Abstract
The chloroplast is a multi-copy cellular organelle that not only performs photosynthesis but also synthesizes amino acids, lipids and phytohormones. The plastid also responds to environmental stimuli such as gravitropism. Biogenesis of chloroplasts is initiated from proplastids in shoot meristems, and involves a series of important events. In the last decade, considerable progress has been made towards understanding various aspects of chloroplast biogenesis at the molecular level, via studies in model systems such as Arabidopsis. This review focuses on two important aspects of chloroplast biogenesis, synthesis/assembly and division/transmission. Chloroplasts originated through endosymbiosis from an ancestor of extant cyanobacteria, and thus contain their own genomes. DNA in chloroplasts is organized into complexes with proteins, and these are called nucleoids. The synthesis of chloroplast proteins is regulated at various steps. However, a majority of proteins are synthesized in the cytosol, and their proper import into chloroplast compartments is a prerequisite for chloroplast development. Fundamental aspects of plastid gene expression/regulation and chloroplast protein transport are described, together with recent proteome analyses of the organelle. Chloroplasts are not de novo synthesized, but instead are propagated from pre-existing plastids. In addition, plastids are transmitted from generation to generation with a unique mode of inheritance. Our current knowledge on the division machinery and the inheritance of plastids is described.
INTRODUCTION
Photosynthesis is undoubtedly the most important process associated with plant life. It converts light energy, captured by pigment-containing light-harvesting antenna, into chemical energy that ultimately sustains plant growth. In plants, photosynthesis occurs exclusively in the chloroplast, the organelle derived through endosymbiosis from a relative of present-day cyanobacteria. It was van Leeuwenhoek in the 1670s who first scientifically described chloroplasts, as green globules in Spirogyra, without completely understanding them (Wise and Hoober, 2006). A chloroplast is defined as a particular type of plastid. These organelles are not synthesized de novo, but are instead propagated from pre-existing plastids via a division process. This division theory was initially hypothesized based on independent cytological analyses performed by Schimper and Meyer in the 1880s. Formation of chloroplasts is initiated from proplastids, an undifferentiated plastid type that is present in the shoot apical meristem (Figure 1A). Responding to light, proplastids develop grana, which are stacks of thylakoid membranes, where the machineries of light harvesting, electron transfer and ATP synthesis are formed. Chloroplasts are not only the site of photosynthesis, but are also responsible for the storage of starch and oil compounds, and for the synthesis of amino acids, lipids and phytohormones. Furthermore, plastids play an active role in environmental sensing, including gravity perception, stomatal opening and closure, and response to pathogen infection.
Figure 1.
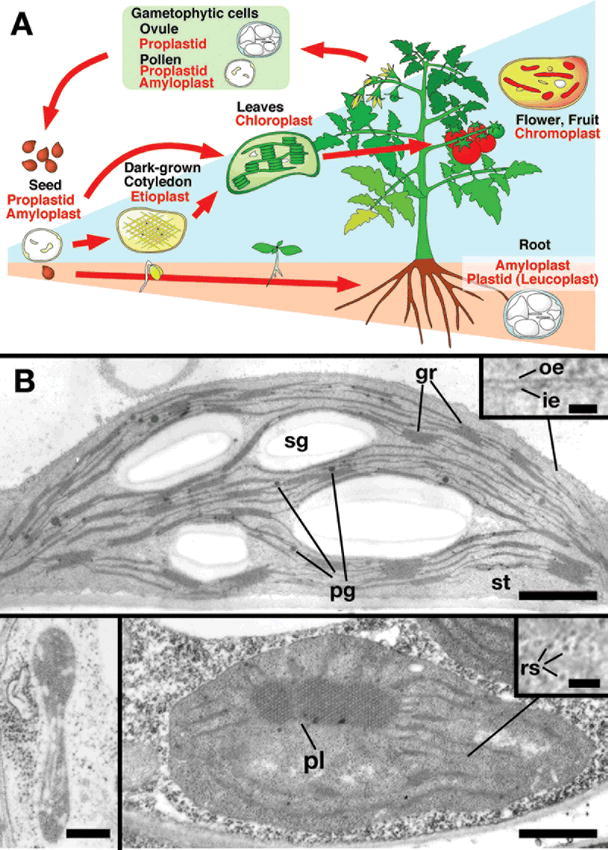
Continuity and differentiation of plastids in plant cells.
(A) Schematic representation of plastid differentiation and of the distribution of several plastid types in different tissues.
(B) Electron micrographs of a chloroplast (upper), a proplastid (lower left) and an etioplast (lower right) in Arabidopsis. gr, grana; ie, inner envelope membrane; oe, outer envelope membrane; pg, plastoglobule; pl, prolamelar body; rs, ribosome; sg, starch granule; st, stroma. Scale bars: upper and lower right panels, 1 μm; lower left panel, 200 nm; inset of upper panel, 50 nm; inset of lower right panel, 100 nm. (B, courtesy of Dr. Chieko Saito in RIKEN).
The first textbook of plastid biology was published in the 1960s by Kirk and Tilney-Bassett (second edition in 1978). It is somewhat surprising that, at that time, very little was stated regarding Arabidopsis (with the exception of the mention of a variegation mutant). Four decades later, however, we have greatly expanded upon the knowledge written in that initial textbook, most notably by utilizing this tiny plant as a model system, since it is highly suitable for modern molecular genetics and systems biology. Here, we focus on several key aspects of plastid biology that are fundamentally important to sustain the organelle's life cycle, including protein import, division, and inheritance. We place emphasis, in principal, on molecular-genetic studies that are primarily related to work in the Arabidopsis model system. We also discuss gene expression in chloroplasts, its regulatory network, and possible signals that are exchanged between the chloroplast and the nucleus. Due to limitations of space, the biogenesis of the photosynthetic apparatus and metabolic pathways are beyond the scope of this review. Plastid biogenesis has also been described in detail in several textbooks and review articles (Kirk and Tilney-Bassett, 1978; Leister, 2003; Daniell and Chase, 2004; Møller, 2005; Wise and Hoober, 2006; Lopez-Juez, 2007).
GENERAL VIEW ON THE ORIGIN, CONTINUITY AND DIFFERENTIATION OF CHLOROPLASTS
Origin and Continuity of Chloroplasts
Mitochondria and chloroplasts are the descendants of serial endosymbiotic events (Cavalier-Smith, 2004). Mitochondria arose first from an α-proteobacterial ancestor that had been engulfed and enslaved by a primitive eukaryotic host. Chloroplasts arose later (around 1-1.5 billion years ago) from a cyanobacterial ancestor engulfed by a eukaryote in which mitochondria had already been established (Cavalier-Smith, 2004; Reyes-Prieto et al., 2007). Most of the bacterial genes were transferred to the nuclear genome or lost, but both modern organelles nevertheless retain metabolic activities, genetic mechanisms, and protein transport complexes that clearly reflect their prokaryotic origins. A single endosymbiosis of a cyanobacterial ancestor gave rise to the chloroplasts of the Glaucophyta (glaucophyte algae), Rhodophyta (red algae) and Viridiplantae (green algae and land plants). In addition to these groups, many other eukaryotic groups, such as stramenopiles (brown algae), euglenids, dinoflagellates and malarian parasites, also have chloroplasts or non-green plastids. The latter groups acquired plastids via secondary endosymbioses of red or green algae, in which their non-photosynthetic eukaryotic progenitors engulfed and enslaved eukaryotic algae (Cavalier-Smith, 2004; Reyes-Prieto et al., 2007).
In unicellular algae, chloroplasts are usually the only type of plastid present. This is consistent with the fact that vegetative cells of cyanobacteria remain blue-green and photosynthetic throughout their life cycle. Thus, from an evolutionary standpoint, the green chloroplast is the origin of the several different types of plastids that are present in land plants. In contrast with unicellular algae, land plants have evolved systems for plastid differentiation, enabling the formation of plastid types specialized for activities other than photosynthesis (Mullet, 1988; Lopez-Juez and Pyke, 2005; Figure 1). In vascular plants, all plastids including chloroplasts are derived from small, non-green proplastids in meristematic cells. Proplastids normally originate maternally during the formation of plant zygotes, and are transmitted from generation to generation (Mullet, 1988; Lopez-Juez and Pyke, 2005; see the section on Inheritance of Plastids).
Structure of Chloroplasts
Among several types of plastid, the best characterized is the chloroplast in vascular plants. The shape and structure of chloroplasts vary depending on the species, tissue and environmental conditions. It should be noted that while some features are common among species and tissues, some other features have evolved in and are specific to vascular plants. In mature leaf cells, chloroplasts are usually lens-shaped, 5–10 μm in diameter and 2–4 μm in thickness (Figure 1). Each leaf cell usually contains 20 to 100 chloroplasts (Mullet, 1988; Lopez-Juez and Pyke, 2005).
Chloroplasts and all other plastid types are surrounded by two membranes, the outer and the inner envelope membranes. In addition to these membranes, chloroplasts have the thylakoids. Thus, chloroplasts have three membrane systems and three aqueous compartments: the intermembrane space (between two envelopes), the stroma (surrounded by the inner envelope), and the thylakoid lumen (surrounded by thylakoid membrane). The inner envelope membrane and thylakoid membrane were descended from the plasma membrane and thylakoid membrane, respectively, of the engulfed cyanobacterium, whereas the origin of the outer envelope membrane is less clear. The presence of galactolipids and carotenoids and the prokaryotic origin of some outer envelope proteins suggest a link with the cyanobacterial outer membrane (Reumann et al., 2005; Inoue, 2007). In contrast, other lipids in the outer envelope membrane suggest a eukaryotic origin (Douce and Joyard, 1990).
The envelope membranes are the sites for lipid biogenesis (Douce and Joyard, 1990; Joyard et al., 1998), the translocation of nucleus-encoded proteins into plastids from the cytosol (Reumann et al., 2005), and the exchange of molecules across the membranes (Weber et al., 2005). Plastids often have tubular extensions of the two envelope membranes called stromules, which interconnect different plastids (Kwok and Hanson, 2004).
The shape of the thylakoids varies depending upon the lineage and tissue. In cyanobacteria and red algal chloroplasts, phycobilisomes uniformly attach to the outside of long thylakoids, and the thylakoids are arrayed at regular intervals. In green algae and plants, which do not have phycobilisomes, the structure of thylakoids is more complex. Thylakoids extend parallel to the chloroplast main axis; some are short, disc-shaped and organized into stacks called grana, while these grana are interconnected by long, stromal thylakoids (Dekker and Boekema, 2005; Figure 1). The thylakoids appear as discrete units under the transmission electron microscope, but actually form an interlinked compartment, enclosing a single lumen in three dimensions. Photosystems (PSI and PSII) exist on the thylakoid membrane. PSII is limited to granal membranes not in contact with the stroma, while PSI exists exclusively in the thylakoids exposed to the stroma (Dekker and Boekema, 2005). Usually, lipoprotein particles called plastoglobules are associated with the thylakoid membranes (Bréhélin et al., 2007; Figure 1).
The stroma corresponds to the cytosol of the original endosymbiont. It contains all the enzymes needed to carry out the carbon reactions of photosynthesis, and therefore contains starch granules. Nucleoids and ribosomes also exist in the stroma. Each chloroplast contains many nucleoids which are attached to the envelope and thylakoid membranes. The number and location of nucleoids changes depending on the type of plastid and species (Sakai et al., 2004).
Other Types of Plastid and Differentiation
The term plastid originated from the organelle's plasticity. The plasticity observed in vascular plants has evolved by the acquisition of mechanisms for the activation or inactivation of particular functions of chloroplasts, according to the requirements of specialized tissues.
In vascular plants, proplastids in meristematic tissues differentiate into several different types of plastid depending on the functions which are needed in particular tissues: yellow etioplasts in dark grown leaves, amyloplasts for starch storage, chromoplasts for pigment synthesis, elaioplasts for storing lipids, and leucoplasts for monoterpene synthesis (Mullet, 1988; Lopez-Juez and Pyke, 2005; Figure 1). The differentiation of plastids is reversible and sequential. Therefore, there are spectra of intermediates and even differences within the same subtype. For example, photosynthetic activities are partitioned between mesophyll chloroplasts and bundle sheath chloroplasts in C4 plants such as maize. Chloroplasts in mesophyll cells have developed granal stacks, while bundle sheath chloroplasts lack grana, are PSII-depleted, and perform most of the reactions of the Calvin cycle (Sheen, 1999).
The best-studied transition is the development of chloroplasts from proplastids (Mullet 1988; Leon et al., 1998). Proplastids in meristematic tissues are colorless, and are usually 0.2–1.0 μm in diameter with very few internal membrane vesicles (Figure 1). These vesicles are the precursors of the thylakoids, and perhaps are derived from parental proplastids or from invagination of the inner envelope (Benning et al., 2006; Aseeva et al., 2007). Each meristematic cell usually contains 10–20 proplastids. During the development and enlargement of mesophyll cells, the number of plastids per cell increases to 50–100, and plastid size also increases greater than 100-fold. The increase in plastid volume results in part from the development of thylakoid membranes and the accumulation of proteins and lipids required for photosynthesis (Mullet 1988; Leon et al., 1998).
Plastid differentiation is largely under nuclear control. The nucleus encodes most of the proteins required for metabolic functions in plastids, and many of those needed for plastid gene expression. Nuclear regulation involves multiple aspects, including the selective targeting of nucleus-encoded plastid proteins, the replication of plastid DNA (ptDNA), and the control of transcription and translation within plastids (Mullet 1988; Leon et al., 1998). In addition to such anterograde control, proper plastid differentiation involves retrograde signaling through which the functional and developmental state of the organelle regulates the expression of nuclear genes (Nott et al., 2006; Woodson and Chory, 2008).
THE PLASTID GENOME
In the 1980s, efforts aimed towards the complete sequencing of plastid genomes were made in several plant species, including liverwort, tobacco and rice (Sugiura, 2003). This scientific venture was notably well ahead of the initiation of Arabidopsis nuclear genome sequencing project. To date, the plastid genome sequences of 122 different species have been determined (Organelle Genome Resources, see Table 1). Comparative analyses have been made between the nuclear and chloroplast genomes of Arabidopsis, and the genomes of cyanobacteria and yeast, and in this way the evolution of the genomes via endosymbiotic events has been elucidated (Martin et al., 2002). Here, we place emphasis on the cytological structure and dynamic behavior of plastid genomes during chloroplast biogenesis. Readers are encouraged to refer to additional reviews which focus more on plastid genome structure and evolution (Sugiura, 1992; Maier and Schmitz-Linneweber, 2004). The plastid genome is often called the ‘plastome’, but we do not use that term and instead refer to either ptDNA or the plastid genome.
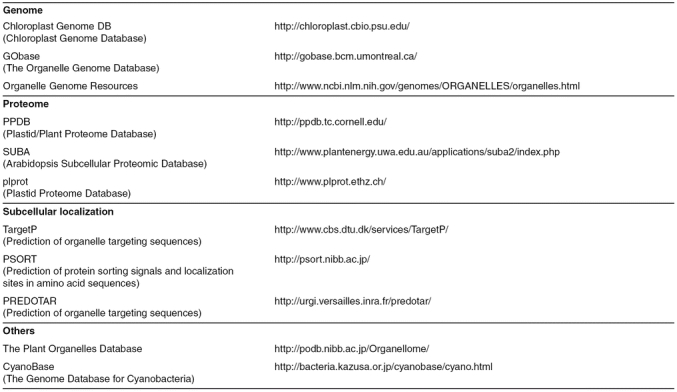
Table 1.
Databases and websites useful for plastid research
Basic Structure
In Arabidopsis, the complete sequence of the ptDNA was reported in 1999 by a group at the Kazusa Institute (Sato et al., 1999). The Arabidopsis plastid genome consists of a circular DNA of 154 kb in length. It retains a highly conserved genome structure consisting of a pair of inverted repeats (26 kb) that split the circular genome into two parts, termed the small (18 kb) and large (84 kb) single copy regions. The Arabidopsis plastid genome contains 45 RNA-coding genes and 87 protein-coding genes. The functional ptDNA gene products are principally involved in: transcription (RNA polymerase), translation (ribosomal and transfer RNAs, ribosomal proteins), photosynthetic electron transfer (subunits of PSI, PSII, the cytochrome b6/f complex and NAD(P)H dehydrogenase [NDH]), and photosynthetic metabolism (subunits of ATP synthase and RubisCO). Exceptions to these aforementioned examples are two photosynthesis-unrelated housekeeping genes, accD and clpP1, which encode subunits of acetyl CoA carboxylase and the Clp (Caseinolytic protease) protease, respectively.
Gene arrangements within the ptDNA are also well conserved between the different species (Sugiura, 1992; Maier and Schmitz-Linneweber, 2004). Since plant chloroplasts are descendent from an ancestral cyanobacterium, many genes have retained prokaryotic features and are organized as operons and are co-transcribed. Processing events of polycistronic transcripts is sometimes complex and leads to an accumulation of various RNA molecules. One of the best characterized examples is the psbB operon, in which five genes are co-transcribed and give rise to many poly- and mono- cistronic RNA molecules (Barkan, 1988; Westhoff and Herrmann, 1988). Genes for rRNAs are also encoded by operons. However, despite the extensive characterization of plastid genomes and their expression, very little is known regarding replication. Several investigations demonstrated that the replication of ptDNA involves an enzyme similar to bacterial DNA polymerase I (Pol-I). In Arabidopsis, two putative Pol-I genes have been identified whose gene products are targeted to chloroplasts (Mori et al., 2005). DNA Pol-I has also been studied in other species, such as rice and tobacco (Kimura et al., 2002; Ono et al., 2007). Collectively, these data suggest that the replication system is shared between chloroplasts and mitochondria, through the dual-targeting of the relevant proteins.
How Plastid DNA Exists in Plastids – Plastid Nucleoids
A single cell contains a variable number of plastids, in which ptD-NAs exist in multiple copies in the stroma. For example, a mesophyll cell in Arabidopsis contains approximately 20–200 chloroplasts, and anywhere from 10 to 500 copies of ptDNA per chloroplast (Fujie et al., 1994; Pyke and Leech, 1994). As a result, the copy number of ptDNAs per cell is highly variable. Nonetheless, total DNA isolated from green leaf tissues may contain approximately 20% of ptDNA.
Several questions arise concerning the spatial organization of the multiple plastid genome copies relative to the intraorganellar compartments. Another question is whether or not ptDNAs are capable of forming a complex with proteins similar to bacterial chromosomes. It is unlikely that homologues of a bacterial DNA-binding protein, HU, are encoded in higher plant genomes (Sato, 2001). On the other hand, through the staining of glutaraldehyde-fixed tissues with 4′, 6-diamidino-2-phenylindole (DAPI), we have detected granulous structures (nucleoids) in plastids (Sato et al., 2003; Sakai et al., 2004) (Figure 2). We have observed these plastid nucleoids as densely-stained small dots in mesophyll chloroplasts, suggesting that ptDNAs are packed with proteins. Such cytological observations demonstrated that nucleoid numbers and morphology change in accordance with chloroplast differentiation in many species. In Arabidopsis, nucleoids have been examined by combining DAPI stain and Technovit thin sections (Fujie et al., 1994). Within the shoot meristem where most cells contain proplastids, nucleoids are observed as an aggregated signal, or a few signals, at the center of the organelle. As the proplastid becomes larger and develops into a mature chloroplast, the nucleoids tend to increase in number, become smaller in size, and are localized along the inner surface of the envelope. In mesophyll cells of mature leaves, the chloroplast is much larger in size and contains a well-developed granal network (Figure 2). At this stage, nucleoids are very dense and are dispersed in the stroma, or are sometimes observed in proximity to the exterior of thylakoid membranes. Thus, morphological alterations of plastid nucleoids correlate with chloroplast development; however, the physiological role of these changes in nucleoid structure requires future investigation.
Figure 2.
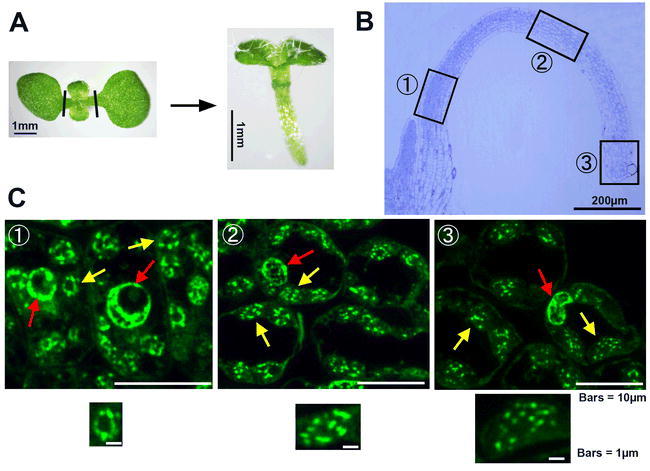
Morphological change of plastid nucleoids in the first true leaves of Arabidopsis.
(A) Plant material used for preparing thin sections. An eight-day-old Arabidopsis seedling (left; a top view is shown) was selected and the two cotyledons were removed (as indicated by black lines). One of the first true leaves of this same plant (right; a side view is shown) was used for the detection of plastid nucleoids.
(B) A thin cross-section of the selected true leaf petiole and lamina, stained by Toluidine blue. The positions indicated by the arrows (1 to 3) were further examined by SYBR-green I staining (as shown in C).
(C) Examination of thin sections by SYRB green I. Signals corresponding to nuclear and plastid DNAs are shown by red and yellow arrows, respectively. Close-up views of the plastids in the respective areas (1 to 3) are shown below each panel.
Biochemical purification of nucleoid proteins has been attempted by several groups, resulting in the successful isolation of such proteins (Murakami et al., 2000; Jeong et al., 2003; Sato et al., 2003). Sulfite reductase (SiR) was somewhat surprisingly shown to be a major component of nucleoids (Sato et al., 2001; Chi-Ham et al., 2002). SiR is an abundant protein in the chloroplasts of land plants, and it apparently functions to induce the reversible compaction of nucleoids. Based on this property, SiR was suggested to negatively affect both transcription and replication. Another novel nucleoid protein is PEND (plastid envelope DNA-binding protein), which was originally isolated from pea (Sato et al., 1998). PEND contains an N-terminal DNA-binding motif and a C-terminal transmembrane domain, and was suggested to reside within the inner envelope membrane. Arabidopsis also contains a PEND homologue. Terasawa and Sato (2005) reported that the N-terminal DNA-binding domain of PEND fused to GFP was co-localized with DAPI signals in Arabidopsis. Using this technology, they were able to observe and characterize the dynamic behavior of nucleoids during chloroplast development.
Is The Amount of Plastid DNA Variable During Chloroplast Differentiation?
