Abstract
Here we have demonstrated that xenobiotic activation of the nuclear receptor CAR (NR1I3) can result in arresting DNA-damaged human hepatocellular carcinoma Huh7 cells at the G2/M phase. Huh7 cells over-expressing CAR were either treated with DMSO, the CAR activator TCPOBOP, or repressor androstenol; these treatments were then followed by adriamycin treatment to damage DNA. FACS analysis revealed that CAR-activation by TCPOBOP increased the rate of arrested Huh7 cells at the G2/M phase (4N DNA content) after DNA damage by adriamycin. This increase correlated with the increase of cell viability in TCPOBOP-treated Huh7 cells, as determined by MTT assays. Real-time PCR analysis determined that, as regulated by CAR, the growth arrest and DNA damage-inducible γ (GADD45γ) and Cyclin G2 genes increased and decreased, respectively, as TCPOBOP increased the number of Huh7 cells arrested at the G2/M phase. Thus, the results suggest that CAR regulates cell cycle, increasing G2/M arrest and delaying the death of DNA-damaged cells.
Keywords: CAR, nuclear receptor, G2/M arrest, Huh7, adriamycin
INTRODUCTION
Nuclear receptor CAR was first characterized as the PB-activated transcriptional factor which regulates cytochrome P4502B10 (CYP2B10) [1, 2]. Soon after, CAR was established as the nuclear receptor, which plays the central role in regulating hepatic genes that encode drug metabolizing enzymes and transporters, including various CYPs and multidrug resistance-associated proteins (MRPs) [3, 4]. Additionally, recent work has extended the functions of CAR to include the regulation of hepatic energy metabolism [5]. While CAR regulates these normal liver functions, it has also been found to act as a key factor in the development of various drug-induced liver injuries and diseases such steatohepatitis, cholestasis and liver tumors [6]. Our previous study demonstrated that CAR is an essential factor in promoting hepatocellular carcinoma (HCC) as demonstrated by the fact that Car−/− mice did not develop HCC after chronic PB treatment for over 34 weeks, while Car+/+ mice developed HCC [7]. In efforts to decipher the molecular mechanism of this tumor promotion, we have demonstrated that CAR can regulate cell death; CAR directly binds to GADD45β to inhibit MKK7-mediated phosphorylation of JNK1 and TNFα-mediated cell death in mouse primary hepatocytes [8]. In an extension of our investigations, here we examine whether or not CAR regulates the cell cycle, utilizing adriamycin treatment of Huh7 cells to damage DNA. Activation of ectopically expressed CAR by TCPOBOP was found to increase G2/M arrest of Huh7 cells, with the concomitant increase and decrease of the expression of GADD45γ and Cyclin G2 genes, respectively. We present experimental considerations to support the hypothesis that CAR can arrest DNA-damaged cells at the G2/M phase.
MATERIALS AND METHODS
Cell culture condition and treatments
Human hepatocellular carcinoma Huh7 cells were cultured in 10% MEM (minimal essential medium supplement with 10% (v/v) fetal bovine serum, antibiotics (100 units/ml penicillin and 100 µg/ml streptomycin) and 2 mM glutamine) in a 5% CO2 atmosphere at 37°C. For flow cytometry analysis or western blot analysis, Huh7 cells were plated onto 60-mm dish and infected with adenovirus-mCAR or adenovirus-β-Gal at 10 MOI (Multiplicity Of Infection). After 24 h infection, the medium was changed to new medium containing DMSO, 250 nM TCPOBOP purchased from Sigma-Aldrich (MO, USA) or 8 µM androstenol purchased from Steraloids (RI, USA) and the cells were incubated for 24h. Then, cells were treated with 0.5 µg/ml of adriamycin purchased from Sigma-Aldrich (MO, USA) for the indicated number of hours (0~24 h).
Recombinant adenoviruses
Adenovirus-mCAR encoding mouse CAR was previously constructed in our laboratory using the AdEasy Vector System kit (Q-BIO gene, Montreal, Canada) as described in the manufacturer’s protocol. Adenovirus-β-Gal encoding β-galactosidase was also constructed previously and used as control. Both adenovirus were propagated in HEK293 cells, and virus titers were measured and calculated using the 50% tissue culture infectious dose assay (TCID50 method) as described in the manufacturer’s application manual.
