Abstract
Background
The objective of this study was to examine the modifying effect of gender on the association between early life trauma and the hypothalamic–pituitary–adrenal (HPA) axis response to a pharmacologic challenge and a social stress task in men and women. Participants (16 men, 23 women) were the control sample of a larger study examining HPA axis function. Individuals with major depressive disorder, posttraumatic stress disorder, bipolar disorder, or psychotic or eating disorders were excluded.
Methods
In two test sessions, subjects received 1 μg/kg of corticotropin-releasing hormone (CRH) intravenously and participated in the Trier Social Stress Test (TSST). Primary outcomes included plasma cortisol and corticotropin levels measured at baseline and more than five time points following the challenges. Predictors included gender and early life trauma, as measured by the Early Trauma Index. Using factor analysis, the domains general trauma, severe trauma, and the effects of trauma were established. Using regression, these constructs were used to predict differential HPA reactivity in men and women following the challenges.
Results
The three factors accounted for the majority of the variance in the ETI. Following the CRH challenge, women had higher overall corticotropin response as dictated by the area under the curve analysis. There were no significant associations between trauma and neuroendocrine response to the TSST.
Conclusions
CRH challenge results indicate that gender differences in the impact of early trauma may help explain the differential gender susceptibility to psychopathology following adverse childhood events. This may help explain gender differences in some stress-sensitive psychiatric disorders.
Keywords: trauma, gender, HPA axis, hypothalamic–pituitary–adrenal axis, cortisol, corticotropin
INTRODUCTION
Accumulating evidence demonstrates that adverse early life experiences increase the risk of the development of psychiatric disorders in adulthood, including major depressive disorder (MDD),[1] posttraumatic stress disorder (PTSD),[2–9] and others.[10] Preclinical and clinical studies demonstrate that repeated early life trauma can lead to alterations in neurobiological systems, particularly in the hypothalamic–pituitary–adrenal (HPA) axis, leading to a pervasive heightened responsiveness to stress.[11,12] Both animal and human studies implicate early life trauma as a developmental determinant of an enduring abnormality in the responsiveness of the HPA axis, independent of psychiatric diagnosis.[13–16] There are notable gender differences in biological systems underlying the stress response, and in HPA axis function may play a role in response to early life trauma. For example, brain circuitry underlying the cognitive processing of stress (including the amygdala and hippocampus) is sexually dimorphic in humans and animals.[17] Furthermore, hormonal regulation contributes to sexual dimorphism in stress responses,[18] with both estrogen, progesterone, and, potentially, testosterone acting as potent modulators of HPA axis stress regulation.[19–22] However, it is notable that at least one study is consistent with the fact that estrogen may not regulate the HPA axis in humans.[23]
Although existing literature clearly implicates the role of early life trauma in the disruption of HPA axis functioning,[11,24–26] there is inconsistency in the type of disruption involved. This inconsistency may reflect differences in the age of trauma, type, and severity of stressor and other environmental variables.[12] Data concerning gender-specific differences in the effect of early life trauma on HPA axis functioning is also lacking and may explain some of the variability on the literature. Heim et al.[27] demonstrated that a history of childhood abuse was a significant predictor of heightened corticotropin response, but remarked that inconsistencies across studies may be explained by gender differences in response or attributed to coexisting disorders. In separate single gender studies, Heim et al.[13,24,27] examined pituitary–adrenal and autonomic reactivity to psychosocial lab stress and endocrine provocation in men and women. In particular, these studies examined the response in men and women with and without MDD and with and without a history of sexual or physical abuse. The results suggest that for both types of provocation, women with a history of childhood abuse without MDD exhibited an exaggerated corticotropin but not cortisol response, and adult men with MDD and a history of childhood abuse exhibited both an exaggerated corticotropin and cortisol response. Although it is possible to make gender comparisons across these studies, it is not ideal because men and women were not included in the same study. Of interest, the findings on cortisol in women are partially consistent with animal studies investigating chronic early life trauma, which show a blunted cortisol and corticotropin responses to stress among females.[28,29]
In a recent study by Carpenter et al.[30] corticotropin and cortisol response to the TSST were measured in men and women reporting early life trauma without MDD or PTSD. As compared to control subjects without a history of childhood maltreatment, subjects with severe childhood maltreatment had significant decreases in both corticotropin and cortisol response to the Trier Social Stress Test (TSST). In a post hoc analysis of gender differences, Carpenter et al. found (1) no gender differences in cortisol response, (2) a significant blunting of corticotropin response for men with a history of maltreatment, and (3) decreased corticotropin concentration throughout the TSST for women with a history of maltreatment. This was one of the first studies to investigate HPA response in individuals with childhood maltreatment and no significant psychopathology, and to directly examine gender comparisons of HPA axis response in individuals with early life trauma. Elzinga et al.[31] reported a blunted corticotropin response in men but not women, to the TSST in early traumatized subjects without significant psychopathology. Considering the rapidly growing literature documenting the influence of sex on brain anatomy, chemistry, and function,[32] further examination of the influence of gender on the response to early life trauma is necessary.
