005a;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005b;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005c;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Cheng G, Zhang X, Gao D, Jiang X, Dong W. Resveratrol inhibits MMP-9 expression by up-regulating PPAR [alpha] expression in an oxygen glucose deprivation-exposed neuron model. Neuroscience Letters. 2009;451:105–108. doi: 10.1016/j.neulet.2008.12.045. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng O, Ostrowski RP, Liu W, Zhang JH. Activation of liver X receptor reduces global ischemic brain injury by reduction of nuclear factor-[kappa]B. Neuroscience. 2010;166:1101–1109. doi: 10.1016/j.neuroscience.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini CC, Escande C, Nin V, Chini EN. HDAC3 Is Negatively Regulated by the Nuclear Protein DBC1. J Biol Chem. 2010;285:40830–40837. doi: 10.1074/jbc.M110.153270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through AKT, BAD, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr Neurovasc Res. 2005;2:271–285. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Enhanced tolerance against early and late apoptotic oxidative stress in mammalian neurons through nicotinamidase and sirtuin mediated pathways. Curr Neurovasc Res. 2008;5:159–170. doi: 10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys. 2010;501:79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimen H, Han M-J, Yang Y, Tong Q, Koc H, Koc EC. Regulation of Succinate Dehydrogenase Activity by SIRT3 in Mammalian Mitochondria. Biochemistry. 2009;49:304–311. doi: 10.1021/bi901627u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C, Frye R, Ploegh H, Kessler BM, Sinclair DA. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell. 2004;13:627–638. doi: 10.1016/s1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Conforti L, Fang G, Beirowski B, Wang MS, Sorci L, Asress S, Adalbert R, Silva A, Bridge K, Huang XP, Magni G, Glass JD, Coleman MP. NAD(+) and axon degeneration revisited: Nmnat1 cannot substitute for Wld(S) to delay Wallerian degeneration. Cell Death Differ. 2007;14:116–127. doi: 10.1038/sj.cdd.4401944. [DOI] [PubMed] [Google Scholar]
- Conforti L, Tarlton A, Mack TG, Mi W, Buckmaster EA, Wagner D, Perry VH, Coleman MP. A Ufd2/D4Cole1e chimeric protein and overexpression of Rbp7 in the slow Wallerian degeneration (WldS) mouse. Proc Natl Acad Sci U S A. 2000;97:11377–11382. doi: 10.1073/pnas.97.21.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Wilbrey A, Morreale G, Janeckova L, Beirowski B, Adalbert R, Mazzola F, Di Stefano M, Hartley R, Babetto E, Smith T, Gilley J, Billington RA, Genazzani AA, Ribchester RR, Magni G, Coleman M. Wld S protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. J Cell Biol. 2009;184:491–500. doi: 10.1083/jcb.200807175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HM, Spelbrink JN. The human SIRT3 protein deacetylase is exclusively mitochondrial. Biochem J. 2008;411:279–285. doi: 10.1042/BJ20071624. [DOI] [PubMed] [Google Scholar]
- Correia SC, Moreira PI. Hypoxia-inducible factor 1: a new hope to counteract neurodegeneration? J Neurochem. 2010;112:1–12. doi: 10.1111/j.1471-4159.2009.06443.x. [DOI] [PubMed] [Google Scholar]
- Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, Perni RB, Riera TV, Szczepankiewicz B, Vlasuk GP, Stein RL. SIRT1 Activation by Small Molecules. Journal of Biological Chemistry. 2010;285:32695–32703. doi: 10.1074/jbc.M110.133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: the ‘magnificent seven’, function, metabolism and longevity. Ann Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proceedings of the National Academy of Sciences. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Murphy EA, Carmichael MD, Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1071–1077. doi: 10.1152/ajpregu.90925.2008. [DOI] [PubMed] [Google Scholar]
- de Boer VC, de Goffau MC, Arts IC, Hollman PC, Keijer J. SIRT1 stimulation by polyphenols is affected by their stability and metabolism. Mech Ageing Dev. 2006;127:618–627. doi: 10.1016/j.mad.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplanque D, Gele P, Petrault O, Six I, Furman C, Bouly M, Nion S, Dupuis B, Leys D, Fruchart JC, Cecchelli R, Staels B, Duriez P, Bordet R. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci. 2003;23:6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Antunes C, Geliang G, Liu ZW, Borok E, Nie Y, Xu AW, Souza DO, Gao Q, Diano S, Gao XB, Horvath TL. Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J Neurosci. 2010;30:11815–11825. doi: 10.1523/JNEUROSCI.2234-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Duclot F, Meffre J, Jacquet C, Gongora C, Maurice T. Mice knock out for the histone acetyltransferase p300/CREB binding protein-associated factor develop a resistance to amyloid toxicity. Neuroscience. 2010;167:850–863. doi: 10.1016/j.neuroscience.2010.02.055. [DOI] [PubMed] [Google Scholar]
- Fan W, Luo J. SIRT1 Regulates UV-Induced DNA Repair through Deacetylating XPA. Molecular Cell. 2010;39:247–258. doi: 10.1016/j.molcel.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Fantini D, Vascotto C, Marasco D, D’Ambrosio C, Romanello M, Vitagliano L, Pedone C, Poletto M, Cesaratto L, Quadrifoglio F, Scaloni A, Radicella JP, Tell G. Critical lysine residues within the overlooked N-terminal domain of human APE1 regulate its biological functions. Nucleic Acids Res. 2010;38:8239–8256. doi: 10.1093/nar/gkq691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnin MS, Donigian JR, Pavletich NP. Structure of the histone deacetylase SIRT2. Nat Struct Biol. 2001;8:621–625. doi: 10.1038/89668. [DOI] [PubMed] [Google Scholar]
- Fitz NF, Cronican A, Pham T, Fogg A, Fauq AH, Chapman R, Lefterov I, Koldamova R. Liver X Receptor Agonist Treatment Ameliorates Amyloid Pathology and Memory Deficits Caused by High-Fat Diet in APP23 Mice. J. Neurosci. 2010;30:6862–6872. doi: 10.1523/JNEUROSCI.1051-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerichs KU, Hallenbeck JM. Hibernation in Ground Squirrels Induces State and Species-Specific Tolerance to Hypoxia and Aglycemia: An In Vitro Study in Hippocampal Slices. J Cereb Blood Flow Metab. 1998;18:168–175. doi: 10.1097/00004647-199802000-00007. [DOI] [PubMed] [Google Scholar]
