Summary
Cyclin-dependent kinases (Cdks) are the central regulators of the cell division cycle. Inhibitors of Cdks ensure proper coordination of cell cycle events and help regulate cell proliferation in the context of tissues and organs. Wee1 homologs phosphorylate a conserved tyrosine to inhibit the mitotic cyclin-dependent kinase Cdk1 [1]. Loss of Wee1 function in fission or budding yeast causes premature entry into mitosis [2, 3]. The importance of metazoan Wee1 homologs for timing mitosis, however, has been demonstrated only in Xenopus egg extracts and via ectopic Cdk1 activation [4, 5]. Here, we report that Drosophila Wee1 (dWee1) regulates Cdk1 via phosphorylation of tyrosine 15 and times mitotic entry during the cortical nuclear cycles of syncytial blastoderm embryos, which lack gap phases. Loss of maternal dwee1 leads to premature entry into mitosis, mitotic spindle defects, chromosome condensation problems, and a Chk2-dependent block of subsequent development, and then embryonic lethality. These findings modify previous models about cell cycle regulation in syncytial embryos [6] and demonstrate that Wee1 kinases can regulate mitotic entry in vivo during metazoan development even in cycles that lack a G2 phase.
Results and Discussion
To assess the function of dWee1 during embryogenesis, we studied embryos from females that are hemizygous for the ES1 allele of dwee1 [7]. This is the strongest extant allele of dwee1 and shows full penetrance: All embryos from dwee1ES1 hemizygous females display abnormal DNA morphology during syncytial blastoderm divisions (cycles 10–13) and die without cellularizing or progressing beyond the syncytial cycles [7]. We report here the basis for failed syncytial divisions. We will refer to embryos derived from dwee1ES1 hemizygous females as “dwee1 mutant embryos” hereafter.
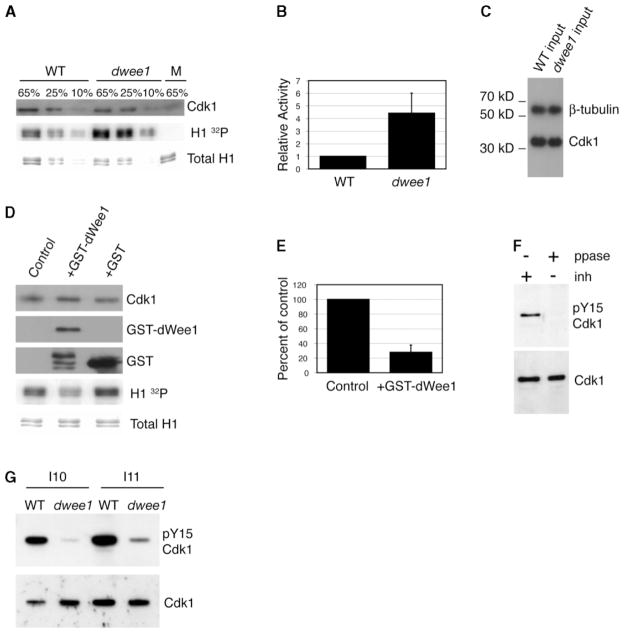
Previous work found no evidence for inhibitory phosphorylation on Cdk1 during syncytial cycles, in which dwee1, paradoxically, has an essential role [6]. To determine if dWee1 regulates Cdk1 at this stage in embryogenesis, we compared Cdk1 activity in wild-type and dwee1 mutant embryos. We immunoprecipitated Cyclin B (and associated Cdk1) from syncytial blastoderm embryos and assayed for kinase activity toward a standard Cdk1 substrate, histone H1 (Figures 1A–1C). We find that Cdk1: Cyclin B activity in dwee1 mutants is increased 4.4-fold over wild-type.
Figure 1. dwee1 Regulates Cdk1 during Syncytial Blastoderm Cycles.
Cyclin B immunoprecipitates from extracts of syncytial blastoderm stage embryos were analyzed for associated Cdk1 and kinase activity toward histone H1 (H1).
(A) Cyclin B immunoprecipitates were processed to detect phosphorylation of H1 (H1 32P) and the amount of H1 added to each reaction (Total H1). Similar amounts of Cdk1 coprecipitated with Cyclin B in wild-type and dwee1 extracts. The percentage of each H1 kinase reaction analyzed is indicated above each lane. M = mock immunoprecipitations from which anti-Cyclin B antibody was omitted.
