Summary
Background
Findings of large randomised trials have shown that lowering LDL cholesterol with statins reduces vascular morbidity and mortality rapidly, but limited evidence exists about the long-term efficacy and safety of statin treatment. The aim of the extended follow-up of the Heart Protection Study (HPS) is to assess long-term efficacy and safety of lowering LDL cholesterol with statins, and here we report cause-specific mortality and major morbidity in the in-trial and post-trial periods.
Methods
20 536 patients at high risk of vascular and non-vascular outcomes were allocated either 40 mg simvastatin daily or placebo, using minimised randomisation. Mean in-trial follow-up was 5·3 years (SD 1·2), and post-trial follow-up of surviving patients yielded a mean total duration of 11·0 years (SD 0·6). The primary outcome of the long-term follow-up of HPS was first post-randomisation major vascular event, and analysis was by intention to treat. This trial is registered with ISRCTN, number 48489393.
Findings
During the in-trial period, allocation to simvastatin yielded an average reduction in LDL cholesterol of 1·0 mmol/L and a proportional decrease in major vascular events of 23% (95% CI 19–28; p<0·0001), with significant divergence each year after the first. During the post-trial period (when statin use and lipid concentrations were similar in both groups), no further significant reductions were noted in either major vascular events (risk ratio [RR] 0·95 [0·89–1·02]) or vascular mortality (0·98 [0·90–1·07]). During the combined in-trial and post-trial periods, no significant differences were recorded in cancer incidence at all sites (0·98 [0·92–1·05]) or any particular site, or in mortality attributed to cancer (1·01 [0·92–1·11]) or to non-vascular causes (0·96 [0·89–1·03]).
Interpretation
More prolonged LDL-lowering statin treatment produces larger absolute reductions in vascular events. Moreover, even after study treatment stopped in HPS, benefits persisted for at least 5 years without any evidence of emerging hazards. These findings provide further support for the prompt initiation and long-term continuation of statin treatment.
Funding
UK Medical Research Council, British Heart Foundation, Merck & Co, Roche Vitamins.
Introduction
The Medical Research Council and British Heart Foundation (MRC/BHF) Heart Protection Study (HPS), among 20 000 patients at high risk of vascular and non-vascular outcomes, was established to assess the long-term efficacy and safety of lowering LDL cholesterol concentrations substantially with statin treatment.1 Collectively, findings of HPS and other major trials of statins provide compelling evidence that lowering LDL cholesterol by about 1 mmol/L reduces vascular mortality and morbidity by about a quarter in a wide range of patients (including elderly people and those with low cholesterol concentrations), without increasing the risk of non-vascular mortality or morbidity (apart from a small myopathy excess) during about 5 years of treatment.2–4 As a result, many people are now prescribed long-term statin treatment to reduce their vascular risk.
During prolonged follow-up in observational epidemiological studies, lower blood cholesterol concentrations have been associated with higher rates of particular types of cancer, and with other non-vascular morbidity and mortality.5,6 It has been suggested, therefore, that lowering LDL cholesterol (particularly to low levels) might produce increases in the rates of cancers and other types of adverse events that take longer than 5 years to emerge.7–9 Only limited evidence about long-term safety has been reported from statin trials.10–14 We aimed to assess the effects of lowering LDL cholesterol on cause-specific mortality and major morbidity, not only during the study treatment period in HPS but also in the longer term, post-trial.
Methods
Patients and randomisation
Details of HPS have been reported previously.1–3 Briefly, between July, 1994, and May, 1997, 20 536 men and women aged about 40–80 years, who were at increased risk of vascular events, were randomly allocated to receive 40 mg simvastatin daily or matching placebo. At the final follow-up (May–October, 2001), participants were encouraged to continue their allocated study treatment (unless it was contraindicated) until the study results were sent to them and their family doctors on Nov 11, 2001. Participants were advised to discuss with their doctors, in light of those results, whether non-trial statin treatment should be prescribed (and study treatment stopped). To allow unbiased assessment of subsequent long-term effects, participants and their doctors were not made aware of their previously allocated study treatment unless there was a particular request to do so, and only 15% of participants were unblinded (18% simvastatin-allocated vs 13% placebo-allocated).
Procedures and follow-up
During the in-trial treatment period, routine follow-up in study clinics was at 4, 8, and 12 months and then every 6 months. Information was sought about any suspected heart attacks, strokes, vascular procedures, cancers, or other serious adverse events. Participants unable or unwilling to attend were contacted by telephone, or follow-up was sought from their family doctors. Post-trial follow-up of serious adverse events was conducted by mailing questionnaires to all surviving participants in late November, 2001, and then annually until November, 2006, with a reminder mailed about 2 months later. Follow-up of participants who did not complete questionnaires was sought from their family doctors. During both the in-trial and post-trial periods, information about the sites of registered cancers and certified causes of deaths was requested from UK national registries for all randomised patients.