- Fritz KS, Galligan JJ, Smathers RL, Roede JR, Shearn CT, Reigan P, Petersen DR. 4-Hydroxynonenal inhibits SIRT3 via thiol-specific modification. Chem Res Toxicol. 2011;24:651–662. doi: 10.1021/tx100355a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J. 2000;349:629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido N, Griparic L, Jokitalo E, Wartiovaara J, van der Bliek AM, Spelbrink JN. Composition and dynamics of human mitochondrial nucleoids. Mol Biol Cell. 2003;14:1583–1596. doi: 10.1091/mbc.E02-07-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso C, Oh S, Douillard GG, Sibille E. Brain molecular aging, promotion of neurological disease and modulation by Sirtuin5 longevity gene polymorphism. Neurobiology of Disease. 2011;41:279–290. doi: 10.1016/j.nbd.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- Grozinger CM, Chao ED, Blackwell HE, Moazed D, Schreiber SL. Identification of a class of small molecule inhibitors of the sirtuin family of NAD-dependent deacetylases by phenotypic screening. J Biol Chem. 2001;276:38837–38843. doi: 10.1074/jbc.M106779200. [DOI] [PubMed] [Google Scholar]
- Guo X, Williams JG, Schug TT, Li X. DYRK1A and DYRK3 promote cell survival through phosphorylation and activation of SIRT1. J Biol Chem. 2010;285:13223–13232. doi: 10.1074/jbc.M110.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proceedings of the National Academy of Sciences. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallows WC, Yu W, Smith BC, Devires MK, Ellinger JJ, Someya S, Shortreed MR, Prolla T, Markley JL, Smith LM, Zhao S, Guan K-L, Denu JM. Sirt3 Promotes the Urea Cycle and Fatty Acid Oxidation during Dietary Restriction. Molecular Cell. 2011;41:139–149. doi: 10.1016/j.molcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Zhou R, Niu J, McNutt MA, Wang P, Tong T. SIRT1 is regulated by a PPAR{gamma}-SIRT1 negative feedback loop associated with senescence. Nucleic Acids Res. 2010;38:7458–7471. doi: 10.1093/nar/gkq609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr., Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisahara S, Chiba S, Matsumoto H, Tanno M, Yagi H, Shimohama S, Sato M, Horio Y. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc Natl Acad Sci U S A. 2008;105:15599–15604. doi: 10.1073/pnas.0800612105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C, van der Veer E, Akawi O, Pickering JG. SIRT1 markedly extends replicative lifespan if the NAD+ salvage pathway is enhanced. FEBS Lett. 2009;583:3081–3085. doi: 10.1016/j.febslet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Ho DJ, Calingasan NY, Wille E, Dumont M, Beal MF. Resveratrol protects against peripheral deficits in a mouse model of Huntington’s disease. Experimental Neurology. 2010;225:74–84. doi: 10.1016/j.expneurol.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Hong LZ, Zhao XY, Zhang HL. p53-mediated neuronal cell death in ischemic brain injury. Neurosci Bull. 2010;26:232–240. doi: 10.1007/s12264-010-1111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- Ichi S, Boshnjaku V, Shen Y-W, Mania-Farnell B, Ahlgren S, Sapru S, Mansukhani N, McLone DG, Tomita T, Mayanil CSK. Role of Pax3 acetylation in the regulation of Hes1 and Neurog2. Mol. Biol. Cell. 2011;22:503–512. doi: 10.1091/mbc.E10-06-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenoue T, Inoki K, Zhao B, Guan KL. PTEN acetylation modulates its interaction with PDZ domain. Cancer Res. 2008;68:6908–6912. doi: 10.1158/0008-5472.CAN-08-1107. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Imai S, Guarente L. Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol Sci. 2010;31:212–220. doi: 10.1016/j.tips.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner LM, Götz J. Amyloid-β and tau — a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12:67–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- Iwahara N, Hisahara S, Hayashi T, Horio Y. Transcriptional activation of NAD+-dependent protein deacetylase SIRT1 by nuclear receptor TLX. Biochemical and Biophysical Research Communications. 2009;386:671–675. doi: 10.1016/j.bbrc.2009.06.103. [DOI] [PubMed] [Google Scholar]
- Iyer NG, Ozdag H, Caldas C. p300//CBP and cancer. Oncogene. 2004;23:4225–4231. doi: 10.1038/sj.onc.1207118. [DOI] [PubMed] [Google Scholar]
- Jackson MD, Schmidt MT, Oppenheimer NJ, Denu JM. Mechanism of Nicotinamide Inhibition and Transglycosidation by Sir2 Histone/Protein Deacetylases. Journal of Biological Chemistry. 2003;278:50985–50998. doi: 10.1074/jbc.M306552200. [DOI] [PubMed] [Google Scholar]
- Jackson RJ. Alternative mechanisms of initiating translation of mammalian mRNAs. Biochem Soc Trans. 2005;33:1231–1241. doi: 10.1042/BST0331231. [DOI] [PubMed] [Google Scholar]
- Jacobs HT, Turnbull DM. Nuclear genes and mitochondrial translation: a new class of genetic disease. Trends in Genetics. 2005;21:312–314. doi: 10.1016/j.tig.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Jeong J, Juhn K, Lee H, Kim SH, Min BH, Lee KM, Cho MH, Park GH, Lee KH. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp Mol Med. 2007;39:8–13. doi: 10.1038/emm.2007.2. [DOI] [PubMed] [Google Scholar]
- Jin Q, Yan T, Ge X, Sun C, Shi X, Zhai Q. Cytoplasm-localized SIRT1 enhances apoptosis. J Cell Physiol. 2007;213:88–97. doi: 10.1002/jcp.21091. [DOI] [PubMed] [Google Scholar]
- Julien C, Tremblay C, Emond V, Lebbadi M, Salem N, Jr., Bennett DA, Calon F. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68:48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakefuda K, Fujita Y, Oyagi A, Hyakkoku K, Kojima T, Umemura K, Tsuruma K, Shimazawa M, Ito M, Nozawa Y, Hara H. Sirtuin 1 overexpression mice show a reference memory deficit, but not neuroprotection. Biochemical and Biophysical Research Communications. 2009;387:784–788. doi: 10.1016/j.bbrc.2009.07.119. [DOI] [PubMed] [Google Scholar]
- Kang H, Jung JW, Kim MK, Chung JH. CK2 is the regulator of SIRT1 substrate-binding affinity, deacetylase activity and cellular response to DNA-damage. PLoS One. 2009;4:e6611. doi: 10.1371/journal.pone.0006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar S, Fan J, Smith MJ, Goedert M, Amos LA. Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J. 2003;22:70–77. doi: 10.1093/emboj/cdg001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppagounder SS, Pinto JT, Xu H, Chen H-L, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochemistry International. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katherine BZM, Donohue JM, Everitt BA. Evidence that core histone H3 is targeted to the mitochondria in Brassica oleracea. Cell Biol Int. 2010;34:997–1003. doi: 10.1042/CBI20090281. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Garduno L, Theodore M, Yang J, Arinze IJ. Acetylation-Deacetylation of the Transcription Factor Nrf2 (nuclear factor erythroid 2-related factor 2) Regulates its Transcriptional Activity and Nucleo-cytoplasmic Localization. Journal of Biological Chemistry. 2011;286:7629–7640. doi: 10.1074/jbc.M110.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007a;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007b;28:277–290. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Kim GW, Noshita N, Sugawara T, Chan PH. Early Decrease in DNA Repair Proteins, Ku70 and Ku86, and Subsequent DNA Fragmentation After Transient Focal Cerebral Ischemia in Mice. Stroke. 2001;32:1401–1407. doi: 10.1161/01.str.32.6.1401. [DOI] [PubMed] [Google Scholar]
- Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583–586. doi: 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang X-J, Zhao Y. Substrate and Functional Diversity of Lysine Acetylation Revealed by a Proteomics Survey. Molecular Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lu HF, Alano CC. Neuronal Sirt3 protects against excitotoxic injury in mouse cortical neuron culture. PLoS One. 2011;6:e14731. doi: 10.1371/journal.pone.0014731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally K, Antonsson B. A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis. 2007;12:857–868. doi: 10.1007/s10495-007-0722-z. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K, et al. ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res. 1990;528:21–24. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, Motoyama N. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med. 2005;16:237–243. [PubMed] [Google Scholar]
- Kolthur-Seetharam U, Dantzer F, McBurney MW, de Murcia G, Sassone-Corsi P. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle. 2006;5:873–877. doi: 10.4161/cc.5.8.2690. [DOI] [PubMed] [Google Scholar]
- Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A, Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120:1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsyi MP, Gouliaeva NA, Kuznetsova EA, Gaziev AI. DNA-binding proteins of mammalian mitochondria. Mitochondrion. 2005;5:35–44. doi: 10.1016/j.mito.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci U S A. 2000;97:5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H. Axonal and neuronal pathology in multiple sclerosis: What have we learnt from animal models. Experimental Neurology. 2010;225:2–8. doi: 10.1016/j.expneurol.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Law IK, Liu L, Xu A, Lam KS, Vanhoutte PM, Che CM, Leung PT, Wang Y. Identification and characterization of proteins interacting with SIRT1 and SIRT3: implications in the anti-aging and metabolic effects of sirtuins. Proteomics. 2009;9:2444–2456. doi: 10.1002/pmic.200800738. [DOI] [PubMed] [Google Scholar]
- Lee J, Kemper JK. Controlling SIRT1 expression by microRNAs in health and metabolic disease. Aging (Albany NY) 2010;2:527–534. doi: 10.18632/aging.100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Calkins MJ, Johnson DA, Johnson JA. Role of Nrf2-dependent ARE-driven antioxidant pathway in neuroprotection. Methods Mol Biol. 2007a;399:67–78. doi: 10.1007/978-1-59745-504-6_6. [DOI] [PubMed] [Google Scholar]
- Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007b;38:2992–2999. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Luo Y, Zhang F, Signore AP, Gobbel GT, Simon RP, Chen J. Ischemic preconditioning in the rat brain enhances the repair of endogenous oxidative DNA damage by activating the base-excision repair pathway. J Cereb Blood Flow Metab. 2005;26:181–198. doi: 10.1038/sj.jcbfm.9600180. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang B, Tang J, Cao Q, Wu Y, Wu C, Guo J, Ling E-A, Liang F. Sirtuin 2, a Mammalian Homolog of Yeast Silent Information Regulator-2 Longevity Regulator, Is an Oligodendroglial Protein That Decelerates Cell Differentiation through Deacetylating {alpha}-Tubulin. J. Neurosci. 2007c;27:2606–2616. doi: 10.1523/JNEUROSCI.4181-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 Deacetylates and Positively Regulates the Nuclear Receptor LXR. Molecular Cell. 2007d;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J-H, Lee Y-M, Chun Y-S, Chen J, Kim J-E, Park J-W. Sirtuin 1 Modulates Cellular Responses to Hypoxia by Deacetylating Hypoxia-Inducible Factor 1[alpha] Molecular Cell. 2010;38:864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Lin JD. Minireview: the PGC-1 coactivator networks: chromatin-remodeling and mitochondrial energy metabolism. Mol Endocrinol. 2009;23:2–10. doi: 10.1210/me.2008-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Khokhlatchev A, Figeys D, Avruch J. Death-associated protein 4 binds MST1 and augments MST1-induced apoptosis. J Biol Chem. 2002;277:47991–48001. doi: 10.1074/jbc.M202630200. [DOI] [PubMed] [Google Scholar]
- Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem. 2005;280:21313–21320. doi: 10.1074/jbc.M413296200. [DOI] [PubMed] [Google Scholar]
- Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular Med. 2009;11:28–42. doi: 10.1007/s12017-009-8058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard DB, Alt FW, Cheng H-L, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr., Weissman S, Verdin E, Schwer B. Mammalian Sir2 Homolog SIRT3 Regulates Global Mitochondrial Lysine Acetylation. Mol. Cell. Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longpre F, Garneau P, Christen Y, Ramassamy C. Protection by EGb 761 against beta-amyloid-induced neurotoxicity: involvement of NF-kappaB, SIRT1, and MAPKs pathways and inhibition of amyloid fibril formation. Free Radic Biol Med. 2006;41:1781–1794. doi: 10.1016/j.freeradbiomed.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Luo Y, Yin W, Signore AP, Zhang F, Hong Z, Wang S, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. J Neurochem. 2006;97:435–448. doi: 10.1111/j.1471-4159.2006.03758.x. [DOI] [PubMed] [Google Scholar]
- Luthi-Carter R, Taylor DM, Pallos J, Lambert E, Amore A, Parker A, Moffitt H, Smith DL, Runne H, Gokce O, Kuhn A, Xiang Z, Maxwell MM, Reeves SA, Bates GP, Neri C, Thompson LM, Marsh JL, Kazantsev AG. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proceedings of the National Academy of Sciences. 2010;107:7927–7932. doi: 10.1073/pnas.1002924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, Wagner D, Thomson D, Gillingwater T, Court F, Conforti L, Fernando FS, Tarlton A, Andressen C, Addicks K, Magni G, Ribchester RR, Perry VH, Coleman MP. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol. 2010;12:842–846. doi: 10.1038/ncb0910-842. [DOI] [PubMed] [Google Scholar]
