Abstract
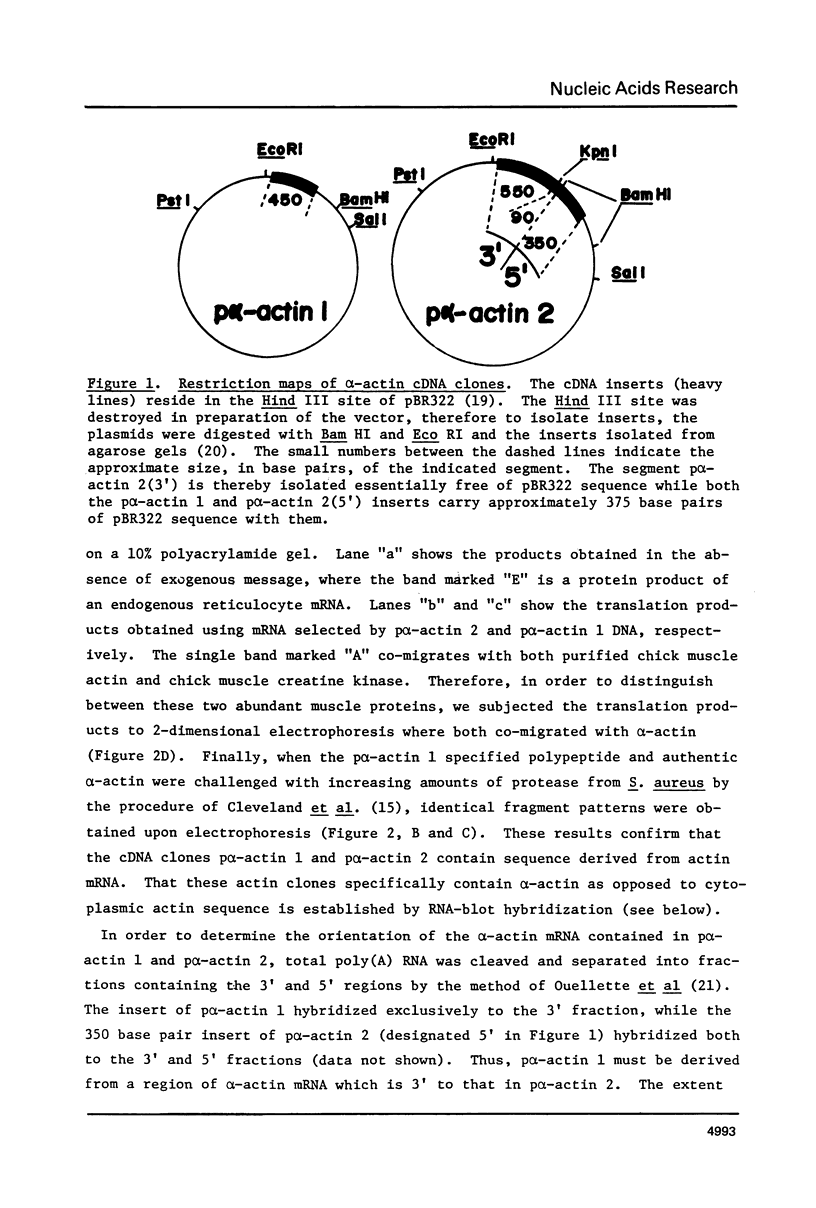
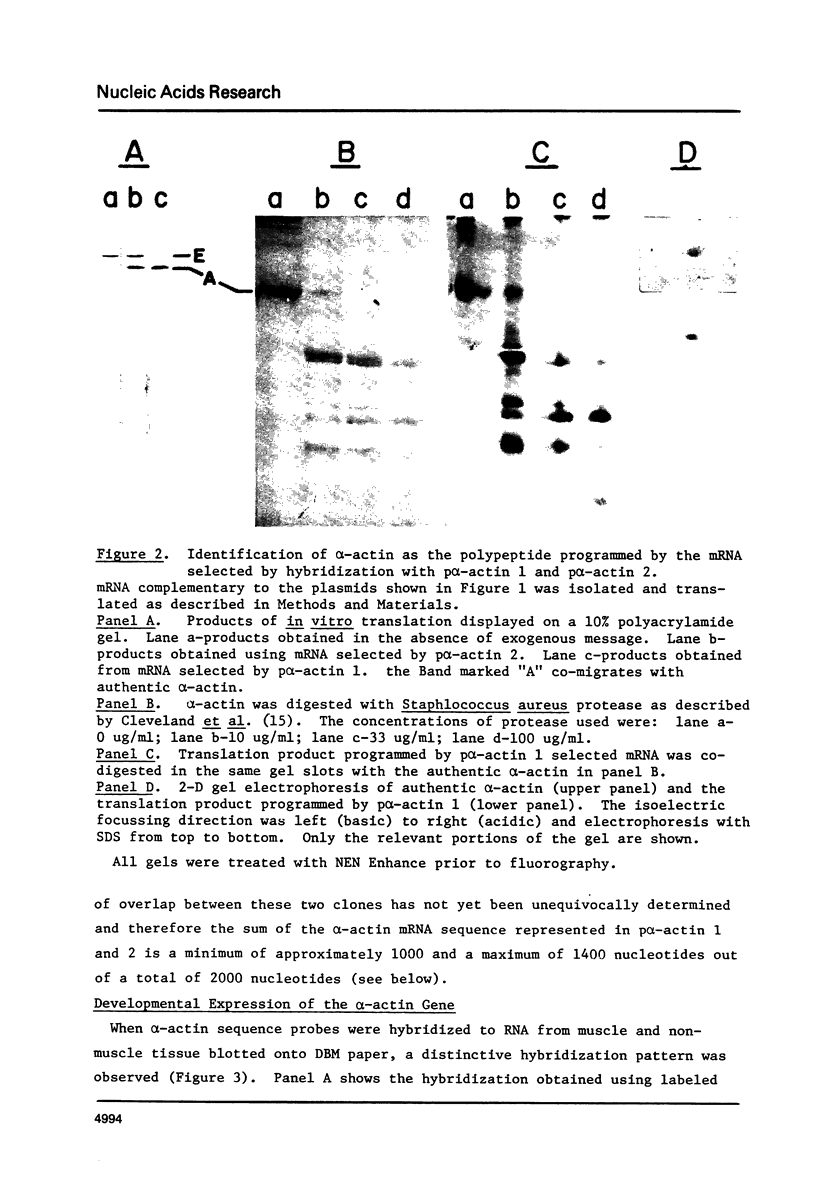
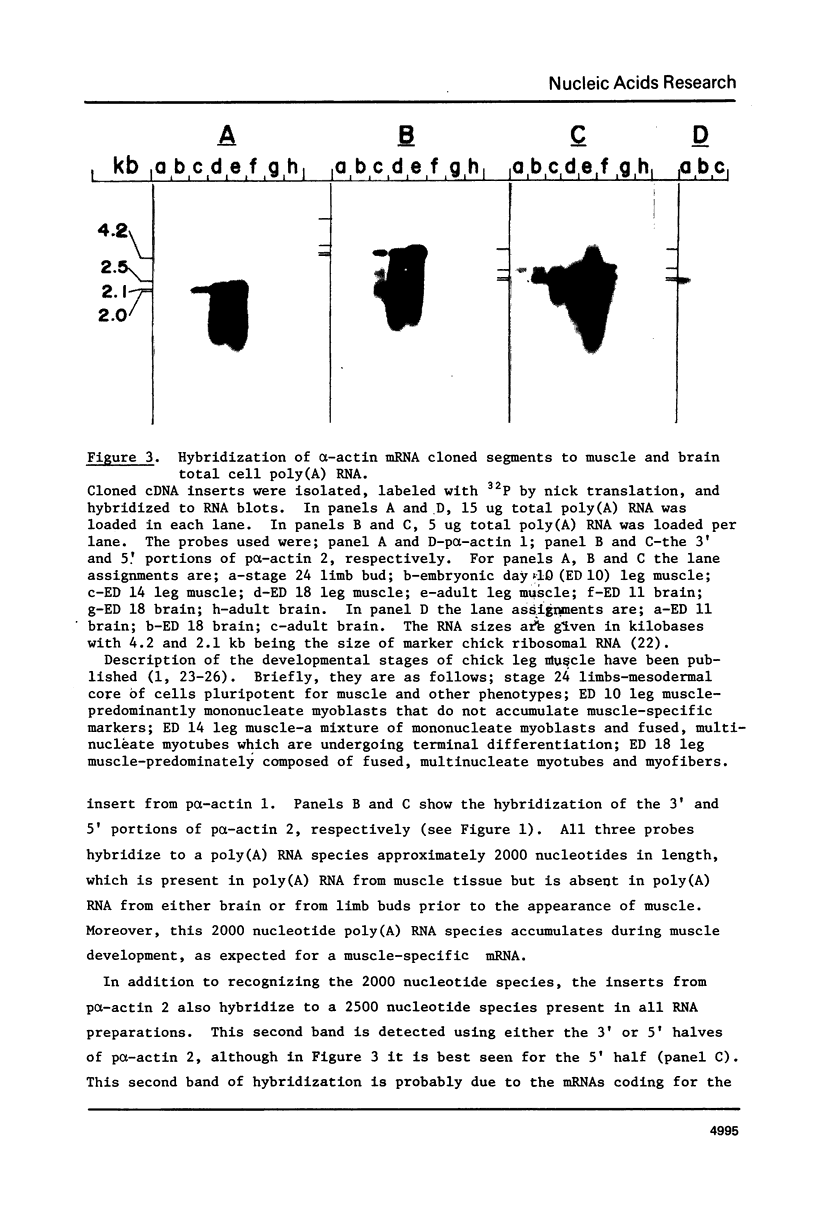
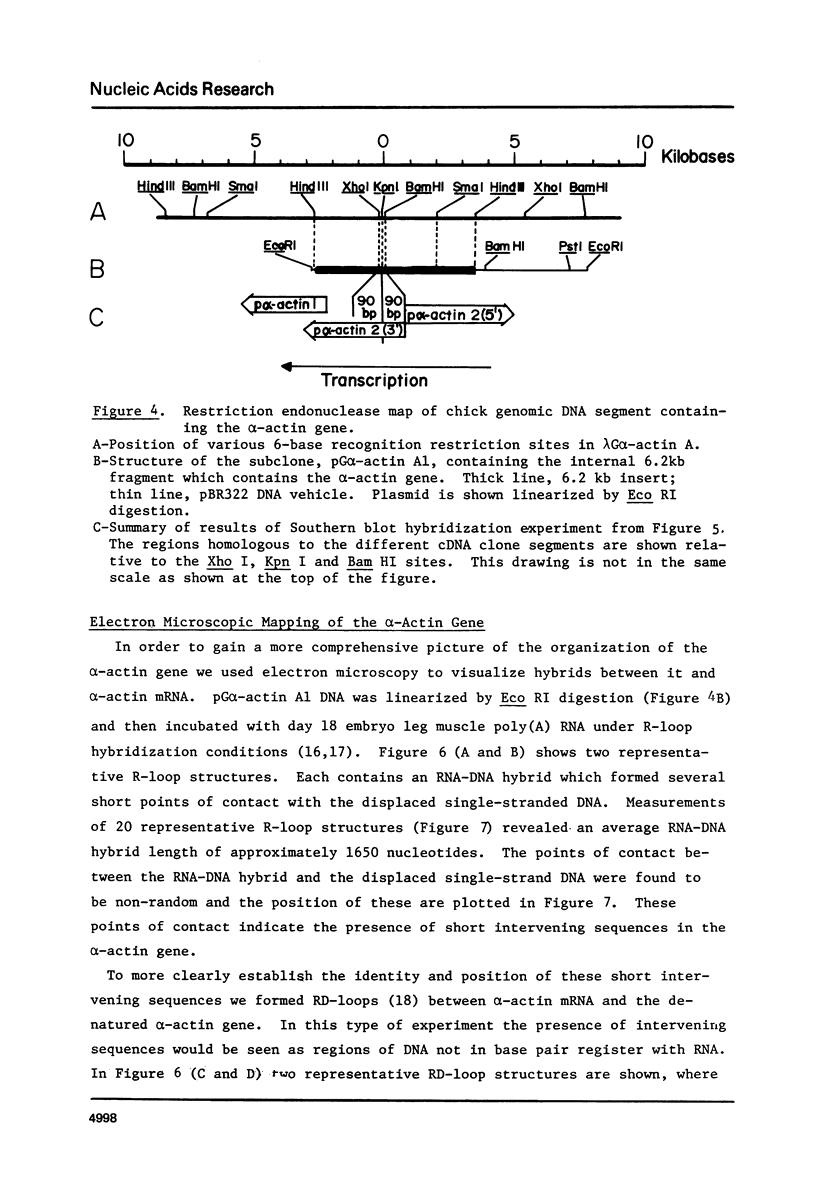
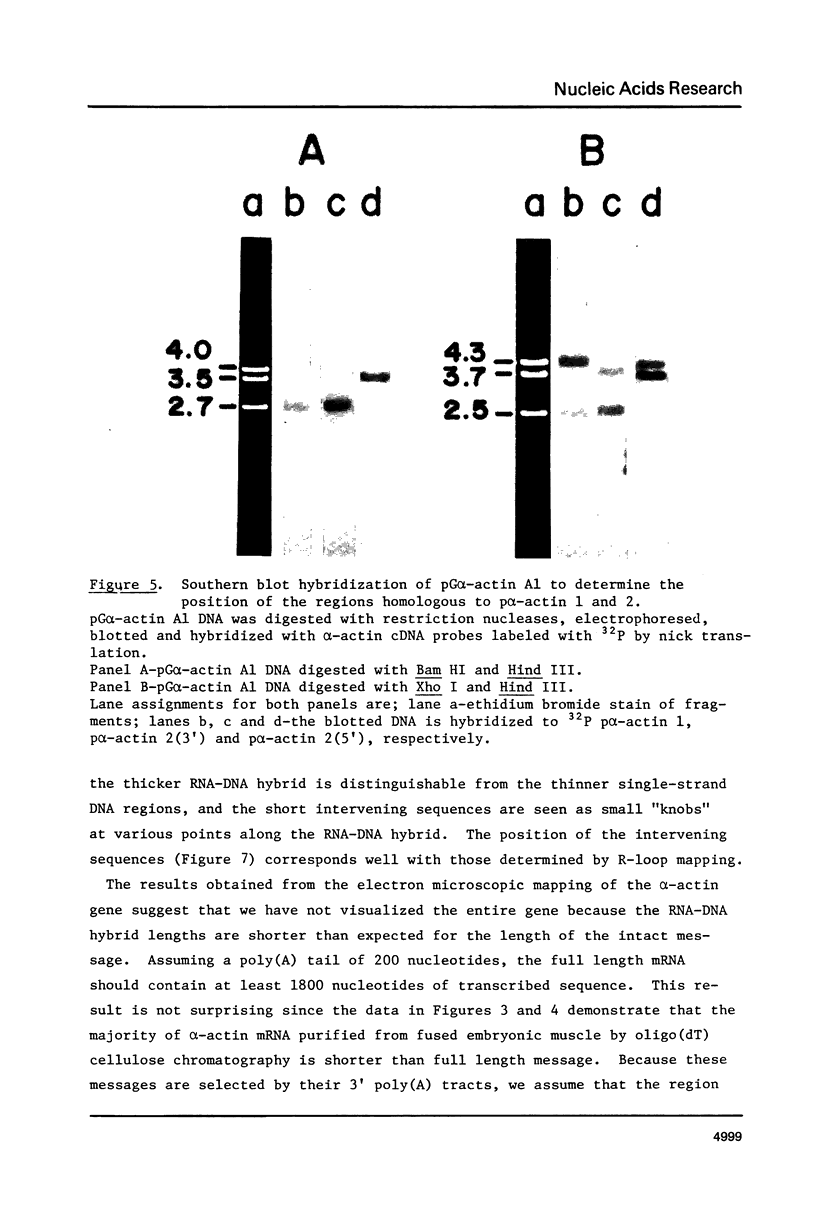
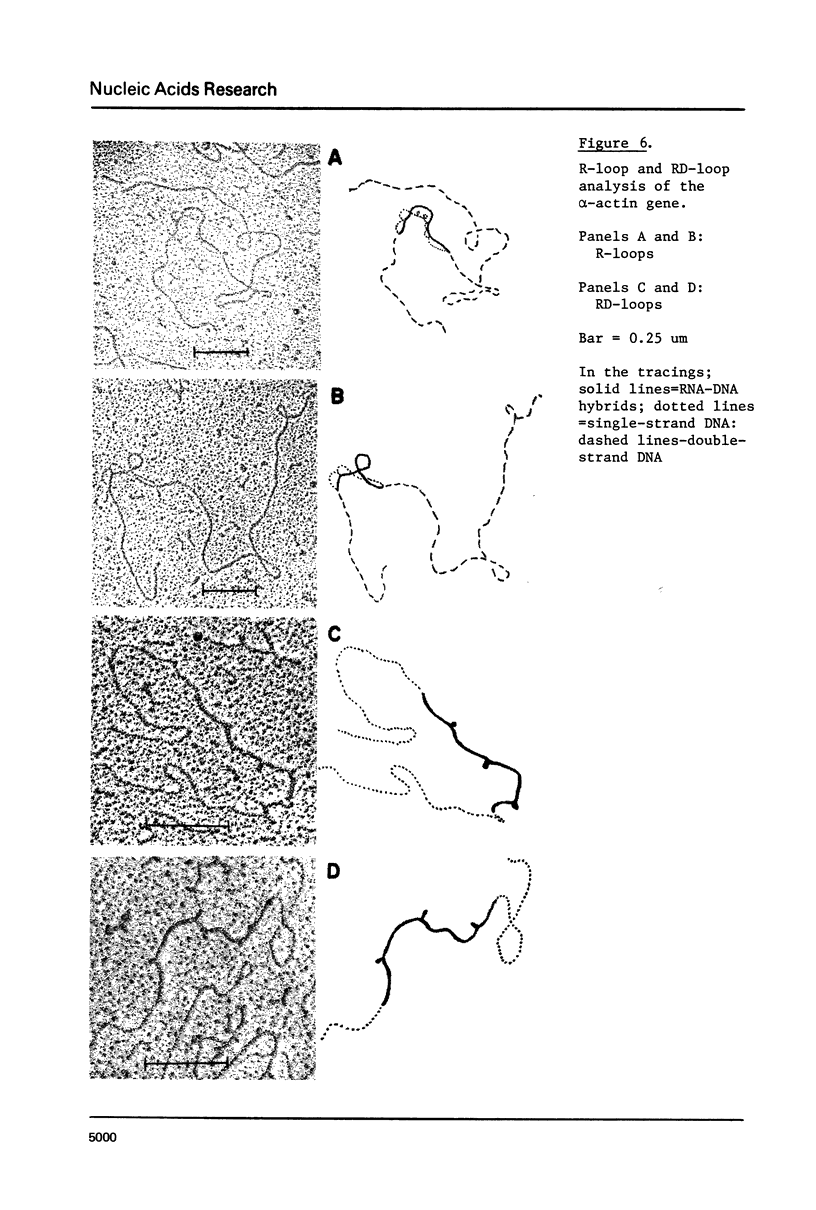
Recombinant DNA clones containing chick alpha-actin mRNA sequence have been isolated and used as probes to analyze the structure and developmental expression of the chick alpha-actin gene. The full length, 2000 nucleotide alpha-actin mRNA is detected in poly(A) RNA at early and late stages of in vivo leg muscle development. As expected, the alpha-actin mRNA is present at very low levels at early myogenic stages but is a high abundance species in terminally differentiated muscle. However, most of the alpha-actin mRNA from fused leg muscle is shorter than 2000 nucleotides, and occurs in relatively discrete size classes. An alpha-actin-like mRNA can be detected in poly(A) RNA from early embryonic brain, indicating that transcription of the alpha-actin gene