Abstract
The molecule responsible for the enzyme activity plasma membrane (PM) aminophospholipid translocase (PM-APLT), which catalyzes phosphatidylserine (PS) translocation from the outer to the inner leaflet of the plasma membrane, is unknown in mammals. A C.elegans study has shown that ablation of Tat-1, which is an ortholog of a mammalian P-type ATPase, Atp8a1, causes PS externalization in the germ cells. We demonstrate here that the hippocampal cells of the dentate gyrus (DG), and Cornu Ammonis (CA1, CA3) in mice lacking Atp8a1 exhibit a dramatic increase in PS externalization. Although their hippocampi showed no abnormal morphology or heightened apoptosis, these mice displayed increased activity and a marked deficiency in hippocampus-dependent learning, but no hyper-anxiety. Such observations indicate that Atp8a1 plays a crucial role in PM-APLT activity in the neuronal cells. In corroboration, ectopic expression of Atp8a1 but not its close homolog, Atp8a2, caused an increase in the population (Vmax) of PM-APLT without any change in its signature parameter Km in the neuronal N18 cells. Conversely, expression of a P-type phosphorylation-site mutant of Atp8a1 (Atp8a1*) caused a decrease in Vmax of PM-APLT without significantly altering its Km. The ATp8a1*-expressing N18 cells also exhibited PS externalization without apoptosis. Together, our data strongly indicate that Atp8a1 plays a central role in the PM-APLT activity of some mammalian cells, such as the neuronal N18 and hippocampal cells.
Keywords: Aminophospholipid translocase, Atp8a1, phosphatidylserine, apoptosis, hippocampus, spatial memory
Introduction
It has been known for almost fifteen years that apoptosis triggers translocation of the inner leaflet phospholipid phosphatidylserine (PS) to the outer leaflet of the plasma membrane (Fadok 1992b, Fadok 1992a). This is a crucial process that PS-labels the apoptotic cells to prepare them for phagocytosis by PS-receptor-bearing macrophages and microglia (Fadok 2001, Adayev 1998, Witting 2000). Still, quite surprisingly, why PS remains in the inner leaflet of the plasma membrane of healthy cells and how it is translocated to the outer leaflet during apoptosis have remained controversial. Ensuing research has led to the identification of a P-type ATPase (Atp8a1), which has been reported to function as an aminophospholipid translocase (APLT) or flippase, to translocate PS and phosphatidylethanolamine (PE) across most lipid bilayers (Tang 1996, Chen 1999, Daleke 2000, Pomorski 2003). Recently, Zhou and Graham reconstituted the yeast Atp8a1 ortholog Drs2p into lipid vesicles to demonstrate its flippase activity (Zhou 2009). Furthermore, in another study, Coleman and coworkers reconstituted the mammalian Atp8a1 homolog Atp8a2 into photoreceptor disc membranes to demonstrate its PS translocase activity (Coleman 2009). Thus, these studies confirm that Atp8a1 catalyzes transbilayer movement of PS. Yet, whether Atp8a1 functions as a flippase in the plasma membrane or in the membrane of an intracellular organelle has remained controversial (Paterson 2006). Thus, Züllig and coworkers reported that RNAi-mediated suppression of TAT-1, which is the C. elegans ortholog of Atp8a1, causes stimulation (rather than inhibition) of plasma membrane flippase activity in germ cells, but Darland-Ransom and coworkers showed the opposite effect in the same cell type (Zullig 2007, Darland-Ransom 2008). Darland-Ransom and coworkers attributed this difference to the relatively weak binding of GFP-Annexin V in vivo to the surface PS in the former study, which was corrected through optimized ex vivo PS staining by Darland-Ransom and coworkers.
Atp8a1, a PS-dependent, vanadate-sensitive Mg2+-ATPase, has been purified and selective antibodies have been prepared for its detection (Daleke 2000, Ding 2000). Our effort to understand the correlation between Atp8a1 and the plasma membrane APLT (PM-APLT) has revealed that stable over-expression of the Atp8a1 cDNA elicits an increase in PM-APLT activity in the hippocampal neuron-derived HN2 cells (Chin 2003). This prompted us to test the hypothesis that the Atp8a1 gene functions as plasma membrane flippase in cells of neural origin. Subsequently, we demonstrated that both human as well as mouse Atp8a1 promoters are highly active in neuroblastoma and glioma cells (Sobocki 2005, Sobocki 2007), and overexpression of Atp8a1 in the neuronal N18 cells causes a significant increase in the Vmax of PM- APLT without affecting the Km value for this PS translocase activity (Levano 2009). This strengthened our hypothesis that Atp8a1 functions as PM-APLT in cells of neural origin. The present study shows that ablation of Atp8a1 in mice causes PS exposure without apoptosis in the hippocampus, simultaneously eliciting a significant delay in hippocampus-dependent learning. It also refines the kinetic parameters for PM-APLT and confirms our postulate via functional inhibition of endogenous Atp8a1 in the N18 cells.
Materials and Methods
(see Supplementary Information, Text S1, for details)
Animals
Equal number of adult C57BL/6 male and female mice (3-8 months old) of both genotypes (WT and Atp8a1-/-) were used for the behavioral as well as Alexafluor488-annexin V staining experiments. All animals were handled and used for surgery following an experimental protocol approved by the Institutional Animal Care Committee (IACUC) of the College of Staten Island (CUNY).
Hematoxylin/Eosin (H&E) and HOECHST33342 staining of brain slices
See Supplementary Text S1 on line.
Dissociation of hippocampal cells
The hippocampus from each mouse was isolated under sterile conditions, oxygenated for 30 min in dissection solution (Mehta 2007), and transverse slices (600-μm thick) were prepared from the entire hippocampus using a tissue chopper. DG and parts of the CA1 and CA3 regions were punched out from the hippocampus using a tissue puncher, dissociated and used for Alexafluor488-annexin V staining as detailed in Supplementary Text S1.
Annexin V staining
Annexin V was detected using the Vybrant® Apoptosis Assay Kit #2 - Alexa Fluor® 488 annexin V/propidium iodide (Invitrogen, CA, USA) according to the manufacturer's instructions. Cells were then washed with 1X annexin-binding buffer and mounted on slides for epifluorescence imaging at 520-nm. Another aliquot containing about 50,000 cells was analyzed flow cytometry. The flow events were first filtered with a forward scatter threshold to remove cell and tissue debris, and then gated to eliminate all clusters and obtain single cells (see Supplementary Text S1).
Behavioral studies
The Morris Water Maze protocol was conducted with wild type (C57BL6) and Atp8a1(-/-) mice (eight mice of each genotype with equal number of male and female mice in each group) following reported procedures (Logue 1997, Vorhees 2006) (see Text S1). Recorded VIDEOs were analyzed using the software ANY-Maze (Stoetling Co., Wood Dale, IL). A mouse open field of 50 cm × 30 cm dimensions was used to compare activity and anxiety levels of eight age-matched mutant and wild type mice (Deacon 2006) using Any-Maze for the analysis of VIDEO images. A mouse Elevated-plus Maze with 60-cm-long and 12.5-cm-wide open and closed arms was used (Walf 2007) (see Text S1). Statistical analysis was performed using two-tailed unpaired t-test with unequal variance.
