Abstract
Despite extensive genetic analysis of the dynamic multi-phase process that transforms a small population of lateral plate mesoderm into the mature limb skeleton, the mechanisms by which signaling pathways regulate cellular behaviors to generate morphogenetic forces are not known. Recently, a series of papers have offered the intriguing possibility that regulated cell polarity fine-tunes the morphogenetic process via orienting cell axes, division planes and cell movements. Wnt5a-mediated non-canonical signaling, which may include planar cell polarity, has emerged as a common thread in the otherwise distinct signaling networks that regulate morphogenesis in each phase of limb development. These findings position the limb as a key model to elucidate how global tissue patterning pathways direct local differences in cell behavior that, in turn, generate growth and form.
Key words: growth, planar cell polarity, Wnt signaling, limb bud, growth plate, morphogenesis, oriented cell division, directional cell movement, tissue polarity
Whether one is interested in studying the mechanisms by which evolutionary processes modify form and function or in applying tissue engineering methods to regenerative medicine, defining the cellular mechanisms that generate tissue architecture and determining the relationship between tissue structure, morphogenesis and function are important goals. The vertebrate skeleton is an emerging model for these studies because of the advantageous fact that many skeletal elements are generated from a single tissue type, the hyaline cartilage, which is molded into highly diverse shapes and sizes, both within an organism (e.g., femur vs. carpal bone of the wrist) and between organisms (e.g., forelimb digits of a human, a bat and a whale).1 Likewise, the growth rates and mechanical properties of each type of element are distinct, even though the development of each element is regulated by a common signaling network. A particularly useful system for studying the mechanisms that direct growth and morphogenesis is the appendicular (limb) skeleton.
The limb skeleton is a product of multiple distinct phases of growth and morphogenetic events that begin with the outgrowth of a small mesodermal population from the flank of the embryo and end with mineralization of the growth plate cartilage at the ends of the long bones. A unique challenge for the developing limb is the extent to which directional growth must occur beyond the torso. Spatial constraints and interactions with surrounding tissues can place limits on the growth of bones in the head and torso. In contrast, each limb develops independently from both the contra-lateral limb and the rest of the body, yet during outgrowth the corresponding limbs are nearly identical in size. For example, lengths of paired skeletal elements are equivalent (± 3%) in 95–97% of chick forelimbs.1 The precision of this process is the result of a tightly regulated signaling network that controls growth via regulation of cell proliferation, cell death and cell size. How these generally isotropic processes give rise to directional growth has been hotly debated, but recently several important breakthroughs have demonstrated a crucial role for regulated cell polarity in defining the vector and scalar components of limb skeleton growth.
Limb Skeleton Morphogenesis
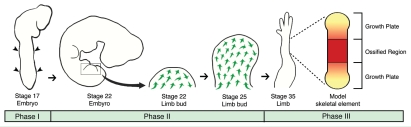
The limb skeleton and associated connective tissue develop from the somatopleure of the lateral plate mesoderm2,3 by sequential application of three distinct growth and patterning phases (Fig. 1). In the first phase, mesenchymal condensations (thickened regions in the flank mesoderm) form at Hamburger-Hamilton (HH) stage 16 in the chick and at embryonic day 8/9 in the mouse.4 Limb bud outgrowth begins at the cervical-thoracic and lumbosacral boundaries between HH stages 16 and 17 in the chick and at embryonic day 9 in mice.4,5 Outgrowth is initially isotropic, but the distal tissue gradually thins in the dorsoventral axis, while the anteroposterior axis expands to form the distinctive paddle shape of the limb bud.4
Figure 1.
Three phases of limb skeleton morphogenesis in the chick. In Phase I, mesenchymal condensations (arrowheads) form in the lateral plate mesoderm of the chick embryo. Limb bud initiation and outgrowth occur in Phase II and are regulated primarily by the apical ectodermal ridge, the zone of polarizing activity and the dorsal ectoderm. As the limb bud continues to elongate, the initially isotropic bud takes on a paddle shape with a bias in tissue mass toward the posterior side. The green arrows indicate local vectors in tissue movement. In Phase III, selective apoptosis delineates the digits and cartilage condensation/differentiation occurs. Subsequently, the cartilage growth plates are established, and the process of chondrocyte maturation begins. This creates a skeletal element with a central, ossified domain surrounded by two growth plates containing chondrocytes that are progressively more mature the closer they are to the ossified domain.
In the second phase of morphogenesis, domains of limb mesenchyme condense and differentiate to form the cartilage anlagen of the future limb skeleton, while adjacent cells generate the perichondrium, a pseudoepithelial layer that regulates cartilage growth and contains precursors of the bone-forming osteoblasts.6 Formation of limb cartilage occurs progressively, beginning with the proximal elements (e.g., humerus) and ending with the most distal elements (e.g., phalangeal elements of the fingers).7 Temporal order is also observed in the anterior-posterior axis as the cartilage templates of the hands and feet develop, resulting in sequential development of the digits.8 At these early stages of skeleton formation, the morphology of the cartilage grossly resembles that of the final bony element.
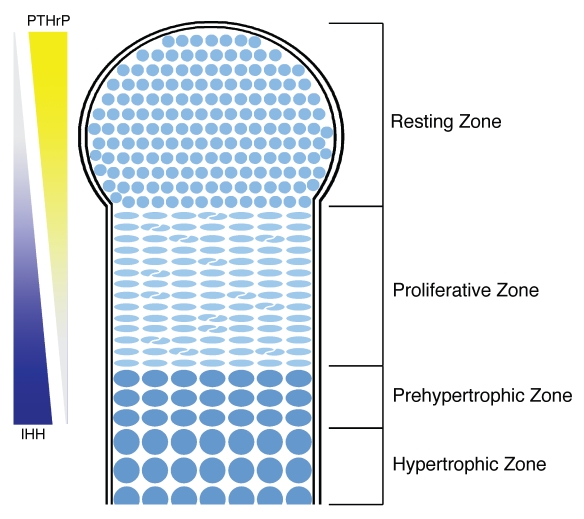
In the third phase of morphogenesis, cells of the anlagen differentiate into chondrocytes (cells that secrete the cartilage matrix) and undergo a maturation process that establishes the growth plate.9 The cartilage growth plate is, from this point onward, the main driving force for bone elongation.10 In the growth plate, chondrocyte maturation occurs non-uniformly and with a distinct polarity within the cartilage element, such that cells closer to the center of the element are more mature than chondrocytes nearer the joint surface (Fig. 2). Under either articular surface reside progenitor cells (resting chondrocytes) that are elliptical and do not show obvious organization within the cartilage matrix. During cartilage maturation, progenitor cells are progressively recruited to a proliferative state that is characterized by the formation of columns of discoid chondrocytes that resemble stacks of coins.11 Subsequently, terminal differentiation of proliferative chondrocytes occurs in two steps: prehypertrophy, in which cells exit the cell cycle and begin to increase in size, and hypertrophy, when chondrocytes increase greatly in size and prepare the element for ossification by secreting a distinct subset of extracellular matrix proteins before undergoing apoptosis.9,12
Figure 2.
A schematic of growth plate architecture and key signaling pathways. The cartilage growth plate found at either end of each limb skeletal element is made up of four zones. Resting chondrocytes are elliptical and lack obvious organization. As resting zone cells are progressively recruited to the proliferative zone, they form clonal columns of flattened cells. Terminal differentiation is gradually achieved by entrance into prehypertrophy (in which cells exit the cell cycle and increase in size) followed by hypertrophy (a further increase in size prior to apoptosis). The size of these zones and the rate of chondrocyte maturation through each state are maintained by opposing signaling molecule gradients of parathyroid hormone-related protein (produced by a subpopulation of resting zone cells adjacent to the articular surface) and Indian Hedgehog (produced by the prehypertrophic cells).