Further details were sought from the participants' family doctors (plus, if necessary, hospital records) about all reports that might relate to major vascular events or deaths. In view of the high confirmation rate (>95%) in central adjudication of cancers reported during the in-trial period, further information was not routinely sought about non-fatal cancers reported during the post-trial period. Events were coded according to prespecified criteria1 by clinical staff in the coordinating centre, who were unaware of the study treatment allocation.
During the in-trial period, compliance with study treatment was assessed at each follow-up by questioning the participant and reviewing their remaining calendar-packed tablets. The effects of treatment allocation on cholesterol concentrations were assessed by assaying blood obtained at study clinics from a sample of about 5% of participants scheduled for follow-up at about the same time each year, and from all participants attending follow-up between August, 2000, and February, 2001.2 During the post-trial period, participants were asked each year about their current statin use, and post-trial lipid profile assays were sought from 1500 randomly selected surviving participants between May, 2004, and August, 2004 (ie, about 3 years after the scheduled treatment period). About 1100 blood samples were obtained by participants' family doctors and were mailed to a central laboratory for assay using previously validated methods.15
Endpoints and statistical analysis
The main comparisons entail log-rank analyses of the first post-randomisation occurrence of particular events during the in-trial period (defined as events occurring up to Nov 11, 2001) and during the post-trial period (defined as events occurring from Nov 11, 2001, until March 31, 2007) among all those originally allocated 40 mg simvastatin daily versus all those allocated matching placebo tablets (ie, intention-to-treat analyses). The results of these in-trial analyses differ slightly from previously published findings2,3 because of the inclusion of events taking place between the participants' final follow-up visit and Nov 11, 2001 (mean extra follow-up of 3·5 months [SD 1·4]). The primary outcome for analyses of prolonged follow-up was prespecified to be the first post-randomisation major vascular event (defined as non-fatal myocardial infarction or coronary death, fatal or non-fatal stroke, coronary or non-coronary revascularisation). Secondary outcomes were: major vascular events during each year of follow-up and in various subcategories of patients; major coronary events (ie, non-fatal myocardial infarction or coronary death), strokes, and revascularisations separately; deaths from vascular and non-vascular causes separately; and cancers at all sites (excluding non-melanoma skin cancer). Analyses are presented of other outcomes, some of which (eg, site-specific cancer, cerebral haemorrhage) were prespecified for the in-trial period whereas some were not (eg, cancer incidence each year). Allowances for multiple comparisons and for the post-hoc nature of such analyses were made in their interpretation.
Role of the funding source
The trial was designed, conducted, analysed, and interpreted by the investigators, independently of all funding sources. The writing committee had full access to the study data and had final responsibility for the decision to submit for publication.
Results
Mean follow-up during the in-trial period for all randomised participants was 5·3 years (SD 1·2), and the mean total follow-up for participants who survived to the end of the post-trial period was 11·0 years (SD 0·6). Of 20 536 participants, 17 519 were still alive at the start of the post-trial follow-up period: 8863 allocated simvastatin versus 8656 placebo (ratio 1·02; because simvastatin reduced mortality).2 Overall, 96 784 person-years of follow-up were available for those originally allocated simvastatin and 95 084 for those allocated placebo (ratio 1·02). Response rates to mailed questionnaires and from family doctors during the post-trial period were high and did not differ by previous statin allocation (table 1). All but 74 participants (42 simvastatin and 32 placebo) could be followed up for cancer reports and death certification through national registries.
Table 1.
Post-trial follow-up of outcomes (other than cancer and death), by year of follow-up
| Simvastatin-allocated | Placebo-allocated | |
|---|---|---|
| Year 0 | 7370/8863 (83%) | 7066/8656 (82%) |
| Year 1 | 7152/8542 (84%) | 6845/8317 (82%) |
| Year 2 | 6525/8181 (80%) | 6284/7950 (79%) |
| Year 3 | 6023/7810 (77%) | 5821/7594 (77%) |
| Year 4 | 6651/7431 (90%) | 6462/7204 (90%) |
| Year 5 | 5375/7063 (76%) | 5165/6860 (75%) |
Data are completed/alive at start of year. Completed=questionnaire or family doctor response (higher response rates during year 4 indicate intensive efforts to contact family doctors).