Atp8a1 and Atp8a2 expression vectors
Mouse brain RNA was isolated using the RNAeasy Mini Kit (QIAGEN, CA, USA) and the cDNA was obtained by using ThermoScript™ RT-PCR System plus Platinum® Taq DNA Polymerase (Invitrogen Corporation, CA, USA). For amplification of Atp8a1 or Atp8a2, primers were designed with the addition of restriction sites for directional cloning. The downstream primer (the antisense primer) included the coding sequence but not the stop codon, to allow insertion into pCDNA6.1/ myc-HisA (for Atp8a1) and/or pcDNA6.1/ myc-His C (for Atp8a2) in-frame with the myc-His sequences to eventually achieve expression of epitope-tagged Atp8a1 and Atp8a2.
Expression vectors for phosphorylation-site mutants of Atp8a1
As judged by Western blotting using Ab-B, we were unsuccessful in our attempts to suppress Atp8a1 expression using transfected siRNA sequences carefully constructed by Dharmacon RNAi Technologies, even though the transfection efficiency was very high. Therefore, we used P-type phosphorylation site mutants to suppress Atp8a1. The aspartate residue in the P-type signature sequence ‘DKTGTL’ was mutated to either lysine (D409→K) or glutamate (D409→E) using the GeneTailor™ Mutagenesis System (Invitrogen, CA, USA) as described in Levano et al. (Levano 2009) and in Supplementary Text S1.
Transfection of cells
N18 neuroblastoma cells, plated in poly-L-lysine-coated six-well plates, were allowed to grow up to 60% confluency. At this point, the cells were transfected using the ExGen transfection reagent (Fermentas, Inc., MD) as described in Supplementary Text S1. For cotransfection of pEGFP or pAsRed2-N1, 2.4 μg of the experimental plasmid was mixed with 0.6 μg of pEGFP or pAsRed2-N1 in 200 μl of 5% NaCl or 5% glucose. The cells were incubated overnight (for a total period of 24 h from transfection) and then subjected to immunostaining or harvested and resuspended in an appropriate buffer for PM-APLT assays for annexin V-alexafluor488 staining.
Plasma Membrane APLT activity
(Also see Supplementary Text S1) The PM-APLT activity was measured in cells using NBD-PS (1-Oleoyl-2-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl) amino]hexanoyl]-sn-Glycero-3-Phospho-L-Serine) by modifying reported procedures (Tyurina 2007, McIntyre 1991, Connor 1992). Cells (~106) in suspension from rapidly dividing healthy cultures at 50% confluence were treated with increasing concentrations of NBD-PS in 1 ml incubation buffer (see Text S1) at 4 °C for 10 min. Unbound NBD-PS was removed by centrifugation, cells resuspended in 1 ml ice-cold incubation buffer and then incubated at 28 °C for 10 min to allow NBD-PS translocation of NBD-PS. Untranslocated NBD-PS remaining in the outer leaflet was modified to a non-fluorescent compound (McIntyre 1991) with sodium dithionite. The fluorescence values were converted to micromolar NBDPS by comparing with a standard curve constructed using 0.1, 0.5, 1, 2.5, and 5 μM NBD-PS in NBDPS/PC vesicles. In order to avoid variations due to differing fluorescence contents of the different lots of NBD-PS, a new standard curve was constructed for each experiment. The values of Km and Vmax were obtained from the substrate saturation curves by using curve-fitting software contained within GraphPad Prism (See Supplementary Text S1 for details).
Immunostaining and immunoblotting
See Supplementary Information Text S1 on line.
Caspase-3/7 assays
It was performed as described in our earlier report (Purkayastha 2009a) a described in Supplementary Information, Text S1.
Results
The Atp8a1(-/-) brains do not show any salient morphological or cellular anomaly
Genotyping identified the Atp8a1(-/-) mice (Fig. 1a), which were bred to obtain a congenic line. Ab-B staining confirmed the expression of Atp8a1 in the wild type but not Atp8a1(-/-) mouse brain (Fig. 1b and c). H&E staining revealed no salient morphological anomaly in the brains of the Atp8a1(-/-) mice (Fig. 1d and e). Similarly, HOECHST33342 staining revealed no nuclear condensation or disintegration that is typically observed in apoptotic cells (Fig. 1f and g) (also see Supporting Information, Text S1).
Fig. 1. Genotyping of Atp8a1(-/-) mice and morphological analysis of wild type and Atp8a1(-/-) brains.
(a) In genotyping analysis, the mutant allele yielded a 437-bp PCR product, whereas wild type allele produced a 253-bp product. (b) and (c) Immunohistochemistry using Ab-B confirmed the expression of Atp8a1 in the whole brain of wild type mice and its absence in the Atp8a1(-/-) mice. (d) and (e) H&E staining showing no morphological aberrance in wild type or Atp8a(-/-) mouse brains. (f) and (g) HOECHST33342 staining revealed clearly visible nucleoli in wild type and Atp8a1(-/-) mice (enlarged view of one arm of the DG layer has been presented here).
Atp8a1 deficiency is associated with PS externalization in the plasma membrane of hippocampal cells
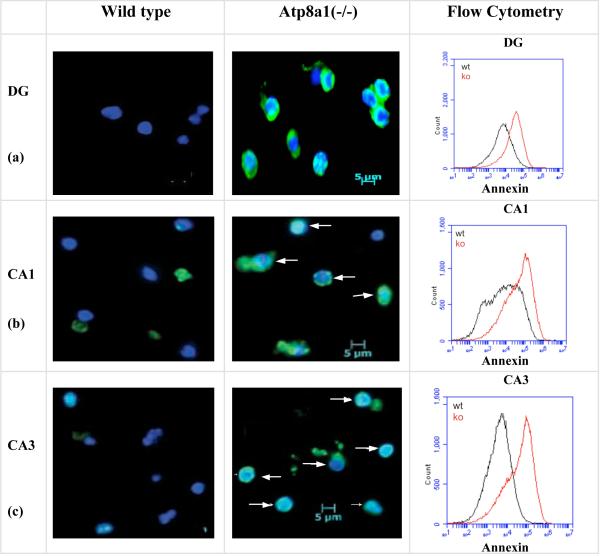
Alexafluor488-annexin V staining of live cells dissociated from the DG, CA1, and CA3 layers of wild type and Atp8a1(-/-) mice revealed pronounced PS externalization in cells from the Atp8a1(-/-) but not wild type mice (Fig. 2a-c). Flow cytometry (50,000 cells) immediately after annexin VAlexafluor488 staining showed that mean fluorescence of the hippocampal cells in the mutant mice was 3.5-10-fold higher than that in the cells from the wild type mice (right panel), thereby indicating the presence of externalized PS in the hippocampus of the Atp8a1(-/-) mice (see Text S1 for methods). This increase in PS externalization compared to wild type was not observed in the hippocampi of heterozygous Atp8a1(+/-) mice (Fig. S1a), therefore was not an artifact of the annexin V assay.
