Abstract
Study Objectives:
In Kleine-Levin Syndrome (KLS), new episodes of hypersomnia are often preceded by an acute flu-like syndrome or upper airway infection 3 to 5 days before onset. This study investigated the relationship between the occurrence of mild upper respiratory tract infections (URIs) in the general population and the occurrence and seasonality and hypersomnic episodes in KLS patients.
Design:
This investigation was a longitudinal clinical study. Based on data obtained from the National Health Research Institutes between 2006 and 2007, the timing of hypersomnic episodes in 30 KLS patients were compared with calendar reports of URI events, and the results compared with age-matched general Taiwanese population.
Measurements:
Clinical symptoms, physical examination, polysomnographic recording, SPECT study, and laboratory tests affirming KLS during both periods of hypersomnic attack and non-attack were collected. Every symptomatic episode was then followed up. The cross-correlation function (CCF) and bivariate correlations analysis were performed to see the relationship between KLS and URIs.
Results:
A positive finding of CCF analysis and significant bivariate correlations were found between KLS episodes and URI in the general population (r = 0.456*). In onset of hypersomnia, significant correlations existed among “acute upper respiratory infections” (r = 0.446*), “acute bronchitis and bronchiolitis” (r = 0.462*), and “pharyngitis and nasopharyngitis” (r = 0.548*) subtypes of infections. A positive correlation between higher reports of symptomatic hypersomnia and URI also existed in a given season. A positive nonsignificant trend for “allergic rhinitis” (r = 0.400) was also found.
Conclusion:
The agent behind URI or its consequence (such as fever) is associated with increased incidence of KLS episodes and may explain periodic symptomatic recurrences.
Citation:
Huang YS; Guilleminault C; Lin KL Hwang FM; Liu FY; Kung YP. Relationship between Kleine-Levin Syndrome and upper respiratory infection in Taiwan. SLEEP 2012;35(1):123-129.
Keywords: Kleine-Levin Syndrome, recurrent hypersomnia, upper respiratory infection, general population peak, seasonality
INTRODUCTION
Kleine-Levin Syndrome (KLS) is a rare disorder with periodic hypersomnia.1–3 It occurs mainly in males, but cases with female middle-aged adults have been reported.4–7 The most common symptoms are periodic hypersomnia, hyperphagia, and various behavioral disturbances.1–3,8,9 According to the International Classification of Sleep Disorders, KLS belongs to the category of recurrent hypersomnias,10 defined as recurrent episodes of excessive daytime sleepiness with at least one of the following symptoms: (i) cognitive or mood disturbances, (ii) megaphagia with compulsive eating; (iii) hypersexuality with inappropriate behaviors; and (iv) abnormal behavior.10 The patient experiences recurrent episodes of excessive sleepiness lasting 2 days to 4 weeks, with recurring episodes at least once a year.10
The etiology of KLS remains unknown and controversial; hypothalamic dysfunction or neurotransmitter imbalance has been noted in previous studies.11–16 There are few articles on brain structural changes. Brain imaging using brain computerized tomography (CT) or brain magnetic resonance imaging (MRI) has been normal in subjets with primary KLS.17,18 Huang et al.19 reported thalamic hypoperfusion on Tc-99m ECD single photon emission tomography (SPECT) analysis in a subgroup of KLS patients during symptomatic periods. This finding was confirmed by another SPECT evaluation.20 In addition, Huang also reported that important changes in sleep occur over time during the symptomatic period, with impairment of slow wave sleep and REM sleep at symptom onset.21
There are few genetic studies about KLS, and most KLS cases are sporadic. Other possible triggers for KLS included head trauma22 and viral infections.23–27 A case series study reported that the first episode of KLS occurred most often during autumn (31.1%) or winter (31.1%) months, peaking in December (14.8%).27,28 The same study also reported that in 43% of the cases, an infection occurred 3 to 5 days before the onset of symptoms. In most cases the infections were trivial flu-like fever or winter-associated upper airway infection.27,28 Recently, the possibility of an autoimmune process has been investigated.29–31 Visscher et al.29 first studied the presence of HLA-DR antigens in KLS. However, HLA-DR1, HLA-DR2, DQA1, and DRB1 typings, tryptophan hydroxylase and catecholomethyl-o-transferase gene polymorphism28–31 investigations did not yield convincing results. Nevertheless, based on the HLA findings, an autoimmune hypothesis was proposed.29–31.
