Abstract
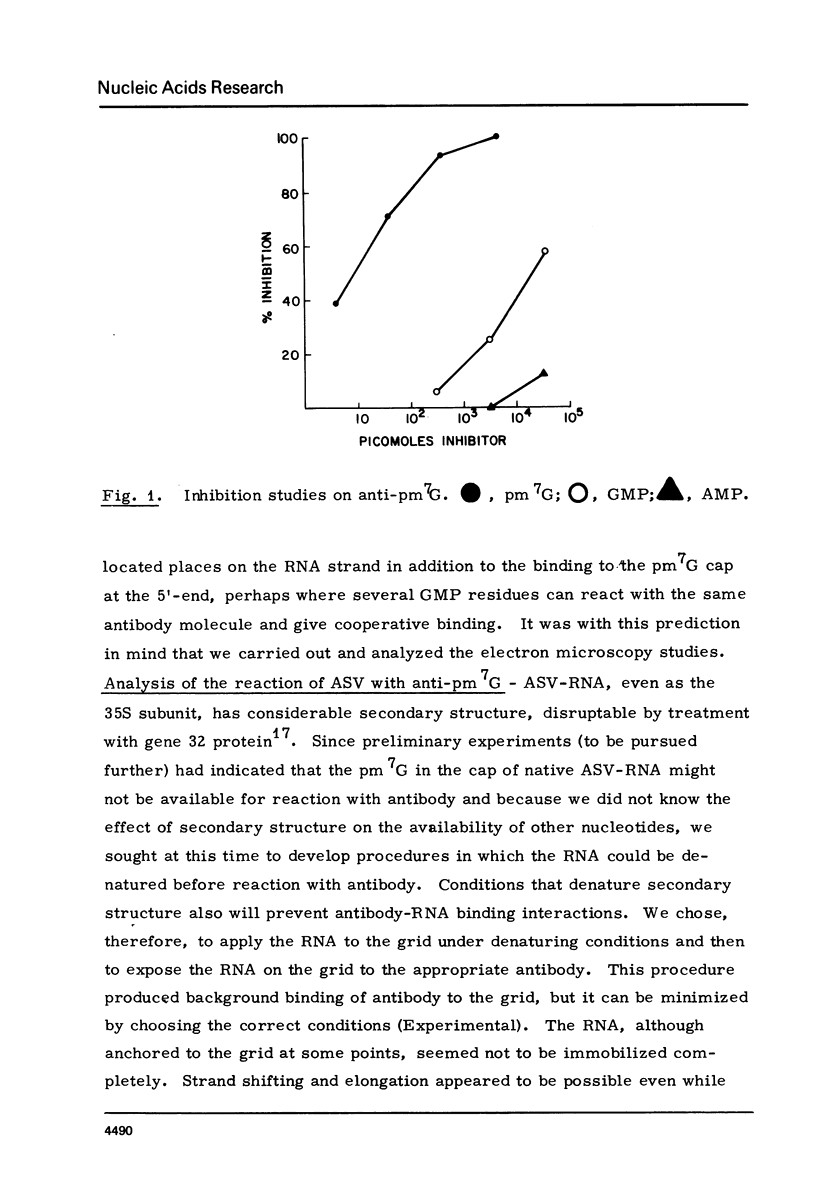
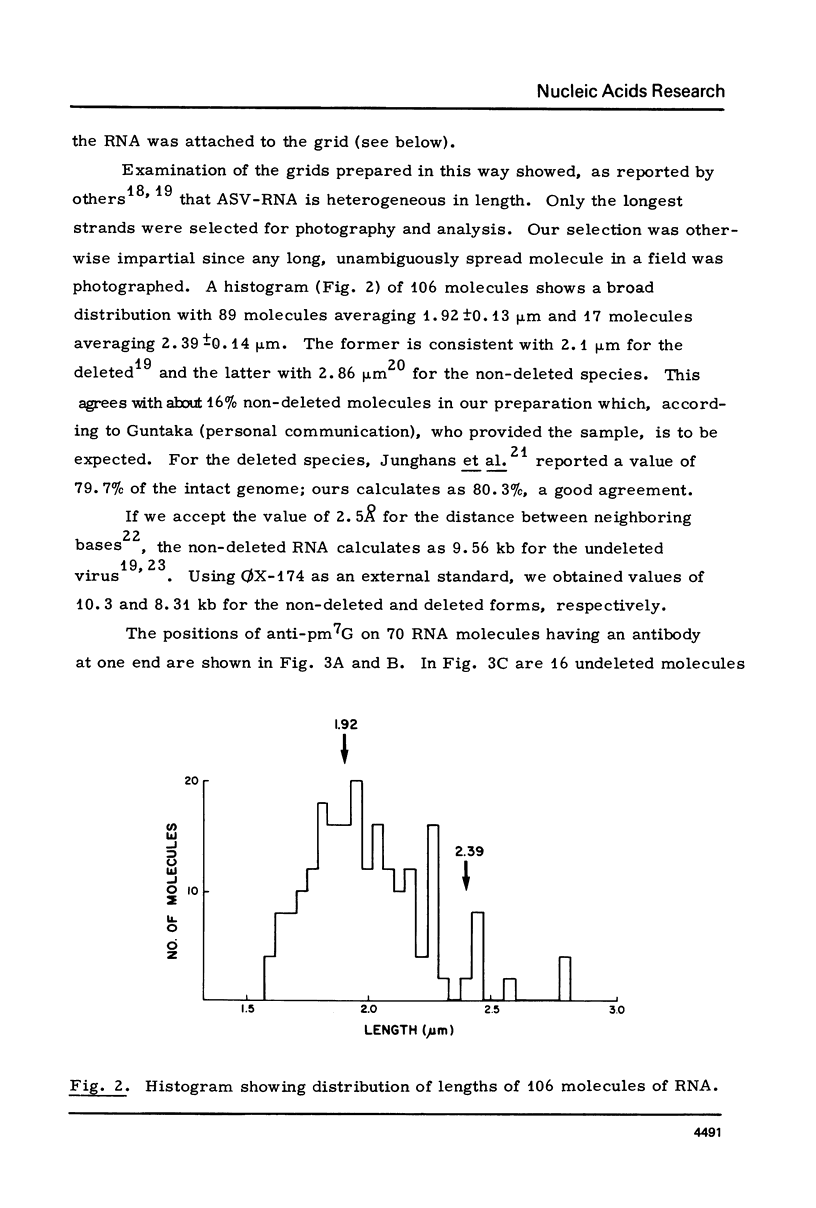
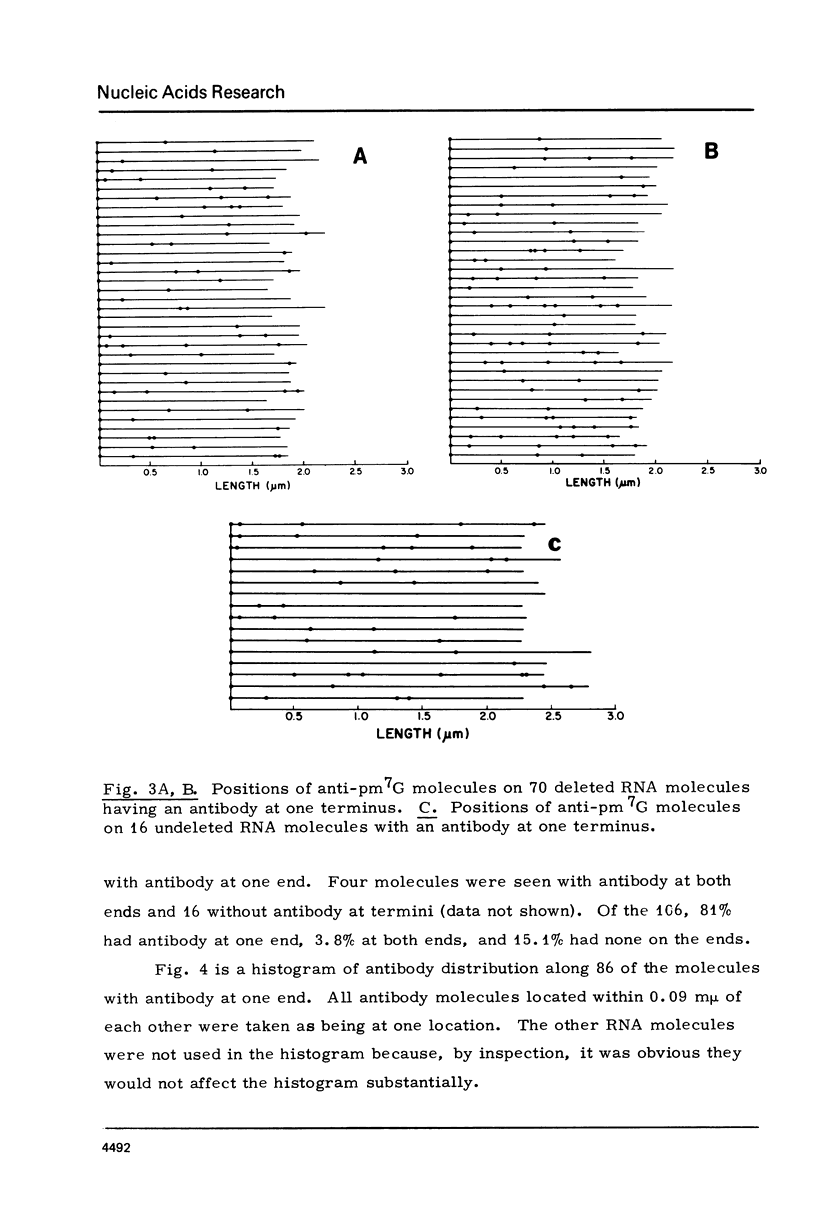
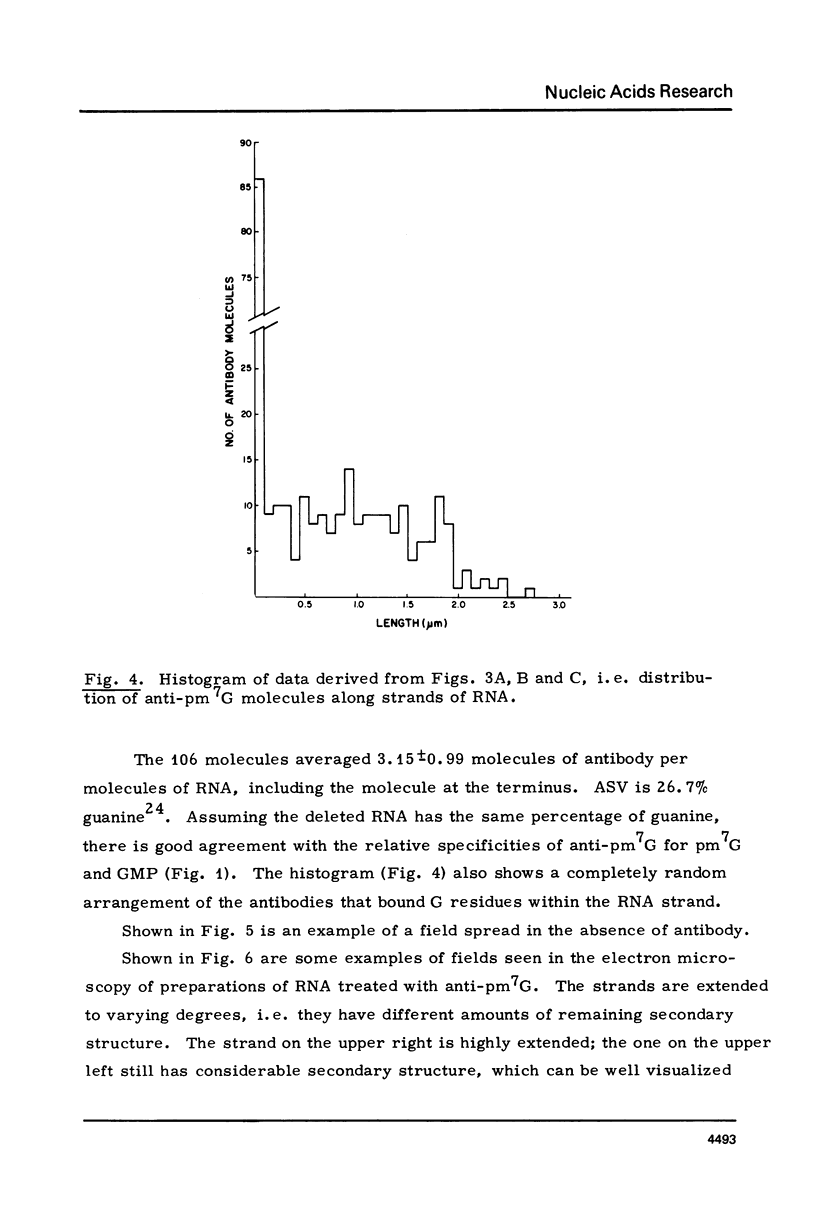
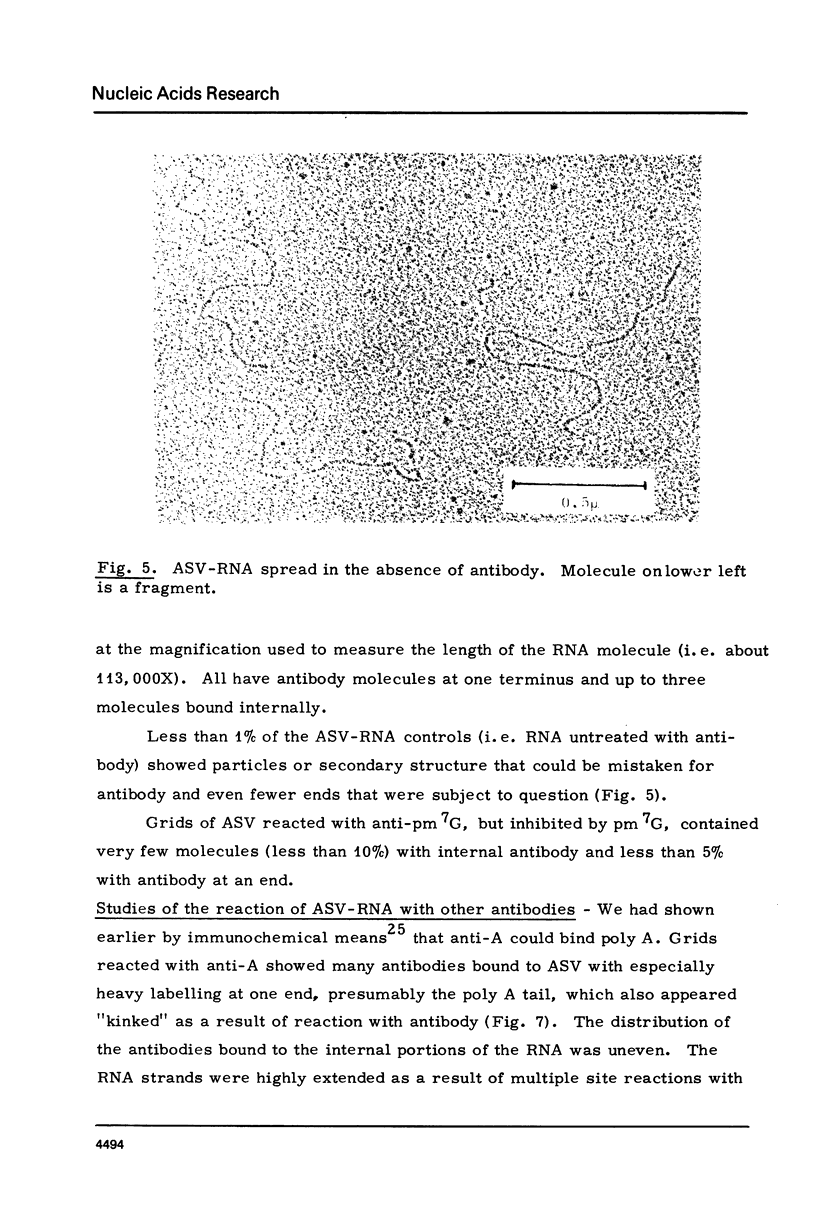
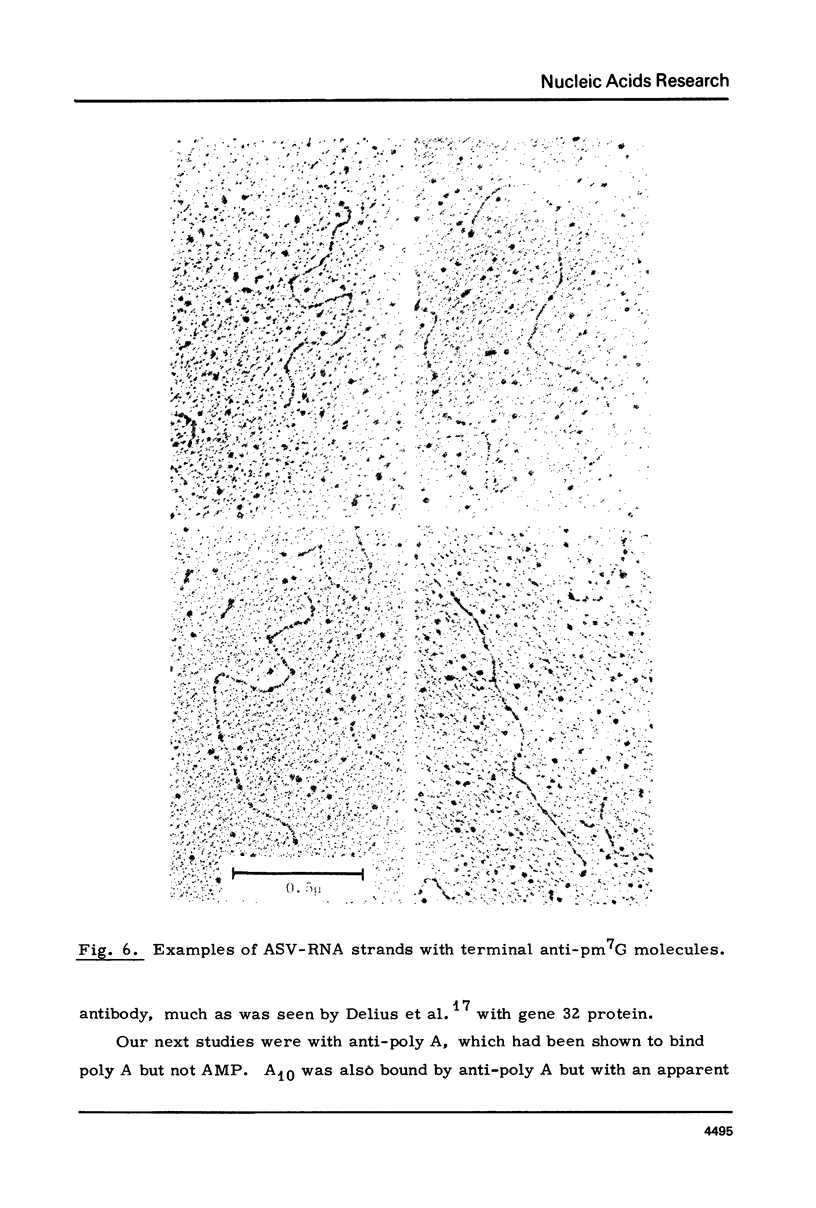
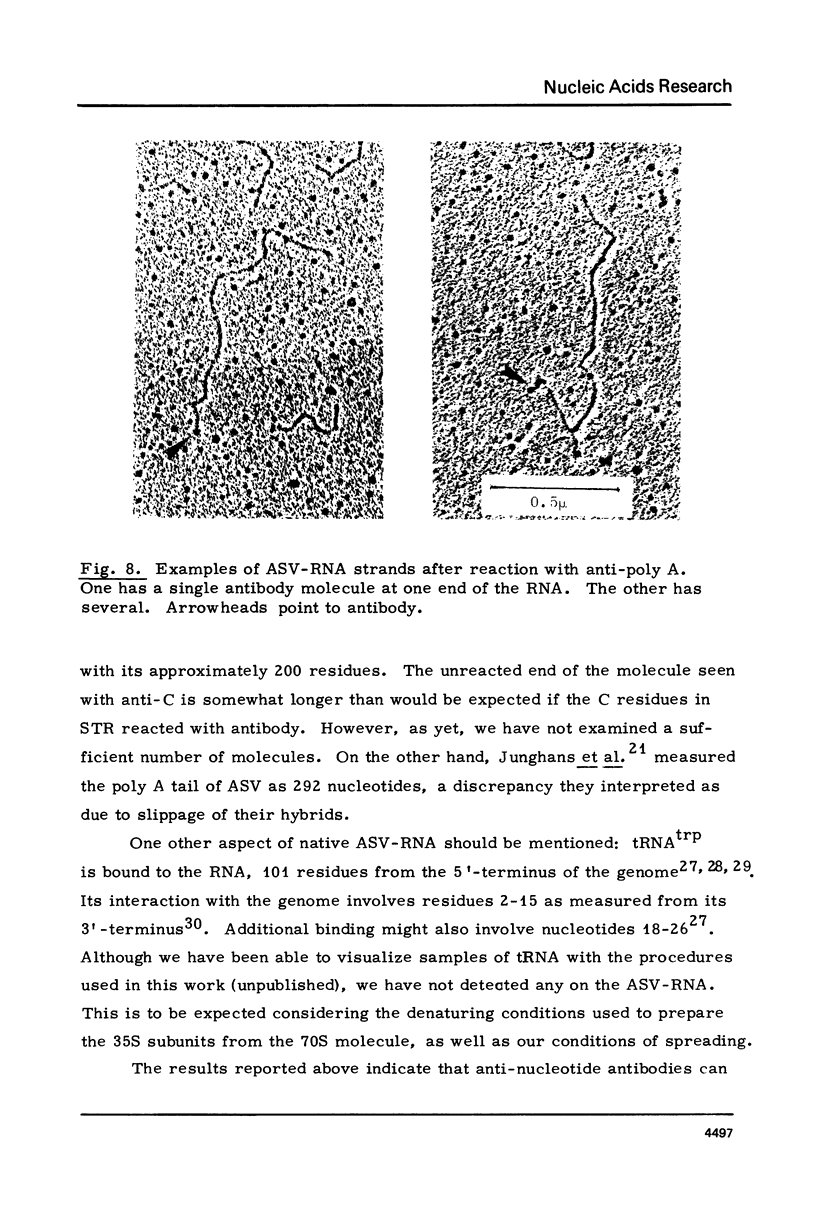
The RNA of a deleted strain (lacking Src gene) of an avian sarcoma virus (ASV) was examined by a newly developed immunoelectron microscopic procedure which uses anti-nucleotide antibodies as probes. After denaturation of the RNA and reaction with a high affinity, highly specific anti-7-methylguanosine-5'-phosphate (anti-pm 7G), 81% of 106 molecules examined were found to have antibody at one terminus, in agreement with the presence of a pm 7G cap in ASV-RNA. Hapten inhibition by pm 7G could be demonstrated. Experiments with anti-A and with anti-poly A gave results consistent with the known structure of ASV-RNA, in particular the