Fig. 2. Spontaneous PS-externalization in the hippocampus of the Atp8a1(-/-) but not the wild type mice.
(a-c) Alexafluor488-Annexin V (green) and HOECHST33342 (blue) staining of cells from the neuronal layers in DG, CA1, and CA3 of control and Atp8a1(-/-) mice. Merged images of annexin V(+) cells were observed in the cells from the Atp8a1(-/-) mice. White arrows show non-apoptotic but annexin V(+) cells. (Flow Cytometry, Right panel) From three experiments, the increases in mean fluorescence were 3.6-fold (DG), 3.9-fold (CA1), and 10.7-fold (CA3) in the Atp8a1(-/-) samples (red traces).
Intriguingly, the Atp8a1(-/-) erythrocytes show no PS exposure, probably because heightened expression of Atp8a2 in these Atp8a1(-/-) cells helps restore the PM-APLT activity (Fig. S1b-d).
The Atp8a1(-/-) mice show marked deficiency in Morris Water Maze (MWM) and hyperactivity, but no hyper anxiety, in Open Field (OF) and Elevated-Plus Maze (EPM) tasks
The PS exposure of hippocampal neurons could trigger increased phagocytosis (Darland-Ransom 2008, Adayev 1998, Das 2003) or aberrant intracellular communication in the hippocampal neurons, thus contributing to behavioral deficits. As shown in Fig. 3, the MWM task showed a significant delay in hippocampus-dependent learning in the Atp8a1(-/-) mice. Some forebrain and hippocampal defects have been linked to elevated anxiety (Ramboz 1998, Sibille 2000, Walf 2007) or hyperactivity in mice (van Praag 1994, Godsil 2005). The Atp8a1(-/-) mice displayed heightened activity in both OF (Fig. 3b) as well as EPM tasks (Fig. 3c and d), but a decreased rate of line crossing in EPM indicated that they were less anxious than the wild type mice (Fig. 3c).
Fig. 3. The Atp8a1(-/-) mice show marked deficiency in Morris Water Maze (MWM) and increased activity in Open Field (OF) and Elevated-Plus Maze (EPM).
(a) MWM: On each day after day 1, there was a significant difference between wild type and mutant mice (p < 0.0001) (six mice/group). (b) OF: the Atp8a1(-/-) mice displayed significantly increased mobility but an insignificant difference from wild type in center entry or time spent in the center zone. (c & d) EPM: The difference between Atp8a1(-/-) and wild type was significant for Open Arm Entry and Open Arm Time. All differences in (d) were significant. Max Speed: p = 0.022; Line Crossing: p = 0.018; Time Mobile: p = 0.014; Time Immobile: p = 0.025. Data were analyzed using two-tailed unpaired t-test with unequal variance.
Analysis of the effect of Atp8a1 and its mutants on the PM-APLT of neuronal N18 cells
In earlier studies, Atp8a1 was isolated from the chromaffin granules and synaptic vesicles (Xie 1989, Moriyama 1988, Hicks 1992), which remain in a dynamic process of fusing to and pinching off the plasma membrane during secretion or neurotransmission. Thus, it is quite likely that Atp8a1 would also be localized to the plasma membrane of the neurosecretory cells. Furthermore, the behavioral deficits of the Atp8a1(-/-) mice indicate that the absence of Atp8a1 elicits aberration in the neuronal circuitry of the hippocampus. If these neuronal cells used Atp8a1 as PM-APLT, then suppression of activity of the protein by using a phosphorylation site mutant that can function as a “dominant negative” would cause PS externalization in these cells (siRNAs were ineffective- see SI Methods).
The ATPase activity of the P-type ATPases critically depends on the phosphorylation of the aspartic acid residue D409 (via formation of a relatively labile –COO-PO42– bond) at the beginning of the signature DKTGT[L,I,V,M][T,I,S] domain, located in a crevasse that is believed to participate with the ATP-binding pocket in trapping Mg2+-ATP (Kuhlbrandt 2004). We expected that a mutation at D409 would eliminate phosphorylation of Atp8a1 and, thereby, its ATPase activity. A mutant lacking ATPase activity could possibly play a dominant negative role by binding to another protein that chaperones Atp8a1 to the PM (see Discussion). Constructs expressing Myc-tagged (Atp8a1-D409→K) (Atp8a1DK) (non-conservative) and (Atp8a1-D409→E) (Atp8a1DE) (conservative) mutants were prepared and expressed in the neuronal N18 cells to test this possibility.
As shown in Fig. S2a, neuronal N18 cells expressing ectopic Atp8a1-Myc displayed high plasma membrane expression of Atp8a1. Similar probing with a Myc antibody demonstrated ectopic expression of both Atp8a1(DK)-Myc and Atp8a1(DE)-Myc in the plasma membrane of the N18 cells (Fig. S2b and c). A few apoptotic cells (Fig. S2c, arrows), as normally observed in any culture, did not display high expression of Atp8a1(DE)-Myc.
Kinetic analyses of the effect of Atp8a1 or its mutants on PM-APLT activity
Kinetic analysis of the effect of ectopically expressed Atp8a1 or its mutant on PM-APLT would require two conditions: (a) high transfection efficiency, which would minimize the effect of untransfected cells in the overall assay; (b) an effective PM-APLT assay that would enable us to obtain reliable kinetic parameters.
Thorough standardization of transfection conditions enabled us to reach ≥80% transfection efficiency (Fig. S3a). A two-step PM-APLT assay was designed for the analysis of this plasma membrane enzyme. The traditional method of determining kinetic parameters uses substrate concentrations maintained in the assay medium/buffer, which also has the enzyme in a dispersed or dissolved state in direct kinetic interactions with the substrate molecules. This satisfies the fundamental condition for Michaelis Menten kinetics. In contrast, the PM-APLT assay used in this study was a two-step process, in which the substrate molecules (NBD-PS) were first allowed to incorporate into the outer leaflet of the PM at 4 °C, following which the substrate-containing buffer was removed, leaving the cells with only those NBD-PS molecules that had been incorporated into the outer leaflet (Fig. S3b, upper panel). Therefore, in sharp contrast to the regular enzyme assays, the PM-APLT assays involve interactions of the PM-cradled APLT molecules with the few NBD-PS molecules that are not in the medium but in the outer leaflet of the PM. Yet, most PM-APLT assays, including our prior assays (Levano 2009), considered the NBD-PS concentrations used in the buffer during the 4-°C incubation for the calculation of kinetic parameters. In the present study, we have refined the kinetic parameters by calculating the outer leaflet NBD-PS content and dividing it by the volume of the outer leaflet of the plasma membrane to obtain the actual NBD-PS concentration (see Text S1, and Fig. S4). This NBD-PS translocation was linear up to 10 min. The Km values for PM-APLT from 10-min incubations were initially obtained as concentrations and then converted into nmole/μm2 surface area to conform to the convention followed in reporting membrane lipid concentrations. For every experiment, comparison with a new standard curve constructed using the same NBD-PS sample that was used for that specific experiment eliminated inter-experiment variations in Km for the control samples. Additionally, passage-dependent changes in the kinetic parameters were further minimized by using N18 cells from the first three passages.