During the in-trial period, the average difference in statin use between simvastatin-allocated versus placebo-allocated patients was 67% (85% vs 17%), resulting in an average LDL cholesterol difference of 1·0 mmol/L (SE 0·02).2 During the post-trial period, self-reported statin use was similar in both groups, rising from about 59% at the end of the first year to 84% by the end of the fifth year (table 2). This similarity in statin use between groups was supported by similar LDL cholesterol concentrations (2·6 [SE 0·03] vs 2·6 [0·03] mmol/L; p=0·7: table 3) after 3·2 years of post-trial follow-up.
Table 2.
In-trial and post-trial statin use (study and non-study), by year of follow-up
| Simvastatin-allocated | Placebo-allocated | |
|---|---|---|
| In-trial | ||
| Year 1 | 8994/10 107 (89%) | 389/10 088 (4%) |
| Year 2 | 8457/9909 (85%) | 889/9826 (9%) |
| Year 3 | 8122/9664 (84%) | 1608/9563 (17%) |
| Year 4 | 7764/9388 (83%) | 2262/9241 (24%) |
| Year 5 | 6058/7370 (82%) | 2345/7225 (32%) |
| Average | 85% | 17% |
| Post-trial | ||
| Year 1 | 4163/7152 (58%) | 4113/6845 (60%) |
| Year 2 | 4555/6525 (70%) | 4381/6284 (70%) |
| Year 3 | 4665/6023 (77%) | 4489/5821 (77%) |
| Year 4 | 5363/6651 (81%) | 5136/6462 (79%) |
| Year 5 | 4527/5375 (84%) | 4294/5165 (83%) |
| Average | 74% | 74% |
Data show statin use/alive (in-trial) and statin use/completed forms (post-trial).
Table 3.
In-trial and post-trial mean (SE) lipid levels
| Simvastatin-allocated | Placebo-allocated | Absolute difference | |
|---|---|---|---|
| Total cholesterol (mmol/L) | |||
| Baseline | 5·9 (0·01) | 5·9 (0·01) | 0·0 (0·01) |
| In-trial | 4·2 (0·01) | 5·4 (0·01) | 1·2 (0·02) |
| Post-trial | 4·3 (0·04) | 4·4 (0·04) | 0·0 (0·06) |
| LDL cholesterol (mmol/L) | |||
| Baseline | 3·4 (0·01) | 3·4 (0·01) | 0·0 (0·01) |
| In-trial | 2·3 (0·01) | 3·3 (0·01) | 1·0 (0·02) |
| Post-trial | 2·6 (0·03) | 2·6 (0·03) | 0·0 (0·05) |
In-trial lipid profiles are study averages based on assays in a selected sample of 5% of participants each year and in all participants during the final year of the scheduled treatment period. Post-trial lipid profiles were measured in a random sample of 1175 participants after 3·2 years of post-trial follow-up.
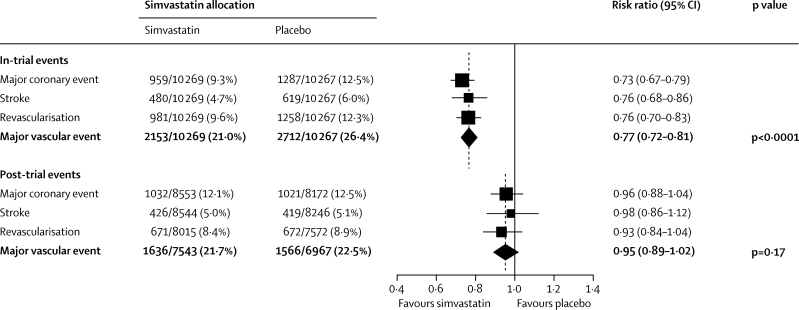
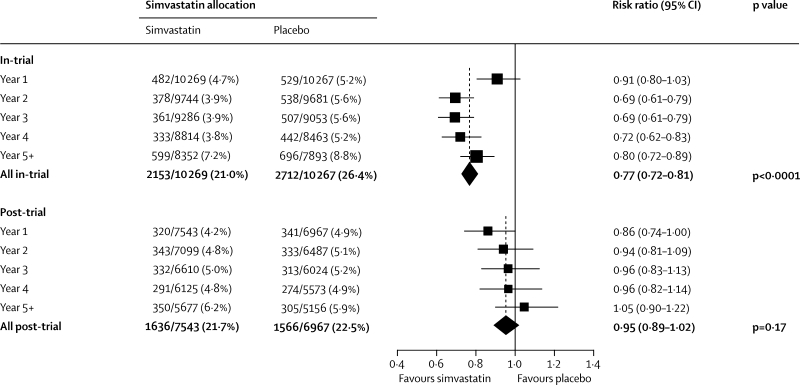
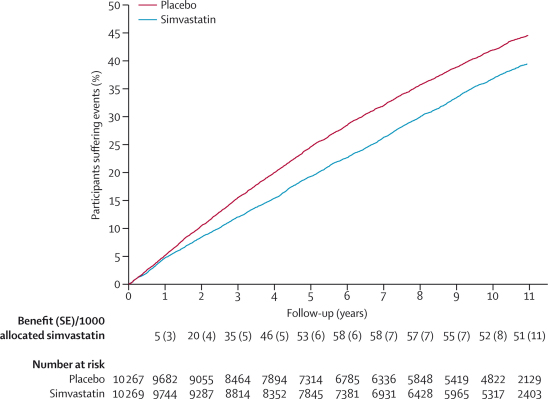
During the in-trial period, 2153 (21·0%) first major vascular events arose in 10 269 participants allocated simvastatin versus 2712 (26·4%) in 10 267 allocated placebo, corresponding to a significant 23% (SE 3) proportional reduction (p<0·0001: figure 1). No significant difference was noted during the first year, but significant reductions of about a quarter were seen during each subsequent in-trial year (figure 2). Among event-free survivors at the start of the post-trial period, 1636 (21·7%) first events arose in patients previously allocated simvastatin versus 1566 (22·5%) in those previously allocated placebo (risk ratio [RR] 0·95 [95% CI 0·89–1·02]; p=0·17). A further decrease of 14% ([0–26]; p=0·05) was