Abstract
Cyclin D1 regulates cell proliferation and is a candidate molecular target for breast cancer therapy. The current work addresses whether Cyclin D1 is indispensable for ErbB2-associated mammary tumor initiation and progression using a breast cancer model in which this cell cycle regulator can be genetically ablated prior to or after neoplastic transformation. Deficiency in Cyclin D1 delayed tumor onset but did not prevent the occurrence of mammary cancer in mice overexpressing wildtype ErbB2. The lack of Cyclin D1 was associated with a compensatory upregulation of Cyclin D3, which explains why the targeted downregulation of Cyclin D1 in established mammary tumors had no effect on cancer cell proliferation. Cyclin D1 and D3 are overexpressed in human breast cancer cell lines and primary invasive breast cancers, and Cyclin D3 frequently exceeded the expression of Cyclin D1 in ErbB2-positive cases. The simultaneous inhibition of both cyclins in mammary tumor cells reduced cancer cell proliferation in vitro and decreased the tumor burden in vivo. Collectively, the results of this study suggest that only the combined inhibition of Cyclin D1 and D3 might be a suitable strategy for breast cancer prevention and therapy.
Keywords: Cyclin D, Gene Targeting, Tetracycline Transactivator, ErbB2, Mammary Gland Development, Breast Cancer
Introduction
D-type cyclins (i.e., Cyclin D1, D2, and D3) are regulators of the Cyclin-dependent kinases 4 and 6 (Cdk4 and Cdk6) and mediate the growth factor-induced progression through the G1-phase of the cell cycle (1, 2). Cyclin D1 is the most extensively studied member of the D-type cyclins due to its suggested pivotal role as a protooncogene in a number of human malignancies including breast cancer (3, 4, 5, 6, 7). The overexpression of Cyclin D1 in the mammary epithelium leads to the formation of tumors in transgenic mice after a latency of more than one year (8), and interference of its nuclear export and proteolytic degradation has been demonstrated to accelerate mammary carcinogenesis (9). Moreover, the targeted ablation of Cyclin D1 or the inhibition of its correct functional association with Cdk4/6 was suggested to completely prevent the onset of ErbB2-associated mammary cancer (10, 11, 12).
In an effort to determine the cellular mechanisms of Cyclin D1 function in mammary tumorigenesis, Jeselsohn and colleagues (12) recently proposed that this cell cycle regulator facilitates the regenerative potential of epithelial progenitors. Earlier reports have suggested that Cyclin D1 is essential for the pregnancy-induced numeric expansion of alveolar progenitors (13, 14) that, as we have demonstrated, are the cellular targets for ErbB2-induced mammary cancer (15). The lactogenic hormone prolactin (PRL), which signals through the Jak2/Stat5 pathway, has been shown to be essential for the proliferation of this epithelial subtype (16, 17, 18, 19, 20). Active Stat5 regulates the transcriptional activation of the Cyclin D1 promoter (21, 22), and our recent work demonstrated that Jak2/Stat5 signaling enhances the expression and activation of Akt1 and the nuclear accumulation of Cyclin D1 (23, 24). Collectively, these findings support the notion that Cyclin D1 is a downstream target of active Jak2/Stat5 signaling that promotes the proliferation of normal mammary epithelial cells in response to lactogenic hormones.
Active Jak2 and Stat5 mediate self-sufficiency in growth signals, and their gain-of-function contributes to neoplastic transformation of mammary epithelial cells (24, 25, 26, 27). Similar to the reported role of Cyclin D1 for mammary tumorigenesis, we have shown recently that Jak2 is essential for ErbB2-associated and PRL-induced mammary carcinogenesis (28, 29). While deficiency in Jak2 prior to neoplastic transformation protected females against the onset of neoplasia, our studies revealed that Jak2/Stat5 signaling was no longer required for the growth of established cancer cells. In conclusion, both studies demonstrated that signaling pathways that facilitate mammary tumor initiation do not necessarily retain a similar importance during tumor maintenance and progression.
Cyclin D1-deficient females overexpressing ErbB2 never developed mammary cancer (10, 11), and it is therefore apparent that the importance of Cyclin D1 in established mammary tumors has not been examined. Consequently, it has never been demonstrated using a genetic model that this cell cycle regulator is a genuine target for the treatment of ErbB2-positive breast cancers. To experimentally address whether Cyclin D1 is required for ErbB2-associated mammary cancer initiation and progression, we generated a mouse model that allows a temporally controlled expression of this cell cycle regulator in the mammary epithelium. To translate the findings obtained from this model to the human disease, we also studied the expression of D-type cyclins in a panel of ErbB2-positive human breast cancer cell lines and in primary human breast cancers.
Materials and Methods
Mouse models
The generation and genotyping of MMTV-tTA and TetO-D1 transgenic strains is described in the supplemental materials and methods. Cyclin D1 knockout (13) and MMTV-neu transgenic mice (30) in an FVB/NJ genetic background were purchased from the Jackson Laboratory. The expression of the luciferase reporter gene was determined using in vivo bioluminescence imaging as described previously (31). All animals used in this study were treated humanely and in accordance with institutional guidelines and federal regulations.
Histological analysis of mammary glands
Protocols for the preparation of mammary gland whole mounts and H&E-stained sections of formalin-fixed tissues were described previously (32). The protocol for immunohistochemistry on histological sections of paraffin-embedded mammary gland specimens can be found elsewhere (20). The primary antibody against Cyclin D1 (clone sp4) was purchased from Abcam. The immunofluorescent staining of CK8 and Wap on mammary gland tissues was performed as described (33, 34).
Immunoprecipitation and western blot analysis
The experimental procedures for immunoprecipitation (IP) and western blot analysis were described in detail elsewhere (23). The following antibodies were used: α-β-actin (I-19), α-Cyclin D1 (72G-13), α-Cyclin D3 (C-16), α-Cyclin E (M-20), α-Cdk4 (C-22) from Santa Cruz Biotechnology; α-Cyclin D1 (sp4) from Abcam; α-tubulin from Epitomics; α-Cyclin D2 (DSC-3.1) from NeoMarkers.
Lentiviral vectors
To generate lentiviral vectors expressing the tetracycline-controlled transactivator, we cloned the tTA cDNA into the NheI site (blunt) of the pPRIME-CMV-GFP-FF3 vector (35). Lentiviral constructs expressing the Cyclin D3 shRNAs were purchased from OpenBiosystems. The pLKO.1-TRC control virus was obtained from Addgene (36). The shRNA lentiviral vectors against the human Cyclin D1 and D3 were kindly provided by Dr. Ming-Sound Tsao (Ontario Cancer Institute).
