Abstract
Efficacy trials of antibody-inducing protein-in-adjuvant vaccines targeting the blood-stage Plasmodium falciparum malaria parasite have so far shown disappointing results. The induction of cell-mediated responses in conjunction with antibody responses is thought to be one alternative strategy that could achieve protective efficacy in humans. Here, we prepared chimpanzee adenovirus 63 (ChAd63) and modified vaccinia virus Ankara (MVA) replication-deficient vectors encoding the well-studied P. falciparum blood-stage malaria antigen merozoite surface protein 1 (MSP1). A phase Ia clinical trial was conducted in healthy adults of a ChAd63-MVA MSP1 heterologous prime-boost immunization regime. The vaccine was safe and generally well tolerated. Fewer systemic adverse events (AEs) were observed following ChAd63 MSP1 than MVA MSP1 administration. Exceptionally strong T-cell responses were induced, and these displayed a mixed of CD4+ and CD8+ phenotype. Substantial MSP1-specific serum immunoglobulin G (IgG) antibody responses were also induced, which were capable of recognizing native parasite antigen, but these did not reach titers sufficient to neutralize P. falciparum parasites in vitro. This viral vectored vaccine regime is thus a leading approach for the induction of strong cellular and humoral immunogenicity against difficult disease targets in humans. Further studies are required to assess whether this strategy can achieve protective efficacy against blood-stage malaria infection.
Introduction
Plasmodium falciparum malaria continues to account for ~0.8 million deaths and over 200 million cases every year.1 Vaccine strategies targeting the blood-stage of malaria infection have almost exclusively aimed to induce high-titer functional antibodies against target antigens that are involved in host red blood cell (RBC) invasion, such as the well-studied merozoite surface protein 1 (MSP1)2,3 and apical membrane antigen 1.4 Sustained efforts over many years have led to the clinical development of a number of candidate blood-stage malaria vaccines that are recombinant proteins formulated in a variety of adjuvants.5 Nonetheless, despite some encouraging clinical immunogenicity and the induction of functional antibodies capable of exerting growth inhibitory activity (GIA) against P. falciparum parasites in vitro,6,7,8 there has been no reported statistically significant efficacy with regard to clinical outcome in any phase IIa/b clinical trial published to date5 and the relationship between in vitro GIA and protective immunity in vivo remains far from clear.9,10 In recent years, experimental studies in human,11,12 nonhuman primate,13 and mouse14,15,16,17,18 malaria challenge models have indicated the potential for a protective contribution of T-cell responses, often independent of antibodies, against the blood-stage parasite. Importantly, no blood-stage malaria vaccine trialed to date has sought to induce effector T-cell responses in addition to protective antibodies against blood-stage malaria antigens, despite calls for such an approach.19
Vectored vaccine platforms were originally developed following the demonstration that these technologies are particularly suited for the induction of T-cell responses.20 Recently, candidate vaccines based on the human adenovirus serotype 5 (AdHu5) have been tested as candidate vaccines against human immunodeficiency virus-1 (HIV-1) in clinical trials.21,22 Despite encouraging levels of cellular immunogenicity, safety concerns in the HIV-1 STEP vaccine trial regarding the use of this vaccine vector in the context of pre-existing AdHu5 immunity in humans23 have led researchers to focus on other vectors. One alternative has been the development of simian adenoviral vaccine vectors,24 some of which can maintain the high levels of immune potency seen with AdHu525 and against which there is little pre-existing immunity in human populations.26 Preclinically, the deployment of such vectors in an adenovirus-poxvirus heterologous prime-boost immunization regime has demonstrated the ability of this approach to stimulate remarkably strong cellular as well as humoral immune responses in mice, rabbits, and nonhuman primates.27,28,29,30,31 This vaccine platform, encoding antigens such as MSP1 and apical membrane antigen 1, can mediate protective efficacy in rodent malaria models against both the blood-27 and liver-stage32 parasites and induces functional antibodies against P. falciparum.28,30,31
Here, we sought to test the safety and immunogenicity of this approach in an open-label dose-escalation phase Ia study using replication-deficient ChAd63 and the attenuated orthopoxvirus-modified vaccinia virus Ankara (MVA) encoding P. falciparum MSP1. MSP1 is synthesized as a large surface glycoprotein that undergoes proteolytic processing by the parasite upon erythrocyte invasion.2 At this time the 42-kDa C-terminus (MSP142) is cleaved into 33-kDa (MSP133) and 19-kDa (MSP119) fragments.33 The ChAd63 and MVA viral vaccines encode an insert that is composed of the conserved blocks of sequence (1, 3, 5, and 12) from P. falciparum MSP1 followed by the two most divergent allelic sequences (3D7 and Wellcome strains) encoding MSP142 fused in tandem28 (Supplementary Figure S1). The MSP1 antigen encoded by the vectors was designed to address antigenic polymorphism and to attempt to induce strain-transcending cellular and humoral immunity.28 Here, we show that this vaccine strategy is safe in malaria-naive adults and, in agreement with the preclinical data, can induce substantial MSP1-specific antibody responses in addition to exceptionally strong T-cell responses.
Results
Study recruitment and vaccinations
In total 16 healthy malaria-naive adult volunteers (9 female and 7 male) from the Oxford area were enrolled and immunized as described (Figure 1). The mean age of volunteers was 22.6 years (range 19–30 years). Vaccinations began in November 2009 and all follow-up visits were completed by September 2010.
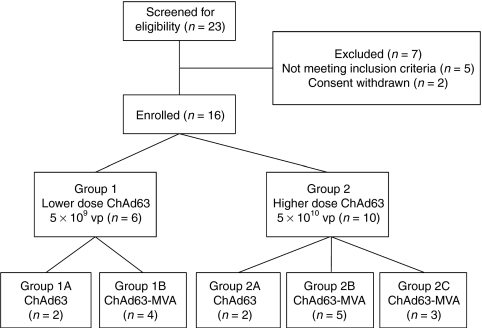
Figure 1.