Flow cytometry
Treated Huh7 cells were trypsinized and harvested in PBS followed by two washes with PBS. Then the cells were resuspended in 70% ethanol overnight at 4°C. The fixed cells were collected by centrifugation, resuspended in RNaseA solution (0.2 mg/ml RNaseA in PBS) and incubated at 37°C for 30 min. The cells were pelleted, incubated in Propidium Iodide (PI) staining solution (20 µg/ml PI in PBS) overnight at 4°C Propidium Iodide was purchased from Sigma-Aldrich. The cells were analyzed by flow cytometer (BD FACSCalibur), and Cell Quest software (BD Biosciences) was used for data acquisition and analysis.
MTT assay
Cell viability and proliferation were determined by the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Huh7 cells were seeded on 96 well plates at densities of 2.0 ×103 cells/well in 10% MEM medium, and then infected with adenovirus, each drug treatment and adriamycin treatment (0.5 µg/ml) described as above. At selected time intervals, 10 µl of MTT working solution (5 mg/ml in ddH2O) was added and incubated at 37°C for 4 h. At the end of the incubation, the media was discarded and 100 µl of DMSO was added to each well to dissolve the dye. After incubation at room temperature for 1 h with gentle shaking, the absorbance of the converted dye was measured using a microplate spectrophotometer (SpectraMax Plus 384; Molecular Device Inc., CA, USA) at 495 nm.
Real-Time PCR
Total RNAs were extracted from treated cells using TRIzol reagent (Invitrogen, CA, USA), and used to synthesize cDNAs using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems Inc. CA, USA). Real-Time PCR was performed with the 7900HT Fast Real-Time PCR System (Applied Biosystems Inc.). Each primer set information follows: Forward and reverse primes for human GADD45α cDNA were: 5’-AGTCAGCGCACGATCACTGT-3’ and 5’-GCAGGCACAACACCACGTTA-3’, for human GADD45β cDNA were: 5’-CGGTGGAGGAGCTTTTGGT-3’ and GCTGTCTGGGTCCACATTCA-3’, for human GADD45γ cDNA were: 5’-GAACGTGGACCCCGACAAT-3’ and 5’-ATGTCGTTCTCGCAGCAGAA-3’. Human Cyclin G2 primer mixture was purchased from Real Time Primers, LLC (PA, USA). PCR amplification was performed with Power SYBR Green Master Mix (Applied Biosystems Inc.). Predesigned TaqMan gene expression assay probe of Human CYP2B6 (Hs00167937_g1), and human B-actin controls were purchased from Applied Biosystems Inc. and PCR amplification was performed with TaqMan Universal PCR Master Mix (Applied Biosystems Inc.). The expression level of each mRNA was normalized to that of human B-actin mRNA.
Statistical analysis
The statistical significance between two groups of data sets was determined by using the Student’s t test (two-tailed) with P<0.05 regarded as statistically significant.