Dynamic changes in the morphology of nucleoids implies that DNA contents in each plastid or chloroplast may differ. Quantification of plastid DAPI signals, estimated by using a video-intensified photon-counting microscope, confirmed that each nucleoid contains ~10 copies of ptDNA (Sato et al., 2003; Sakai et al., 2004). These observations also determined that the overall DNA level per plastid increases 5-fold during chloroplast development (Kuroiwa, 1991; Fujie et al., 1994). Furthermore, within mature leaves containing fully-expanded mesophyll cells, there are occasionally few or no detectable DAPI signals (Oldenburg and Bendich, 2004; Rowan et al., 2004). Based on these observations, it is suggested that the amount of ptDNA per organelle decreases within mature, old and/or senescing chloroplasts. While the fluctuations in ptDNA levels would be expected to occur to an extent that is detectable via multiple experimental methods, the reduction in old leaves seems to reveal contradictory results. Using fluorescence microscopy, Rowan et al. (2004) showed that ptDNA levels per chloroplast vary significantly, but decrease in older Arabidopsis leaves. Conversely, Li et al. (2006) reported that this reduction was never observed when a DNA hybridization method was employed. It appears that the contradiction is due to technical limitations for the quantitative detection of DNA. Although DNA blot analysis may be a reliable method, it is not sensitive to subtle changes. Whereas DAPI stain is a very sensitive, qualitative method for visualizing DNA, it may not result in an accurate estimation of DNA quantity; in particular, a lack of signal should not be taken to indicate that there is no DNA. Hopefully an accurate, novel method that can reliably measure ptDNA content in each chloroplast can be developed in the future. In contrast to leaves, our current studies indicate that ptDNA levels drastically decline during pollen maturation.
PLASTID GENE EXPRESSION AND REGULATION MECHANISMS
Historically, gene expression in chloroplasts has been extensively studied using in vitro systems. These studies have enabled scientists to identify the basic machineries involved in transcription, RNA processing and maturation, and translation (Daniell and Chase, 2004; Cahoon et al., 2006). Such biochemical analyses, which have been conducted since the 1980s, have primarily utilized spinach as a model system. Meanwhile, molecular-genetic analyses in the unicellular alga, Chlamydomonas, and transposon mutagenesis in maize have pioneered methods for deciphering the numerous regulatory factors involved, which mostly act post-transcriptionally and in a gene-specific manner (Barkan and Goldschmidt-Clermont, 2000; Rochaix, 2006). In addition, recent systematic forward- and reverse-genetic approaches in Arabidopsis have enabled us to draw a blueprint of gene expression networks in chloroplasts (Leister, 2003; Leister and Pesaresi, 2005). Here, we briefly overview plastid gene expression, placing particular emphasis on transcriptional and post-transcriptional regulation.
Transcriptional Regulation
RNA Polymerases.
Two types of RNA polymerase are known to promote transcription in chloroplasts (Kanamaru and Tanaka, 2004; Shiina et al., 2005; Cahoon et al., 2006) (Figure 3). One is a bacterial type polymerase, as might be expected considering the cyanobacterial origin of chloroplasts, and is indeed encoded by the plastid genome; this polymerase is termed PEP (plastid-encoded polymerase). PEP is a holoenzyme consisting of four subunits (α, β, β′1 and β′2) encoded by the plastidic genes, rpoA, rpoB, rpoC1 and rpoC2, respectively. Eubacterial polymerases such as PEP require an additional factor (‘sigma’) which facilitates promoter recognition (see below). To date, no sigma-like factors have been shown to be encoded in chloroplast genomes. Instead, a nuclear gene coding for a sigma factor was reported (Isono et al., 1997), and was later shown to represent a small gene family in higher plants (Tanaka et al., 1997). In Arabidopsis, six sigma factors (SIG1 to SIG6) are localized in chloroplasts (Shiina et al., 2005; Cahoon et al., 2006).
Figure 3.
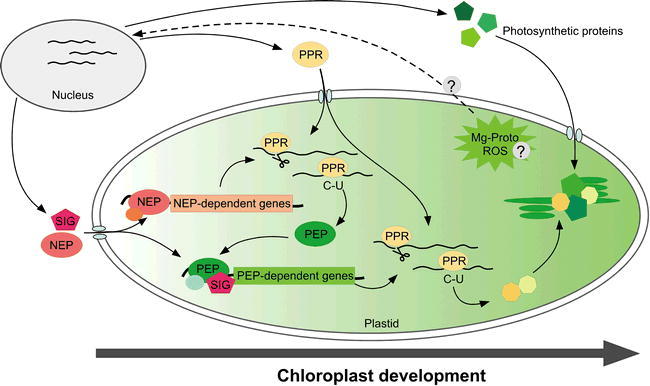
A regulatory network of nuclear and chloroplast gene expression.
This schematic view represents chloroplast gene expression and the assembly of photosynthetic proteins. The process is governed by the coordinated transcription mediated by the NEP and PEP polymerases, and by the post-transcriptional regulatory steps mediated by PPR proteins. A time-course of chloroplast development is illustrated spatially, from left to right. The flow of gene products (NEP, SIG, PPR, and photosynthetic proteins) is indicated by arrows. At an initial stage, NEP and SIG are synthesized and imported into proplastids. These molecules drive the subsequent expression of NEP-dependent genes, including PEP, and lead to the ‘switching-on’ of chloroplast transcription. Numerous PPR proteins are concomitantly imported from the cytosol, and play roles in RNA processing, editing and translation. The products of photosynthetic genes in the chloroplast genome are finally assembled into complexes with other subunits encoded by the nuclear genome, the latter components having been synthesized in the cytosol and imported. To enable coordinated regulation between the nuclear and chloroplast genomes, Mg-protoporphyrin-IX (Mg-proto) and ROS act as possible retrograde signals (indicated by the dotted line); the precise nature of these retrograde signaling pathways is not clear at the present time.
The second polymerase is a bacteriophage-type polymerase, consisting of a single subunit and bearing similarity to a mitochondrial polymerase (Hedtke et al., 1997). This polymerase is encoded in the nuclear genome, and hence is termed NEP (nucleus-encoded polymerase). The presence of NEP activity in chloroplasts has been suggested for a long time. This hypothesis was substantiated by using transgenic tobacco plants in which one of the PEP subunits was inactivated by chloroplast transformation (Allison et al., 1996); the mutant plants clearly accumulated chloroplast transcripts that were attributable to NEP activity (Hajdukiewicz et al., 1997). In Arabidopsis, three genes encoding NEP have been identified: RpoT3 (or RpoTp) for plastids, RpoT1 (RpoTm) for mitochondria, and RpoT2 (RpoTmp) for both organelles. As a consequence, the transcription of ptDNA is driven by three different polymerases. Presently, any differences between the roles of RpoT2 and RpoT3 remain unclear.
By analogy with eubacterial σ70 promoters, PEP in principle recognizes a conserved promoter motif consisting of −10 and −35 sequence elements. In contrast, NEP recognizes an AT-rich promoter sequence similar to mitochondrial consensus promoters (Shiina et al., 2005; Cahoon et al., 2006). In vitro transcription studies in species other than Arabidopsis have identified additional important promoter cis-elements. Various experimental approaches, such as knockout and microarray analyses, have contributed towards elucidating the distinct physiological functions of PEP and NEP (Figure 3). A generally accepted concept for the differential roles of the polymerases during chloroplast development is as follows. At an early stage, NEP is highly expressed. In turn, this induction of NEP initiates transcription of a set of genes encoding PEP subunits (rpo genes), ribosomal RNAs (e.g. rrn16), ribosomal proteins (e.g. rps12), and other ‘house-keeping’ proteins (e.g. clpP1). Machineries for protein synthesis and degradation can be constructed at this stage. As the chloroplast develops, PEP is now activated and becomes the polymerase exhibiting the highest activity. Thus, PEP in combination with various SIG factors acts to drive the expression of genes involved in the formation of the photosynthetic machineries, and the concomitant formation of thylakoid membranes during the process of greening (Figure 3). Interestingly, there is no evidence that NEP primes transcription in chloroplasts of red and green algae. Thus, the implication that the evolutionary acquisition of NEP may be associated with light-dependent chloroplast biogenesis is not fully supported at this time.
Sigma Factors.
Once PEP is employed, one can ask how the transcriptional regulation is fine-tuned. One important regulatory mechanism is accomplished by the activities of multiple sigma factors, whose differential roles have been implicated in several species. Since the functions of the six Arabidopsis SIG proteins have been extensively studied using T-DNA insertion and RNAi mutant lines, the functional roles of different SIGs are best characterized in Arabidopsis (Kanamaru and Tanaka, 2004; Shiina et al., 2005). To date, no sig1 homozygous null mutant has been reported, whereas knockout lines have been isolated for all other SIGs. Therefore, it is likely that SIG1 may have an essential function that cannot be compensated for by other SIGs during chloroplast development. Pale green leaf and cotyledon phenotypes were observed in sig2 and sig6 mutants, respectively (Shirano et al., 2000; Ishizaki et al., 2005). Thus, SIG2 and SIG6 are proposed to play important roles in an early step of chloroplast development. While different SIGs act redundantly in the transcription of the PEP-dependent genes, the induction of particular genes by specific SIGs has been demonstrated. For example, it appears that SIG2 acts on several tRNA genes, and thus its mutant affects global protein synthesis (Kanamaru et al., 2001). Moreover, transfer RNAGLU is a precursor of tetrapyrrole, and so the expression of SIG2 indirectly influences tetrapyrrole biosynthesis. SIG5 activates psbA and the novel blue-light-responsive promoter of psbD (encoding the D2 reaction center protein of PSII) (Tsunoyama et al., 2004). Similarly, SIG3 and SIG4 are suggested to activate psbN and ndhF (Favory et al., 2005; Zghidi et al., 2007). Thus, two mutually interacting events – successive initiation of transcription by NEP and then PEP, and the activation of a subset of genes by specific SIGs – seem to orchestrate plastid development at the level of transcription.
Post-transcriptional Regulation
Important roles of post-transcriptional control in chloroplasts have been implicated since the 1980s, when in vivo analyses by run-on transcription and protein pulse labeling revealed that the rate of protein synthesis does not always parallel that of transcription (Gruissem et al., 1988). Since chloroplast genes are transcribed as operons, the generation of various mono- and poly-cistronic RNA molecules was presumed to be regulated by complex mechanisms, likely at the level of RNA processing. The importance of post-transcriptional regulation has been reinforced by pioneering genetic works in model organisms such as Chlamydomonas and maize, in which many post-transcriptional factors were identified in a gene-specific manner (Barkan and Gold-schmidt-Clermont, 2000; Rochaix, 2006). Those works were accomplished through the characterization of non-photosynthetic mutants, and, for example, maize mutants showing high chlorophyll fluorescence (hcf). In Arabidopsis, the systematic characterization of hcf and other chlorophyll fluorescence mutants in several laboratories has enabled the identification of some novel factors acting on chloroplast gene expression at the post-transcriptional level (Meurer et al., 1996; Shikanai et al., 1999).
The isolation of photosynthetic mutants by chlorophyll fluorescence is based on the fact that the emitted fluorescence reflects the pigment's photochemical status. If photosynthetic electron flow occurs normally, then the excitation energy of chlorophyll molecules after illumination is used to oxidize water and to drive the passage of electrons through the photosystems and ultimately to NADP. Any mutation that blocks proper electron transfer causes over-reduction of the plastoquinone pool. As a result, the excess excitation energy in chlorophyll cannot be used for photosynthesis and is instead emitted as fluorescence. Thus, tracing the quenching of chlorophyll fluorescence allows a high-throughput screening approach for identifying mutants of interest. A collection of such mutants, showing various fluorescence patterns including hcf, was therefore a good resource for the identification of nucleus-encoded factors that mediate post-transcriptional control in a gene-specific manner (Figure 3). In fact, most of the nuclear factors that were identified as regulators of one or a few chloroplast genes turned out to play roles in RNA processing (including RNA splicing, endo-processing and editing) or translation. Due to limitations of space, we can only refer to the few examples listed below.
The psbB operon is one of the most complex operons conserved in the chloroplast genomes of many higher plants, since it co-transcribes five genes (psbB-psbT-psbH-petB-petD) (Barkan, 1988; Westhoff and Herrmann, 1988). Once transcribed, at least three steps – RNA splicing (in the cases of petB and petD), processing (to generate the psbB-psbT, psbH, petB and petD RNA species), and editing (in the case of petB) – are required to generate transcripts that are competent for translation. Forward-genetic analyses have identified three factors involved in the processing of this transcript: HCF107 acts on the cleavage between psbT and psbH (Sane et al., 2005), while HCF152 acts between psbH and petB (Meierhoff et al., 2003) and CRP1 (chloroplast RNA processing1) acts between petB and petD (Fisk et al., 1999). Molecular cloning of the corresponding loci revealed that HCF152 and CRP1 encode proteins that belong to a penta-tricopeptide repeat (PPR) protein family (see below and Figure 3). In a separate study, Shikanai's group characterized a category of mutants that exhibit defects in the NDH complex (Yamazaki et al., 2004). Among these, CRR4 (chlororespiratory reduction4) has been shown to encode a PPR protein that plays a role in gene-specific RNA editing (Kotera et al., 2005). RNA editing is a mechanism by which a specific cytidine residue in a primary transcript is edited to uridine. In Arabidopsis chloroplasts, 19 editing sites are known, and CRR4 is involved in one of them, generating an initiation codon (ACG to AUG) in the ndhD gene. In addition, some PPRs affect translation rather than RNA processing. Together, these observations (particularly the discovery of PPR proteins) provide a great body of functional evidence that highlights the importance of post-transcriptional regulation in plastids, as was implicated in earlier studies.
The presence of the PPR family was first implicated via a bioinformatics approach (Lurin et al., 2004; Saha et al., 2007). The PPR domain is characterized by a signature PPR motif that consists of a degenerate 35 residue sequence. Depending on the protein, this motif exists in tandem repeats ranging in number from 2 to 27. Genes encoding PPRs are predominantly found in plant genomes, although a few PPR proteins have been detected in Drosophila and C. elegans. In fact, bioinformatic analysis revealed that 450 and 655 PPR proteins are present in Arabidopsis and rice, respectively. Most of these seem to have N-terminal targeting signals that are predicted to target them to chloroplasts or mitochondria. PPR repeat motifs are predicted to form a structure that serves as a binding site for a single-stranded RNA molecule. In rare cases, PPR domains are suggested to interact with DNA. The PPR family is therefore considered to control organellar gene expression in mitochondria as well as chloroplasts. The abundance of PPRs in plants raises the intriguing question of how this regulatory system has been acquired in the nuclear genome.
Besides PPR proteins and other gene-specific factors, general components in RNA processing and translation were also identified by forward- and reverse-genetic approaches. In chloroplast genomes, ribosomal RNA genes are clustered as an operon and are co-transcribed. After transcription, their respective gene messages are processed. Two types of exonucleases —polynucleotide phosphorylase (PNPase), and RNase R homologues (RNR)—were shown to participate in this process (Kishine et al., 2004). PNPase seems to simultaneously act on 3′-end maturation of certain mRNAs and tRNA turnover (Walter et al., 2002; Bollenbach et al., 2005). Nuclear factors involved in the splicing of group II introns have been identified (Barkan, 2004; Asakura and Barkan, 2006). Genes encoding ribosomal proteins are found in both the nuclear and plastid genomes. A viable mutant lacking one of the nucleus-encoded plastidic ribosomal proteins has been reported (Pesaresi et al., 2006a). In addition, general factors for translation (sharing similarity with prokaryotic factors) have been identified in Arabidopsis chloroplasts, including: translation initiation factor 2 (cpIF2) (Miura et al., 2007), elongation factor G (cpEF-G) (Albrecht et al., 2006), and peptide release factors (cpRF) 1 and 2 (Meurer et al., 2002; Motohashi et al., 2007). Complete loss of such factors results in an embryolethal or albino phenotype, but several mutant lines with leaky mutations have been reported. Chloroplast genomes in higher plants seems to contain all the tRNAs necessary for translating chloroplast mRNAs. In contrast, certain tRNAs are missing in mitochondria, and thus must be imported from the cytosol. All of the enzymes required for the aminoacylation of each tRNA are imported from the cytosol. Interestingly, most of the aminoacyl synthases seem to be shared between chloroplasts and mitochondria (Duchêne et al., 2005).
Retrograde Signaling
Because of the partitioning of genetic information, chloroplast development and functions necessarily require input from two different genomes. For example, the multiprotein complexes of photosynthesis are mixtures of nucleus- and chloroplast-encoded subunits; to ensure their proper, stoichiometric assembly, and enable their reorganization in response to developmental or environmental cues, the activities of the nuclear and chloroplast genomes must be coordinated through intracellular signaling.
The pre-eminence of the nucleus in this inter-organellar exchange is beyond doubt. The import of nucleus-encoded proteins itself constitutes a massive flow of information (Jarvis, 2008). Moreover, as discussed earlier, numerous nucleus-encoded regulators mediate stringent, predominantly post-transcriptional control of the expression of chloroplast genes (Rochaix, 2006). Nevertheless, it is also clear that signals emitted by chloroplasts (so-called “retrograde” signals; Figure 3) have profound effects on events in the nucleus (Nott et al., 2006; Pesaresi et al., 2007; Woodson and Chory, 2008). Redox balance within the photosynthetic electron transport (PET) chains, the accumulation of reactive oxygen species (ROS), and the perturbation of plastid gene expression or chlorophyll biosynthesis, all influence nuclear gene expression.
Tetrapyrrole Signaling and gun Mutants.
Arabidopsis mutants with defects in retrograde signaling were identified in a reporter-based, forward-genetic screen (Susek et al., 1993). The screening strategy hinged on observations that the transcription of nuclear genes for chloroplast proteins is strongly repressed if chloroplast development is blocked through photooxidative damage (Oelmuller, 1989). The herbicide norflurazon inhibits the formation of photoprotective carotenoids, leading to the photodestruction of the chloroplast interior whilst leaving the rest of the cell intact. Under these circumstances, genes such as those encoding light-harvesting chlorophyll a/b-binding proteins (Lhcb) are strongly repressed. Fusion of an Lhcb promoter to a selectable-marker gene enabled the identification of mutants no longer able to repress Lhcb expression upon norflurazon treatment.
Five independent genomes uncoupled (gun) mutants were identified, four of which (gun2-gun5) interfere with the chlorophyll biosynthetic pathway (Mochizuki et al., 2001; Larkin et al., 2003). Analyses of these mutants culminated in the identification of the tetrapyrrole intermediate, Mg-protoporphyrin-IX, as a key instigator of one particularly important retrograde signaling pathway (Strand et al., 2003). Wild-type plants accumulate Mg-protoporphyrin-IX following norflurazon treatment, triggering a signaling response, whereas the gun2-gun5 mutants are unable to build up sufficient quantities of the intermediate.
The effect of gun1 is somewhat different from that of the other gun mutations (Vinti et al., 2000; Mochizuki et al., 2001). The GUN1 protein is a chloroplast-localized PPR domain protein that binds to DNA and localizes at sites of active transcription, but its exact function remains uncertain (Koussevitzky et al., 2007). While the gun2-gun5 mutations block the Mg-protoporphyrin-IX signaling pathway specifically, the effect of gun1 extends to the plastid gene expression-dependent and redox-related pathways as well. This suggests that GUN1 acts downstream in all of these pathways, and that its role is to integrate information from multiple sources.
An ACGT motif was found to be substantially overrepre-sented in the promoters of retrograde-regulated genes (Koussevitzky et al., 2007). This motif forms the core of the abscisic acid (ABA) response element, as well as of the light-responsive G-box, suggesting possible convergence of retrograde and ABA signaling pathways. When this possibility was investigated, the ABA-insensitive 4 (abi4) mutant was found to be phenotypically similar to gun1, with defects in all tested retrograde pathways. The ABI4 protein is an AP2-type nuclear transcriptional regulator.
A G-box element in the Lhcb promoter, termed CUF1, was previously shown to mediate responses to plastid signals, as well as light induction (Strand et al., 2003). Two partially-overlapping binding motifs exist in CUF1, suggesting a model in which ABI4 and a light-responsive G-box-binding factor (GBF) compete for access to the promoter (Koussevitzky et al., 2007). This would explain how negative retrograde signals from plastids (mediated by ABI4) are able to override positive light-induced signals (mediated by GBF). However, not all retrograde-regulated promoters contain both motifs, implying that this may not be a universal mechanism.
A more complex picture of retrograde signaling emerges upon consideration of transcriptome responses to genetic lesions or environmental factors that impinge on chloroplast function. While some treatments or conditions trigger the en masse up- or down-regulation of nuclear genes for chloroplast proteins (suggesting the existence of a “master switch” that perhaps corresponds to ABI4), many others cause more complex patterns of gene expression involving the simultaneous up- and down-regulation of different sets of genes (Biehl et al., 2005). One example is provided by the ppi1 mutant (see section on Protein Transport Systems), which lacks the chloroplast protein import receptor, atToc33 (Figure 5); this mutation triggers the down-regulation of nuclear photosynthetic genes specifically, suggesting that retrograde signaling mechanisms exist to prevent the futile expression of proteins not able to reach their final destination (Kubis et al., 2003).
Figure 5.
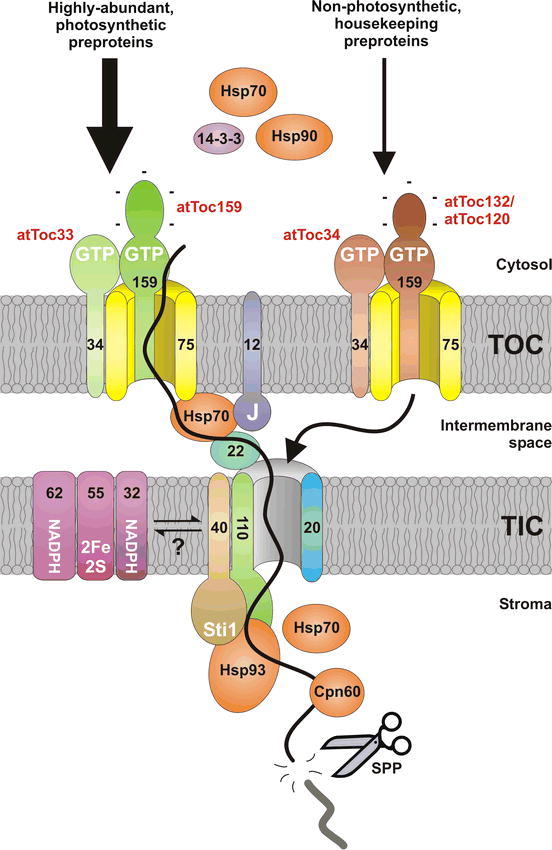
The TOC/TIC protein import machinery.
Diagram showing the main components implicated in the import of proteins into chloroplasts. Outer envelope membrane components form the TOC complex, while inner envelope membrane components form the TIC complex. Components are identified by their predicted molecular weights (black text), and some key functional domains are indicated (white text). The TOC core-complex is formed by Toc159, Toc34 and Toc75. The former two proteins are receptors that together control preprotein recognition, while Toc75 forms the translocation channel. Different isoforms of the receptors exist in Arabidopsis (red text), and these associate preferentially to form distinct TOC complexes with substrate specificity. This may prevent the bulk flow of abundant precursors from out-competing the import of relatively scarce preproteins during the (potentially rate-limiting) early stages of import; once this potential bottleneck has been passed, the import pathways may converge at a common TIC machinery. Cytosolic 14-3-3, Hsp70 and Hsp90 proteins may form ‘guidance complexes’ that direct preproteins to the TOC apparatus. It has been suggested that Toc12, Hsp70 and Tic22 act to facilitate the passage of preproteins across the intermembrane space. The inner membrane translocation channel may be formed by Tic110 and/or Tic20. The former protein is also thought to coordinate late events in import by recruiting stromal chaperones to import sites; Tic110 has been proposed to collaborate with Tic40 and Hsp93 in a putative stromal import motor complex. Upon arrival in the interior, the transit peptide is cleaved by SPP, and other chaperones (Cpn60 or Hsp70) may assist in the folding or onward transport of the mature domain. Finally, the Tic62, Tic55 and Tic32 components may enable the regulation of import in response to redox signals; these components might only be recruited to import sites under certain conditions or for certain preproteins.