Flow chart of the study. All vaccinations were administered intramuscularly. Chimpanzee adenovirus 63 (ChAd63) merozoite surface protein 1 (MSP1) dose-escalation was assessed (groups 1 versus 2), as well as the effect of modified vaccinia virus Ankara (MVA) MSP1 boosting (groups 1A versus 1B, and groups 2A versus 2B+C). The dose of MVA MSP1 was 5 × 108 plaque forming units (pfu). The three volunteers in group 2C (otherwise identical to group 2B) were subsequently recruited into a phase IIa sporozoite challenge safety and efficacy study (S.H. Sheehy et al., manuscript in preparation) at the day 84 time-point. Safety and immunogenicity data are included for these three volunteers up until that time-point.
Safety and reactogenicity
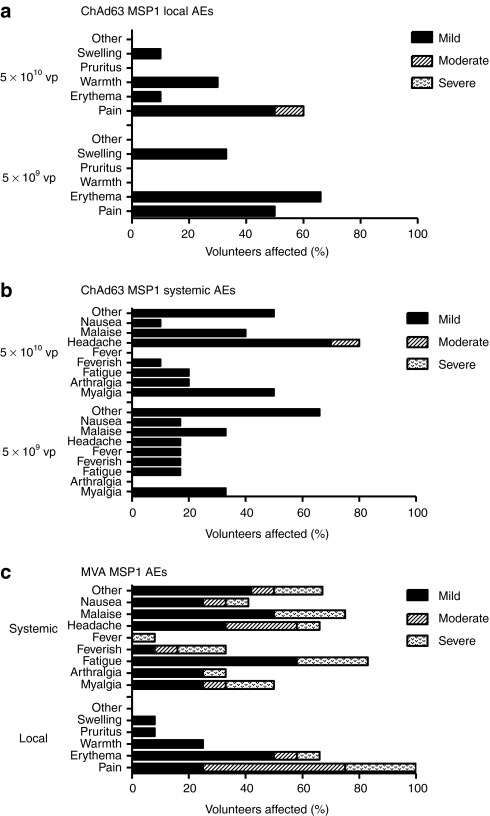
No unexpected or serious adverse events (AEs) occurred during the study and no volunteers were withdrawn due to AEs. ChAd63 MSP1 demonstrated an excellent safety profile at both 5 × 109 viral particles and 5 × 1010 viral particles doses; with the vast majority of local and systemic AEs mild in severity (97%) and all resolving completely (Figure 2a,b). MVA MSP1, administered at the relatively high poxviral dose of 5 × 108 plaque-forming units (pfu), was more reactogenic than ChAd63 MSP1 (Figure 2c); all vaccines experienced injection site pain, which was moderate or severe in 75% of volunteers. Other local AEs were mild in severity with the exception of two cases of delayed onset (3–5 days post vaccination) moderate or severe erythema, developing distal to, and not including the vaccine sites. All vaccinees described one or more systemic AEs post-MVA MSP1; whereas the majority of these were mild in severity, three volunteers (25%) experienced a constellation of severe systemic AEs (including rigors, malaise, myalgia, fatigue, and feverishness) which developed within 24 hours of vaccination and fully resolved within 5 days. Two volunteers received 5 × 108 pfu MVA MSP1 administered as two separate injections of 2.5 × 108 pfu in each deltoid to assess if split-dosing reduced local site pain and systemic reactogenicity. However, both these volunteers experienced severe systemic AEs and either moderate or severe local pain.
Figure 2.
Systemic and local adverse events (AEs) deemed definitely, probably, or possibly related to immunization. Only the highest intensity of each AE per subject is listed. Local and systemic reactogenicity was evaluated at clinic visits and graded for severity (mild, moderate, severe), outcome and association to vaccination as per the criteria outlined in Supplementary Tables S1–S4. Data are combined for all AEs for all volunteers receiving the same vaccine at the stated dose. There were no immunization related serious AEs. Immunizations took place between November 2009 and January 2010 (during a time of high local incidence of upper respiratory tract infections). (a) Local and (b) systemic AEs post-chimpanzee adenovirus 63 (ChAd63) merozoite surface protein 1 (MSP1). (c) Local and systemic AEs post-modified vaccinia virus Ankara (MVA) MSP1. “Other” AEs are detailed in Supplementary Materials and Methods.
ChAd63-MVA MSP1 T-cell immunogenicity assessed by ex-vivo IFN-γ ELISPOT
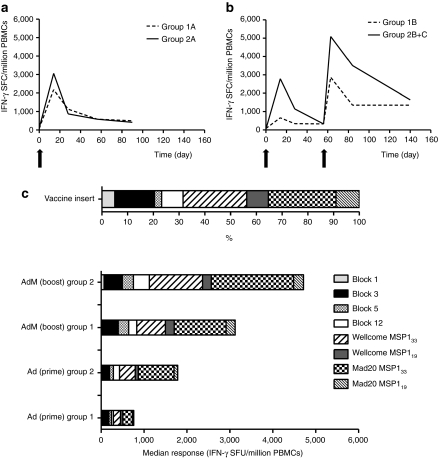
T-cell responses to the MSP1 vaccine insert were assessed over time by ex-vivo interferon-γ (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT). Group medians are shown in Figure 3a,b, with individual responses shown in Supplementary Figure S2. Following the ChAd63 MSP1 prime, there was a trend for stronger median responses in the higher dose group at the peak of the response on day 14 (median 2,785 versus 979 spot-forming units (SFU)/million peripheral blood mononuclear cells (PBMC) in groups 2 versus 1, respectively, n = 10 versus 6, P = 0.07) (Figure 3a). Responses contracted by day 56 and were maintained at day 90 in groups 1A and 2A. Administration of MVA MSP1 at day 56 significantly boosted these responses in all volunteers as measured 1 week later. The median response in group 2B+C was higher at day 63 in comparison to group 1B but this did not reach significance (median 5,090 versus 2,868 SFU/million PBMC in groups 2B+C versus 1B, respectively, n = 8 versus 4, P = 0.37) (Figure 3b). Responses again contracted but were maintained at high levels at the end of the study period (day 140) with significantly higher responses in group 2B in comparison to group 1B (median 1,640 versus 1,347 SFU/million PBMC in groups 2B versus 1B, respectively, n = 5 versus 4, P = 0.02).