Furthermore, research to date has typically focused on a binary measure of trauma. In a recent large-scale study of early life adversity,[33] childhood adversity impacted psychiatric and physical health in a “dose-dependent” manner with greater adversity associated with more severe pathology in adulthood. Therefore, some measurement of trauma severity may be important in examining the lasting effects of trauma, particularly in individuals without mood and anxiety disorders for whom these effects may be more subtle.
The purpose of this study, therefore, was to examine the influence of gender on the effect of early life trauma on HPA axis response to pharmacological and psychosocial stress provocations in individuals without mood or anxiety disorders. Measures of early life trauma were derived from the Early Trauma Inventory (ETI;[35]) and analyzed as continuous variables.
METHODS
PARTICIPANTS
Participants (16 men and 23 women) were part of a larger study examining HPA axis functioning in individuals within a healthy control population. Participants were recruited primarily via media and newspaper advertisements. Written informed consent was obtained before study assessments were administered and all procedures were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki, and received Institutional Review Board approval. Exclusion criteria included (1) current MDD or PTSD; (2) psychotic, eating, or bipolar affective disorders; (3) history of or current medical conditions that might interfere with the safe conduct of the study or affect HPA activity; (4) synthetic glucocorticoid or exogenous steroid therapy within 1 month of testing; (5) pregnancy, nursing, or ineffective means of birth control; or (6) a body mass index ≥ 35. The study group did not meet current criteria for any substance abuse or dependence disorders (except caffeine/nicotine).
ASSESSMENTS
Patients meeting preliminary screening criteria were evaluated for study eligibility with the Structured Clinical Interview for the DSM-IV, which permits diagnosis of lifetime and current psychiatric disorders.[34] A history and physical examination, including electrocardiogram, was completed to assess for medical exclusions. Following baseline assessment, eligible participants were then scheduled to complete the inpatient laboratory procedure.
The ETI,[35] a 56-item self-report form, was used to evaluate early life trauma, including physical, sexual, emotional, and general forms of trauma. The ETI is a structured interview that assesses the number, frequency, duration, and subjective impact (i.e., functional, emotional, and relational) of traumatic experiences. High test–retest reliability, internal consistency, and external validity have been reported for the ETI.[35]
LABORATORY PROCEDURES
Participants were admitted to the General Clinical Research Center at theMedical University of South Carolina at approximately 2000 h the evening before testing to allow for the control of extraneous variables (e.g., sleep, nicotine and caffeine intake, nutrition) that could potentially affect stress reactivity. Individuals who smoked cigarettes were provided with a nicotine patch upon admission. Nicotine replacement therapy was continuously maintained throughout the hospital stay (≥20 cigarettes/day = 21 mg; 10–19 cigarettes/day = 14 mg patch; 5–9 cigarettes/day = 7 mg patch). In addition, breathalyzer tests (AlcoSensor III, Intoximeters, Inc., St. Louis, MO) and urine toxicology screens (Roche Diagnostics, Indianapolis, IN) were used to verify that participants had not used alcohol or drugs before hospital admission.
During the 2-day hospital stay, subjects completed a corticotropin-releasing hormone (CRH) pharmacological provocation and the TSST. A standard breakfast was provided at 0830 h on both mornings before testing. Sedentary activities, such as reading, were then allowed until testing began. At 1150 h, an indwelling intravenous catheter was inserted in the nondominant forearm. A standard lunch was provided at 1200 h.
Between 1340 and 1400 h, the subjects completed testing procedures including the CRH administration and TSST. Ovine CRH (1μg/kg to a maximum dose of 100 mg; Ferring Pharmaceuticals, Saint Prex, Switzerland) was administered via intravenous push over a 1 min period. For the TSST, participants were asked to spend 5 min preparing and then 5 min delivering an impromptu speech before an audience of three research staff members unknown to the participant. Immediately after the speech, participants were asked to complete serial subtractions out loud for an additional 5 min before the same audience. Paper and writing instruments were prohibited (except during the first 5 min when participants prepared to give the speech). Audience members were trained to withhold any verbal or nonverbal reinforcement (e.g., head nodding, smiling) during the TSST. The uncontrollable and social-evaluative aspects of the TSST have been shown to reliably evoke an HPA axis response.[36] Blood samples were obtained immediately prior, immediately after, and at multiple time points post CRH and TSST from an indwelling catheter (5, 15, 30, and 60 min).
NEUROENDOCRINE ASSAYS
Blood samples were collected in EDTA-prepared tubes and immediately iced. The samples were centrifuged under refrigeration and the serum was frozen at −70°C until assayed. Rockefeller University personnel performed all of the assays; all samples from each subject were run in the same assay. The corticotropin was assayed in duplicate using Allegro HS corticotropin system which has an intra-assay coefficient of variation (CV) of 6% with a sensitivity of 1 pg/ml (Nichols Institute Diagnostics, San Juan Capistrano, CA). For cortisol, Roche Diagnostics Elecys 2010 immunoassay analyzer and kits based on electrochemiluminescence competitive immunoassay with a functional sensitivity (lowest reportable concentration) of 8.0 nmol/l (0.29 ug/dl) and intra-assay reproducibility CV of less than 2% were used.
