Abstract
Background
Recently, portable extracorporeal membrane oxygenation (ECMO) machines have become commercially available. This creates the potential to utilize extracorporeal life support (ECLS) for the treatment of sudden cardiac arrest in the emergency department, and potentially in the out-of-hospital setting.
Objective
We sought to determine the feasibility of installing the ECMO circuit during delivery of mechanical chest compression CPR.
Methods
We used 5 mixed-breed domestic swine with a mean mass of 26.0 kg. After induction of anesthesia, animals were instrumented with micromanometer-tipped transducers placed in the aorta and right atrium via the left femoral artery and vein. Ventricular fibrillation (VF) was induced electrically with a transthoracic shock and left untreated for 8 minutes. Then, mechanical chest compressions were begun (LUCAS, Jolife, Lund, Sweden) and manual ventilations were performed to maintain ETCO2 between 35-45 torr. Compressions continued until ECMO flow was started. Ten minutes after induction of VF, drugs were given (epinephrine, vasopressin, and propranolol). ECMO installation was started via cutdown on the right external jugular vein and right femoral artery for placement of venous and arterial catheters while chest compressions continued. ECMO installation start time varied from 17 to 30 minutes after start of compressions and continued until ECG indicated a shockable rhythm. First rescue shocks were given at 22, 32, 35, 44, and 65 minutes.
Results
ECMO was successfully installed in all five animals without incident. It was necessary to briefly discontinue chest compressions during the most delicate part of inserting the catheters into the vessels. ECMO also allowed for very rapid cooling of the animals and facilitated post-resuscitation hemodynamic support. Only the 65-minute animal did not attain return of spontaneous circulation (ROSC).
Conclusion
Mechanical chest compression may be a suitable therapeutic bridge to the installation of ECMO and does not interfere with ECMO catheter placement.
Introduction
There are approximately 295,000 cases of out-of-hospital cardiac arrest that are treated by Emergency Medical Services (EMS) personnel in the United States each year.1 Survival to hospital discharge for patients experiencing out-of-hospital cardiac arrest is on the order of 7.9% in North America.2
Extracorporeal life support (ECLS) using extracorporeal membrane oxygenation (ECMO) perfusion has been used as a therapeutic modality for refractory cardiac arrest, but mostly in pediatrics in the in-hospital setting.3-9 While its use in emergency pediatric events has met with some success, ECMO is not without complications.10,11 Several small series have examined the use of ECLS for the treatment of adult out-of-hospital cardiac arrest, but each of these initiated ECLS in the Emergency Department.12-14 The reported times from collapse to start of ECLS ranged from 61 to 105 minutes, and the rates of survival to hospital discharge ranged from 10% to 24%.12-14
Recently, portable ECMO devices have become commercially available.15,16 This raises the possibility that ECLS could be started more quickly in the emergency department, and potentially in the prehospital setting, which might greatly reduce the time from collapse to initiation of ECLS and thereby increase the effectiveness of the intervention. One case report utilized mechanical chest compression for 1.5 hours as a bridge to starting cardiopulmonary bypass for a profoundly hypothermic cardiac arrest patient.17 This patient was successfully resuscitated, but mechanical chest compressions were not begun until arrival at the emergency department. Another case report demonstrated that portable ECMO could be started in the out-of-hospital arena. 18 This was a case of a nine year old girl who had drowned (submerged for 20 minutes), and had 50 minutes of failed advanced life support before arrival of the air medical ECMO team. ECMO was started, the girl had pulses restored and she was admitted to the hospital. While she did day on day 1 in-hospital after support was withdrawn, the case does illustrate that using ECMO in the field may be possible.
We sought to determine the feasibility of initiating ECLS while external mechanical chest compressions were being done, using an established porcine model of out-of-hospital cardiac arrest. Our primary outcome was successful installation of the ECMO circuit during mechanical chest compression CPR. Our secondary outcomes were numbers of complications, body temperature, and return of spontaneous circulation (ROSC) after varying durations of mechanical chest compression prior to onset of ECLS.