Primary Cell Cultures and orthotopic transplants
TetO-D1 transgenic MEFs were infected with a pBabe-rtTA-puro retrovirus and selected in 7 μg/ml puromycin. To induce expression of the Flag-tagged Cyclin D1, cells were treated with 1 μg/ml doxycycline for 48 hrs. Normal and neoplastic primary mammary epithelial cells were derived and cultured as described (28). Two days after infection of cells with the lentivirus expressing the tTA, the expression of luciferase was verified using bioluminescence imaging. 5 × 105 cells were transplanted into cleared mammary fat pads of recipient females (a total of 14 for the uninfected control cells and 30 for tTA-expressing cells).
For a stable knockdown of Cyclin D3, MMTV-neu and MMTV-neu/CyclinD1−/− mammary cancer cells were infected with Cyclin D3 shRNA or the pLKO.1-TRC control vectors. Cells were selected in complete medium containing increasing concentrations (1–5μg/ml) of puromycin (Sigma). To establish orthotopic transplant models, 1 × 106 MMTV-neu/CyclinD1−/−mammary cancer cells with and without stable knockdown of Cyclin D3 were injected into the # 4 mammary glands of athymic nude females (NCr strain). Tumor volumes were measured as described previously (28).
Examination of Cyclin D1/D3 expression in human breast cancer cell lines and primary breast cancer
A panel of human breast cancer cell lines was obtained from ATCC with financial support from the Integrative Cancer Biology Program (NCI). A subset of these cell lines that overexpress ErbB2 were expanded using media and supplements recommended by ATCC. Immunoblots against Cyclin D1 and D3 where performed as described above.
Deidentified FFPE tissues representing normal human breast (N=40) and invasive breast cancer specimens (N=100) were obtained under institutional guidelines from the Thomas Jefferson University pathology archives and organized in a tissue microarray as previously described (37). The staining and quantitative analysis of the expression of Cyclin D1/D3 and ErbB2 is described in the supplemental materials and methods.
Results
Cyclin D1 is largely dispensable for the proliferation and differentiation of alveolar cells that are cellular targets for ErbB2-induced mammary cancer
Mammary cancers in females that overexpress wildtype ErbB2 (MMTV-neu) occur in the FVB strain whereas C57/Bl6 females are refractory to tumorigenesis (30, 38). We therefore obtained MMTV-neu transgenic and Cyclin D1 knockout mice that carry the transgene and the targeted Cyclin D1 allele in an FVB genetic background. Unexpectedly, Cyclin D1 deficiency in this strain led to a substantial reduction in spermatogenesis and infertility (Suppl. Fig. S1). It was therefore necessary to use a much less effective heterozygous breeding scheme to generate females that carry multiple transgenes in a Cyclin D1−/− background.
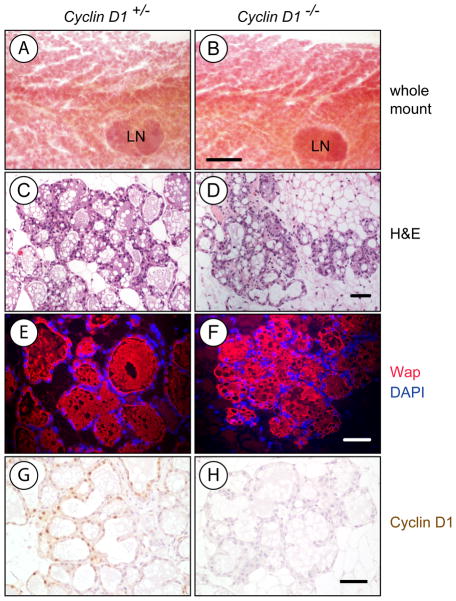
We have shown previously that ErbB2-induced mammary cancers predominantly originate from alveolar cells (15), but lack of Cyclin D1 in a 129/C57 mixed genetic background was reported to cause impaired alveologenesis (13, 14). In contrast to 129/C57 mice, Cyclin D1 is largely dispensable for the proliferation and differentiation of secretory alveoli in postpartum FVB females (Fig. 1). Mammary gland whole mounts from Cyclin D1 knockout mice were indistinguishable from wildtype controls (Fig. 1A, 1B), and differences in the extent of alveolar expansion were only detectable in a few selected areas of H&E-stained histological sections (Fig. 1C, 1D). Overall, Cyclin D1 deficiency did not adversely affect the terminal differentiation of the secretory epithelium as demonstrated by immunofluorescence staining of the late milk protein Wap (Fig. 1E-1H). Despite fairly normal development and occasionally some milk in the stomach of pups, Cyclin D1 knockout females did not exhibit a normal nursing behavior and failed to rear the offspring. Pups from knockout females could be successfully fostered by wildtype dams. Regardless of the ability of Cyclin D1-deficient females to sustain their litters, the examination of the developing mammary gland in these females clearly demonstrated that, unlike recently reported (12), the target cell population for ErbB2-induced mammary tumor formation is present in the FVB genetic background.
Fig. 1. Cyclin D1 deficiency in the FVB strain background does not adversely affect alveologenesis in the mammary gland.