Plastid Gene Expression and Redox Signaling.
Inhibition of plastid gene expression, either through the use of genetic mutations or the treatment of plants with inhibitors (e.g. lincomycin), also triggers the repression of nuclear genes for chloroplast proteins (Gray et al., 2003; Pesaresi et al., 2006b). This response is light-independent, and is especially (but not exclusively) important in young seedlings. While plastid gene expression signals are thought to be distinct from tetrapyrrole signals, the absence of a normal response in the gun1 mutant implies that this pathway eventually converges with that triggered by Mg-protoporphyrin-IX. The identity of signaling intermediates (other than GUN1) in the plastid gene expression pathway remain elusive.
Redox poise within the photosynthetic machinery and ROS accumulation both impact on the expression of nuclear genes. The redox state of the plastoquinone pool (which links PSII with the cytochrome b6/f complex) is thought to be a key determinant of PET-mediated retrograde signaling, but other positions along the PET chain are also responsive (Escoubas et al., 1995; Shao et al., 2006). During excessive stimulation of the photosynthetic machinery, reduced forms of PET components predominate, and ROS formation occurs. These ROS include singlet oxygen, hydrogen peroxide and superoxide, which seem to trigger different response pathways (Karpinski et al., 1999; Laloi et al., 2007). Retrograde signaling via the various redox- and ROS-dependent pathways (e.g. under strong light) causes the down-regulation of photosynthetic genes and the up-regulation of anti-oxidative defense genes in the nucleus. Signaling components in these pathways have been identified (Lee et al., 2007).
Plastid Proteases
Chloroplast proteins are either synthesized within chloroplasts or transported from the cytosol. Once delivered and/or assembled in a proper sub-compartment, they must be maintained by ‘protein quality control’, in which proteases play an essential role. Thus, regulated proteolysis can be regarded as fine-tuning at the last step of gene expression. In addition, chloroplastic proteins become sensitive to the inevitable photooxidative damage, which is often caused by excess light energy. For example, the D1 protein of the PSII reaction center is considered to be a main target of photodamage (Aro et al., 1993). As a consequence, D1 is turned over very rapidly by the repair cycle, in which some proteases (see below) were shown to play very important roles (Nixon et al., 2005). Also, some proteases processively degrade partially assembled and mis-folded proteins (Adam et al., 2006; Sakamoto, 2006). Furthermore, pre-proteins from the cytosol (containing N-terminal transit peptides) or precursor proteins synthesized in stroma (e.g. D1 and cytochrome f) undergo maturation by endoproteolytic processing enzymes. Proteases involved in these processing and maturation events have been identified, including stromal processing peptidase (SPP), thylakoid processing peptidase (TPP), transit peptide-degrading zinc-metalloprotease (termed Zn-MP or PreP), the carboxyterminal protease of D1 (CtpA), and the type I signal peptidase (SPaseI for Toc75 maturation).
As exemplified by the ubiquitin-dependent degradation pathway through the 26S proteasome in the cytosol, proteolysis is generally accepted to be an important regulatory pathway. While chloroplasts do not have this pathway, studies in the last decade revealed that chloroplasts instead contain many prokaryotic-type proteases, some of which are ATP-dependent and evolutionarily related to the 26S proteasome. The best studied are Clp (Caseinolytic protease) and FtsH (Filamentous temperature sensitive H) (Adam et al., 2006; Sakamoto, 2006). Clp is a serine-protease present in stroma, and is composed of the proteolytic complex ClpP/R/S/T and the chaperone complex ClpB/C/D. The whole complex comprises more than twenty subunits and isomers (Peltier et al., 2004). For example, four ClpP subunits are present in chloroplasts (ClpP1 is encoded in the chloroplast genome, while ClpP3-6 are nucleus-encoded). Each isomer seems to be essential for plant viability, suggesting unique rather than redundant roles for each isomer. A study with a knockdown line of ClpP6 revealed possible substrates for Clp: based on these substrates, Clp is suggested to play a role in the quality control of housekeeping proteins processively, rather than the proteins for photosynthesis and other metabolic functions (Sjögren et al., 2006). A recent genetic study in Arabidopsis indicates that Clp may degrade chlorophyllide a oxygenase, an enzyme involved in chlorophyll b synthesis, and thus regulate the chlorophyll degradation pathway (Nakagawara et al., 2007).
FtsH is a zinc-metalloprotease present in the thylakoid membrane, and its protease and chaperone domains exist within a single polypeptide. It is embedded in the thylakoid membrane via its N-terminal transmembrane domains, while the C-terminal protease domain is present at the stromal side. Nine FtsH isomers are present in chloroplasts: FtsH2 and FtsH5 are the major isomers forming a hetero-complex (Sakamoto et al., 2003), and the loss of either one results in a leaf variegation phenotype in Arabidopsis (Chen et al., 2000; Sakamoto et al., 2002). As represented by these two isomers, there are two types of FtsH, Type A (FtsH1/5) and Type B (FtsH2/8), that are functionally distinguishable (Zaltsman et al., 2005). Interestingly, the co-existence of two types of FtsH is highly conserved within photosynthetic organisms. Within each type, however, the functions seem interchangeable (Yu et al., 2005). Accumulating studies in Arabidopsis and cyanobacteria demonstrate that FtsH is a major protease involved in the PSII repair cycle (Nixon et al., 2005). Degradation of the photodamaged D1 by FtsH is light-dependent, but the recognition mechanism of the damaged D1 is currently unclear. Recently, preproteins of Clp and FtsH subunits (ClpP4 and FtsH1) were shown to be substrates for ubiquitination in vitro (Shen et al., 2007). Although further studies seem necessary, this observation raises an intriguing possibility that the level of chloroplastic proteases may be controlled in the cytosol through the utiquitine-proteasome pathway.
Besides Clp and FtsH, a serine-protease, Deg, is known to be present in chloroplasts, and four isomers were so far characterized in Arabidopsis chloroplasts. Deg1, Deg5 and Deg8 are peripherally attached to the lumenal side of the thylakoid membrane, and they were recently demonstrated to play a role in degrading photodamaged D1, and thus in PSII repair (Shen et al., 2007). In contrast, Deg2 is attached to the stromal side of the thylakoid membrane; however, its precise role is unclear (Huesgen et al., 2006). Unlike the situation in chloroplasts, Deg proteins do not appear to play a role in PSII repair in Synechocystis (Barker et al., 2006). How lumenal Deg proteases work on photodamaged D1 in concert with FtsH is an interesting question for future research. Lon is a stromal ATP-dependent protease that belongs to the AAA protein family (ATPase associated with various cellular activities). It is structurally related to FtsH, but does not contain the trans-membrane domains. Recently, one of the four Lon proteins present in Arabidopsis, Lon4, was shown to be dual-targeted into mitochondria and chloroplasts (Ostersetzer et al., 2007). Several other proteases have been identified in chloroplasts, but revealing their functions in regulating chloroplast development and homeostasis awaits further research.
PROTEIN TRANSPORT SYSTEMS
The plastid genome is greatly reduced, encoding just ~100 different proteins. Thus, >90% of the ~3000 different proteins present in mature plastids are encoded on nuclear DNA and synthesized in the cytosol. Because all plastids within an organism contain the same limited complement of genes, it is the imported proteins that define the developmental fate of the organelle (which may include chloroplast, amyloplast or chromoplast formation).
Nucleus-encoded chloroplast proteins are synthesized in precursor form – each one bearing an amino-terminal targeting signal called a transit peptide – and are imported into the organelle in an active, post-translational targeting process (Soll and Schleiff, 2004; Kessler and Schnell, 2006; Jarvis, 2008). This process is mediated by molecular machines in the outer and inner envelope membranes, termed TOC and TIC (Translocon at the outer/inner envelope membrane of chloroplasts), respectively. Upon arrival in the stroma, the transit peptide is removed and the protein either takes on its final conformation or is sorted to one of several internal compartments in a separate targeting process (Figure 4).
Figure 4.
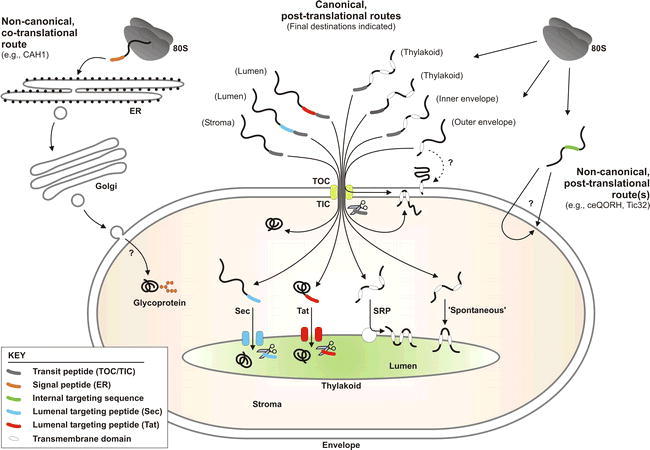
Overview of the protein import and routing systems of chloroplasts.
Most proteins access the chloroplast interior via the TOC/TIC machinery (yellow; centre of figure). Examples of proteins that utilize this canonical pathway are shown schematically, and their final destinations are indicated parenthetically. Transit peptides (see key) mediate envelope translocation, and are cleaved by SPP (represented by scissors) on arrival in the stroma. Then, imported proteins may either adopt their final conformation, or engage one of several internal sorting pathways. Lumenal proteins cross the thylakoid membrane via the Sec pathway (blue) or the Tat pathway (red). Distinct Sec and Tat lumenal targeting peptides engage the respective translocation machineries, and are cleaved by the thylakoidal processing peptidase (TPP; represented by scissors) in the lumen. Most thylakoid membrane proteins do not possess a cleavable targeting signal. Some of these proteins are targeted by the SRP machinery (white), whereas others insert ‘spontaneously’ into the membrane. Similarly, most outer envelope membrane proteins are targeted without the aid of a cleavable targeting signal; while it has been proposed that their insertion occurs spontaneously (see dotted line), recent evidence suggests that such proteins utilize TOC component(s) during their insertion. Two different TOC/TIC-based pathways mediate targeting to the inner envelope membrane: in the ‘post-import’ pathway, complete translocation into the stroma is followed by export to the inner membrane; in the ‘stop-transfer’ pathway, transmembrane domains within the mature part of the protein cause lateral exit from the TIC machinery. Recently, non-canonical, TOC/TIC-independent pathways for chloroplast protein targeting have been identified. In the first of these (right side of figure), proteins with non-cleavable, internal targeting signals are directed to the inner membrane by one or more novel pathways. Such import is energy dependent, but the translocon component(s) have not been identified. In the second (left side of figure), proteins are synthesized with a signal peptide for ER translocation. These proteins follow a pathway through the ER and Golgi, where they may become glycosylated; exactly how such proteins traverse the envelope membranes is not known. This figure has been adapted from Jarvis (2008).
Envelope Translocation
Transit Peptides.
For most nucleus-encoded proteins of the chloroplast interior, protein import is dependent upon the presence of a transit peptide (Bruce, 2001). Transit peptides engage the translocation machinery directly, and are sufficient to mediate the import of heterologous passenger proteins, such as GFP. As they emerge on the stromal side of the envelope, they are cleaved at a weakly conserved processing site by the stromal processing peptidase (SPP) – a metalloendopeptidase related to the βsubunit of the mitochondrial processing peptidase – and then degraded.
Because mistargeting of chloroplast precursor proteins (preproteins) would compromise cellular functionality, it is essential that proteins are sorted efficiently and specifically, avoiding other organelles that also accept cytosolically-translated precursor proteins (e.g. mitochondria, peroxisomes and the ER). Thus, one might expect chloroplast transit peptides to share well-defined primary or secondary structural motifs. However, transit peptides are remarkably heterogeneous (Bruce, 2001). They vary in length from 20 to >100 residues, and share no obvious sequence conservation. In fact, their only shared properties appear to be a profusion of hydroxylated residues and a lack of acidic residues, giving them an overall positive charge. In this regard, transit peptides resemble the presequences that mediate import into mitochondria. Interestingly, some preproteins are dual-targeted to both chloroplasts and mitochondria (Duchêne et al., 2005; Millar et al., 2006), clearly indicating functional similarity between the two types of targeting signal.
The lack of conservation amongst transit peptides makes their identification rather difficult. Nevertheless, several programs have been developed that enable their detection with reasonable accuracy (Table 1) (Emanuelsson et al., 2007). Analysis of the Arabidopsis genome sequence using these programs led to estimations of the chloroplast proteome ranging from ~2,000 to >4,000 proteins.
Cytosolic Factors.
Since chloroplast protein import is a post-translational process (cf. co-translational transport into the ER), it is likely that soluble, cytosolic factors facilitate the routing of precursors from the ribosome to the chloroplast surface. Preproteins are threaded through the envelope membranes in unfolded conformation, and so cytosolic chaperones are thought necessary to prevent their folding or aggregation. Indeed, it is well-documented that Hsp70 chaperones are able to interact with chloroplast transit peptides (Jackson-Constan et al., 2001). It has been suggested that 14-3-3, Hsp70 and Hsp90 proteins facilitate the cytosolic steps of chloroplast targeting, by forming so-called ‘guidance complexes’ (Qbadou et al., 2006), but these ideas have not been supported by in vivo studies in Arabidopsis and so their significance remains unclear.
Stages of Envelope Translocation.
Based on energetic requirements determined in vitro, chloroplast protein import can be divided into three steps (Olsen and Keegstra, 1992; Young et al., 1999). First, the transit peptide reversibly interacts with receptor components of the TOC complex; this is energy-independent binding. Second, the preprotein becomes deeply inserted into the TOC complex and makes contact with the TIC machinery. Progression to this early import intermediate stage requires low ATP concentrations (~100 μM) in the intermembrane space and GTP, and is irreversible. Finally, the preprotein is completely translocated into the stroma, and the transit peptide is cleaved by SPP. Progression through this step requires high ATP concentrations (~1 mM) in the stroma. Unlike mitochondrial protein import, chloroplast import does not utilize a transmembrane protonmotive force. Preproteins likely pass through these different steps seamlessly in vivo. Translocation through the two envelope membranes occurs simultaneously, at locations called ‘contact sites’ where they are held in close proximity.
Recognition and Outer Membrane Translocation.
Preprotein recognition and outer membrane translocation are the two main functions of the TOC machine. The TOC core-complex comprises three proteins, termed Toc159, Toc34 and Toc75 (Figure 5). Toc159 and Toc34 are anchored in the outer membrane by C-terminal domains, and project homologous GTPase domains into the cytosol; in addition to its membrane and GTPase domains, Toc159 possesses an N-terminal acidic domain of unknown function. These two proteins control preprotein recognition, and are regarded as receptors. By contrast, Toc75 is deeply embedded in the membrane and, like the functionally equivalent Tom40 protein of mitochondria, it possesses a β-barrel structure. It forms a translocation pore that is ~14 Å in diameter, sufficient to accept only unfolded preproteins.
The exact mechanism of TOC receptor action is debated, and two different models have emerged. In the first model, a soluble, cytosolic form of Toc159 is the initial point of contact for the transit peptide (Hiltbrunner et al., 2001; Smith et al., 2004). Once formed, the cytosolic Toc159-preprotein complex docks at Toc34 in the outer membrane, through a homotypic GTPase domain interaction, and the preprotein cargo is transferred to the Toc75 channel; this leaves the Toc159 receptor is free to disengage and initiate another targeting cycle in the cytosol. This model is reminiscent of peroxisomal import, which also employs cycling soluble receptors, and SRP-dependent ER translocation, which is initiated following a similar interaction between GTPase receptors at the target membrane. The crystal structure of Toc34 revealed a dimeric configuration, supporting the notion that Toc159 and Toc34 may undergo heterodimerization in vivo (Yeh et al., 2007).
In the second model, membrane-bound Toc34 is the initial point of contact for incident transit peptides (Becker et al., 2004a). Proponents of this model suggest that the soluble Toc159 form observed by others is an experimental artefact, and argue that Toc159 remains stably associated with the membrane throughout the import mechanism. Electron microscopic analysis of purified TOC core-complexes revealed a toroid structure comprising four putative translocation channels surrounding a central finger-like domain (Schleiff et al., 2003b). The four channels are proposed to each contain one Toc75 unit and one Toc34 unit, and the central region is proposed to comprise a single Toc159 molecule. Centrally located Toc159 might rotate about its axis to accept preproteins from different Toc34 primary receptors, and act as a GTP-driven motor to push them through the Toc75 channels using a ‘sewing machine’ mechanism (Schleiff et al., 2003a).
The two models seem to be very different, but it is possible that the mechanism actually employed in vivo incorporates elements of both.
Substrate-Specific Protein Import Pathways.
Most components of the import apparatus were identified through biochemical analysis of isolated pea chloroplasts. More recently, Arabidopsis has been widely adopted as an alternative model system (Jarvis et al., 1998; Bauer et al., 2000), due mainly to the availability of its genome sequence. Interestingly, when the genome was scanned for TOC homologues, many components were found to be represented by multiple genes (Jackson-Constan and Keegstra, 2001). For example, Toc34 is encoded by two Arabidopsis genes, termed atTOC33 and atTOC34 (Jarvis et al., 1998), while Toc159 is encoded by four genes, termed atTOC159, atTOC132, atTOC120 and atTOC90 (Bauer et al., 2000). Careful analyses revealed that these gene families encode different receptor isoforms with distinct functions.
Characterization of an Arabidopsis atToc33 knockout mutant, plastid protein import 1 (ppi1), provided the first in vivo verification of the role of a biochemically-identified translocon component (Jarvis et al., 1998). Later studies on an atToc159 mutant, termed ppi2, yielded the attractive hypothesis that atToc159 is a receptor with specificity for highly-abundant, photosynthetic proteins (Bauer et al., 2000). The ppi2 mutant is albino due to a block in chloroplast differentiation, and, while photosynthetic proteins are deficient in ppi2, non-photosynthetic proteins seem to accumulate normally. During establishment of the photosynthetic apparatus, the import machinery must accommodate massive increases in the expression of key photosynthetic proteins. Existence of a separate receptor system for such proteins would prevent their bulk flow from out-competing the import of less abundant, but equally important non-photosynthetic, housekeeping proteins (Figure 5).
Studies on mutants lacking other receptor isoforms, such as atToc132, atToc120 and atToc34, indicated that these are more important for the biogenesis of non-photosynthetic plastids (Constan et al., 2004; Ivanova et al., 2004; Kubis et al., 2004). The existence of distinct TOC complexes was supported biochemically (Ivanova et al., 2004): the atToc33 isoform of Toc34 predominates in atToc159-containing complexes, whereas the atToc34 isoform predominates in atToc132/atToc120-containing complexes. This accounts nicely for the fact that the ppi1 phenotype is qualitatively similar to that of ppi2 (Kubis et al., 2003). Operation of these substrate-specific import pathways might contribute to the differentiation of different plastid types.
Inner Membrane Translocation.
Several putative components of the TIC complex have been identified, but there is considerable disagreement concerning their roles (Figure 5). Preproteins most likely encounter Tic22 first of all, since it resides in the inter-membrane space. It may facilitate the passage of preproteins from TOC to TIC, perhaps functioning in association with an Hsp70 and the inwardly facing J-domain protein, Toc12 (Becker et al., 2004b). The most basic function of the TIC machinery is channel formation, and yet even this function is unclear, since both Tic110 and Tic20 have been proposed to fulfil this role (Chen et al., 2002; Heins et al., 2002). Perhaps both proteins participate in pore formation.
A major component of the TIC machinery is Tic110, which projects a sizeable domain into the stroma (Kessler and Blobel, 1996; Jackson et al., 1998). This stromal domain binds transit peptides, and probably functions to recruit molecular chaperones to the complex (Akita et al., 1997; Inaba et al., 2003). By analogy with the Hsp70-based ‘motors’ that drive transport into mitochondria and the ER, it is thought that stromal chaperones bind to emerging preproteins to ensure unidirectional movement, in a ratchet-type mechanism (Jackson-Constan et al., 2001). In chloroplasts, the relevant chaperone is probably the Hsp100 homologue, Hsp93/ClpC (Nielsen et al., 1997; Kovacheva et al., 2005). The Tic40 protein is an Sti1-domain co-chaperone, and is proposed to control the activity of the Hsp93-based import motor (Chou et al., 2006; Bédard et al., 2007).
Other proteins (Tic62, Tic55 and Tic32) possess redox-related motifs, suggesting roles in the regulation of import in response to redox status (Stengel et al., 2008). It is well documented that chloroplast redox signals influence gene expression, so it would not be surprising if chloroplast import is demonstrated to receive similar control. That the import of some preproteins is influenced by light is consistent with this hypothesis (Hirohashi et al., 2001), since these effects might be mediated by redox signals. However, the precise roles of these redox-related proteins remains unclear.
Non-Canonical Chloroplast Targeting Pathways.
For many years, TOC/TIC-mediated import of preproteins with transit peptides was regarded as the unique route for entry into the chloroplast interior. Recent data indicate that alternative targeting signals and pathways exist (Figure 4). For example, proteins lacking cleavable amino-terminal targeting signals are found associated with the inner envelope membrane (Nada and Soll, 2004; Miras et al., 2007). In each case, targeting was shown to proceed without assistance from the TOC machinery. Another exciting development has been the identification of a chloroplast protein targeting pathway involving the endomembrane system (Villarejo et al., 2005). Clients of this pathway possess signal peptides for co-translational transport to the ER, from whence they pass the Golgi prior to final arrival in the chloroplast. These pathways were revealed in part by proteomic analysis, and so are discussed in the next section.
Targeting To The Envelope System
Several mechanisms exist for protein targeting to the chloroplast envelope (Hofmann and Theg, 2005). Most proteins of the outer membrane do not have cleavable targeting signals. Instead, targeting information resides within hydrophobic transmembrane domains. It was originally thought that such proteins insert ‘spontaneously’ into the membrane bilayer, without assistance from an import apparatus. However, a cytosolic sorting factor (AKR2) that mediates transport to the chloroplast surface was recently identified, while other data indicate that insertion employs the Toc75 channel protein, possibly dissociated from other TOC components (Tu et al., 2004; Bae et al., 2008).
One exceptional outer membrane protein is Toc75. This protein possesses a bipartite targeting signal, comprising a standard transit peptide and, immediately downstream of that, an intraorganellar targeting peptide. The latter functions as a ‘stop-transfer’ signal, arresting translocation so that the preprotein can disengage from the translocon and undergo membrane integration (Inoue and Keegstra, 2003).
With the exception of the non-canonical examples mentioned above, proteins of the inner envelope membrane possess a transit peptide and engage the TOC/TIC machinery. Such proteins follow two different targeting routes, referred to as the ‘post-import’ (or ‘conservative sorting’) and ‘stop-transfer’ pathways (Li and Schnell, 2006; Tripp et al., 2007). The former is a two-step process; complete translocation into the stroma is followed by membrane integration of the soluble intermediate in a separate event. This is reminiscent of ‘conservative sorting’ to the mitochondrial inner membrane, so-called because the second-step event is mediated by components of bacterial origin. Mediators of inner membrane insertion in chloroplasts have not been identified. In the ‘stop-transfer’ pathway, hydrophobic transmembrane domains mediate lateral exit from the TIC translocon and membrane integration; this route may be particularly important for polytopic proteins that might otherwise be prone to aggregation.