(B) Quantification of 32P incorporation into H1 in (A). Data from three independent experiments are shown. In each case, 32P signal from dwee1 mutant immunoprecipitate (dwee1) was normalized to the 32P signal from wild-type immunoprecipitate (WT). Cyclin B-associated kinase activity toward H1 is 4.4-fold higher in precipitates from dwee1 extracts (p < .05).
(C) Cdk1 is present at similar levels in extracts from wild-type and dwee1 mutant embryos. Western blotting for γ-tubulin confirms equal loading (10% input shown).
(D) Cyclin B immunoprecipitates were incubated with GST-dwee1, GST, or buffer (control) before being processed for H1 kinase activity. Relative amounts of Cdk1, GST-dWee1, GST, and histone H1 in each reaction are shown.
(E) Quantification of 32P incorporation into H1 in (D). Data from each of three independent experiments were expressed as percent of buffer control. GST-dWee1 significantly inhibited Cdk1: Cyclin B kinase activity (p= .001).
(F) Detection of pY15-Cdk1 by a phosphospecific antibody is phosphatase sensitive. Wild-type embryos in interphase of cycles 10–12 were treated with either phosphatase inhibitors (“inh +,” lane 1) or phosphatase (“ppase +,” lane 2). After being probed with anti-pY15-Cdk1 antibody, blots were stripped and reprobed with nonphosphospecific anti-cdk1 as a loading control.
(G) dwee1 mutants have reduced levels of pY15-Cdk1. Wild-type embryos (WT) in interphase of cycle 10 (I10) and cycle 11 (I11) were compared to dwee1 mutants of the same stage.
We also asked whether dWee1 could inhibit Cdk1 from syncytial stage embryos in vitro. Purified recombinant GST-dWee1 autophosphorylates and can phosphorylate recombinant Cdk1: Cyclin B (data not shown). We found that GST-dWee1 inhibits the H1 kinase activity of Cdk1: Cyclin B complexes immunoprecipitated from syncytial embryos (Figures 1D and 1E). Collectively, these data indicate that dWee1 is an important regulator of Cyclin B: Cdk1 activity in the syncytial blastoderm.
The above data do not tell us how dWee1 inhibits Cdk1. dWee1, however, can phosphorylate Cdk1 in vitro on a conserved tyrosine [8]. Phosphorylation of this tyrosine inhibits Cdk1 activity, at least in cellularized embryos [6]. Therefore, we hypothesized that phosphorylation of Cdk1 is the mechanism by which dWee1 inhibits Cdk1. Accordingly, we found that a phosphospecific antibody raised against human tyrosine 15-phosphorylated Cdk1 (pY15-Cdk1) was able to detect inhibitory phosphorylation of Cdk1 in individually staged embryos during interphase of cycle 10–12 (I10–I12) (Figure 1F). The pY15cdk1 signal from cellularized I14 embryos is much stronger than in syncytial embryos (not shown). This could explain why pY15-Cdk1 was previously reported to be undetectable before cycle 14 [8]. We also routinely loaded ten embryos/lane, whereas previous studies examined protein from a single embryo. The pY15-Cdk1 signal was phosphatase sensitive (Figure 1F) and showed retarded electrophoretic mobility relative to that of unphosphorylated Cdk1 under the appropriate electrophoresis conditions (not shown), confirming the specificity of the antibody. Importantly, pY15-Cdk1 antigenicity was diminished in dwee1 mutant embryos (Figure 1G). The residual low pY15-Cdk1 levels that remain in dwee1 mutants may be due to the activity of a redundant kinase, such as dMyt1, that can also phosphorylate Cdk1 [9–11].
dwee1 Mutants Enter Mitosis Prematurely
We next asked whether dwee1 regulates the timing of mitotic entry during syncytial blastoderm cycles. A transgenic strain isolated from a GFP intron trap screen was used for live analysis of cortical syncytial cycles 11–13 [12]. In this strain, GFP is inserted into a protein of unknown function, CG17238, which colocalizes with centrosomes and microtubules. This stock (to be referred to as “17238-GFP”) is homozygous-viable and has no apparent defects in cell cycle progression during syncytial cycles when compared to those in embryos visualized by injecting labeled tubulin [13] (Table 1; see also Movie 1 in the Supplemental Data available with this article online). We find that interphase in dwee1 mutant embryos is significantly shorter than in control 17238-GFP embryos from cycle 11 on; that is, mutants enter mitosis prematurely (Table 1; Movie 2). We conclude that the function of Wee1-related kinases in timing mitotic entry is conserved during Drosophila embryogenesis.