Figure 3.
Cellular immunogenicity of chimpanzee adenovirus 63 (ChAd63) merozoite surface protein 1 (MSP1) and ChAd63-modified vaccinia virus Ankara (MVA) MSP1 immunization regimes. Median ex-vivo interferon-γ (IFN-γ) ELISPOT responses in peripheral blood mononuclear cells (PBMC) to the MSP1 insert (summed response across all the individual peptide pools) are shown over time for (a) groups 1A and 2A, and (b) group 1B and groups 2B+C (d0–d84 time-points include data combined for groups 2B+2C, d140 time-point includes only data from group 2B). (c) The percentage of the amino acid sequence within the MSP1 vaccine insert (Supplementary Figure S1) that is attributable to each block is shown (top). Data show the median total response to each block of sequence within the MSP1 insert according to group (1 or 2) and immunization regime (Ad = ChAd63, AdM = ChAd63-MVA) at the peak time-point (d14 after ChAd63 and d63/d84 after ChAd63-MVA).
Breadth of the MSP1 T-cell response
T-cell responses in all volunteers were detected in multiple peptide pools spanning the entire MSP1 vaccine insert in the ELISPOT assay. Individual responses are shown according to magnitude of the response (Supplementary Figure S3) or as a percentage of the total summed ELISPOT response (Supplementary Figure S4). Irrespective of whether the responses are analyzed after the priming immunization or following the MVA MSP1 boosting immunization, the individual and median responses broadly mirror the composition of the vaccine antigen (Figure 3c). These data indicate that no single immunodominant region exists within the MSP1 transgene insert.
MSP1 T-cell multifunctionality
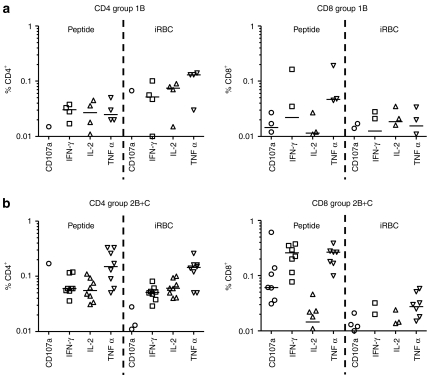
Antigen-specific CD3+ T-cell functionality was assayed at the d84 time-point (Figure 4). Following peptide restimulation, detectable MSP1-specific CD3+ T-cells consisted of a mixed CD4+ and CD8+ phenotype, with stronger median responses seen for both subsets in group 2B+C in comparison to group 1B. CD8+ T cells upregulated CD107a expression (marker of degranulation), and produced IFN-γ and tumor necrosis factor-α but only negligible levels of interleukin (IL)-2. In comparison the CD4+ T cells produced IFN-γ, tumor necrosis factor-α, and IL-2, but did not upregulate CD107a expression. Following restimulation with cryopreserved iRBCs, comparable CD4+ T-cell responses were evident to those seen following peptide restimulation but, in contrast, CD8+ T-cell responses were barely detectable. Distinct populations of CD4+ and CD8+ T cells expressing 1+, 2+, 3+, or 4+ functional markers/cytokines were evident following a boolean gate analysis (Supplementary Figure S5).
Figure 4.
Multifunctionality of the CD3+ T-cell responses was assessed by polychromatic flow cytometry and intracellular cytokine staining (ICS) following chimpanzee adenovirus 63 (ChAd63)-modified vaccinia virus Ankara (MVA) immunization at d84. PBMC from (a) group 1B and (b) group 2B+C were restimulated with a pool of merozoite surface protein 1 (MSP1) peptides or cryopreserved iRBCs. Individual data points and the median are shown for the % CD4+ and CD8+ T cells positive for CD107a, interferon-γ (IFN-γ), interleukin-2 (IL-2), or tumor necrosis factor-α (TNF-α). Responses <0.01% are not shown.
ChAd63-MVA MSP1 antibody immunogenicity
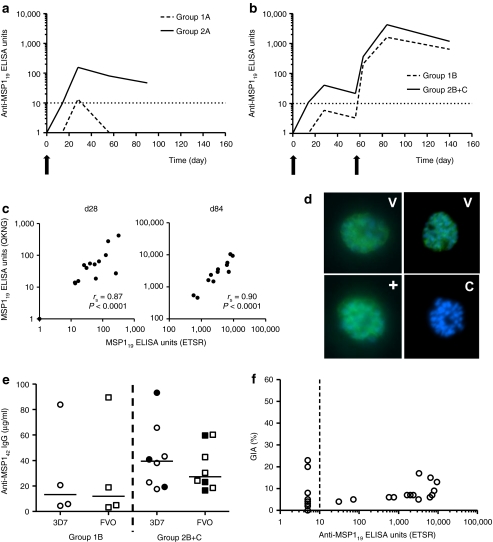
Serum immunoglobulin G (IgG) antibody responses against MSP119 were assessed by enzyme-linked immunosorbent assay (ELISA). Group geomeans are shown in Figure 5a,b, with individual responses shown in Supplementary Figure S6. Significantly stronger responses were measured in the higher dose group at the peak of the primary response on day 28 (geomean titer 53.1 versus 7.8 MSP1 AU in groups 2 versus 1, respectively, n = 10 versus 6, P = 0.03) (Figure 5a). Responses subsequently declined by day 56 and were only maintained above the detection limit at day 90 in group 2A. Administration of MVA MSP1 at day 56 significantly boosted these responses in all volunteers, with serum IgG responses peaking 4 weeks later as measured on day 84 (Figure 5b). At this time-point, MSP119-specific IgG responses only tended to be stronger in group 2B+C in comparison to group 1B (geomean titer 4,266 versus 1,618 MSP1 AU in groups 2B+C versus 1B, respectively, n = 8 versus 4, P = 0.21) (Figure 5b). Responses again declined over time but were maintained at high levels at the end of the study period (day 140) with responses in group 2B again tending to be stronger than those in group 1B. Serum IgG responses against the Wellcome allele of MSP119 (which differs from the 3D7/Mad20 allele by four amino acids) were assessed by ELISA, and a strong correlation was evident between the responses against the two alleles (Figure 5c). Recognition of native parasites by sera of all ChAd63-MVA MSP1 immunized volunteers was confirmed by immunofluorescence against 3D7 strain P. falciparum schizonts (Figure 5d).