presence of a 3' poly A tail. These studies illustrate the feasibility of using anti-nucleotide antibodies in a combined immunochemical and electron microscopic study of the fine structure of nucleic acids.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beiser S. M., Erlanger B. F. Antibodies which react with nucleic acids. Cancer Res. 1966 Sep;26(9):2012–2017. [PubMed] [Google Scholar]
- Cordell B., Stavnezer E., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence that binds primer for DNA synthesis to the avian sarcoma virus genome. J Virol. 1976 Aug;19(2):548–558. doi: 10.1128/jvi.19.2.548-558.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Duesberg P. H., Mangel W. F. Electron microscope measurements of rous sarcoma virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):835–843. doi: 10.1101/sqb.1974.039.01.097. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., BEISER S. M. ANTIBODIES SPECIFIC FOR RIBONUCLEOSIDES AND RIBONUCLEOTIDES AND THEIR REACTION WITH DNA. Proc Natl Acad Sci U S A. 1964 Jul;52:68–74. doi: 10.1073/pnas.52.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Garapin A. C., Levinson W. E., Bishop J. M., Goodman H. M. Characterization of the low-molecular-weight RNAs associated with the 70S RNA of Rous sarcoma virus. J Virol. 1973 Aug;12(2):334–342. doi: 10.1128/jvi.12.2.334-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. DNA structure: evidence from electron microscopy. Science. 1978 Aug 11;201(4355):525–527. doi: 10.1126/science.663672. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A. B., Bromley P. A. Molecular weight of RNA subunits of Rous sarcoma virus determined by electron microscopy. J Virol. 1975 Jan;15(1):161–166. doi: 10.1128/jvi.15.1.161-166.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Hu S., Knight C. A., Davidson N. Heteroduplex analysis of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1977 Feb;74(2):477–481. doi: 10.1073/pnas.74.