Signaling Pathways Generate Tissue Polarity in the Developing Limb
Directional outgrowth and local differences in skeletal morphogenesis during limb development reflect developmental and structural polarities within limb tissues that are generated by regional differences in cell signaling. During limb bud formation and elongation, tissue polarity is established along three axes (anterior-posterior, proximal-distal and dorsal-ventral) by the interactions of several signaling pathways. The proximodistal axis is initially established when Hox genes initiate expression of fibroblast growth factor (FGF) and Wingless/int-1 (Wnt) genes (that encode secreted signaling molecules) in the limb field mesenchyme, which, in turn, activates FGF expression in the overlying ectoderm.13–15 Interactions between the mesoderm and ectoderm of the limb bud induce formation of the apical ectodermal ridge (AER), an epithelial structure that promotes limb bud outgrowth and sequentially expands the cell populations that form distinct segments of the limb skeleton.7 Distal FGF signaling is opposed by proximal retinoic acid, a small molecule produced by the flank mesenchyme.16 Retinoic acid diffuses into the limb bud and is degraded by distal mesenchyme cells, thus creating a proximal-to-distal morphogen gradient.16–18 The importance of these morphogen gradients to limb patterning was recently underscored by in vitro studies demonstrating that proximodistal identity is expressed when limb mesenchyme cells experience retinoic acid signaling after leaving the distal FGF signaling domain.19,20 However, although these signals define the proximodistal axis, alone they do not promote limb bud outgrowth.
Limb outgrowth is driven by cell proliferation that requires FGF and Wnt5a signaling in the distal mesenchyme.21–23 This distal domain depends on a feedback loop between a posterior signaling domain in the mesenchyme, the zone of polarizing activity (ZPA) and the AER. The morphogen Sonic Hedgehog (SHH) produced by the ZPA maintains FGF4 expression in the AER, which signals back to the ZPA to maintain SHH expression.24,25 Together, these signals maintain function of the AER and the undifferentiated, proliferative population of mesenchyme at the distal tip of the limb bud that are required for limb bud outgrowth. SHH secretion by the ZPA also forms a concentration gradient across the limb bud, which patterns the mesenchyme along the anteroposterior axis.8,26–28 Dorsoventral polarity of the limb bud requires expression of WNT7a in the dorsal ectoderm and bone morphogenetic protein (BMP) signaling and is closely tied to AER formation.29,30
Following induction of skeleton formation, a new signaling network is employed to establish and maintain tissue polarity in the cartilage template. Two key signaling molecules at the foundation of this network are parathyroid hormone-related protein (PTHrP) and Indian Hedgehog (IHH).31–33 Opposing concentration gradients of PTHrP (secreted by resting chondrocytes) and IHH (secreted from prehypertrophic chondrocytes) interact through a signaling feedback loop to regulate chondrocyte maturation. IHH signaling promotes two antagonistic processes in chondrocytes, proliferation and hypertrophy (the terminal phase of chondrocyte differentiation). 34–36 IHH signaling also promotes PTHrP expression, which, in turn, maintains the pool of resting chondrocytes and limits hypertrophy.31,34,37 The output of this central PTHrP/IHH feedback loop is modified by the actions of other graded and regional secreted signaling molecules, such as FGFs, Wnts and transforming growth factorá/bone morphogenetic protein superfamily factors (TGFá/BMPs). Together, these signaling pathways regulate the spatial and temporal properties of chondrocyte maturation and thereby generate the characteristic domain structure of the growth plate cartilage.
Cell Polarity Mirrors Tissue Polarity in the Developing Limb
Numerous genetic and surgical manipulations that perturb the networks described above have demonstrated the importance of short- and long-range signaling in establishing directional growth as well as tissue polarity and tissue pattern. One important question is how the unique signaling domains generated by graded distribution of these secreted factors establish both the scalar and the vector components of tissue growth. Early models proposed that the limb bud was a mass of unordered mesenchyme that was shaped via local differences in cell proliferation and cell-cell adhesion.38 Indeed, this model might accurately represent the earliest stages of limb specification after Hox genes designate an area of the lateral plate mesoderm as a prospective limb field.14,39 At this stage, mesenchyme cells are characterized by increased cell proliferation and greater tissue cohesiveness compared with cells of the adjacent flank mesoderm.40,41 Damon, et al. propose that the increase in limb bud cohesiveness and changes in the mechanical response of the tissue to compression, both of which occur in response to FGF8, together could explain the separation of the two tissues and the initial budding of the limb.42 However, as the limb bud elongates, mathematical models suggest that regional differences in isotropic growth properties cannot easily account for the observed morphogenetic process.43
Although mesenchyme cells were not thought to be ordered, recent evidence from quantitative histological analysis and live imaging experiments suggests that specific oriented cell behaviors might underlie anisotropic growth during skeletal development. During limb bud formation, mesenchyme cells exhibit coordinated bulk movement from the lateral plate mesoderm toward the distal-posterior region.44 Following formation of the AER, mesenchyme cells at the margins of the bud move toward the ectoderm.45 At both stages, oriented cell movements and the associated growth vectors roughly align with the long axis of the cell and with the orientation of the mitotic spindle. Together, these observations suggest that regulation of cell shape, oriented cell division and directional cell movement might promote growth and morphogenesis in the limb bud.44,45
Likewise, oriented cell behaviors are evident in the growth plate cartilage (Fig. 3). In the proliferative zone, cells are organized into clonal columns of flattened, discoid cells.11 From histological sections, Dodds surmised that these columns form in a two-step process from cells in the resting zone.11 In the first step, elliptical resting chondrocytes without oriented cell behaviors progressively flatten and display planar alignment of the mitotic spindle and the cell axes.46 In the second phase, daughter cells that were displaced laterally by cell division reorient 90° to form a column parallel to the long axis of the skeletal element.11,46 Interestingly, the organization of proliferative chondrocytes is also evident at the subcellular level in the planar alignment of the primary cilium of each chondrocyte at the center of the column.47 Thus, as in the limb bud, cartilage growth is associated with planar alignment of cell axes, oriented cell division and oriented cell movements.
Figure 3.
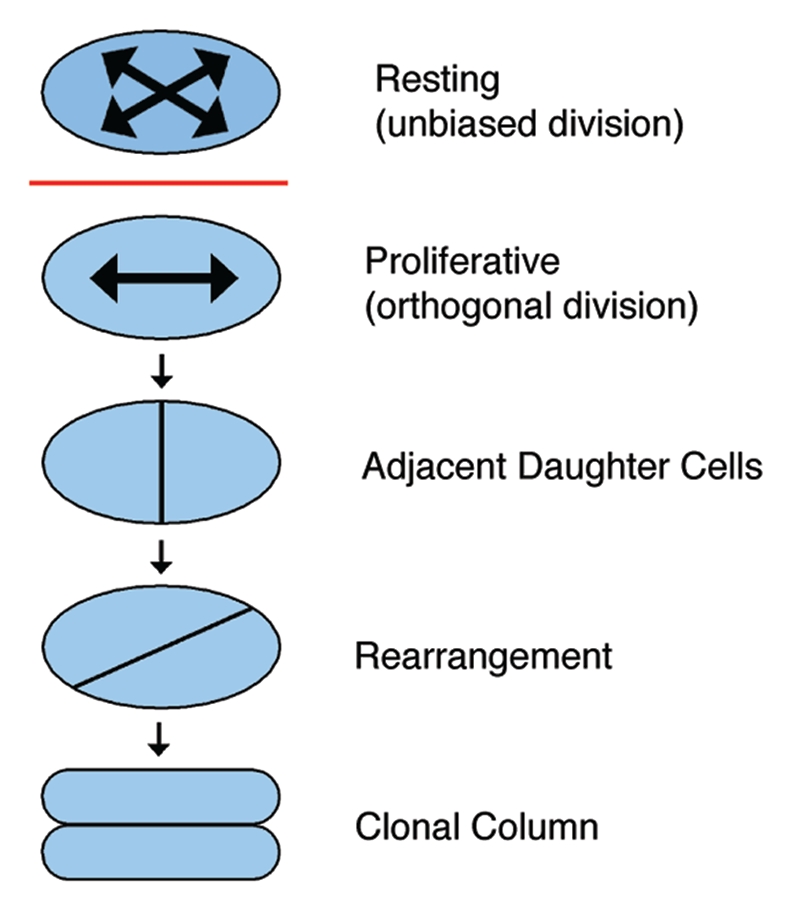
Model of chondrocyte column formation. Chondrocytes in the resting zone are elliptical and do not exhibit a bias in the orientation of cell division. After being recruited into the proliferative zone, however, chondrocytes demonstrate planes of cell division that are perpendicular to the long axis of the skeletal element. The resultant daughter cells then rearrange to form a clonal column parallel to the long axis of the growth plate.
Wnt5a regulates multiple steps in skeletal development.
The regulation of cell shape, alignment of division planes and oriented cell movements are characteristics of skeletal formation from the earliest stages of limb patterning through postnatal elongation of bones. Such similar cell behaviors in these different structures suggest the possibility that the distinct signaling networks in each tissue regulate polarity through common effector pathways. Recent papers have solidified a prominent role for Wnt5a signaling in regulating oriented cell behaviors throughout development of the limb skeleton.