recorded in the first post-trial year in patients originally allocated simvastatin, but little difference was seen between treatment groups thereafter. As a result, the cumulative proportions of participants who had major vascular events diverged throughout the in-trial period, and this separation then persisted roughly unchanged throughout the post-trial period (figure 3). The effects of statin allocation on major vascular events during the in-trial and post-trial periods were unaffected by age or pretreatment lipid profiles (webappendix p 3).
Figure 1.
First major vascular event during in-trial and post-trial follow-up
Analyses are of numbers of participants having a first post-randomisation event of each type during follow-up, so there is some non-additivity between different types of event. Denominators during the post-trial period are the numbers of randomised patients who had not had the particular outcome or died during the in-trial period. Risk ratios (RRs) are plotted (black squares with area proportional to amount of statistical information in each subdivision) comparing outcome among the participants allocated 40 mg simvastatin daily to that among those allocated placebo, along with their 95% CIs (horizontal lines; ending with arrow head when CI extends beyond scale). For particular subtotals and totals, the result and its 95% CI are represented by a diamond, with the relative risk reduction (and 95% CI) and statistical significance given alongside. A broken vertical line indicates the overall RR.
Figure 2.
First major vascular event by year during in-trial and post-trial follow-up
Conventions as in figure 1. Denominators are the numbers of patients at risk of a first post-randomisation major vascular event at the start of each year.
Figure 3.
First major vascular event during total follow-up period
Life-table plot of the effects of simvastatin allocation on percentage of major vascular events during the in-trial and post-trial periods.
Similar patterns were seen for each component of major vascular events (figure 1). For major coronary events, a 27% (SE 4) reduction was noted during the in-trial period (p<0·0001). During the post-trial period, the incidence rates were similar in both treatment groups (RR 0·96 [95% CI 0·88–1·04]; p=0·31), although a further reduction was recorded in first non-fatal myocardial infarction (413 [4·8%] vs 465 [5·7%]; p=0·01). For strokes, a 24% (SE 5) reduction was seen during the in-trial period (p<0·0001), reflecting a 29% (SE 6) reduction in definite ischaemic stroke and no difference in haemorrhagic stroke (51 [0·5%] vs 56 [0·5%]; p=0·59). During the post-trial period, the incidence rates were similar in both treatment groups (0·98 [0·86–1·12]; p=0·77), with no adverse effect on haemorrhagic stroke (38 [0·4%] vs 51 [0·6%]; p=0·13). For revascularisation procedures, a significant 24% (SE 4) reduction was seen during the in-trial period (p<0·0001). During the post-trial period, the incidence rates were similar in both treatment groups (0·93 [0·84–1·04]; p=0·20).