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana Z. E., Erlanger B. F. Immunochemical study of the structure of poly(adenylic acid). Biochemistry. 1980 Jan 22;19(2):320–324. doi: 10.1021/bi00543a011. [DOI] [PubMed] [Google Scholar]
- Kung H. J., Bailey J. M., Davidson N., Vogt P. K., Nicolson M. O., McAllister R. M. Electron microscope studies of tumor virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):827–834. doi: 10.1101/sqb.1974.039.01.096. [DOI] [PubMed] [Google Scholar]
- Lubit B. W., Pham T. D., Miller O. J., Erlanger B. F. Localization of 5-methylcytosine in human metaphase chromosomes by immunoelectron microscopy. Cell. 1976 Dec;9(4 Pt 1):503–509. doi: 10.1016/0092-8674(76)90032-5. [DOI] [PubMed] [Google Scholar]
- Mangel W. F., Delius H., Duesberg P. H. Structure and molecular weight of the 60-70S RNA and the 30-40S RNA of the Rous sarcoma virus. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4541–4545. doi: 10.1073/pnas.71.11.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith R. D., Erlanger B. F. Isolation and characterization of rabbit anti-m7G-5'-P antibodies of high apparent affinity. Nucleic Acids Res. 1979;6(6):2179–2191. doi: 10.1093/nar/6.6.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller O. J., Erlanger B. F. Immunochemical probes of human chromosome organization. Pathobiol Annu. 1975;5:71–103. [PubMed] [Google Scholar]
- Miller O. J., Schnedl W., Allen J., Erlanger B. F. 5-Methylcytosine localised in mammalian constitutive heterochromatin. Nature. 1974 Oct 18;251(5476):636–637. doi: 10.1038/251636a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Sawicki D. L., Erlanger B. F., Beiser S. M. Immunochemical detection of minor bases in nucleic acids. Science. 1971 Oct 1;174(4004):70–72. doi: 10.1126/science.174.4004.70. [DOI] [PubMed] [Google Scholar]