Wnt5a was first identified as a morphogenetic factor in the limb for its role in the regulation of cell proliferation. For example, loss of Wnt5a function in mice decreases the BrdU labeling index of limb mesenchyme and growth plate chondrocytes.21,48 In chondrocytes, this effect on cell proliferation was confirmed using immunofluorescence detection to show a decrease in the cell cycle-promoting proteins cyclin A and cyclin D and an increase in repressive proteins of the Rb family, such as p130.48 In both the limb bud and in the growth plate, reduced cell proliferation is accompanied by decreased growth in the proximodistal axis, as well as altered growth in other axes.
An additional role for Wnt5a signaling in regulating cell morphology and cell organization was evident from studies of the growth plate cartilage. In Wnt5a mutants, proliferative chondrocytes fail to adopt discoid morphology, and clonal expansion of these cells does not result in obvious columns.48 Similarly, ectopic expression of Wnt5a in the chick prevents flattening of proliferative chondrocytes and column formation (Li and Dudley, unpublished observations). One caveat is the potential for these experimental approaches that perturb a particular signaling pathway to alter the properties of proliferative chondrocytes by indirectly affecting the tissue-level signaling network. However, the presence of a similar cell morphology phenotype in the early limb bud supports the idea that these effects on cell shape and axis orientation are Wnt5a-specific. Thus, Gros et al. showed that in the absence of Wnt5a function, limb mesenchyme cells adopted an elliptical shape, and the long axis of the cell was not primarily oriented toward the ectoderm.44 In fact, the change in cell morphology from elongated to elliptical is a common aspect of Wnt5a mutant phenotypes in mesenchymal tissues throughout development.49 Together, these observations suggest that Wnt5a signaling is an important regulator of cell shape.
Wnt5a signaling is also important for orienting the division plane and promoting directional cell movement/migration. Thus, in Wnt5a mutants, limb mesenchyme cells show arbitrary division planes and multidirectional cell movement that occurs with low coherence between cells.45 Similar defects in cell movement disrupt convergent extension in zebrafish mutant for Wnt5 or Wnt11.49,50 The Wnt pathway as a regulator of cell division or cell movement has also been proposed in neurite extension as well as in the division of neuroblasts in Drosophila and vulval precursor cells in C. elegans.51,52 In some asymmetric cell divisions, activation of Frizzled receptors and the intracellular signaling molecule Dishevelled by Wnt ligands localizes a complex of Pins/LGN, Mud/NUMA and the G protein Gαi, among others, to orient the mitotic spindle.53,54 However, it is not yet established whether Wnt5a activates this pathway to regulate the presumed symmetric divisions during limb development. Moreover, it is not known whether orientation of cell division and cell movement/migration in limb mesenchyme represent distinct roles for Wnt5a signaling or if these events are a consequence of cell shape. It has been known for over one hundred years that the mitotic spindle orients in the plane of the longest axis of a cell, an observation known as Sach's and Hertwig's rule.55 Modern three-dimensional image analysis has confirmed this observation and has provided mechanistic insights by demonstrating roles for cell-matrix adhesion via integrin receptors and for tension on the cortical actin cytoskeleton in determining cell shape and spindle orientation.56 Thus, Wnt5a regulation of cell shape might bias the orientation of cell division and cell migration rather than directly regulate these processes.
How does Wnt5a signaling regulate cell polarity?
Wnt ligands activate multiple pathways that were historically categorized as canonical and non-canonical pathways.57 In the canonical pathway, Wnt activation of Frizzled (Fzd) receptors acts through the intracellular mediator Dishevelled (Dvl) to prevent proteolytic degradation of cytosolic β-catenin, which then localizes to the nucleus to promote expression of target genes via interaction with the TCF/LEF family of transcriptional regulators. By contrast, the non-canonical pathways function independent of β-catenin, though many act through Dvl. Depending on the cellular context, non-canonical Wnt signaling can function by regulating intracellular Ca2+ and/or activating proteins such as the GTPase Rac and the intracellular kinases Src, calcium/calmodulin-depenent kinase II (CAMKII) or Jun N-terminal kinase (JNK). These pathways regulate diverse cellular processes, including cell polarity, dynamic organization of the cytoskeleton and gene expression.57
One non-canonical Wnt pathway that regulates cell polarity is the planar cell polarity pathway (PCP; reviewed in this issue).58–62 PCP signaling is regulated by Fzd activity through Dvl but also involves the actions of distinct core components that include Vangl1/2, four-pass transmembrane proteins that interact with Fzd; Flamingo, a seven-pass transmembrane cadherin; and Prickle, an intracellular protein. The net result of PCP signaling is the integration of global patterning cues (mediated in part through the atypical cadherins Fat and Dachsous) to generate asymmetric distribution of Fzd activity at the cell membrane. This is accomplished through differential localization of Fzd/Dvl and Vangl/Prickle and provides directional information to orient subcellular structures, cell morphology and cell movement. Output of the PCP pathway is tissue-specific despite involvement of a common set of core proteins. Thus, PCP signaling generates planar alignment of both stereocilia in the inner ear and hairs on a fly wing as well as promoting directional migration of neurites and cell intercalation at a midline that promotes axis extension (convergent extension cell movements).
Currently, specificity of pathway activation is not thought to reside in the ligand, but rather to depend on the presence of an ever-increasing number of co-receptors (such as LRP5/6 and the Ror and Ryk family receptor tyrosine kinases) and other interacting molecules (e.g., the Sfrp family of secreted Wnt antagonists and Cthrc1).63–69 However, Wnt5a is largely regarded as a non-canonical Wnt because of its effects on β-catenin activity. In chondrocytes and many other cells, Wnt5a signaling alone does not promote stabilization of β-catenin, but instead decreases β-catenin activity induced by a canonical Wnt signal.70,71 While these findings are important, they do not provide extensive insight into the mechanism of Wnt5a action, because non-canonical signaling is composed of diverse pathways. However, evidence is now mounting that Wnt5a signaling via the Ror1/2 kinases is an important component in the vertebrate planar cell polarity pathway (PCP), which regulates oriented cell behaviors and directional cell movements in many tissues.72–74
Several lines of evidence have implicated Wnt5a and PCP signaling in oriented cell behaviors during limb skeleton development. First, in the zebrafish, loss of Knypek (Kny) function suppresses convergent extension movements during gastrulation and results in reduced elongation of particular cartilage elements in the head.75 Specifically, chondrocytes that are normally flattened and stacked in columns, as in the growth plate, are obviously rounder and show disorganized packing in Kny mutants. The net result is that mutant cartilage is shorter and wider than wild type. Kny interacts with Wnt11 (silberblick) in gastrulation and with Wnt5 (pipetail) in axis elongation, two processes in which mesenchymal cell movements are regulated by the PCP pathway. Consistent with a model in which Kny plays a role in PCP, Kny interacts with trilobite (Vangl/Stbm) in convergent extension cell movements.76–78 Interestingly, Kny also interacts genetically with Wnt5 (pipetail) in cartilage morphogenesis, suggesting that the columnar organization of chondrocytes might result from PCP signaling.75 Kny is a member of the glypican family of heparan sulfate proteoglycans that interact with multiple signaling ligands, and therefore, the phenotype might result from pleiotropic effects on signaling pathways activated by other heparan sulfate binding factors.79 However, in the context of convergent extension, Kny does not appear to regulate Hedgehog, fibroblast growth factor or bone morphogenetic protein signaling.75,76 Glypican-4, a homolog of Knypek, has also been shown to act in conjunction with Wnt11 and Dishevelled to regulate convergent extension cell movements in Xenopus via non-canonical Wnt signaling.80 Interestingly, glypicans are anchored to the cell surface by a glycosyl-phosphatidylinositol (GPI) moiety, and mutations in Piga, an essential enzyme for GPI biosynthesis, produce a Knypek-like phenotype in the growth plates of mice.81 Although a specific role for glypicans in regulating cell polarity via Wnt signaling has not been determined, genetic interactions suggest that Kny might bind Wnt5 and Wnt11, while other studies in zebrafish provide evidence that Kny stabilizes Frizzled receptors on the cell surface.82
Second, experiments in the chick using a dominant-negative Wnt receptor (dnFzd7) and a dominant-negative Dishevelled (dnDvl) showed a cell autonomous requirement for Wnt signaling to orient division planes and to generate columns in proliferative chondrocytes.46 Neither gain nor loss of β-catenin function in chick or in mouse, respectively, prevent column formation, suggesting that non-canonical Wnt signaling, rather than canonical Wnt signaling, regulates oriented cell behaviors in proliferative chondrocytes.46,81 Failure to align division planes and organize into columns in dnFzd7- and dnDvl-expressing chondrocytes is likely the result of interfering with PCP signaling because introduction of a point mutation analogous to the Drosophila mutant dsh1 (which specifically interferes with PCP signaling) into the C-terminal DEP domain rendered dnDvl inactive.46,83
Third, recent work demonstrated dose-dependent genetic interactions between the secreted ligand Wnt5a, the receptor tyrosine kinase Ror2 and PCP pathway gene in developing digits.70 Moreover, asymmetrical membrane localization of Vangl2, a hallmark of PCP in epithelial sheets, is disturbed in the developing radius, ulna and digits of Wnt5a and Ror2 mutants.70 Together, these findings strongly suggest that Wnt5a, Ror2 and Vangl2 are important in skeletal development but fall short of defining a single pathway, since observation of genetic interactions is also consistent with models composed of parallel pathways. Going from genetic interaction to detailed mechanism requires precise biochemical tools. Recently, Gao et al. produced a major breakthrough that could provide robust tools for the analysis of PCP signaling in the developing skeleton and other mesenchyme-derived tissues.70 Building on results from genetic studies, these authors showed that Ror2 and Vangl2 form a stable complex on the plasma membrane in transfected cells. Within this context, Wnt5a/Ror2 promotes phosphorylation of Vangl2. Multiple potential serine/threonine phosphorylation sites exist in two clusters in the N terminus of Vangl2. Mutagenesis studies showed that serines 82 and 84 in cluster I and serine 5 in cluster II are important “seed” residues that are required for achieving more than basal levels of Vangl2 phosphorylation. The full extent of Vangl2 phosphorylation is crucial for PCP signaling, as shown by the potency of different alanine mutants and glutamate-based phosphomimetics in rescuing convergent extension cell movements in trilobite (Vangl2) mutant zebrafish embryos. Finally, and perhaps most importantly, Wnt5a signaling enhanced Vangl2 phosphorylation in a dose-dependent manner that required Ror2.70 Together, these data provide very strong evidence that Wnt5a acts through a Ror2/Vangl2 complex to regulate oriented cell behaviors via the PCP pathway and provide important biochemical tools to analyze PCP signaling in vivo.