Table 1.
Quantification of Cell Cycle Times in dwee1 Embryos
| Cycle | Wild-Type (min) (n = 6) | dwee1 (min) (n = 5) |
|---|---|---|
| Interphase 11 | 5.8 ± 0.9 | 3.8 ± 0.1 |
| Mitosis 11 | 5.9 ± 0.7 | 6.2 ± 0.1 |
| Interphase 12 | 8.2 ± 0.7 | 4.8 ± 0.6 |
| Mitosis 12 | 6.1 ± 0.6 | 6.1 ± 0.9 |
| Interphase 13 | 14.2 ± 0.8 | 5.9 ± 0.5 |
| Mitosis 13 | 6.6 ± 0.7 | 11.4 ± 5.1 |
Times (in min) were determined from analysis of 17238-GFP. Mitosis = nuclear envelope breakdown to nuclear envelope formation. Interphase = nuclear envelope formation to nuclear envelope breakdown. Nuclear envelope breakdown was defined as the time in which 17238-GFP was seen entering the nuclear interior, and nuclear envelope formation was defined as the point that 17238-GFP was excluded from the nucleus. n = number of embryos assayed.
Previous work in Xenopus eggs showed that depletion of maternally supplied Wee1 with morpholino oligonucleotides led to increased mitotic index and gastrulation defects, but whether this is due to accelerated entry into mitosis or a block during mitosis was not determined [14]. Therefore, this is the first report that a Wee1 homolog times mitotic entry during metazoan development.
dwee1 Mutants Show Mitotic Spindle Defects
The timing of mitotic entry in syncytial cycles that lack G2 is thought to occur both through changes in mitotic cyclin levels and via a checkpoint that monitors the completion of DNA replication [13, 15, 16]. In checkpoint mutants that enter mitosis prematurely [grp (Drosophila Chk1) and mei-41 (Drosophila ATR)] or in wild-type embryos that enter mitosis in the presence of drugs that inhibit DNA replication, centrosomes become inactivated, spindles fail to segregate chromosomes, and central spindles do not form [13, 17]. To determine if premature entry into mitosis in dwee1 mutants also leads to centrosome inactivation and chromosome segregation failures, we examined mitotic spindles.
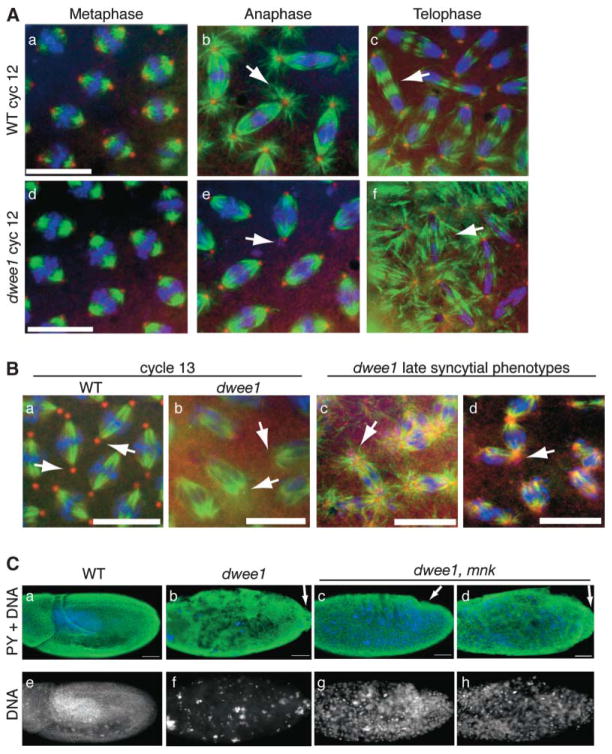
Immunostaining for microtubules and components of the γ-tubulin ring complex (γTuRC) showed no difference between dwee1 mutant embryos and the wild-type during the first cortical mitosis in cycle 11. During mitosis of cycle 12 (M12), prophase and metaphase figures in dwee1 mutants also appear similar to those in the wild-type (Figure 2A). In anaphase and telophase of M12, however, astral microtubules are severely diminished, and the central spindle becomes disorganized in dwee1 mutants (Figure 2A). DNA still segregates apparently normally during M12 in these embryos, and γ-tubulin is still localized to spindle poles. Two characteristic features of centrosome inactivation, loss of astral microtubules and γTuRC components from the spindle poles, become more apparent during M13 (Figure 2B). On the basis of nuclei number, dwee1 mutant embryos also appear to undergo an extra syncytial division, and centrosome inactivation is apparent in this cycle as well (data not shown).