- Shine J., Czernilofsky A. P., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence at the 5' terminus of the avian sarcoma virus genome. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1473–1477. doi: 10.1073/pnas.74.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll E., Billeter M. A., Palmenberg A., Weissmann C. Avian myeloblastosis virus RNA is terminally redundant: implications for the mechanism of retrovirus replication. Cell. 1977 Sep;12(1):57–72. doi: 10.1016/0092-8674(77)90185-4. [DOI] [PubMed] [Google Scholar]
- Szafran H., Beiser S. M., Erlanger B. F. The use of egg albumin conjugates for the purification of antibody. J Immunol. 1969 Nov;103(5):1157–1158. [PubMed] [Google Scholar]
- Vollenweider H. J., James A., Szybalski W. Discrete length classes of DNA depend on mode of dehydration. Proc Natl Acad Sci U S A. 1978 Feb;75(2):710–714. doi: 10.1073/pnas.75.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider H. J., Koller T., Parello J., Sogo J. M. Superstructure of linear duplex DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4125–4129. doi: 10.1073/pnas.73.11.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider H. J., Sogo J. M., Koller T. A routine method for protein-free spreading of double- and single-stranded nucleic acid molecules. Proc Natl Acad Sci U S A. 1975 Jan;72(1):83–87. doi: 10.1073/pnas.72.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. Properties and location of poly(A) in Rous sarcoma virus RNA. J Virol. 1974 Dec;14(6):1515–1529. doi: 10.1128/jvi.14.6.1515-1529.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst R. M., Vournakis J. N., Maxam A. M. Structure mapping of 5'-32P-labeled RNA with S1 nuclease. Biochemistry. 1978 Oct 17;17(21):4493–4499. doi: 10.1021/bi00614a021. [DOI] [PubMed] [Google Scholar]