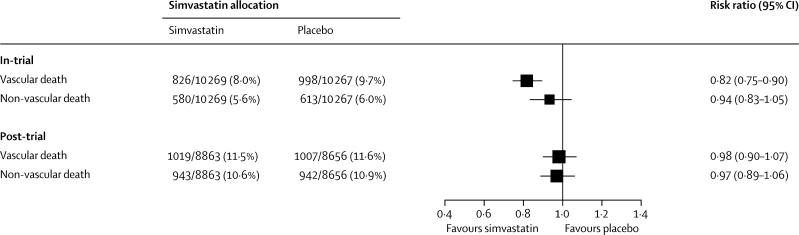
Vascular mortality during the in-trial period accounted for 826 (8·0%) deaths in participants allocated simvastatin versus 998 (9·7%) in those allocated placebo, corresponding to an 18% (SE 4) proportional reduction (p<0·0001, figure 4; webappendix, p 4). During the post-trial period, vascular mortality rates were similar in both treatment groups (1019 [11·5%] vs 1007 [11·6%]; RR 0·98 [95% CI 0·90–1·07]; p=0·71), so in-trial survival gains persisted. The effects of statin allocation on vascular mortality in both the in-trial and post-trial periods were unaffected by age or pretreatment lipid profiles (webappendix, p 5).
Figure 4.
Vascular and non-vascular mortality during in-trial and post-trial follow-up
Conventions as in figure 1.
Non-vascular mortality during the in-trial period accounted for 580 (5·6%) deaths in participants allocated simvastatin versus 613 (6·0%) in those allocated placebo (p=0·25; figure 4). A marginally significant reduction in deaths attributed to respiratory disease was noted in simvastatin-allocated participants (95 [0·9%] vs 124 [1·2%]; p=0·04), but deaths from cancer, or other prespecified categories of non-vascular death, did not differ significantly (webappendix, p 4). During the post-trial period, non-vascular mortality rates were similar in both treatment groups (943 [10·6%] vs 942 [10·9%]; RR 0·97 [95% CI 0·89–1·06]; p=0·55), with no differences reported in deaths from cancer, respiratory disease, or non-medical causes. The apparent reduction in deaths attributed to other medical causes in participants originally allocated simvastatin (200 [2·3%] vs 239 [2·8%]) was only marginally significant (p=0·03) and involved no material differences in deaths due to renal or hepatic disease and only a non-significant difference in a miscellaneous group of other non-vascular causes. Despite previous concerns about the safety of lipid-lowering treatment in elderly people and those with below-average cholesterol concentrations,7,8 there was no evidence of any adverse effect on non-vascular mortality in statin-allocated participants aged 70 or older at baseline, or those with pretreatment total cholesterol concentrations less than 5·0 mmol/L (webappendix, p 6). When the 11 years of in-trial and post-trial follow-up are considered together, allocation to about 5 years of statin treatment was not associated with any increase in non-vascular mortality, either overall (1523 [14·8%] vs 1555 [15·1%]; RR 0·96 [95% CI 0·89–1·03]; p=0·24) or for any prespecified category of death.
The 14% (SE 3) proportional reduction in all-cause mortality (p=0·0001) seen during the in-trial period reflects the combination of an 18% (SE 4) proportional reduction in vascular mortality and little effect on non-vascular mortality (webappendix p 4). During the post-trial period, there was no further reduction in vascular mortality and no emergence of any adverse effect on non-vascular mortality; the proportions of participants who died were similar in both treatment groups (1962 [22·1%] vs 1949 [22·5%]; RR 0·98 [95% CI 0·92–1·04]; p=0·49). As a result, the absolute reduction in all-cause mortality that emerged with simvastatin allocation during the in-trial period persisted roughly unchanged after 11 years of follow-up.