Methods
This study was approved by the University of Pittsburgh Institutional Animal Care and Use Committee (protocol 0806787), and was performed in complete compliance with the Guide for the Care and Use of Laboratory Animals.19 Five female mixed-breed domestic swine (sus scrofa) with a mean mass of 26.0 kgs were used. We sedated the animals with intramuscular ketamine (10 mg/kg) and xylazine (4 mg/kg), and anesthetized them with intravenous alpha-chloralose (40 mg/kg bolus, followed by 10 mg/kg/hr continuous infusion). We intubated the animals by direct laryngoscopy and ventilated them with room air mechanically with an Ohmeda 7000 ventilator (BOC Health Care, Madison, WI) at a rate of 12 breaths per minute with an inspiration:expiration ratio of 50%. After securing the airway, we paralyzed the animals with pancuronium (4mg bolus, 2mg additional as needed), and a cut-down was performed on the left-side groin to expose the femoral artery and vein. We placed micro-manometer tipped pressure catheters (Mikro-Tip Catheter Transducers SPR-471A and SPC-370-S, Millar Instruments, Houston, Texas) in the aorta and right atrium for monitoring of central blood pressures. We continuously monitored the electrocardiogram (Lead II ECG) and recorded it along with central pressure data with a high speed digital data collection system (Powerlab, AD Instruments NSW, Australia). Temperature was measured with an esophageal probe placed approximately 10 cm beyond the glottis opening. Baseline blood gas measurements were obtained with an I-STAT Portable Clinical Analyzer (Heska Corporation, Waukesha, WI).
After instrumentation was completed, we induced ventricular fibrillation (VF) with a single 3 second, 60 Hz, 100 mA transthoracic shock. We confirmed VF by the ECG and the aortic pressure tracing, and did not treat it for 8 minutes, at which time mechanical chest compressions were begun (LUCAS, Jolife, Lund, Sweden) and manual ventilations were performed to maintain ETCO2 between 35-45 torr. After two minutes of CPR, we administered intravenous epinephrine (0.10 mg/kg), vasopressin (40 U), and propranolol (1 mg), and continued mechanical chest compression CPR.
The ECMO circuit was composed of one arterial input catheter (to the animal) and one venous output catheter (to the ECMO pump-head/oxygenator). In real-time with chest compressions ongoing, the right femoral artery and right external jugular vein were exposed by cut-down. A 14F stainless steel-tipped catheter was placed in the femoral artery and an 18F coil-reinforced catheter was placed in the external jugular vein. The catheters were flushed and clamped off with tube occluding forceps until the initiation of ECMO flow. The bypass circuit was driven by a centrifugal pump head in line with a hollow fiber oxygenator (Affinity NT, Medtronic, Inc, Brooklyn Park, MN). The circuit was primed with 0.9% normal saline, and the air mix delivered to the oxygenator was 50% oxygen. The circuit was pre-heparinized with 225U heparin per kg body mass. In order to maintain clinical relevance, the animals did not receive any heparin prior to the induction of VF. The approximate total circuit priming volume was 800mL. A water-circulating heat exchanger provided temperature control for the bypass circuit, and the priming solution was maintained at a temperature of 34C. Control of all circuit components was managed through a Bard CPS Cardiopulmonary Bypass System (C.R. Bard, Inc). In order to preliminarily explore the outer limits of when ECLS might still be effective, we varied the time when ECMO flow was begun between 17 and 30 minutes, and the first rescue shocks were delivered 22, 32, 35, 44, and 65 minutes after VF was induced based on the visual inspection of the ECG waveform. Though we can typically install ECMO in 10 minutes or less, we intentionally varied the start of flow-time to more accurately represent the time it may take clinically in patients with cardiovascular and/or peripheral vascular disease.
We defined successful installation of ECMO as uneventful placement of the femoral and jugular catheters and the ability to maintain adequate blood flow. We defined complications as surgical misadventures (vessels torn or transected), the inability to advance the catheters deep enough within the blood vessels, and the inability to maintain adequate chest compressions. We defined ROSC as an organized ECG with a systolic blood pressure ≥80 mmHg sustained for 20 minutes. Animals that attained ROSC were euthanized with a rapid intravenous injection of 40 mEq of KCl.
As this is a preliminary study, no comparative statistics were calculated. Temperatures and flow are reported as means and standard deviations. Data were summarized using Stata (version 11, College Station, Texas).