As an additional measure of precaution, successful kinetic analysis requires that the ATP content of the cells is high enough to be at the saturation levels for the concerned ATP-binding enzyme. Therefore, rapidly dividing and healthy N18 cells from 50% confluent plates containing fresh growth medium were used in each assay.
Transient overexpression of Atp8a1 in N18 cells causes an increase in Vmax without altering the Km for the PM-APLT
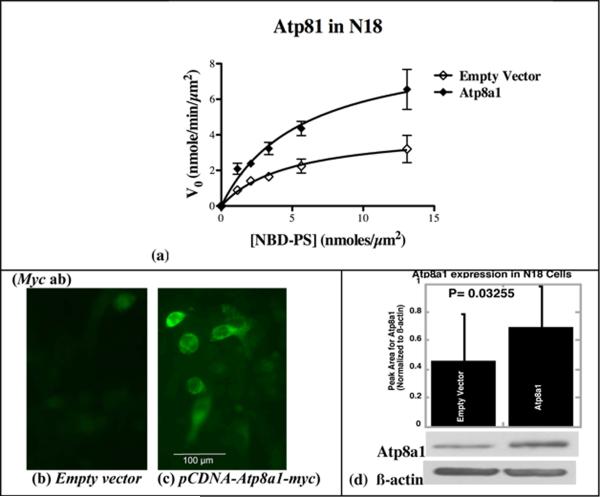
As shown in Fig. 4a and Table I (mean of three independent experiments, each performed with triplicate samples) expression of Atp8a1-Myc in the N18 cells caused no significant change in the Km for PM-APLT (p = 0.327). However, curve fitting to the Michaelis-Menten equation indicated a significant increase the Vmax for this enzyme (1.7-fold; p = 0.004) in the Atp8a1-Myc expressing cells (Fig. 4a and Table I). A similar increase in Atp8a1 (1.7-fold) was observed also by Western blotting (4d). This strongly supported the possibility that Atp8a1 is indeed the plasma membrane APLT of the N18 cells (the same Km value) and that the process of this transfection causes an overall increase in the number of PM-APLT molecules in these cells, thus augmenting the Vmax value for the enzyme.
Fig. 4. Transient Expression of Atp8a1 in the mouse neuroblastoma cell line N18 causes an increase in Vmax without any change in Km.
(a) Kinetic parameters obtained with GraphPad Prism from substrate saturation curves indicate a significant increase (1.7-fold) in Vmax of PM-APLT without any change in Km in N18 cells expressing ectopic Atp8a1 (see Table I). (b and c) Immunostaining of the pCDNA-Atp8a1-myc transfected N18 cells with a Myc antibody shows plasma membrane localization of Atp8a1-Myc (Scale: 100 μm). (d) Immunoblot analysis of enriched membrane fractions using Ab-B shows a 1.7-fold increase in Atp8a1 expression (Atp8a1 bands were normalized to β-actin, from three independent experiments and analyzed using paired t-test). (Also see Figures S2, S3 and S5, Table S1, and Text S1).
Table I. Atp8a1 overexpression causes an increase in Vmax for the PM-APLT activity in the N18 cells.
(paired t-test was used for the analysis of Vmax and Km values from three experiments)
| Exp | Empty Vector in N18 | Atp8a1 in N18 | ||||||
|---|---|---|---|---|---|---|---|---|
| Km (nmole/μm2) |
Km(nmole/μm2) Mean± Std.Dev. |
Vmax (nmole/ min/μm2) |
Vmax (nmole/ min/μm2) Mean± Std.Dev. |
Km (nmole/μ m2) |
Km(nmole/μm2) Mean± Std.Dev. |
Vmax (nmole/ min/μm2) |
Vmax (nmole/ min/μm2) Mean± Std.Dev. |
|
| 1 | 4.9 | 3.9±0.9 | 4.32 | 5.1±0.81 | 5.7 |
4.4±1 p=0.3269 (vector vs. Atp8a1) |
9.23 |
8.3±0.8 p=0.004112 (vector vs. Atp8a1) |
| 2 | 3.8 | 4.96 | 4.2 | 7.92 | ||||
| 3 | 3.1 | 5.94 | 3.2 | 7.76 | ||||
| Exp | Fold Increase in Km in the presence of Atp8a1 | Fold Increase in Vmax in the presence of Atp8a1 | ||
|---|---|---|---|---|
| Fold Increase | Mean± Std.Dev | Fold Increase | Mean± Std.Dev | |
| 1 | 1.16 | 1.1±0.066 | 2.14 | 1.7±0.42 |
| 2 | 1.11 | 1.60 | ||
| 3 | 1.03 | 1.31 | ||
Transient overexpression of Atp8a2 in N18 cells does not cause an increase in Vmax for the PM-APLT
Overexpression of an Atp8a1 homolog, Atp8a2, which is also a putative APLT that bears 65% sequence identity to Atp8a1 (Coleman 2009) did not cause an increase in the Vmax for PM-APLT (Fig. S5 and Table S1). For reasons that are not clear yet, it caused a significant decrease in both Vmax and Km. Data shown in Fig. 4, Table I and Fig. S5 are consistent with our hypothesis that the ectopically expressed Atp8a1 does indeed produce a specific effect by increasing the number of PM-APLT molecules and thereby, the Vmax of the enzyme in N18 cells.
Transient expression of an Atp8a1 phosphorylation-site mutant in N18 cells causes a decrease in Vmax without affecting the value of Km for the PM-APLT
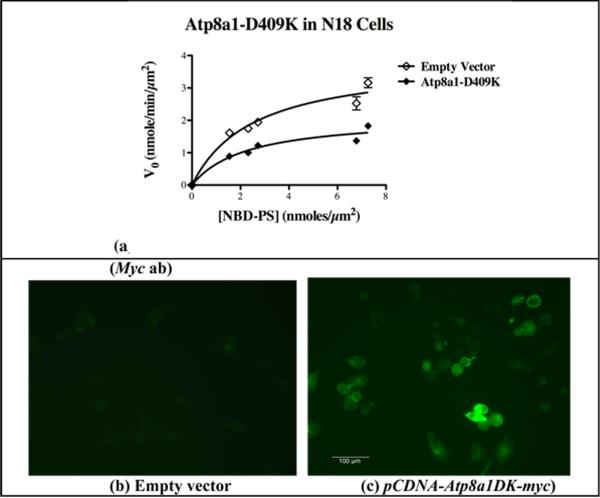
Expression of the Atp8a1(DK) mutant in the N18 cells caused a significant decrease in Vmax (p = 0.004) without any significant change in Km (p = 0.272) (Fig. 5a and Table II). The expression of this Myc-tagged mutant protein was confirmed by immunostaining with an anti-Myc antibody (Fig. 5b and c). These data further supported our postulate that Atp8a1 is the PM-APLT of the N18 cells.