In this study, we had the opportunity to follow up a group of well-documented KLS patients. Despite the rarity of the syndrome, our subject group was considered large because our division is the referral center for neurological sleep disorders for the entire island of Taiwan. Matched for age, the relationship between the occurrence of upper respiratory tract infection in the general Taiwanese population and KLS patients were compared. The occurrence and seasonality of hypersomnic episodes were also compared.
METHODS
This is a prospective cohort study using a well-defined group of KLS patients. We systematically collected KLS patients' data from 2004 to 2007.
KLS Subjects
The subjects were selected based on strict criteria for KLS as outlined in the International Classification of Sleep Disorders, 2nd edition.10 The criteria included having been clinically followed by one neurologist and one sleep specialist for ≥ 2 years to confirm the diagnosis, and having been seen during symptomatic and asymptomatic periods. In addition, during both periods, the patients underwent polysomnographic recording, SPECT study, and laboratory tests, as well as having well-structured documentation of clinical status from their family prior to any new clinical episode. Each subject and immediate family member had access to a direct cell-phone number to immediately contact a sleep specialist and pediatric neurologist at the onset of any suspicious symptomatic episode. Subjects were seen in the specialized sleep unit within 24 h of clinical onset. All subjects and parents of minors provided informed consent. The Institutional Review Board of Chang-Gung Memorial Hospital (Taiwan) approved the use of all collected data for research purposes.
Controls
Since Taiwan is covered by national health insurance, approximately 99% of the citizens in Taiwan are covered under the state's National Health Insurance system. Any physician visit is recorded for payment purpose. In addition, the health system is government controlled and free, and therefore accessible. Education is likewise accessible, thereby producing highly educated parents and the social habit of having children and teenagers seen in school clinics and pediatric clinics for any suspicion of change in health status, even if mild. According to figures from school healthcare centers, > 90% of children who did not feel well received a medical examination or medical treatment from places such as the school clinic, local clinics, or hospitals. Therefore, the Taiwan National Health Research Institutes were able to provide 4 years of clinical records (2004 to 2007) for control data from its 23 million Taiwan National Health Insurance (NHI) enrollees. The calendar reports of URI events were obtained from the Taiwan National Health Insurance Research Database (NHIRD) on age-matched Taiwanese general population subjects for the years selected for the investigation (2006 to 2007). There were 538,701 patients seen in 2006 and 548,502 in 2007. The NHIRD performed a systematic random sampling procedure for public-health purposes with sample of 1/500. We requested access to information from the systematic sampling on subjects age 9 to 19 years. There were 73,490 subjects in 2006 and 77,250 in 2007.
Analysis
The mean, median, standard deviation, minimum, and maximum values of the observations for each KLS subject were tabulated. Frequency and percentages of incidences, the time of the year, and seasonal relationship were indicated for each symptomatic attack. The following metrics were delineated from the NHIRD: “acute upper respiratory infections,” “acute bronchitis and bronchiolitis,” “pharyngitis and nasopharyngitis,” and “allergic rhinitis.” Other infectious diseases such as “enterogastritis” were used as a control diagnostic category. Following the National Health Service nomenclature, we subdivided reports obtained from KLS patients into “acute upper respiratory infections,” “acute bronchitis and bronchiolitis,” “pharyngitis and nasopharyngitis,” “allergic rhinitis,” and “enterogastritis.”
Two-tailed bivariate correlation and cross-correlation function (CCF) analysis were performed to compare the occurrence of symptomatic events in KLS patients and the various subdivisions of acute respiratory events reported in the general population from 2006 to 2007. The CCF quantifies any linear relationship that exists between successive data in 2 paired time series. The analysis of the CCF was set in the 2 time series for time shifts (lags) between −3 and 3 periods. For example, a lag of 0 means there is no time shift between the 2 series, while a lag of 1 means that KLS precedes the different subdivisions of acute respiratory events by 1 month. We used SPSS version 18.0 for all statistical calculations.