RESULTS
G2/M arrest and cell viability
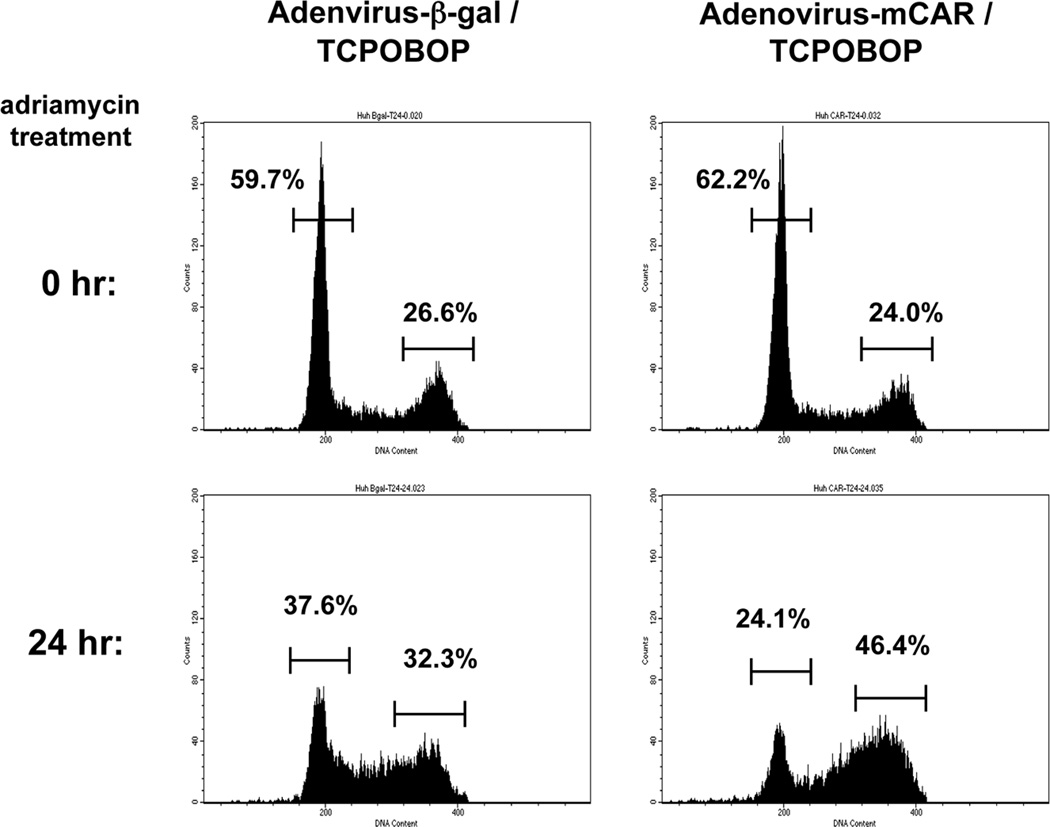
Mouse CAR (mCAR) was over-expressed using an adenovirus-based infection in Huh7 cells. After activating mCAR with TCPOBOP, these cells were treated with adriamycin to damage DNA and were subjected to FACS analysis. TCPOBOP treatment was found to increase the number of DNA-damaged Huh 7 cells at the G2/M phase, relative to what was observed with DNA-damaged Huh7 cells infected with adenovirus-β-Gal, instead of adenovirus-mCAR (Figure 1). Given this finding, we extended the FACS analysis to include additional controls; non-infected Huh7 cells, treatments with DMSO and a mCAR repressor androstenol. The results from these FACS analyses are summarized in Table 1. Adriamycin treatment for 24 hr increased the number of adenovirus-mCAR infected and TCPOBOP treated Huh7 cells at the G2/M phase by approximately 22% over corresponding levels prior to adriamycin treatment. In comparison, DMSO and androstenol treatments increased this number by only 14% and 8%. And a roughly 7% increase at the G2/M phase was also observed in no-infected or adenovirus β-Gal-infected Huh7 cells. The observed enhancement of G2/M arrest by activated CAR appeared to occur specifically when DNA was damaged by adriamycin, since damaging DNA by H2O2 treatment and UV irradiation did not show any changes of cell cycle status between any treatment in CAR dependent manner (data not shown).
Figure 1.
CAR enhances cell cycle arrest at the G2/M phase after adriamycin treatment. Huh7 cells were seeded, infected with adenovirus-β-Gal or adenovirus-mCAR at 10 MOI and following treated with TCPOBOP (CAR activator). Cells were harvested before and after treatment of adriamycin for 24 h, and the DNA content was analyzed by flow cytometry to determine the cell cycle distribution. The bars and numbers in figure show the percentage of each fraction.
Table 1.