Histological analysis of the postpartum mammary glands (lactation day 1) of a homozygous Cyclin D1 knockout female (Cyclin D1−/−) and her heterozygous littermate control (Cyclin D1+/−) A, B. Whole mounts; bar represents 1mm; LN, lymph node. C, D. H&E-stained tissue sections. E, F. Immunofluorescence staining against Wap. G, H. Immunohistochemical staining for Cyclin D1; slides were counterstained with hematoxylin; bars in panels C.-H. represents 50 μm
Development of transgenic strains that allow a ligand-controlled expression of Cyclin D1 in the developing mammary gland
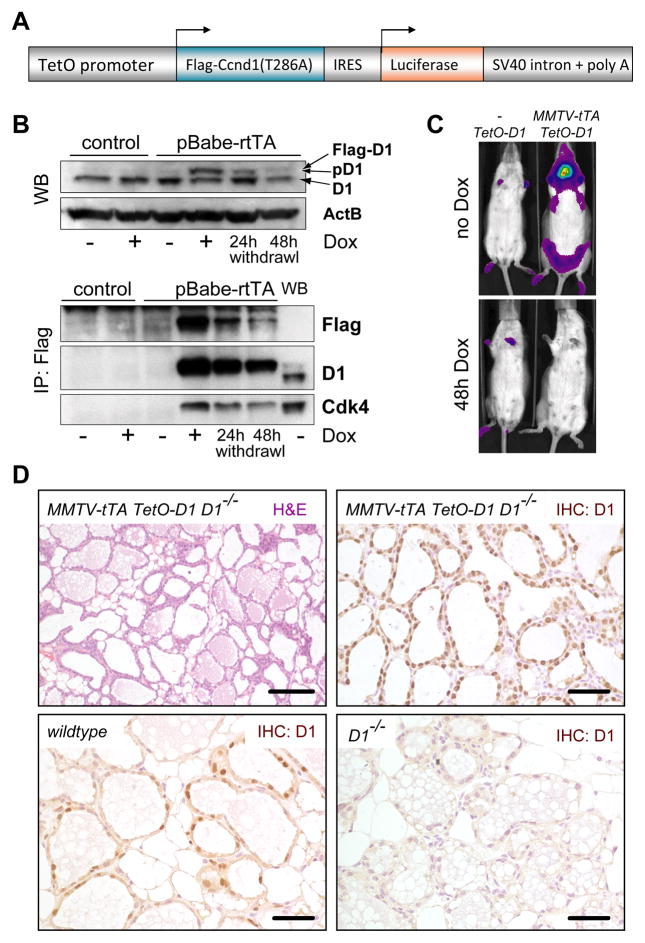
As a first step of developing an animal model that allows a downregulation of Cyclin D1 in progressing mammary cancers, we generated a transgenic strain (TetO-D1), in which the expression of exogenous Cyclin D1 and luciferase can be targeted to the developing mammary gland in a doxycycline (Dox)-regulatable manner (Fig. 2A). In order to facilitate a higher degree of functionality of Cyclin D1, we utilized a Flag-tagged mutant (T286A) that has a delayed proteolytic turnover (39). To determine the correct Dox-controlled expression of the TetO-D1 transgene, we derived primary MEFs and infected those with a retrovirus expressing the reverse transactivator (rtTA). Expression of Cyclin D1 was only detected when these cells were treated with Dox, and the levels declined following the withdrawal of the ligand (Fig. 2B). The binding of Cdk4 to the Flag-tagged Cyclin D1 (T286A) was indicative of the proper functionality of the transgene. To target the TetO-D1 transgene to the developing mammary gland, we developed a novel MMTV-tTA strain that exhibits a stringent expression of the transactivator protein in the mammary epithelium and salivary gland in the absence of Dox (Fig. 2C). Using bioluminescence imaging, we determined that a) the TetO-D1 transgene does not exhibit any leaky expression in the absence of the transactivator, and b) the transgene can be downregulated within 48 hours of Dox administration. Next, we generated female mice that express exogenous Cyclin D1 in a Cyclin D1 null background (MMTV-tTA TetO-D1 Cyclin D1−/−). The histological analysis of the postpartum mammary gland of these mice revealed that nuclear Cyclin D1 was abundant in our experimental animals, and the alveolar compartment in these mice was fully developed and comparable to an advanced stage of lactation (Fig. 2D). Regardless of the presence of milk in these alveoli, the mice did not lactate. This clearly supports our previous assumption that the lactation defect in Cyclin D1 knockout mice might be a complex phenotype and is not simply the result of impaired alveologenesis as previously suggested.
Fig. 2. Development of a genetic model that allows a doxycycline-controlled expression of exogenous Cyclin D1 in the mammary gland of Cyclin D1 knockout mice.
A. Schematic outline of the TetO-D1 transgenic vector that mediates the co-expression of active Cyclin D1 (T286A) and luciferase under the control of the doxycycline (Dox)-controlled transactivator. B. Western blot (WB, upper panel) and IP/Western blot (lower panel) on transgenic MEFs that were infected with a retroviral vector expressing the reverse tet-controlled transactivator (rtTA). Note expression of D1 (T286A) is induced by the administration of Dox. C. Bioluminescence imaging on an MMTV-tTA/TetO-D1 double transgenic female and TetO-D1 littermate control; in contrast to panel B, the expression of exogenous Cyclin D1 in this mouse model is repressed through administration of Dox. D. H&E and immunohistochemical staining for Cyclin D1 (upper panels) on mammary gland tissue sections from a postpartum female expressing exogenous Cyclin D1 in a Cyclin D1−/−background. Tissue sections from postpartum wildtype and Cyclin D1 knockout mice were used as controls, bars represent 50 μm.
Downregulation of Cyclin D1 has no effect on the growth of ErbB2-induced mammary cancer cells
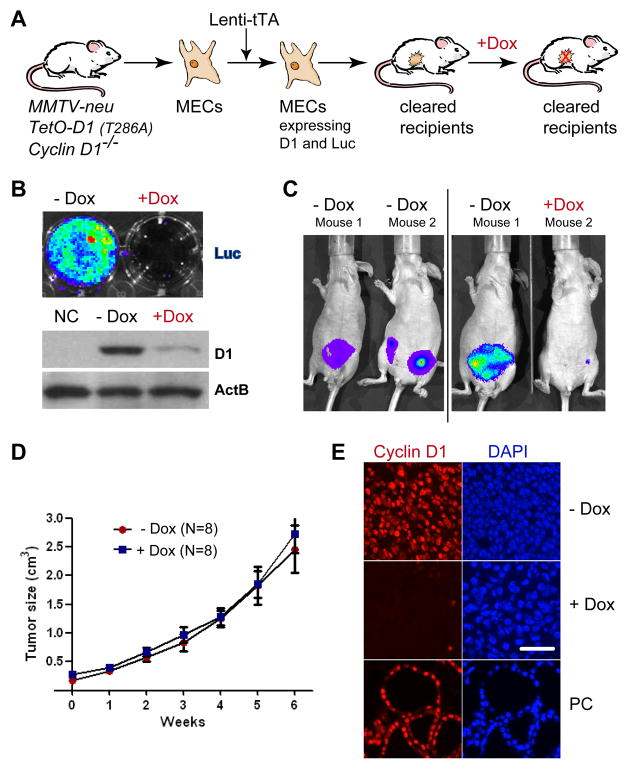
Although it was reported that Cyclin D1 deficient mice are resistant to ErbB2-induced mammary carcinogenesis (10, 11), it has never been examined whether the ablation of this cell cycle regulator has an impact on the growth of established tumors. To address this issue, we utilized our TetO-D1 transgenic strain in combination with the Cyclin D1 knockout to generate an animal model that allows a downregulation of Cyclin D1 prior to or following neoplastic transformation. Male and female homozygous Cyclin D1 knockout mice have reproductive impairments, and it is highly inefficient to breed heterozygous mice to generate sufficient knockout females that also carry at least three additional transgenes (MMTV-tTA, TetO-D1, and MMTV-neu). We therefore simplified the model design by using a lentiviral gene transfer of the transactivator (Lenti-tTA) combined with a mammary epithelial transplantation approach (Fig. 3A). We derived normal MECs from MMTV-neu TetO-D1 Cyclin D1−/− females and infected those with a lentivirus expressing the tTA. After having verified the activation of the TetO-D1 transgene, infected cells and their uninfected controls were transplanted into the cleared #4 mammary fat pads of FVB or Athymic nude recipient mice (Suppl. Fig. S2). Following the development of sporadic mammary tumors, we isolated and cultured cancer cells ex vivo and the Dox-controlled expression of Cyclin D1 and luciferase was examined using bioluminescence imaging and western blotting (Fig. 3B). Next, we orthotopically transplanted tumor cells into athymic nude recipients to establish a cohort of females that carry syngeneic tumors that allow for a direct comparison of the effects of Cyclin D1 ablation. After engraftment and growth of secondary tumors to 0.5 cm in diameter (3–5 months), the continuous activation of the TetO-D1 transgene in the growing cancer cells were assessed using in vivo imaging (Fig. 3C). Half of the recipient mice were then treated with Dox to downregulate the expression of the TetO-D1 transgene for a period of 6 weeks (Fig. 3C). The weekly analyses of the tumor sizes showed that a downregulation of Cyclin D1 did not slow the growth of the neoplasms (Fig. 3D). The expression or absence of Cyclin D1 in the cancer cells of both experimental cohorts was verified using immunofluorescence staining (Fig. 3E). Collectively, this line of investigation revealed that a downregulation of Cyclin D1 has no effect on the growth of ErbB2-induced mammary cancer cells in vivo.