Why polarize cells in the developing limb?
A commonality between limb bud development, digit elongation and growth plate function is the correlation between cell polarity and growth. Thus, in each system, experimental manipulations that alter cell shape, cell arrangement or oriented cell movement also alter growth vectors and result in limb structures that are shorter and wider (thicker) than in wild type. For example, in the growth plate, loss of oriented cell behaviors leads to loss of proliferative column structure and generates bones that are reduced in length and greater in width.46,81 One simple model derived from these observations is that oriented cell behaviors provide a driving force for tissue elongation in the developing skeleton,46,84 analogous to a model previously proposed for events in zebrafish gastrulation.85
In the context of the limb, this model has only been empirically tested in the growth plate, a tissue in which precise measurements of elongation have been performed. Growth rate is a measure of the increase in tissue mass per unit time along a defined axis. In the growth plate, cell proliferation (number of chondrocytes per column) and hypertrophy (cell size) are two factors that influence growth rate. Of these two, hypertrophy is more significant, since the height of chondrocytes increases by 4-fold in the transition from proliferation to hypertrophy.86 In fact, studies of cartilage growth demonstrated a linear relationship between the growth rate of long bones and the volume of hypertrophic chondrocytes.10 This correlation was consistent in the growth plates of various bones throughout the limb, including those that that grow at different rates, supporting the idea that hypertrophy generates the driving force for bone elongation.
If hypertrophy rather than column formation drives bone growth, then what is the relationship between column integrity and bone elongation? One perspective is that column formation in discoid proliferative chondrocytes serves to maximize cell density in the longitudinal axis while regulating cell density (i.e., column number) in the lateral axis.46 The net effect of this arrangement is to constrain the growth-promoting effects of hypertrophy to the longitudinal axis. Similarly, although chondrocytes of the elongating digits do not appear to form columns and do not undergo hypertrophy in the early stages, cell polarity might still play a role in digit lengthening. The maintenance of cell shape and orientation of cell division could generate a driving force for digit elongation, even if together these cell behaviors simply limit lateral expansion of the developing digit cartilage. However, additional studies will be required to determine if growth is the primary output of oriented cell behaviors in developing digits.
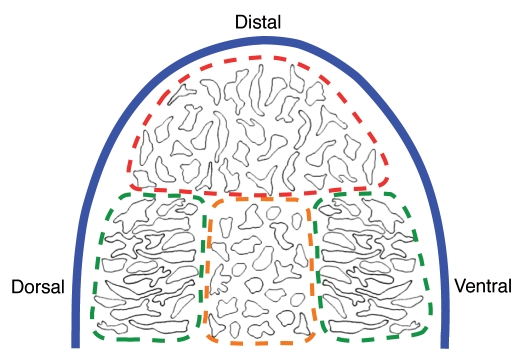
Unlike in cartilage, in which cell division and cell movement occur in orthogonal planes, cell shape, cell division and cell migration all occur in the same plane in the early limb bud, an observation consistent with a direct role for cell polarity in promoting outgrowth. As the limb bud emerges from the flank, oriented cell divisions and the associated bulk movement of mesenchyme cells to the posterior-distal region could populate the early bud with mesenchyme and promote outgrowth.45 As the limb bud elongates, dynamic local fine-tuning of cell polarity could then shape the emerging limb by defining the vectors along which tissue expansion occurs. Although this simple, elegant model is appealing, there is no compelling reason to assume that all oriented cell behaviors in the early limb bud must contribute directly to the vectors of tissue expansion. In fact, several lines of evidence are difficult to reconcile with this model. For example, only cells in the distal region of the limb bud show oriented cell behaviors that are aligned with the primary vector of growth (distal).45 Cells in the dorsal and ventral regions are oriented toward the overlying ectoderm and roughly orthogonal to the proximodistal axis (Fig. 4). While it is possible that this reflects different growth vectors in different regions of the limb bud, cell lineage analyses have not uncovered a predominant dorsal-ventral expansion of clones in the proximal limb bud, and it is not clear how outward directed cell movements could lead to thinning along the dorsal-ventral axis at later stages of development.87–89 One model consistent with the observed cell orientation is that cells in the dorsal and ventral domains undergo convergent extension-type cell movements that promote cell stacking in the proximodistal axis to drive limb bud elongation. Unfortunately, the random labeling methods used by Gros et al. are not amenable to the analysis of nearest neighbor interactions that drive convergent extension movements, and the narrow window of observation is insufficient to determine quantitatively if the rate of limb outgrowth is proportional to the rate of cell migration into the dorsal and ventral domains.44 Therefore, further analysis will be required to validate this model in limb outgrowth.
Figure 4.
This schematic summarizes data compiled from the images of Gros et al.45 Mesenchymal cells in different regions of the developing chick limb bud exhibit distinct morphologies. Cells in the dorsal and ventral domains (green dashed boundaries) appear more spindle-like and organized than cells in the central and distal domains (orange and red dashed boundaries, respectively). Cell division and cell migration in the dorsal and ventral domains are aligned to the ectoderm (blue line). Cells in the central domain appear rounder with fewer and less active protrusions than cells in the distal domain.
As an alternative to generating forces for growth, the planar alignment of cells in the dorsal and ventral regions could establish unique cellular domains that differ in mechanical properties. For example, it is apparent from the images of Gros et al. that cells in the different regions of the limb bud display distinct morphologies (Fig. 4).45 In particular, cells in the dorsal and ventral domains appear more spindle-like and show more organized packing than cells in the central and distal domains. Cells in the central domain appear rounder with fewer and less active protrusions than cells in the distal domain. This distinction is important, because cells in the central domain will presumably form chondrocytes; cells in the distal domain are mesenchymal progenitors, and cells in the dorsal and ventral domains do not contribute to the cartilaginous skeleton, though they probably contain precursors of connective tissue, such as tendon. In this case, stacking of dorsal and ventral cells might serve to separate these domains from the prechondrogenic mesenchyme of the central region. An alternative, but not mutually exclusive, possibility is that stacking of the dorsal and ventral cells provides a mechanical barrier to direct forces generated by mesenchyme growth distally and prevent retrograde movement of the rapidly expanding distal mesenchyme population (which would produce isotropic growth). In this way, local differences in tissue structure would promote distal extrusion of a highly proliferative mesenchyme population, much like toothpaste from a tube. This model would suggest that constriction of the dorsal-ventral axis as the autopod (future hand/foot) develops could result from narrowing of the “spout” by changes in the structure of the dorsal and ventral domains. Finally, stacking of dorsal and ventral mesenchyme could also double as physical barriers that restrict migratory tracts for ingressing cells of the nervous and vascular systems.