Cell cycle status before and after adriamycin treatment
| adriamycin | G0/G1 | S | G2/M | ||
|---|---|---|---|---|---|
| 0 h | DMSO | 56.5±1.7% | 16.8±0.5% | 26.7±1.2% | |
| No-infection | TCPOBOP | 57.6±3.3% | 16.2±1.2% | 26.2±2.1% | |
| androstenol | 60.2±1.6% | 16.0±0.2% | 23.8±1.7% | ||
| DMSO | 59.3±3.9% | 14.0±0.8% | 26.6±3.5% | ||
| adenovirus-β-Gal | TCPOBOP | 59.0±3.2% | 13.9±0.6% | 27.1±2.6% | |
| androstenol | 60.1±2.9% | 13.1±0.2% | 26.8±2.8% | ||
| DMSO | 59.7±3.3% | 14.0±0.8% | 26.3±2.6% | ||
| adenovirus-mCAR | TCPOBOP | 61.1±3.7% | 13.4±0.4% | 25.5±3.7% | |
| androstenol | 60.6±2.9% | 13.1±0.5% | 26.3±2.6% | ||
| 24 h | DMSO | 40.3±4.7% | 27.7±2.9% | 32.0±2.5% | |
| No-infection | TCPOBOP | 39.4±1.9% | 27.5±1.3% | 33.1±2.2% | |
| androstenol | 38.2±3.2% | 28.3±4.2% | 33.4±1.3% | ||
| DMSO | 39.6±3.6% | 26.2±3.2% | 34.2±2.4% | ||
| adenovirus-β-Gal | TCPOBOP | 38.4±4.3% | 26.9±3.4% | 34.7±3.1% | |
| androstenol | 37.5±1.7% | 27.1±3.8% | 35.4±2.9% | ||
| DMSO | 30.7±5.7% | 28.5±3.7% | 40.8±3.0% | ||
| adenovirus-mCAR | TCPOBOP | 25.8±2.1% | 27.1±2.3% | 47.1±0.8% | |
| androstenol | 37.5±3.8% | 28.0±3.6% | 34.4±3.0% | ||
Huh7 cells were infected adenovirus-β-Gal or adenovirus-mCAR, following treated with DMSO, TCPOBOP or androstenol for 24hr. DNA contents were analyzed by flow cytometry assay before or after of 24hr adriamycin treatment. These experiments were repeated three times and the averaged percentage±S.D. are shown.
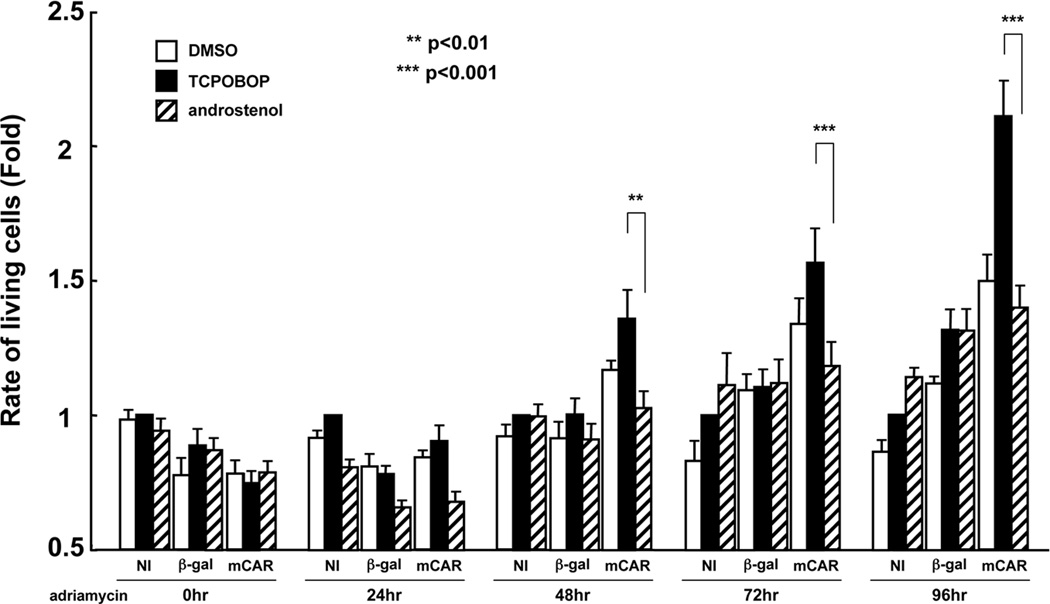
Since we found that CAR activation enhanced cell cycle arrest at the G2/M phase, next we examined the effects on cell viability in DNA-damaged Huh7 cells. Only the adenovirus mCAR-infected Huh7 cells exhibited a statistically significant difference in specific increase of cell viability by TCPOBOP after from 48 to 96 hr of adriamycin treatment (Figure 2). These results indicate that CAR can increase viability of DNA-damaged Huh7 cells as well as arrest them at the G2/M phase.