Targeting To The Thylakoids
The thylakoids contain highly-abundant protein complexes of the photosynthetic light reactions, which comprise both chloroplast-and nucleus-encoded subunits. The latter are first translocated across the chloroplast envelope via the TOC/TIC system, and then subsequently engage one of four different pathways for thylakoid targeting (Figure 4) (Jarvis and Robinson, 2004; Gutensohn et al., 2006; Schünemann, 2007).
Thylakoid lumenal proteins are targeted via the so-called Sec and twin-arginine translocase (Tat) pathways. Proteins engaging these translocation systems possess bipartite targeting signals: a standard transit peptide, followed by a lumenal targeting peptide similar to the signal peptides that mediate inner membrane transport in bacteria. The Sec pathway is powered by ATP (consumed by the SecA motor protein) and accepts only unfolded proteins. By contrast, the Tat pathway is proposed to be driven by the thylakoidal proton gradient, in an antiporter mechanism, and is able to accommodate fully-folded substrates; it may serve to deliver proteins that must acquire their final conformation in the stroma, through co-factor binding or oligomerization. Costs estimated for Tat translocation are remarkably high (~80,000 protons per protein) (Alder and Theg, 2003), but the energetics of the system have been questioned by its apparent lack ofΔpH-dependence in vivo (Finazzi et al., 2003).
Thylakoid membrane proteins utilize either the signal recognition particle (SRP)-dependent pathway or the so-called ‘spontaneous’ insertion pathway. While the latter proceeds without energy consumption or the involvement of a proteinaceous transport machinery, the former consumes GTP as a consequence of a critical interaction between the SRP (a complex of SRP54 and SRP43, the latter being unique to chloroplasts) and its membrane receptor, FtsY, and is principally concerned with the insertion of polytopic proteins of the light-harvesting complexes. Studies on Arabidopsis mutants lacking components of the SRP pathway have played a key role in its elucidation (Tzvetkova-Chevolleau et al., 2007).
Unlike the TOC/TIC pathway, which shares no clear homology with other translocation systems, at least three of these thylakoid targeting pathways are closely related to protein transport systems of the bacterial inner membrane. They nicely exemplify the ‘conservative sorting’ concept, since the thylakoidal transport events have been retained (or conserved) from the organelle's prokaryotic origins (hence the name Sec, which is an abbreviation of Secretory). All four pathways are essential for the biogenesis of the photosynthetic machinery, and so have been studied intensively. Most available information relates to nucleus-encoded proteins, but it should be noted that these pathways also target proteins encoded by the plastid genome. For example, a variation on the SRP pathway described above mediates the co-translational insertion of the D1 protein of PSII (Schünemann, 2007)
Finally, the possibility exists that certain other proteins are brought to the thylakoids in transport vesicles that bud off from the inner envelope membrane. While there is no direct evidence for such vesicle-mediated protein traffic, there is considerable evidence that the lipids needed for thylakoid formation are indeed transported in this way (Benning et al., 2006; Aseeva et al., 2007). Bearing in mind that some chloroplast proteins arrive at the organelle in cytosolic transport vesicles (Villarejo et al., 2005), it would not be surprising if similar mechanisms exist for onward transport to the photosynthetic membranes themselves.
PROTEOME ANALYSIS
Completion of genome sequencing projects for Arabidopsis, rice and other species, together with method development for protein identification by mass spectrometry, precipitated the onset of the proteomic era. Because of the extreme complexity of cellular proteomes, and the dynamic-range limitations associated with analyses on such complex mixtures (i.e. the tendency of highly-abundant proteins to mask the presence of others), proteomic studies have mostly focused on isolated subcellular components. In this regard, chloroplasts have received considerable attention (Baginsky and Gruissem, 2004; van Wijk, 2004; Jarvis, 2007). Proteomic analysis can confirm the expression and structure of genes predicted by genome analysis in silico, it can determine subcellular and suborganellar protein localizations, it can provide estimates of protein abundance, and it can even yield information on post-translational modification and multiprotein complex composition. Such information is invaluable, since up to 50% of the ~27,000 protein-coding genes in Arabidopsis are presently of unknown function (Swarbreck et al., 2008).
As discussed earlier, most chloroplast proteins possess an amino-terminal targeting signal, or transit peptide (Jarvis, 2008). Because transit peptides share certain characteristics, it is possible to identify candidate chloroplast proteins in silico by sequence analysis (the TargetP program is a popular choice; Table 1) (Emanuelsson et al., 2007). However, a lack of conservation amongst transit peptides and their similarity to mitochondrial presequences mean that such in silico methods are not totally reliable. Thus, the only dependable method for chloroplast protein identification is direct experimentation. Computational methods suggest that there are ~2,000–4,000 different proteins in chloroplasts, but presently there are just ~1,000 experimentally-verified, Arabidopsis plastid proteins in the PPDB and SUBA databases (Friso et al., 2004; Heazlewood et al., 2007, see Table 1 for the websites). This further emphasizes the need for additional proteome analysis.
Proteome Catalogues
Cataloguing aims to identify all proteins within a particular cellular or organellar compartment. While the chloroplast proteome is substantially smaller than that of an entire cell, it nevertheless comprises several thousand proteins. Thus, many cataloguing experiments have focused on a particular suborganellar compartment (e.g. the thylakoids, envelope, stroma or plastoglobules). Nonetheless, some studies on whole organelles have been conducted, and these have focused on different plastid types (e.g. chloroplasts, amyloplasts and etioplasts).
TheThylakoids.
The thylakoid membranes themselves harbor the four multiprotein complexes of the photosynthetic light reactions (PSI, PSII, the cytochrome b6/f complex, and ATP synthase), but also function to form a central aqueous compartment called the lumen. Difficulties associated with the analysis of highly-hydrophobic integral membrane proteins led many thylakoid proteome studies to focus on lumenal proteins, or proteins associated peripherally with the membranes (Kieselbach and Schröder, 2003). Yet even this is challenging, since the molar ratio between the most and least abundant lumenal proteins may be as high as 106, presenting a major dynamic-range barrier to comprehensive analysis (Peltier et al., 2002).
Lumenal studies revealed an unanticipated level of complexity. Relatively few Arabidopsis lumen proteins were identified experimentally, but information from these enabled in silico estimations for the whole lumenal proteome, ranging from ~80 to ~400 proteins (Peltier et al., 2002; Kieselbach and Schröder, 2003; Westerlund et al., 2003); the actual size presumably lies somewhere between these estimates. In addition to the expected photosynthetic proteins, and a significant proportion of unknown function, substantial numbers of candidate lumenal proteins mediate protein folding, processing and proteolysis, anti-oxidative defense, and non-photosynthetic redox reactions; these may serve to repair and maintain normal functionality of the photosynthetic machinery, which experiences substantial redox stress. Interestingly, up to 50% were predicted to be substrates of the Tat pathway, which as discussed earlier is able to transport fully-folded proteins.
The thylakoid membrane itself is dominated by the four photosynthetic complexes, which together comprise ~100 different proteins. Nevertheless, the membrane also contains many proteins associated with the assembly, maintenance and regulation of the complexes. Despite the difficulties that hydrophobic membrane proteins present, proteomic studies achieved near complete coverage of the photosynthetic complexes, and also identified low-abundance components such as those involved in cyclic electron flow around PSI and chlororespiration (Friso et al., 2004; Peltier et al., 2004a).
By combining data from proteomic studies and other reports, the total number of experimentally-verified, thylakoid-associated proteins in Arabidopsis was found to be ~400 (Peltier et al., 2004a; van Wijk, 2004). Of these, ~30% are involved in photosynthesis, ~25% are of unknown function (including proteins with tetratricopeptide repeat [TPR], pentatricopeptide repeat [PPR], DnaJ and rhodanese domains), ~20% mediate protein translocation, folding, processing and proteolysis, and ~10% are involved in oxidative stress defense.
The Envelope System.
The envelope is a double-membrane system that forms a semi-permeable barrier between the cytosol and the stroma. It contains the machinery responsible for importing nucleus-encoded proteins (Jarvis, 2008), as well as transporters that exchange ions and metabolites (Weber et al., 2005). It also possesses a unique biochemical machinery (e.g. for the synthesis of plastid membrane components and other lipids), and participates in the communication between plastids and the nucleus (López-Juez and Pyke, 2005; Nott et al., 2006).
Like the thylakoid membrane, the envelope proteome is dominated by hydrophobic integral membrane proteins (Ferro et al., 2003; Froehlich et al., 2003; Rolland et al., 2003). Combined data from proteomic studies and other experiments identified a total of ~400 envelope-associated proteins in Arabidopsis (Peltier et al., 2004a; van Wijk, 2004). Of these, ~30% are of unknown function, 13% mediate protein translocation, folding, processing or degradation, 10% participate in lipid or fatty acid metabolism, and 9% are small molecule transporters. Thus, the proteome clearly reflects the main functions of the envelope, which are quite different from those of the thylakoids. The high proportion of proteins of unknown function indicates that envelope functions are not yet fully understood. Intriguingly, proteins similar to components of the mitochondrial protein import machinery were identified, suggesting that novel protein transport systems operate in the envelope (Ferro et al., 2003).
Protein targeting to the two envelope membranes, and to the intermembrane space, is not well understood (Hofmann and Theg, 2005). Thus, it is difficult to make in silico predictions concerning the composition of the three individual proteomes. Nonetheless, evidence suggests that the outer membrane is characterized by beta-barrel proteins, and that the inner membrane is dominated by polytopic, alpha-helical transporters (Koo and Ohlrogge, 2002; Schleiff et al., 2003c).
By analyzing carefully collected sets of integral proteins from the inner envelope and thylakoid membranes (identified on the basis of published information), the respective proteomes were found to have quite different characteristics (Sun et al., 2004). On average, thylakoid proteins were smaller and more acidic than envelope proteins, and contained fewer cysteine residues. The larger average size of the envelope proteins probably reflects the presence of numerous transporters with multiple membrane spans (Weber et al., 2005), while the pI differences may be linked to pH differences between the compartments (protons are accumulated in the thylakoid lumen during photosynthesis). Cysteines are able to engage directly in redox reactions, and so their deficiency in thylakoidal proteins might be a measure to reduce oxidative damage.
The Stroma.
The carbon reactions of photosynthesis (the Calvin cycle) and other major metabolic pathways are located in the stroma, as are components of the plastid genetic system. One in silico study estimated that the stroma contains up to ~80% of the total chloroplast proteome (Sun et al., 2004). However, it should be noted that many of these proteins may associate permanently or transiently with the thylakoids or the inner envelope membrane, through protein-protein, electrostatic or hydrophobic interactions, or via lipid anchors. Interestingly, the acetyl-coenzyme A carboxylase complex appears to be envelope-associated, placing it near the site of fatty acid use or export (Rolland et al., 2003). Such metabolic channelling has obvious advantages, and there may be many other similar examples.
Over 200 stromal proteins were identified in one study (Peltier et al., 2006). Of these, 26% mediate protein synthesis, folding, proteolysis and sorting, 12% are involved in primary carbon metabolism, including Calvin cycle enzymes, 11% are of unknown function, while 7%, 6%, 4% and 4% mediate the biosynthesis of amino acids, tetrapyrroles, nucleotides and lipids, respectively. Interestingly, proteins of primary carbon metabolism constituted most (~75%) of the total stromal mass; others responsible for protein synthesis, biogenesis and fate represented ~10% of mass, whereas those involved in nitrogen and sulphur assimilation made up ~8%. Other biosynthetic pathways each represented less than 1% of the total mass.
Other studies on the stromal compartment were more focused. For example, all proteins of the plastidic 70S ribosome were identified (Yamaguchi and Subramanian, 2000; Yamaguchi et al., 2000). The ribosome comprises 59 different proteins; 53 are orthologues of bacterial ribosomal proteins, while six are plastid specific. The latter may mediate functions unique to plastid translation and its regulation, such as protein targeting to the thylakoids and control by nuclear factors.
Interestingly, two ribosomal proteins were identified as targets for regulation by the stromal thioredoxin system (Balmer et al., 2003). This system is composed of ferredoxin, ferredoxin-thiore-doxin reductase, and thioredoxin, and it links light to the regulation of photosynthetic enzymes and processes such as lipid biosynthesis. Electrons flow from ferredoxin to thioredoxin, which in its reduced state regulates the activity of target proteins by reducing specific disulphides. Proteomic strategies were employed to extend the list of targets of the system (Hisabori et al., 2007). Proteins identified in this way included components of established thioredoxin-regulated pathways (e.g. the Calvin cycle, nitrogen and sulphur metabolism, and protein synthesis) and others not previously recognized as thioredoxin targets (e.g. tetrapyrrole biosynthesis, protein folding, assembly and degradation, starch degradation, DNA replication and transcription, and plastid division). Remarkably, this mainly stromal regulatory network even extends into the thylakoid lumen, where it targets the photosynthetic electron transport chains.
Plastoglobules.
Plastoglobules are lipid-containing bodies in chloroplasts, chromoplasts and other plastids. They contain various lipidic compounds (e.g. galactolipids, fatty acids, carotenoids, tocopherols and plastoquinone), and were previously thought to function as simple lipid stores. However, proteomic analyses identified ~30 different proteins in plastoglobules, revealing a much more complex set of activities (Bréhélin et al., 2007). In addition to the plastoglobulin family of structural proteins, which bind to the surface of the globules and prevent their coalescence, a number of enzymes were identified. Plastoglobules in chloroplasts actively participate in the synthesis of their lipophilic constituents (e.g. alpha-tocopherol [vitamin E], an important anti-oxidant in thylakoids); similarly, those in chromoplasts contain carotenoid biosynthetic enzymes. In chloroplasts, plastoglobules are directly coupled to the thylakoids, suggesting that their contents are in equilibrium and that they contribute to the synthesis of thylakoidal constituents (Austin et al., 2006).
Whole Organelles.
An extensive study of the whole Arabidopsis chloroplasts identified ~600 different proteins (Kleffmann et al., 2004). Almost complete coverage was achieved for major metabolic pathways (e.g. the Calvin cycle), while coverage for less abundant pathways was only partial. Interestingly, over 30% of the identified proteins were of unknown function. Parallel RNA profiling revealed a correlation between transcript levels and protein abundances in some metabolic pathways, but not others, implying that distinct regulatory mechanisms operate in different pathways.
Proteome studies on amyloplasts identified most enzymes of starch biosynthesis, as expected, but also revealed a surprisingly broad spectrum of biosynthetic capabilities (Balmer et al., 2006a; Stensballe et al., 2008). Like chloroplasts, amyloplasts possess enzymes for nitrogen and sulphur assimilation, and the biosynthesis of amino acids, fatty acids and tetrapyrroles. In comparison with chloroplasts, amyloplasts contain a higher proportion of proteins of carbon, nitrogen and sulphur metabolism, and transport processes. By contrast, chloroplasts contain proportionally more proteins of unknown function, presumably reflecting their more complex activities. While components of the TOC/TIC machinery were identified in amyloplasts, no ribosomal proteins were detected, suggesting that most amyloplast proteins are nucleus-encoded. This makes sense, since the plastid genome is dominated by genes for photosynthetic components. Interestingly, a thioredoxin regulatory network also operates in amyloplasts (Balmer et al., 2006b).
Analyses of etioplasts and chromoplasts have also been conducted (von Zychlinski et al., 2005; Siddique et al., 2006). Proteome comparisons revealed that both have metabolic functions typical of heterotrophic plastids, but that etioplasts nevertheless share significant similarities with chloroplasts – the organelles into which they ultimately develop.
Protein Targeting Issues
Surprisingly, when the ~600 proteins identified in the whole chloroplast proteome study mentioned above were analyzed using the TargetP program, only ~60% were predicted to have a transit peptide (Kleffmann et al., 2004). While a more recent study suggests that this may overestimate the number of chloroplast proteins lacking a canonical transit peptide (Zybailov et al., 2008), it is nevertheless interesting to note that considerable numbers were predicted to have putative mitochondrial presequences, signal peptides for ER translocation, or no cleavable targeting signal at all. It is possible that some of these proteins were contaminants from other cellular compartments, or had received incorrect TargetP predictions, but the data nevertheless suggest that protein targeting to chloroplasts is more complex than was previously envisaged (Jarvis, 2008). Until recently, all nucleus-encoded proteins of the chloroplast interior were thought to have a transit peptide for TOC/TIC engagement.
Initial evidence for a more complicated picture of chloroplast protein biogenesis was provided by studies on the Arabidopsis envelope proteome (Ferro et al., 2003). A protein named ceQORH (chloroplast envelope quinone oxidoreductase homologue) was found in the inner membrane, in spite of its lack of a transit peptide. Interestingly, an internal sequence of ~40 residues controls ceQORH localization, in a process that is not mediated by the TOC/TIC machinery (Figure 4) (Miras et al., 2007). Another inner membrane protein, Tic32, was also found to lack a transit peptide, and to be targeted in TOC-independent fashion (Nada and Soll, 2004). Whether Tic32 follows the same import pathway as ceQORH remains to be determined. Nonetheless, it should be noted that Tic32 targeting information resides at its N-terminus, and that the energetic requirements for Tic32 targeting are lower than for ceQORH.
The identification of chloroplast proteins with signal peptides for ER translocation was surprising (Kleffmann et al., 2004). Chloroplast protein traffic through the endomembrane system is well documented in algae and apicomplexan parasites, which have complex plastids (Nassoury and Morse, 2005). While such targeting makes sense in these organisms, due to the complex nature of their plastids and the likely autogenous origin of the outer organellar membrane, it would seem unnecessary in higher plants. Nonetheless, evidence for such a targeting pathway was recently presented (Villarejo et al., 2005). The Arabidopsis carbonic anhydrase 1 (CAH1) protein was found in the stroma, in spite of its predicted signal peptide. Intriguingly, CAH1 could not be imported directly by chloroplasts, but was instead taken up co-translationally by ER microsomes. Moreover, stromal CAH1 is glycosylated; because the relevant glycans are only added in the Golgi, a chloroplast protein transport pathway through the Golgi was inferred (Figure 4). Indeed, application of brefeldin A (an agent that interferes with Golgi-mediated vesicle traffic) obstructed the transport of CAH1, causing its arrest within the endomembrane system. What happens once the vesicles arrive at the chloroplast is surface is less clear, but it is interesting to note that there is considerable evidence for vesicle budding at the inner envelope membrane (Benning et al., 2006; Aseeva et al., 2007). More recently, another chloroplast protein was shown to follow a similar pathway (Nanjo et al., 2006). The CAH1 pathway may be the vestige of an ancestral targeting mechanism that prevailed during early evolution, and which for some reason has been retained for a few proteins.
That some proteins in chloroplasts are predicted to have mitochondrial presequences is much less surprising, since the dual-targeting of certain proteins to both chloroplasts and mitochondria is well documented (Duchêne et al., 2005; Millar et al., 2006). Overall, the emerging picture of chloroplast protein targeting is rather complex, demonstrating that transit peptide prediction in silico cannot provide a complete description of the organellar proteome.
Comparative Proteomics
In addition to its role in cataloguing experiments, proteomics has been employed in comparative studies with considerable success. For example, changes in the organellar proteome were studied during de-etiolation or greening (Lonosky et al., 2004; Kleffmann et al., 2007), while the responses of lumenal, stromal and plastoglobular proteomes to low temperature or light stress were characterized (Giacomelli et al., 2006; Goulas et al., 2006; Ytterberg et al., 2006). In another example, chloroplasts from Arabidopsis mutants lacking different TOC receptor isoforms were analyzed (Kubis et al., 2003). Different groups of chloroplast proteins were selectively deficient in the mutants, indicating that the different TOC receptor isoforms possess preprotein recognition specificity (Jarvis, 2008). The data suggested that at least two different import pathways operate in plastids (Figure 5), as discussed in the previous section.
Oligomerization and Modification
Many proteins do not function in isolation, but as part of multiprotein complexes. Thus, experimental procedures that maintain oligomeric status are desirable, since they preserve important interaction information. Several complexes of chloroplasts have been purified to homogeneity and analyzed individually; examples include the photosystems, the cytochrome b6/f complex (Whitelegge, 2003), the ribosomal subunits (see earlier), and a Clp complex (Peltier et al., 2004b). Additionally, the oligomeric state of the stromal proteome was systematically analysed by Peltier et al. (2006).
Protein modification is another important issue that must be taken into consideration. The molecular mass of an intact protein defines its native covalent state, and so its accurate measurement can reveal modifications mediated either post-transcriptionally, through processes like RNA editing, or post-translationally (Whitelegge, 2003; van Wijk, 2004). Several studies revealed covalent modifications of plastidic proteins, including acetylation, glycosylation, palmitoylation, phosphorylation, and N-terminal methionine excision. Such modifications may influence the activity, interactions or stability of the protein, or anchor it to a membrane.
PLASTID DIVISION
Like their free-living ancestors, both chloroplasts and mitochondria divide, thus enabling their continued inheritance by daughter cells after cell division (Boffey and Lloyd, 1988; Kuroiwa et al., 1998; Figure 6). However, most of the ancestral bacterial genes were either lost or transferred to the host nuclear genome during evolution, so that the genomes of both organelles lack sufficient information to mediate their own biogenesis and division. Therefore, unlike in bacteria, division of these organelles is performed and controlled by the eukaryotic nuclear genome (Kuroiwa et al. 1998; Pyke, 1999). Recent structural and molecular-genetic studies have allowed us to understand the mechanisms of plastid division, and in particular the division apparatus including ring structures at the division site (Kuroiwa et al. 1998) and GTPase proteins that are strongly related to components of the bacterial division apparatus (Miyagishima et al., 2003; Osteryoung and Nunnari, 2003; Figure 6). Our current knowledge on plastid division comes from studies in chloroplasts, but it should be noted that some plastid-type-specific mechanisms might exist. As explained below, molecular-genetic works in Arabidopsis have significantly contributed to this field. In addition, plastid division has been studied in ancestral algal chloroplasts. In light of these studies, the division machinery appears to be shared between chloroplasts and other plastid types.
Figure 6.
Plastid division and the division machinery.
(A) Chloroplasts dividing in Arabidopsis hypocotyl cells.
(B) Localization of GFP-tagged DRP5B dynamin protein at the cytosolic side of the division site in Arabidopsis mesophyll cell chloroplasts. (C) Schematic representation of the plastid division machinery. A ‘bacterial’ division complex based on FtsZ forms first at the division site. This is then followed by the formation of the inner and outer PD rings, and finally the recruitment of DRP5B dynamin. Constriction at the vision site then initiates. Scale bars in (A) and (B), 10 μm.