Figure 2. dwee1 Mutant Embryos Show Spindle and Centrosome Defects.
(A–B) Wild-type and dwee1 mutant 1–2 hr embryos were fixed and stained for DNA with Hoechst (blue), α-tubulin (green), and either γ-tubulin (A) or Dgrip84 (B) (red). (A) M12 dwee1 mutants lack astral microtubules in anaphase (compare arrows in [b] and [e]) and form disorganized central spindles in telophase (compare arrows in [c] and [f]). (B) (a and b) Spindle poles from WT but not dwee1 mutant embryos in M13 contain Dgrip84 (arrows). (c and d) dwee1 mutants show spindle poles with many small γ-tubulin foci (arrow in [c]) and broad γ-tubulin-stained poles associated with multiple spindles (arrow in [d]).
(C) Analysis of cellularization in the wild-type, dwee1 mutants, and dwee1,mnk double mutants. Embryos 4–19 hr old were fixed and stained with an antibody to phosphorylated tyrosine (PY), which stains cell membranes, and with Hoechst to visualize DNA. Wild-type embryos (WT) show normal nuclear and cellular morphology. dwee1 mutants show no evidence of cell membranes, and DNA appears mostly internalized or in large bodies. In contrast, dwee1,mnk double mutants contain cell membranes and DNA at the embryo cortex. Two representative samples are shown to illustrate the range of phenotypes in double mutants (embryo in [c] exhibits further development and more complete cellularization than embryo in [d]). Pole cells in dwee1 mutants remain at the posterior end of embryos (arrow in [b]), whereas pole cells in some dwee1,mnk double mutants (~60%) can be seen in the process of becoming internalized (arrow in [c]). The scale bar represents 10 μm in (A) and (B) and 50 μm in (C).
Older (>2 hr) dwee1 mutant embryos display terminal phenotypes that include abnormal aggregates of DNA and more severe microtubule defects, such as spindle poles that contain multiple foci of γ-tubulin and broad γ-tubulin foci that appear to nucleate multiple chromosome-containing spindles (Figure 2B). To better understand how these defects arose, we studied live embryos.
Live Analysis of dwee1 Mutant Embryos
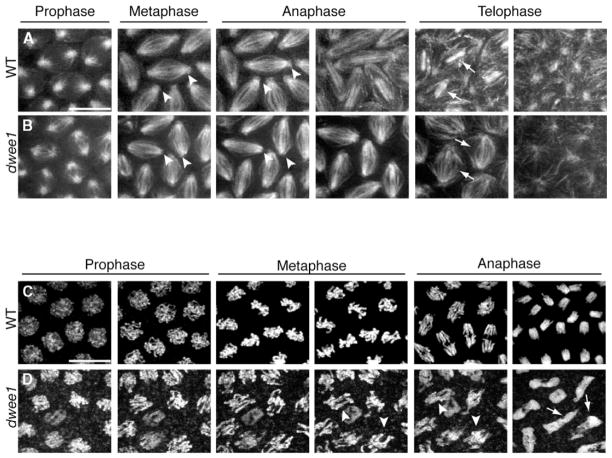
Time-lapse analysis of dwee1 mutants carrying the 17238-GFP transgene confirmed our observations in fixed embryos. First, it confirmed that M11 was apparently normal in dwee1 mutant embryos with respect to mitotic spindle formation (Movie 2). Next, it revealed a plethora of microtubule- and centrosome-related defects during cycles 12 and 13 (Figures 3A and 3B; Movie 2). These include the two defects documented in fixed embryos: (1) diminished astral microtubules and GFP signal at spindle poles and (2) disorganized central spindles (Figure 3B). Loss of GFP signal from the spindle poles during M12 and M13 is consistent with the γTuRC component loss observed in M13 of fixed embryos (Figure 2B). The mutant nuclei underwent an additional syncytial cycle before becoming arrested with large clumps of abnormal microtubule networks similar to those seen in fixed embryos (Figure 2B).