Figure 2.
Cell proliferation analysis in CAR activated Huh7 cells. Huh7 cells were seeded, infected with adenovirus-β-Gal or adenovirus-mCAR at 10 MOI. NI means No-Infection. After following treatment with DMSO, TCPOBOP (CAR activator) or androstenol (CAR repressor), MTT assay was carried out 0, 24, 48, 72 and 96 h after adriamycin treatment (0.5 µg/ml). Relative rate of living cells was expressed in each time group by taking those in NI sample with TCPOBOP treatment as equal to one. The results are reported as mean ± SD of six independent experiments. **, p<0.01 and ***, p*<0.001 for CAR activated group by TCPOBOP versus CAR inactivated group by androstenol.
CAR regulates the GADD45γ and cyclin G2 genes
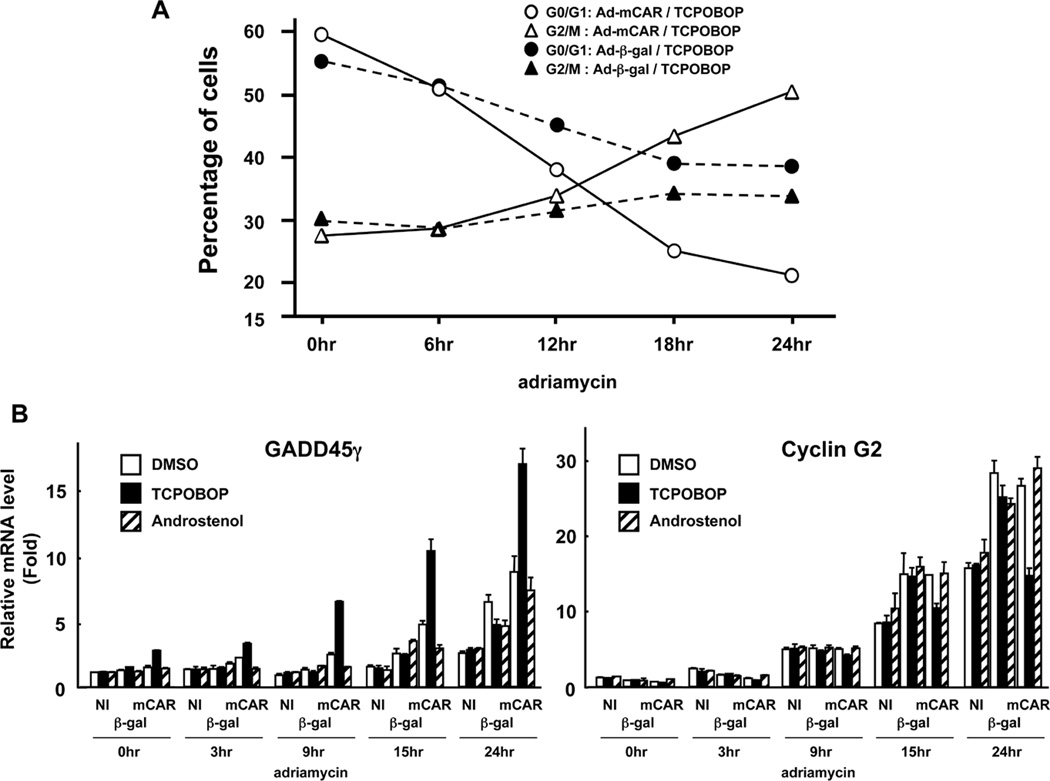
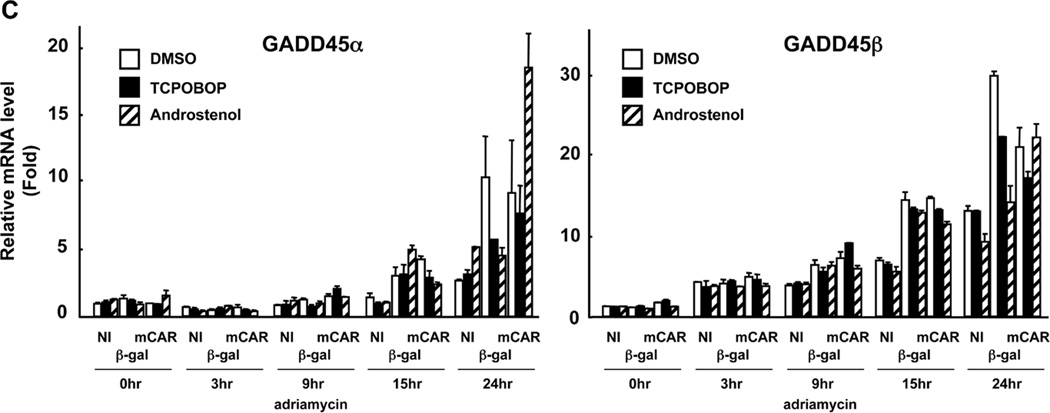
First, we determined the TCPOBOP-dependent changes of cell cycles in time-dependent manner after adriamycin treatment (Figure 3A). The number of Huh 7 cells at the G2/M phase began to increase 12 hrs after treatment and continued to do so until levels had increased by approximately 50% after 24 hrs. Conversely, the corresponding number of cells at the G0/G1 phase gradually decreased after adriamycin treatment and continued after being overtaken by the reciprocal increase of cells at the G2/M phase after 12 hrs. With adenovirus β-Gal-infected Huh7 cells, the degrees of these changes in cell cycle were less significant and the levels of cells at the G2/M phase were lower than those at the G0/G1 phase. Real-time PCR was employed to screen various genes which are known to be involved in cell cycle regulation during the increase of DNA-damaged cells at the G2/M phase. As a result, two genes were identified as CAR-regulated genes, the expressions of which correlated with increasing arrest at the G2/M phase: GADD45γ and cyclin G2. GADD45γ mRNA was increased by TCPOBOP in adenovirus mCAR-infected Huh7 cells without adriamycin treatment. This increase was greatly enhanced as the DNA of Huh7 cells damaged (Figure 3B). Contrary to TCPOBOP, androstenol