Unanswered Questions
It has been said that good experiments yield more questions than answers. Clearly, great gains have been made recently in elucidating the cell biological mechanisms of skeletal growth and morphogenesis and establishing a link between cell polarity pathways and skeletal growth. However, it is also important to acknowledge that many unknowns exist that could fundamentally alter the models on which future directions are founded.
Do skeletal phenotypes result directly from cell polarity defects?
There is now extensive data in the developing limb showing that cells exhibit oriented behaviors; that Wnt5a signaling regulates these behaviors, and that disturbing oriented behaviors results in growth defects. Moreover, there is compelling evidence that Wnt5a signaling through a Ror2-Vangl2 complex regulates oriented cell behaviors in the developing skeleton. For example, biochemical analysis demonstrates that Wnt5a regulates Ror2 to promote phosphorylation of Vangl2, and loss of Ror2 function reduces or eliminates asymmetric localization of Vangl2 in the early limb skeleton.70 Furthermore, the increase in severity of the limb phenotype in Ror1/Ror2 double mutants suggests that a substantial fraction (but perhaps not all) of Wnt5a signaling in limb development occurs through Ror receptors.90 However, it is also important to note that in addition to regulating cell polarity, Wnt5a signaling also regulates other processes, such as the cell cycle.21 This is important, because it was previously established that formation of cartilage primordia requires a threshold number of progenitor cells, and that growth potential is related to the initial primordium size.91,92 Thus, defects in mesenchyme proliferation and/or cell polarity in the limb bud could result in decreased growth and altered morphogenesis of mutant skeletons independent of a direct role for PCP signaling.
To what extent, then, are the growth defects directly related to Wnt5a/Ror/PCP-induced changes in cell shape/polarity? In other PCP models, such as the inner ear, the loss of Vangl2 function presents a more severe phenotype than mutations in Wnt5a or Ror2.70,90,93 In the context of the limb, by contrast, loss of Ror2 or Vangl2 function individually results in a substantially weaker phenotype than observed in Wnt5a mutants.70 Although Ror2 and Vangl2 interact genetically in the limb, the double-mutant phenotype is not equivalent to that of Wnt5a mutants.70,90,93 Whether these differences can be explained by redundancy, as shown for Vangl1 and Vangl2 in the inner ear,94 remains to be determined. However, the available data leaves open the possibility that the terminal skeletal phenotype results in part from actions of Wnt5a in the early limb bud and/or Wnt5a signaling through alternative pathways in addition to PCP.90
If Wnt5a function is a summation of the effects of multiple pathways, one possibility is that Wnt5a interaction with distinct receptor complexes activates different downstream pathways. Wnt5a might induce distinct signals when bound to the cognate receptor of the Frizzled family as opposed to Ror2 or as a result of interaction with different co-receptors such as Cthrc1, Dickkopf or glypicans.69,75,95 Alternatively, crosstalk with other Wnt pathways could modify the Wnt5a/PCP signal. For example, asymmetric Wnt/PCP signaling could arise from the interaction of multiple signaling complexes and/or from cross-regulation by non-Wnt pathways. In C. elegans, opposite orientation of cell division generates vulva precursor cells (VPC) that display mirror-image symmetry. This arrangement of VPCs is dependent on two distinct Wnt regulated pathways. First, “ground” polarity is established in both cells by EGL-20 (Wnt) signaling through CAM-1 (Ror-like kinase) and VANG-1 (Van Gogh/Vangl). Second, MOM-2 and LIN-44 (two WNT molecules) expressed in the VPCs signal through LIN-17 (Fzd) and LIN-18 (Ryk tyrosine kinase) to reverse the orientation of cell division in the posterior VPC cell division relative to the anterior cell via a β-catenin-dependent pathway. This specific example of crosstalk could occur in the limb mesenchyme but is not likely to function in the growth plate, since defects in canonical Wnt signaling do not block chondrocyte column formation in the growth plate.46,81 However, it remains to be determined whether important roles exist for Ryk or for the PCP-associated tyrosine kinase Ptk7 in modulating the non-canonical Wnt signal during limb development.96,97
Is cell polarity in the limb regulated by Wnt5a gradients?
As a secreted signaling molecule, Wnt5a may regulate cell polarity by generating morphogen gradients in the developing skeleton. In the limb bud, Wnt5a is strongly expressed in the apical ectodermal ridge (AER) at the distal tip of the limb bud and in a graded manner in the distal mesenchyme, decreasing proximally.21,66,98 Distal expression is maintained in the developing hand plate and localized expression is observed in each growth plate.48,66 From these expression data, it is likely that a gradient of Wnt5a signaling exists at each phase of limb development. Importantly, evidence suggests that diverse cells can respond to gradients of Wnt5a signaling. For example, limb mesenchyme cells, neurites and developing palate cells all migrate toward sources of Wnt5a.45,99–101 Recently, Gao et al. demonstrated dose-dependent Vangl2 phosphorylation by Wnt5a in transfected CHO cells, providing a mechanism for regulating cell polarity in the limb via concentration gradients of Wnt5a.70
Although cells can respond to a graded Wnt5a signal, it is not certain whether polarization of all cells in the developing limb occurs in response to a Wnt5a concentration gradient. The key difference is that in the (simple) polarity model, the cell uses the difference in signaling across the cell surface to asymmetrically localize factors that produce functionally distinct outcomes into opposite domains of the cells. One problem is that the hypothetical morphogen gradient is ill defined spatially and temporally in many tissues. For example, models proposed by several laboratories assume that Wnt5a concentration gradients are a product of simple diffusion from a single source. However, in the limb bud mesenchyme, the gradient of Wnt5a signaling appears to be, at least partially, the product of a gradient of transcriptional regulation.21 Thus, the cells that are predicted to respond to the concentration gradient may also be producers of Wnt5a. It is not certain whether ligand-producing cells can sense the direction of the gradient.
An additional issue with the simple gradient model is that Wnt5a expression is dynamic in the developing limb; it is first expressed in the ectodermal jacket (predominately ventral) of the forming limb bud, but it is later expressed in the distal ectoderm and mesenchyme as discussed above.21,98 Distal expression of Wnt5a continues in the developing hand plate but is excluded from the mesenchyme condensates that differentiate into the cartilage templates for the metacarpal/metatarsals and the digits. The mesenchymal condensates are partially surrounded by Wnt5a-expressing mesenchyme at the distal tip of the limb, and therefore, it is possible that cells in this region do not experience a simple distal-to-proximal gradient of Wnt5a. The picture is similarly complicated in the developing growth plate cartilage, where Wnt5a is expressed in a domain of mature chondrocytes and within the perichondrium that surrounds the cartilage element.48 Potentially, proliferative chondrocytes at different positions in the growth plate are presented with concentration gradients of Wnt5a that have different vectors and slopes, yet all cells display similar planar alignment. Additionally, the simple gradient model is further challenged in the limb bud by the dynamic expression of Wnt antagonists and other Wnt molecules in either the ectodermal jacket or in the mesenchyme, which might directly or indirectly modulate Wnt5a/Ror2 signaling.98
Perhaps by understanding the shape of the Wnt concentration gradients or how cells interpret the Wnt signal, we can make better sense of the findings of Gros et al. in mouse limb buds. At the stage analyzed, Wnt5a expression is strong at the distal tip, graded in the limb mesenchyme and absent from the ectoderm (Fig. 3 in Gros et al.). Yet, the orientation of cell bodies and the vector of directed cell movements appear directed toward the ectoderm and not the presumed Wnt5a concentration gradient. Thus, the long axis of distal cells is aligned with the proximodistal axis of the limb bud, and cells move distally up the predicted concentration gradient. By contrast, dorsal/ventral cells are aligned to and move toward the ectoderm in the dorsoventral axis, an orientation that is roughly orthogonal to the predicted concentration gradient of Wnt5a. Moreover, in Wnt5a mutant mouse embryos, directed cell movements and the coherence of cell movement are most severely affected in the dorsal and ventral mesenchyme domains, where the slope of the morphogen gradient is likely very shallow, rather than in the distal domain where the expression of Wnt5a is greatest.45 Compensation by other Wnt ligands could reduce the severity of defects in Wnt5a mutant limb buds. Indeed, many Wnt ligands are expressed in the ectodermal jacket and AER of the limb bud.66 However, one confounding factor is that several Wnt antagonists are expressed in limb mesenchyme, predominately in the proximal region.66 Whether these antagonists can regulate cell polarity in limb mesenchyme by shaping concentration gradients or by activating non-canonical Wnt signaling via reducing canonical signaling activity remains to be determined.