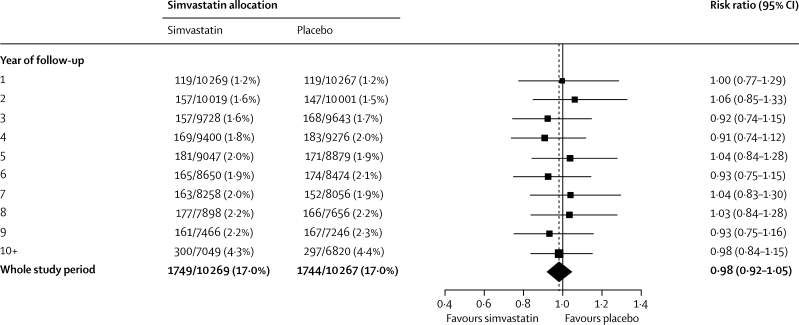
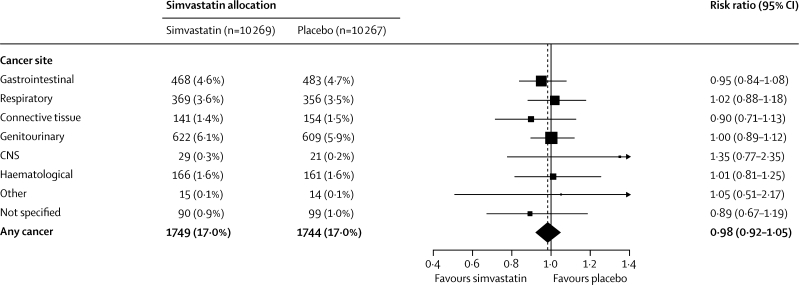
The incidence of a first diagnosis of any type of cancer (excluding, as prespecified, non-melanoma skin cancer) was similar throughout the in-trial and post-trial periods combined (1749 [17·0%] allocated simvastatin vs 1744 [17·0%] allocated placebo; RR 0·98 [0·92–1·05]; p=0·60; figure 5). Indeed, even during the later years of this prolonged follow-up, no suggestion was noted of any emerging difference in the overall incidence of cancer. If cholesterol lowering with statin treatment had effects on cancer then these might be expected to be restricted to particular types.9,16 The large numbers of incident cancers recorded during the combined in-trial and post-trial periods allow reliable assessment of the effects of a substantial 5-year reduction in cholesterol on 11-year risks of the commoner types of cancer. The incidence of genitourinary, gastrointestinal, respiratory, haematological, or any other malignant disease did not differ significantly (figure 6), even in patients aged 70 years or older at baseline or with below-average pretreatment cholesterol concentrations (webappendix, p 7).
Figure 5.
First incident cancer by year during total follow-up period
Conventions as in figure 1.
Figure 6.
Incidence of site-specific cancer during total follow-up period
Conventions as in figure 1. Not including non-melanoma skin cancer, which was prospectively to be considered separately (561 [5·5%] vs 512 [5·0%]; risk ratio 1·08 [0·96–1·21]; p=0·22).
Discussion
Prolonged post-trial follow-up of participants in HPS shows that the substantial reduction in vascular mortality and morbidity produced during an average 5-year reduction in LDL cholesterol of 1 mmol/L with simvastatin persisted largely unchanged during the subsequent 6 years. Reassuringly, there was no evidence that any adverse effect on particular causes of non-vascular mortality or major morbidity (including site-specific cancers) was emerging during this prolonged follow-up period (panel).
Panel. Research in context.
Systematic review
Electronic searches of Medline, PubMed, and Scopus, supplemented by hand searches of reference lists of meta-analyses and other review articles, identified reports from five large randomised trials of statin therapy with post-trial follow-up of clinical outcomes. These previous reports included limited numbers of major vascular events, incident cancers, and non-vascular deaths during prolonged post-trial follow-up, making reliable assessment of persistence of benefit or emergence of any hazard difficult.
Interpretation
Prolonged follow-up in the Heart Protection Study now shows that reduction of about a quarter in vascular mortality and morbidity—produced by an average 1 mmol/L reduction in LDL cholesterol with 5 years of statin therapy—persisted largely unchanged during the subsequent 6 years, despite similar LDL cholesterol concentrations and statin use in both treatment groups. Reassuringly, no adverse effects on particular causes of non-vascular mortality or major morbidity (including site-specific cancers) were seen to emerge during prolonged follow-up. These findings support prompt initiation and long-term continuation of statin treatment in people at increased risk of vascular events.
Study treatment was not routinely unblinded at the end of the in-trial period of HPS, and no differences between treatment groups were recorded in statin use or LDL cholesterol concentrations during post-trial follow-up. In each year of post-trial follow-up, about a fifth of the surviving participants did not return their postal questionnaire, but information about non-fatal clinical events was obtained from family doctors for about half of these non-responders, and the completeness of follow-up was similar in the two treatment groups. Furthermore, information on site-specific cancers and cause-specific mortality was obtained from national registries for almost all randomised patients throughout both the in-trial and the post-trial periods. As a result, although the power to detect any effects might have been reduced slightly by some events having been missed, this occurrence should not have introduced any bias. Hence, the large numbers of major vascular events that were recorded during the post-trial period of follow-up in HPS provide robust evidence about the persistence of the cardiovascular benefits produced by previous statin treatment. Similarly, the large numbers of other types of outcome recorded during prolonged follow-up provide compelling evidence that 5 years of statin therapy is not associated with excesses of any particular type of non-vascular death, site-specific cancer, or other major non-vascular morbidity. Moreover, although 11 years might still not be long enough for deleterious effects on cancer to emerge fully, no adverse trend was noted, even during the later years of post-trial follow-up.