Fig. 5. Expression of the Atp8a1(DK) mutant causes an inhibition of Vmax without any significant change in Km for the PM-APLT activity in the N18 cells.
(a) Kinetic parameters obtained with GraphPad Prism from substrate saturation curves for PM-APLT activity indicate a significant decrease in Vmax in N18 cells expressing Atp8a1(DK) without any alteration in the value of Km (see Table II). (b and c) Immunostaining of the pCDNA-Atp8a1(DK)-myc transfected N18 cells with a Myc antibody showed plasma membrane localization of Atp8a1(DK)-Myc. (Scale: 100 μm).
Table II. Mutant Atp8a1 causes a decrease in Vmax for PM-APLT in the N18 cells.
(paired t-test was used for the analysis of Km and Vmax)
| Exp | Empty Vector in N18 | Atp8a1DK in N18 | ||||||
|---|---|---|---|---|---|---|---|---|
| Km (nmole/μ m2) |
Km(nmole/μm2) Mean± Std.Dev. |
Vmax (nmole/ min/μm2) |
Vmax (nmole/ min/μm2) Mean± Std.Dev. |
Km (nmole/μ m2) |
Km(nmole/μm2) Mean± Std.Dev. |
Vmax (nmole/ min/μm2) |
Vmax (nmole/ min/μm2)Mean± Std.Dev. |
|
| 1 | 2.5 | 3.3±0.6 | 3.86 | 4.1±0.3 | 2.3 |
2.9±0.6 p=0.2721 (vector vs. Atp8a1) |
2.13 |
2.4±0.5 p=0.00426 (vector vs. Atp8a1) |
| 2 | 3.6 | 4.44 | 3.1 | 2.26 | ||||
| 3 | 3.70 | 4.02 | 2.9 | 2.48 | ||||
| Exp | Fold Increase in Km in the presence of Atp8a1DK | Fold Increase in Vmax in the presence of Atp8a1DK | ||
|---|---|---|---|---|
| Fold Increase | Mean± Std.Dev | Fold Increase | Mean± Std.Dev | |
| 1 | 0.89 | 0.89±0.04 | 0.55 | 0.61±0.14 |
| 2 | 0.86 | 0.51 | ||
| 3 | 0.93 | 0.76 | ||
Transient expression of Atp8a1 phosphorylation-site mutants in N18 cells leads to PS externalization
As shown in Fig. 6a, the Atp8a1(DK)-expressing cells displayed pronounced PS externalization in the N18 cells. Similarly, the Atp8a1(DE)-expressing cells also showed wide-spread PS externalization (Fig. 6b and Fig. S6). Enlarged images of the cells in Fig. 6b show colocalization of Alexafluor488-annexin V (green) and co-expressed AsRed2 (red) fluorescence. The number of annexin V(+) cells was normalized to the number of AsRed2(+) cells for quantification (Fig. 6c).
Fig. 6. Transient expression of Atp8a1 mutants causes PS externalization without apoptosis.
(a) PS externalization (annexin V staining) occurs in the N18 cells expressing the DK mutant (or DE; Fig. S6) but not wild type Atp8a1. Co-expressed AsRed2 (red) marks the transfected cells (shown by white arrows). (Scale: 100 μm). (b) Merged (enlarged) images showing localization of annexin V staining in the AsRed2-expressing transfected cells. (c) Graphical view of the increase in annexin V(+) cells (normalized to AsRed2(+) cell number) expressing the DE or DK mutants. (also see Fig. S6). (d) Transfection with pCDNA6-Myc (vector) or pCDNA6-Atp8a1(DK)-Myc yields no apoptotic cells after 24 h, but curcumin treatment yields dark, round apoptotic N18 cells (Scale: 200 μm). (e) The curcumin-treated N18 cells but not the Atp8a1(DK) expressing cells show a dramatic increase in caspase-3/7 activity (p < 0.0001 versus all other sets). Statistical analysis was performed using ANOVA.
As shown in Fig. 6d, ectopic expression of Atp8a1(DK) caused no appreciable change in the morphology of the N18 cells. Relative to the vector or sham transfected (only transfection reagents) cells, no increase in the apoptosis-associated enzymes caspase-3/7 activity was observed in the Atp8a1(DK)-expressing cells (Fig. 6e). A dramatic increase in caspase-3/7 activity and the presence of condensed, apoptotic N18 cells were observed following a 24-h treatment with 50 μM curcumin according to our earlier report (Fig. 6d and e) (Purkayastha 2009a).
Discussion
An externalized PS molecule has been identified as a universal “eat me” signal (Venegas 2007, Hirt 2000, Fadok 2000). Interactions of PS with its specific receptor/s on phagocytic cells elicits an anti-inflammatory signal and inhibits immune responses while removing the PS-displaying cells by phagocytosis (Hoffmann & Jordan 2005). Based on our earlier studies (Adayev 1998, Das 2003), we expected that most PS-labeled hippocampal cells in the Atp8a1(-/-) mice will be recognized by the scavenger cells of the brain and eliminated by phagocytosis. However, PS externalization may not be the only signal for phagocytosis. Recent studies have indicated that the apoptotic cells also secrete “find me” signals, in the form of molecules like lysophosphatidylcholine, ATP, and UTP, to attract scavenger cells (Bratton 2007, Ravichandran 2007, Elliott et al. 2009). It is not clear if the PS-displaying but nonapoptotic cells in the hippocampus of the Atp8a1(-/-) mice would also secrete such molecular signals to recruit phagocytic cells. Furthermore, multiple receptors and hinging molecules are required to mediate PS binding to phagocytes (Bratton 2007, Ravichandran 2007). Thus, PS externalization may initiate phagocytosis of a cell only if a scavenger cell with the appropriate receptor and/or hinging molecule is juxtaposed to it. Data shown in the current report demonstrate the absence of any salient morphological anomaly in the brain of the Atp8a1(-/-) mice, which refutes the possibility of widespread phagocytosis in the hippocampus.
It is possible that PS externalization or the absence of Atp8a1 per se produces some functional defects in the hippocampal cells. Earlier mouse studies have shown that most hippocampal lesions give rise to hyperactivity (van Praag 1994, Godsil 2005) and impairment in spatial memory (van Praag 1994, Broadbent 2006). The Atp8a1(-/-) mice displayed increased activity, impaired hippocampus-dependent memory (Remondes & Schuman 2004, Broadbent 2006), but no consistent sign of hyper anxiety (Walf 2007). It is quite likely that in the brain, the loss of Atp8a1 yields cell type-dependent differential changes in the glial and neuronal cells of the hippocampus. Although the behavioral impairment observed in the Atp8a1(-/-) mice strongly indicates aberrance in the circuitry of the hippocampal neurons, further studies are required to clearly define the cell type-specific role of Atp8a1 in the functional activity of the hippocampal neurons and glia.