The inconsistencies between the presumed Wnt5a concentration gradient and the predominant sites of Wnt5a action might reflect our lack of understanding of the distribution of Wnt ligands in limb mesenchyme, but could also suggest that cells do not detect Wnt5a as a graded signal. Indeed, directional cell movement (chemotaxis) of some cancer cells in culture appears dependent on Wnt5a signaling but not a gradient of Wnt5a. In WM239A melanoma cells, exposure to Wnt5a results in localization of Fzd3, F-actin, myosin IIb and the cell adhesion molecule MCAM in intracellular structures.102 In response to a gradient of the chemokine CXCL12, solitary melanoma cells redistribute the contents of these structures to the cell surface in a polarized manner. In this way, Wnt5a signaling might be required but not sufficient to establish cell polarity that promotes oriented cell behaviors in response to other graded signals.
Where does Wnt5a/PCP signaling act?
In light of the extensive evidence demonstrating roles for Wnt5a and PCP signaling throughout development of the limb skeleton, it is easy to lose sight of the fact that few of the experiments performed definitively show when and where signaling is required. For example, during limb bud initiation, oriented cell behaviors in both the lateral plate mesoderm and the expanding limb mesenchyme are affected by loss of Wnt5a function.44,45 However, unexpectedly, Wnt5a signaling is more important for oriented cell behaviors in the lateral plate mesoderm than in the expanding limb bud mesenchyme in both the mouse and the zebrafish.44 Thus, it is not obvious whether directional cell movements of mesenchyme into the early limb bud are the result of current cell polarity signaling or a consequence of earlier cell polarity decisions in the lateral plate mesoderm.
In the growth plate, the question of where Wnt signaling is required has been addressed using mosaic analysis, an essential tool for demonstrating the site of action of a signaling pathway. A cell autonomous requirement for non-canonical Fzd signaling for column formation in proliferative chondrocytes has been established via mosaic analysis using viral transduction of wild-type and dominant-negative molecules.46 In these studies, infected chondrocytes, but not uninfected neighbors, showed defects in cell orientation and in planar cell divisions when Wnt signaling was affected at the level of Fzd, Dvl and Vangl2 activity. These mosaic studies provide a level of confidence that the phenotype results from defects in non-canonical Wnt signaling in the proliferative chondrocytes.
The demonstration of a cell autonomous requirement for non-canonical Wnt signaling in the growth plate cartilage is not necessarily relevant to Wnt function in digit formation. Defects in cell arrangement and the loss of Vangl2 asymmetry in Wnt5a mutants (and partial loss in Ror2 mutants) support the idea that PCP signaling promotes elongation of the digit templates and provide a mechanism that accounts for the skeletal phenotype.70 However, this conclusion may be premature. Examination of Wnt5a mutants suggests that development of the hand plate is especially slowed during phalanx formation.21,70 Specifically, while the proximal skeletal elements appear normal initially, the metacarpals/metatarsals show abnormal morphology, and the proximal and middle phalanges are absent.21 A similar phenotype is observed in Ror2/Vangl2 double mutants,70 and a severe reduction in phalanx size results in embryos that express a putative dominant-negative form of Ror2.104 Together, these observations are potentially significant, because development of the phalangeal elements appears to use a developmental mechanism distinct from that of the remaining cartilaginous skeleton.103,104 As phalangeal elements elongate, a domain of cells called the phalanx-forming region (PFR) is observed immediately distal to the element.103,104 Decreased size of the domain correlates with reduction of phalanx size in some experimental models, suggesting that the PFR regulates digit growth.104 The PFR exhibits high levels of BMP signaling and is contained within a domain of strong Ror2 expression.103,104 Ror2 activity was presumed to activate non-canonical Wnt pathways to oppose β-catenin activity, which is an antagonist of BMP signaling and chondrogenesis.104–106 Consistent with this model, disruption of Ror2 function using a putative dominant-negative molecule results in distal activation of canonical Wnt signaling (as shown by the Axin-lacZ reporter allele), downregulation of BMP signaling and reduced digit elongation.104 Interestingly, upregulation of β-catenin activity was also observed in Ror2/Vangl2 double mutants, but not either single mutant, using the TOPGAL reporter allele.70,104 This intriguing finding with the double mutants suggests at least two models for Wnt5a/Ror2 signaling in digit elongation, in addition to a role for PCP regulated cell polarity in growth of the cartilage template. In one model, Wnt5a/Ror2 signaling might regulate PCP signaling in the PFR to maintain structure or to promote recruitment of mesenchyme cells into the growing digit ray. As another model, it is possible that Vangl2 function in the PFR is to provide robustness to ensure that non-canonical Wnt5a/Ror2 signaling maintains strong repression of β-catenin activity to preserve PFR function. This latter role could conceivably occur independent of Vangl2 function in PCP.104 Thus, while it is clear that loss of Wnt5a, Ror2 and Vangl2 results in defects in digit formation, it is not obvious if, when or where planar cell polarity is required for this process.
Conclusions
Since the beginning of modern biology over century ago, questions about the mechanisms of morphogenesis have motivated the work of many laboratories. Over time, models based on physical forces were supplanted by molecular descriptions of cell signaling and gene transcription. However, these new models lacked the intermediary cell biological mechanisms that enable signaling pathways to generate appropriate morphogenetic forces. Recent work provides compelling evidence that cell polarity provides the key to quantitative fine-tuning of the scalar and vector properties of forces that generate growth and form.
Acknowledgements
We thank all of the individuals whose data contributed to this review but were not cited due to space constraints. This work was supported by the Cellular Molecular Basis of Disease Training Grant (S.M.R.) and the National Institutes of Health/NIAMS (AR054857). The authors do not have conflicts of interest associated with this work.
