Abstract
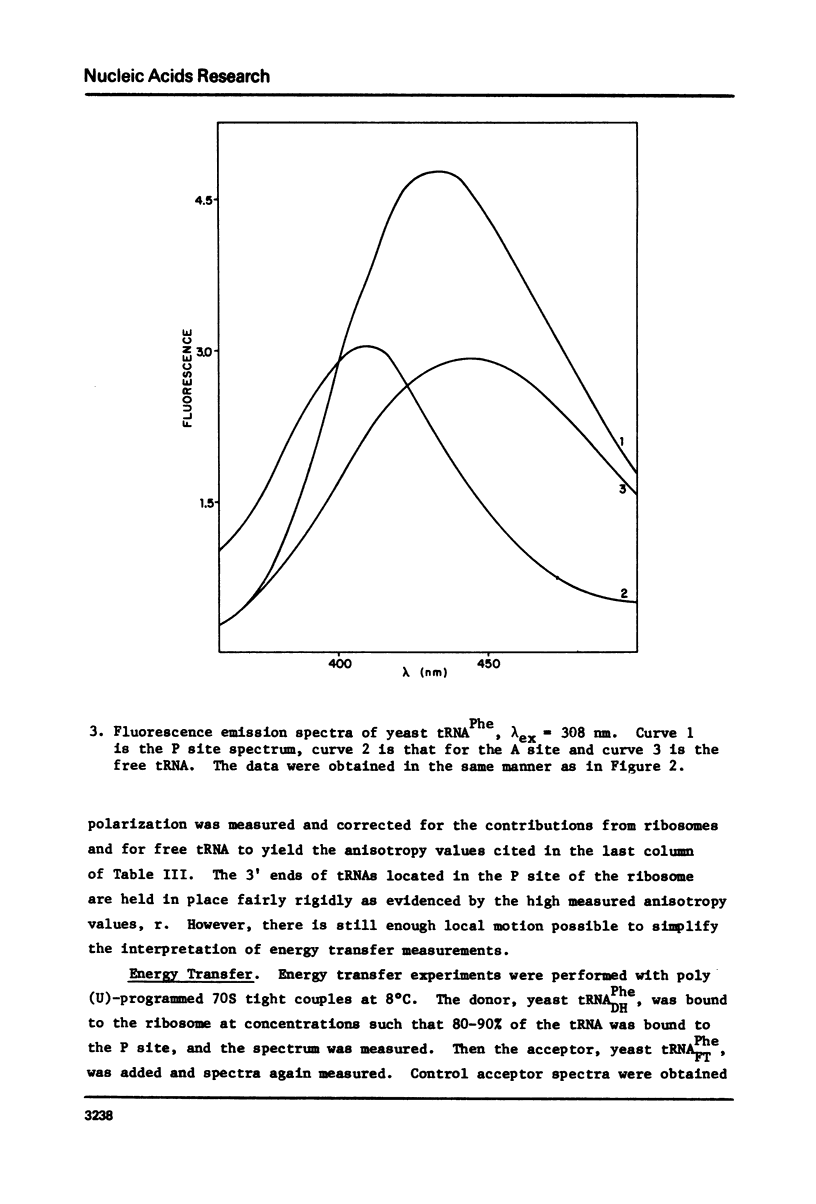
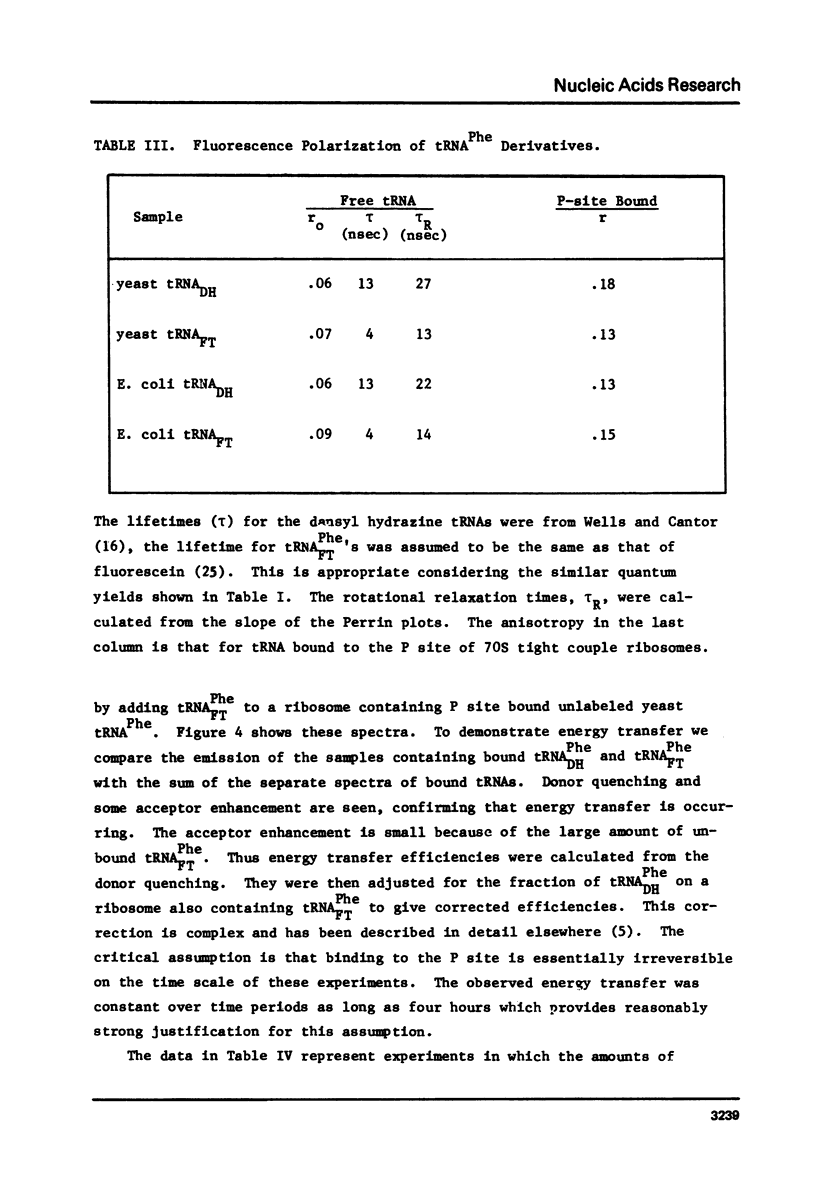
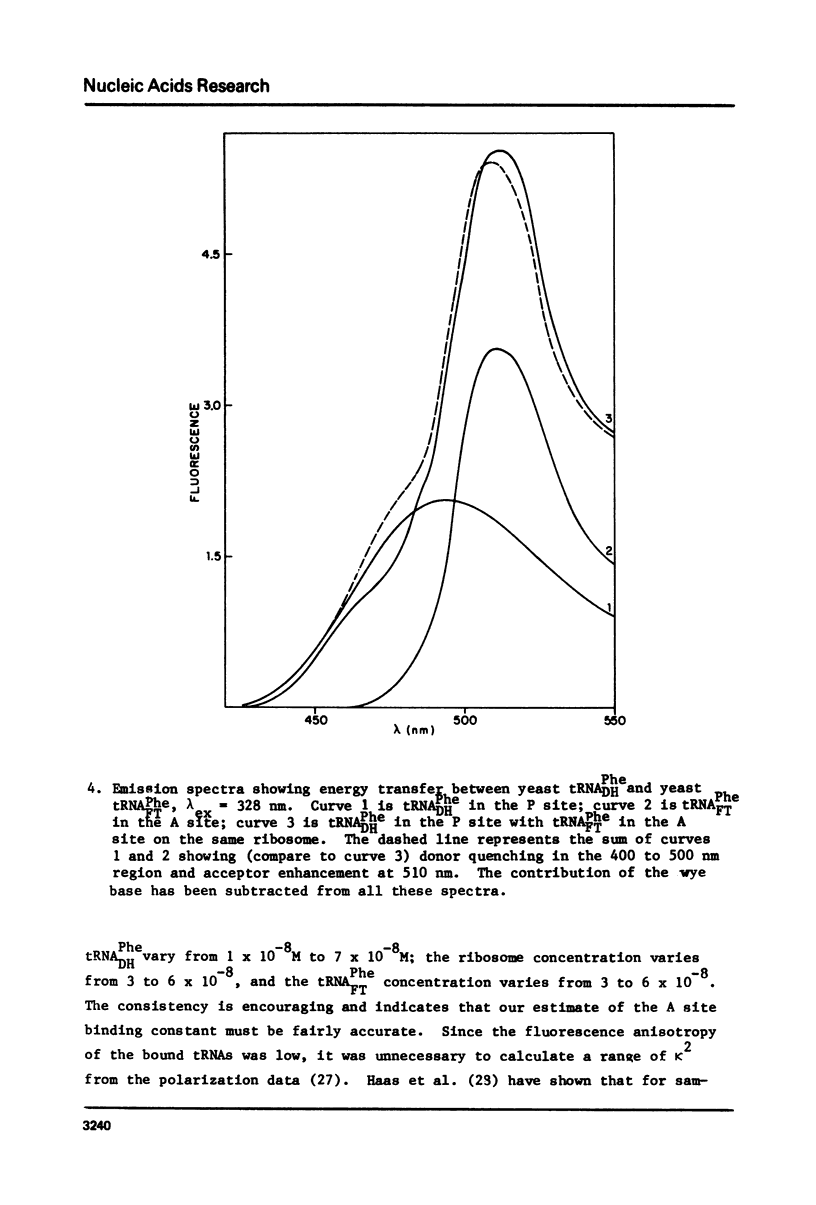
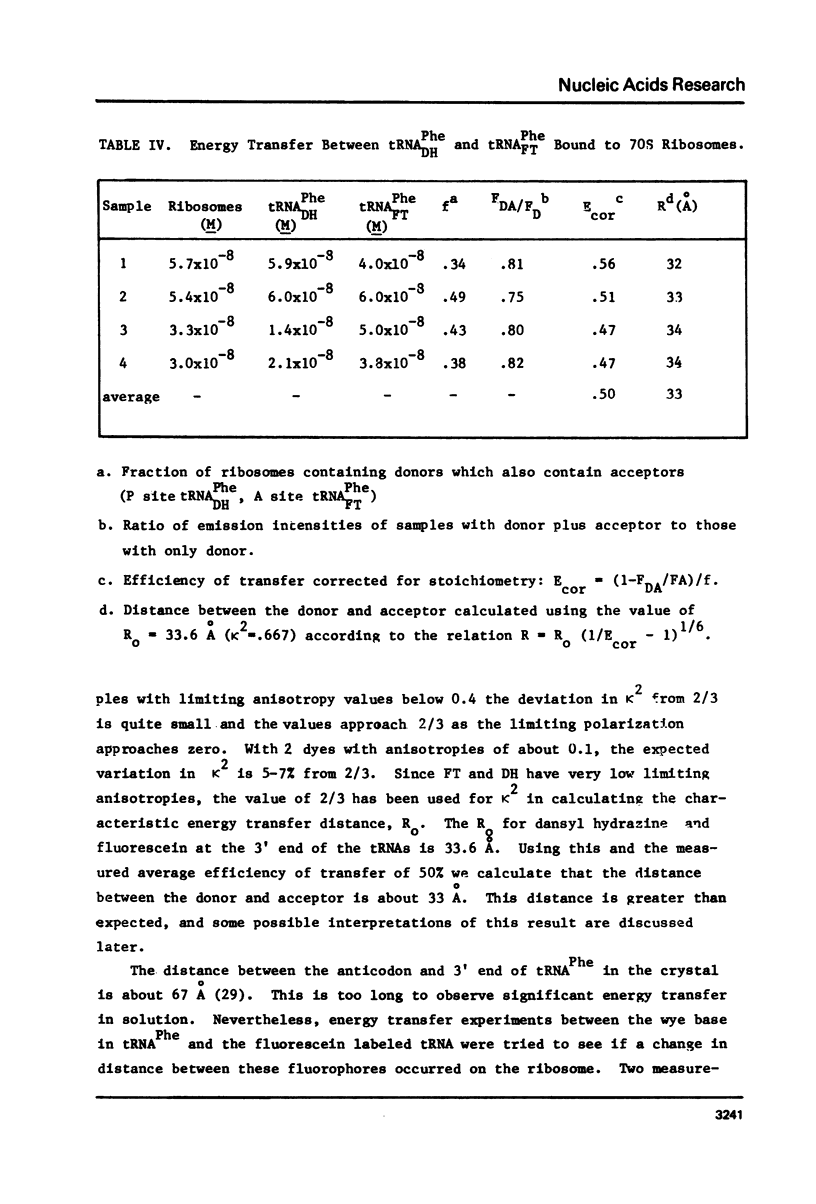
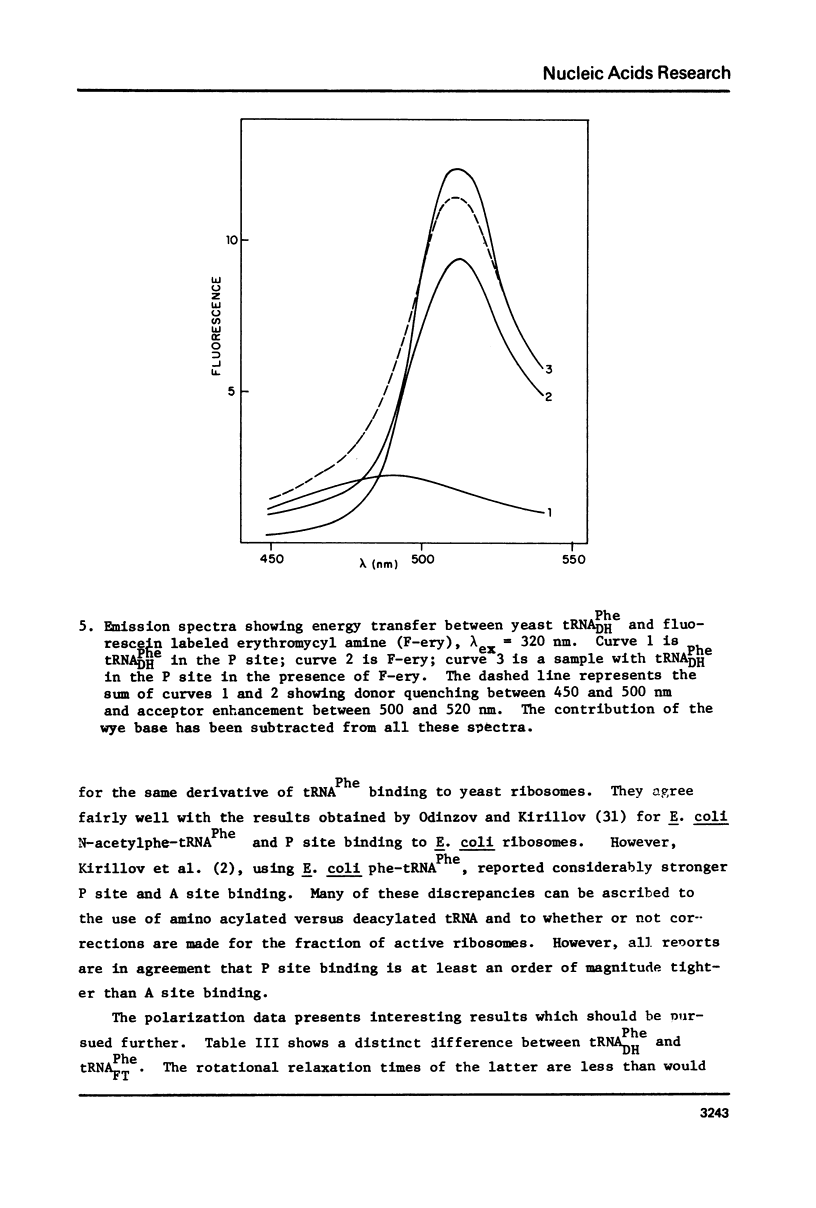
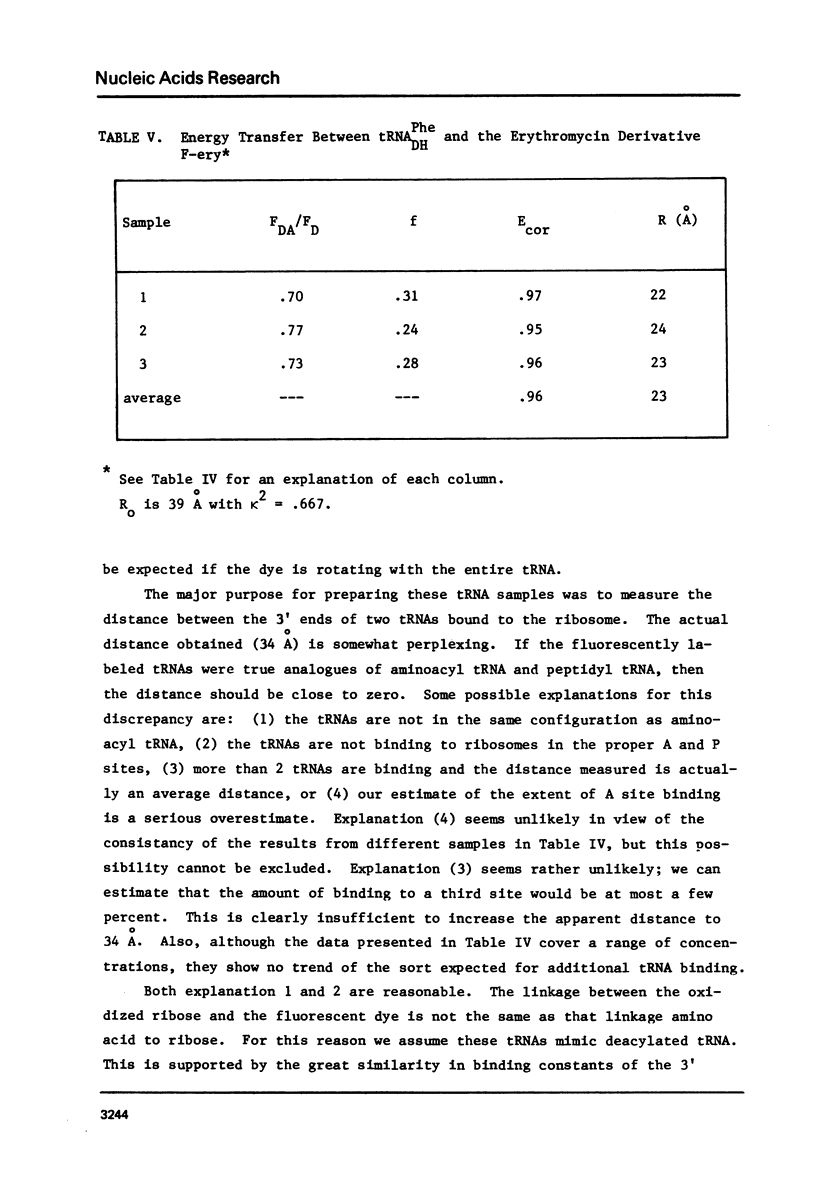
Yeast and E. coli tRNAPhe samples were oxidized and labeled at the 3' end with dansyl hydrazine or fluorescein thiosemicarbazide. These tRNAs can bind to poly(U)-programmed E. coli 70S tight couple ribosomes in 25 mM magnesium at 8 degrees C. Two binding sites with binding constants of about 1 X 10(9) M-1 (P) and 3 X 10(7) M-1 (A) were determined for the yeast tRNAPhe derivatives. With E. coli tRNAPhe the A site affinity is similar to yeast tRNAPhe but the P site affinity is 5-fold weaker. Singlet-singlet energy transfer showd that the distance from the 3' end of tRNAPhe in the P site to a fluorescein derivative of erythromycin is 23 A. This supports in vitro studies suggesting that erythromycin binds near the peptide moiety of peptidyl tRNA. A distance of 34 A between the 3' ends of 2 tRNAs bound simulatneously on the ribosome was also