RESULTS
Thirty KLS patients (26 males and 4 females) were included in the study. Their ages ranged from 9 to 19 years olds, with a mean of 13.23 years. All were followed ≥ 24 months, with a maximum of 6 years, and experienced ≥ 2 episodes a year, with a maximum of 7 episodes a year.
Table 1 presents the clinical manifestations of the KLS subjects, showing the frequency of symptoms reported during the initial symptomatic episode and the frequency during at least one episode in the second year. Symptoms and the severity of problems were different for each symptomatic episode; however, 50% to 80% manifested increase in disinhibition, 40% to 50% hypersexuality, 23% to 40% depressive symptoms, and 15% to 33% compulsion during long-term follow-up. Nonetheless, the overall frequency of hypersomnic episodes per year decreased with time.
Table 1.
Frequency of symptoms reported in the initial symptomatic episode and during at least one episode in the second year in 30 Taiwanese KLS patients
| Clinical symptoms | Y1(%) | Y2(%) |
|---|---|---|
| • Hypersomnia | 100 | 100 |
| • Disinhibition | 50 | 80 |
| • Eating behavior disturbance | 75 | 83 |
| Megaphagia | 57 | 56 |
| Decreased appetite | 37 | 40 |
| Increased drinking | 0 | 0 |
| Craving for sweets | 30 | 23 |
| Hyperphagia after episode | 17 | 33 |
| • Hypersexuality | 40 | 50 |
| • Cognitive impairment | 100 | 93 |
| Impaired concentration | 95 | 92 |
| Apathy | 83 | 87 |
| Derealization | 93 | 83 |
| Confusion | 10 | 8 |
| Dreamy feeling | 73 | 68 |
| Abnormal speech | 33 | 17 |
| Mutism | 70 | 77 |
| Slow response | 20 | 15 |
| Amnesia | 27 | 30 |
| Psychological change | 90 | 88 |
| Personality changes | 97 | 87 |
| Childishness | 60 | 73 |
| Irritability | 67 | 63 |
| Anxiety | 33 | 38 |
| Depressive symptoms | 23 | 40 |
| Negative thoughts | 13 | 27 |
| Impulsive thoughts | 23 | 30 |
| Fearfulness | 40 | 40 |
| Decreased motivation | 33 | 30 |
| Compulsion | 15 | 33 |
| Aggressive tendency | 33 | 23 |
| Impatience | 67 | 40 |
| • Psychotic signs/symptoms | 60 | 40 |
| Auditory hallucination | 40 | 13 |
| Visual hallucination | 30 | 10 |
| delusion | 40 | 13 |
| Sleep disturbances | 47 | 50 |
| Intense dreaming | 60 | 67 |
| Sleep talking | 8 | 6 |
| Sleep terror | 13 | 12 |
Data from 30 KLS patients. %, percentage of symptoms reported by KLS patients; BW, body weight (change during hypersomnic period). Y1: The frequency of symptoms reported in the initial symptomatic episode. Y2: The frequency of symptoms reported during at least one episode in the second year.
None of the patients presented EEG or imaging abnormalities on brain CT and MRI. Table 2 presents SPECT and HLA findings. This is the largest group of KLS patients receiving systematic symptomatic-asymptomatic SPECT analysis. Results showed that during symptomatic episodes only, 66.7% of the cases showed asymmetric hypoperfusion in the left thalamus and 11.1% in the right thalamus, and 11.1% in the left basal ganglia and 22.2% in the right basal ganglia. These findings are similar to those previously reported by us19 and Hong et al.,20 but involving a much larger group.
Table 2.