Results
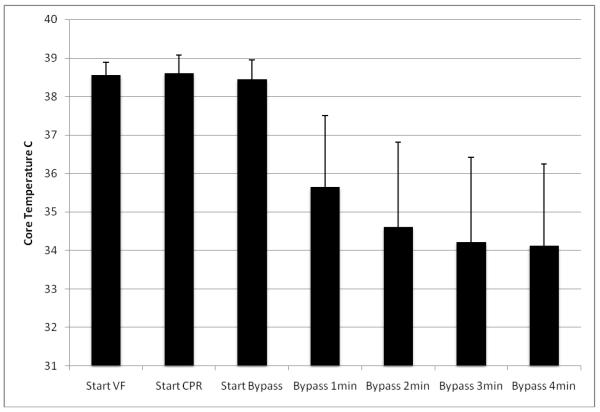
The ECMO circuit was successfully placed in all five animals. There were no complications during any of the procedures. While chest compressions did not interfere with installation of the ECMO circuit, it was necessary to briefly cease chest compressions during the most delicate point in the placement of these large bore catheters. An illustrative example of one of the resuscitations is shown in Figure 1. Four of the five animals attained ROSC, with only the 65 minute animal failing to do so. The longest duration from onset of VF to ROSC was 44 minutes. The animals’ temperatures dropped rapidly with the onset of ECMO, going from 38.5° C (± 0.3) at the onset of VF to 34.1° C (± 2.1) after 4 minutes of ECMO perfusion (see figure 2). Mean flow was 2.7 (± 0.5) L/min which approached normal resting blood flow (normal resting cardiac output for pigs this size is 2.94 L/min20). Peak flow was commonly achieved in 30-60 seconds. No additional pressors were given, but additional fluid was added to the circuit as needed to maintain flow.
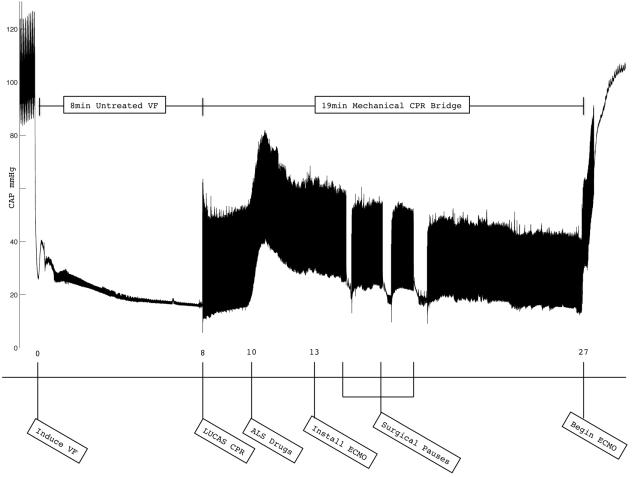
Figure 1.
This is an illustrative example of a VF cardiac arrest and resuscitation with continuous mechanical chest compression and ECMO, with intentional delay of the start of flow until 27 minutes after the induction of VF. Central arterial pressure (CAP) is shown in mmHg. Note the three brief pauses in chest compressions while the ECMO catheters were being inserted into the artery.
Figure 2.
This graph shows the rapid reduction in temperature with the onset of ECMO (unit of measure is degrees C), and shows means and standard deviations for the five animals.
Discussion
There is increasing interest in the potential use of ECLS for the treatment of out-of-hospital cardiac arrest. Studies to date have had limited success, with survival to hospital discharge rates ranging from 10% to 20%.12-14 One reason for this limited success may be that it is taking too long to initiate ECLS. In the study by Kagawa, et al., the survival rate for patients having in-hospital cardiac arrest was 26% while the survival rate for those having out-of-hospital cardiac arrest was only 10%.14 It is worth noting that the time it took to begin ECLS reperfusion was, on average, 24 minutes longer for the out-of-hospital patients. The study by Massetti, et al., had an inclusion criterion that patients had to have 45 minutes of failed resuscitation before they could be treated with ECLS.13 This resulted in their time-from-collapse to time-to-start-ECLS interval of 105 minutes, and yet they still had a survival rate of 20% (though only 5/40 of their patients had out-of-hospital cardiac arrest). Finally, the study that had the highest survival rate also had the fastest time from collapse to start of ECLS (61 minutes, and 24% survival).12
The advent of portable ECMO devices coupled with mechanical chest compression devices might enable EMS systems to play an important role in decreasing the time it takes to initiate ECLS. The use of mechanical chest compressions would free EMS personnel to initiate the surgical preparation of the patient, and perhaps even cannulate the external jugular in the ambulance. Simultaneously, a physician and perfusionist could be en route while priming a portable ECMO device for installation in the field. The recent case report in which a nine year old drowning victim was resuscitated using portable ECMO is illustrative of this possibility.20 Alternatively, EMS could notify emergency department personnel to ready the portable ECMO device while they are en route to the hospital. Thus, the patient would arrive prepared for cannulation and the ECMO device would already be present and primed. Theoretically, decreasing the time to the start of ECLS would increase survival.