measured. This long distance may mean that the deacylated fluorescent tRNA binds to the A site in an orientation like that in the stringent response rather than in protein synthesis.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amils R., Matthews E. A., Cantor C. R. An efficient in vitro total reconstitution of the Escherichia coli 50S ribosomal subunit. Nucleic Acids Res. 1978 Jul;5(7):2455–2470. doi: 10.1093/nar/5.7.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A. D., Uhlenbeck O. C., Tinoco I., Jr Circular dichroism study of nine species of transfer ribonucleic acid. Biochemistry. 1972 Aug 15;11(17):3248–3256. doi: 10.1021/bi00767a019. [DOI] [PubMed] [Google Scholar]
- Chinali G., Horowitz J., Ofengand J. Replacement of pseudouridine in transfer RNA by 5-fluorouridine does not affect the ability to stimulate the synthesis of guanosine 5'-triphosphate 3'-diphosphate. Biochemistry. 1978 Jul 11;17(14):2755–2760. doi: 10.1021/bi00607a009. [DOI] [PubMed] [Google Scholar]
- Chinali G., Liou R., Ofengand J. Role of the aminoacyl end of transfer RNA in the allosteric control of guanosine pentaphosphate synthesis by the stringent factor-ribosome complex of Escherichia coli. Biochemistry. 1978 Jul 11;17(14):2761–2768. doi: 10.1021/bi00607a010. [DOI] [PubMed] [Google Scholar]
- De Groot N., Panet A., Lapidot Y. The binding of purified Phe-tRNA and peptidyl-tRNA Phe to Escherichia coli ribosomes. Eur J Biochem. 1971 Dec 10;23(3):523–527. doi: 10.1111/j.1432-1033.1971.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Fairclough R. H., Cantor C. R. An energy transfer equilibrium between two identical copies of a ribosome-bound fluorescent transfer RNA analogue: implications for the possible structure of codon-anticodon complexes. J Mol Biol. 1979 Aug 25;132(4):587–601. doi: 10.1016/0022-2836(79)90376-0. [DOI] [PubMed] [Google Scholar]
- Fairclough R. H., Cantor C. R. The distance between the anticodon loops of two tRNAs bound to the 70 S Escherichia coli ribosome. J Mol Biol. 1979 Aug 25;132(4):575–586. doi: 10.1016/0022-2836(79)90375-9. [DOI] [PubMed] [Google Scholar]