Findings of SPECT and HLA typing of 30 Taiwanese KLS patients
| N = 30 | N (%) | |
|---|---|---|
| Age of onset (mean: y/o) | 13.23 (range: 9-19 y/o) | |
| Sex (n = 30) | Male: n = 26 (86.7%), female: n = 4 | |
| SPECT finding during SMP (n = 27) | L thalamus hypoperfusion | 18 (66.7) |
| R thalamus hypoperfusion | 3 (11.1) | |
| L basal ganglia hypoperfusion | 3 (11.1) | |
| R basal ganglia hypoperfusion | 6 (22.2) | |
| R cerebellum hypoperfusion | 2 (7.4) | |
| HLA typing finding during SMP (n = 28) | HLA DQ 0602 positive | 3 (11.1) |
| HLA DQ 0601 positive | 7 (25) | |
| HLA DQ 0301 positive | 4 (14.3) | |
| HLA DQ 0302 positive | 3 (11.1) | |
| HLA DQ 3032 positive | 7 (25) | |
| HLA DQ 03 positive | 5 (17.9) | |
| HLA DQ 0201 positive | 4 (14.3) | |
| HLA DQ 05 positive | 7 (25) | |
| HLA DR 15 positive | 7 (25) | |
| Patient with the precipitating factors were reported during at least one episode in the first year (n = 30) | Stress | 7 (23.3) |
| Nasal allergy | 2 (6.7) | |
| Encephalitis symptoms | 1 (3.3) | |
| Influenza/URI symptoms | 23 (76.7) | |
| Fever | 14 (46.7) | |
| None | 3 (10) |
Data from 30 KLS patients. y/o, years old. Hypoperfusion in SPECT: defined as the decreasing perfusion of more than 10%.
Compared to the normal control group (n = 50), HLA typing results (KLS n = 28) showed variable HLA typing with positive HLADQ 0602 in n = 3 (11.1% versus 4% in control), HLA DQ0601 in n = 7 (25% versus 14% in control), HLA DQ3032 in n = 7 (25% versus 21.3%in control), HLA DQ 05 in n = 7 (25% versus 28.3%in control), and HLA DR 15 in n = 7 (25% versus 23.1% in control).
Table 2 also notes health changes just prior to the onset of a symptomatic episode. All the subjects were asymptomatic during all interim periods. Analysis of data from Tables 1 and 2 indicate that 96.6% of first episodes of KLS occurred after URI symptoms (n = 23), fever (n = 14), or encephalitis (n = 1). All the subjects were referred to us from neurologists, pediatricians, and local hospitals. Moreover, the following episodes in the second year were triggered by infection (65%), fever (20%), psychological events and stress (5%), in addition to sleep deprivation (5%). In about 5% of recurring hypersomnic episodes, no precipitating factor was detected. Vaccinations never induced an onset.
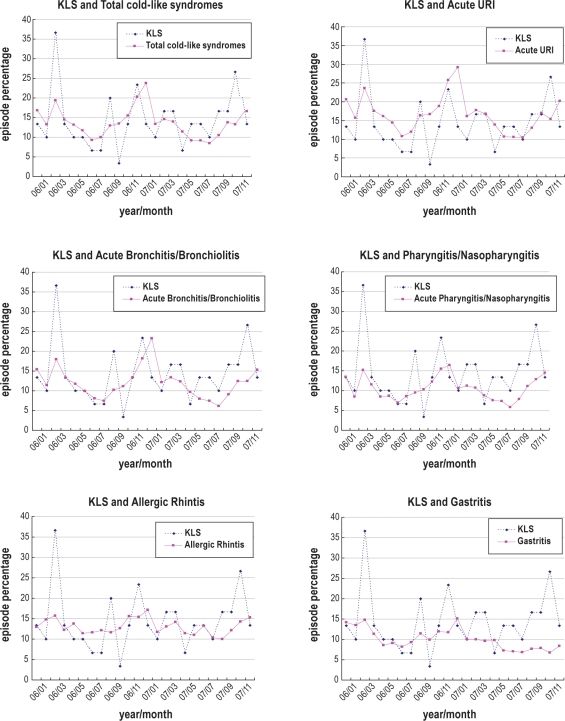
Figure 1 presents the percentage occurrences of KLS symptomatic episodes, and the percentage number of months whereby the various NHIRD subdivisions of acute respiratory events occurred for the years 2006 and 2007. Due to the large sample size, the percentage occurrences of the various subdivisions of acute respiratory events were small. Therefore, even if our KLS sample was one of the largest collected at a single location, it comprised only 30 patients, thus rendering comparison more difficult. To compensate for this limitation, the percentage of total cold-like syndromes was weighted by 5 times, acute URI by 10, acute bronchitis and bronchiolitis by 20, pharyngitis and nasopharyngitis by 100, allergic rhinitis by 50, and gastritis by 100. This statistical manipulation did not influence CCF analysis, which was performed in terms of percentage occurrences of KLS symptomatic episodes using the original rather than the weighted percentage of the various subdivisions of acute respiratory events.