treatment did not increase the GADD45γ mRNA levels prior to or after DNA damage. Moreover, TCPOBOP-dependent increase of GADD45γ mRNA was not observed in adenovirus β-Gal-infected Huh7 cells with or without DNA damage. However the other GADD45 members GADD45α and GADD45β were not regulated by CAR (Figure 3C). Cyclin G2 mRNA, on the other hand, was decreased in a CAR-dependent manner in only TCPOBOP treated Huh7 cells after adriamycin treatment (Figure 3B). Thus, the increase of CAR-mediated G2/M arrest correlated with the increase and decrease of GADD45γ and cyclin G2 mRNAs, respectively
Figure 3.
Transition of the cell cycle status and the candidate GADD45γ and Cyclin G2 mRNA expression after adriamycin treatment. (A) Huh7 cells were infected with adenovirus-β-Gal or adenovirus-mCAR at 10 MOI and following treated with TCPOBOP. Then, treated cells were collected after indicated time course of adriamycin treatment and the DNA content was analyzed by flow cytometry to determine the cell cycle distribution. The percentage of cells at the G0/G1 and at the G2/M phase in each group was summarized. This experiment was reproducibly repeated three times. (B) Treated Huh7 cells were cultured on duplicated dishes and were harvested after indicated time course of adriamycin treatment. Total RNAs were prepared from cells and were subjected to real-time PCR, and normalized to that of human B-actin mRNA. The relative levels of GADD45β and Cyclin G2 mRNA were shown relative to those of corresponding mRNAs in NI / DMSO / adriamycin 0 hr treatment sample set at one. The data are reported as mean ± SD, n=2. (C) Treated Huh7 cells were cultured on duplicated dishes and were harvested after indicated time course of adriamycin treatment. Total RNAs were prepared from cells and were subjected to real-time PCR, and normalized to that of human B-actin mRNA. The relative levels of GADD45α and GADD45β mRNA were shown relative to those of corresponding mRNAs in NI / DMSO / adriamycin 0 hr treatment sample set at one. The data are reported as mean ± SD, n=2.