Results of the larger study have been reported in Brady et al.[37] where both pharmacological and stressful stimulation was shown to induce significant increases in neuroendocrine response.
STATISTICAL METHODS
Our primary hypothesis was that gender modifies the effect of early life trauma on stress reactivity, as measured by cortisol and corticotropin response to CRH provocation and the TSST. Chi square and Wilcoxon rank sum tests were first used to assess gender differences in demographic and trauma variables. Demographic variables with significantly different gender distributions were adjusted for in all regression analyses (e.g., age, employment). To address the hypothesis, a two-stage analytical approach was used. The first stage reduced the ETI by summarizing the seven variables deduced from the inventory into meaningful constructs. The second stage utilized these constructs to predict differential stress reactivity in men and women.
To reduce the number of variables and identify meaningful constructs, we applied a factor analysis to all available subjects using the principal components method of estimation. The number of factors retained for model development was determined both by the size of the eigenvalues as well as their interpretability; factors that are not interpretable would not elucidate which types of trauma are important predictors of HPA axis dysregulation. To enhance interpretability, factors were rotated using the varimax rotation. Factor scores were calculated for each subject using least squares and factor loadings greater than .50 were considered meaningful.
In the second stage, the primary outcome variables (determined a priori) were: baseline and area under the curve (AUC) for cortisol and corticotropin. Secondary-related outcomes that we considered were mean change and longitudinal analysis. The main purpose of these secondary analyses was to demonstrate consistency with AUC results; thus, Bonferonni corrections were not used. To assess the impact of trauma on baseline neuroendocrine measures, multiple linear regression models were fit with the factor scores, a binary indicator of gender, and the interactions of each factor score with gender and age as predictors. To assess gender differences in the impact of trauma on neuroendocrine response to CRH and TSST, similar multiple linear regression models were fit with AUC as the outcome. Regression models were also fit with mean change as outcomes, and linear mixed models were fit with longitudinal cortisol and corticotropin as outcomes. Backwards model selection was performed to arrive at the best predictive model as determined by a significant change in log likelihood. If an interaction remained in the model, all lower order covariates were also forced to remain the model. Non-normality of residuals was addressed via log10 transformations of AUC and longitudinal response variables. Where factor × gender interactions were significant, analyses stratified by gender were performed for ease of graphical demonstration.
P-values for main effects less than .05 were considered significant findings. P-values for main effects .05 < P < .10 were considered trends. To detect meaningful interactions, interaction P-values of .10 or less were considered significant, whereas .10 < P < .15 were considered trends and were retained in the adjusted models.[38] Where descriptive statistics are presented, they represent the mean ± standard deviation or n (%) where appropriate. A single point was deemed to be an influential observation for the baseline analysis of corticotropin and was removed from the corresponding baseline regression models. No adjustments for multiple testing were applied as all hypotheses were generated a priori. All analyses were performed in SAS (SAS/STAT software, Version 9.1.3, SAS Institute Inc., Cary, NC).
RESULTS
Table 1 displays the gender distribution of demographic variables and variables commonly deduced from the ETI. Age and employment were significantly different for men and women. Women were significantly older (40.7 ± 11.0 versus 31.7 ± 11.5; P = .01) and more likely to be currently employed (91.3% versus 56.3%; P = .02). Baseline cortisol levels measured in men were higher than those measured in women. These variables were therefore controlled in all regression analyses.
TABLE 1.
Demographics and early trauma variables by gender (controls only)
| Characteristic | Gender
|
Statistica | P-value | |
|---|---|---|---|---|
| Men n = 16 | Women n = 23 | |||
| Age | 31.8 ± 11.8 | 40.7 ± 11.0 | −2.41 | .016 |
| Education (% some college) | 14 (87.5) | 19 (86.4) | 0.01 (1) | .919 |
| Employment (% employed) | 9 (56.3) | 21 (91.3) | 6.53 (1) | .019 |
| Race (% Caucasian)b | 13 (81.3) | 13 (56.5) | 2.60 (1) | .107 |
| Marital status (% married)c | 2 (12.5) | 9 (39.1) | 3.30 (1) | .086 |
| Smoking status (% smokers) | 11 (64.7) | 12 (52.2) | 0.63 (1) | .428 |
| Baseline cortisol | 8.7 ± 2.7 | 7.3 ± 4.0 | 2.16 | .031 |
| Baseline corticotropin (ACTH) | 23.0 ± 8.7 | 17.5 ± 6.8 | 1.61 | .107 |
| Past major depressive disorder (% MDD) | 1 (6.3) | 6 (26.1) | 2.52 | .206 |
| ETI frequencies | ||||
| Total ETI items | 12.9 ± 8.6 | 12.6 ± 10.3 | 0.36 | .720 |
| General ETI items | 6.3 ± 4.4 | 6.7 ± 5.7 | 0.16 | .875 |
| Emotional ETI items | 1.9 ± 2.5 | 1.7 ± 2.4 | 0.43 | .670 |
| Physical ETI items | 3.4 ± 1.8 | 2.4 ± 1.9 | 1.98 | .048 |
| Sexual ETI items | 1.4 ± 2.5 | 1.9 ± 2.9 | −0.91 | .363 |
| Emotional effect | 3.2 ± 0.5 | 2.9 ± 0.5 | 1.38 | .167 |
| Functional effect | 3.1 ± 0.3 | 3.1 ± 0.3 | 0.04 | .969 |
| Relational effect | 3.0 ± 0.3 | 2.9 ± 0.4 | 0.26 | .798 |
Mean ± standard deviation is listed unless otherwise noted. Mean and SD represent the mean and standard deviation of the total number of childhood traumas reported by the ETI-SR. P-values represent Wilcoxon rank sum two sided normal approximation tests or chi square tests.