Figure 1.
Time series data of KLS and the various subdivisions of acute respiratory events. In order to compare KLS and the various subdivisions of acute respiratory events in terms of time lag trend in the chart, we weighted the percentage of acute URI by 10 times, acute bronchitis and bronchiolitis by 20, pharyngitis and nasopharyngitis by 100, allergic rhinitis by 50, and gastritis by 100.
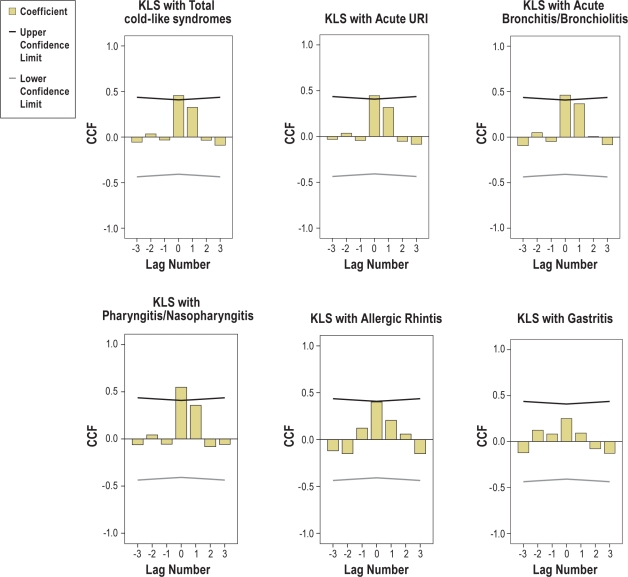
Figure 2 shows the results of the CCF, where a significant positive cross-correlation indicates that an increase in KLS percentage and occurrence of symptomatic KLS episodes were accompanied by an increase in the various subdivisions of acute respiratory events, and vice versa. In addition, significant positive cross-correlations also occurred between KLS and “total cold-like syndromes” (r = 0.456*), KLS and “acute upper respiratory infections” (r = 0.446*), KLS and “acute bronchitis and bronchiolitis” (r = 0.462*), and KLS and “pharyngitis and nasopharyngitis” (r = 0.548*) in a time lag of 0. In other words, between 2006 and 2007, KLS demonstrated a similar trend with these infectious syndromes. A nonsignificant correlation existed between KLS and “allergic rhinitis”(r = 0.400), and no significant cross-correlation was found between KLS and gastritis (r = 0.251). These results are consistent with the bivariate correlation between incidents of KLS symptomatic episodes and total upper respiratory infection (r = 0.418, P = 0.042), and likewise subdivisions of acute respiratory events.
Figure 2.
Cross-correlation function results for KLS and the various subdivisions of acute respiratory events. It shows significant positive cross-correlations between KLS and URI subdivisions in a lag of 0; i.e., KLS showed similar trends with these syndromes from 2006 to 2007.
No obvious seasonality for hypersomnic episodes and URI was found. During 2006, the highest frequency of new hypersomnic episodes was in spring (March); and during 2007, in winter (November).
Two subgroups of 14 (1 female) and 21 KLS patients (2 females) who were already followed in 2004 and 2005 were included in the 30-patient group in 2006 and 2007. Despite the even smaller sample size, similar analysis was performed since the time range from 2004 to 2007 provided a longer time period for measuring this subgroup against the NHIRD. Again, significant results were found not only for the occurrences of KLS symptomatic episodes and total upper respiratory infections in the Taiwan general population (P = 0.047*), but also when comparing the onset of hypersomnic episodes with subdivisions of acute respiratory events, “acute upper respiratory infections” (P = 0.043*), “acute bronchitis and bronchiolitis” (P = 0.047*), and “pharyngitis and nasopharyngitis” (P = 0.019*).