DISCUSSION
Xenobiotic activation of CAR has now been demonstrated to arrest DNA-damaged Huh7 cells at the G2/M phase, with cell survival being increased. The two CAR-regulated genes GADD45γ and cyclin G2 are found to correlate with this G2/M arrest. Thus, CAR may be capable of regulating cell cycle when cells incur injuries such as DNA damage. We also performed the same type of experiments with H2O2 treatment and UV irradiation instead of adriamycin treatment, but we could not detect any CAR-dependent changes in cell cycles (data not shown). Thus, the type of DNA damages may be critical in the CAR-dependent arrest of Huh7 cell cycle at the G2/M phase.
GADD45 proteins are involved in the checkpoint of G2/M phase cell cycle [9–11]. GADD45γ, a member of the GADD45 protein family, is recently suggested to induce HepG2 cells to arrest at the G2/M phase via p38 and JNK signaling pathways [12]. Given the fact that CAR activation arrested DNA-damaged Huh7 cells at the G2/M phase, the activation of the GADD45γ gene is consistent with these known roles of GADD45γ. Various signal pathways have been reported to be involved in the arrest of G2/M phase, including the p38-JNK pathway and the cyclin B1/cdc2-mediated strength of G2/M checkpoint. However, clear CAR dependent changes of expression and phosphorylation levels of these signal molecules after DNA damage treatment were not found during the CAR-induced G2/M arrest (Supplemental Figure 1). Thus, CAR may utilize a yet unknown mechanism to arrest DNA damaged Huh7 cells at the G2/M phase. Cyclin G2 is a homologue of cyclin G1 [13]. Although a regulatory role of cyclin G2 in cell cycles has not yet been confirmed, its role in cell growth arrest has been demonstrated. Cyclin G2 mRNA was induced in B cells treated with growth inhibitors, such as transforming growth factor β1 (TGFβ1) or Dexamethasone [14]. Ectopic over-expression of cyclin G2 repressed the proliferation of oral cancer cells [15]. The down-regulation of cyclin G2 may have been involved in the increased cell viability which occurred during the CAR-induced G2/M arrest.
Ongoing studies have been undertaken in an effort to understand the molecular mechanisms by which xenobiotic activation of CAR promotes HCC development. CAR-mediated DNA methylation was reported to occur during promotion [16]. CAR was found to interact with GADD45β and repress MKK7-mediated phosphorylation of JNK1, down-regulating cell death in TNFα-treated mouse primary hepatocytes [8]. The CAR-mediated G2/M arrest has now been demonstrated to occur in Huh7 cells after treatment with adriamycin. Neither methylation nor apoptosis, nor G2/M arrest is directly connected to phenobarbital promotion of HCC development at the present time. While G2/M arrest is considered to be a defense mechanism against damaged cells, the unexpectedly prolonged arrest may cause negative effects to this defense mechanism. Although methylation, cell death and arrest have not been directly connected with CAR-induced promotion of HCC development, they provide the experimental basis for future investigations.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the National Institutes of Health, and National Institute of Environmental Health Sciences, Z01ES1005-01. We would like to acknowledge the efforts of all of the staff of the NIEHS Flow Cytometry Center. We especially thank to all our lab members for valuable discussions.