Continuous variables used is z approximation of Mann–Whitney U statistic, where z = (U − mU)/σU and discrete statistic is usual chi square (df) or Fisher’s Exact Test where applicable.
Caucasian group contains two pacific islanders; subjects not included in this category are African American.
Includes married but separated.
FACTOR ANALYSIS
Table 2 shows meaningful factor loadings (r > .50) as determined by rotated factor analysis. These results demonstrate that Factor 1 loaded moderate-to-high on the emotional, functional, and relational effects (e.g., r = .86, .85, and .88), Factor 2 loaded high on the number of sexual, emotional, and physical traumas (e.g., r = .58, .84, and .86), and Factor 3 loaded high on the number of general traumas alone (e.g., r = .93). As a result of these correlations between the original variables and factors, the three factors were labeled: trauma effects, severe trauma, and general trauma, respectively. The three factors accounted for the majority of the total observed variance (37, 31, and 10%, respectively) and the loadings implied that the factors had clear interpretations. Scores for each factor were calculated for each subject using the eigenvectors that gave maximum variation.
TABLE 2.
Factor pattern extracted from rotated principle components analysis
| Early trauma inventory groupings | Factor 1 (effects) | Factor 2 (severe) | Factor 3 (general) |
|---|---|---|---|
| General ETI items | .9281 | ||
| Emotional ETI items | .8361 | ||
| Physical ETI items | .8572 | ||
| Sexual ETI items | .5758 | ||
| Emotional effect | .8570 | ||
| Functional effect | .8468 | ||
| Relational effect | .8780 |
EFFECTS OF EARLY LIFE TRAUMA ON BASELINE CORTISOL AND CORTICOTROPIN
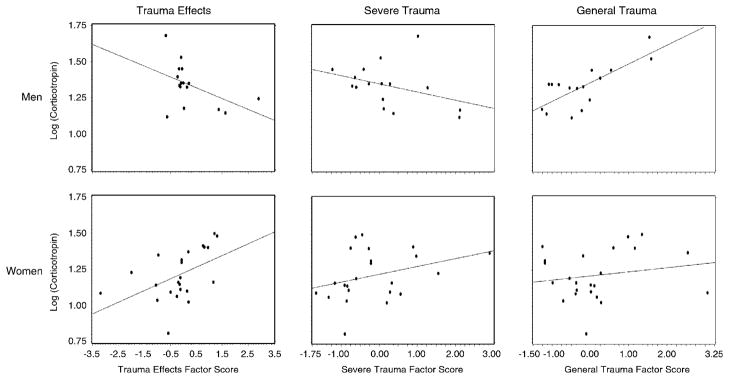
Adjusting for potential confounders, significant interactions between gender and the severe trauma factor (F1,29 = 9.41; P = .005), gender and the trauma effects factor (F1,29 = 9.65; P = .004), and a trend toward gender and the general trauma factor (F1,29 = 3.15; P = .09) indicated that gender modifies the effect of trauma on baseline corticotropin. Figure 1 shows the regression lines stratified by gender. Specifically, reported trauma effects and the severity of trauma were both highly positively associated with baseline corticotropin in women but negatively associated with baseline corticotropin in men. General trauma was highly positively associated with baseline corticotropin in men and women. Examination of baseline cortisol failed to demonstrate a significant interaction between gender and any of the factors tested; however, the severe trauma factor main effect was negatively associated with baseline cortisol levels (F1,33 = 4.25; P = .05). It is notable that baseline cortisol and corticotropin were not highly correlated with one another (ρ = .27; P = .101).
Figure 1.
Relationship between baseline corticotropin and factor scores stratified by gender. Adjusting for potential confounders, significant interactions between gender, and the severe trauma factor (F = 7.46; P = .011), gender and the general trauma factor (F = 5.36; P = .028), and gender and the trauma effects factor (F = 8.96; P = .006) indicated that gender modifies the effect of trauma on baseline corticotropin. Specifically, reported trauma effects and the severity of trauma were both highly positively associated with baseline corticotropin in women but negatively associated with baseline corticotropin in men.