Figure 3. Time-Lapse Confocal Analysis of dwee1 Mutant Spindles and Chromosomes.
(A) Live wild-type (WT) and (B) dwee1 mutant (dwee1) embryos expressing 17238-GFP were analyzed by time-lapse confocal microscopy. In cycle 13 dwee1 mutant embryos, GFP signal is not detected at spindle poles or on astral microtubules (compare arrowheads in [A] and [B]), and central spindles appear disorganized (compare arrows in [A] and [B]).
(C) Live WT and (D) dwee1 mutant embryos expressing histone H2A-GFP (H-GFP) were analyzed by time-lapse confocal microscopy. In dwee1 mutants, anaphase fails in cycle 13, leading to formation of polyploid nuclei (arrows in [D]). Chromosomes in dwee1 mutants attempt anaphase in a much less condensed state than chromosomes in wild-type embryos (arrowheads in [D]). The scale bar represents 10 μm.
Live studies also revealed, in cycles 12 and 13, additional spindle defects that were unique to dwee1 mutants and not previously reported in mutants that enter mitosis prematurely. We are currently working to understand the basis for these phenotypes, and we speculate that dWee1 may have additional roles.
Another consequence of premature entry into mitosis in the syncytium is an attempt to align chromosomes before they are fully condensed [18]. Indeed, chromosome condensation defects, instead of or in addition to centrosome inactivation, were proposed to be the cause of chromosome segregation failure in grp mutants. Using a histone-GFP to visualize DNA [19], we also observed DNA condensation defects beginning with M13 in dwee1 mutant embryos (Figure 3D; Movies 3 and 4). Chromosomes in dwee1 mutant embryos appear less condensed than in the wild-type when anaphase begins and are not fully segregated when nuclei exit mitosis (Figures 3C and 3D).
These data suggest that dwee1 is required for coordinating multiple events that constitute mitosis. Truncation of interphases is seen starting with I11, but spindle defects become widespread only in M12, and chromosome condensation and segregation problems occur in M13. The accumulation of abnormalities in each cell cycle could be explained if premature entry into mitosis becomes increasingly detrimental as cortical cycles progress, leading to more profound consequences in later cycles.
Partial Rescue of dwee1 Mutant Embryos by Chk2 Mutations
Embryos that lack grp and mei-41 enter mitosis prematurely [13, 15]. In these mutants, embryogenesis does not progress beyond syncytial stages, transcription of zygotic genes does not commence, and embryos do not cellularize. These responses, as well as the centrosome inactivation described above, require Drosophila Chk2 [20]. DmChk2 is also required independently to cull damaged nuclei from the embryo cortex via exile into the embryo interior. Thus, mutations in mnk, which encodes DmChk2, partially rescue zygotic transcription and cellularization defects in grp and mei-41 mutants. Furthermore, in mnk mutants, damaged nuclei remain at the embryo cortex.
To determine if DmChk2-dependent checkpoints are responsible for some dwee1 mutant phenotypes, we examined dwee1,mnk double mutants. dwee1 single mutant embryos lack evidence of cellularization, and most of the DNA is internalized or in large aggregates (Figure 2C). In addition, pole cells remain at the posterior end of the embryo, as is typical for syncytial blastoderm stages (Figure 2C). We find that cellularization and cortical retention of DNA are partially restored in dwee1,mnk double mutants (Figure 2C). Some (76 of 131, or ~60%) dwee1,mnk double mutant embryos also display evidence of morphogenetic changes, such as the migration of pole cells toward the embryo anterior, that normally occur in cellularized embryos (Figure 2C). The extent of “rescue” varies from embryo to embryo (the extreme cases are shown in Figure 2C), and although nearly all embryos showed some sign of cellularization, none survived to hatching (n > 1000). The distribution and appearance of DNA in dwee1,mnk embryos is also abnormal (Figure 2C), presumably because repeated rounds of replication errors caused by truncated interphases were not rescued by removal of DmChk2 function. We conclude that at least some dwee1 phenotypes can be explained by the Chk2-mediated checkpoint.