DISCUSSION
This study is the first longitudinal clinical study on a well-defined group of KLS patients that included not only continuous clinical follow-up, but also imaging studies during symptomatic and asymptomatic periods. The results showed a strong positive correlation between higher URI reports in the general age-matched Taiwanese population and the occurrence of symptomatic episodes in KLS patients. Despite being the referral center for the island and therefore being a large concentrated group of regularly followed KLS patients, the sample size was small due to the rarity of the syndrome; this is a limitation of the study. However, the cohort was very systematically followed at a single location by a group of specialists who ensured appropriate documentation of every episode by maintaining close and very regular contact with each patient and family by providing direct cell-phone access to contact a sleep specialist at the onset of any suspicious symptom, thereby allowing accurate and well-documented records of timing and information on the onset any new episode. As such, despite the sample size of 30, results of CCF and two-tailed bivariate analysis were significant. These follow-up data allowed us to not only look with confidence at the 2006 and 2007 records, but also the five years of documentation for a smaller subgroup, comprising even fewer subjects than the 2006 and 2007 groups whose results were significant.
Although, the very good health insurance system in Taiwan (99% of the citizens in Taiwan are covered under the state's National Health Insurance system, and over 90% of children who did not feel well received a medical examination or medical treatment), the sample size of the URI control group in this study is extremely big (nearly the whole population of a nation) and the strictest random sampling procedure was employed. (Please refer to Internet: English and Chinese NHIRD) The reliability of this study could be acceptable. Unfortunately this study is unable to be completely thorough; there is still a limitation, because of variability that we could not control in health system capture of true incident infections.
Follow-up data analysis showed that the symptoms and severity associated with every symptomatic episode were different, and that the overall frequency of hypersomnic episode per year decreased over time.
The results confirmed for the first time a strong positive correlation between higher URI reports in the general age-matched Taiwanese population and the occurrence of symptomatic events in KLS patients. In addition, a significant correlation between higher URI reports in a given season and higher reports of symptomatic hypersomnia was also found. Arnulf et al.'s compilation of cases from different origins with different geographic locations indicated that first episodes of KLS occurred mostly during autumn or winter, peaking in December.27,28 However, whether looking at first episodes in the 2004-2005 subjects or those in 2006-2007, our results did not demonstrate such seasonality in terms of peaks in KLS events or URI; for example, in 2006 peaking occurred in March (spring) while in 2007, it occurred in November (winter). It may be due to the timing of URI incidents per year in Taiwan.
To date the etiology of KLS remains unknown and explanations for acute hypoperfusion in the thalamus and basal ganglia ambiguous. Our replication, which was on a much larger patient group than all other previous studies,19,20 indicated repetitive brain syndrome, thereby suggesting the presence of an immune disorder.30,31 However, our HLA typing was not very instructive, and the fact that only 30 subjects were collected did not allow further depth of explanation.
Several hypotheses can be proposed. An immune related dysfunction may be an etiological factor in the symptomatic recurrence of KLS patients.30,31 Our study showed that most first attacks of KLS occurred after URI symptoms or fever, or as in one case, encephalitis. Moreover, our finding of significant correlation between URI and KLS symptomatic episodes, in addition to reports from our KLS subjects that infectious symptoms often preceded periods of recurrent hypersomnia, combine to suggest that URI may have been an important precipitating factor for KLS. Therefore our hypothesis is consistent with a previous idea that KLS may be an autoimmune syndrome.30,31 In other words, URI may not be the only direct cause of KLS; rather, one may hypothesize that the a mild infection such as fever may modify the permeability of the blood-brain-barrier. Concurrent with a certain genetic background, this may lead to recurrence of symptoms for a short period of time. However, this is the extent of our hypothesis given the limitations of our study.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was supported by the National Science Council: NSC 98-2314-B-182A-040-MY3. The authors thank the National-Health-Research-Institutes of Taiwan (NHIRD) for allowing the use of data and information. The authors also thank Dr. Hsiang-Heng Chen for his help with statistical analysis.