EFFECTS OF EARLY LIFE TRAUMA ON STRESS REACTIVITY TO CRH
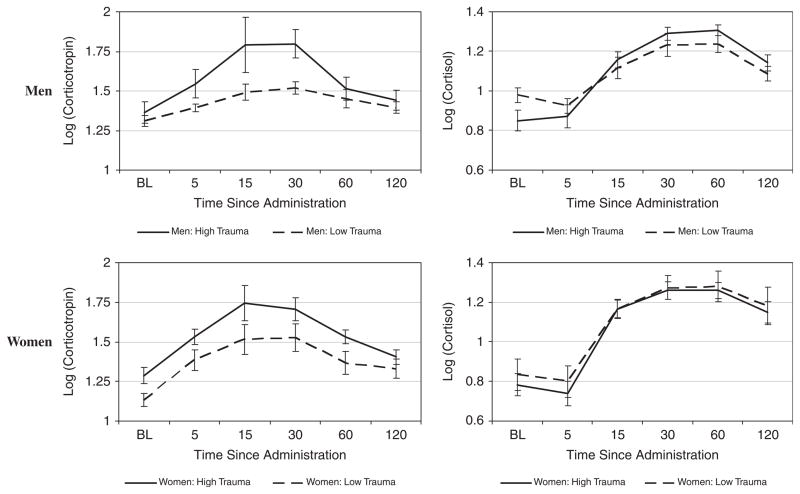
Longitudinal corticotropin and cortisol responses to CRH infusion by median split of total number of traumas are shown stratified by gender in Figure 2. This Figure is shown for descriptive purposes only (i.e., to demonstrate a response to CRH), as all statistical results are presented for continuous trauma factors, and not median split. There was a significant gender by severe trauma interaction on corticotropin AUC (F1,33 = 5.31; P = .03). There was a trend (although insignificant) toward a gender by severe trauma interaction on the cortisol AUC (F1,32 = 2.50; P = .12). Findings were upheld by the analysis of the mean response. To clarify the interaction, Table 3 shows the gender-stratified correlations of the severe trauma factor score with AUC and mean responses of corticotropin and cortisol. In this gender stratified analysis, there was a significant association between severe trauma score and corticotropin AUC in men (ρ = .26), but no correlation in women (ρ = .01). Model R-squared values were very large indicating good predictive ability of the model. These findings were also upheld by a longitudinal analysis using all the post-CRH infusion time points, controlling for baseline. This analysis resulted in a similar gender by severe trauma interaction for corticotropin (F1,33 = 5.42; P = .03) and a trend toward a significant interaction for cortisol (F1,33 = 2.60; P = .12), where the effect was in the same direction. Thus, controlling for baseline levels, men exhibited a higher corticotropin response to severe trauma throughout the CRH challenge. It is noted that these gender-specific trends did not change throughout the course of the experiment (i.e., there was no significant gender × factor score × time interaction for either corticotropin: F1,33 = 0.00; P = .95 or cortisol: F1,33 = 0.12; P = .73).
Figure 2.
Corticotropin and cortisol response to CRH stimulation. Data are shown as mean ± standard error of the mean (SEM) of the base 10 logarithm transformed data. High and low trauma groups were determined using the median split of the total number of ETI occurring in the sample data. The median (range, IQR) number of total ETI occurring in the sample is 11 (range: 2–48, IQR: 5–18). Of the 39 total subjects, 20 are considered to have a high number of total ETI (median: 18, range: 11–48, IQR: 14–24) and 19 are considered to have a low number of total ETI (median: 5, range: 2–9, IQR: 4–7).
TABLE 3.
Gender interactions with the severe trauma factor
| Hormone | Measure | Gender-specific correlation
|
Interaction parameter estimate (β) Women versus Men | Interaction P-value | Model R2 | |
|---|---|---|---|---|---|---|
| Men | Women | |||||
| Corticotropin | AUC | 0.26 | 0.01 | −.349 | .029 | .398 |
| Mean | 0.18 | 0.09 | −.286 | .030 | .536 | |
| Cortisol | AUC | −0.04 | −0.13 | −.204 | .302 | .040 |
| Mean | 0.09 | −0.30 | −.179 | .108 | .240 | |
EFFECTS OF EARLY LIFE TRAUMA ON STRESS REACTIVITY TO TRIER SOCIAL STRESS TASK
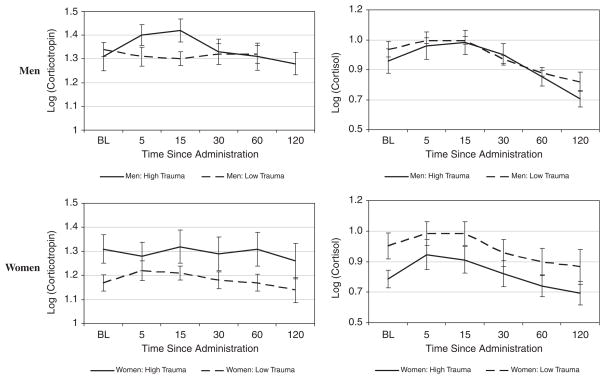
Longitudinal corticotropin and cortisol responses to the TSST by median split of total number of traumas are shown stratified by gender in Figure 3 This figure is shown for descriptive purposes only (i.e., to demonstrate a response to the TSST), as all statistical results are presented for continuous trauma factors and not median split. There were no gender interactions with severe trauma, general trauma, or the effect of trauma on corticotrophin or cortisol AUC or mean AUC following the TSST (P > .15 for all interactions). These findings were upheld by longitudinal analysis; there was no significant interaction between gender and any type of trauma on corticotrophin and cortisol response profiles (P > .15 for all interactions).