Figure 5.
Antibody immunogenicity of chimpanzee adenovirus 63 (ChAd63) merozoite surface protein 1 (MSP1) and ChAd63-modified vaccinia virus Ankara (MVA) MSP1 immunization regimes. (a) Total immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) responses against 3D7 PfMSP119 (ETSR/Mad20 allele) as measured in the serum over time following immunization. The geometric mean response is shown for each group and the dotted line represents the limit of detection of the assay. (a) groups 1A and 2A, and (b) group 1B and groups 2B+C (d0–d84 time-points include data combined for groups 2B+2C, d140 time-point includes only data from group 2B). (c) Spearman's correlation of serum IgG ELISA titers against PfMSP119 for the 3D7 ETSR versus Wellcome QKNG alleles after ChAd63 MSP1 priming at d28, n = 16 (left panel), and at the peak time-point (d84) after ChAd63-MVA MSP1 immunization, n = 12 (right panel). (d) Immunofluorescence (IFA) showing the recognition of 3D7 strain P. falciparum schizonts by immunoglobulin G (IgG) (green) in the sera of ChAd63-MVA MSP1 vaccinated volunteers. Day 84 sera from all 12 volunteers in groups 1B and 2B+C tested positive, but two representative results from vaccines (V) are shown (top row). DNA was counterstained with DAPI (blue). A representative control (C) testing pooled preimmunization sera was negative (bottom right), and a human monoclonal antibody (mAb) specific for PfMSP119 was included as a positive control (+) (bottom left). (e) ELISA titers against 3D7 and FVO PfMSP142 in µg/ml for group 1B (n = 4) and groups 2B+C (n = 8) at the peak time-point (d84). Individual data points and the median are shown. Groups 1B and 2B (open symbols), group 2C (closed symbols). (f) Relationship between 3D7 strain % GIA using purified immunoglobulin G (IgG) at 10 mg/ml and serum 3D7 PfMSP119-specific IgG ELISA titer. Samples testing less than the minimal detection level by ELISA were set at 5.0 ELISA units. All individual data points for all time-points assayed are shown (n = 30).
Antibody responses against the other regions of MSP1 were assayed by ELISA (Supplementary Figure S7). Responses were dominated by the MSP142 region, with no antibodies detectable against the MSP183 and MSP138 regions also present in the vaccine antigen. Concentrations of serum IgG specific for both alleles of MSP142 were quantified by ELISA at the peak time-point (d84), with median titers of 39.5 and 27.3 µg/ml anti-MSP142 3D7 and FVO IgG, respectively, in group 2B+C (Figure 5e). In agreement with published data for other MSP1-based protein vaccines,7,34 IgG responses of this magnitude did not induce functional GIA above baseline against the 3D7 strain of P. falciparum in vitro (Figure 5f and Supplementary Figure S8).
Discussion
In this phase Ia dose-escalation and safety study, we have shown in healthy adult volunteers that a recombinant ChAd63-MVA heterologous prime-boost immunization regime can induce substantial antigen-specific antibody responses in addition to exceptionally strong T-cell responses—an attribute unparalleled to date by other subunit vaccination strategies. Although both vectors were safe, the MVA MSP1 vaccine was less well tolerated than ChAd63 MSP1, an effect likely related to the relatively high dose of MVA used. A subsequent study has now shown comparable immunogenicity with a more acceptable tolerability profile when MVA MSP1 is used at a lower dose of 2 × 108 pfu (S.H. Sheehy, C.J.A. Duncan, S.C. Elias, K.A. Collins, A.V.S. Hill, S.J. Draper et al., manuscript in preparation).
A growing body of data now indicates the potential for a protective contribution of T-cell responses to blood-stage malaria immunity.19 Despite the fact that parasites reside within RBCs, which lack MHC molecules capable of directly presenting parasite antigen, it is suggested that T helper 1 (Th1)-type CD4+ T-cells may activate macrophages in the spleen leading to enhanced opsonization of infected RBCs. Alternatively, Th1-type CD4+ T cells may bias the induction of cytophilic, rather than neutralizing, antibodies from B cells that can mediate antiparasitic neutrophil respiratory activity35 or antibody-dependent cellular cytotoxicity via monocytes.36 Moreover, CD8+ T-cell responses against classical “blood-stage” antigens, including MSP1, can target the preceding late liver-stage parasite forms (given such antigens are expressed by parasites towards the end of hepatic development).32,37,38 The ChAd63-MVA delivery platform was thus developed to induce effector T-cell responses in addition to functional antibodies against a target blood-stage malaria antigen in humans. The data presented here show that MSP1-specific T cell responses peaked at a median level of >5,000 SFU/million PBMC in the full-dose ChAd63-MVA group. This median response is, to our knowledge, the highest yet reported following immunization with any vectored, or other type of, vaccine regimen.20 Data for candidate HIV-1 vaccines have shown average T-cell responses of lower magnitude following the use of recombinant human adenoviruses in prime-boost regimes in primates or humans.22,39,40 Given recent concerns surrounding the use of AdHu5 in humans,20 it remains encouraging that such strong T-cell immunogenicity can be achieved in adult volunteers by using just two immunizations and a nonhuman serotype chimpanzee adenovirus (ChAd) and MVA vaccine vectors.
T-cell responses in mice and humans vaccinated with MSP142 have frequently been reported in the MSP133 region32,41 and these can provide essential CD4+ T cell help for B cell responses against MSP119, as well as CD8+ T-cell responses that can reduce the liver-stage parasite burden in the Plasmodium yoelii mouse model.32 In this study, T-cell responses as measured by ELISPOT were detected across the entire 3.33 kbp MSP1 antigen insert. Although a large portion of this response was focused on the two allelic regions of MSP133 contained within the vaccine antigen, responses were also detected against the more conserved blocks of sequence. This is in contrast to preclinical mouse data,28 but in agreement with limited data from naturally exposed individuals.42 T-cell responses can thus be induced by vaccination in humans against relatively conserved epitopes, as well as against naturally dimorphic sequences contained within the same antigenic insert, and these data bode well for the potential to induce cellular immune responses that may contribute to strain-transcending immunity in the field.