REFERENCES
- 1.Kleine W. Periodische Schlafsucht. Monatsschrift fur Psychiatrie und Neurologie. 1925;57:285–320. [Google Scholar]
- 2.Levin M. Periodic somnolence and morbid hunger: a new syndrome. Brain. 1936;59:494–504. [Google Scholar]
- 3.Billiard M. The Kleine-Levin syndrome. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: WB Saunders; 1989. pp. 377–8. [Google Scholar]
- 4.Kesler A, Gadoth N, Vainstein G, Peled R, Lavie P. Kleine-Levin syndrome (KLS) in young females. Sleep. 2000;23:563–7. [PubMed] [Google Scholar]
- 5.Reynolds CF, Black RS, Coble P, Holzer B, Kuper DJ. Similarities in EGG sleep finding for Kleine-Levin syndrome and unipolar depression. Am J Psychiatry. 1980;137:116–7. doi: 10.1176/ajp.137.1.116. [DOI] [PubMed] [Google Scholar]
- 6.Pike M, Stores G. Kleine-Levin syndrome: a cause of diagnostic confusion. Arch Dis Child. 1994;71:355–7. doi: 10.1136/adc.71.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peimao R, Shimizu MH. Kleine-Levin syndrome: clinical course, polysomnography and multiple sleep latency test. Arq Neuropsiquiar. 1998;56:650–4. doi: 10.1590/s0004-282x1998000400021. [DOI] [PubMed] [Google Scholar]
- 8.Critchley M. Periodic hypersomnia and megaphagia in adolescent males. Brain. 1962;85:627–56. doi: 10.1093/brain/85.4.627. [DOI] [PubMed] [Google Scholar]
- 9.Critchley M, Hoffman H. The syndrome of periodic somnolence and morbic hunger (Kleine-Levin syndrome) BMJ. 1942;1:137–9. doi: 10.1136/bmj.1.4230.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders, 2nd ed: diagnostic and coding manual. [Google Scholar]
- 11.Gadoth N, Dickerman Z, Bechar M, Laron Z, Lavie P. Episodic hormone secretion during sleep in Kleine-Levin syndrome: evidence for hypothalamic dysfunction. Brain Dev. 1987;9:309–15. doi: 10.1016/s0387-7604(87)80051-7. [DOI] [PubMed] [Google Scholar]
- 12.Chesson AL, Jr., Levine SN, Kong LS, Lee SC. Neuroendocrine evaluation in Kleine-Levin syndrome: evidence of reduced dopaminergic tone during periods of hypersomnolence. Sleep. 1991;14:226–32. [PubMed] [Google Scholar]
- 13.Fernandez JM, Lara I, Gila L, O'Neill of Tyrone A, Tovar J, Gimeno A. Disturbed hypothalamic-pituitary axis in idiopathic recurring hypersomnia syndrome. Acta Neurol Scand. 1990;82:361–3. doi: 10.1111/j.1600-0404.1990.tb03317.x. [DOI] [PubMed] [Google Scholar]
- 14.Malhotra S, Das MK, Gupta N, Muralidharan R. A clinical study of Kleine-Levin syndrome with evidence for hypothalamic-pituitary axis dysfunction. Biol Psychiatry. 1997;42:299–301. doi: 10.1016/s0006-3223(97)00252-7. [DOI] [PubMed] [Google Scholar]
- 15.Koerber RK, Torkelson R, Haren G, Donaldson J, Cohen SM, Case M. Increased cerebrospinal fluid 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in Kleine-Levin syndrome. Neurology. 1984;34:1597–600. doi: 10.1212/wnl.34.12.1597. [DOI] [PubMed] [Google Scholar]
- 16.Mayer G, Leonhard E, Krieg J, Meier-Ewert K. Endocrinological and polysomnographic finding in Kleine-Levin Syndrome: No evidence for hypothalamic and circadian dysfunction. Sleep. 1998;21:278–84. doi: 10.1093/sleep/21.3.278. [DOI] [PubMed] [Google Scholar]