Figure 3.
Corticotropin and cortisol response to the Trier Social Stress Task. Data are shown as mean ± standard error of the mean (SEM) of the base 10 logarithm transformed data. High and low trauma groups were determined using the median split of the total number of ETI occurring in the sample data. The median (range, IQR) number of total ETI occurring in the sample is 11 (range: 2–48, IQR: 5–18). Of the 39 total subjects, 20 are considered to have a high number of total ETI (median: 18, range: 11–48, IQR: 14–24) and 19 are considered to have a low number of total ETI (median: 5, range: 2–9, IQR: 4–7).
DISCUSSION
In this study, the modifying effect of gender on the impact of early life trauma on HPA response to provocation, as measured by HPA response to CRH and the TSST, was assessed. The study allowed for examination of gender differences in HPA response in a group of individuals who had experienced a considerable amount and varied types of early life trauma, but who had no concurrent MDD or PTSD.
In this article, a novel approach to summarizing the ETI was undertaken in order to derive continuous-scale trauma domains. To our knowledge, this was a unique approach that incorporates many of the trauma items and allows trauma to be measured on a continuous scale. The derivation of a continuous measure of trauma is important, as studies have clearly shown relationships between the number of experienced childhood traumas and general mental health problems in adulthood;[10,33,39] yet, most studies addressing the association between early life trauma and stress reactivity use a binary measure of early life trauma.[1,13,24,27] This approach to measurement of early life adversity might be particularly important in the investigation of individuals without mood and anxiety disorders, because the impact of early life trauma on HPA axis function in these individuals may be more subtle.
The latent factors severe trauma and trauma effects, were shown to be strongly positively associated with baseline corticotropin levels in men and less so in women. Similarly, severe trauma was strongly positively associated with corticotropin response to CRH in men but not women. Finally, when considering the entire vector of response measures, severe trauma was positively associated with corticotropin response to CRH in men but not women. Trend level findings in the same direction were observed for cortisol.
Neuroendocrine response to CRH infusion has been examined in separate studies of women[13] and men,[27] with and without MDD and with and without histories of early life trauma. Using a 2 × 2 factorial design of MDD by early life trauma, Heim et al. found that women with early life trauma did not have increased cortisol in response to pharmacological challenge, but men did.[13,24,27] The results of this study showed a similar relationship for corticotropin but not for cortisol, regardless of the presence of mood or anxiety disorders.
Neuroendocrine response to the TSST has previously been examined in studies of women and men experiencing early life trauma. In repeated measures analysis, cortisol response to the stress challenge was lower among those with self-reported history of moderate-to-severe childhood maltreatment.[40] Similarly, women reporting childhood physical abuse displayed a significantly blunted cortisol response to the TSST compared to controls without physical abuse.[41] Our findings did not confirm this for any of our trauma measures; potentially the power to detect the interaction observed with CRH was not large enough as the TSST did not produce nearly as robust a neuroendocrine response as CRH.
CRH findings imply that a blunting of HPA axis response to stress may be more substantial in the long-term consequences of severe early life trauma for women as compared to men without MDD or PTSD. Furthermore, this blunting effect of early life trauma on HPA axis response in women occurs in a dose–response fashion, with the level of neuroendocrine response decreasing with increases in the amount of early life trauma endured. To our knowledge, this is the first study to characterize a decrease in the overall corticotropin response with increasing levels of severe trauma in women. In the study by Carpenter et al.[30] there were subtle gender differences found in the corticotropin response to TSST, with men showing a blunting of peak response and women demonstrating a blunted corticotropin level throughout testing, as compared to a control group. Our CRH results are consistent with those observed for women; however, we note that our TSST failed to provide as robust a response as CRH stimulation.
The gender differences and dose–response relationship in neuroendocrine response to pharmacological and social stressors in relationship to severe early life trauma, but not in relation to general traumas or reported trauma effects, are of interest. The severe trauma factor loaded high on sexual, emotional, and physical traumas, which include items, such as rape, neglect, physical abuse by a primary care taker, or serious accident or injury. A number of animal studies demonstrate a gender differential in the impact of some of these early life experiences (such as neglect and physical intimidation), including stress, on brain structure and function in later life.[42–44] In both rats and monkeys, chronic stress causes damage in the hippocampus of male rats and monkeys, but the magnitude is less in females.[43] In clinical and animal studies, gender differences in amygdala function during the processing of emotional memories have been demonstrated.[45,46] Several studies have demonstrated that the corpus callosum of boys is particularly vulnerable to the effects of neglect as compared to girls.[44,47] This is of particular interest because of emerging findings concerning significant sex differences in brain laterality, particularly in response to emotional stimuli.[32] Diminished corpus callosum development might exaggerate hemispheric specialization in males and exaggerate differences in response to emotional stimuli. The findings in this study contribute to the literature suggesting gender differences in the nature, timing, and extent of vulnerability to the impact of early trauma that may help explain the differential gender susceptibility to psychopathology following adverse childhood events.