Abbreviation
- AER
apical ectodermal ridge
- BMP
bone morphogenetic protein
- Dvl
dishevelled
- FGF
fibroblast growth factor
- Fzd
frizzled
- GPI
glycosylphosphatidylinositol
- HH
hamburger-hamilton
- IHH
indian hedgehog
- JNK
Jun N-terminal kinase
- Kny
Knypek
- PTHrP
parathyroid hormone-related protein
- PFR
phalangeal forming region
- PCP
planar cell polarity
- SHH
sonic hedgehog
- TGFβ
transforming growth factorβ
- VPC
vulva precursor cells
- WNT
wingless/int-1
- ZPA
zone of polarizing activity
References
- 1.Summerbell D. Evidence for regulation of growth, size and pattern in the developing chick limb bud. J Embryol Exp Morphol. 1981;65:129–150. [PubMed] [Google Scholar]
- 2.Kieny M. On the relations between somatic and somatopleural mesoderm before and during primary induction of chick embryo limbs. C R Acad Sci Hebd Seances Acad Sci D. 1969;268:3183–3186. [PubMed] [Google Scholar]
- 3.Dhouailly D, Kieny M. Participation of the somatopleural mesoderm of the side to the building of an additional limb in birds. C R Acad Sci Hebd Seances Acad Sci D. 1970;271:519–522. [PubMed] [Google Scholar]
- 4.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo 1951. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- 5.Burke AC, Nelson CE, Morgan BA, Tabin C. Hox genes and the evolution of vertebrate axial morphology. Development. 1995;121:333–346. doi: 10.1242/dev.121.2.333. [DOI] [PubMed] [Google Scholar]
- 6.Fell HB. The histogenesis of cartilage and bone in the long bones of the embryonic fowl. J Morphol. 2005;40:417–459. doi: 10.1002/jmor.1050400302. [DOI] [Google Scholar]
- 7.Saunders JW., Jr The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J Exp Zool. 1948;108:363–403. doi: 10.1002/jez.1401080304. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Drossopoulou G, Chuang PT, Duprez D, Marti E, Bumcrot D, et al. Relationship between dose, distance and time in Sonic Hedgehog-mediated regulation of anteroposterior polarity in the chick limb. Development. 1997;124:4393–4404. doi: 10.1242/dev.124.21.4393. [DOI] [PubMed] [Google Scholar]
- 9.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/S1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 10.Breur GJ, VanEnkevort BA, Farnum CE, Wilsman NJ. Linear relationship between the volume of hypertrophic chondrocytes and the rate of longitudinal bone growth in growth plates. J Orthop Res. 1991;9:348–359. doi: 10.1002/jor.1100090306. [DOI] [PubMed] [Google Scholar]
- 11.Dodds GS. Row formation and other types of arrangement of cartilage cells in endochondral ossification. The Anatomical Record. 2005;46:385–399. doi: 10.1002/ar.1090460409. [DOI] [Google Scholar]
- 12.Hatori M, Klatte KJ, Teixeira CC, Shapiro IM. End labeling studies of fragmented DNA in the avian growth plate: evidence of apoptosis in terminally differentiated chondrocytes. J Bone Miner Res. 1995;10:1960–1968. doi: 10.1002/jbmr.5650101216. [DOI] [PubMed] [Google Scholar]
- 13.Ohuchi H, Nakagawa T, Yamamoto A, Araga A, Ohata T, Ishimaru Y, et al. The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development. 1997;124:2235–2244. doi: 10.1242/dev.124.11.2235. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal P, Wylie JN, Galceran J, Arkhitko O, Li C, Deng C, et al. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 2003;130:623–633. doi: 10.1242/dev.00191. [DOI] [PubMed] [Google Scholar]
- 15.Fallon JF, Lopez A, Ros MA, Savage MP, Olwin BB, Simandl BK. FGF-2: apical ectodermal ridge growth signal for chick limb development. Science. 1994;264:104–107. doi: 10.1126/science.7908145. [DOI] [PubMed] [Google Scholar]
- 16.Mercader N, Leonardo E, Piedra ME, Martinez AC, Ros MA, Torres M. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development. 2000;127:3961–3970. doi: 10.1242/dev.127.18.3961. [DOI] [PubMed] [Google Scholar]
- 17.Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dolle P. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development. 2002;129:3563–3574. doi: 10.1242/dev.129.15.3563. [DOI] [PubMed] [Google Scholar]
- 18.Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, et al. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb. Dev Cell. 2004;6:411–422. doi: 10.1016/S1534-5807(04)00062-0. [DOI] [PubMed] [Google Scholar]
- 19.Cooper KL, Hu JK, ten Berge D, Fernandez-Teran M, Ros MA, Tabin CJ. Initiation of proximal-distal patterning in the vertebrate limb by signals and growth. Science. 2011;332:1083–1086. doi: 10.1126/science.1199499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosello-Díez A, Ros MA, Torres M. Diffusible signals, not autonomous mechanisms, determine the main proximodistal limb subdivision. Science. 2011;332:1086–1088. doi: 10.1126/science.1199489. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi TP, Bradley A, McMahon AP, Jones SA. Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 22.Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nat Genet. 2000;26:460–463. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Mariani FV, Martin GR. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature. 2002;418:501–508. doi: 10.1038/nature00902. [DOI] [PubMed] [Google Scholar]
- 24.Niswander L, Jeffrey S, Martin GR, Tickle C. A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature. 1994;371:609–612. doi: 10.1038/371609a0. [DOI] [PubMed] [Google Scholar]
- 25.Laufer E, Nelson CE, Johnson RL, Morgan BA, Tabin C. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell. 1994;79:993–1003. doi: 10.1016/0092-8674(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 26.Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25:1–47. doi: 10.1016/S0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- 27.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 28.Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB., 3rd BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development. 2001;128:4449–4461. doi: 10.1242/dev.128.22.4449. [DOI] [PubMed] [Google Scholar]
- 30.Pizette S, Abate-Shen C, Niswander L. BMP controls proximodistal outgrowth, via induction of the apical ectodermal ridge and dorsoventral patterning in the vertebrate limb. Development. 2001;128:4463–4474. doi: 10.1242/dev.128.22.4463. [DOI] [PubMed] [Google Scholar]
- 31.Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 32.Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 33.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kronenberg HM, Chung U. The parathyroid hormone-related protein and Indian hedgehog feedback loop in the growth plate. Novartis Found Symp. 2001;232:144–152. doi: 10.1002/0470846658.ch10. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi T, Soegiarto DW, Yang Y, Lanske B, Schipani E, McMahon AP, et al. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J Clin Invest. 2005;115:1734–1742. doi: 10.1172/JCI24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo J, Chung UI, Yang D, Karsenty G, Bringhurst FR, Kronenberg HM. PTH/PTHrP receptor delays chondrocyte hypertrophy via both Runx2-dependent and -independent pathways. Dev Biol. 2006;292:116–128. doi: 10.1016/j.ydbio.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 37.Mak KK, Kronenberg HM, Chuang PT, Mackem S, Yang Y. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy. Development. 2008;135:1947–1956. doi: 10.1242/dev.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niswander L, Tickle C, Vogel A, Booth I, Martin GR. FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell. 1993;75:579–587. doi: 10.1016/0092-8674(93)90391-3. [DOI] [PubMed] [Google Scholar]
- 39.Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, et al. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn. 1996;206:379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 40.Janners MY, Searls RL. Effect of removal of the apical ectodermal ridge on the rate of cell division in the subridge mesenchyme of the embryonic chick wing. Dev Biol. 1971;24:465–476. doi: 10.1016/0012-1606(71)90060-1. [DOI] [PubMed] [Google Scholar]
- 41.Heintzelman KF, Phillips HM, Davis GS. Liquid-tissue behavior and differential cohesiveness during chick limb budding. J Embryol Exp Morphol. 1978;47:1–15. [PubMed] [Google Scholar]
- 42.Damon BJ, Mezentseva NV, Kumaratilake JS, Forgacs G, Newman SA. Limb bud and flank mesoderm have distinct “physical phenotypes” that may contribute to limb budding. Dev Biol. 2008;321:319–330. doi: 10.1016/j.ydbio.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Boehm B, Westerberg H, Lesnicar-Pucko G, Raja S, Rautschka M, Cotterell J, et al. The role of spatially controlled cell proliferation in limb bud morphogenesis. PLoS Biol. 2010;8:1000420. doi: 10.1371/journal.pbio.1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wyngaarden LA, Vogeli KM, Ciruna BG, Wells M, Hadjantonakis AK, Hopyan S. Oriented cell motility and division underlie early limb bud morphogenesis. Development. 2010;137:2551–2558. doi: 10.1242/dev.046987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gros J, Hu JK, Vinegoni C, Feruglio PF, Weissleder R, Tabin CJ. WNT5A/JNK and FGF/MAPK pathways regulate the cellular events shaping the vertebrate limb bud. Curr Biol. 2010;20:1993–2002. doi: 10.1016/j.cub.2010.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Dudley AT. Non-canonical frizzled signaling regulates cell polarity of growth plate chondrocytes. Development. 2009;136:1083–1092. doi: 10.1242/dev.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGlashan SR, Haycraft CJ, Jensen CG, Yoder BK, Poole CA. Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpk mice lacking the primary cilia protein polaris. Matrix Biol. 2007;26:234–246. doi: 10.1016/j.matbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130:1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- 49.Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev. 2003;120:467–476. doi: 10.1016/S0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 50.Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 51.Green JL, Inoue T, Sternberg PW. Opposing Wnt pathways orient cell polarity during organogenesis. Cell. 2008;134:646–656. doi: 10.1016/j.cell.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roegiers F, Younger-Shepherd S, Jan LY, Jan YN. Two types of asymmetric divisions in the Drosophila sensory organ precursor cell lineage. Nat Cell Biol. 2001;3:58–67. doi: 10.1038/35050568. [DOI] [PubMed] [Google Scholar]