In this preliminary study, we successfully installed and started ECLS while mechanical chest compressions were being done. There we no surgical misadventures or complications. Four of the five animals (80%) attained ROSC, with only the most prolonged case (65 minutes) failing. ECLS also allowed for very rapid cooling. So ECLS has the added advantage of rapidly inducing therapeutic hypothermia.
Limitations
There are several limitations to this early work. First, we used healthy young swine that were free of vascular disease. Cannulation of these major vessels may have been easier than it might be with older humans who might have both cardiovascular and peripheral vascular disease. Second, as we were primarily interested in whether chest compressions would interfere with ECMO circuit placement we did not perform necropsy on these animals, nor did we survive them to determine their neurologic status. Third, we did not actually use one of the recently available portable ECMO devices. Though cannulae for insertion would be the same, the priming volume needed for a portable device would be considerably less than what we used here. Finally, we realize that there will be logistical challenges not addressed here but again see this as a proof of concept study.
Conclusion
Mechanical chest compression may be a suitable therapeutic bridge to ECLS and does not interfere with ECMO catheter placement. ECLS may extend the therapeutic window for the treatment of cardiac arrest that is refractory to standard treatments. EMS involvement in the deployment of these technologies might decrease the time to onset of ECLS therapy.
Acknowledgments
Supported in part by National Heart, Lung, and Blood Institute grant 1 R01 HL80483 to Dr. Menegazzi.
Footnotes
Conflict of Interest Statement The LUCAS device that was used in this study was loaned to Dr. Menegazzi by Jolife. None of the investigators have any financial interest in Jolife. No Jolife representative had any participation in the conduct of this study or in manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented at the American Heart Association Scientific Sessions 2009, Orlando Florida, and at the NAEMSP Scientific Assembly 2010, Phoenix, Arizona.
References
- 1.American Heart Association [accessed March 16, 2010];Heart and Stoke Statistics 2010. http://www.americanheart.org/downloadable/heart/1265665152970DS-3241%20HeartStrokeUpdate_2010.pdf.
- 2.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiel I. Resuscitation Outcomes Consortium Investigators. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duncan BW, Ibrahim AE, Hraska V, del Nido PJ, Laussen PC, Wessel DL, Mayer JE, Jr, Bower LK, Jonas RA. Use of rapid-deployment extracorporeal membrane oxygenation for the resuscitation of pediatric patients with heart disease after cardiac arrest. J Thorac Cardiovasc Surg. 1998;116:305–311. doi: 10.1016/s0022-5223(98)70131-x. [DOI] [PubMed] [Google Scholar]
- 4.Morris MC, Wernovsky G, Nadkarni VM. Survival outcomes after extracorporeal cardiopulmonary resuscitation instituted during active chest compressions following refractory in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2004;5:440–446. doi: 10.1097/01.pcc.0000137356.58150.2e. [DOI] [PubMed] [Google Scholar]
- 5.Allan CK, Thiagarajan RR, Armsby LR, del Nido PJ, Laussen PC. Emergent use of extracorporeal membrane oxygenation during pediatric cardiac catheterization. Pediatr Crit Care Med. 2006;7:212–219. doi: 10.1097/01.PCC.0000200964.88206.B0. [DOI] [PubMed] [Google Scholar]
- 6.Thiagarajan RR, Laussen PC, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. 2007;116:1693–1700. doi: 10.1161/CIRCULATIONAHA.106.680678. [DOI] [PubMed] [Google Scholar]