Limitations of the study must be considered. First, the sample size was moderate. Second, it was not feasible to standardize the menstrual cycle phase for women at the time of testing. Previous studies have shown differences in response to CRH and TSST according to menstrual phase.[17,48] Because this study was part of a larger study in which oral contraceptives and hormone replacement therapy were allowed, findings must be considered in light of this. Several studies have shown that exogenous horomone administration alters the HPA hormonal response to stress.[49–51] However, only two women in this study were on oral contraceptives and one woman was on hormone replacement therapy. With only three people on exogenous steroid therapy, it was not possible to discern the effects of receiving hormone therapy. In addition, such a small group would be unlikely to affect the findings.
These findings add to the literature in several important ways. Notably, this study elaborates on previous investigations by focusing on gender-specific effects of early life trauma on stress response in a dose–response fashion in a group without concurrent mood or anxiety disorders. It is clear that early life trauma can cause lasting abnormalities in the stress responsive neural circuitry and alter the set point of the HPA axis to later stressful events. Gender and severity of trauma may be important determinants of the nature of these lasting changes, which should be carefully controlled and investigated in future studies. In this study, mean age differed significantly for men (32 years) and women (41 years). We cannot exclude the possibility that age differentially impacts HPA axis reactivity as a function of sex, which would require the inclusion of a three-way interaction, something that our study is not powered to test. However, we note that the three-way interaction, in the above described analyses, was not significant. Another important question is why general trauma and trauma effects did not reveal similar findings to severe traumas. Examples of general events include personal illness, experiencing the death of a caretaker, separation of parents, etc. Although these are traumatic events, they were very likely to be endorsed in this sample. On average, 6.4 of these were endorsed; however, this is in stark comparison to the lesser endorsement of emotional, physical, and sexual items (see Table 1). A high prevalence of early life general traumas in this healthy population who did not develop PTSD or MDD may be an indication of their resilience and, therefore, the effects of these types of traumas on stress might be masked in this particularly resilient sample. One reason that trauma effects may not have predicted neuroendocrine response is a limitation of the scale—the instrument may not be capable of picking up wide variations in trauma effects because the range of responses was small in our sample, with most people reporting “no effect.” It is also quite possible that some participants misunderstood the directionality of the effects scale. For example, one participant reported being sexually assaulted and then reported that this had a “moderately positive” effect on them today. Such measurement error that is inherent in self-report can mask effects. Our dose–response approach to measurement of early life adversity might be particularly important in the investigation of individuals without mood and anxiety disorders, because the impact of early life trauma on HPA axis function in these individuals may be more subtle.
Acknowledgments
We acknowledge and thank Samantha Friedenberg and Stephanie Shaftman for their work in preparing the manuscript.
Footnotes
The authors report they have no financial relationships within the past 3 years to disclose.
References
- 1.Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- 2.Agid O, Shapira B, Zislin J, et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- 3.Bradley RG, Binder EB, Epstein MP, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremner J, Southwick S, Johnson D, Yehuda R, Charney D. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. Am J Psychiatry. 1993;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- 5.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 6.Kendler K, Kessler R, Neale M, Heath A, Eaves L. The prediction of major depression in women: toward an integrated etiologic model. Am J Psychiatry. 1993;150:1139–1148. doi: 10.1176/ajp.150.8.1139. [DOI] [PubMed] [Google Scholar]
- 7.McCauley J, Kern DE, Kolodner K, et al. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. J Am Med Assoc. 1997;277:1362–1368. [PubMed] [Google Scholar]
- 8.Mullen PE, Martin JL, Anderson JC, Romans SE, Herbison GP. The long-term impact of the physical, emotional, and sexual abuse of children: a community study. Child Abuse Neglect. 1996;20:7–21. doi: 10.1016/0145-2134(95)00112-3. [DOI] [PubMed] [Google Scholar]
- 9.Stein M, Walker J, Anderson G, et al. Childhood physical and sexual abuse in patients with anxiety disorders and in a community sample. Am J Psychiatry. 1996;153:275–277. doi: 10.1176/ajp.153.2.275. [DOI] [PubMed] [Google Scholar]
- 10.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) study. Am J Prevent Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 11.Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav. 2003;79:471–478. doi: 10.1016/s0031-9384(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 12.Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65:18–28. [PubMed] [Google Scholar]