MSP1-specific CD3+ T cells consisted of a mixed CD4+ and CD8+ phenotype, and in vitro restimulation of these cells with MSP1 peptides confirmed the presence of multifunctional subsets. Given the wealth of phenotypic and functional markers that can now be assayed, it will remain an important area of work within the field to ascertain the protective contribution, if any, of such cellular phenotypes against P. falciparum malaria. Interestingly, restimulation of PBMC with iRBC showed comparable CD4+ but not CD8+ T-cell responses in immunized volunteers. It remains to be determined whether this is due to impaired or insufficient antigen processing and crosspresentation of native parasite MSP1 onto class I MHC molecules, and whether in vitro analysis using peptide or parasite restimulation is most relevant to the elucidation of T cell functions in vivo.
The ChAd63-MVA prime-boost regime also induced substantial MSP1-specific serum IgG antibody responses, as predicted by animal models.27,28 Unlike studies in rabbits28 and naturally exposed adults,43 antibodies were almost exclusively detected against the MSP142 region of the antigen. Within MSP142, antibody responses against MSP119, but not MSP133, are associated with protection against malaria incidence in naturally exposed individuals,44 and have also been shown to account for the protective immunity induced by this antigen in animal models.45,46 The vaccine-induced MSP119-specific antibodies were broadly crossreactive by ELISA against both alleles of MSP119 and were capable of recognizing native parasite MSP1 when tested by immunofluorescence, however, purified IgG failed to show detectable GIA in vitro. This is in agreement with published data suggesting titers of >600 µg/ml MSP1-specific human IgG are required to achieve 50% GIA against 3D7 strain parasites in this assay.34 When quantified by a standardized ELISA, the concentrations of MSP142-specific IgG induced by ChAd63-MVA at the peak of the response (median 39.5 µg/ml against the 3D7 strain antigen) were comparable to or higher than MSP142 protein vaccines formulated in Alum7 or AS02,8 but three- to fourfold lower than Alum + CpG.7 These data confirm that antibody titers, comparable to some protein-in-adjuvant formulations, can be generated in humans by an adenovirus-MVA vectored vaccine regime. Further studies are now essential to ascertain whether the induction of strong cellular immunity, in conjunction with this level of antibody response, can enhance vaccine efficacy against blood-stage infection in malaria challenge phase IIa studies.
Overall, vaccine developers targeting challenging diseases such as HIV-1, malaria, and cancer have struggled for many years to translate promising immunogenicity in animal models into high magnitude immune responses in humans. This work indicates that the ChAd63-MVA viral vectored vaccine regimen now provides a safe and clinically relevant strategy for the development of highly immunogenic vaccines where strong cellular and/or humoral immune responses are likely to be required for protection.
Materials and Methods
Study design, participants, vaccination, and follow-up. Full details relating to trial protocol are provided in Supplementary Materials and Methods. Sixteen volunteers were recruited for the study under a protocol approved by the UK Gene Therapy Advisory Committee (GTAC 166) and the UK Medicines and Healthcare products Regulatory Agency (Ref: 21584/0253/001-0001). All volunteers gave written informed consent before participation. The study was conducted according to the principles of the Declaration of Helsinki and in accordance with Good Clinical Practice. The trial was registered with ClinicalTrials.gov (Ref: NCT01003314). Volunteers were not screened for pre-existing neutralizing antibodies to the ChAd63 vector,26 and there was no selection of volunteers on the basis of low neutralizing antibodies titers. Six volunteers (group 1) were vaccinated with 5 × 109 viral particles ChAd63 MSP1 intramuscularly and four of these were subsequently vaccinated with 5 × 108 pfu MVA MSP1 intramuscularly 56 days later. Another 10 volunteers (group 2) were vaccinated with 5 × 1010 viral particles ChAd63 MSP1 intramuscularly and eight of these were subsequently vaccinated with 5 × 108 pfu MVA MSP1 intramuscularly 56 days later (Figure 1).
Volunteers attended clinical follow-up at days 2, 14, 28, 56, and 90 following ChAd63 MSP1 immunization (groups 1A and 2A), and days 2, 14, 28, 56, 58, 63, 84, and 140 following ChAd63-MVA MSP1 immunization (groups 1B and 2B+C). A time window ranging between 1 and 14 days was allowed for vaccination and follow-up visits. Throughout the paper, study day refers to the nominal time-point for a group and not the actual day of sampling.
Vaccines. The ChAd63 (previously termed AdCh63) and MVA vaccines encode an insert previously termed PfM128.28 Recombinant ChAd63 MSP128 and markerless recombinant MVA MSP129 were manufactured under Good Manufacturing Practice conditions by the Clinical Biomanufacturing Facility, University of Oxford (ChAd63 MSP1), and IDT Biologika, Rosslau, Germany (MVA MSP1). Each vaccine lot underwent comprehensive quality control analysis to ensure that the purity, identity, and integrity of the virus met predefined specifications.
Ex-vivo IFN-γ ELISPOT. Detailed methodologies for all immunological analyses are provided in Supplementary Materials and Methods. Briefly, fresh PBMC were prepared and used in all ELISPOT assays as previously described,47 except that 50 µl/well MSP1 peptide pools (Supplementary Table S5) (final concentration each peptide 5 µg/ml) were added to test wells. Results are expressed as IFN-γ SFU per million PBMC. Background responses in unstimulated control wells were almost always <20 spots, and were subtracted from those measured in peptide-stimulated wells.