Fig. 3. Cyclin D1 is not required for the maintenance of ErbB2-induced mammary cancer.
A. Schematic outline of the experiment. B. Bioluminescence imaging and western blot analysis to assess the Dox-controlled expression of luciferase and exogenous Cyclin D1 (T286A) in MMTV-neu transgenic mice that lack both endogenous Cyclin D1 alleles. C. Bioluminescence imaging on wildtype recipient mice that developed tumors following the transplantation of cancer cells expressing Cyclin D1. Both panels show the same mice before and 72 hrs after administration of Dox. D. Growth curve of mammary cancers in untreated or Dox-treated recipients (i.e., with or without Cyclin D1). E. Immunofluorescence staining of Cyclin D1 (red) in tumors of recipient mice. PC, positive control tissue from a postpartum female expressing exogenous Cyclin D1 in a Cyclin D1−/−background, bar represents 50 μm.
Cyclin D1 deficiency delays tumor initiation but does not protect against ErbB2-induced mammary cancer
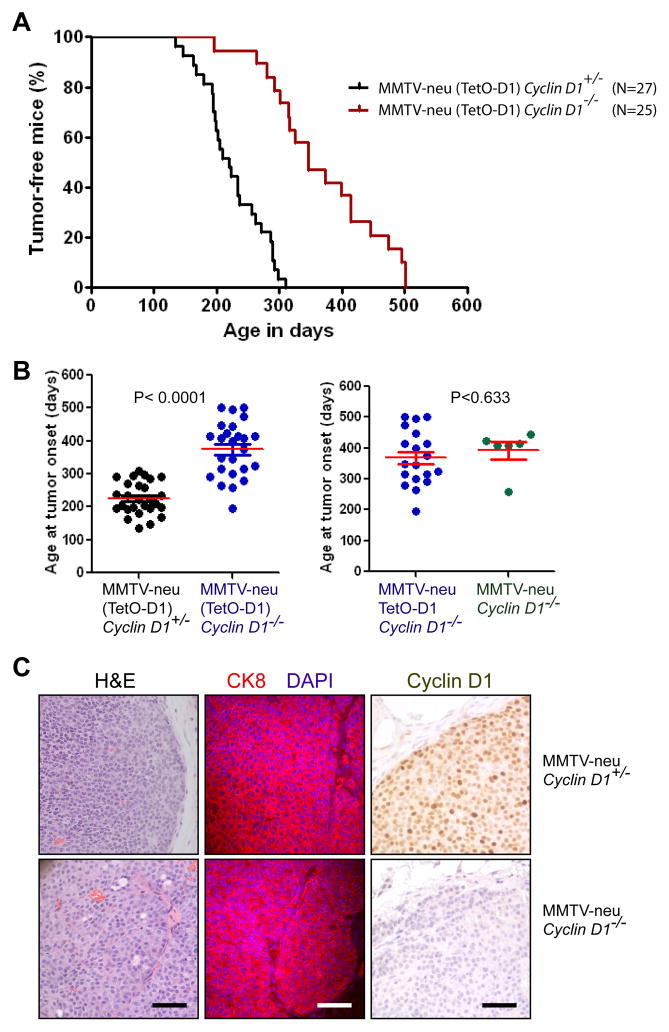
Unexpectedly, a number of recipient females that were engrafted with normal MECs not carrying the tTA and not expressing luciferase developed mammary cancers (data not shown). We also observed that some of the parous females from the MMTV-neu TetO-D1 Cyclin D1−/− donor cohort developed mammary cancer, and we therefore decided to monitor these females for a prolonged period to revisit the current paradigm that Cyclin D1 is essential for ErbB2-induced cancer initiation (10). Although we never detected any leaky expression of the TetO-D1 construct (Fig. 2B, 2C), we maintained a subset of females without this transgene (MMTV-neu Cyclin D1−/−) as an additional control within the experimental cohort. As shown in Fig. 4A and 4B (left panel), Cyclin D1 deficiency delayed the development of palpable tumors by about 3 to 4 months. However, the ablation of this D-type Cyclin did not protect against ErbB2-indcuced mammary carcinogenesis as reported previously. Tumorigenesis occurred at a similar latency regardless of the presence of the unexpressed TetO-D1 transgene (Fig. 4B, right panel). The lack of the Cyclin D1 protein in mammary tumors of Cyclin D1−/− females was confirmed by immunohistochemistry, and the functional ablation of this cell cycle regulator did not noticeably alter the histopathological appearance and expression of the luminal marker CK8 (Fig. 4C).
Fig. 4. Cyclin D1 deficiency delays but does not prevent the initiation of ErbB2-induced mammary carcinogenesis.
A. Kaplan-Meier curve illustrating the tumor-free survival of females expressing wildtype ErbB2 (MMTV-neu) in the presence or absence of Cyclin D1 (Cyclin D1+/− and Cyclin D1−/−). Some animals also carried the TetO-D1 transgene (indicated by parentheses), which is not expressed in the absence of the transactivator. B. Mean age of tumor onset (horizontal bars). Each marker represents the age of onset of the first palpable tumor per mouse (P value, t test). The left graph represents the entire cohort of mice shown in panel A, the right graph compares the age of tumor onset within the cohort of Cyclin D1 knockout mice with and without the TetO-D1 transgene. C. H&E and immunostaining against cytokeratin 8 (CK8) and Cyclin D1 in ErbB2-induced mammary cancers that originated in the presence or absence of Cyclin D1, bars represent 50 μm.