Cytological Aspects
A large number of cytological studies have shown that: (1) plastids multiply by division along with the duplication and separation of their nucleoids; (2) different plastid types, including proplastids, etioplasts, chloroplasts and amyloplasts, are capable of dividing themselves (Boffey and Lloyd, 1988). Electron microscopic observations have also shown that plastids divide by simultaneous constriction of the inner and outer envelopes. Plastids usually multiply by binary fission, but multiple fission has been observed in some species and tissues. Some primitive algae have only one or a few chloroplasts per cell, and the chloroplasts divide synchronously with the cell cycle. In higher plants, however, cells generally contain large numbers of plastids, and the plastids divide non-synchronously, even within the same cell (Figure 6). In addition, plastids continue to divide in developing tissues in which cells expand but do not divide. Many earlier studies demonstrated that the plastid number per cell varies considerably depending on the cell type, developmental stage, and environmental conditions (Boffey and Lloyd, 1988; Pyke, 1999). In spite of these observations, little is understood about how plastid number per cell is controlled at the molecular level, or how it is related to the rate of plastid division.
Importantly, electron microscopic studies have identified electrondense ring structures encircling the constriction furrow of dividing plastids. This ring structure, called the plastid-dividing (PD) ring, has been observed in several lineages of algae and plants including Arabidopsis (Mita et al., 1986; Hashimoto, 1986; Kuroiwa et al., 1998). In most cases, the PD ring was detected as a double-ring structure, with one ring (the outer PD ring) on the cytosolic face of the outer envelope membrane, and the other ring (the inner PD ring) on the stromal face of the inner envelope membrane. In the red alga, Cyanidioschyzon merolae, a middle PD ring was also identified in the intermembrane space. The structure and behavior of the two (or three) rings are different, suggesting that each ring has distinct functions and is composed of distinct sets of proteins (Kuroiwa et al., 1998; Miyagishima et al., 2003). Attempts to identify proteins associated with or comprising the PD ring have been made. In fact, several proteins were shown to exist at the plastid division site (see below), but none of these proteins appears to be an actual component of the PD ring. Thus, the protein components of the PD ring remain unclear.
FtsZ Descended From The Cyanobacterial Endosymbiont
The first protein shown to play a role in chloroplast division was a plant homologue of FtsZ, the key bacterial division protein (Osteryoung and Vierling, 1995; Osteryoung et al., 1998; Strepp et al., 1998). FtsZ is a bacterial GTPase that is structurally similar to tubulin, and which self-assembles into a ring structure beneath the cytoplasmic membrane at the division site. Formation of the FtsZ ring is an initial event at the division site, and initiates the recruitment of other proteins that constitute the bacterial division complex. Of all the proteins involved in bacterial cell division, FtsZ is thought to play an especially important role (Harry et al., 2006). A gene encoding a chloroplast-targeted FtsZ protein was found in the Arabidopsis nuclear genome, and, subsequently, similar FtsZ homologues have been reported for other photosynthetic eukaryotes. Plant FtsZ proteins are most closely related to their cyanobacterial counterparts, which supports an endosymbiotic origin of chloroplasts. Analogous to the bacterial protein, plant FtsZ is localized at the stromal side of the chloroplast division site.
Whereas most bacteria (including cyanobacteria) have only one ftsZ gene, plants have more than two genes that are clustered into two phylogenetic groups: FtsZ1 and FtsZ2. It remains unclear as to why plants contain two types of FtsZ. Nevertheless, depletion of either protein in Arabidopsis disrupts plastid division, suggesting that, instead of being redundant, FtsZ1 and FtsZ2 may have distinct functions. Interestingly, both FtsZ1 and FtsZ2 were shown to co-localize in plastids, even when the localization of FtsZ filaments was altered experimentally (McAndrew et al., 2001; Kuroiwa et al., 2002). A comparison of their primary structures revealed that FtsZ2 contains a short stretch of conserved amino acids at the C-terminus (the C-terminal core domain) very similar to an equivalent region in bacterial FtsZ; by contrast, FtsZ1 does not contain this core domain (Osteryoung et al., 1998; McAndrew et al., 2001). A recent study showed that another plastid division protein, ARC6 (accumulation and replication of chloroplasts 6, see below), specifically interacts with FtsZ2, but not with FtsZ1. ARC6 interacts with FtsZ2 via the core domain, which is absent in FtsZ1, suggesting functional differences between these FtsZ proteins (Maple and Møller, 2007).
In addition to FtsZ1 and FtsZ2, a third FtsZ-like protein (ARC3) was identified in Arabidopsis by map-based cloning of arc3 mutations. In the arc mutants, the number and size of chloroplasts in leaf mesophyll cells differs from that in wild-type plants. Among them, arc2, arc3 and arc5 through to arc12 all have mesophyll cells with reduced numbers of enlarged chloroplasts, suggesting that chloroplast division is defective in these mutants (Pyke, 1999). ARC3 encodes a protein that has an FtsZ-like N-terminal region and a C-terminal domain homologous to a region of phosphatidylinositol-4-phosphate 5-kinase (Shimada et al., 2004). ARC3 is localized on the stromal side of the chloroplast division site (Maple et al., 2007), and forms a complex with at least FtsZ1, FtsZ2 and ARC6 (McAndrew et al., 2008). Functional studies suggest that ARC3 is involved in the placement of the FtsZ ring in chloroplasts (Maple et al., 2007).
Other Division Proteins Descended From The Cyanobacterial Endosymbiont
Given the successful discovery of plastidic FtsZ proteins, reversegenetic approaches have been taken to identify other plastid division proteins homologous to bacterial components. In addition, conventional forward-genetic approaches were taken using chloroplast division mutants such as the arc mutants. These studies enabled the identification of novel plastid division genes that are likely to be of cyanobacterial origin. Homologues of minD (mini-cell D) (Colletti et al., 2000) and minE (Itoh et al., 2001), which determine the site forming the FtsZ ring in bacteria, have been found in plant nuclear genomes. Involvement of these genes in the positioning of the chloroplast division site was confirmed by characterizing the corresponding mutants in Arabidopsis (Colletti et al., 2000; Itoh et al., 2001). In a separate work, the arc6 mutation in Arabidopsis was identified by map-based cloning. It was revealed that the ARC6 locus is orthologous to a cyanobacterial division gene, ftn2. ARC6 contains a DnaJ-like domain and is localized at the chloroplast division site spanning the inner envelope membrane. ARC6 is thus suggested to play a role in stabilizing FtsZ filaments (Vitha et al., 2003). Identification of these bacterial-type division genes, in addition to ftsZ, confirms the hypothesis that much (if not all) of the chloroplast division apparatus is derived from the cyanobacterial ancestor, and that the corresponding genes have been transferred to the nuclear genome during evolution. However, comparative studies between cyanobacteria and plants indicated that that the majority of bacterial cell division genes were lost after endosymbiosis (Miyagishima et al., 2005).
Dynamin and PDV Proteins Originated From The Eukaryotic Host
In addition to cyanobacteria-derived mechanisms, plastid division requires additional components evolved from the eukaryotic host cell, as represented by a member of dynamin family (Miyagishima et al., 2003; Osteryoung and Nunnari, 2003). Dynamin family proteins contain a GTPase domain and are specifically found in eukaryotic organisms, although a recent study showed that eubacteria do have proteins distantly related to the eukaryotic dynamin family. The best characterized example in eukaryotes is the dynamin protein that self-assembles into a ring at the neck of clathrin-coated pits. During this process, the dynamin plays a predominant role in pinching off vesicles from the plasma membrane. To date, several proteins have been included in the dynamin family owing to their structural similarity, and each member has been shown to play roles in fission or fusion of distinct eukaryotic membrane systems, for example in mitochondrial division (Praefcke and McMahon, 2004). One member of the dynamin family, named DRP5B (dynamin-related protein 5B), was shown to be involved in plastid division in the red alga, C. merolae (Miyagishima et al., 2003), and Arabidopsis (Gao et al., 2003). In Arabidopsis, DRP5B was identified through forward genetics: map-based cloning of the arc5 locus revealed that the loss of DRP5B results in the arc phenotype. Analogous to conventional dynamins at the plasma membrane, DRP5B localizes at the cytosolic side of the plastid division site. In Arabidopsis arc5 mutants, chloroplast divisions are arrested at the stage of division-site constriction, supposedly after the FtsZ and PD rings are formed (Pyke, 1999). Recent analyses of Arabidopsis mutants showing a phenotype similar to arc5 led to identification of PDV1 (plastid division 1), an integral protein of the outer envelope membrane. Database searches found a protein paralogous to PDV1, termed PDV2. Similar to DRP5B, PDV1 is localized to a discontinuous ring at the plastid division site. Based on the mutant phenotypes of these genes, we hypothesize that PDV1 and PDV2 act co-ordinately and recruit DRP5B to the division site (Miyagishima et al., 2006).
Relationship Between Plastid Division Components
As aforementioned, many factors of eukaryotic and prokaryotic origin have been identified as components of the chloroplast division machinery in the last decade. Precise roles of these division proteins remain unclear and further characterization is necessary. Nevertheless, combining cytological observations (mainly performed in the red alga, C. merolae) with molecular-genetic studies (using available mutants in Arabidopsis) has uncovered (at least part of) the spatio-temporal relationship amongst these components, as follows (Marrison et al., 1999; Miyagishima et al., 2003; Osteryoung and Nunnari, 2003; Maple et al., 2005; Glynn et al., 2007; Maple and Møller, 2007; Figure 6). The FtsZ, PD and dynamin rings form in this order at the division site. Small dynamin patches are discontinuously localized at the division site at the onset of constriction. After this, constriction commences and, at later stages of constriction, dynamin forms a continuous ring structure (Miyagishima et al., 2003). The FtsZ and inner PD rings disappear just before the completion of division. In contrast, remnants of the outer PD ring remain between the daughter chloroplasts, and remnants of the dynamin ring remain clinging to only one daughter chloroplast. Following the completion of division, these remnants of the PD and dynamin rings eventually disappear and dissolve in the cytosol (Miyagishima et al., 2003).
Studies in Arabidopsis suggest that MinD and MinE regulate the positioning of FtsZ ring formation, as in bacteria. ARC3 also appears to play a role in this positioning process. ARC6 is suggested to be involved in the formation or stabilization of the FtsZ ring (Glynn et al., 2007; Maple and Møller, 2007). PDV1 and PDV2 recruit the ARC5/DRP5B dynamin to the division site. A very recent study using the chloroplast division apparatus isolated from C. merolae suggested that the rings are connected by unknown factors that span across the two envelope membranes. Interestingly, it was also shown that the isolated complex (the various rings) is capable of constriction after stretching, most likely by the function of the dynamin protein (Yoshida et al., 2006).
Other Proteins Implicated in Chloroplast Division and Its Regulation
Disruption of three other genes of cyanobacterial origin, ALB4 (albino 4), CRL (crumpled leaf), AtSulA/GC1 (giant chloroplast 1), and a single gene of eukaryotic origin, FZL (FZO-like), were shown to impair chloroplast division leading to altered chloroplast morphologies in Arabidopsis (reviewed in Glynn et al., 2007; Maple and Møller, 2007). It is currently unclear, however, whether these proteins are directly involved in chloroplast division or how these proteins are involved in the division process. In addition, genes related to the cyanobacterial pathway for peptidoglycan synthesis are present in plants, and these possibly affect chloroplast division. In the moss, Physcomitrella patens, disruption of these genes causes defects in chloroplast division, while in Arabidopsis the development of chloroplasts was perturbed in the corresponding mutants. How these genes are involved in chloroplast division awaits further research.
Additional factors, MSL (MscS-like protein) and CDT1 (cdc10-dependent transcript 1), were identified as regulators of chloroplast division through reverse-genetic approaches. Arabidopsis MSL proteins are homologous to the bacterial mechanosensitive ion channel protein, MscS. In bacteria, MscS proteins are involved in regulating osmotic potential across the cell membrane in response to increased membrane tension induced by osmotic shock. Arabidopsis MSL2 and MSL3 are localized to plastid membranes, and the mesophyll cells in msl2 msl3 double mutants have a smaller number of large chloroplasts than those in the wild type. Based on these results, it is suggested that the MSL system plays a role in the density dependent control of chloroplast numbers and volume in mesophyll cells (Haswell and Meyerowitz, 2006).
CDT1 is a member of the pre-replication complex in eukaryotes, and acts as a key regulator of nuclear DNA replication. Arabidopsis CDT1a was shown to be dually targeted to the nucleus and chloroplasts. Interestingly, AtCDT1-RNAi plants show defects in plastid division. This study suggested that AtCDT1a is a component of the nuclear pre-replication complex as in other eukaryotes, and that its additional plastidic form regulates FtsZ ring formation through interaction with ARC6 (Raynaud et al., 2005).
INHERITANCE OF PLASTIDS
As implicated in earlier works in the 1900s by Baur and Corens, which were published shortly after the re-discovery of Mendelian inheritance, some genetic traits (in this case exemplified by leaf variegation in Mirabilis) were demonstrated to show maternal transmission (Hagemann, 2000). At the present time, a large body of data regarding the mode of inheritance of chloroplasts has been accumulated (Kirk and Tilney-Bassett, 1978; Hagemann and Schrödoer, 1989; Kuroiwa, 1991; Birky, 1995; Mogensen, 1996; Hagemann, 2004). In plants, chloroplasts and mitochondria are the subject of organelle genetics, since both organelles were derived from endosymbiosis and both contain their own genomes (Birky, 2001). Despite such a long-lasting interest, very little is known about how ptDNA is distributed into daughter plastids upon plastid division. Likewise, we know very little about the molecular mechanisms of plastid inheritance, although many cytological analyses strongly indicate that the behavior of plastids in male gametes is a key issue. In this review, we consider the inheritance mode of plastids, and place particular emphasis on the fate of plastid and ptDNAs during pollen development. We also focus on knowledge related to Arabidopsis, comparing it with other species.
Types of Plastid Inheritance – Maternal Inheritance Is Common But Not Absolute
To begin this area of investigation, it is logical to ask the question “What happens if two different types of plastid co-exist in the same cell?” Since no event of plastid fusion has been previously demonstrated in higher plants, either one of the plastid types remains, or both types co-exist. We can test these hypotheses by performing protoplast fusions with two different cell types, and then following their plastid types during subsequent plant regeneration (Perl et al., 1991; Wolters et al., 1993). Such experiments suggest that only one type remains, and that the other type is excluded. Based upon these observations, which are sometimes referred to as ‘vegetative segregation’, we assume that two plastid types can co-exist, but it appears that either one or the other is eventually sorted out or lost (leading to ‘homoplasmy’). This ‘sort-out’ mechanism is also applicable during fertilization, if a zygotic cell formed by fertilization contains plastids from both male and female gametes (Birky, 2001). If these two types co-exist, then this condition is called ‘heteroplasmy’.
Plastid inheritance after fertilization in an F1 cross might be: (1) uniparental maternal type; (2) uniparental paternal type; or (3) biparental type. In biparental plants, vegetative segregation leads to the sorting-out of either plastid type, resulting in maternal or paternal homoplasmy in the following generations. This mode of plastidic inheritance has been genetically identified in many angiosperms, and examples of each of the other aforementioned types have also been reported. However, there is an apparent bias in the proportion of their occurrence (Kuroiwa, 1991; Mogensen, 1996; Hagemann, 2004). In angiosperms, maternal inheritance dominates over the others: cytological studies (see below) suggest that approximately 80% of all species show maternal inheritance, and that the remaining species show biparental inheritance (Corriveau and Coleman, 1988; Zhang et al., 2003). In contrast, uniparental paternal inheritance of plastids is very rare, and is only reported in the kiwi plant (Actinidia deliciosa). Thus, one can assume that the behavior of plastids (or ptDNA) during pollen development and fertilization is relevant to plastid inheritance.
Plastid Behavior During Pollen Development
Mature angiosperm pollen grains are composed of two or three male reproductive cells (McCormick, 2004). A tetrad formed by meiosis of pollen mother cells gives rise to four microspores. Each microspore undergoes pollen mitosis I (PMI) to divide into a larger vegetative cell and a smaller generative cell. Subsequently, the generative cell undergoes pollen mitosis II (PMII) which results in the formation of two sperm cells. The timing of PMII varies among species, sometimes occurring within the anther, although more commonly it occurs during pollen tube growth. In Arabidopsis, mature pollen is comprised of one vegetative and two sperm cells (PMII completed before pollen maturation, Figure 7). How do plastids behave during this maturation process? At the present time, it appears that there are at least several important steps which determine plastid inheritance by including or excluding plastids. Here we focus on species showing maternal inheritance, and follow Hagemann (2004) in an attempt to categorize these species.
Figure 7.
Plastid inheritance in Arabidopsis.
(A) A schematic representation illustrating the transmission of plastids and mitochondria and their nucleoids during pollen development. Note that organelle DNAs are represented as nucleoids detected by DAPI and other fluorescent dyes. Note also that the signals disappear in mature pollen.
(B) Micrographs of a microspore (left) and a mature pollen grain (right) stained by DAPI (ecotype Columbia). Arrowheads indicate the prominent DAPI-stained signals, representing plastid nucleoids.
(C) An example of maternal inheritance revealed by nuclear and plastid DNA polymorphisms. F1 plants were generated by reciprocal crosses between the ecotypes Columbia (Col) and Cape Verde Islands (Cvi). Total DNAs from the parents and the F1 plants were subjected to CAPS (Cleaved Amplified Polymorphic Sequence) analyses, to detect both nuclear and plastid DNA polymorphisms (top and bottom panels, respectively). DNA fragments representing Col and Cvi genotypes are indicated by magenta and cyan arrowheads, respectively. The CAPS markers used were G4711 for nuclear DNA, and ndhG for plastid DNA.
Careful examination of pollen tissues by transmission electron microscopy reveals organelle behavior throughout PMI and PMII. According to the classification described by Hagemann (2004), the exclusion of pollen plastids occurs at different steps. The first step occurs at the stage of PMI, where the asymmetric division results in the formation of a generative cell which is devoid of plastids (Figure 7). This type of exclusion occurs in Lycopersicon, and so the relevant plants are referred to as ‘Lycopersicon type’ species. The second step at which exclusion may occur is within the generative cell, in which the incorporated plastids are degraded by unknown mechanisms. This occurs in Solanum, and so the relevant plants are termed ‘Solanum type’ species. The last step occurs at the onset of fertilization, and is related to the fact that sperm cells are much smaller in volume than egg cells. As a consequence of this disparity, any plastids present in the sperm will occupy only a limited amount of cytoplasm, and so will not contribute to fertilization and are excluded from the zygote. This type of exclusion occurs in Triticum, and so the relevant plants are termed ‘Triticum type’ species. Studies performed by our research group and by other investigators have demonstrated that Arabidopsis is a ‘Lycopersicon type’ species, and that it transmits plastids maternally (Martínez et al., 1997; Nagata et al., 1999) (Figure 7). In some species, while plastids are obviously dispensable in the sperm cells, many plastids can nevertheless be observed in the cytoplasm of the vegetative cell of the male gametophyte. In contrast with the maternal inheritance species, plastids containing ptDNAs are detectable in the pollen of biparental species. The latter include Pelagonium, and so these are termed ‘Pelagonium type’ species.
Strictness of Maternal Inheritance
The aforementioned cytological and genetic studies demonstrated that maternal inheritance is a dominant way of plastid transmission in angiosperms. However, this maternal inheritance mode is not absolute, even in the species that were experimentally demonstrated to show maternal inheritance. Specifically, at a relatively low frequency, some paternal plastids are not excluded or degenerated during pollen maturation, and so may be transmitted into the next generation (Azhagiri and Maliga, 2007; Ruf et al., 2007; Svab and Maliga, 2007). Paternal transmission of plastids should be detectable at a certain frequency by following a phenotype (such as yellow or variegated leaves) linked to the paternal plastids in the F1 generation (Hagemann, 2004). Given its occurrence at an extremely low frequency, the detection of paternal inheritance is technically very challenging. Thus, a plastid phenotype which allows for positive and strong selection, such as antibiotic resistance encoded by the plastid genome, can greatly facilitate detailed assessments of the leakage of paternal plastids. In Arabidopsis, plastids are maternally transmitted (‘Lycopersicon type’). Fortunately, a strong selection by spectinomycin resistance is possible due to a mutation which resides in the 16S rRNA gene. Thus, paternally transmitted ptDNA can be detected at a frequency of 3.9 × 10−5 (Azhagiri and Maliga, 2007). These results imply that, despite a very low frequency, paternal plastids can overcome all of the aforementioned exclusion steps and still be transmitted into subsequent generations.
Do Plastid DNAs Decrease During Pollen Development?
The mode of plastid inheritance (particularly maternal inheritance) is in large part the consequence of plastid exclusion or degeneration during pollen maturation and fertilization. However, another interesting question is whether the amount of ptDNA decreases prior to pollen maturation, and whether this reduction is associated with plastid inheritance. A change in the amount of ptDNA has been characterized in cytological studies which detect organelle DNAs through the usage of a fluorescent dye such as DAPI in pollen (Kuroiwa, 1991; Sato et al., 2003; Sakai et al., 2004). Using this method, ptDNAs can be observed as aggregates with variable size, likely representing DNA-protein complexes as plastid nucleoids (described earlier; Figures 2 and 7). Among species showing biparental plastid inheritance, we have frequently observed plastids with detectable DAPI signals (corresponding to nucleoids) in generative or sperm plastids (Nagata et al., 1999). In contrast, such DAPI signals are not detected in maternal inheritance species (Miyamura et al., 1987). This disparity serves as a convenient method for assessing the ‘potential’ for biparental inheritance (Corriveau and Coleman, 1988; Zhang et al., 2003). In fact, it has enabled us to estimate that 80% of angiosperms exhibit maternal inheritance. Although the presence of organellar DAPI signals in sperm cells does not always guarantee biparental inheritance, this observation implies that the amount of ptDNAs that are retained in sperm plastids is controlled, either by preferential replication or by degradation.
We must be cautious when making conclusions that are based upon these observations. DAPI signals (even signals with other fluorescent dyes) do not represent the quantity of DNAs. On the contrary, they tend to show the physiological state in which ptDNA is condensed with associating proteins. Likewise, the absence of DAPI signals does not mean that organelle DNAs are completely missing, as evidenced by several exceptional species showing biparental inheritance. Nevertheless, it should be noted that the ptDNA signals detected by DAPI are missing even in the vegetative cells which are unlike the sperm cells and do not contribute to fertilization (Miyamura et al., 1987; Corriveau, 1991). We recently characterized DAPI-detectable ptDNAs during pollen maturation in Arabidopsis (Figure 7). These DAPI signals were abundant at the initial stage of pollen maturation, were reduced at and around PMII, and then finally disappeared in the vegetative cell of mature pollen (manuscript in preparation). Again, the disappearance of DAPI signals may not represent the actual degradation of DNA. However, at the least it can be concluded that ptDNAs undergo a physiological change during pollen development. We place particular emphasis on this change of ptDNA status, because it may be somewhat related to male-specific degradation of chloroplast DNAs seen in the lower unicellular alga, Chlamydomonas (Sager and Lane, 1972). Sears and VanWinkle-Swift (1994) proposed that the degradation of organelle DNAs plays an important role in gametogenesis by acting partly as a salvage pathway for nucleotide synthesis. Although the diminishment of organelle DNAs detected by DAPI in higher plant pollen may not directly correlate with the mode of plastid inheritance, it may reveal some of the important issues regarding the metabolism of organellar DNA.