- 53.Segalen M, Bellaiche Y. Cell division orientation and planar cell polarity pathways. Semin Cell Dev Biol. 2009;20:972–977. doi: 10.1016/j.semcdb.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 54.Ségalen M, Johnston CA, Martin CA, Dumortier JG, Prehoda KE, David NB, et al. The Fz-Dsh planar cell polarity pathway induces oriented cell division via Mud/NuMA in Drosophila and zebrafish. Dev Cell. 2010;19:740–752. doi: 10.1016/j.devcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson EB. The Cell in Development and Inheritance. Norwood, MA: The Macmillian Company, Norwood Press; 1900. [Google Scholar]
- 56.Toyoshima F, Nishida E. Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent manner. EMBO J. 2007;26:1487–1498. doi: 10.1038/sj.emboj.7601599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McNeill H, Woodgett JR. When pathways collide: collaboration and connivance among signalling proteins in development. Nat Rev Mol Cell Biol. 2010;11:404–413. doi: 10.1038/nrm2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 59.Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009;19:295–305. doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klein TJ, Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- 61.Bayly R, Axelrod JD. Pointing in the right direction: new developments in the field of planar cell polarity. Nat Rev Genet. 2011;12:385–391. doi: 10.1038/nrg2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vladar EK, Antic D, Axelrod JD. Planar cell polarity signaling: the developing cell's compass. Cold Spring Harb Perspect Biol. 2009;1:2964. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, et al. Canonical and non-canonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andersson ER, Bryjova L, Biris K, Yamaguchi TP, Arenas E, Bryja V. Genetic interaction between Lrp6 and Wnt5a during mouse development. Dev Dyn. 2010;239:237–245. doi: 10.1002/dvdy.22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Witte F, Dokas J, Neuendorf F, Mundlos S, Stricker S. Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. Gene Expr Patterns. 2009;9:215–223. doi: 10.1016/j.gep.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Wang X, Sommer RJ. Antagonism of LIN-17/Frizzled and LIN-18/Ryk in Nematode Vulva Induction Reveals Evolutionary Alterations in Core Developmental Pathways. PLoS Biol. 2011;9:1001110. doi: 10.1371/journal.pbio.1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. :2003–2054. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, Yonemura S, et al. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15:23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 70.Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kimmel AR. An orphan nuclear receptor finds a home. Mol Cell. 2010;37:155–157. doi: 10.1016/j.molcel.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y, Rubin B, Bodine PV, Billiard J. Wnt5a induces homodimerization and activation of Ror2 receptor tyrosine kinase. J Cell Biochem. 2008;105:497–502. doi: 10.1002/jcb.21848. [DOI] [PubMed] [Google Scholar]
- 74.Nomachi A, Nishita M, Inaba D, Enomoto M, Hamasaki M, Minami Y. Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. J Biol Chem. 2008;283:27973–27981. doi: 10.1074/jbc.M802325200. [DOI] [PubMed] [Google Scholar]
- 75.Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, et al. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–264. doi: 10.1016/S1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 76.Marlow F, Zwartkruis F, Malicki J, Neuhauss SC, Abbas L, Weaver M, et al. Functional interactions of genes mediating convergent extension, knypek and trilobite, during the partitioning of the eye primordium in zebrafish. Dev Biol. 1998;203:382–399. doi: 10.1006/dbio.1998.9032. [DOI] [PubMed] [Google Scholar]
- 77.Henry CA, Hall LA, Burr Hille M, Solnica-Krezel L, Cooper MS. Somites in zebrafish doubly mutant for knypek and trilobite form without internal mesenchymal cells or compaction. Curr Biol. 2000;10:1063–1066. doi: 10.1016/S0960-9822(00)00677-1. [DOI] [PubMed] [Google Scholar]
- 78.Yin C, Solnica-Krezel L. Convergence and extension movements mediate the specification and fate maintenance of zebrafish slow muscle precursors. Dev Biol. 2007;304:141–155. doi: 10.1016/j.ydbio.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 79.Fico A, Maina F, Dono R. Fine-tuning of cell signaling by glypicans. Cell Mol Life Sci. 2011;68:923–929. doi: 10.1007/s00018-007-7471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohkawara B, Yamamoto TS, Tada M, Ueno N. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2003;130:2129–2138. doi: 10.1242/dev.00435. [DOI] [PubMed] [Google Scholar]
- 81.Ahrens MJ, Li Y, Jiang H, Dudley AT. Convergent extension movements in growth plate chondrocytes require gpi-anchored cell surface proteins. Development. 2009;136:3463–3474. doi: 10.1242/dev.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shao M, Liu ZZ, Wang CD, Li HY, Carron C, Zhang HW, et al. Loss of mRor1 enhances the heart and skeletal abnormalities in mRor2-deficient mice: redundant and pleiotropic functions of mRor1 and mRor2 receptor tyrosine kinases. Mol Cell Biol. 2001;21:8329–8335. doi: 10.1128/MCB.21.24.8329-35.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–18. doi: 10.1016/S0092-8674(00)81226-X. [DOI] [PubMed] [Google Scholar]
- 84.Hopyan S, Sharpe J, Yang Y. Budding behaviors: Growth of the limb as a model of morphogenesis. Dev Dyn. 2011;240:1054–62. doi: 10.1002/dvdy.22601. [DOI] [PubMed] [Google Scholar]
- 85.Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–93. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- 86.Hunziker EB, Schenk RK, Cruz-Orive LM. Quantitation of chondrocyte performance in growthplate cartilage during longitudinal bone growth. J Bone Joint Surg Am. 1987;69:162–73. [PubMed] [Google Scholar]
- 87.Vargesson N, Clarke JD, Vincent K, Coles C, Wolpert L, Tickle C. Cell fate in the chick limb bud and relationship to gene expression. Development. 1997;124:1909–18. doi: 10.1242/dev.124.10.1909. [DOI] [PubMed] [Google Scholar]
- 88.Dudley AT, Ros MA, Tabin CJ. A re-examination of proximodistal patterning during vertebrate limb development. Nature. 2002;418:539–44. doi: 10.1038/nature00945. [DOI] [PubMed] [Google Scholar]
- 89.Pearse RV, 2nd, Scherz PJ, Campbell JK, Tabin CJ. A cellular lineage analysis of the chick limb bud. Dev Biol. 2007;310:388–400. doi: 10.1016/j.ydbio.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nomi M, Oishi I, Kani S, Suzuki H, Matsuda T, Yoda A, et al. Loss of mRor1 enhances the heart and skeletal abnormalities in mRor2-deficient mice: redundant and pleiotropic functions of mRor1 and mRor2 receptor tyrosine kinases. Mol Cell Biol. 2001;21:8329–35. doi: 10.1128/MCB.21.24.8329-35.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22:138–147. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 92.Wolpert L, Tickle C, Sampford M. The effect of cell killing by x-irradiation on pattern formation in the chick limb. J Embryol Exp Morphol. 1979;50:175–193. [PubMed] [Google Scholar]
- 93.Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, et al. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–382. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Caneparo L, Huang YL, Staudt N, Tada M, Ahrendt R, Kazanskaya O, et al. Dickkopf-1 regulates gastrulation movements by coordinated modulation of Wnt/beta catenin and Wnt/PCP activities, through interaction with the Dally-like homolog Knypek. Genes Dev. 2007;21:465–480. doi: 10.1101/gad.406007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 97.Shnitsar I, Borchers A. PTK7 recruits dsh to regulate neural crest migration. Development. 2008;135:4015–4024. doi: 10.1242/dev.023556. [DOI] [PubMed] [Google Scholar]
- 98.Parr BA, Shea MJ, Vassileva G, McMahon AP. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993;119:247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- 99.Shafer B, Onishi K, Lo C, Colakoglu G, Zou Y. Vangl2 promotes Wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance. Dev Cell. 2011;20:177–191. doi: 10.1016/j.devcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blakely BD, Bye CR, Fernando CV, Horne MK, Macheda ML, Stacker SA, et al. Wnt5a regulates midbrain dopaminergic axon growth and guidance. PLoS ONE. 2011;6:18373. doi: 10.1371/journal.pone.0018373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He F, Xiong W, Yu X, Espinoza-Lewis R, Liu C, Gu S, et al. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated non-canonical pathway in mammalian palate development. Development. 2008;135:3871–3879. doi: 10.1242/dev.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 2008;320:365–369. doi: 10.1126/science.1151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suzuki T, Hasso SM, Fallon JF. Unique SMAD1/5/8 activity at the phalanx-forming region determines digit identity. Proc Natl Acad Sci USA. 2008;105:4185–4190. doi: 10.1073/pnas.0707899105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Witte F, Chan D, Economides AN, Mundlos S, Stricker S. Receptor tyrosine kinase-like orphan receptor 2 (ROR2) and Indian hedgehog regulate digit outgrowth mediated by the phalanx-forming region. Proc Natl Acad Sci USA. 2010;107:14211–14216. doi: 10.1073/pnas.1009314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fischer L, Boland G, Tuan RS. Wnt signaling during BMP-2 stimulation of mesenchymal chondrogenesis. J Cell Biochem. 2002;84:816–831. doi: 10.1002/jcb.10091. [DOI] [PubMed] [Google Scholar]