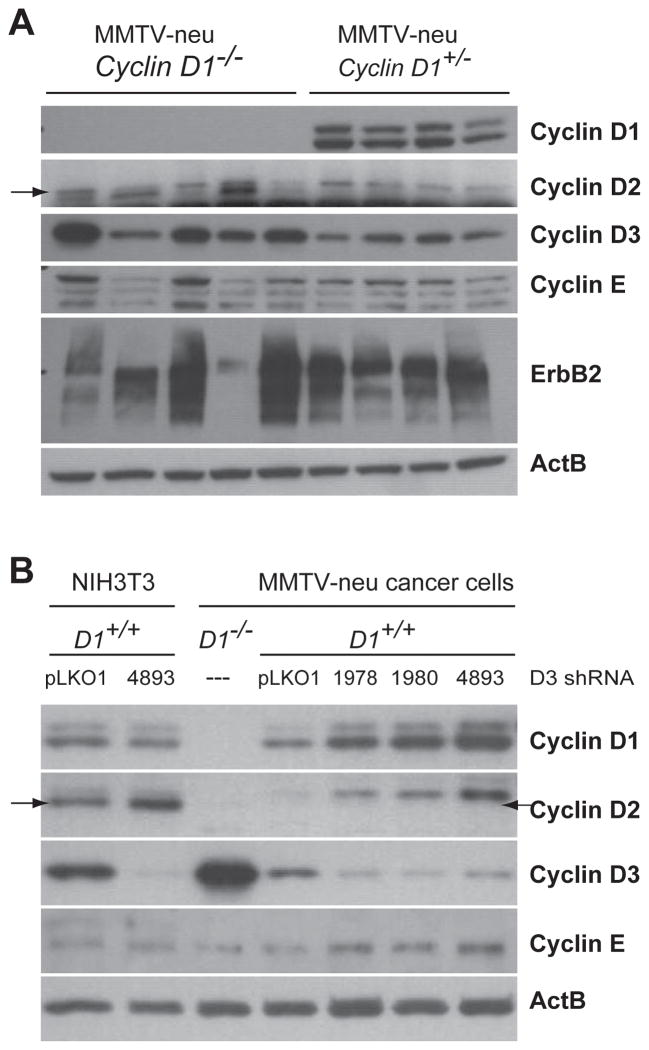
Compensatory expression of D-type Cyclins in ErbB2-expressing mammary cancers
Cyclin D1 is the most abundant member of the family of D-type cyclins in ErbB2-indcued mammary cancers of MMTV-neu transgenic mice (Fig. 5A), but these tumors also express substantial amounts of Cyclin D3. Interestingly, the expression of Cyclin D3 was clearly elevated in the majority of mammary cancers that lack Cyclin D1. The analysis of six additional Cyclin D1-deficient mammary cancers revealed that in total 73% exhibited a higher expression of Cyclin D3 compared to wildtype tumors. The levels of Cyclin D2, on the other hand, were quite low in all mammary cancers. Along with the increase in Cyclin D3, we detected only a slight elevation in Cyclin E in three of the mammary tumors lacking Cyclin D1. To assess whether Cyclin D3 is biologically relevant in mammary cancers expressing Cyclin D1, we performed a knockdown of Cyclin D3 in explanted cancer cells (Fig. 5B). After testing a panel of eight different lentiviral shRNA constructs in NIH3T3 cells, we found that three of them were capable of stably knocking down the expression of Cyclin D3 to barely detectable levels. These three shRNA vectors were then used to downregulate Cyclin D3 in ErbB2-induced mammary cancer cells, but unlike in fibroblasts, Cyclin D3 could never be fully ablated in these cells. More importantly, a knockdown of Cyclin D3 resulted in a compensatory upregulation of Cyclin D1 and only a slight elevation in Cyclin E.
Fig. 5. Consequential upregulation of Cyclin D3 in ErbB2-induced mammary tumors that lack expression of Cyclin D1.
A. Western blot analysis of D-type cyclins and Cyclin E in ErbB2-indcued primary mammary tumors that originated in the presence or absence of endogenous Cyclin D1. B. Western blot on explanted MMTV-neu-induced mammary tumor cells that were infected with three different Cyclin D3 shRNA knockdown vectors. pLKO1, control vector; cancer cells from a Cyclin D1 knockout mouse (D1−/−) were used as a positive/negative control for cyclins D1 and D3. NIH3T3 cells with and without one of the shRNA vectors served as an additional control. Note that Cyclin D2, which migrates below the D1, is absent in ErbB2-expressing cancer cells.
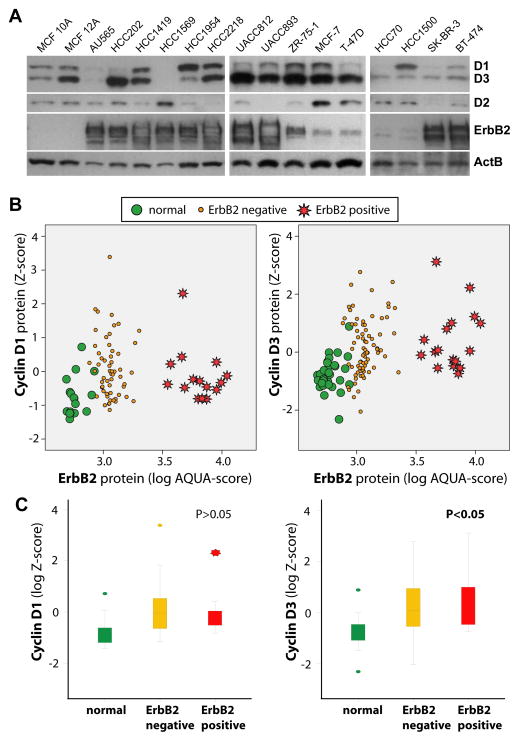
Cyclin D3 is significantly upregulated in human breast cancers and frequently exceeds the expression of Cyclin D1 in ErbB2-positive cases
To assess whether a similar reciprocal expression of D-type cyclins occurs in human breast cancers, we initially examined their levels in ten ErbB2-positive cancer cell lines (Fig. 6A). We also included two untransformed (MCF10A and 12A) and five ERα-positive breast cancer lines (including MCF-7 and T-47D) into the analysis. Due to distinct size differences between Cyclin D1 and D3 and quite similar binding affinities of the primary antibodies (Suppl. Fig. S3), we were able to simultaneously assess the expression of these two proteins using the same secondary antibody and identical blotting conditions. The results of this study revealed that both cyclins are simultaneously upregulated in 67% of all breast cancer lines, and Cyclin D3 exceeds the expression of Cyclin D1 in six of the ten ErbB2-positive lines. Cyclin D1 was more abundant in only two cases overall and only one ErbB2-positive cell line (HCC1954).
Fig. 6. Cyclin D3 is significantly upregulated in human breast cancer cell lines and primary human breast cancer specimens.
A. Western blot analysis to assess the expression of D-type cyclins in a panel of predominantly ErbB2-positive breast cancer cell lines. B. Quantitative analysis of the intensity of immunofluorescence straining of Cyclin D1 and D3 in combination with ErbB2 in normal human breast tissue (green circle), ErbB2-negative breast tumors (yellow circle), and ErbB2-positive breast cancers (red stars). C. Graphic illustration of the mean differences in the expression of Cyclin D1 and D3 (P, t-test).