The Web site of Deltagen Inc. (http://www.informatics.jax.org/external/ko/deltagen/1902.html), which created the knockout line, reported no anomalies in the Atp8a1(-/-) mice in a battery of tests that did not involve those included in the current report. In addition to the anomalies documented in Fig. 3, the Atp8a1(-/-) females also display poor nesting ability and maternal behavior, which has been observed before in the Pet-1 deficient mice that display aberrance in the development of serotonergic neurotransmission (Lerch-Haner 2008). Thus, future experiments may reveal other anomalies in the Atp8a1(-/-) mice.
In our kinetics and annexin V binding experiments, newly expressed molecules (Atp8a1, Atp8a1DK, or Atp8a2) were expected to enter the plasma membrane of the transfected N18 cells. As can be seen in the Atp8a2-transfected cells (Fig. S5 and Table S1), ectopic expression of Atp8a2 does not cause an increase in the Vmax of NBD-PS translocation in the plasma membrane. Only identical or almost identical molecules like Atp8a1 or Atp8a1DK would be expected to cause no conformational change of the resident PM-APLT, thus leaving the value of Km unaltered while changing the value of Vmax, which represents the total number active enzyme molecules under one fixed assay condition and excess substrate. Our observation receives support from recent studies by Darland-Ransom and coworkers in C.elegans and Soupene and coworkers in red blood cells indicating that mammalian Atp8a1 and its C.elegans ortholog TAT-1 function as PM-APLT (Darland-Ransom 2008, Soupene 2008). As mentioned earlier, only rapidly dividing N18 cells at 50% confluence in fresh growth medium were used for the kinetic analysis, thus ensuring the presence of high ATP levels in the cells.
Relative to the hippocampal cells from the wild type mice, the hippocampal cells from the heterozygous Atp8a1(+/-) mice did not show any increase Alexafluor488-annexin V staining (fig. S1a). This indicated that the normal PS asymmetry in the hippocampal cells could be maintained by half of the wild-type level of Atp8a1. Intriguingly, the Atp8a1(-/-) mice express the homologous flippase Atp8a2 in 5-times more erythrocytes (Fig. S1c-e), which, unlike the hippocampal cells, do not display PS (Fig. S1b). Although an earlier study suggested Atp8a1 to be the PM-APLT of the erythrocytes (Soupene 2008), our results indicate that in the absence of Atp8a1, induced Atp8a2 could compensate for the lack of Atp8a1 in the Atp8a1(-/-) mouse erythrocytes, thereby inhibiting PS externalization. It would be of great interest to investigate the expression and role of Atp8a2 in the hippocampal cells.
As shown by our results, the phosphorylation-site mutants DK and DE function as dominant negative mutants and a mechanism for this inhibitory activity is suggested here. Dominant negative mutants can efficiently inhibit any functional consequence of the corresponding wild type enzyme if the enzymatic reaction is limited by the concentration of a substrate or protein that critically regulates the processing or activation of the enzyme (Herskowitz 1987). Prior studies have indicated that an interaction between Atp8a1 (or its homolog Atp8b1) and a chaperone-like protein CDC50 is required for its translocation from the endoplasmic reticulum (ER) to the plasma membrane and even phosphorylation at the D409 residue (Lenoir 2009, Paulusma 2008, Saito 2004, Bryde et al. 2010). The Atp8a1 phosphorylation-site mutants could sequester the mammalian homolog of CDC50, thus preventing this chaperone from targeting the wild-type Atp8a1 molecules to the plasma membrane. Consequently, the Atp8a1-catalyzed PS translocation to the inner leaflet of the plasma membrane would be inhibited, thus exposing PS molecules on the surface by the overpowering action of scramblase, which translocates membrane phospholipids both ways in a head group-independent manner (Zwaal 1997, Daleke 2007, Devaux 2008, Bratton 1997). Future investigations will test this hypothesis.
As mentioned earlier, comparison with an NBD-PS standard curve constructed using the same NBD-PS sample that was used for a specific experiment eliminated most inter-experiment variation in the Km for the control samples (see Text S1 for methods). Thus, all the control Km values obtained for PM-APLT from the three experiments were not widely different from one another (Fig. 4, 5, S5, and Tables I, II, and S1). Furthermore, conducting all experiments within the first three passages of the N18 cells helped minimize passage-dependent changes in the kinetic parameters.
In summary, this study establishes that Atp8a1 plays an essential role in plasma membrane APLT activity in a neuronal cell line and also provides direct evidence showing that deletion of Atp8a1 in mice is associated with PS externalization in hippocampal cells and various hippocampus-associated behavioral anomalies in adult mice.
Supplementary Material
Acknowledgment
Fellowships from Alliance for Minority Participation (AMP) and MAGNET-AGEP to KL and grant support from NIH (CA77803-03) are gratefully acknowledged here. We thank Ms. Sreyashi Samaddar, Dr. Sara Guariglia, and Mr. Jonathan Blaize for valuable assistance and Dr. Yu-Wen Hwang for thoughtful advice. None of the authors have any conflict of interest in the publication of this manuscript.
Footnotes
Present address of Kelly Levano and Sudarshana Purkayastha: Albert Einstein College of Medicine of Yeshiva University, Jack and Pearl Resnick Campus, 1300 Morris Park Avenue, Bronx, NY 10461.