- Fairclough R. H., Cantor C. R., Wintermeyer W., Zachau H. G. Fluorescence studies of the binding of a yeast tRNAPhe derivative to Escherichia coli ribosomes. J Mol Biol. 1979 Aug 25;132(4):557–573. doi: 10.1016/0022-2836(79)90374-7. [DOI] [PubMed] [Google Scholar]
- Gavrilova L. P., Kostiashkina O. E., Koteliansky V. E., Rutkevitch N. M., Spirin A. S. Factor-free ("non-enzymic") and factor-dependent systems of translation of polyuridylic acid by Escherichia coli ribosomes. J Mol Biol. 1976 Mar 15;101(4):537–552. doi: 10.1016/0022-2836(76)90243-6. [DOI] [PubMed] [Google Scholar]
- Gualerzi C., Risuleo G., Pon C. L. Initial rate kinetic analysis of the mechanism of initiation complex formation and the role of initiation factor IF-3. Biochemistry. 1977 Apr 19;16(8):1684–1689. doi: 10.1021/bi00627a025. [DOI] [PubMed] [Google Scholar]
- Haas E., Katchalski-Katzir E., Steinberg I. Z. Effect of the orientation of donor and acceptor on the probability of energy transfer involving electronic transitions of mixed polarization. Biochemistry. 1978 Nov 14;17(23):5064–5070. doi: 10.1021/bi00616a032. [DOI] [PubMed] [Google Scholar]