To determine the expression of Cyclin D1 and D3 in primary human breast cancers, we performed a quantitative analysis of the level of immunofluorescent staining against these cell cycle regulators in addition to ErbB2. We examined 40 normal breast tissues along with 100 invasive ductal carcinomas. Evaluable levels of both D1 and D3 could be quantified for 76 cases, and among those, 17 were identified as ErbB2-positive. Images of representative cases that overexpress Cyclin D1 and/or Cyclin D3 are shown in Suppl. Fig. S4. The quantitative analysis of the staining intensity revealed that both D-type cyclins are upregulated individually or together in over 70% of all breast cancer cases, and among those, 26.3% exhibited a high expression of Cyclin D3 but not Cyclin D1. More importantly, only the expression of Cyclin D3 was significantly elevated in ErbB2-positive cases (Fig. 6B and Fig 6C; P < 0.05). Collectively, the analyses performed on the cell lines and primary cancer specimens using different methods for protein quantification indicate that the levels of Cyclin D3 are elevated to a greater extent than Cyclin D1 in ErbB2-positive human breast cancers.
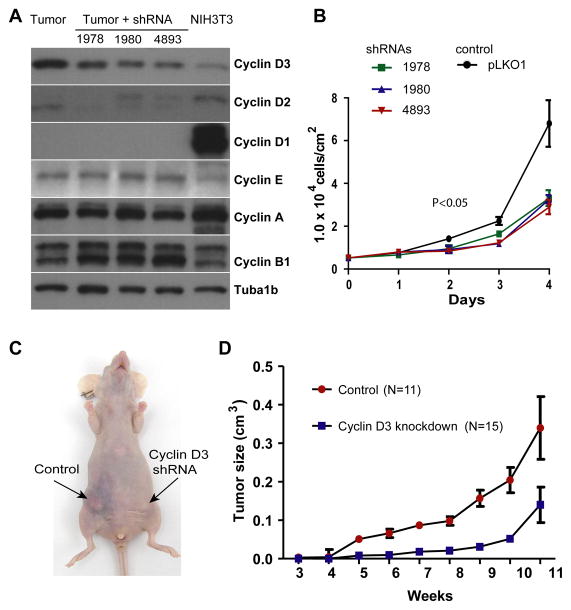
Knockdown of Cyclin D3 has a significant impact on the growth of Cyclin D1-deficient mammary tumor cells
In mammary cancers in humans and mice, the pool of the Cyclin D3 and D1 proteins appears to be regulated in a concordant manner and might therefore be critical for tumor cell proliferation. To experimentally address this issue, we first attempted to downregulate the expression of Cyclin D1 and D3 using a panel of published shRNAs (40) in an ErbB2-positve breast cancer cell line that expresses both cyclins (Suppl. Fig. S5). Although only one shRNA construct resulted in a sustained downregulation of Cyclin D3, a comparison of these knockdown cells to their controls showed that loss of Cyclin D3 led to a compensatory upregulation of Cyclin D1. This supports the notion that, like in mouse mammary cancers, the levels of both cyclins are regulated in a concordant manner. To assess whether the combined pool of D-type cyclins is critical for ErbB2-induced mammary cancers, we derived tumor cells from Cyclin D1-deficient mice and infected them with three different Cyclin D3 shRNA vectors (Fig. 7A). Since only Cyclin D3 is present in these cells in a significant quantity, a knockdown of this protein would create tumor cells that lack all three D-type cyclins. Following puromycin selection, Cyclin D3 levels declined, but the protein could never be ablated in the surviving cancer cells in a comparable manner to cancer cells that express Cyclin D1 (compare Figs. 7A and 5B). More importantly, Cyclin E was not upregulated and consequently the knockdown of Cyclin D3 significantly impaired cell growth (Fig. 7A, 7B). Next, we orthotopically transplanted similar numbers of knockdown cells and their controls into immunocompromised animals (Fig. 7C). Compared to the knockdown cells, the controls expressing Cyclin D3 exhibited a much better engraftment and faster growth (Fig. 7D). Smaller tumors that eventually appeared in recipient mice carrying the D3 knockdown cells were mainly a result of a clonal expansion of cells that had regained expression of Cyclin D3 (Suppl. Fig. S6). In conclusion, the results of this study demonstrate that the pool of Cyclin D1 and D3 are critical for the growth of ErbB2-expressing mammary cancer cells in vitro and in vivo.
Fig. 7. Ablation of Cyclin D1 and Cyclin D3 impairs the proliferation of ErbB2-induced cancer cells.
A. Western blot analysis of D-type cyclins, Cyclin E, Cyclin A, and Cyclin B in primary ErbB2-induced mammary cancer cells that lack expression of endogenous Cyclin D1. Tubulin (Tuba1b) was used as a loading control. Mammary tumor cells were infected with lentiviral vectors that express three different shRNAs to knock down the expression of Cyclin D3. B. viable cell count over a 4-d period to determine growth rates of three Cyclin D1/D3-deficient cancer cell lines compared to control cells that lack only Cyclin D1 (pLKO1); P, U-test. C. Recipient female that developed mammary tumors after orthotopic transplantation of Cyclin D1-single (control) or Cyclin D1/D3-double deficient cells. D. Growth curve of Cyclin D1-deficient mammary tumors in the presence (control) or absence of Cyclin D3.
Discussion
The mammary glands of Cyclin D1-deficient females in an FVB background exhibit extensive alveologenesis during pregnancy and consequently must possess alveolar progenitors that, as we have reported previously (15), are the main targets for ErbB2-induced neoplastic transformation. Although lack of Cyclin D1 extended the tumor-free survival in our study, Cyclin D1 deficiency did not prevent the onset of mammary cancer as reported previously (10, 11). Following neoplastic transformation, Cyclin D1-deficient mammary tumors exhibited the same sporadic occurrence, growth, and histopathological features compared to cancers that arose in females expressing Cyclin D1. The difference in the outcome of our study from that of others can be explained by the effects of diverse strain backgrounds. The previous reports were based on the maintenance of the MMTV-neu transgene in a predominantly 129/C57 mixed genetic background. While Cyclin D1-deficient females carrying C57 alleles lack alveolar progenitors and therefore the cancer-initiating cell type, there is also compelling evidence from a study by Rowes and colleagues (38) that suggests that the genetic background has a profound impact on the tumor latency in MMTV-neu mice. While the underlying mechanism for this phenomenon remains unknown, it is evident that genetic studies related to MMTV-neu-induced mammary tumorigenesis should be performed in the FVB strain.