Genetic studies place dwee1 downstream of mei-41 during syncytial divisions [7]. Our data implicate dwee1 as a regulator of Cdk1 activity and, like replication checkpoint genes, an essential regulator of mitotic entry in syncytial cycles. Mammalian embryogenesis is proposed to consist of rapid cell cycles that are similar to syncytial divisions [21]. Interestingly, ATR and Chk1 are essential at this stage of mammalian embryogenesis, as grp and mei-41 are in syncytial cycles [21]. These findings raise the intriguing possibility that inhibition of Cdk1 via ATR/Chk1/Wee1 has a conserved and essential function in metazoan development. We suggest that understanding mitotic entry in syncytial embryos could have a direct bearing on cell cycle regulation in mammalian embryos.
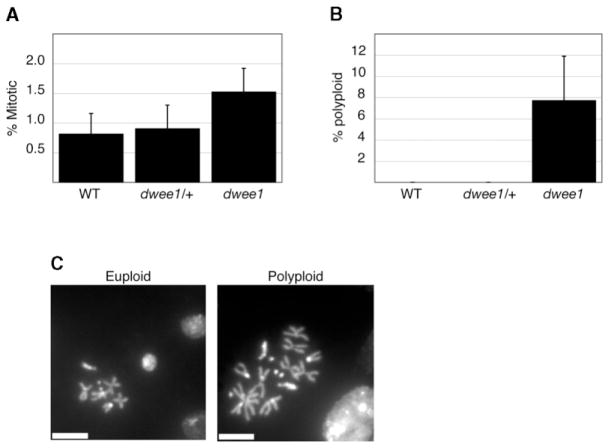
The role of dWee1 beyond the syncytial cycles remains to be determined, but it is clearly not essential; dwee1 hemizygotes survive to adulthood [7]. However, we detect an increase in mitotic index and polyploid nuclei in larval neuroblasts from dwee1ES1 hemizygotes (Figure 4). Thus, loss of dwee1 function may affect mitosis in somatic cells. We speculate that dwee1’s dispensability during somatic cycles could be due to compensation by another protein, such as the Myt1 kinase [9–11].
Figure 4. Mitotic Defects in dwee1 Mutant Larval Neuroblasts.
(A) The percentage of mitotic cells in larval brains from wild-type (WT), dwee1ES1, or Df(2L)dwee1wo5 heterozygotes (dwee1/+) and dwee1ES1 hemizygous mutant larvae (dwee1) was determined. Mitotic index is significantly elevated in dwee1 mutant brains when compared to those of the wild-type (p < .05).
(B) A significant percentage of mitotic neuroblasts in dwee1 mutant brains are polyploid (p < .05).
(C) Examples of euploid and polyploid neuroblasts from a dwee1 mutant brain. The scale bar represents 5 μm.
In conclusion, Drosophila Wee1 regulates Cdk1 activity and helps time mitosis in cortical syncytial cycles. The failure to do so results in premature entry into mitosis, spindle defects, chromosome condensation problems, and a Chk2-dependent block of subsequent development, and then embryonic lethality. In addition, Wee1 also ensures successful cell division in larval cell cycles. These data enhance our understanding about the role of Wee1 homologs in timing mitosis during metazoan development.
Supplementary Material
Acknowledgments
We thank A. Debec, M. Brodsky, and R. Saint for fly stocks, Y. Zheng and P. O’Farrell for antibodies, and Doug Kellogg for critical reading of the manuscript. This work was supported by a grant from the National Institutes of Health (RO1 GM66441) to T.T.S., a National Institutes of Health STCCR (signal transduction and cell cycle regulation) training grant to J.S., a fellowship from the American Cancer Society to T.D. through the University of Colorado Cancer Center, and a grant from the Canadian Institutes of Health Research to E.H and S.D.C.
Footnotes
Detailed Experimental Procedures and several movies are available at http://www.current-biology.com/cgi/content/full/14/23/2143/DC1/.