- Maier CM, Ahern K. v., Cheng ML, Lee JE, Yenari MA, Steinberg GK, Kirsch JR. Optimal Depth and Duration of Mild Hypothermia in a Focal Model of Transient Cerebral Ischemia : Effects on Neurologic Outcome, Infarct Size, Apoptosis, and Inflammation. Stroke. 1998;29:2171–2180. doi: 10.1161/01.str.29.10.2171. • Editorial Comment: Effects on Neurologic Outcome, Infarct Size, Apoptosis, and Inflammation. [DOI] [PubMed] [Google Scholar]
- Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A, Seluanov A, Gorbunova V. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332:1443–1446. doi: 10.1126/science.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert CD, Kim E, Gifondorwa DJ, Childers MK, Milligan CE. A single-dose resveratrol treatment in a mouse model of amyotrophic lateral sclerosis. J Med Food. 2010;13:1081–1085. doi: 10.1089/jmf.2009.0243. [DOI] [PubMed] [Google Scholar]
- Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: Cancer’s stygian link to the “Warburg effect” and a pivotal target for effective therapy. Seminars in Cancer Biology. 2009;19:17–24. doi: 10.1016/j.semcancer.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellert HS, McMahon SB. Biochemical pathways that regulate acetyltransferase and deacetylase activity in mammalian cells. Trends in Biochemical Sciences. 2009;34:571–578. doi: 10.1016/j.tibs.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G, Lauro R, Federici M. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, Meyers D, Cole PA, Ott M, Gan L. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming M, Shea CR, Guo X, Li X, Soltani K, Han W, He YY. Regulation of global genome nucleotide excision repair by SIRT1 through xeroderma pigmentosum C. Proc Natl Acad Sci U S A. 2010;107:22623–22628. doi: 10.1073/pnas.1010377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojsilovic-Petrovic J, Nedelsky N, Boccitto M, Mano I, Georgiades SN, Zhou W, Liu Y, Neve RL, Taylor JP, Driscoll M, Clardy J, Merry D, Kalb RG. FOXO3a is broadly neuroprotective in vitro and in vivo against insults implicated in motor neuron diseases. J Neurosci. 2009;29:8236–8247. doi: 10.1523/JNEUROSCI.1805-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F. Poly(ADP-ribose)polymerase 1 (PARP-1) and postischemic brain damage. Current Opinion in Pharmacology. 2008;8:96–103. doi: 10.1016/j.coph.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Morris KC, Lin HW, Thompson JW, Perez-Pinzon MA. Pathways for ischemic cytoprotection: Role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab. 2011 doi: 10.1038/jcbfm.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Mysiorek C, Culot M, Dehouck L, Derudas B, Staels B, Bordet R, Cecchelli R, Fenart L, Berezowski V. Peroxisome-proliferator-activated receptor-alpha activation protects brain capillary endothelial cells from oxygen-glucose deprivation-induced hyperpermeability in the blood-brain barrier. Curr Neurovasc Res. 2009;6:181–193. doi: 10.2174/156720209788970081. [DOI] [PubMed] [Google Scholar]
- Nadtochiy SM, Redman E, Rahman I, Brookes PS. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovasc Res. 2011;89:643–649. doi: 10.1093/cvr/cvq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 Deacetylates Carbamoyl Phosphate Synthetase 1 and Regulates the Urea Cycle. Cell. 2009;137:560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Ogura M, Tanaka D, Inagaki N. Localization of mouse mitochondrial SIRT proteins: shift of SIRT3 to nucleus by co-expression with SIRT5. Biochem Biophys Res Commun. 2008;366:174–179. doi: 10.1016/j.bbrc.2007.11.122. [DOI] [PubMed] [Google Scholar]
- Napper AD, Hixon J, McDonagh T, Keavey K, Pons JF, Barker J, Yau WT, Amouzegh P, Flegg A, Hamelin E, Thomas RJ, Kates M, Jones S, Navia MA, Saunders JO, DiStefano PS, Curtis R. Discovery of indoles as potent and selective inhibitors of the deacetylase SIRT1. J Med Chem. 2005;48:8045–8054. doi: 10.1021/jm050522v. [DOI] [PubMed] [Google Scholar]
- Nasrin N, Kaushik VK, Fortier E, Wall D, Pearson KJ, de Cabo R, Bordone L. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One. 2009;4:e8414. doi: 10.1371/journal.pone.0008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- Neugebauer RC, Sippl W, Jung M. Inhibitors of NAD+ dependent histone deacetylases (sirtuins) Curr Pharm Des. 2008;14:562–573. doi: 10.2174/138161208783885380. [DOI] [PubMed] [Google Scholar]
- Niizuma K, Yoshioka H, Chen H, Kim GS, Jung JE, Katsu M, Okami N, Chan PH. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim Biophys Acta. 2010;1802:92–99. doi: 10.1016/j.bbadis.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- Ogura M, Nakamura Y, Tanaka D, Zhuang X, Fujita Y, Obara A, Hamasaki A, Hosokawa M, Inagaki N. Overexpression of SIRT5 confirms its involvement in deacetylation and activation of carbamoyl phosphate synthetase 1. Biochemical and Biophysical Research Communications. 2010;393:73–78. doi: 10.1016/j.bbrc.2010.01.081. [DOI] [PubMed] [Google Scholar]
- Okawara M, Katsuki H, Kurimoto E, Shibata H, Kume T, Akaike A. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochemical Pharmacology. 2007;73:550–560. doi: 10.1016/j.bcp.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Okumura K, Mendoza M, Bachoo RM, DePinho RA, Cavenee WK, Furnari FB. PCAF modulates PTEN activity. J Biol Chem. 2006;281:26562–26568. doi: 10.1074/jbc.M605391200. [DOI] [PubMed] [Google Scholar]
- Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, Eto M, Kozaki K, Akishita M, Ouchi Y, Kaneki M. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25:176–185. doi: 10.1038/sj.onc.1209049. [DOI] [PubMed] [Google Scholar]
- Outeiro TF, Grammatopoulos TN, Altmann S, Amore A, Standaert DG, Hyman BT, Kazantsev AG. Pharmacological inhibition of PARP-1 reduces [alpha]-synuclein- and MPP+-induced cytotoxicity in Parkinson’s disease in vitro models. Biochemical and Biophysical Research Communications. 2007a;357:596–602. doi: 10.1016/j.bbrc.2007.03.163. [DOI] [PubMed] [Google Scholar]
- Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet J-C, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG. Sirtuin 2 Inhibitors Rescue α-Synuclein-Mediated Toxicity in Models of Parkinson’s Disease. Science. 2007b;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas M, Pizarro JG, Gutierrez-Cuesta J, Crespo-Biel N, Alvira D, Tajes M, Yeste-Velasco M, Folch J, Canudas AM, Sureda FX, Ferrer I, Camins A. Modulation of SIRT1 expression in different neurodegenerative models and human pathologies. Neuroscience. 2008;154:1388–1397. doi: 10.1016/j.neuroscience.2008.04.065. [DOI] [PubMed] [Google Scholar]
- Pallos J, Bodai L, Lukacsovich T, Purcell JM, Steffan JS, Thompson LM, Marsh JL. Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington’s disease. Hum Mol Genet. 2008;17:3767–3775. doi: 10.1093/hmg/ddn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Neri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- Parsons JL, Dianova II, Dianov GL. APE1 is the major 3′-phosphoglycolate activity in human cell extracts. Nucleic Acids Res. 2004;32:3531–3536. doi: 10.1093/nar/gkh676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W, Proud CG, Mies G. Shut-down of translation, a global neuronal stress response: mechanisms and pathological relevance. Curr Pharm Des. 2007;13:1887–1902. doi: 10.2174/138161207780858401. [DOI] [PubMed] [Google Scholar]
- Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol Aging. 2005;26:995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Penberthy WT, Tsunoda I. The importance of NAD in multiple sclerosis. Curr Pharm Des. 2009;15:64–99. doi: 10.2174/138161209787185751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K, Li Y, Long L, Li D, Jia Q, Wang Y, Shen Q, Tang Y, Wen L, Kung HF, Peng Y. Knockdown of FoxO3a induces increased neuronal apoptosis during embryonic development in zebrafish. Neurosci Lett. 2010;484:98–103. doi: 10.1016/j.neulet.2010.07.068. [DOI] [PubMed] [Google Scholar]
- Perry VH, Lunn ER, Brown MC, Cahusac S, Gordon S. Evidence that the Rate of Wallerian Degeneration is Controlled by a Single Autosomal Dominant Gene. Eur J Neurosci. 1990;2:408–413. doi: 10.1111/j.1460-9568.1990.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17:1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, de Oliveira R. Machado, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-[gamma] Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzi M, Sarnico I, Lanzillotta A, Battistin L, Spano P. Post-ischemic brain damage: NF-kappaB dimer heterogeneity as a molecular determinant of neuron vulnerability. FEBS J. 2009;276:27–35. doi: 10.1111/j.1742-4658.2008.06767.x. [DOI] [PubMed] [Google Scholar]
- Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schroter F, Ninnemann O, Siegert E, Bendix I, Brustle O, Nitsch R, Zipp F, Aktas O. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10:385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-Specific Deletion of SIRT1 Alters Fatty Acid Metabolism and Results in Hepatic Steatosis and Inflammation. Cell Metabolism. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Chachich M, Lane M, Roth G, Bryant M, de Cabo R, Ottinger MA, Mattison J, Ingram D, Gandy S, Pasinetti GM. Calorie restriction attenuates Alzheimer’s disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus) J Alzheimers Dis. 2006a;10:417–422. doi: 10.3233/jad-2006-10411. [DOI] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006b;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie Restriction Reduces Oxidative Stress by SIRT3-Mediated SOD2 Activation. Cell Metabolism. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Rajamohan SB, Pillai VB, Gupta M, Sundaresan NR, Birukov KG, Samant S, Hottiger MO, Gupta MP. SIRT1 Promotes Cell Survival under Stress by Deacetylation-Dependent Deactivation of Poly(ADP-Ribose) Polymerase 1. Mol. Cell. Biol. 2009;29:4116–4129. doi: 10.1128/MCB.00121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci. 2008;28:9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, Vatner DE, Vatner SF, Abdellatif M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879–886. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Perez-Pinzon MA. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2006;26:1141–1147. doi: 10.1038/sj.jcbfm.9600262. [DOI] [PubMed] [Google Scholar]
- Raval AP, Lin HW, Dave KR, Defazio RA, Della Morte D, Kim EJ, Perez-Pinzon MA. Resveratrol and ischemic preconditioning in the brain. Curr Med Chem. 2008;15:1545–1551. doi: 10.2174/092986708784638861. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;364:362. doi: 10.1038/364362c0. [DOI] [PubMed] [Google Scholar]
- Sabri M, Ai J, Knight B, Tariq A, Jeon H, Shang X, Marsden PA, Macdonald R. Loch. Uncoupling of endothelial nitric oxide synthase after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2011;31:190–199. doi: 10.1038/jcbfm.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto J, Miura T, Shimamoto K, Horio Y. Predominant expression of Sir2alpha, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Lett. 2004;556:281–286. doi: 10.1016/s0014-5793(03)01444-3. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Maier B, Koclega KD, Chruszcz M, Gluba W, Stukenberg PT, Minor W, Scrable H. Phosphorylation regulates SIRT1 function. PLoS One. 2008;3:e4020. doi: 10.1371/journal.pone.0004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BPS, Baloh RH, Milbrandt J. Transgenic Mice Expressing the Nmnat1 Protein Manifest Robust Delay in Axonal Degeneration In Vivo. J. Neurosci. 2009;29:6526–6534. doi: 10.1523/JNEUROSCI.1429-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Brace CS, Ben-Josef G, West T, Wozniak DF, Holtzman DM, Herzog ED, Imai S. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J Neurosci. 2010;30:10220–10232. doi: 10.1523/JNEUROSCI.1385-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders LR, Sharma AD, Tawney J, Nakagawa M, Okita K, Yamanaka S, Willenbring H, Verdin E. miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging (Albany NY) 2010;2:415–431. doi: 10.18632/aging.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve AA, Celic I, Avalos J, Deng H, Boeke JD, Schramm VL. Chemistry of gene silencing: the mechanism of NAD+-dependent deacetylation reactions. Biochemistry. 2001;40:15456–15463. doi: 10.1021/bi011858j. [DOI] [PubMed] [Google Scholar]
- Sauve AA, Schramm VL. Sir2 regulation by nicotinamide results from switching between base exchange and deacetylation chemistry. Biochemistry. 2003;42:9249–9256. doi: 10.1021/bi034959l. [DOI] [PubMed] [Google Scholar]
- Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annu Rev Biochem. 2006;75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- Sawada M, Hayes P, Matsuyama S. Cytoprotective membrane-permeable peptides designed from the Bax-binding domain of Ku70. Nat Cell Biol. 2003;5:352–357. doi: 10.1038/ncb955. [DOI] [PubMed] [Google Scholar]
- Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CFW, Steegborn C. Substrates and Regulation Mechanisms for the Human Mitochondrial Sirtuins Sirt3 and Sirt5. Journal of Molecular Biology. 2008;382:790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Schwer B, Bunkenborg J, Verdin RO, Andersen JS, Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proceedings of the National Academy of Sciences. 2006;103:10224–10229. doi: 10.1073/pnas.0603968103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B, Schumacher B, Lombard DB, Xiao C, Kurtev MV, Gao J, Schneider JI, Chai H, Bronson RT, Tsai LH, Deng CX, Alt FW. Neural sirtuin 6 (Sirt6) ablation attenuates somatic growth and causes obesity. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1016306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta N, Seto E. Regulation of histone deacetylase activities. J Cell Biochem. 2004;93:57–67. doi: 10.1002/jcb.20179. [DOI] [PubMed] [Google Scholar]
- Seo J-S, Moon M-H, Jeong J-K, Seol J-W, Lee Y-J, Park B-H, Park S-Y. SIRT1, a histone deacetylase, regulates prion protein-induced neuronal cell death. Neurobiology of Aging. 2011 doi: 10.1016/j.neurobiolaging.2010.09.019. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman JM, Stone EM, Freeman-Cook LL, Brachmann CB, Boeke JD, Pillus L. The conserved core of a human SIR2 homologue functions in yeast silencing. Mol Biol Cell. 1999;10:3045–3059. doi: 10.1091/mbc.10.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shie FS, Nivison M, Hsu PC, Montine TJ. Modulation of microglial innate immunity in Alzheimer’s disease by activation of peroxisome proliferator-activated receptor gamma. Curr Med Chem. 2009;16:643–651. doi: 10.2174/092986709787458399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler KS, Ventura E, Dutt M, Elliott P, Fitzgerald DC, Rostami A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J Neuroophthalmol. 2010;30:328–339. doi: 10.1097/WNO.0b013e3181f7f833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler KS, Ventura E, Rex TS, Elliott P, Rostami A. SIRT1 activation confers neuroprotection in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2007;48:3602–3609. doi: 10.1167/iovs.07-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulga N, Wilson-Smith R, Pastorino JG. Sirtuin-3 deacetylation of cyclophilin D induces dissociation of hexokinase II from the mitochondria. J Cell Sci. 2010;123:894–902. doi: 10.1242/jcs.061846. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, Avalos JL, Escalante-Semerena JC, Grubmeyer C, Wolberger C, Boeke JD. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci U S A. 2000;97:6658–6663. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon JM, Pasupuleti R, Xu L, McDonagh T, Curtis R, DiStefano PS, Huber LJ. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol. 2006;26:28–38. doi: 10.1128/MCB.26.1.28-38.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 Mediates Reduction of Oxidative Damage and Prevention of Age-Related Hearing Loss under Caloric Restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorolla MA, Nierga C, Rodriguez-Colman MJ, Reverter-Branchat G, Arenas A, Tamarit J, Ros J, Cabiscol E. Sir2 is induced by oxidative stress in a yeast model of Huntington disease and its activation reduces protein aggregation. Arch Biochem Biophys. 2011;510:27–34. doi: 10.1016/j.abb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Srivastava DK, Berg BJ, Prasad R, Molina JT, Beard WA, Tomkinson AE, Wilson SH. Mammalian abasic site base excision repair. Identification of the reaction sequence and rate-determining steps. J Biol Chem. 1998;273:21203–21209. doi: 10.1074/jbc.273.33.21203. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. The Lancet. 2003;362:1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- Stetler RA, Gao Y, Zukin RS, Vosler PS, Zhang L, Zhang F, Cao G, Bennett MV, Chen J. Apurinic/apyrimidinic endonuclease APE1 is required for PACAP-induced neuroprotection against global cerebral ischemia. Proc Natl Acad Sci U S A. 2010;107:3204–3209. doi: 10.1073/pnas.1000030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strum JC, Johnson JH, Ward J, Xie H, Feild J, Hester A, Alford A, Waters KM. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol Endocrinol. 2009;23:1876–1884. doi: 10.1210/me.2009-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Noshita N, Lewen A, Kim GW, Chan PH. Neuronal Expression of the DNA Repair Protein Ku 70 After Ischemic Preconditioning Corresponds to Tolerance to Global Cerebral Ischemia. Stroke. 2001;32:2388–2393. doi: 10.1161/hs1001.097109. [DOI] [PubMed] [Google Scholar]
- Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. The Journal of Clinical Investigation. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 Is a Stress-Responsive Deacetylase in Cardiomyocytes That Protects Cells from Stress-Mediated Cell Death by Deacetylation of Ku70. Mol. Cell. Biol. 2008;28:6384–6401. doi: 10.1128/MCB.00426-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Koike T. Mammalian Sir2-related protein (SIRT) 2-mediated modulation of resistance to axonal degeneration in slow Wallerian degeneration mice: A crucial role of tubulin deacetylation. Neuroscience. 2007;147:599–612. doi: 10.1016/j.neuroscience.2007.04.059. [DOI] [PubMed] [Google Scholar]
- Sydow A, Van der Jeugd A, Zheng F, Ahmed T, Balschun D, Petrova O, Drexler D, Zhou L, Rune G, Mandelkow E, D’Hooge R, Alzheimer C, Mandelkow EM. Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic Tau mutant. J Neurosci. 2011;31:2511–2525. doi: 10.1523/JNEUROSCI.5245-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira N, Nihira K, Yamaguchi T, Miki Y, Yoshida K. DYRK2 is targeted to the nucleus and controls p53 via Ser46 phosphorylation in the apoptotic response to DNA damage. Mol Cell. 2007;25:725–738. doi: 10.1016/j.molcel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Tang BL. Sirt1’s complex roles in neuroprotection. Cell Mol Neurobiol. 2009;29:1093–1103. doi: 10.1007/s10571-009-9414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL. Resveratrol is neuroprotective because it is not a direct activator of Sirt1-A hypothesis. Brain Res Bull. 2010;81:359–361. doi: 10.1016/j.brainresbull.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Tanner KG, Landry J, Sternglanz R, Denu JM. Silent information regulator 2 family of NAD- dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc Natl Acad Sci U S A. 2000;97:14178–14182. doi: 10.1073/pnas.250422697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- Tanny JC, Moazed D. Coupling of histone deacetylation to NAD breakdown by the yeast silencing protein Sir2: Evidence for acetyl transfer from substrate to an NAD breakdown product. Proc Natl Acad Sci U S A. 2001;98:415–420. doi: 10.1073/pnas.031563798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, McDonald W. Hayes, Olivier AK, Spitz DR, Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AMR. Neurodegeneration in xeroderma pigmentosum. Brain. 2008;131:1967–1968. doi: 10.1093/brain/awn153. [DOI] [PubMed] [Google Scholar]