Multiparameter flow cytometry. Cytokine secretion by PBMC was assayed by intracellular cytokine staining followed by flow cytometry. Frozen PBMC were restimulated for 18 hours in the presence of MSP1 vaccine insert-specific peptides or with cryopreserved RBCs infected with schizont/late trophozoite stage 3D7 strain P. falciparum parasites (iRBC). Cells were stained with a live/dead maker as well as antihuman CD4, CD14, CD20, CD8α, CD3, IFN-γ, tumor necrosis factor-α, CD107a, and IL-2 (Supplementary Figure S9) before analysis using a LSRII Flow Cytometer (BD Biosciences, Franklin Lakes, NJ), FlowJo v8.8 (Tree Star, Ashland, OR), and SPICE v5.1, downloaded from http://exon.niaid.nih.gov/spice.48 Background responses in unstimulated peptide and uninfected RBC control cells were subtracted from the MSP1 peptide and iRBC-stimulated responses, respectively.
Total IgG ELISA. The production of recombinant GST-PfMSP119 (either ETSR 3D7/Mad20 allele or the QKNG Wellcome/K1 allele) or GST control protein for ELISA assays has been described elsewhere.28 Diluted sera were tested for PfMSP119-specific antibody responses according to published standardized ELISA methodology.49 All sera tested against the GST control protein were less than the minimal detection level of the assay (data not shown). Antibodies against PfMSP142 (3D7 and FVO alleles) were assayed by the GIA Reference Center, National Institutes of Health, as previously described,49 and these OD-based ELISA units were converted to µg/ml also as described previously.34 Antibodies against the PfMSP183, PfMSP130, PfMSP138, and PfMSP142 regions of MSP1 were assayed as previously described.43
Immunofluorescence assay. Cultured P. falciparum (3D7 strain) parasites were smeared onto glass slides, fixed, permeabilized, quenched, and then blocked. Volunteer sera were diluted 1:100 and allowed to bind, before the addition of goat antihuman IgG-Alexa 488 conjugate (Invitrogen, Paisley, UK). A negative control was included, consisting of a pool of preimmunization sera. A positive control was included consisting of a human PfMSP119-specific monoclonal antibody (a human IgG3 recognizing epitope C150) tested at 2 µg/ml (a kind gift from Prof Richard Pleass, Liverpool School of Tropical Medicine, Liverpool, UK). DNA was counterstained with DAPI, and the slides were viewed under a Leica DMI3000 microscope (Leica Microsystems, Milton Keynes, UK).
In vitro GIA assay. The ability of induced anti-MSP1 antibodies to inhibit growth of P. falciparum 3D7 parasites was assessed by a standardized GIA assay using purified IgG as previously described.34 Briefly, each test IgG (10 mg/ml in a final test well) was incubated with synchronized P. falciparum parasites for ~48 hours and relative parasitemia levels were quantified by biochemical determination of parasite lactate dehydrogenase.
Statistical analysis. Data were analyzed using GraphPad Prism version 5.03 for Windows (GraphPad Software, La Jolla, CA). Geometric mean or median responses are shown for each group. Significance testing of differences between two group means used the two-tailed Mann–Whitney U-test. Correlations were analyzed using Spearman's rank correlation coefficient (rs) for nonparametric data. A value of P < 0.05 was considered significant in all cases.
SUPPLEMENTARY MATERIAL Figure S1. MSP1 vaccine insert. Figure S2. Individual ex-vivo IFN-γ ELISPOT data. Figure S3. Breakdown of ex-vivo IFN-γ ELISPOT data according to total response. Figure S4. Breakdown of ex-vivo IFN-γ ELISPOT data according to % response. Figure S5. T-cell multifunctionality following ChAd63-MVA MSP1 immunization. Figure S6. Individual IgG ELISA data. Figure S7. IgG ELISA against all regions of MSP1. Figure S8. Functional GIA of purified IgG. Figure S9. Gating strategy for analysis of MSP1-specific T-cell responses. Table S1. Assessment of severity. Table S2. Assessment of local AEs. Table S2. Assessment of local AEs. Table S3. Assessment of outcome. Table S4. Assessment of relatedness. Table S5. MSP1 overlapping peptides. Materials and Methods.
Acknowledgments
We thank C. Bateman, M. Smith, J. Meyer, and P. Lillie for clinical assistance; L. Dinsmore and K. Gantlett for logistical support; M. Cottingham, S. Saurya, J. Furze, N. Edwards, C. Oliveira, E. Bolam, P. Bird, E. Mukhopadhyay, A. Crook, A. Turner, and N. Green for assistance with vaccine manufacture; the Jenner Institute Flow Cytometry and Vector Core Facilities for technical assistance; H. Zhou and G. Tullo for technical support performing the GIA assays; A. Holder for comments on the manuscript; and all the study volunteers. This work was supported by the UK Medical Research Council (MRC) (grant number G0700735), the European Malaria Vaccine Development Association (EMVDA), a European Commission FP6-funded consortium (LSHP-CT-2007-037506), the UK National Institute of Health Research through the Oxford Biomedical Research Centre (A91301 Adult Vaccine) and the Wellcome Trust [084113/Z/07/Z]. The GIA work was supported by the PATH Malaria Vaccine Initiative (MVI) and the Intramural Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases. S.C.G., A.V.S.H., and S.J.D. are Jenner Investigators; A.V.S.H. was supported by a Wellcome Trust Principal Research Fellowship (45488/Z/05) and S.J.D. is a MRC Career Development Fellow (G1000527). A.R.W., S.C.G., A.V.S.H., and S.J.D. are named inventors on patent applications covering malaria vectored vaccines and immunization regimes. Authors from Okairòs are employees of and/or shareholders in Okairòs which is developing vectored vaccines for malaria and other diseases.
Supplementary Material
MSP1 vaccine insert.
Individual ex-vivo IFN-γ ELISPOT data.
Breakdown of ex-vivo IFN-γ ELISPOT data according to total response.
Breakdown of ex-vivo IFN-γ ELISPOT data according to % response.
T-cell multifunctionality following ChAd63-MVA MSP1 immunization.
Individual IgG ELISA data.
IgG ELISA against all regions of MSP1.
Functional GIA of purified IgG.
Gating strategy for analysis of MSP1-specific T-cell responses.
Assessment of severity.
Assessment of local AEs.
Assessment of outcome.
Assessment of relatedness.
MSP1 overlapping peptides.
