Abstract
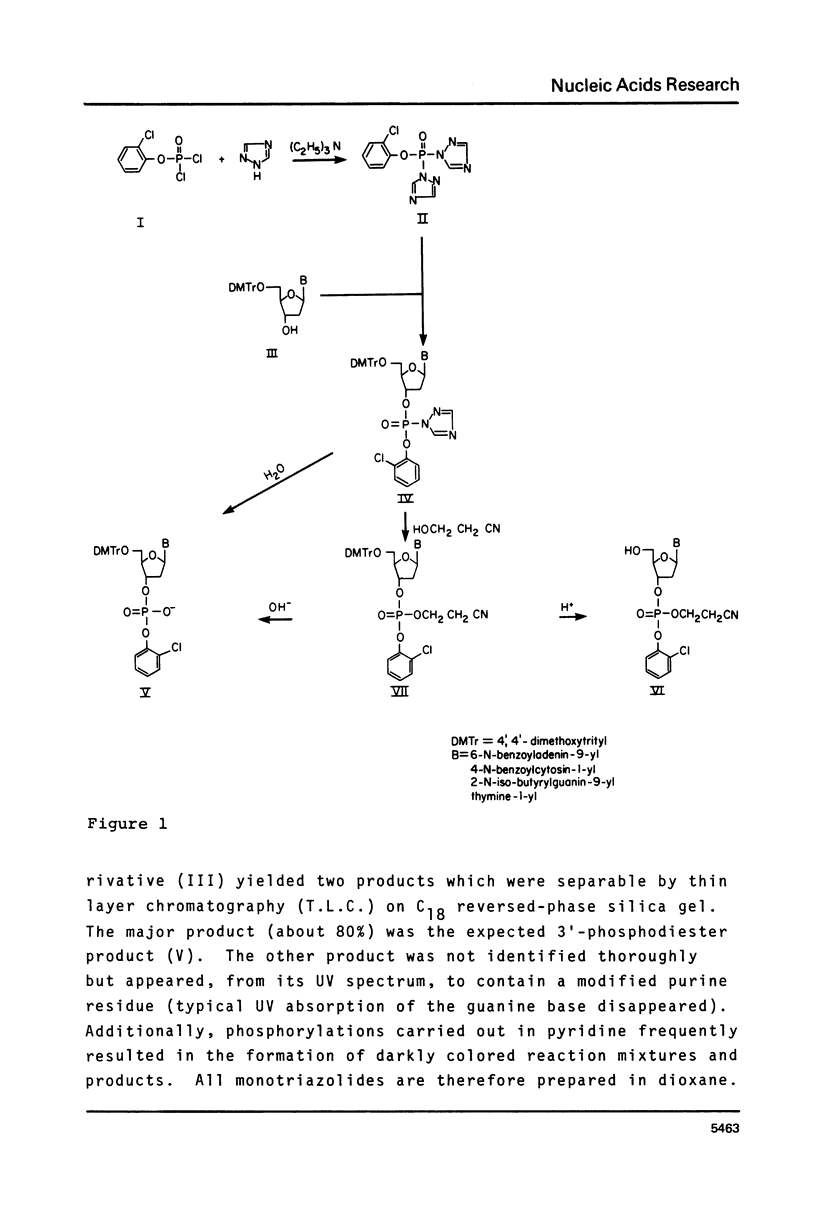
A rapid and convenient procedure has been developed for the synthesis of fully protected mono, di and trideoxyribonucleotides utilizing an aryl phosphoroditriazolide. It affords advantages over coupling strategies employing condensing reagents, such as 2,4,6-triisopropylbenzenesulfonyl tetrazolide in preparing small oligonucleotides and is relatively free of the drawbacks inherent in other approaches using bifunctional phosphorylating reagents. In particular, the synthesis of trinucleotide blocks without purification at the dimer stage is described.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Riftina F. Chemical synthesis of a self-complementary octanucleotide, dG-G-T-T-A-A-C-C by a modified triester method. Nucleic Acids Res. 1978 Aug;5(8):2809–2823. doi: 10.1093/nar/5.8.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin J. C., Cramer F. Deoxy oligonucleotide synthesis via the triester method. J Org Chem. 1973 Jan 26;38(2):245–250. doi: 10.1021/jo00942a011. [DOI] [PubMed] [Google Scholar]
- Gough G. R., Collier K. J., Weith H. L., Gilham P. T. The use of barium salts of protected deoxyribonucleoside-3' p-chlorophenyl phosphates for construction of oligonucleotides by the phosphotriester method: high-yield synthesis of dinucleotide blocks. Nucleic Acids Res. 1979 Dec 11;7(7):1955–1964. doi: 10.1093/nar/7.7.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung H. M., Brousseau R., Michniewicz J., Narang S. A. Synthesis of human insulin gene. Part I. Development of reversed-phase chromatography in the modified triester method. Its application in the rapid and efficient synthesis of eight deoxyribooligonucleotides fragments constituting human insulin A DNA. Nucleic Acids Res. 1979 Apr;6(4):1371–1385. doi: 10.1093/nar/6.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura K., Katagiri N., Bahl C. P., Wightman R. H., Narang S. A. Improved triester approach for the synthesis of pentadecathymidylic acid. J Am Chem Soc. 1975 Dec 10;97(25):7327–7332. doi: 10.1021/ja00858a020. [DOI] [PubMed] [Google Scholar]
- Itakura K., Katagiri N., Bahl C. P., Wightman R. H., Narang S. A. Improved triester approach for the synthesis of pentadecathymidylic acid. J Am Chem Soc. 1975 Dec 10;97(25):7327–7332. doi: 10.1021/ja00858a020. [DOI] [PubMed] [Google Scholar]
- Stawinski J., Hozumi T., Narang S. A., Bahl C. P., Wu R. Arylsulfonyltetrazoles, new coupling reagents and further improvements in the triester method for the synthesis of deoxyribooligonucleotides. Nucleic Acids Res. 1977 Feb;4(2):353–371. doi: 10.1093/nar/4.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boom J. H., Burgers P. M., van der Marel G., Verdegaal C. H., Wille G. Synthesis of oligonucleotides with sequences identical with or analogous to the 3'-end of 16S ribosomal RNA of Escherichia coli: preparation of A-C-C-U-C-C via the modified phosphotriester method. Nucleic Acids Res. 1977 Apr;4(4):1047–1063. doi: 10.1093/nar/4.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]