may not be strictly muscle-specific at all stages of development. We have identified at least 3, very short (< 100 base pairs) intervening sequences in the alpha-actin gene which was isolated from a chick genomic library. The structure of the chick alpha-actin gene differs, therefore, from the structures of actin genes from yeast and Drosophila, both of which contain a single, relatively long, intervening sequence.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Caplan A. I., Ordahl C. P. Irreversible gene repression model for control of development. Science. 1978 Jul 14;201(4351):120–130. doi: 10.1126/science.351805. [DOI] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Kirschner M. W., Cowan N. J. Isolation of separate mRNAs for alpha- and beta-tubulin and characterization of the corresponding in vitro translation products. Cell. 1978 Nov;15(3):1021–1031. doi: 10.1016/0092-8674(78)90286-6. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Kindle K. L., Davidson N., Kindle K. L. The actin genes of Drosophila: a dispersed multigene family. Cell. 1980 Feb;19(2):365–378. doi: 10.1016/0092-8674(80)90511-5. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Sures I. Structure of a split yeast gene: complete nucleotide sequence of the actin gene in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 May;77(5):2546–2550. doi: 10.1073/pnas.77.5.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H., Heywood S. M., Marchok A. C. Reconstruction of muscle development as a sequence of macromolecular synthesis. Curr Top Dev Biol. 1970;5:181–234. [PubMed] [Google Scholar]
- Hitchcock S. E. Regulation of motility in nonmuscle cells. J Cell Biol. 1977 Jul;74(1):1–15. doi: 10.1083/jcb.74.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Garrels J. I. Characterization of the mRNAs for alpha-, beta- and gamma-actin. Cell. 1977 Nov;12(3):767–781. doi: 10.1016/0092-8674(77)90276-8. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katcoff D., Nudel U., Zevin-Sonkin D., Carmon Y., Shani M., Lehrach H., Frischauf A. M., Yaffe D. Construction of recombinant plasmids containing rat muscle actin and myosin light chain DNA sequences. Proc Natl Acad Sci U S A. 1980 Feb;77(2):960–964. doi: 10.1073/pnas.77.2.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle K. L., Firtel R. A. Identification and analysis of Dictyostelium actin genes, a family of moderately repeated genes. Cell. 1978 Nov;15(3):763–778. doi: 10.1016/0092-8674(78)90262-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lauer J., Shen C. K., Maniatis T. The chromosomal arrangement of human alpha-like globin genes: sequence homology and alpha-globin gene deletions. Cell. 1980 May;20(1):119–130. doi: 10.1016/0092-8674(80)90240-8. [DOI] [PubMed] [Google Scholar]
- Leder A., Miller H. I., Hamer D. H., Seidman J. G., Norman B., Sullivan M., Leder P. Comparison of cloned mouse alpha- and beta-globin genes: conservation of intervening sequence locations and extragenic homology. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6187–6191. doi: 10.1073/pnas.75.12.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchok A. C., Herrmann H. Studies of muscle development. I. Changes in cell proliferation. Dev Biol. 1967 Feb;15(2):129–155. doi: 10.1016/0012-1606(67)90010-3. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- McKeown M., Taylor W. C., Kindle K. L., Firtel R. A., Bender W., Davidson N. Multiple, heterogeneous actin genes in Dictyostelium. Cell. 1978 Nov;15(3):789–800. doi: 10.1016/0092-8674(78)90264-7. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Leder A., Leder P. Unusual alpha-globin-like gene that has cleanly lost both globin intervening sequences. Proc Natl Acad Sci U S A. 1980 May;77(5):2806–2809. doi: 10.1073/pnas.77.5.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ordahl C. P., Kioussis D., Tilghman S. M., Ovitt C. E., Fornwald J. Molecular cloning of developmentally regulated, low-abundance mRNA sequences from embryonic muscle. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4519–4523. doi: 10.1073/pnas.77.8.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette A. J., Reed S. L., Malt R. A. Short-lived methylated messenger RNA in mouse kidney. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2609–2613. doi: 10.1073/pnas.73.8.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Rubenstein P. A., Spudich J. A. Actin microheterogeneity in chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Jan;74(1):120–123. doi: 10.1073/pnas.74.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Leder P. Purification and cloning of a mouse ribosomal gene fragment in coliphage lambda. Gene. 1977;2(3-4):173–191. doi: 10.1016/0378-1119(77)90016-6. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978 Dec 25;126(4):783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]
- White R. L., Hogness D. S. R loop mapping of the 18S and 28S sequences in the long and short repeating units of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):177–192. doi: 10.1016/0092-8674(77)90213-6. [DOI] [PubMed] [Google Scholar]