- Tejedor FJ, Hammerle B. MNB/DYRK1A as a multiple regulator of neuronal development. FEBS J. 2011;278:223–235. doi: 10.1111/j.1742-4658.2010.07954.x. [DOI] [PubMed] [Google Scholar]
- Teng FY, Tang BL. NF-kappaB signaling in neurite growth and neuronal survival. Rev Neurosci. 2010;21:299–313. doi: 10.1515/revneuro.2010.21.4.299. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Tong L, Denu JM. Function and metabolism of sirtuin metabolite O-acetyl-ADP-ribose. Biochim Biophys Acta. 2010;1804:1617–1625. doi: 10.1016/j.bbapap.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S-K, Hung L-M, Fu Y-T, Cheng H, Nien M-W, Liu H-Y, Zhang FB-Y, Huang S-S. Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. Journal of Vascular Surgery. 2007;46:346–353. doi: 10.1016/j.jvs.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Valerio A, Dossena M, Bertolotti P, Boroni F, Sarnico I, Faraco G, Chiarugi A, Frontini A, Giordano A, Liou HC, De Simoni MG, Spano P, Carruba MO, Pizzi M, Nisoli E. Leptin is induced in the ischemic cerebral cortex and exerts neuroprotection through NF-kappaB/c-Rel-dependent transcription. Stroke. 2009;40:610–617. doi: 10.1161/STROKEAHA.108.528588. [DOI] [PubMed] [Google Scholar]
- van der Horst A, Tertoolen LGJ, de Vries-Smits LMM, Frye RA, Medema RH, Burgering BMT. FOXO4 Is Acetylated upon Peroxide Stress and Deacetylated by the Longevity Protein hSir2SIRT1. Journal of Biological Chemistry. 2004;279:28873–28879. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- Varon R, Vissinga C, Platzer M, Cerosaletti KM, Chrzanowska KH, Saar K, Beckmann G, Seemanova E, Cooper PR, Nowak NJ, Stumm M, Weemaes CM, Gatti RA, Wilson RK, Digweed M, Rosenthal A, Sperling K, Concannon P, Reis A. Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome. Cell. 1998;93:467–476. doi: 10.1016/s0092-8674(00)81174-5. [DOI] [PubMed] [Google Scholar]
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger A, Perdomo J, Crossley M. Modification with SUMO. A role in transcriptional regulation. EMBO Rep. 2003;4:137–142. doi: 10.1038/sj.embor.embor738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Chen L, Hou X, Li Z, Kabra N, Ma Y, Nemoto S, Finkel T, Gu W, Cress WD, Chen J. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol. 2006;8:1025–1031. doi: 10.1038/ncb1468. [DOI] [PubMed] [Google Scholar]
- Wang F, Nguyen M, Qin FX-F, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007a;6:505–514. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, Maniar K, Dolios G, Wang R, Hof PR, Pasinetti GM. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2005a;19:659–661. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, He Z. A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol. 2005b;170:349–355. doi: 10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang L, Chen Z-B, Wu J-Y, Zhang X, Xu Y. Icariin enhances neuronal survival after oxygen and glucose deprivation by increasing SIRT1. European Journal of Pharmacology. 2009a;609:40–44. doi: 10.1016/j.ejphar.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Wang P, Xu T-Y, Guan Y-F, Tian W-W, Viollet B, Rui Y-C, Zhai Q-W, Su D-F, Miao C-Y. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate–activated kinase pathway. Annals of Neurology. 2011;69:360–374. doi: 10.1002/ana.22236. [DOI] [PubMed] [Google Scholar]
- Wang S, Vosler PS, Gan Y, Zhang W, Cao G, Chen J. NAD protects neurons from NMDA-induced toxicity in vitro by a SIRT1-dependent mechanism. 39th annual meeting of SFN; Chicago, IL. 2009b. p. Online. 2009 Neuroscience Meeting. [Google Scholar]
- Wang S, Xing Z, Vosler PS, Yin H, Li W, Zhang F, Signore AP, Stetler RA, Gao Y, Chen J. Cellular NAD replenishment confers marked neuroprotection against ischemic cell death: role of enhanced DNA repair. Stroke. 2008;39:2587–2595. doi: 10.1161/STROKEAHA.107.509158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Gu J, Wu P-F, Wang F, Xiong Z, Yang Y-J, Wu W-N, Dong L-D, Chen J-G. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-[kappa]B pathways and inhibition of intracellular ROS/RNS generation. Free Radical Biology and Medicine. 2009c;47:229–240. doi: 10.1016/j.freeradbiomed.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Wang Y-Q, Guo X, Qiu M-H, Feng X-Y, Sun F-Y. VEGF overexpression enhances striatal neurogenesis in brain of adult rat after a transient middle cerebral artery occlusion. Journal of Neuroscience Research. 2007b;85:73–82. doi: 10.1002/jnr.21091. [DOI] [PubMed] [Google Scholar]
- Wareski P, Vaarmann A, Choubey V, Safiulina D, Liiv J, Kuum M, Kaasik A. PGC-1{alpha} and PGC-1{beta} regulate mitochondrial density in neurons. J Biol Chem. 2009;284:21379–21385. doi: 10.1074/jbc.M109.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick A, Wick W, Waltenberger J, Weller M, Dichgans J, Schulz JB. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J Neurosci. 2002;22:6401–6407. doi: 10.1523/JNEUROSCI.22-15-06401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Woodhouse BC, Dianova II, Parsons JL, Dianov GL. Poly(ADP-ribose) polymerase-1 modulates DNA repair capacity and prevents formation of DNA double strand breaks. DNA Repair (Amst) 2008;7:932–940. doi: 10.1016/j.dnarep.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Xiong S, Salazar G, Patrushev N, Alexander RW. FoxO1 Mediates an Autofeedback Loop Regulating SIRT1 Expression. Journal of Biological Chemistry. 2011;286:5289–5299. doi: 10.1074/jbc.M110.163667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T, DeRicco J, Naqvi A, Hoffman TA, Mattagajasingh I, Kasuno K, Jung S-B, Kim C-S, Irani K. SIRT1 deacetylates APE1 and regulates cellular base excision repair. Nucleic Acids Research. 2010;38:832–845. doi: 10.1093/nar/gkp1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. 