- Kirillov S. V., Makhno V. I., Odinzov V. B., Semenkov Y. P. The mechanism of codon-anticodon interaction in ribosomes. Heterogeneity of tRNA complexes with 70-S ribosomes of Escherichia coli. Eur J Biochem. 1978 Aug 15;89(1):305–313. doi: 10.1111/j.1432-1033.1978.tb20928.x. [DOI] [PubMed] [Google Scholar]
- Kirillov S. V., Makhno V. I., Semenkov Y. P. The mechanism of codon-anticodon interaction in ribosomes. Quantitative study of codon-dependent binding of tRNA to the 30-S ribosomal subunits of Escherichia coli. Eur J Biochem. 1978 Aug 15;89(1):297–304. doi: 10.1111/j.1432-1033.1978.tb20927.x. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Hunston D. L. Properties of graphical representations of multiple classes of binding sites. Biochemistry. 1971 Aug 3;10(16):3065–3069. doi: 10.1021/bi00792a013. [DOI] [PubMed] [Google Scholar]
- Langlois R., Lee C. C., Cantor C. R., Vince R., Pestka S. The distance between two functionally significant regions of the 50 S Escherichia coli ribosome: the erythromycin binding site and proteins L7/L12. J Mol Biol. 1976 Sep 15;106(2):297–313. doi: 10.1016/0022-2836(76)90087-5. [DOI] [PubMed] [Google Scholar]