- 13.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary–adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 14.Kajantie E, Phillips DIW. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- 16.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) and mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 17.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Patchev VK, Almeida OF. Gender specificity in the neural regulation of the response to stress: new leads from classical paradigms. Mol Neurobiol. 1998;16:63–77. doi: 10.1007/BF02740603. [DOI] [PubMed] [Google Scholar]
- 19.Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic–pituitary–adrenal regulation in the female rat. J Endocrinol. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- 20.Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo–pituitary–adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 21.Windle RJ, Gamble LE, Kershaw YM, Wood SA, Lightman SL, Ingram CD. Gonadal steroid modulation of stress-induced hypothalamo–pituitary–adrenal activity and anxiety behavior: role of central oxytocin. Endocrinology. 2006;147:2423–2431. doi: 10.1210/en.2005-1079. [DOI] [PubMed] [Google Scholar]
- 22.Handa R, Weiser W, Zuloaga D. A role for the androgen metabolite, 5alpha-androstane-3beta,17beta-diol, in modulating oestrogen receptor beta-mediated regulation of hormonal stress reactivity. J Neuroendocrinol. 2009;21:351–358. doi: 10.1111/j.1365-2826.2009.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roca C, Schmidt P, Altemus M, et al. Differential menstrual cycle regulation of hypothalamic–pituitary–adrenal axis in women with premenstrual syndrome and controls. J Clin Endocrinol Metabol. 2003;88:3057–3063. doi: 10.1210/jc.2002-021570. [DOI] [PubMed] [Google Scholar]
- 24.Heim C, Newport DJ, Heit S, et al. Pituitary–adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 25.Meaney MJ, Aitken DH, Viau V, Sharma S, Sarrieau A. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology. 1989;50:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- 26.SantaAna EJ, Saladin ME, Back SE, et al. PTSD and the HPA axis: differences in response to the cold pressor task among individuals with child vs. adult trauma. Psychoneuroendocrinology. 2006;31:501–509. doi: 10.1016/j.psyneuen.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol Psychiatry. 2008;63:398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Saltzman W, Hogan BK, Abbott DH. Diminished cortisol levels in subordinate female marmosets are associated with altered central drive to the hypothalamic–pituitary–adrenal axis. Biol Psychiatry. 2006;60:843–849. doi: 10.1016/j.biopsych.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Saltzman W, Prudom SL, Schultz-Darken NJ, Abbott DH. Reduced adrenocortical responsiveness to adrenocorticotropic hormone (ACTH) in socially subordinate female marmoset monkeys. Psychoneuroendocrinology. 2000;25:463–477. doi: 10.1016/s0306-4530(00)00003-2. [DOI] [PubMed] [Google Scholar]
- 30.Carpenter LL, Carvalho JP, Tyrka AR, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elzinger B, Roelofs K, Tollenaar M, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events: a study among healthy young subjects. Psychoneuroendocrinology. 2008;22:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 33.Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160:1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- 34.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for Axes I DSM-IV Disorders (Version 2.0) New York, NY: New York State Psychiatric Institute, Biometrics Research Department; 1994. [Google Scholar]
- 35.Bremner J, Vermetten E, Mazure C. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 36.Dickerson S, Kemenyk M. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 37.Brady KT, McRae AL, Moran-Santa Maria MM, et al. Response to corticotropin-releasing hormone infusion in cocaine-dependent individuals. Arch Gen Psychiatry. 2009;66:422–430. doi: 10.1001/archgenpsychiatry.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selvin S. Statistical Analysis of Epidemiologic Data. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 39.Spinhoven P, Elzinger B, Hovens J, et al. The specificity of childhood adversities and negative life events across the life span to anxiety and depressive disorders. J Affect Disord. 2010;126:103–112. doi: 10.1016/j.jad.2010.02.132. [DOI] [PubMed] [Google Scholar]
- 40.Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-2007-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Juraska JM, Fitch JM, Henderson C, Rivers N. Sex differences in the dendritic branching of dentate granule cells following differential experience. Brain Res. 1985;333:73–80. doi: 10.1016/0006-8993(85)90125-8. [DOI] [PubMed] [Google Scholar]
- 43.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 44.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 45.Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: an FMRI investigation. Learn Mem. 2004;11:261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 47.DeBellis MD, Keshavan MS. Sex differences in brain maturation in maltreatment-related pediatric posttraumatic stress disorder. Neurosci Behav Rev. 2003;27:103–117. doi: 10.1016/s0149-7634(03)00013-7. [DOI] [PubMed] [Google Scholar]
- 48.Kirschbaum C, Kudielka B, Gaab J, Schommer NC, Hellhammper DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Burgess LHHR. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- 50.Komesaroff PASK, Esler MD. Effects of estrogen on stress responses in women. J Clin EndocrinolMetab. 1999;81:4292–4293. doi: 10.1210/jc.84.11.4292-a. [DOI] [PubMed] [Google Scholar]
- 51.Kudielka BMS-RA, Hellhammer DH, Kirschbaum C. Psychological and endocrine responses to psychosocial stress and dexamethasone/corticotropin-releasing hormone in healthy postmenopausal women and young controls: the impact of age and a two-week estradiol treatment. Neuroendocrinology. 1999;70:422–430. doi: 10.1159/000054504. [DOI] [PubMed] [Google Scholar]