2007;21:1745–1755. doi: 10.1210/me.2007-0079. [DOI] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007a;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26:5310–5318. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cimen H, Han M-J, Shi T, Deng J-H, Koc H, Palacios OM, Montier L, Bai Y, Tong Q, Koc EC. NAD+-dependent Deacetylase SIRT3 Regulates Mitochondrial Protein Synthesis by Deacetylation of the Ribosomal Protein MRPL10. Journal of Biological Chemistry. 2010;285:7417–7429. doi: 10.1074/jbc.M109.053421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Fu W, Chen J, Olashaw N, Zhang X, Nicosia SV, Bhalla K, Bai W. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol. 2007b;9:1253–1262. doi: 10.1038/ncb1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hou H, Haller EM, Nicosia SV, Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J. 2005;24:1021–1032. doi: 10.1038/sj.emboj.7600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J, Luo J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim Biophys Acta. 2010;1804:1684–1689. doi: 10.1016/j.bbapap.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W, Alano CC, Garnier P, Swanson RA. NAD+ as a metabolic link between DNA damage and cell death. Journal of Neuroscience Research. 2005;79:216–223. doi: 10.1002/jnr.20289. [DOI] [PubMed] [Google Scholar]
- Ying W, Wei G, Wang D, Wang Q, Tang X, Shi J, Zhang P, Lu H. Intranasal administration with NAD+ profoundly decreases brain injury in a rat model of transient focal ischemia. Front Biosci. 2007;12:2728–2734. doi: 10.2741/2267. [DOI] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:830–839. [PubMed] [Google Scholar]
- Yuan F, Xie Q, Wu J, Bai Y, Mao B, Dong Y, Bi W, Ji G, Tao W, Wang Y, Yuan Z. MST1 promotes apoptosis through regulating Sirt1-dependent p53 deacetylation. Journal of Biological Chemistry. 2011;286:6940–6945. doi: 10.1074/jbc.M110.182543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Lehtinen MK, Merlo P, Villen J, Gygi S, Bonni A. Regulation of neuronal cell death by MST1-FOXO1 signaling. J Biol Chem. 2009;284:11285–11292. doi: 10.1074/jbc.M900461200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Zhang X, Sengupta N, Lane WS, Seto E. SIRT1 Regulates the Function of the Nijmegen Breakage Syndrome Protein. Molecular Cell. 2007;27:149–162. doi: 10.1016/j.molcel.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh A, Schneider RJ. Translation by ribosome shunting on adenovirus and hsp70 mRNAs facilitated by complementarity to 18S rRNA. Genes Dev. 2000;14:414–421. [PMC free article] [PubMed] [Google Scholar]
- Zhan L, Wang T, Li W, Xu ZC, Sun W, Xu E. Activation of Akt/FoxO signaling pathway contributes to induction of neuroprotection against transient global cerebral ischemia by hypoxic pre-conditioning in adult rats. Journal of Neurochemistry. 2010;114:897–908. doi: 10.1111/j.1471-4159.2010.06816.x. [DOI] [PubMed] [Google Scholar]
- Zhang F, Chen J. Leptin protects hippocampal CA1 neurons against ischemic injury. J Neurochem. 2008;107:578–587. doi: 10.1111/j.1471-4159.2008.05645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Signore AP, Zhou Z, Wang S, Cao G, Chen J. Erythropoietin protects CA1 neurons against global cerebral ischemia in rat: potential signaling mechanisms. J Neurosci Res. 2006;83:1241–1251. doi: 10.1002/jnr.20816. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke. 2007;38:2329–2336. doi: 10.1161/STROKEAHA.107.482786. [DOI] [PubMed] [Google Scholar]
- Zhang F, Xing J, Liou AK, Wang S, Gan Y, Luo Y, Ji X, Stetler RA, Chen J, Cao G. Enhanced Delivery of Erythropoietin Across the Blood-Brain Barrier for Neuroprotection against Ischemic Neuronal Injury. Transl Stroke Res. 2010;1:113–121. doi: 10.1007/s12975-010-0019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Yin W, Chen J. Apoptosis in cerebral ischemia: executional and regulatory signaling mechanisms. Neurol Res. 2004;26:835–845. doi: 10.1179/016164104X3824. [DOI] [PubMed] [Google Scholar]
- Zhang T, Berrocal JG, Frizzell KM, Gamble MJ, DuMond ME, Krishnakumar R, Yang T, Sauve AA, Kraus WL. Enzymes in the NAD+ salvage pathway regulate SIRT1 activity at target gene promoters. J Biol Chem. 2009;284:20408–20417. doi: 10.1074/jbc.M109.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Kraus WL. SIRT1-dependent regulation of chromatin and transcription: Linking NAD(+) metabolism and signaling to the control of cellular functions. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbapap.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang X, Park TS, Gidday JM. Cerebral endothelial cell apoptosis after ischemia-reperfusion: role of PARP activation and AIF translocation. J Cereb Blood Flow Metab. 2005;25:868–877. doi: 10.1038/sj.jcbfm.9600081. [DOI] [PubMed] [Google Scholar]
- Zhao K, Harshaw R, Chai X, Marmorstein R. Structural basis for nicotinamide cleavage and ADP-ribose transfer by NAD+-dependent Sir2 histone/protein deacetylases. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8563–8568. doi: 10.1073/pnas.0401057101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Li J, Chen AF. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. American Journal of Physiology - Endocrinology And Metabolism. 2010;299:E110–E116. doi: 10.1152/ajpendo.00192.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587–590. doi: 10.1038/nature06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26:884–892. doi: 10.1179/016164104X2357. [DOI] [PubMed] [Google Scholar]
- Zhu HR, Wang ZY, Zhu XL, Wu XX, Li EG, Xu Y. Icariin protects against brain injury by enhancing SIRT1-dependent PGC-1alpha expression in experimental stroke. Neuropharmacology. 2010;59:70–76. doi: 10.1016/j.neuropharm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Zschoernig B, Mahlknecht U. SIRTUIN 1: regulating the regulator. Biochem Biophys Res Commun. 2008;376:251–255. doi: 10.1016/j.bbrc.2008.08.137. [DOI] [PubMed] [Google Scholar]
- Zschoernig B, Mahlknecht U. Carboxy-terminal phosphorylation of SIRT1 by protein kinase CK2. Biochem Biophys Res Commun. 2009;381:372–377. doi: 10.1016/j.bbrc.2009.02.085. [DOI] [PubMed] [Google Scholar]
- Zu Y, Liu L, Lee MYK, Xu C, Liang Y, Man RY, Vanhoutte PM, Wang Y. SIRT1 Promotes Proliferation and Prevents Senescence Through Targeting LKB1 in Primary Porcine Aortic Endothelial Cells. Circulation Research. 2010;106:1384–U1184. doi: 10.1161/CIRCRESAHA.109.215483. [DOI] [PubMed] [Google Scholar]