Abbreviation
- CAR
constitutive active/androstane receptor
- GADD45γ
growth arrest and DNA-damage-inducible 45 γ
- PB
phenobarbital
- DMSO
dimethyl sulfoxide
- TCPOBOP
1, 4-bis[2-(3,5-dichloropyridyloxy)]benzene
- androstenol
16,(5α)-Androsten-3α-OL
- PCR
polymerase chain reaction
REFERENCES
- 1.Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998;18:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6. J Biol Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- 3.Kodama S, Negishi M. Phenobarbital confers its diverse effects by activating the orphan nuclear receptor CAR. Drug Metab Rev. 2006;38:75–87. doi: 10.1080/03602530600569851. [DOI] [PubMed] [Google Scholar]
- 4.Urquhart BL, Tirona RG, Kim RB. Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: implications for inter- individual variability in response to drugs. J Clin Pharmacol. 2007;47:566–578. doi: 10.1177/0091270007299930. [DOI] [PubMed] [Google Scholar]
- 5.Kodama S, Koike C, Negishi M, Yamamoto Y. Nuclear receptors CAR and PXR cross talk with FoxO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol. 2004;24:7931–7940. doi: 10.1128/MCB.24.18.7931-7940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kakizaki S, Yamazaki Y, Takizawa D, Negishi M. New insights on the xenobiotic-sensing nuclear receptors in liver diseases –CAR and RXR-- Curr Drug Metab. 2008;9:614–621. doi: 10.2174/138920008785821666. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto Y, Moore R, Goldsworthy TL, Negishi M, Maronpot RR. The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res. 2004;64:7197–7200. doi: 10.1158/0008-5472.CAN-04-1459. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto Y, Moore R, Flavell RA, Lu B, Negishi M. Nuclear receptor CAR repressed TNFα-induced cell death by interacting with the anti-apoptotic GADD45B. PLoS ONE. 2010;5:e10121. doi: 10.1371/journal.pone.0010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin S, Antinore MJ, Lung FD, et al. The Gadd 45 inhibition of Cdc2 kinase correlates with Gadd 45-mediated growth suppression. Biol Chem. 2000;275:16602–16608. doi: 10.1074/jbc.M000284200. [DOI] [PubMed] [Google Scholar]
- 10.Zhan Q, Antinore MJ, Wang XW, et al. Association with Cdc2 and inhibition of Cdc2/Cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene. 1999;18:2892–2900. doi: 10.1038/sj.onc.1202667. [DOI] [PubMed] [Google Scholar]
- 11.Vairapandi M, Balliet AG, Hoffman B, Liebermann DA. GADD45β and GADD45γ are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J Cell Physiol. 2002;192:327–338. doi: 10.1002/jcp.10140. [DOI] [PubMed] [Google Scholar]
- 12.Zhu N, Shao Y, Xu L, Yu L, Sun L. Gadd45-α and Gadd45-γ utilize p38 and JNK signaling pathways to induce cell cycle G2/M arrest in Hep-G2 hepatoma cells. Mol Biol Rep. 2009;36:2075–2085. doi: 10.1007/s11033-008-9419-9. [DOI] [PubMed] [Google Scholar]
- 13.Horne MC, Goolsby GL, Donaldson KL, Tran D, Neubaur M, Wahl AF. Cyclin G1 and cyclin G2 comprise a new family of cyclins with contrasting tissue-specific and cell cycle-regulated expression. J Biol Chem. 1996;271:6050–6061. doi: 10.1074/jbc.271.11.6050. [DOI] [PubMed] [Google Scholar]
- 14.Horne MC, Donaldson KL, Goolsby GL, et al. Cyclin G2 is up-regulated during growth inhibition and B cell antigen receptor-mediated cell cycle arrest. J Biol Chem. 1997;272:12650–12661. doi: 10.1074/jbc.272.19.12650. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y, Shintani S, Kohno Y, Zhang R, Wong DT. Cyclin G2 dysregulation in human oral cancer. Cancer Res. 2004;64:8980–8986. doi: 10.1158/0008-5472.CAN-04-1926. [DOI] [PubMed] [Google Scholar]
- 16.Phillips JM, Yamamoto Y, Negishi M, Maronpot RR, Goodman JI. Orphan nuclear receptor constitutive active/androstane receptor-mediated alterations in DNA methylation during phenobarbital promotion of liver tumorigenesis. Toxicol Sci. 2007;96:72–82. doi: 10.1093/toxsci/kfl188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.