- Levin J. G. Codon-specific binding of deacylated transfer ribonucleic acid to ribosomes. J Biol Chem. 1970 Jun;245(12):3195–3202. [PubMed] [Google Scholar]
- Litman D. J., Lee C. C., Cantor C. R. Evidence for a conformational change in the 30 S E. coli ribosomal subunit upon formation of 70 S particles. FEBS Lett. 1974 Oct 15;47(2):268–271. doi: 10.1016/0014-5793(74)81027-6. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J. Protein biosynthesis. Annu Rev Biochem. 1971;40:409–448. doi: 10.1146/annurev.bi.40.070171.002205. [DOI] [PubMed] [Google Scholar]
- Odinzov V. B., Kirillov S. V. Interaction of N-acetyl-phenylalanyl-tRNAPhe with 70S ribosomes of Escherichia coli. Nucleic Acids Res. 1978 Oct;5(10):3871–3879. doi: 10.1093/nar/5.10.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom O. W., Craig B. B., Hardesty B. A. The conformation of the anticodon loop of yeast tRNAPhe in solution and on ribosomes. Biopolymers. 1978 Dec;17(12):2909–2931. doi: 10.1002/bip.1978.360171212. [DOI] [PubMed] [Google Scholar]
- Robertson J. M., Kahan M., Wintermeyer W., Zachau H. G. Interactions of yeast tRNAPhe with ribosomes from yeast and Escherichia coli. A fluorescence spectroscopic study. Eur J Biochem. 1977 Jan 3;72(1):117–125. doi: 10.1111/j.1432-1033.1977.tb11231.x. [DOI] [PubMed] [Google Scholar]
- Vince R., Weiss D., Pestka S. Binding of N-substituted erythromycyclamines to ribosomes. Antimicrob Agents Chemother. 1976 Jan;9(1):131–136. doi: 10.1128/aac.9.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells B. D., Cantor C. R. A strong ethidium binding site in the acceptor stem of most or all transfer RNAs. Nucleic Acids Res. 1977;4(5):1667–1680. doi: 10.1093/nar/4.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. H., Söll D. Studies of transfer RNA tertiary structure of singlet-singlet energy transfer. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2838–2842. doi: 10.1073/pnas.71.7.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir A., Miskin R., Elson D. Inactivation and reactivation of ribosomal subunits: amino acyl-transfer RNA binding activity of the 30 s subunit of Escherichia coli. J Mol Biol. 1971 Sep 14;60(2):347–364. doi: 10.1016/0022-2836(71)90299-3. [DOI] [PubMed] [Google Scholar]