- 17.Servan J, Marchand F, Garna L, Rancurel G, Willme JC. Two new cases of Kleine-Levin syndrome associated with CT scan abnormalities. Abstract, 15th world congress of Neurology. Can J Neurol Sci. 1993;20(suppl. 4):5137. [Google Scholar]
- 18.Poryazova R, Schnepf B, Boesiger P, Bassetti CL. Magnetic resonance spectroscopy in a patient with Kleine-Levin syndrome. J Neurol. 2007;254:1445–6. doi: 10.1007/s00415-007-0531-x. [DOI] [PubMed] [Google Scholar]
- 19.Huang YS, Guilleminault C, Kao PF, Lin FY. SPECT findings in the Kleine-Levin syndrome. Sleep. 2005;28:955–60. doi: 10.1093/sleep/28.8.955. [DOI] [PubMed] [Google Scholar]
- 20.Hong BS, Joo EY, Tae WS, et al. Episodic diencephalic hypoperfusion in Kleine-Levin Syndrome. Sleep. 2006;29:1091–3. doi: 10.1093/sleep/29.8.1091. [DOI] [PubMed] [Google Scholar]
- 21.Huang YS, Lin YH, Guilleminault C. Polysomnography in Kleine-Levin syndrome. Neurology. 2009;70:795–801. doi: 10.1212/01.wnl.0000304133.00875.2b. [DOI] [PubMed] [Google Scholar]
- 22.Will RG, Young JPR, Thamas DJ. Report of two cases with onset of symptoms precipitated by head trauma. Br J Psychiatry. 1988;152:410–20. doi: 10.1192/bjp.152.3.410. [DOI] [PubMed] [Google Scholar]
- 23.Merriam AE. Kleine-Levin Syndrome following acute viral encephalitis. Biol Psychiatry. 1986;21:1301–4. doi: 10.1016/0006-3223(86)90313-6. [DOI] [PubMed] [Google Scholar]
- 24.Salter MS, White PD. A variant of the Kleine-Levin Syndrome precipitated by both Epstein- Barr and varicella- zoster virus infections. Biol Psychiatry. 1993;33:388–90. doi: 10.1016/0006-3223(93)90329-c. [DOI] [PubMed] [Google Scholar]
- 25.Carpenter S, Yassa R, Ochs R. A pathologic basis for Kleine-Levin syndrome. Arch Neurol. 1982;39:25–8. doi: 10.1001/archneur.1982.00510130027005. [DOI] [PubMed] [Google Scholar]
- 26.Fenzi F, Simonati A, Crosato F, Ghersini L, Rizzuto N. Clinical features of Kleine-Levin syndrome with localized encephalitis. Neuropediatrics. 1993;24:292–5. doi: 10.1055/s-2008-1071559. [DOI] [PubMed] [Google Scholar]
- 27.Arnulf I, Zeitzer JM, File J, Farber N, Mignot E. Kleine-Levin Syndrome: a systematic review of 186 cases in the literature. Brain. 2005;128:2763–76. doi: 10.1093/brain/awh620. [DOI] [PubMed] [Google Scholar]
- 28.Arnulf I, Lin L, Gadoth N, et al. Kleine-Levin Syndrome: a systematic study of 108 patients. Ann Neurol. 2008;63:482–92. doi: 10.1002/ana.21333. [DOI] [PubMed] [Google Scholar]
- 29.Visscher F, van der Horst AR, Smith LM. HLA-DR antigens in Kleine-Levin syndrome. Ann Neurol. 1990;28:195. doi: 10.1002/ana.410280216. [DOI] [PubMed] [Google Scholar]
- 30.Manni R, Martinetti M, Ratti MT, Tartara A. Electrophysiological and immunogenetic findings in recurrent monosymptomatic type hypersomnia: a study of two unrelated Italian cases. Acta Neurol Scand. 1993;88:293–5. doi: 10.1111/j.1600-0404.1993.tb04239.x. [DOI] [PubMed] [Google Scholar]
- 31.Dauvilliers Y, Mayer G, Lecendreux M, et al. Kleine-Levin syndrome: an autoimmune hypothesis based on clinical and genetic analyses. Neurology. 2002;59:1739–45. doi: 10.1212/01.wnl.0000036605.89977.d0. [DOI] [PubMed] [Google Scholar]