A delay or lack of tumor formation in a knockout mouse expressing an oncogene may not be an appropriate indicator for whether the targeted ablation of a gene or its encoded protein is also relevant for therapeutic approaches in humans and animals (41). Cancer cells that evolve though selective mechanisms frequently shift signaling networks and employ alternative pathways to optimize growth and survival as demonstrated recently for the Jak/Stat pathway (28, 29). A suitable experimental design to assess the importance of a protein during cancer progression is to repress its expression within the cancer cells of an established neoplasm. Based on this concept, we have generated a genetic model that allows the targeted downregulation of Cyclin D1 in progressing ErbB2-positive mammary cancers. Using this tumor model, we demonstrated that this cell cycle regulator is not essential for the proliferation of cancer cells, and consequently Cyclin D1 may not be a primary candidate target to treat ErbB2-associated breast cancer as suggested previously.
While Cyclin D1 has long been recognized as an oncogene in breast carcinogenesis, considerably fewer studies included an examination of the expression and functionality of the other two D-type cyclins. The first immunohistochemical analysis of Cyclin D3 expression in human malignancies by Bartkova et al. (42) revealed an overabundance of this particular cell cycle regulator in breast cancer specimens. A synchronous upregulation of Cyclin D1 and D3 in a subset of human breast cancers was also reported by Russell and colleagues (43), and the authors proposed that this was, in part, a consequence of defective proteolysis. A subsequent study by the same research team demonstrated that overexpression of Cyclin D3 is sufficient to induce the development of squamous carcinomas in the mammary gland of transgenic mice (44). Using western blot analysis and quantitative immunofluorescence (i.e., two independent assays with different antibodies) on breast cancer cell lines and primary tumor specimens, we show in this report that the extent of Cyclin D3 upregulation in human breast cancer cells often exceeds that of Cyclin D1. In particular, ErbB2-positive cases that are known to have a poor prognosis express significantly more Cyclin D3 compared to Cyclin D1. Our findings are in line with an empirical study by Wong et al. (45) that shows that Cyclin D3 was more abundant than Cyclin D1 in high-grade breast cancers.
In this study, we provide several lines of evidence that the expression of D-type cyclins is concordantly regulated in mammary cancer cells, and only the combined inhibition of Cyclin D1 and D3 had a profound impact on cancer cell proliferation. We did not observe a compensatory upregulation or a gain-of-function of Cyclin E that was sufficient to bypass the importance of the D-type cyclins as proposed previously for ErbB2-associated mammary cancer (46) and normal MECs expressing exogenous ERα (47). Collectively, our findings suggest that targeting the combined functions of Cyclin D1 and D3 might be a suitable strategy for breast cancer therapy. These D-type cyclins regulate both Cdk4 and Cdk6, and it has been shown recently that a highly specific and well-tolerated Cdk4/6 dual inhibitor (PD-0332991) can effectively block the proliferation of selected ERα-positive and ERα-negative breast cancer cells that have normal Rb function (48). In the light of these encouraging findings, our study suggests extending the use of this Cdk4/6 inhibitor or similar agents to treat ErbB2-positive breast cancer cases.
Supplementary Material
Acknowledgments
Financial support: PHS grants CA117930 (K.-U.W.) and CA118740 (H.R.) from the National Cancer Institute; the S.G. Komen for the Cure Promise Grant KG091116 (H.R.); the Nebraska Cancer and Smoking Disease Research Program NE DHHS LB506 2009-45 (K.-U.W.); and the Susan G Komen Breast Cancer Foundation fellowship PDF0600835 (K.S.).
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- 1.Sherr CJ. D-type cyclins. Trends Biochem Sci. 1995;20:187–90. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- 2.Diehl JA. Cycling to cancer with cyclin D1. Cancer Biol Ther. 2002;1:226–31. doi: 10.4161/cbt.72. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki R, Kuroda H, Komatsu H, et al. Selective usage of D-type cyclins in lymphoid malignancies. Leukemia. 1999;13:1335–42. doi: 10.1038/sj.leu.2401485. [DOI] [PubMed] [Google Scholar]
- 4.Dickson C, Fantl V, Gillett C, et al. Amplification of chromosome band 11q13 and a role for cyclin D1 in human breast cancer. Cancer Lett. 1995;90:43–50. doi: 10.1016/0304-3835(94)03676-a. [DOI] [PubMed] [Google Scholar]
- 5.Sutherland RL, Musgrove EA. Cyclins and breast cancer. J Mammary Gland Biol Neoplasia. 2004;9:95–104. doi: 10.1023/B:JOMG.0000023591.45568.77. [DOI] [PubMed] [Google Scholar]
- 6.Knudsen KE, Diehl JA, Haiman CA, Knudsen ES. Cyclin D1: polymorphism, aberrant splicing and cancer risk. Oncogene. 2006;25:1620–8. doi: 10.1038/sj.onc.1209371. [DOI] [PubMed] [Google Scholar]
- 7.Lee YM, Sicinski P. Targeting cyclins and cyclin-dependent kinases in cancer: lessons from mice, hopes for therapeutic applications in human. Cell Cycle. 2006;5:2110–4. doi: 10.4161/cc.5.18.3218. [DOI] [PubMed] [Google Scholar]
- 8.Wang TC, Cardiff RD, Zukerberg L, et al. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–71. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 9.Lin DI, Lessie MD, Gladden AB, et al. Disruption of cyclin D1 nuclear export and proteolysis accelerates mammary carcinogenesis. Oncogene. 2008;27:1231–42. doi: 10.1038/sj.onc.1210738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–21. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- 11.Landis MW, Pawlyk BS, Li T, Sicinski P, Hinds PW. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell. 2006;9:13–22. doi: 10.1016/j.ccr.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Jeselsohn R, Brown NE, Arendt L, et al. Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell. 2010;17:65–76. doi: 10.1016/j.ccr.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sicinski P, Donaher JL, Parker SB, et al. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–30. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 14.Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–72. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- 15.Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene. 2004;23:6980–5. doi: 10.1038/sj.onc.1207827. [DOI] [PubMed] [Google Scholar]
- 16.Horseman ND, Zhao W, Montecino-Rodriguez E, et al. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997;16:6926–35. doi: 10.1093/emboj/16.23.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Robinson GW, Wagner KU, et al. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–86. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 18.Teglund S, McKay C, Schuetz E, et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–50. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 19.Shillingford JM, Miyoshi K, Robinson GW, et al. Jak2 is an essential tyrosine kinase involved in pregnancy-mediated development of mammary secretory epithelium. Mol Endocrinol. 2002;16:563–70. doi: 10.1210/mend.16.3.0805. [DOI] [PubMed] [Google Scholar]