PERSPECTIVES
Chloroplast biogenesis is one of the most important subjects in plant biology. Complete and comprehensive coverage of this subject area could consume the space of an entire textbook, and so we have elected to focus on several key aspects within this article. In the last two decades, we have seen considerable progress that has significantly increased our understanding of chloroplast biogenesis. Scientists working within the field have benefited significantly from the collective advancements achieved within the Arabidopsis research community. Nevertheless, there are several fundamental questions that remain to be answered. For example, early events associated with thylakoid biogenesis are poorly understood. Thylakoid membranes are considered to be derived from the inner envelope membrane, but the molecular events that underlie their biogenesis are not fully appreciated. It is likely that conventional molecular-genetic studies may be difficult in this case, since fundamental defects in thylakoid formation would likely be detrimental to plant survival. Additionally, the mechanisms by which thylakoids are distributed between daughter cells upon plastid division awaits elucidation in future research. The processes involved in the distribution of chloroplasts (during cell division) or chloroplastic components such as ptDNA (during organellar division) are also poorly understood. It is possible that experiments which aim to shed light on the continuity or maintenance of plastids, rather than their biogenesis or formation, may be the direction of research endeavors in the next decade. Systematic investigations of genome information, gene products, and their tissue-specific patterns of expression and activity will enable us to address unresolved questions within this field.
Acknowledgments
We thank Chieko Saito, Ryo Matsushima, Yusuke Kato, and Yasuyuki Uno for their help in preparing figures. Research in the authors' laboratories was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (to W.S.), and by the Royal Society Rosenheim Fellowship and the Biotechnology and Biological Sciences Research Council (to P.J.).
Footnotes
Citation: Sakamoto W., Miyagishima S., and Jarvis P. (2008) Chloroplast Biogenesis: Control of Plastid Development, Protein Import, Division and Inheritance. The Arabidopsis Book 6:e0110. doi:10.1199/tab.0110
elocation-id: e0110
Published on: July 22, 2008
REFERENCES
- Adam Z., Rudella A., van Wijk K. J. Recent advances in the study of Clp, FtsH and other proteases located in chloroplasts. Curr. Opin. Plant Biol. 2006;96(1):234–240. doi: 10.1016/j.pbi.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Akita M., Nielsen E., Keegstra K. Identification of protein transport complexes in the chloroplastic envelope membranes via chemical cross-linking. J. Cell Biol. 1997;1366(1):983–994. doi: 10.1083/jcb.136.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht V., Ingenfeld A., Apel K. Characterization of the snowy cotyledon 1 mutant of Arabidopsis thaliana: the impact of chloroplast elongation factor G on chloroplast development and plant vitality. Plant Mol. Biol. 2006;606(1):507–518. doi: 10.1007/s11103-005-4921-0. [DOI] [PubMed] [Google Scholar]
- Alder N. N., Theg S. M. Energetics of protein transport across biological membranes: a study of the thylakoid ΔpH-dependent/cpTat pathway. Cell. 2003;1126(1):231–242. doi: 10.1016/s0092-8674(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Allison L. A., Simon L. D., Maliga P. Deletion of rpoB reveals a second distinct transcription system in plastids of higher plants. EMBO J. 1996;156(1):2802–2809. [PMC free article] [PubMed] [Google Scholar]
- Aro E. M., Virgin I., Andersson B. Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;11436(1):113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Asakura Y., Barkan A. Arabidopsis orthologs of maize chloroplast splicing factors promote splicing of orthologous and species-specific group II introns. Plant Physiol. 2006;1426(1):1656–1663. doi: 10.1104/pp.106.088096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aseeva E., Ossenbuhl F., Sippel C., Cho W. K., Stein B., Eichacker L. A., Meurer J., Wanner G., Westhoff P., Soll J., Vothknecht U. C. Vipp1 is required for basic thylakoid membrane formation but not for the assembly of thylakoid protein complexes. Plant Physiol. Biochem. 2007;456(1):119–128. doi: 10.1016/j.plaphy.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Austin J. R., 2nd, Frost E., Vidi P. A., Kessler F., Staehelin L. A. Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell. 2006;186(1):1693–1703. doi: 10.1105/tpc.105.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhagiri A. K., Maliga P. Exceptional paternal inheritance of plastids in Arabidopsis suggests that low-frequency leakage of plastids via pollen may be universal in plants. Plant J. 2007;526(1):817–823. doi: 10.1111/j.1365-313X.2007.03278.x. [DOI] [PubMed] [Google Scholar]
- Bae W., Lee Y. J., Kim D. H., Lee J., Kim S., Sohn E. J., Hwang I. AKR2A-mediated import of chloroplast outer membrane proteins is essential for chloroplast biogenesis. Nat. Cell Biol. 2008;106(1):220–227. doi: 10.1038/ncb1683. [DOI] [PubMed] [Google Scholar]
- Baginsky S., Gruissem W. Chloroplast proteomics: potentials and challenges. J. Exp. Bot. 2004;556(1):1213–1220. doi: 10.1093/jxb/erh104. [DOI] [PubMed] [Google Scholar]
- Balmer Y., Koller A., del Val G., Manieri W., Schurmann P., Buchanan B. B. Proteomics gives insight into the regulatory function of chloroplast thioredoxins. Proc. Natl. Acad. Sci. USA. 2003;1006(1):370–375. doi: 10.1073/pnas.232703799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer Y., Vensel W. H., DuPont F. M., Buchanan B. B., Hurkman W. J. Proteome of amyloplasts isolated from developing wheat endosperm presents evidence of broad metabolic capability. J. Exp. Bot. 2006a;576(1):1591–1602. doi: 10.1093/jxb/erj156. [DOI] [PubMed] [Google Scholar]
- Balmer Y., Vensel W. H., Cai N., Manieri W., Schurmann P., Hurkman W. J., Buchanan B. B. A complete ferre-doxin/thioredoxin system regulates fundamental processes in amyloplasts. Proc. Natl. Acad. Sci. USA. 2006b;1036(1):2988–2993. doi: 10.1073/pnas.0511040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A. Proteins encoded by a complex chloroplast transcription unit are each translated from both monocistronic and poly-cistronic mRNAs. EMBO J. 1988;76(1):2637–2644. doi: 10.1002/j.1460-2075.1988.tb03116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A. Intron splicing in plant organelles. In: Daniell H., Chase C., editors. Molecular Biology and Biotechnology of plant organelles. 1. Vol. 6. Dortrecht: Springer; 2004. pp. 295–322. [Google Scholar]
- Barkan A., Goldschmidt-Clermont M. Participation of nuclear genes in chloroplast gene expression. Biochimie. 2000;826(1):559–572. doi: 10.1016/s0300-9084(00)00602-7. [DOI] [PubMed] [Google Scholar]
- Barker M., de Vries R., Nield J., Komenda J., Nixon P. J. The deg proteases protect Synechocystis sp. PCC 6803 during heat and light stresses but are not essential for removal of damaged D1 protein during the photosystem two repair cycle. J. Biol. Chem. 2006;2816(1):30347–30355. doi: 10.1074/jbc.M601064200. [DOI] [PubMed] [Google Scholar]
- Bauer J., Chen K., Hiltbunner A., Wehrli E., Eugster M., Schnell D., Kessler F. The major protein import receptor of plastids is essential for chloroplast biogenesis. Nature. 2000;4036(1):203–207. doi: 10.1038/35003214. [DOI] [PubMed] [Google Scholar]
- Becker T., Jelic M., Vojta A., Radunz A., Soll J., Schleiff E. Preprotein recognition by the Toc complex. EMBO J. 2004a;236(1):520–530. doi: 10.1038/sj.emboj.7600089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T., Hritz J., Vogel M., Caliebe A., Bukau B., Soll J., Schleiff E. Toc12, a novel subunit of the intermembrane space preprotein translocon of chloroplasts. Mol. Biol. Cell. 2004b;156(1):5130–5144. doi: 10.1091/mbc.E04-05-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard J., Kubis S., Bimanadham S., Jarvis P. Functional similarity between the chloroplast translocon component, Tic40, and the human co-chaperone, Hsp70-interacting protein (Hip). J. Biol. Chem. 2007;2826(1):21404–21414. doi: 10.1074/jbc.M611545200. [DOI] [PubMed] [Google Scholar]
- Benning C., Xu C., Awai K. Non-vesicular and vesicular lipid trafficking involving plastids. Curr. Opin. Plant Biol. 2006;96(1):241–247. doi: 10.1016/j.pbi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Biehl A., Richly E., Noutsos C., Salamini F., Leister D. Analysis of 101 nuclear transcriptomes reveals 23 distinct regulons and their relationship to metabolism, chromosomal gene distribution and co-ordination of nuclear and plastid gene expression. Gene. 2005;3446(1):33–41. doi: 10.1016/j.gene.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Birky C. W., Jr. Uniparental inheritance of mitochondrial and chloroplast genes: mechanisms and evolution. Proc. Natl. Acad. Sci. USA. 1995;926(1):11331–11338. doi: 10.1073/pnas.92.25.11331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky C. W., Jr. The inheritance of genes in mitochondria and chloroplasts: laws, mechanisms, and models. Annu. Rev. Genet. 2001;356(1):125–148. doi: 10.1146/annurev.genet.35.102401.090231. [DOI] [PubMed] [Google Scholar]
- Boffey S. A., Lloyd D. Division and Segregation of Organelles. Cambridge, UK: Cambridge University Press; 1988. [Google Scholar]
- Bollenbach T. J., Lange H., Gutierrez R., Erhardt M., Stern D. B., Gagliardi D. RNR1, a 3′–5′ exoribonuclease belonging to the RNR superfamily, catalyzes 3′ maturation of chloroplast ribosomal RNAs in Arabidopsis thaliana. Nucleic Acids Res. 2005;336(1):2751–2763. doi: 10.1093/nar/gki576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bréhélin C., Kessler F., van Wijk K. J. Plastoglobules: versatile lipoprotein particles in plastids. Trends Plant Sci. 2007;126(1):260–266. doi: 10.1016/j.tplants.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Bruce B. D. The paradox of plastid transit peptides: conservation of function despite divergence in primary structure. Biochim. Biophys. Acta. 2001;15416(1):2–21. doi: 10.1016/s0167-4889(01)00149-5. [DOI] [PubMed] [Google Scholar]
- Cahoon A. B., Komine Y., Stern D. B. Plastid transcriptioin: competetion, regulation, and promotion by plastid- and nuclear-encoded polymerases. In: Wise R. R., Hoober J. K., editors. The structure and function of plastids. 1. Vol. 6. Dortrecht: Springer; 2006. pp. 167–181. [Google Scholar]
- Cavalier-Smith T. Only six kingdoms of life. Proc. Biol. Sci. 2004;2716(1):1251–1262. doi: 10.1098/rspb.2004.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Choi Y., Voytas D. F., Rodermel S. Mutations in the Arabidopsis VAR2 locus cause leaf variegation due to the loss of a chloroplast FtsH protease. Plant J. 2000;226(1):303–313. doi: 10.1046/j.1365-313x.2000.00738.x. [DOI] [PubMed] [Google Scholar]
- Chen X., Smith M. D., Fitzpatrick L., Schnell D. J. In vivo analysis of the role of atTic20 in protein import into chloroplasts. Plant Cell. 2002;146(1):641–654. doi: 10.1105/tpc.010336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi-Ham C. L., Keaton M. A., Cannon G. C., Heinhorst S. The DNA-compacting protein DCP68 from soybean chloroplasts is ferredoxin:sulfite reductase and co-localizes with the organellar nucleoid. Plant Mol. Biol. 2002;496(1):621–631. doi: 10.1023/a:1015500431421. [DOI] [PubMed] [Google Scholar]
- Chou M. L., Chu C. C., Chen L. J., Akita M., Li H. M. Stimulation of transit-peptide release and ATP hydrolysis by a cochaperone during protein import into chloroplasts. J. Cell Biol. 2006;1756(1):893–900. doi: 10.1083/jcb.200609172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constan D., Patel R., Keegstra K., Jarvis P. An outer envelope membrane component of the plastid protein import apparatus plays an essential role in Arabidopsis. Plant J. 2004;386(1):93–106. doi: 10.1111/j.1365-313X.2004.02024.x. [DOI] [PubMed] [Google Scholar]
- Corriveau J. L., Coleman A. W. Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. Am. J. Bot. 1988;756(1):1443–1458. [Google Scholar]
- Corriveau J. L., Coleman A. W. Monitoring by epifluorescence microscopy of organelle DNA fate during pollen development in five angiosperm species. Dev. Biol. 1991;1476(1):271–280. doi: 10.1016/s0012-1606(05)80024-7. [DOI] [PubMed] [Google Scholar]
- Daniell H., Chase C. Chloroplasts and mitochondria. Dortrecht: Springer; 2004. Molecular biology and biotechnology of plant organelles. [Google Scholar]
- Dekker J. P., Boekema E. J. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta. 2005;17066(1):12–39. doi: 10.1016/j.bbabio.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Douce R., Joyard J. Biochemistry and function of the plastid envelope. Annu. Rev. Cell Biol. 1990;66(1):173–216. doi: 10.1146/annurev.cb.06.110190.001133. [DOI] [PubMed] [Google Scholar]
- Duchêne A. M., Giritch A., Hoffmann B., Cognat V., Lancelin D., Peeters N. M., Zaepfel M., Marechal-Drouard L., Small I. D. Dual targeting is the rule for organellar aminoacyl-tRNA synthetases in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2005;1026(1):16484–16489. doi: 10.1073/pnas.0504682102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O., Brunak S., von Heijne G., Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc. 2007;26(1):953–971. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
- Escoubas J. M., Lomas M., LaRoche J., Falkowski P. G. Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proc. Natl. Acad. Sci. USA. 1995;926(1):10237–10241. doi: 10.1073/pnas.92.22.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favory J. J., Kobayshi M., Tanaka K., Peltier G., Kreis M., Valay J. G., Lerbs-Mache S. Specific function of a plastid sigma factor for ndhF gene transcription. Nucleic Acids Res. 2005;336(1):5991–5999. doi: 10.1093/nar/gki908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro M., Salvi D., Brugiere S., Miras S., Kowalski S., Louwagie M., Garin J., Joyard J., Rolland N. Proteomics of the chloroplast envelope membranes from Arabidopsis thaliana. Mol. Cell. Proteomics. 2003;26(1):325–345. doi: 10.1074/mcp.M300030-MCP200. [DOI] [PubMed] [Google Scholar]
- Finazzi G., Chasen C., Wollman F. A., de Vitry C. Thylakoid targeting of Tat passenger proteins shows no ΔpH dependence in vivo. EMBO J. 2003;226(1):807–815. doi: 10.1093/emboj/cdg081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk D. G., Walker M. B., Barkan A. Molecular cloning of the maize gene crp1 reveals similarity between regulators of mitochondrial and chloroplast gene expression. EMBO J. 1999;186(1):2621–2630. doi: 10.1093/emboj/18.9.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friso G., Giacomelli L., Ytterberg A. J., Peltier J. B., Rudella A., Sun Q., Wijk K. J. In-depth analysis of the thylakoid membrane proteome of Arabidopsis thaliana chloroplasts: new proteins, new functions, and a plastid proteome database. Plant Cell. 2004;166(1):478–499. doi: 10.1105/tpc.017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich J. E., Wilkerson C. G., Ray W. K., McAndrew R. S., Osteryoung K. W., Gage D. A., Phinney B. S. Proteomic study of the Arabidopsis thaliana chloroplastic envelope membrane utilizing alternatives to traditional two-dimensional electrophoresis. J. Proteome Res. 2003;26(1):413–425. doi: 10.1021/pr034025j. [DOI] [PubMed] [Google Scholar]
- Fujie M., Kuroiwa H., Kawano S., Mutoh S., Kuroiwa T. Behavior of oeganelles and their nucleoids in the shoot apical meristem during leaf development in Arabidopsis thaliana L. Planta. 1994;1946(1):395–405. [Google Scholar]
- Gao H., Kadirjan-Kalbach D., Froehlich J. E., Osteryoung K. W. ARC5, a cytosolic dynamin-like protein from plants, is part of the chloroplast division machinery. Proc. Natl. Acad. Sci. USA. 2003;1006(1):4328–4333. doi: 10.1073/pnas.0530206100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli L., Rudella A., van Wijk K. J. High light response of the thylakoid proteome in Arabidopsis wild type and the ascorbate-deficient mutant vtc2-2. A comparative proteomics study. Plant Physiol. 2006;1416(1):685–701. doi: 10.1104/pp.106.080150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn J. M., Miyagishima S., Yoder D. W., Osteryoung K. W., Vitha S. Chloroplast division. Traffic. 2007;86(1):451–461. doi: 10.1111/j.1600-0854.2007.00545.x. [DOI] [PubMed] [Google Scholar]
- Goulas E., Schubert M., Kieselbach T., Kleczkowski L. A., Gardestrom P., Schröder W., Hurry V. The chloroplast lumen and stromal proteomes of Arabidopsis thaliana show differential sensitivity to short- and long-term exposure to low temperature. Plant J. 2006;476(1):720–734. doi: 10.1111/j.1365-313X.2006.02821.x. [DOI] [PubMed] [Google Scholar]
- Gray J. C., Sullivan J. A., Wang J. H., Jerome C. A., MacLean D. Coordination of plastid and nuclear gene expression. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;3586(1):135–144. doi: 10.1098/rstb.2002.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruissem W., Barkan A., Deng X. W., Stern D. Transcriptional and post-transcriptional control of plastid mRNA levels in higher plants. Trends Genet. 1988;46(1):258–263. doi: 10.1016/0168-9525(88)90033-9. [DOI] [PubMed] [Google Scholar]
- Gutensohn M., Fan E., Frielingsdorf S., Hanner P., Hou B., Hust B., Klösgen R. B. Toc, Tic, Tat et al.: structure and function of protein transport machineries in chloroplasts. J. Plant Physiol. 2006;1636(1):333–347. doi: 10.1016/j.jplph.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Hagemann R. Erwin Baur or Carl Correns: who really created the theory of plastid inheritance. J. Hered. 2000;916(1):435–440. doi: 10.1093/jhered/91.6.435. [DOI] [PubMed] [Google Scholar]
- Hagemann R. The sexual inheritance of plant organelles. In: Daniell H., Chase C., editors. Molecular biology and biotechnology of plant organelles. 1. Vol. 6. Dortrecht: Springer; 2004. pp. 93–113. [Google Scholar]
- Hagemann R., Schrödoer M-B. The cytological basis of the plastid inheritance in angiosperms. Protoplasma. 1989;1526(1):57–64. [Google Scholar]
- Hajdukiewicz T. J., Allison L. A., Maliga P. The two RNA polymerases encoded by the nuclear and the plastid compartments transcribe distinct groups of genes in tobacco plastids. EMBO J. 1997;166(1):4041–4048. doi: 10.1093/emboj/16.13.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry E., Monahan L., Thompson L. Bacterial cell division: the mechanism and its precision. Int. Rev. Cytol. 2006;2536(1):27–94. doi: 10.1016/S0074-7696(06)53002-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto H. Double-ring structure around the constricting neck of dividing plastids of Avena sativa. Protoplasma. 1986;1356(1):166–172. [Google Scholar]
- Haswell E. S., Meyerowitz E. M. MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr. Biol. 2006;166(1):1–11. doi: 10.1016/j.cub.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Heazlewood J. L., Verboom R. E., Tonti-Filippini J., Small I., Millar A. H. SUBA: the Arabidopsis Subcellular Database. Nucleic Acids Res. 2007;356(1):D213–D218. doi: 10.1093/nar/gkl863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedtke B., Borner T., Weihe A. Mitochondrial and chloroplast phage-type RNA polymerases in Arabidopsis. Science. 1997;2776(1):809–811. doi: 10.1126/science.277.5327.809. [DOI] [PubMed] [Google Scholar]
- Heins L., Mehrle A., Hemmler R., Wagner R., Küchler M., Hörmann F., Sveshnikov D., Soll J. The preprotein conducting channel at the inner envelope membrane of plastids. EMBO J. 2002;216(1):2616–2625. doi: 10.1093/emboj/21.11.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltbrunner A., Bauer J., Vidi P. A., Infanger S., Weibel P., Hohwy M., Kessler F. Targeting of an abundant cytosolic form of the protein import receptor atToc159 to the outer chloroplast membrane. J. Cell Biol. 2001;1546(1):309–316. doi: 10.1083/jcb.200104022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohashi T., Hase T., Nakai M. Maize non-photosynthetic ferredoxin precursor is mis-sorted to the intermembrane space of chloroplasts in the presence of light. Plant Physiol. 2001;1256(1):2154–2163. doi: 10.1104/pp.125.4.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisabori T., Motohashi K., Hosoya-Matsuda N., Ueoka-Nakanishi H., Romano P. G. Towards a functional dissection of thioredoxin networks in plant cells. Photochem. Photobiol. 2007;836(1):145–151. doi: 10.1562/2006-02-27-IR-816. [DOI] [PubMed] [Google Scholar]
- Hofmann N. R., Theg S. M. Chloroplast outer membrane protein targeting and insertion. Trends Plant Sci. 2005;106(1):450–457. doi: 10.1016/j.tplants.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Inaba T., Li M., Alvarez-Huerta M., Kessler F., Schnell D. J. atTic110 functions as a scaffold for coordinating the stromal events of protein import into chloroplasts. J. Biol. Chem. 2003;2786(1):38617–38627. doi: 10.1074/jbc.M306367200. [DOI] [PubMed] [Google Scholar]
- Inoue K., Keegstra K. A polyglycine stretch is necessary for proper targeting of the protein translocation channel precursor to the outer envelope membrane of chloroplasts. Plant J. 2003;346(1):661–669. doi: 10.1046/j.1365-313x.2003.01755.x. [DOI] [PubMed] [Google Scholar]
- Inoue K. The chloroplast outer envelope membrane: the edge of light and excitement. J. Integr. Plant Biol. 2007;496(1):1100–1111. [Google Scholar]
- Ishizaki Y., Tsunoyama Y., Hatano K., Ando K., Kato K., Shinmyo A., Kobori M., Takeba G., Nakahira Y., Shiina T. A nuclear-encoded sigma factor, Arabidopsis SIG6, recognizes sigma-70 type chloroplast promoters and regulates early chloroplast development in cotyledons. Plant J. 2005;426(1):133–144. doi: 10.1111/j.1365-313X.2005.02362.x. [DOI] [PubMed] [Google Scholar]