REFERENCES
- World Health Organization Aregawi M, Cibulskis RE, Otten M, Williams R, WHO Global Malaria Programme . World Malaria Report 2009. World Health Organization: Geneva; 2009. Surveillance Monitoring and Evaluation Unit. [Google Scholar]
- Holder AA. The carboxy-terminus of merozoite surface protein 1: structure, specific antibodies and immunity to malaria. Parasitology. 2009;136:1445–1456. doi: 10.1017/S0031182009990515. [DOI] [PubMed] [Google Scholar]
- Holder AA, Lockyer MJ, Odink KG, Sandhu JS, Riveros-Moreno V, Nicholls SC.et al. (1985Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites Nature 317270–273. [DOI] [PubMed] [Google Scholar]
- Remarque EJ, Faber BW, Kocken CH., and, Thomas AW. Apical membrane antigen 1: a malaria vaccine candidate in review. Trends Parasitol. 2008;24:74–84. doi: 10.1016/j.pt.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Goodman AL., and, Draper SJ. Blood-stage malaria vaccines - recent progress and future challenges. Ann Trop Med Parasitol. 2010;104:189–211. doi: 10.1179/136485910X12647085215534. [DOI] [PubMed] [Google Scholar]
- Spring MD, Cummings JF, Ockenhouse CF, Dutta S, Reidler R, Angov E.et al. (2009Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A PLoS ONE 4e5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RD, Martin LB, Shaffer D, Long CA, Miura K, Fay MP.et al. (2010Phase 1 trial of the Plasmodium falciparum blood stage vaccine MSP1(42)-C1/Alhydrogel with and without CPG 7909 in malaria naive adults PLoS ONE 5e8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogutu BR, Apollo OJ, McKinney D, Okoth W, Siangla J, Dubovsky F, MSP-1 Malaria Vaccine Working Group et al. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS ONE. 2009;4:e4708. doi: 10.1371/journal.pone.0004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton PD, Miura K, Traore B, Kayentao K, Ongoiba A, Weiss G.et al. (2010In vitro growth-inhibitory activity and malaria risk in a cohort study in mali Infect Immun 78737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murhandarwati EE, Wang L, de Silva HD, Ma C, Plebanski M, Black CG.et al. (2010Growth-inhibitory antibodies are not necessary for protective immunity to malaria infection Infect Immun 78680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ.et al. (2009Protection against a malaria challenge by sporozoite inoculation N Engl J Med 361468–477. [DOI] [PubMed] [Google Scholar]
- Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, Cloonan N.et al. (2002Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum Lancet 360610–617. [DOI] [PubMed] [Google Scholar]
- Weiss WR, Kumar A, Jiang G, Williams J, Bostick A, Conteh S.et al. (2007Protection of rhesus monkeys by a DNA prime/poxvirus boost malaria vaccine depends on optimal DNA priming and inclusion of blood stage antigens PLoS ONE 2e1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzon-Charry A, McPhun V, Kienzle V, Hirunpetcharat C, Engwerda C, McCarthy J.et al. (2010Low doses of killed parasite in CpG elicit vigorous CD4+ T cell responses against blood-stage malaria in mice J Clin Invest 1202967–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Hodder AN, Yan H, Crewther PE, Anders RF., and, Good MF. CD4+ T cells acting independently of antibody contribute to protective immunity to Plasmodium chabaudi infection after apical membrane antigen 1 immunization. J Immunol. 2000;165:389–396. doi: 10.4049/jimmunol.165.1.389. [DOI] [PubMed] [Google Scholar]
- Wipasa J, Hirunpetcharat C, Mahakunkijcharoen Y, Xu H, Elliott S., and, Good MF. Identification of T cell epitopes on the 33-kDa fragment of Plasmodium yoelii merozoite surface protein 1 and their antibody-independent protective role in immunity to blood stage malaria. J Immunol. 2002;169:944–951. doi: 10.4049/jimmunol.169.2.944. [DOI] [PubMed] [Google Scholar]
- Makobongo MO, Riding G, Xu H, Hirunpetcharat C, Keough D, de Jersey J.et al. (2003The purine salvage enzyme hypoxanthine guanine xanthine phosphoribosyl transferase is a major target antigen for cell-mediated immunity to malaria Proc Natl Acad Sci USA 1002628–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Shen J, Chou B, Duan X, Tu L, Tetsutani K.et al. (2010Involvement of CD8+ T cells in protective immunity against murine blood-stage infection with Plasmodium yoelii 17XL strain Eur J Immunol 401053–1061. [DOI] [PubMed] [Google Scholar]
- Good MF., and, Engwerda C. Defying malaria: Arming T cells to halt malaria. Nat Med. 2011;17:49–51. doi: 10.1038/nm0111-49. [DOI] [PubMed] [Google Scholar]
- Draper SJ., and, Heeney JL. Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol. 2010;8:62–73. doi: 10.1038/nrmicro2240. [DOI] [PubMed] [Google Scholar]
- Catanzaro AT, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, Vaccine Research Center 006 Study Team et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–1649. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Step Study Protocol Team et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Step Study Protocol Team et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AV, Reyes-Sandoval A, O'Hara G, Ewer K, Lawrie A, Goodman A.et al. (2010Prime-boost vectored malaria vaccines: progress and prospects Hum Vaccin 678–83. [DOI] [PubMed] [Google Scholar]
- Sridhar S, Reyes-Sandoval A, Draper SJ, Moore AC, Gilbert SC, Gao GP.et al. (2008Single-dose protection against Plasmodium berghei by a simian adenovirus vector using a human cytomegalovirus promoter containing intron A J Virol 823822–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva M, Andrews L, Gilbert SC, Bejon P, Marsh K, Mwacharo J.et al. (2009Prevalence of serum neutralizing antibodies against chimpanzee adenovirus 63 and human adenovirus 5 in Kenyan children, in the context of vaccine vector efficacy Vaccine 273501–3504. [DOI] [PubMed] [Google Scholar]