- 7.Alsoufi B, Al-Radi OO, Nazer RI, Gruenwald C, Foreman C, Williams WG, Coles JG, Caldarone CA, Bohn DG, Van Arsdell GS. Survival outcomes after rescue extracorporeal cardiopulmonary resuscitation in pediatric patients with refractory cardiac arrest. J Thorac Cardiovasc Surg. 2007;134:952–959. doi: 10.1016/j.jtcvs.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 8.Huang SC, Wu ET, Chen YS, Chang CI, Chiu IS, Wang SS, Lin FY, Ko WJ. Extracorporeal membrane oxygenation rescue for cardiopulmonary resuscitation in pediatric patients. Crit Care Med. 2008;36:1607–1613. doi: 10.1097/CCM.0b013e318170b82b. [DOI] [PubMed] [Google Scholar]
- 9.Kelly RB, Harrison RE. Outcome Predictors of Pediatric Extracorporeal Cardiopulmonary Resuscitation. Pediatr Cardiol. 2010;31:626–633. doi: 10.1007/s00246-010-9659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cenqiz P, Seidel K, Rycus PT, Brogan TV, Roberts JS. Central nervous system complications during pediatric extracorporeal life support: incidence and risk factors. Crit Care Med. 2005;33:2854–2855. doi: 10.1097/01.ccm.0000189940.70617.c3. [DOI] [PubMed] [Google Scholar]
- 11.Chen YS, Chao A, Yu HY, Ko WJ, Wu IH, Chen RJ, Huang SC, Lin FY, Wang SS. Analysis and results of prolonged resuscitation in cardiac patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol. 2003;41:197–203. doi: 10.1016/s0735-1097(02)02716-x. [DOI] [PubMed] [Google Scholar]
- 12.Nagao K, Hayashi N, Kanmatsuse K, Arima K, Ohtsuki J, Kikushima K, Watanabe I. Cardiopulmonary cerebral resuscitation using emergency cardiopulmonary bypass, coronary reperfusion therapy and mild hypothermia in patients with cardiac arrest outside the hospital. Journal of the American College of Cardiology. 2000;36(3):776–83. doi: 10.1016/s0735-1097(00)00779-8. [DOI] [PubMed] [Google Scholar]
- 13.Massetti M, Tasle M, Le Page O, Deredec R, Babatasi G, Buklas D, Thuaudet S, Charbonneau P, Hamon M, Grollier G, Gerard JL, Khayat A. Back from irreversibility: extracorporeal life support for prolonged cardiac arrest. Ann Thorac Surg. 2005;79:178–183. doi: 10.1016/j.athoracsur.2004.06.095. [DOI] [PubMed] [Google Scholar]
- 14.Kagawa E, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Kurisu S, Nakam Y, Dai K, Takayuki O, Ikenaga H, Morimoto Y, Ejiri K, Oda N. Assessment of outcomes and differences between in- and out-of-hospital cardiac arrest patients treated with cardiopulmonary resuscitation using extracorporeal life support. Resuscitation. 2010;Volume 81(Issue 8):968–973. doi: 10.1016/j.resuscitation.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 15.Maunz O, Horisberger J, von Segesser L. Bridge to life: the Lifebridge B2T extracorporeal life support system in an in vitro trial. Perfusion. 2008;23(5):279–82. doi: 10.1177/0267659109104259. [DOI] [PubMed] [Google Scholar]
- 16.Arlt M, Philipp A, Zimmermann M, Voelkel S, Hilker M, Hobbhahn J. Schmid C First experiences with a new miniaturised life support system for mobile percutaneous cardiopulmonary bypass. Resuscitation. 2008;77:345–50. doi: 10.1016/j.resuscitation.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Wik L, Kiil S. Use of an automatic mechanical chest compression device (LUCAS) as a bridge to establishing cardiopulmonary bypass for a patient with hypothermic cardiac arrest. Resuscitation. 2008;66(3):391–4. doi: 10.1016/j.resuscitation.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Arlt M, Philipp A, Voelkel S, Graf BM, Scmid C, Hilker M. Out-of-hospital extracorporeal life support for cardiac arrest- A case report. Resuscitation. 2011 doi: 10.1016/j.resuscitation.2011.03.022. DOI: 0.1016/j.resuscitation.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Laboratory Animal Research. Commission on Life Sciences. National Research Council [Accessed March 21, 2011];Guide for the Care and Use of Laboratory Animals. http://www.nap.edu/catalog/5140.html.
- 20.Lee CH, Wang JY, Huang KL, Chiu HW, Fei TC, Chen TS, Chang H. Unreliability of Pulse Contour-Derived Cardiac Output in Piglets Simulating Acute Hemorrhagic Shock and Rapid Volume Expansion. J Trauma. 2010;68:1357–1361. doi: 10.1097/TA.0b013e3181d7685a. [DOI] [PubMed] [Google Scholar]