References
- 1.Kellogg DR. Wee1-dependent mechanisms required for coordination of cell growth and cell division. J Cell Sci. 2003;116:4883–4890. doi: 10.1242/jcs.00908. [DOI] [PubMed] [Google Scholar]
- 2.Nurse P. Genetic control of cell size at cell division in yeast. Nature. 1975;256:547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- 3.Harvey SL, Kellogg DR. Conservation of mechanisms controlling entry into mitosis: Budding yeast wee1 delays entry into mitosis and is required for cell size control. Curr Biol. 2003;13:264–275. doi: 10.1016/s0960-9822(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 4.Heald R, McLoughlin M, McKeon F. Human wee1 maintains mitotic timing by protecting the nucleus from cytoplasmically activated Cdc2 kinase. Cell. 1993;74:463–474. doi: 10.1016/0092-8674(93)80048-j. [DOI] [PubMed] [Google Scholar]
- 5.Walter SA, Guadagno SN, Ferrell JE., Jr Activation of Wee1 by p42 MAPK in vitro and in cycling Xenopus egg extracts. Mol Biol Cell. 2000;11:887–896. doi: 10.1091/mbc.11.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edgar BA, Sprenger F, Duronio RJ, Leopold P, O’Farrell PH. Distinct molecular mechanism regulate cell cycle timing at successive stages of Drosophila embryogenesis. Genes Dev. 1994;8:440–452. doi: 10.1101/gad.8.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price D, Rabinovitch S, O’Farrell PH, Campbell SD. Drosophila wee1 has an essential role in the nuclear divisions of early embryogenesis. Genetics. 2000;155:159–166. doi: 10.1093/genetics/155.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell SD, Sprenger F, Edgar BA, O’Farrell PH. Drosophila Wee1 kinase rescues fission yeast from mitotic catastrophe and phosphorylates Drosophila Cdc2 in vitro. Mol Biol Cell. 1995;6:1333–1347. doi: 10.1091/mbc.6.10.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: A membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 1995;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- 10.Booher RN, Holman PS, Fattaey A. Human Myt1 is a cell cycle-regulated kinase that inhibits Cdc2 but not Cdk2 activity. J Biol Chem. 1997;272:22300–22306. doi: 10.1074/jbc.272.35.22300. [DOI] [PubMed] [Google Scholar]
- 11.Liu F, Stanton JJ, Wu Z, Piwnica-Worms H. The human Myt1 kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi complex. Mol Cell Biol. 1997;17:571–583. doi: 10.1128/mcb.17.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morin X, Daneman R, Zavortink M, Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc Natl Acad Sci USA. 2001;98:15050–15055. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sibon OC, Stevenson VA, Theurkauf WE. DNA-replication checkpoint control at the Drosophila midblastula transition. Nature. 1997;388:93–97. doi: 10.1038/40439. [DOI] [PubMed] [Google Scholar]
- 14.Murakami MS, Moody SA, Daar IO, Morrison DK. Morphogenesis during Xenopus gastrulation requires Wee1-mediated inhibition of cell proliferation. Development. 2004;131:571–580. doi: 10.1242/dev.00971. [DOI] [PubMed] [Google Scholar]
- 15.Sibon OC, Laurencon A, Hawley R, Theurkauf WE. The Drosophila ATM homologue Mei-41 has an essential checkpoint function at the midblastula transition. Curr Biol. 1999;9:302–312. doi: 10.1016/s0960-9822(99)80138-9. [DOI] [PubMed] [Google Scholar]
- 16.Ji JY, Squirrell JM, Schubiger G. Both cyclin B levels and DNA-replication checkpoint control the early embryonic mitoses in Drosophila. Development. 2004;131:401–411. doi: 10.1242/dev.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fogarty P, Kalpin RF, Sullivan W. The Drosophila maternal-effect mutation grapes causes a metaphase arrest at nuclear cycle 13. Development. 1994;120:2131–2142. doi: 10.1242/dev.120.8.2131. [DOI] [PubMed] [Google Scholar]
- 18.Yu KR, Saint RB, Sullivan W. The Grapes checkpoint coordinates nuclear envelope breakdown and chromosome condensation. Nat Cell Biol. 2000;2:609–615. doi: 10.1038/35023555. [DOI] [PubMed] [Google Scholar]
- 19.Clarkson M, Saint R. A His2AvDGFP fusion gene complements a lethal His2AvD mutant allele and provides an in vivo marker for Drosophila chromosome behavior. DNA Cell Biol. 1999;18:457–462. doi: 10.1089/104454999315178. [DOI] [PubMed] [Google Scholar]
- 20.Takada S, Kelkar A, Theurkauf WE. Drosophila checkpoint kinase 2 couples centrosome function and spindle assembly to genomic integrity. Cell. 2003;113:87–99. doi: 10.1016/s0092-8674(03)00202-2. [DOI] [PubMed] [Google Scholar]
- 21.O’Farrell PH, Stumpff J, Su TT. Embryonic cleavage cycles: How is a mouse like a fly? Curr Biol. 2004;14:R35–R45. doi: 10.1016/j.cub.2003.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.