- Draper SJ, Moore AC, Goodman AL, Long CA, Holder AA, Gilbert SC.et al. (2008Effective induction of high-titer antibodies by viral vector vaccines Nat Med 14819–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AL, Epp C, Moss D, Holder AA, Wilson JM, Gao GP.et al. (2010New candidate vaccines against blood-stage Plasmodium falciparum malaria: prime-boost immunization regimens incorporating human and simian adenoviral vectors and poxviral vectors expressing an optimized antigen based on merozoite surface protein 1 Infect Immun 784601–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas AD, de Cassan SC, Dicks MD, Gilbert SC, Hill AV., and, Draper SJ. Tailoring subunit vaccine immunogenicity: maximizing antibody and T cell responses by using combinations of adenovirus, poxvirus and protein-adjuvant vaccines against Plasmodium falciparum MSP1. Vaccine. 2010;28:7167–7178. doi: 10.1016/j.vaccine.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper SJ, Biswas S, Spencer AJ, Remarque EJ, Capone S, Naddeo M.et al. (2010Enhancing blood-stage malaria subunit vaccine immunogenicity in rhesus macaques by combining adenovirus, poxvirus, and protein-in-adjuvant vaccines J Immunol 1857583–7595. [DOI] [PubMed] [Google Scholar]
- Biswas S, Dicks MD, Long CA, Remarque EJ, Siani L, Colloca S.et al. (2011Transgene Optimization, Immunogenicity and In Vitro Efficacy of Viral Vectored Vaccines Expressing Two Alleles of Plasmodium falciparum AMA1 PLoS ONE 6e20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper SJ, Goodman AL, Biswas S, Forbes EK, Moore AC, Gilbert SC.et al. (2009Recombinant viral vaccines expressing merozoite surface protein-1 induce antibody- and T cell-mediated multistage protection against malaria Cell Host Microbe 595–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder AA, Sandhu JS, Hillman Y, Davey LS, Nicholls SC, Cooper H.et al. (1987Processing of the precursor to the major merozoite surface antigens of Plasmodium falciparum Parasitology 94 (Pt 2)199–208. [DOI] [PubMed] [Google Scholar]
- Miura K, Zhou H, Diouf A, Moretz SE, Fay MP, Miller LH.et al. (2009Anti-apical-membrane-antigen-1 antibody is more effective than anti-42-kilodalton-merozoite-surface-protein-1 antibody in inhibiting plasmodium falciparum growth, as determined by the in vitro growth inhibition assay Clin Vaccine Immunol 16963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos C, Marrama L, Polson HE, Corre S, Diatta AM, Diouf B.et al. (2010Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies PLoS ONE 5e9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T., and, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata Y, Udono H, Honma K, Ueda M, Mukae H, Kadota J.et al. (2002Merozoite surface protein 1-specific immune response is protective against exoerythrocytic forms of Plasmodium yoelii Infect Immun 706075–6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belnoue E, Voza T, Costa FT, Grüner AC, Mauduit M, Rosa DS.et al. (2008Vaccination with live Plasmodium yoelii blood stage parasites under chloroquine cover induces cross-stage immunity against malaria liver stage J Immunol 1818552–8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM.et al. (2009Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys Nature 45787–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup RA, Roederer M, Lamoreaux L, Fischer J, Novik L, Nason MC, VRC 009 Study Team; VRC 010 Study Team et al. Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PLoS ONE. 2010;5:e9015. doi: 10.1371/journal.pone.0009015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huaman MC, Martin LB, Malkin E, Narum DL, Miller LH, Mahanty S.et al. (2008Ex vivo cytokine and memory T cell responses to the 42-kDa fragment of Plasmodium falciparum merozoite surface protein-1 in vaccinated volunteers J Immunol 1801451–1461. [DOI] [PubMed] [Google Scholar]
- Lee EA, Flanagan KL, Odhiambo K, Reece WH, Potter C, Bailey R.et al. (2001Identification of frequently recognized dimorphic T-cell epitopes in plasmodium falciparum merozoite surface protein-1 in West and East Africans: lack of correlation of immune recognition and allelic prevalence Am J Trop Med Hyg 64194–203. [DOI] [PubMed] [Google Scholar]
- Woehlbier U, Epp C, Kauth CW, Lutz R, Long CA, Coulibaly B.et al. (2006Analysis of antibodies directed against merozoite surface protein 1 of the human malaria parasite Plasmodium falciparum Infect Immun 741313–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowkes FJ, Richards JS, Simpson JA., and, Beeson JG. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS Med. 2010;7:e1000218. doi: 10.1371/journal.pmed.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlborg N, Ling IT, Howard W, Holder AA., and, Riley EM. Protective immune responses to the 42-kilodalton (kDa) region of Plasmodium yoelii merozoite surface protein 1 are induced by the C-terminal 19-kDa region but not by the adjacent 33-kDa region. Infect Immun. 2002;70:820–825. doi: 10.1128/IAI.70.2.820-825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen D, Leung WH, Cheung R, Hashimoto C, Ng SF, Ho W.et al. (2007Antigenicity and immunogenicity of the N-terminal 33-kDa processing fragment of the Plasmodium falciparum merozoite surface protein 1, MSP1: implications for vaccine development Vaccine 25490–499. [DOI] [PubMed] [Google Scholar]
- Berthoud TK, Hamill M, Lillie PJ, Hwenda L, Collins KA, Ewer KJ.et al. (2011Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1 Clin Infect Dis 521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer M, Nozzi JL., and, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A., and, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26:193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh RS, Shi J, Jennings RM, Chappel JC, de Koning-Ward TF, Smith T.et al. (2007The importance of human FcgammaRI in mediating protection to malaria PLoS Pathog 3e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MSP1 vaccine insert.
Individual ex-vivo IFN-γ ELISPOT data.
Breakdown of ex-vivo IFN-γ ELISPOT data according to total response.
Breakdown of ex-vivo IFN-γ ELISPOT data according to % response.
T-cell multifunctionality following ChAd63-MVA MSP1 immunization.
Individual IgG ELISA data.
IgG ELISA against all regions of MSP1.
Functional GIA of purified IgG.
Gating strategy for analysis of MSP1-specific T-cell responses.
Assessment of severity.
Assessment of local AEs.
Assessment of outcome.
Assessment of relatedness.
MSP1 overlapping peptides.