References
- Adayev T, Estephan R, Meserole S, Mazza B, Yurkow EJ, Banerjee P. Externalization of phosphatidylserine may not be an early signal of apoptosis in neuronal cells, but only the phosphatidylserine-displaying apoptotic cells are phagocytosed by microglia. J. Neurochem. 1998;71:1854–1864. doi: 10.1046/j.1471-4159.1998.71051854.x. [DOI] [PubMed] [Google Scholar]
- Adayev T, Ray I, Sondhi R, Sobocki T, Banerjee P. The G protein-coupled 5-HT1A receptor causes suppression of caspase-3 through MAPK and protein kinase Cα. Biochim. Biophys. Acta. 2003;1640:85–96. doi: 10.1016/s0167-4889(03)00023-5. [DOI] [PubMed] [Google Scholar]
- Arms K, Camp P. Biology. Harcourt Brace College Publishers; New York: 1995. [Google Scholar]
- Bratton DL, Hensen PM. Apoptotic Cell Recognition: Will the Real Phosphatidylserine Receptor(s) Please Stand up? Current Biology. 2007;18:R76–R79. doi: 10.1016/j.cub.2007.11.024. [DOI] [PubMed] [Google Scholar]
- Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA, Henson PM. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated non-specific flip-flop and is enhanced by loss of the aminophospholipid translocase. J. Biol. Chem. 1997;272:26159–26165. doi: 10.1074/jbc.272.42.26159. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Reversible hippocampal lesions disrupt water maze performance during both recent and remote memory tests. Learning and Memory. 2006;13:187–191. doi: 10.1101/lm.134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryde S, Hennrich H, Verhulst PM, Devaux PF, Lenoir G, Holthuis JCM. CDC50 Proteins Are Critical Components of the Human Class-1 P4-ATPase Transport Machinery. Journal of Biological Chemistry. 2010;285:40562–40572. doi: 10.1074/jbc.M110.139543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Ingram MF, Rosal PH, Graham TR. Role for Drs2p, a P-type ATPase and Potential Aminophospholipid Translocase, in Yeast Late Golgi Function. J. Cell Biology. 1999;147:1223–1236. doi: 10.1083/jcb.147.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin G, El-Sherif Y, Jayman F, Estephan R, Wieraszko A, Banerjee P. Appearance of voltage-gated calcium channels following overexpression of ATPase II cDNA in neuronal HN2 cells. Molecular Brain Research. 2003;117:109–115. doi: 10.1016/s0169-328x(03)00210-9. [DOI] [PubMed] [Google Scholar]
- Coleman JA, Kwok MCM, Molday RS. Localization, Purification, and Functional Reconstitution of the P4-ATPase Atp8a2, a Phosphatidylserine Flippase in Photoreceptor Disc Membranes. J Biol Chem. 2009;284:32670–32679. doi: 10.1074/jbc.M109.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J, Pak CH, Zwaal RFA, Schroit AJ. Bidirectional Transbilayer Movement of Phospholipid Analogs in Human Red Blood Cells. journal of Biological Chemistry. 1992;267:19412–19417. [PubMed] [Google Scholar]
- Connor J, Schroit AJ. Transbilayer movement of phosphatidylserine in nonhuman erythrocytes: evidence that the aminophospholipid transporter is a ubiquitous membrane protein. Biochemistry. 1989;28:9680–9685. doi: 10.1021/bi00451a021. [DOI] [PubMed] [Google Scholar]
- Curtis H, Barnes SN. Biology. Worth Publishers; New York: 1989. [Google Scholar]
- Daleke D, Lyles J. Identification and purification of aminophospholipid flippases. Biochim. Biophy. Acta. 2000;1486:108–127. doi: 10.1016/s1388-1981(00)00052-4. [DOI] [PubMed] [Google Scholar]
- Daleke DL. Phospholipid Flippases. Journal of Biological Chemistry. 2007;282:821–825. doi: 10.1074/jbc.R600035200. [DOI] [PubMed] [Google Scholar]
- Darland-Ransom M, Wang X, Sun C-L, Mapes J, Gengyo-Ando K, Mitani S, Xue D. Role of C. elegans TAT-1 protein in maintaining plasma membrane phosphatidylserine asymmetry. Science. 2008;320:528–531. doi: 10.1126/science.1155847. [DOI] [PubMed] [Google Scholar]
- Das P, Estephan R, Banerjee P. Apoptosis is associated with an inhibition of aminophospholipid translocase (APTL) in CNS-derived HN2-5 and HOG cells and phosphatidylserine is a recognition molecule in microglial uptake of the apoptotic HN2-5 cells. Life Sci. 2003;72:2617–2627. doi: 10.1016/s0024-3205(03)00163-2. [DOI] [PubMed] [Google Scholar]
- Deacon MJR. Housing, husbandry and handling of rodents for behavioral experiments. Nature Protocols. 2006;1:936–946. doi: 10.1038/nprot.2006.120. [DOI] [PubMed] [Google Scholar]
- Devaux PF, Herrmann A, Ohlwein N, Kozlov MM. How lipid flippases can modulate membrane structure. Biochimica Biophysica Acta. 2008;1778:1591–1600. doi: 10.1016/j.bbamem.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Ding J, Wu Z, Crider BP, Ma Y, Li X, Slaughter C, Gong L, Xie X-S. Identification and functional expression of four isoforms of ATPase II, the putative aminophospholipid translocase. J. Biol. Chem. 2000;275:23378–23386. doi: 10.1074/jbc.M910319199. [DOI] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz AB, Henson PM. A receptor for phosphatidylserine-specificc clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Cathelineau AD, Daleke DL, Henson PM, Bratton DL. Loss of Phospholipid Asymmetry and Surface Exposure of Phosphatidylserine Is Required for Phagocytosis of Apoptotic Cells by Macrophages and Fibroblasts. J. Biol. Chem. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA, Henson PM. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J. Immunol. 1992a;149:4029–4035. [PubMed] [Google Scholar]
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Hensen PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992b;148:2207–2216. [PubMed] [Google Scholar]
- Godsil BP, Stefanacci L, Fanselow MS. Bright light suppresses hyperactivity induced by excitotoxic dorsal hippocampus lesions in the rat. Bahavioral Neuroscience. 2005;119:1339–1352. doi: 10.1037/0735-7044.119.5.1339. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987;329:219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Hicks BW, Parsons SM. Characterization of the P-type and V-type ATPases of cholinergic synaptic vesicles and coupling of nucleotide hydrolysis to acetylcholine transport. Journal of Neurochemistry. 1992;58:1211–1220. doi: 10.1111/j.1471-4159.1992.tb11331.x. [DOI] [PubMed] [Google Scholar]
- Hine R. “Membrane”. The Facts on File Dictionary of Biology. Checkmark; New York: 1999. [Google Scholar]
- Hirt UA, Ganter F, Leist M. Phagocytosis of Nonapoptotic Cells Dying by Caspase-Independent Mechanisms. J. Immunol. 2000;164:6520–6529. doi: 10.4049/jimmunol.164.12.6520. [DOI] [PubMed] [Google Scholar]
- Hoffmann PR, Kench JA, Vondracek A, Kruk E, Daleke DL, Jordan M, Marrack P, Henson PM, Fadok*† Valerie A. Interaction between Phosphatidylserine and the Phosphatidylserine Receptor Inhibits Immune Responses In Vivo. J. Immunol. 2005;174:1393–1404. doi: 10.4049/jimmunol.174.3.1393. [DOI] [PubMed] [Google Scholar]
- Kuchel PW, Ralston GB. Theory and Problems of Biochemistry. Schaum's Outline/McGraw-Hill; New York: 1988. [Google Scholar]
- Kuhlbrandt W. Biology, Structure and Mechanism of P-type ATPases. Nature Reviews. 2004;5:282–295. doi: 10.1038/nrm1354. [DOI] [PubMed] [Google Scholar]