- Isono K., Shimizu M., Yoshimoto K., Niwa Y., Satoh K., Yokota A., Kobayashi H. Leaf-specifically expressed genes for polypeptides destined for chloroplasts with domains of σ70 factors of bacterial RNA polymerases in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 1997;946(1):14948–14953. doi: 10.1073/pnas.94.26.14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova Y., Smith M. D., Chen K., Schnell D. J. Members of the Toc159 import receptor family represent distinct pathways for protein targeting to plastids. Mol. Biol. Cell. 2004;156(1):3379–3392. doi: 10.1091/mbc.E03-12-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. T., Froehlich J. E., Keegstra K. The hydrophilic domain of Tic110, an inner envelope membrane component of the chloroplastic protein translocation apparatus, faces the stromal compartment. J. Biol. Chem. 1998;2736(1):16583–16588. doi: 10.1074/jbc.273.26.16583. [DOI] [PubMed] [Google Scholar]
- Jackson-Constan D., Keegstra K. Arabidopsis genes encoding components of the chloroplastic protein import apparatus. Plant Physiol. 2001;1256(1):1567–1576. doi: 10.1104/pp.125.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Constan D., Akita M., Keegstra K. Molecular chaperones involved in chloroplast protein import. Biochim. Biophys. Acta. 2001;15416(1):102–113. doi: 10.1016/s0167-4889(01)00148-3. [DOI] [PubMed] [Google Scholar]
- Jarvis P. The proteomes of chloroplasts and other plastids. In: Šamaj J., Thelen J. J., editors. Plant proteomics. 1. Vol. 6. Heidelberg: Springer-Verlag; 2007. pp. 207–225. [Google Scholar]
- Jarvis P. The targeting of nucleus-encoded proteins to chloroplasts in plants (Tansley Review). New Phytol. 2008;1796(1):257–285. doi: 10.1111/j.1469-8137.2008.02452.x. [DOI] [PubMed] [Google Scholar]
- Jarvis P., Robinson C. Mechanisms of protein import and routing in chloroplasts. Curr. Biol. 2004;146(1):R1064–R1077. doi: 10.1016/j.cub.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Jarvis P., Chen L. J., Li H., Peto C. A., Fankhauser C., Chory J. An Arabidopsis mutant defective in the plastid general protein import apparatus. Science. 1998;2826(1):100–103. doi: 10.1126/science.282.5386.100. [DOI] [PubMed] [Google Scholar]
- Jeong S. Y., Rose A., Meier I. MFP1 is a thylakoid-associated, nucleoid-binding protein with a coiled-coil structure. Nucleic Acids Res. 2003;316(1):5175–5185. doi: 10.1093/nar/gkg693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Teyssier E., Miege C., Berny-Seigneurin D., Marechal E., Block M. A., Dorne A. J., Rolland N., Ajlani G., Douce R. The biochemical machinery of plastid envelope membranes. Plant Physiol. 1998;1186(1):715–723. doi: 10.1104/pp.118.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamaru K., Tanaka K. Roles of chloroplast RNA polymerase sigma factors in chloroplast development and stress response in higher plants. Biosci Biotechnol Biochem. 2004;686(1):2215–2223. doi: 10.1271/bbb.68.2215. [DOI] [PubMed] [Google Scholar]
- Kanamaru K., Nagashima A., Fujiwara M., Shimada H., Shirano Y., Nakabayashi K., Shibata D., Tanaka K., Takahashi H. An Arabidopsis sigma factor (SIG2)-dependent expression of plastid-encoded tRNAs in chloroplasts. Plant Cell Physiol. 2001;426(1):1034–1043. doi: 10.1093/pcp/pce155. [DOI] [PubMed] [Google Scholar]
- Karpinski S., Reynolds H., Karpinska B., Wingsle G., Creissen G., Mullineaux P. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science. 1999;2846(1):654–657. doi: 10.1126/science.284.5414.654. [DOI] [PubMed] [Google Scholar]
- Kessler F., Blobel G. Interaction of the protein import and folding machineries of the chloroplast. Proc. Natl. Acad. Sci. USA. 1996;936(1):7684–7689. doi: 10.1073/pnas.93.15.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler F., Schnell D. J. The function and diversity of plastid protein import pathways: a multilane GTPase highway into plastids. Traffic. 2006;76(1):248–257. doi: 10.1111/j.1600-0854.2005.00382.x. [DOI] [PubMed] [Google Scholar]
- Kieselbach T., Schröder W. P. The proteome of the chloroplast lumen of higher plants. Photosynth. Res. 2003;786(1):249–264. doi: 10.1023/B:PRES.0000006913.86689.f1. [DOI] [PubMed] [Google Scholar]
- Kimura S., Uchiyama Y., Kasai N., Namekawa S., Saotome A., Ueda T., Ando T., Ishibashi T., Oshige M., Furukawa T., Yamamoto T., Hashimoto J., Sakaguchi K. A novel DNA polymerase homologous to Escherichia coli DNA polymerase I from a higher plant, rice (Oryza sativa L.). Nucleic Acids Res. 2002;306(1):1585–1592. doi: 10.1093/nar/30.7.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J. T. O., Tilney-Bassett R. A. E. Their chemistry, structure, growth, and inheritance. Amsterdam: Elsevier/North-Holland Biomedical Press; 1978. The plastids. [Google Scholar]
- Kishine M., Takabayashi A., Munekage Y., Shikanai T., Endo T., Sato F. Ribosomal RNA processing and an RNase R family member in chloroplasts of Arabidopsis. Plant Mol. Biol. 2004;556(1):595–606. doi: 10.1007/s11103-004-1507-1. [DOI] [PubMed] [Google Scholar]
- Kleffmann T., Russenberger D., von Zychlinski A., Christopher W., Sjolander K., Gruissem W., Baginsky S. The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Curr. Biol. 2004;146(1):354–362. doi: 10.1016/j.cub.2004.02.039. [DOI] [PubMed] [Google Scholar]
- Kleffmann T., von Zychlinski A., Russenberger D., Hirsch-Hoffmann M., Gehrig P., Gruissem W., Baginsky S. Proteome dynamics during plastid differentiation in rice. Plant Physiol. 2007;1436(1):912–923. doi: 10.1104/pp.106.090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo A. J., Ohlrogge J. B. The predicted candidates of Arabidopsis plastid inner envelope membrane proteins and their expression profiles. Plant Physiol. 2002;1306(1):823–836. doi: 10.1104/pp.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotera E., Tasaka M., Shikanai T. A pentatricopeptide repeat protein is essential for RNA editing in chloroplasts. Nature. 2005;4336(1):326–330. doi: 10.1038/nature03229. [DOI] [PubMed] [Google Scholar]
- Koussevitzky S., Nott A., Mockler T. C., Hong F., Sachetto-Martins G., Surpin M., Lim J., Mittler R., Chory J. Signals from chloroplasts converge to regulate nuclear gene expression. Science. 2007;3166(1):715–719. [PubMed] [Google Scholar]
- Kovacheva S., Bédard J., Patel R., Dudley P., Twell D., Ríos G., Koncz C., Jarvis P. In vivo studies on the roles of Tic110, Tic40 and Hsp93 during chloroplast protein import. Plant J. 2005;416(1):412–428. doi: 10.1111/j.1365-313X.2004.02307.x. [DOI] [PubMed] [Google Scholar]
- Kubis S., Baldwin A., Patel R., Razzaq A., Dupree P., Lilley K., Kurth J., Leister D., Jarvis P. The Arabidopsis ppi1 mutant is specifically defective in the expression, chloroplast import, and accumulation of photosynthetic proteins. Plant Cell. 2003;156(1):1859–1871. doi: 10.1105/tpc.012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa H., Mori T., Takahara M., Miyagishima S., Kuroiwa T. Chloroplast division machinery as revealed by immunofluorescence and electron microscopy. Planta. 2002;2156(1):185–190. doi: 10.1007/s00425-002-0734-4. [DOI] [PubMed] [Google Scholar]
- Kuroiwa T. The replication, differentiation, and inheritance of plastids with emphasis on the concept of organelle nuclei. Int. Rev. Cytol. 1991;1286(1):1–62. [Google Scholar]
- Kuroiwa T., Kuroiwa H., Sakai A., Takahashi H., Toda K., Itoh R. The division apparatus of plastids and mitochondria. Int. Rev. Cytol. 1998;1816(1):1–41. doi: 10.1016/s0074-7696(08)60415-5. [DOI] [PubMed] [Google Scholar]
- Laloi C., Stachowiak M., Pers-Kamczyc E., Warzych E., Murgia I., Apel K. Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2007;1046(1):672–677. doi: 10.1073/pnas.0609063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin R. M., Alonso J. M., Ecker J. R., Chory J. GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science. 2003;2996(1):902–906. doi: 10.1126/science.1079978. [DOI] [PubMed] [Google Scholar]
- Lee K. P., Kim C., Landgraf F., Apel K. EXECUTER1-and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2007;1046(1):10270–10275. doi: 10.1073/pnas.0702061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leister D. Chloroplast research in the genomic age. Trends Genet. 2003;196(1):47–56. doi: 10.1016/s0168-9525(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Leister D., Pesaresi P. The genomic era of chloroplast research. In: Møller S. G., editor. Plastids, Annual Plant Reviews. 1. Vol. 13. Vol. 6. Oxford: Blackwell; 2005. pp. 1–29. In. ed. pp. [Google Scholar]
- Leon P., Arroyo A., Mackenzie S. Nuclear control of plastid and mitochondrial development in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;496(1):453–480. doi: 10.1146/annurev.arplant.49.1.453. [DOI] [PubMed] [Google Scholar]
- Li M., Schnell D. J. Reconstitution of protein targeting to the inner envelope membrane of chloroplasts. J. Cell Biol. 2006;1756(1):249–259. doi: 10.1083/jcb.200605162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Ruf S., Bock R. Constancy of organellar genome copy numbers during leaf development and senescence in higher plants. Mol. Genet. Genomics. 2006;2756(1):185–192. doi: 10.1007/s00438-005-0075-7. [DOI] [PubMed] [Google Scholar]
- Lonosky P. M., Zhang X., Honavar V. G., Dobbs D. L., Fu A., Rodermel S. R. A proteomic analysis of maize chloroplast biogenesis. Plant Physiol. 2004;1346(1):560–574. doi: 10.1104/pp.103.032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Juez E., Pyke K. A. Plastids unleashed: their development and their integration in plant development. Int. J. Dev. Biol. 2005;496(1):557–577. doi: 10.1387/ijdb.051997el. [DOI] [PubMed] [Google Scholar]
- Lopez-Juez E. Plastid biogenesis, between light and shadows. J. Exp. Bot. 2007;586(1):11–26. doi: 10.1093/jxb/erl196. [DOI] [PubMed] [Google Scholar]
- Lurin C., Andres C., Aubourg S., Bellaoui M., Bitton F., Bruyere C., Caboche M., Debast C., Gualberto J., Hoffmann B., Lecharny A., Le Ret M., Martin-Magniette M. L., Mireau H., Peeters N., Renou J. P., Szurek B., Taconnat L., Small I. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell. 2004;166(1):2089–2103. doi: 10.1105/tpc.104.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. M., Schmitz-Linneweber C. Plastid genomes. In: Daniell H., Chase C., editors. Molecular biology and biotechnology of plant organelles. 1. Vol. 6. Dortrecht: Springer; 2004. pp. 115–150. [Google Scholar]
- Maple J., Møller S. G. Plastid division coordination across a double-membraned structure. FEBS Lett. 2007;5816(1):2162–2167. doi: 10.1016/j.febslet.2007.02.062. [DOI] [PubMed] [Google Scholar]
- Maple J., Aldrige C., Møller S. G. Plastid division is mediated by combinatorial assembly of plastid division proteins. Plant J. 2005;436(1):811–823. doi: 10.1111/j.1365-313X.2005.02493.x. [DOI] [PubMed] [Google Scholar]
- Maple J., Vojta L., Soll J., Møller S. G. ARC3 is a stromal Z-ring accessory protein essential for plastid division. EMBO Rep. 2007;86(1):293–299. doi: 10.1038/sj.embor.7400902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrison J. L., Rutherford S. M., Robertson E. J., Lister C., Dean C., Leech R. M. The distinctive roles of five different ARC genes in the chloroplast division process in Arabidopsis. Plant J. 1999;186(1):651–662. doi: 10.1046/j.1365-313x.1999.00500.x. [DOI] [PubMed] [Google Scholar]
- Martin W., Rujan T., Richly E., Hansen A., Cornelsen S., Lins T., Leister D., Stoebe B., Hasegawa M., Penny D. Evolutionary analysis of Arabidopsis, cyanobacterial, and chloroplast genomes reveals plastid phylogeny and thoudands of cyanobacterial genes in the nucleus. Proc. Natl. Acad. Sci. USA. 2002;996(1):12246–12251. doi: 10.1073/pnas.182432999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez P., López C., Roldán M., Sabater B., Martín M. Plastid DNA of five ecotypes of Arabidopsis thaliana: sequence of ndhG gene and maternal inheritance. Plant Sci. 1997;1236(1):113–122. [Google Scholar]
- McAndrew R. S., Froehlich J. E., Vitha S., Stokes K. D., Osteryoung K. W. Colocalization of plastid division proteins in the chloroplast stromal compartment establishes a new functional relationship between FtsZ1 and FtsZ2 in higher plants. Plant Physiol. 2001;1276(1):1656–1666. [PMC free article] [PubMed] [Google Scholar]
- McAndrew R. S., Olson B. J., Kadirjan-Kalbach D., Chi-Ham C. L., Vitha S., Froehlich J. E., Osteryoung K. W. In vivo quantitative relationship between plastid division proteins FtsZ1 and FtsZ2 and identification of ARC6 and ARC3 in a native FtsZ complex. Biochem J. 2008. doi:10.1042/BJ20071354. [DOI] [PubMed]
- McCormick S. Control of male gametophyte development. Plant Cell. 2004;16 Suppl6(1):S142–153. doi: 10.1105/tpc.016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meierhoff K., Felder S., Nakamura T., Bechtold N., Schuster G. HCF152, an Arabidopsis RNA binding pentatricopeptide repeat protein involved in the processing of chloroplast psbB-psbT-psbH-petB-petD RNAs. Plant Cell. 2003;156(1):1480–1495. doi: 10.1105/tpc.010397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurer J., Meierhoff K., Westhoff P. Isolation of high-chlorophyll-fluorescence mutants of Arabidopsis thaliana and their characterisation by spectroscopy, immunoblotting and northern hybridisation. Planta. 1996;1986(1):385–396. doi: 10.1007/BF00620055. [DOI] [PubMed] [Google Scholar]
- Meurer J., Lezhneva L., Amann K., Godel M., Bezhani S., Sherameti I., Oelmuller R. A peptide chain release factor 2 affects the stability of UGA-containing transcripts in Arabidopsis chloroplasts. Plant Cell. 2002;146(1):3255–3269. doi: 10.1105/tpc.006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar A. H., Whelan J., Small I. Recent surprises in protein targeting to mitochondria and plastids. Curr. Opin. Plant Biol. 2006;96(1):610–615. doi: 10.1016/j.pbi.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Miras S., Salvi D., Piette L., Seigneurin-Berny D., Grunwald D., Reinbothe C., Joyard J., Reinbothe S., Rolland N. Toc159- and Toc75-independent import of a transit sequenceless precursor into the inner envelope of chloroplasts. J. Biol. Chem. 2007;2826(1):29482–29492. doi: 10.1074/jbc.M611112200. [DOI] [PubMed] [Google Scholar]
- Mita T., Kanbe T., Tanaka K., Kuroiwa T. A ring structure around the dividing plane of the Cyanidium caldarium chloroplast. Protoplasma. 1986;1306(1):211–213. [Google Scholar]
- Miura E., Kato Y., Matsushima R., Albrecht V., Laalami S., Sakamoto W. The balance between protein synthesis and degradation in chloroplasts determines leaf variegation in Arabidopsis yellow variegated mutants. Plant Cell. 2007;196(1):1313–1328. doi: 10.1105/tpc.106.049270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagishima S., Nishida K., Mori T., Matsuzaki M., Higashiyama T., Kuroiwa H., Kuroiwa T. A plant-specific dynamin-related protein forms a ring at the chloroplast division site. Plant Cell. 2003a;156(1):655–665. doi: 10.1105/tpc.009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagishima S., Nishida K., Kuroiwa T. An evolutionary puzzle: Chloroplast and mitochondrial division rings. Trends Plant Sci. 2003b;86(1):432–438. doi: 10.1016/S1360-1385(03)00193-6. [DOI] [PubMed] [Google Scholar]
- Miyagishima S., Wolk C. P., Osteryoung K. W. Identification of cyanobacterial cell division genes by comparative and mutational analyses. Mol. Microbiol. 2005;566(1):126–143. doi: 10.1111/j.1365-2958.2005.04548.x. [DOI] [PubMed] [Google Scholar]
- Miyagishima S., Froehlich J. E., Osteryoung K. W. PDV1 and PDV2 mediate recruitment of the dynamin-related protein ARC5 to the plastid division site. Plant Cell. 2006;186(1):2517–2530. doi: 10.1105/tpc.106.045484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura S., Kuroiwa T., Nagata T. Disappearance of plastids and mitochondrial nucleoids during the formation of generative cells of higher plants revealed by fluorescence microscopy. Protoplasma. 1987;1416(1):149–159. [Google Scholar]
- Mogensen H. L. The hows and whys of cytoplasmic inheritance in seed plants. Am. J. Bot. 1996;836(1):383–404. [Google Scholar]
- Møller S. G. Plastids. Oxford: Blackwell; 2005. [Google Scholar]
- Mochizuki N., Brusslan J. A., Larkin R., Nagatani A., Chory J. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc. Natl. Acad. Sci. USA. 2001;986(1):2053–2058. doi: 10.1073/pnas.98.4.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y., Kimura S., Saotome A., Kasai N., Sakaguchi N., Uchiyama Y., Ishibashi T., Yamamoto T., Chiku H., Sakaguchi K. Plastid DNA polymerases from higher plants, Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2005;3346(1):43–50. doi: 10.1016/j.bbrc.2005.06.052. [DOI] [PubMed] [Google Scholar]
- Motohashi R., Yamazaki T., Myouga F., Ito T., Ito K., Satou M., Kobayashi M., Nagata N., Yoshida S., Nagashima A., Tanaka K., Takahashi S., Shinozaki K. Chloroplast ribosome release factor 1 (AtcpRF1) is essential for chloroplast development. Plant Mol. Biol. 2007;646(1):481–497. doi: 10.1007/s11103-007-9166-7. [DOI] [PubMed] [Google Scholar]
- Mullet J. E. Chloroplast development and gene expression. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988;396(1):475–502. [Google Scholar]
- Murakami S., Kondo Y., Nakano T., Sato F. Protease activity of CND41, a chloroplast nucleoid DNA-binding protein, isolated from cultured tobacco cells. FEBS Lett. 2000;4686(1):15–18. doi: 10.1016/s0014-5793(00)01186-8. [DOI] [PubMed] [Google Scholar]
- Nada A., Soll J. Inner envelope protein 32 is imported into chloroplasts by a novel pathway. J. Cell Sci. 2004;1176(1):3975–3982. doi: 10.1242/jcs.01265. [DOI] [PubMed] [Google Scholar]
- Nagata N., Saito C., Sakai A., Kuroiwa H., Kuroiwa T. The selective increase or decrease of organellar DNA in generative cells just after pollen mitosis one controls cytoplasmic inheritance. Planta. 1999;2096(1):53–65. doi: 10.1007/s004250050606. [DOI] [PubMed] [Google Scholar]
- Nakagawara E., Sakuraba Y., Yamasato A., Tanaka R., Tanaka A. Clp protease controls chlorophyll b synthesis by regulating the level of chlorophyllide a oxygenase. Plant J. 2007;496(1):800–809. doi: 10.1111/j.1365-313X.2006.02996.x. [DOI] [PubMed] [Google Scholar]
- Nanjo Y., Oka H., Ikarashi N., Kaneko K., Kitajima A., Mitsui T., Muñoz F. J., Rodríguez-López M., Baroja-Fernández E., Pozueta-Romero J. Rice plastidial N-glycosylated nucleotide pyrophosphatase/phosphodiesterase is transported from the ER-golgi to the chloroplast through the secretory pathway. Plant Cell. 2006;186(1):2582–2592. doi: 10.1105/tpc.105.039891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassoury N., Morse D. Protein targeting to the chloroplasts of photosynthetic eukaryotes: getting there is half the fun. Biochim. Biophys. Acta. 2005;17436(1):5–19. doi: 10.1016/j.bbamcr.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Nielsen E., Akita M., Davila-Aponte J., Keegstra K. Stable association of chloroplastic precursors with protein translocation complexes that contain proteins from both envelope membranes and a stromal Hsp100 molecular chaperone. EMBO J. 1997;166(1):935–946. doi: 10.1093/emboj/16.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon P. J., Barker M., Boehm M., de Vries R., Komenda J. FtsH-mediated repair of the photosystem II complex in response to light stress. J Exp Bot. 2005;566(1):357–363. doi: 10.1093/jxb/eri021. [DOI] [PubMed] [Google Scholar]
- Nott A., Jung H. S., Koussevitzky S., Chory J. Plastid-to-nucleus retrograde signaling. Annu. Rev. Plant Biol. 2006;576(1):739–759. doi: 10.1146/annurev.arplant.57.032905.105310. [DOI] [PubMed] [Google Scholar]
- Oelmuller R. Photooxidative destruction of chloroplasts and its effect on nuclear gene expression and extraplastidic enzyme levels. Photochem. Photobiol. 1989;496(1):229–239. [Google Scholar]
- Oldenburg D. J., Bendich A. J. Changes in the structure of DNA molecules and the amount of DNA per plastid during chloroplast development in maize. J. Mol. Biol. 2004;3446(1):1311–1330. doi: 10.1016/j.jmb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Olsen L. J., Keegstra K. The binding of precursor proteins to chloroplasts requires nucleoside triphosphates in the intermembrane space. J. Biol. Chem. 1992;2676(1):433–439. [PubMed] [Google Scholar]
- Ono Y., Sakai A., Takechi K., Takio S., Takusagawa M., Takano H. NtPolI-like1 and NtPolI-like2, bacterial DNA polymerase I homologs isolated from BY-2 cultured tobacco cells, encode DNA polymerases engaged in DNA replication in both plastids and mitochondria. Plant Cell Physiol. 2007;486(1):1679–1692. doi: 10.1093/pcp/pcm140. [DOI] [PubMed] [Google Scholar]
- Ostersetzer O., Kato Y., Adam Z., Sakamoto W. Multiple intracellular locations of Lon protease in Arabidopsis: evidence for the localization of AtLon4 to chloroplasts. Plant Cell Physiol. 2007;486(1):881–885. doi: 10.1093/pcp/pcm052. [DOI] [PubMed] [Google Scholar]
- Osteryoung K. W., Nunnari J. The division of endosymbiotic organelles. Science. 2003;3026(1):1698–1704. doi: 10.1126/science.1082192. [DOI] [PubMed] [Google Scholar]
- Osteryoung K. W., Vierling E. Conserved cell and organelle division. Nature. 1995;3766(1):473–474. doi: 10.1038/376473b0. [DOI] [PubMed] [Google Scholar]