The findings of HPS for persistence of vascular benefits are generally consistent with the results for follow-up beyond the scheduled treatment period from four other large (>1000 participants) randomised trials of statin treatment (webappendix p 1; one other such trial has only reported the results for the in-trial and post-trial period combined).14 In 4S,10 in which over 4000 people with coronary disease were studied, vascular mortality during 5 years of post-trial follow-up was non-significantly higher among those originally allocated simvastatin, but non-fatal vascular events were not reported. Perhaps due to routine unblinding at the end of the in-trial period, use of cholesterol-lowering drugs (usually simvastatin) after 3 years of post-trial follow-up was slightly higher among participants originally allocated simvastatin (86% vs 82%). In LIPID,11 in which over 9000 people with coronary disease were included, vascular mortality during 2 years of post-trial follow-up was significantly (p=0·02) lower among those originally allocated pravastatin. After routine unblinding at the end of the in-trial period, both groups were encouraged to take pravastatin 40 mg daily and similar proportions commenced it (88% vs 86%), and blood lipid levels were similar during each of the post-trial years. In WOSCOPS,12 in which over 6000 men without known coronary disease were enrolled, significantly fewer non-fatal myocardial infarctions or coronary deaths (p=0·02) and non-significantly fewer strokes (p=0·22) were seen during 10 years of post-trial follow-up. Again, perhaps due to routine unblinding, a slightly higher proportion of those originally allocated pravastatin were taking statins during the first 5 years of post-trial follow-up (29% vs 24% at 1 year and 39% vs 35% at 5 years). In ASCOT-LLA,13 which involved 10 000 people with hypertension, the incidence of non-fatal myocardial infarction or coronary death during 2 years of post-trial follow-up was significantly (p=0·005) lower among those originally allocated atorvastatin. At the end of the lipid-lowering treatment period, atorvastatin was offered to all participants (who were not routinely unblinded) and a slightly higher proportion of those originally allocated atorvastatin were taking statin treatment at the end of the post-trial period (67% vs 63%), although lipid profiles were broadly similar.
Concerns have been raised that lower blood cholesterol concentrations could be associated with an increased incidence of cancer or some other non-vascular outcomes.5,6,9,16 The Cholesterol Treatment Trialists' meta-analysis of about 130 000 patients in 21 trials of statin versus control (including HPS), which included about 7000 incident cancers and 3000 non-vascular deaths during an average 5-year LDL cholesterol reduction of 1 mmol/L, did not find any excess of particular types of cancer or non-vascular death.4 But, if adverse effects were caused by lowering cholesterol then they might only emerge after longer term follow-up. Previously, the numbers and types of non-vascular outcomes reported from prolonged post-trial follow-up in statin trials have been limited (webappendix p 2). In ASCOT-LLA,13 only mortality during 2 years of post-trial follow-up was reported, with previous allocation to atorvastatin not associated with an excess in non-vascular mortality. Furthermore, the LIPID study group has only reported 2 years of post-trial follow-up,11 with no significant differences in deaths from all types of non-vascular cause combined or in incidence of cancers at all sites combined. 4S has reported 5 years of post-trial follow-up,10 with no apparent differences in all types of non-vascular mortality or cancer incidence at all sites. 10 years of post-trial follow-up has been reported in WOSCOPS,12 with no apparent differences in total non-vascular mortality or cancer incidence. In WOSCOPS, more of the men originally allocated pravastatin did have prostate cancer diagnosed during the combined follow-up period (89 [2·7%] vs 59 [1·8%]), but there were few such cancers and the difference was not statistically convincing (p=0·03). The much larger numbers of men in HPS who were found to have developed prostate cancer (367 [4·7%] simvastatin vs 348 [4·5%] placebo) during 11 years of follow-up show that substantial reductions in cholesterol with statin treatment do not materially increase its incidence (1·03 [0·89–1·20]; p=0·67).
In HPS, among participants allocated simvastatin, fewer major vascular events were noted in the first year after randomisation and significant reductions of about a quarter were seen in each separate year of the in-trial period. These findings suggest that the absolute benefits of prolonged statin treatment are likely to be much greater than is indicated by analyses restricted merely to in-trial periods of statin trials. Moreover, even after in-study statin treatment stopped in HPS, the benefits persisted for several years. As well as providing reliable evidence about the long-term benefits of statin therapy, the large numbers of other major health outcomes recorded during prolonged follow-up in HPS provide considerable reassurance—both to prescribers and to patients—about the long-term safety of lowering LDL cholesterol substantially for about 5 years. These findings provide further support for the prompt initiation and long-term continuation of statin treatment in people at increased risk of vascular events.
Acknowledgments
Acknowledgments
We thank participants in the study and the doctors, nurses, and administrative staff in hospitals and general practices throughout the UK who assisted with its conduct (see reference 2 for a full list); and Mark Corbett and Irene Boller who helped make this post-trial follow-up successful.