- Lenoir G, Williamson P, Puts CF, Holthuis JCM. Cdc50p plays a vital role in the ATPase reaction cycle of the putative aminophospholipid transporter Drs2p. Journal of Biological Chemistry. 2009;284:17956–17967. doi: 10.1074/jbc.M109.013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nature Neuroscience. 2008;11:1001–1003. doi: 10.1038/nn.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levano K, Sobocki T, Jayman F, Debata PR, Sobocka MB, Banerjee P. A genetic strategy involving a glycosyltransferase promoter and a lipid translocating enzyme to eliminate cancer cells. Glycoconjugate Journal. 2009;26:739–748. doi: 10.1007/s10719-009-9233-1. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris Water Maze and conditioned-fear task. Behavioral Neuroscience. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- McIntyre JC, Sleight RG. Fluorescence Assay for Phospholipid Membrane Asymmetry. Biochemistry. 1991;20:11819–11827. doi: 10.1021/bi00115a012. [DOI] [PubMed] [Google Scholar]
- Mehta M, Ahmed Z, Fernando SS, Cano-Sanchez P, Adayev T, Ziemnicka D, Wieraszko A, Banerjee P. Plasticity of 5-HT1A receptor-mediated signaling during early postnatal brain development. Journal of Neurochemistry. 2007;101:918–928. doi: 10.1111/j.1471-4159.2007.04448.x. [DOI] [PubMed] [Google Scholar]
- Moriyama Y. a. N., N. Purification and properties of a Vanadate- nd N-Ethylmaleimide-sensitive ATPase from Chromaffin Granule Membranes. J. Biol. Chem. 1988;263:8521–8527. [PubMed] [Google Scholar]
- Paterson JK, Renkema K, Burden L, Halleck MS, Schlegel RA, Williamson P, Daleke DL. Lipid Specific Activation of the Murine P4-ATPase Atp8a1 (ATPase II). Biochemistry. 2006;45:5367–5376. doi: 10.1021/bi052359b. [DOI] [PubMed] [Google Scholar]
- Paulusma CC, Folmer DE, Ho-Mok KS, de Waart DR, Hilarius PM, Verhoeven AJ, Oude Elferink RP. ATP8B1 requires an accessory protein for endoplasmic reticulum exit and plasma membrane lipid flippase activity. Hepatology. 2008;47:268–278. doi: 10.1002/hep.21950. [DOI] [PubMed] [Google Scholar]
- Pomorski R, Lombardi R, Riezman H, Devaux PF, van Meer G, Holthuis JC. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Molecular Biology of the Cell. 2003;14:1240–1254. doi: 10.1091/mbc.E02-08-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkayastha S, Berliner A, Fernando SS, Ranasinghe B, Ray I, Tariq H, Banerjee P. Curcumin Blocks Brain Tumor Formation. Brain Research. 2009a;1266C:130–138. doi: 10.1016/j.brainres.2009.01.066. [DOI] [PubMed] [Google Scholar]
- Purkayastha S, Fernando SS, Diallo S, Cohen L, Levano K, Banerjee P. Regulation of Protein Kinase C Isozymes During Early Post-Natal Hippocampal Development. Brain Research. 2009b;1288:29–41. doi: 10.1016/j.brainres.2009.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor1A knockout: An animal model of anxiety-related disorder. Proc. Natl. Acad. Sci. USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nature. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- Saito K, Fujimura-Kamada K, Furuta N, Kato U, Umeda M, Tanaka K. Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:3418–3432. doi: 10.1091/mbc.E03-11-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille E, Pavlides C, Benke D, Toth M. Genetic Inactivation of the Serotonin1A Receptor in Mice Results in Downregulation of Major GABAA Receptor alpha Subunits, Reduction of GABAA Receptor Binding and Benzodiazepine-Resistant Anxiety. J. Neurosci. 2000;20:2758–2765. doi: 10.1523/JNEUROSCI.20-08-02758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobocki T, Jayman F, Sobocka MB, Duchatellier R, Banerjee P. Isolation, Sequencing, and Functional Analysis of the TATA-less Human ATPase II Promoter. Biochimica Biophysica Acta. 2005;1728:186–198. doi: 10.1016/j.bbaexp.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Sobocki T, Jayman F, Sobocka MB, Marmur J, Banerjee P. Isolation, Sequencing, and Functional Analysis of the TATA-less Murine ATPase II Promoter and Structural Analysis of the ATPase II Gene. Biochimica Biophysica Acta. 2007;1769:61–75. doi: 10.1016/j.bbaexp.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupene E, Kemaladewi DU, Kuypers FA. ATP8A1 activity and phosphatidylserine transbilayer movement. J. Receptor Ligand Channel Res. 2008;1:1–10. doi: 10.2147/jrlcr.s3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kamakura M, Morii M, Takeguchi N. The phospholipid flippase activity of gastric vesicles. J Biol Chem. 1997;272:10429–10434. doi: 10.1074/jbc.272.16.10429. [DOI] [PubMed] [Google Scholar]
- Tang X, Halleck MS, Schlegel RA, Williamson P. A Subfamily of P-Type ATPases with Aminophospholipid Transporting Activity. Science. 1996;272:1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- Tyurina YY, Basova LV, Konduru NV, Tyurin VA, Potapovich AI, Cai P, Bayir HI, Stoyanovsky D, Pitt BR, Shvedova AA, Fadeel B, Kagan VE. Nitrosative Stress Inhibits the Aminophospholipid Translocase Resulting in Phosphatidylserine Externalization and Macrophage Engulfment. journal of Biological Chemistry. 2007;282:8498–8509. doi: 10.1074/jbc.M606950200. [DOI] [PubMed] [Google Scholar]
- van Praag H, Dreyfus CF, Black IB. Dissociation of motor hyperactivity and spatial memory deficits by selective hippocampal lesions in the neonatal rat. Journal of Cognitive Neuroscience. 1994;6:321–331. doi: 10.1162/jocn.1994.6.4.321. [DOI] [PubMed] [Google Scholar]
- Venegas V, Zhou Z. Two alternative mechanisms that regulate the presentation of apoptotic cell engulfment signal in Caenorhabditis elegans. Molecular Biology of the Cell. 2007;18:3180–3192. doi: 10.1091/mbc.E07-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature Protocols. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf A, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature Protocols. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting A, Müller P, Herrmann A, Kettenmann H, Nolte C. Phagocytic clearance of apoptotic neurons by microglia/brain macrophages in vitro: involvement of lectin-, integrin-, and phosphatidylserine-mediated recognition. J. Neurochem. 2000;75:1060–1070. doi: 10.1046/j.1471-4159.2000.0751060.x. [DOI] [PubMed] [Google Scholar]
- Xie X-S, Stone DK, Racker E. Purification of a vanadate-sensitive ATPase from clathrin-coated vesicles of bovine brain. J. Biol. Chem. 1989;264:1710–1714. [PubMed] [Google Scholar]
- Zhou X, Graham TR. Reconstitution of phospholipid translocase activity with purified Drs2p, a type-IV P-type ATPase from budding yeast. Proceedings of the National Academy of Sciences USA. 2009;106:16586–16591. doi: 10.1073/pnas.0904293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullig S, Neukomm LJ, Jovanovic M, Charette SJ, Lyssenko NN, Halleck M, Reutelingsperger S, Chris PM, Schlegel RA, Hengartner MO. Aminophospholipid Translocase TAT-1 Promotes Phosphatidylserine Exposure during C. elegans Apoptosis. Current Biology. 2007;17:994–999. doi: 10.1016/j.cub.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Zwaal RFA, Schroit RA. Pathophysiologic Implications of Membrane Phospholipid Asymmetry in Blood Cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.