- 20.Wagner KU, Krempler A, Triplett AA, et al. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol Cell Biol. 2004;24:5510–20. doi: 10.1128/MCB.24.12.5510-5520.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brockman JL, Schroeder MD, Schuler LA. PRL activates the cyclin D1 promoter via the Jak2/Stat pathway. Mol Endocrinol. 2002;16:774–84. doi: 10.1210/mend.16.4.0817. [DOI] [PubMed] [Google Scholar]
- 22.Brockman JL, Schuler LA. Prolactin signals via Stat5 and Oct-1 to the proximal cyclin D1 promoter. Mol Cell Endocrinol. 2005;239:45–53. doi: 10.1016/j.mce.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakamoto K, Creamer BA, Triplett AA, Wagner KU. The Janus kinase 2 is required for expression and nuclear accumulation of cyclin D1 in proliferating mammary epithelial cells. Mol Endocrinol. 2007;21:1877–92. doi: 10.1210/me.2006-0316. [DOI] [PubMed] [Google Scholar]
- 24.Creamer BA, Sakamoto K, Schmidt JW, et al. Stat5 promotes survival of mammary epithelial cells through transcriptional activation of a distinct promoter in Akt1. Mol Cell Biol. 2010;30:2957–70. doi: 10.1128/MCB.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iavnilovitch E, Groner B, Barash I. Overexpression and forced activation of stat5 in mammary gland of transgenic mice promotes cellular proliferation, enhances differentiation, and delays postlactational apoptosis. Mol Cancer Res. 2002;1:32–47. [PubMed] [Google Scholar]
- 26.Iavnilovitch E, Cardiff RD, Groner B, Barash I. Deregulation of Stat5 expression and activation causes mammary tumors in transgenic mice. Int J Cancer. 2004;112:607–19. doi: 10.1002/ijc.20484. [DOI] [PubMed] [Google Scholar]
- 27.Vafaizadeh V, Klemmt P, Brendel C, et al. Mammary epithelial reconstitution with gene-modified stem cells assigns roles to Stat5 in luminal alveolar cell fate decisions, differentiation, involution, and mammary tumor formation. Stem Cells. 2010;28:928–38. doi: 10.1002/stem.407. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto K, Lin WC, Triplett AA, Wagner KU. Targeting janus kinase 2 in Her2/neu-expressing mammary cancer: Implications for cancer prevention and therapy. Cancer Res. 2009;69:6642–50. doi: 10.1158/0008-5472.CAN-09-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakamoto K, Triplett AA, Schuler LA, Wagner KU. Janus kinase 2 is required for the initiation but not maintenance of prolactin-induced mammary cancer. Oncogene. 2010;29:5359–69. doi: 10.1038/onc.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guy CT, Webster MA, Schaller M, et al. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992;89:10578–82. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Creamer BA, Triplett AA, Wagner KU. Longitudinal analysis of mammogenesis using a novel tetracycline-inducible mouse model and in vivo imaging. Genesis. 2009;47:234–45. doi: 10.1002/dvg.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner KU, Young WS, Liu X, et al. Oxytocin and milk removal are required for post- partum mammary-gland development. Genes and Function. 1997;1:233–44. doi: 10.1046/j.1365-4624.1997.00024.x. [DOI] [PubMed] [Google Scholar]
- 33.Triplett AA, Sakamoto K, Matulka LA, et al. Expression of the whey acidic protein (Wap) is necessary for adequate nourishment of the offspring but not functional differentiation of mammary epithelial cells. Genesis. 2005;43:1–11. doi: 10.1002/gene.20149. [DOI] [PubMed] [Google Scholar]
- 34.Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303:29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 35.Stegmeier F, Hu G, Rickles RJ, Hannon GJ, Elledge SJ. A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc Natl Acad Sci U S A. 2005;102:13212–7. doi: 10.1073/pnas.0506306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moffat J, Grueneberg DA, Yang X, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–98. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 37.Tran TH, Lin J, Sjolund AB, Utama FE, Rui H. Protocol for constructing tissue arrays by cutting edge matrix assembly. Methods Mol Biol. 2010;664:45–52. doi: 10.1007/978-1-60761-806-5_5. [DOI] [PubMed] [Google Scholar]
- 38.Rowse GJ, Ritland SR, Gendler SJ. Genetic modulation of neu proto-oncogene-induced mammary tumorigenesis. Cancer Res. 1998;58:2675–9. [PubMed] [Google Scholar]
- 39.Alt JR, Cleveland JL, Hannink M, Diehl JA. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2000;14:3102–14. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radulovich N, Pham NA, Strumpf D, et al. Differential roles of cyclin D1 and D3 in pancreatic ductal adenocarcinoma. Mol Cancer. 2010;9:24. doi: 10.1186/1476-4598-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matulka LA, Wagner KU. Models of breast cancer. Drug Discovery Today: Disease Models. 2005;2:1–6. [Google Scholar]
- 42.Bartkova J, Zemanova M, Bartek J. Abundance and subcellular localisation of cyclin D3 in human tumours. Int J Cancer. 1996;65:323–7. doi: 10.1002/(SICI)1097-0215(19960126)65:3<323::AID-IJC8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 43.Russell A, Thompson MA, Hendley J, et al. Cyclin D1 and D3 associate with the SCF complex and are coordinately elevated in breast cancer. Oncogene. 1999;18:1983–91. doi: 10.1038/sj.onc.1202511. [DOI] [PubMed] [Google Scholar]
- 44.Pirkmaier A, Dow R, Ganiatsas S, et al. Alternative mammary oncogenic pathways are induced by D-type cyclins; MMTV-cyclin D3 transgenic mice develop squamous cell carcinoma. Oncogene. 2003;22:4425–33. doi: 10.1038/sj.onc.1206488. [DOI] [PubMed] [Google Scholar]
- 45.Wong SC, Chan JK, Lee KC, Hsiao WL. Differential expression of p16/p21/p27 and cyclin D1/D3, and their relationships to cell proliferation, apoptosis, and tumour progression in invasive ductal carcinoma of the breast. J Pathol. 2001;194:35–42. doi: 10.1002/path.838. [DOI] [PubMed] [Google Scholar]
- 46.Bowe DB, Kenney NJ, Adereth Y, Maroulakou IG. Suppression of Neu-induced mammary tumor growth in cyclin D1 deficient mice is compensated for by cyclin E. Oncogene. 2002;21:291–8. doi: 10.1038/sj.onc.1205025. [DOI] [PubMed] [Google Scholar]
- 47.Frech MS, Torre KM, Robinson GW, Furth PA. Loss of cyclin D1 in concert with deregulated estrogen receptor alpha expression induces DNA damage response activation and interrupts mammary gland morphogenesis. Oncogene. 2008;27:3186–93. doi: 10.1038/sj.onc.1210974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dean JL, McClendon AK, Stengel KR, Knudsen ES. Modeling the effect of the RB tumor suppressor on disease progression: dependence on oncogene network and cellular context. Oncogene. 2010;29:68–80. doi: 10.1038/onc.2009.313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.