Contributors
RC and JA coordinated and, with SP and RP, designed the study. KW analysed the data and RB and LB contributed to the running of the study, data collection, and interpretation. RB, JA, and RC wrote the report and all members of the writing committee critically reviewed it.
MRC/BHF Heart Protection Study collaborative group
Writing committee—R Bulbulia, L Bowman, K Wallendszus, S Parish, J Armitage, R Peto, R Collins. Steering committee—R Collins (principal investigator), T Meade (chairman), P Sleight (vice-chairman), J Armitage (clinical coordinator), S Parish and R Peto (statisticians), L Youngman (laboratory director), M Buxton, D de Bono (deceased), C George, J Fuller, A Keech, A Mansfield, B Pentecost, D Simpson, C Warlow; J McNamara and L O'Toole (MRC observers). Data monitoring committee—R Doll (chairman, deceased), L Wilhelmsen (vice-chairman), K M Fox, C Hill, P Sandercock. Collaborators—listed in reference 2.
Conflicts of interest
The Clinical Trial Service Unit has a staff policy of not accepting honoraria or other payments from the pharmaceutical industry, except for the reimbursement of costs to participate in scientific meetings. Members of the writing committee have, therefore, only had such costs reimbursed.
Web Extra Material
References
- 1.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol-lowering therapy and of antioxidant vitamin supplementation in a wide range of patients at increased risk of coronary heart disease death: early safety and efficacy experience. Eur Heart J. 1999;20:725–741. doi: 10.1053/euhj.1998.1350. [DOI] [PubMed] [Google Scholar]
- 2.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 3.Heart Protection Study Collaborative Group The effects of cholesterol lowering with simvastatin on cause-specific mortality and on cancer incidence in 20,536 high-risk people: a randomised placebo-controlled trial. BMC Med. 2005;3:6. [Google Scholar]
- 4.Cholesterol Treatment Trialists' (CTT) Collaboration Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs D, Blackburn H, Higgins M. Report of the conference on low blood cholesterol: mortality associations. Circulation. 1992;86:1046–1060. doi: 10.1161/01.cir.86.3.1046. [DOI] [PubMed] [Google Scholar]
- 6.Neaton JD, Blackburn H, Jacobs D. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor Intervention Trial. Arch Intern Med. 1992;152:1490–1500. [PubMed] [Google Scholar]
- 7.Oliver MF. Might treatment of hypercholesterolaemia increase non-cardiac mortality? Lancet. 1991;337:1529–1531. doi: 10.1016/0140-6736(91)93208-q. [DOI] [PubMed] [Google Scholar]
- 8.Davey Smith G, Pekkanen J. Should there be a moratorium on the use of cholesterol lowering drugs? BMJ. 1992;304:431–434. doi: 10.1136/bmj.304.6824.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. JAMA. 1996;275:55–60. [PubMed] [Google Scholar]
- 10.Strandberg TE, Pyorala PK, Cook TJ. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S) Lancet. 2004;364:771–777. doi: 10.1016/S0140-6736(04)16936-5. [DOI] [PubMed] [Google Scholar]
- 11.The Lipid Study Group Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up. Lancet. 2002;359:1379–1387. doi: 10.1016/S0140-6736(02)08351-4. [DOI] [PubMed] [Google Scholar]
- 12.Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane PW, Cobbe SM. Long-term follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med. 2007;357:1477–1486. doi: 10.1056/NEJMoa065994. [DOI] [PubMed] [Google Scholar]
- 13.Sever PS, Poulter NR, Dahlof B, on behalf of the ASCOT Investigators The Anglo-Scandinavian Cardiac Outcomes Trial lipid lowering arm: extended observations 2 years after trial closure. Eur Heart J. 2008;29:499–508. doi: 10.1093/eurheartj/ehm583. [DOI] [PubMed] [Google Scholar]
- 14.Holdaas H, Fellstrom B, Cole E. Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: the ALERT extension study. Am J Transplant. 2005;5:2929–2936. doi: 10.1111/j.1600-6143.2005.01105.x. [DOI] [PubMed] [Google Scholar]
- 15.Clark S, Youngman LD, Palmer A, Parish S, Peto R, Collins R. Stability of plasma analytes after delayed separation of whole blood: implications for epidemiological studies. Int J Epidemiol. 2003;32:125–130. doi: 10.1093/ije/dyg023. [DOI] [PubMed] [Google Scholar]
- 16.Farwell WR, Scranton RE, Lawler EV. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst. 2008;100:134–139. doi: 10.1093/jnci/djm286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.