- Osteryoung K. W., Stokes K. D., Rutherford S. M., Percival A. L., Lee W. Y. Chloroplast division in higher plants requires members of two functionally divergent gene families with homology to bacterial ftsZ. Plant Cell. 1998;106(1):1991–2004. doi: 10.1105/tpc.10.12.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier J. B., Emanuelsson O., Kalume D. E., Ytterberg J., Friso G., Rudella A., Liberles D. A., Soderberg L., Roepstorff P., von Heijne G., van Wijk K. J. Central functions of the lumenal and peripheral thylakoid proteome of Arabidopsis determined by experimentation and genome-wide prediction. Plant Cell. 2002;146(1):211–236. doi: 10.1105/tpc.010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier J. B., Ytterberg A. J., Sun Q., van Wijk K. J. New functions of the thylakoid membrane proteome of Arabidopsis thaliana revealed by a simple, fast, and versatile fractionation strategy. J. Biol. Chem. 2004a;2796(1):49367–49383. doi: 10.1074/jbc.M406763200. [DOI] [PubMed] [Google Scholar]
- Peltier J. B., Ripoll D. R., Friso G., Rudella A., Cai Y., Ytterberg J., Giacomelli L., Pillardy J., van Wijk K. J. Clp protease complexes from photosynthetic and non-photosynthetic plastids and mitochondria of plants, their predicted three-dimensional structures, and functional implications. J. Biol. Chem. 2004b;2796(1):4768–4781. doi: 10.1074/jbc.M309212200. [DOI] [PubMed] [Google Scholar]
- Peltier J. B., Cai Y., Sun Q., Zabrouskov V., Giacomelli L., Rudella A., Ytterberg A. J., Rutschow H., van Wijk K. J. The oligomeric stromal proteome of Arabidopsis thaliana chloroplasts. Mol. Cell. Proteomics. 2006;56(1):114–133. doi: 10.1074/mcp.M500180-MCP200. [DOI] [PubMed] [Google Scholar]
- Perl A., Aviv D., Galun E. Nuclear-organelle interactioin in Solanum: Interspecific cybridizations and their correlation with a plastome dendrogram. Mol. Gen. Genet. 1991;2286(1):193–200. doi: 10.1007/BF00282465. [DOI] [PubMed] [Google Scholar]
- Pesaresi P., Masiero S., Eubel H., Braun H. P., Bhushan S., Glaser E., Salamini F., Leister D. Nuclear photosynthetic gene expression is synergistically modulated by rates of protein synthesis in chloroplasts and mitochondria. Plant Cell. 2006;186(1):970–991. doi: 10.1105/tpc.105.039073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaresi P., Schneider A., Kleine T., Leister D. Interorganellar communication. Curr. Opin. Plant Biol. 2007;106(1):600–606. doi: 10.1016/j.pbi.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Praefcke G. J., McMahon H. T. The dynamin superfamily: universal membrane tubulation and fission molecules. Nat. Rev. Mol. Cell Biol. 2004;56(1):133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- Pyke K. A. Plastid division and development. Plant Cell. 1999;116(1):549–556. doi: 10.1105/tpc.11.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke K. A., Leech R. M. A genetic analysis of chloroplast division and expansion in Arabidopsis thaliana. Plant Physiol. 1994;1046(1):201–207. doi: 10.1104/pp.104.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qbadou S., Becker T., Mirus O., Tews I., Soll J., Schleiff E. The molecular chaperone Hsp90 delivers precursor proteins to the chloroplast import receptor Toc64. EMBO J. 2006;256(1):1836–1847. doi: 10.1038/sj.emboj.7601091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud C., Perennes C., Reuzeau C., Catrice O., Brown S., Bergounioux C. Cell and plastid division are coordinated through the prereplication factor AtCDT1. Proc. Natl. Acad. Sci. USA. 2005;1026(1):8216–8221. doi: 10.1073/pnas.0502564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann S., Inoue K., Keegstra K. Evolution of the general protein import pathway of plastids. Mol. Membr. Biol. 2005;226(1):73–86. doi: 10.1080/09687860500041916. [DOI] [PubMed] [Google Scholar]
- Reyes-Prieto A., Weber A. P., Bhattacharya D. The origin and establishment of the plastid in algae and plants. Annu. Rev. Genet. 2007;416(1):147–168. doi: 10.1146/annurev.genet.41.110306.130134. [DOI] [PubMed] [Google Scholar]
- Rochaix J-D. The role of nucleus- and cytoplasmic-encoded factors in the synthesis of the photosynthetic apparatus. In: Wise R. R., Hoober J. K., editors. The structure and function of plastids. 1. Vol. 6. Dortrecht: Springer; 2006. pp. 145–165. [Google Scholar]
- Rolland N., Ferro M., Seigneurin-Berny D., Garin J., Douce R., Joyard J. Proteomics of chloroplast envelope membranes. Photosynth. Res. 2003;786(1):205–230. doi: 10.1023/B:PRES.0000006891.12416.6c. [DOI] [PubMed] [Google Scholar]
- Rowan B. A., Oldenburg D. J., Bendich A. J. The demise of chloroplast DNA in Arabidopsis. Curr. Genet. 2004;466(1):176–181. doi: 10.1007/s00294-004-0515-7. [DOI] [PubMed] [Google Scholar]
- Ruf S., Karcher D., Bock R. Determining the transgene containment level provided by chloroplast transformation. Proc. Natl. Acad. Sci. USA. 2007;1046(1):6998–7002. doi: 10.1073/pnas.0700008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Lane D. Molecular basis of maternal inheritance. Proc. Natl. Acad. Sci. USA. 1972;696(1):2410–2413. doi: 10.1073/pnas.69.9.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha D., Prasad A. M., Srinivasan R. Pentatricopeptide repeat proteins and their emerging roles in plants. Plant Physiol Biochem. 2007;456(1):521–534. doi: 10.1016/j.plaphy.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Sakai A., Takano H., Kuroiwa T. Organelle nuclei in higher plants: structure, composition, function, and evolution. Int. Rev. Cytol. 2004;2386(1):59–118. doi: 10.1016/S0074-7696(04)38002-2. [DOI] [PubMed] [Google Scholar]
- Sakamoto W. Protein degradation machineries in plastids. Annu. Rev. Plant Biol. 2006;576(1):599–621. doi: 10.1146/annurev.arplant.57.032905.105401. [DOI] [PubMed] [Google Scholar]
- Sakamoto W., Tamura T., Hanba-Tomita Y., Murata M. The VAR1 locus of Arabidopsis encodes a chloroplastic FtsH and is responsible for leaf variegation in the mutant alleles. Genes Cells. 2002;76(1):769–780. doi: 10.1046/j.1365-2443.2002.00558.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto W., Zaltsman A., Adam Z., Takahashi Y. Coordinated regulation and complex formation of yellow variegated1 and yellow variegated2, chloroplastic FtsH metalloproteases involved in the repair cycle of photosystem II in Arabidopsis thylakoid membranes. Plant Cell. 2003;156(1):2843–2855. doi: 10.1105/tpc.017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sane A. P., Stein B., Westhoff P. The nuclear gene HCF107 encodes a membrane-associated R-TPR (RNA tetratri-copeptide repeat)-containing protein involved in expression of the plastidial psbH gene in Arabidopsis. Plant J. 2005;426(1):720–730. doi: 10.1111/j.1365-313X.2005.02409.x. [DOI] [PubMed] [Google Scholar]
- Sato N. Was the evolution of plastid genetic machinery discontinuous>. Trends Plant Sci. 2001;66(1):151–155. doi: 10.1016/s1360-1385(01)01888-x. [DOI] [PubMed] [Google Scholar]
- Sato N., Nakayama M., Hase T. The 70-kDa major DNA-compacting protein of the chloroplast nucleoid is sulfite reductase. FEBS Lett. 2001;4876(1):347–350. doi: 10.1016/s0014-5793(00)02342-5. [DOI] [PubMed] [Google Scholar]
- Sato N., Ohshima K., Watanabe A., Ohta N., Nishiyama Y., Joyard J., Douce R. Molecular characterization of the PEND protein, a novel bZIP protein present in the envelope membrane that is the site of nucleoid replication in developing plastids. Plant Cell. 1998;106(1):859–872. doi: 10.1105/tpc.10.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Terasawa K., Miyajima K., Kabeya Y. Organization, developmental dynamics, and evolution of plastid nucleoids. Int. Rev. Cytol. 2003;2326(1):217–262. doi: 10.1016/s0074-7696(03)32006-6. [DOI] [PubMed] [Google Scholar]
- Sato S., Nakamura Y., Kaneko T., Asamizu E., Tabata S. Complete structure of the chloroplast genome of Arabidopsis thaliana. DNA Res. 1999;66(1):283–290. doi: 10.1093/dnares/6.5.283. [DOI] [PubMed] [Google Scholar]
- Schleiff E., Eichacker L. A., Eckart K., Becker T., Mirus O., Stahl T., Soll J. Prediction of the plant beta-barrel proteome: a case study of the chloroplast outer envelope. Protein Sci. 2003a;126(1):748–759. doi: 10.1110/ps.0237503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiff E., Jelic M., Soll J. A GTP-driven motor moves proteins across the outer envelope of chloroplasts. Proc. Natl. Acad. Sci. USA. 2003b;1006(1):4604–4609. doi: 10.1073/pnas.0730860100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiff E., Soll J., Küchler M., Kuhlbrandt W., Harrer R. Characterization of the translocon of the outer envelope of chloroplasts. J. Cell Biol. 2003c;1606(1):541–551. doi: 10.1083/jcb.200210060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schünemann D. Mechanisms of protein import into thylakoids of chloroplasts. Biol. Chem. 2007;3886(1):907–915. doi: 10.1515/BC.2007.111. [DOI] [PubMed] [Google Scholar]
- Shao N., Vallon O., Dent R., Niyogi K. K., Beck C. F. Defects in the cytochrome b6/f complex prevent light-induced expression of nuclear genes involved in chlorophyll biosynthesis. Plant Physiol. 2006;1416(1):1128–1137. doi: 10.1104/pp.106.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. C4 gene expression. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;506(1):187–217. doi: 10.1146/annurev.arplant.50.1.187. [DOI] [PubMed] [Google Scholar]
- Shen G., Adam Z., Zhang H. The E3 ligase AtCHIP ubiquitylates FtsH1, a component of the chloroplast FtsH protease, and affects protein degradation in chloroplasts. Plant J. 2007;526(1):309–321. doi: 10.1111/j.1365-313X.2007.03239.x. [DOI] [PubMed] [Google Scholar]
- Shiina T., Tsunoyama Y., Nakahira Y., Khan M. S. Plastid RNA polymerases, promoters, and transcription regulators in higher plants. Int. Rev. Cytol. 2005;2446(1):1–68. doi: 10.1016/S0074-7696(05)44001-2. [DOI] [PubMed] [Google Scholar]
- Shikanai T., Munekage Y., Shimizu K., Endo T., Hashimoto T. Identification and characterization of Arabidopsis mutants with reduced quenching of chlorophyll fluorescence. Plant Cell Physiol. 1999;406(1):1134–1142. doi: 10.1093/oxfordjournals.pcp.a029498. [DOI] [PubMed] [Google Scholar]
- Shimada H., Koizumi M., Kuroki K., Mochizuki M., Fujimoto H., Ohta H., Masuda T., Takamiya K. ARC3, a chloroplast division factor, is a chimera of prokaryotic FtsZ and part of eukaryotic phosphatidylinositol-4-phosphate 5-kinase. Plant Cell Physiol. 2004;456(1):960–967. doi: 10.1093/pcp/pch130. [DOI] [PubMed] [Google Scholar]
- Shirano Y., Shimada H., Kanamaru K., Fujiwara M., Tanaka K., Takahashi H., Unno K., Sato S., Tabata S., Hayashi H., Miyake C., Yokota A., Shibata D. Chloroplast development in Arabidopsis thaliana requires the nuclear-encoded transcription factor sigma B. FEBS Lett. 2000;4856(1):178–182. doi: 10.1016/s0014-5793(00)02216-x. [DOI] [PubMed] [Google Scholar]
- Siddique M. A., Grossmann J., Gruissem W., Baginsky S. Proteome analysis of bell pepper (Capsicum annuum L.) chromoplasts. Plant Cell Physiol. 2006;476(1):1663–1673. doi: 10.1093/pcp/pcl033. [DOI] [PubMed] [Google Scholar]
- Sjögren L. L., Stanne T. M., Zheng B., Sutinen S., Clarke A. K. Structural and functional insights into the chloroplast ATP-dependent Clp protease in Arabidopsis. Plant Cell. 2006;186(1):2635–2649. doi: 10.1105/tpc.106.044594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Rounds C. M., Wang F., Chen K., Afitlhile M., Schnell D. J. atToc159 is a selective transit peptide receptor for the import of nucleus-encoded chloroplast proteins. J. Cell Biol. 2004;1656(1):323–334. doi: 10.1083/jcb.200311074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J., Schleiff E. Protein import into chloroplasts. Nat. Rev. Mol. Cell Biol. 2004;56(1):198–208. doi: 10.1038/nrm1333. [DOI] [PubMed] [Google Scholar]
- Stengel A., Benz P., Balsera M., Soll J., Bölter B. Tic62 - redox-regulated translocon composition and dynamics. J. Biol. Chem., Epub ahead of print. 2008;PMID6(1):18180301. doi: 10.1074/jbc.M706719200. [DOI] [PubMed] [Google Scholar]
- Stensballe A., Hald S., Bauw G., Blennow A., Welinder K. G. The amyloplast proteome of potato tuber. FEBS J. 2008. in press. [DOI] [PubMed]
- Strand A., Asami T., Alonso J., Ecker J. R., Chory J. Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature. 2003;4216(1):79–83. doi: 10.1038/nature01204. [DOI] [PubMed] [Google Scholar]
- Strepp R., Scholz S., Kruse S., Speth V., Reski R. Plant molecular gene knockout reveals a role in plastid division for the homolog of the bacterial cell division protein FtsZ, an ancestral tubulin. Proc. Natl. Acad. Sci. USA. 1998;956(1):4368–4373. doi: 10.1073/pnas.95.8.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M. The chloroplast genome. Plant Mol. Biol. 1992;196(1):149–168. doi: 10.1007/BF00015612. [DOI] [PubMed] [Google Scholar]
- Sugiura M. History of chloroplast genomics. Photosynth. Res. 2003;766(1):371–377. doi: 10.1023/A:1024913304263. [DOI] [PubMed] [Google Scholar]
- Sun Q., Emanuelsson O., van Wijk K. J. Analysis of curated and predicted plastid subproteomes of Arabidopsis. Subcellular compartmentalization leads to distinctive proteome properties. Plant Physiol. 2004;1356(1):723–734. doi: 10.1104/pp.104.040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek R. E., Ausubel F. M., Chory J. Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell. 1993;746(1):787–799. doi: 10.1016/0092-8674(93)90459-4. [DOI] [PubMed] [Google Scholar]
- Svab Z., Maliga P. Exceptional transmission of plastids and mitochondria from the transplastomic pollen parent and its impact on transgene containment. Proc. Natl. Acad. Sci. USA. 2007;1046(1):7003–7008. doi: 10.1073/pnas.0700063104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarbreck D., Wilks C., Lamesch P., Berardini T. Z., Garcia-Hernandez M., Foerster H., Li D., Meyer T., Muller R., Ploetz L., Radenbaugh A., Singh S., Swing V., Tissier C., Zhang P., Huala E. The Arabidopsis Information Resource (TAIR): gene structure and function annotation. Nucleic Acids Res. 2008;366(1):D1009-D1014. doi: 10.1093/nar/gkm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Tozawa Y., Mochizuki N., Shinozaki K., Nagatani A., Wakasa K., Takahashi H. Characterization of three cDNA species encoding plastid DNA polymerase sigma factors in Arabidopsis thaliana: evidence for the sigma factor heterogeneity in higher plant plastids. FEBS Lett. 1997;4136(1):309–313. doi: 10.1016/s0014-5793(97)00906-x. [DOI] [PubMed] [Google Scholar]
- Tripp J., Inoue K., Keegstra K., Froehlich J. E. A novel serine/proline-rich domain in combination with a transmembrane domain is required for the insertion of AtTic40 into the inner envelope membrane of chloroplasts. Plant J. 2007;526(1):824–838. doi: 10.1111/j.1365-313X.2007.03279.x. [DOI] [PubMed] [Google Scholar]
- Tsunoyama Y., Ishizaki Y., Morikawa K., Kobori M., Nakahira Y., Takeba G., Toyoshima Y., Shiina T. Blue light-induced transcription of plastid-encoded psbD gene is mediated by a nuclear-encoded transcription initiation factor, AtSig5. Proc. Natl. Acad. Sci. USA. 2004;1016(1):3304–3309. doi: 10.1073/pnas.0308362101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S. L., Chen L. J., Smith M. D., Su Y. S., Schnell D. J., Li H. M. Import pathways of chloroplast interior proteins and the outer-membrane protein OEP14 converge at Toc75. Plant Cell. 2004;166(1):2078–2088. doi: 10.1105/tpc.104.023952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzvetkova-Chevolleau T., Hutin C., Noel L. D., Goforth R., Carde J. P., Caffarri S., Sinning I., Groves M., Teulon J. M., Hoffman N. E., Henry R., Havaux M., Nussaume L. Canonical signal recognition particle components can be bypassed for post-translational protein targeting in chloroplasts. Plant Cell. 2007;196(1):1635–1648. doi: 10.1105/tpc.106.048959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk K. J. Plastid proteomics. Plant Physiol. Biochem. 2004;426(1):963–977. doi: 10.1016/j.plaphy.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Villarejo A., Buren S., Larsson S., Dejardin A., Monne M., Rudhe C., Karlsson J., Jansson S., Lerouge P., Rolland N., von Heijne G., Grebe M., Bako L., Samuelsson G. Evidence for a protein transported through the secretory pathway en route to the higher plant chloroplast. Nat. Cell Biol. 2005;76(1):1124–1131. doi: 10.1038/ncb1330. [DOI] [PubMed] [Google Scholar]
- Vinti G., Hills A., Campbell S., Bowyer J. R., Mochizuki N., Chory J., López-Juez E. Interactions between hy1 and gun mutants of Arabidopsis, and their implications for plastid/nuclear signalling. Plant J. 2000;246(1):883–894. doi: 10.1046/j.1365-313x.2000.00936.x. [DOI] [PubMed] [Google Scholar]
- Vitha S., Froehlich J. E., Koksharova O., Pyke K. A., van Erp H., Osteryoung K. W. ARC6 is a J-domain plastid division protein and an evolutionary descendant of the cyanobacterial cell division protein Ftn2. Plant Cell. 2003;156(1):1918–1933. doi: 10.1105/tpc.013292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zychlinski A., Kleffmann T., Krishnamurthy N., Sjolander K., Baginsky S., Gruissem W. Proteome analysis of the rice etioplast: metabolic and regulatory networks and novel protein functions. Mol. Cell. Proteomics. 2005;46(1):1072–1084. doi: 10.1074/mcp.M500018-MCP200. [DOI] [PubMed] [Google Scholar]
- Walter M., Kilian J., Kudla J. PNPase activity determines the efficiency of mRNA 3′-end processing, the degradation of tRNA and the extent of polyadenylation in chloroplasts. EMBO J. 2002;216(1):6905–6914. doi: 10.1093/emboj/cdf686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. P., Schwacke R., Flügge U. I. Solute transporters of the plastid envelope membrane. Annu. Rev. Plant Biol. 2005;566(1):133–164. doi: 10.1146/annurev.arplant.56.032604.144228. [DOI] [PubMed] [Google Scholar]
- Westerlund I., Von Heijne G., Emanuelsson O. LumenP - a neural network predictor for protein localization in the thylakoid lumen. Protein Sci. 2003;126(1):2360–2366. doi: 10.1110/ps.0306003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff P., Herrmann R. G. Complex RNA maturation in chloroplasts. The psbB operon from spinach. Eur. J. Biochem. 1988;1716(1):551–564. doi: 10.1111/j.1432-1033.1988.tb13824.x. [DOI] [PubMed] [Google Scholar]
- Whitelegge J. P. Thylakoid membrane proteomics. Photosynth. Res. 2003;786(1):265–277. doi: 10.1023/B:PRES.0000006828.65688.0d. [DOI] [PubMed] [Google Scholar]
- Wise R. R., Hoober J. K. The structure and function of plastids. Dortrecht: Springer; 2006. [Google Scholar]
- Wolters A. A., Vergunst A. C., van der Werff F., Koorneef M. Analysis of nuclear and organellar DNA of somatic hybrid calli and plants between Lycopersicon spp. and Nicotiana spp. Mol. Gen. Genet. 1993;2416(1):707–718. doi: 10.1007/BF00279915. [DOI] [PubMed] [Google Scholar]
- Woodson J. D., Chory J. Coordination of gene expression between organellar and nuclear genomes. Nature Rev. Genet. 2008;96(1):383–395. doi: 10.1038/nrg2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Subramanian A. R. The plastid ribosomal proteins. Identification of all the proteins in the 50 S subunit of an organelle ribosome (chloroplast). J. Biol. Chem. 2000;2756(1):28466–28482. doi: 10.1074/jbc.M005012200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., von Knoblauch K., Subramanian A. R. The plastid ribosomal proteins. Identification of all the proteins in the 30 S subunit of an organelle ribosome (chloroplast). J. Biol. Chem. 2000;2756(1):28455–28465. doi: 10.1074/jbc.M004350200. [DOI] [PubMed] [Google Scholar]
- Yamazaki H., Tasaka M., Shikanai T. PPR motifs of the nucleus-encoded factor, PGR3, function in the selective and distinct steps of chloroplast gene expression in Arabidopsis. Plant J. 2004;386(1):152–163. doi: 10.1111/j.1365-313X.2004.02035.x. [DOI] [PubMed] [Google Scholar]
- Yeh Y. H., Kesavulu M. M., Li H. M., Wu S. Z., Sun Y. J., Konozy E. H., Hsiao C. D. Dimerization is important for the GTPase activity of chloroplast translocon components atToc33 and psToc159. J. Biol. Chem. 2007;2826(1):13845–13853. doi: 10.1074/jbc.M608385200. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Kuroiwa H., Misumi O., Nishida K., Yagisawa F., Fujiwara T., Nanamiya H., Kawamura F., Kuroiwa T. Isolated chloroplast division machinery can actively constrict after stretching. Science. 2006;3136(1):1435–1438. doi: 10.1126/science.1129689. [DOI] [PubMed] [Google Scholar]
- Young M. E., Keegstra K., Froehlich J. E. GTP promotes the formation of early-import intermediates but is not required during the translocation step of protein import into chloroplasts. Plant Physiol. 1999;1216(1):237–244. doi: 10.1104/pp.121.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ytterberg A. J., Peltier J. B., van Wijk K. J. Protein profiling of plastoglobules in chloroplasts and chromoplasts. A surprising site for differential accumulation of metabolic enzymes. Plant Physiol. 2006;1406(1):984–997. doi: 10.1104/pp.105.076083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Park S., Rodermel S. R. Functional Redundancy of AtFtsH Metalloproteases in Thylakoid Membrane Complexes. Plant Physiol. 2005;1386(1):1957–1966. doi: 10.1104/pp.105.061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaltsman A., Ori N., Adam Z. Two types of FtsH protease subunits are required for chloroplast biogenesis and Photosystem II repair in Arabidopsis. Plant Cell. 2005;176(1):2782–2790. doi: 10.1105/tpc.105.035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zghidi W., Merendino L., Cottet A., Mache R., Lerbs-Mache S. Nucleus-encoded plastid sigma factor SIG3 transcribes specifically the psbN gene in plastids. Nucleic Acids Res. 2007;356(1):455–464. doi: 10.1093/nar/gkl1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Liu Y., Sodmergen Examination of the cytoplasmic DNA in male reproductive cells to determine the potential for cytoplasmic inheritance in 295 angiosperm species. Plant Cell Physiol. 2003;446(1):941–951. doi: 10.1093/pcp/pcg121. [DOI] [PubMed] [Google Scholar]
- Zybailov B., Rutschow H., Friso G., Rudella A., Emanuelsson O., Sun Q., van Wijk K. J. Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS ONE. 2008;36(1):e1994. doi: 10.1371/journal.pone.0001994. [DOI] [PMC free article] [PubMed] [Google Scholar]


