Abstract
Pancreatic cancer (PC) is a complex disease harboring a myriad of genetic and epigenetic changes. The dismal survival of patients diagnosed with PC is in part due to de novo and acquired resistance to conventional therapeutics, resulting from deregulated signaling including aberrant expression of small nc miRNAs. Emerging research in this area has lead to the identification and characterization of deregulated miRNAs, which have generated a renewed interest and hope in that novel targeting of miRNAs may lead to a better clinical outcome for patients diagnosed with PC. However, recent evidence suggests that miRNAs are also under a highly coordinated system of epigenetic regulation emphasizing the fact that the design of miRNAs as targeted therapy may not be as simple as originally anticipated. For a successful miRNA-based therapeutic regimen, a holistic integrated approach may be required to take into account because of these emerging epigenetic regulatory mechanisms. In this article, we will discuss miRNA epigenetics, it's significance in PC and the use of a systems science to identify these aberrant epigenetically groomed miRNAs, and we believe that such knowledge would likely benefit further research to realize the dream of miRNA-based targeted therapy for human malignancies.
Keywords: drug design and development, drug resistance, epigenetics, miRNA, network modeling, pancreatic cancer, personalized medicine, systems biology
Pancreatic cancer (PC) is by far an incurable disease with an estimated 168,800 annual fatalities worldwide translating to approximately 20 deaths every hour (Global Cancer Statistics 2011) [1]. PC is often called ‘the silent killer’ because early stages of the disease often does not cause any symptoms so this leads to PC being diagnosed at a very late stage when it is not amenable to surgery or standard chemotherapy [2]. The median survival is 6 months, and thus the overall therapeutic response rate is less than 5% [3]. These dismal statistics suggest that newer diagnostic biomarkers and therapeutically druggable targets (newer avenues of treatment) need to be urgently identified. PC is among the most complex and heterogeneous of all known malignancies involving multiple deregulatory signaling mechanisms [4]. Among the various critically deregulated signaling pathways found in PC, the deregulated expression of the miRNA system is one of them [5]. miRNAs comprise of a class of short nc RNAs that are approximately 20–25 nucleotides in length found in all animal and plant cells [6]. In 1993, the first miRNAs were recognized in Caenorhabditis elegans by Lee et al. [7]. Later on, in the year 2001, various small regulatory RNAs were discovered in plants and mammals and designated as ‘miRNA’ [8–10]. Even though there are thousands of miRNAs registered in the miRNAbase miRNA database; only approximately 1200 have been characterized for their different biological functions [11]. miRNAs have been extensively studied for their involvement in RNAi mechanisms that regulates gene expression post-transcriptionally and contributes to diverse physiological and pathophysiological functions, including the regulation of developmental timing and pattern formation, restriction of differentiation potential [10], cell signaling [11], cardiovascular diseases [12] and carcinogenesis [13]. The biogenesis and RNAi functions of miRNA (i.e., how miRNAs are generated and processed into a mature form, and how they regulate gene expression) have been intensely investigated and well described (Figure 1A) [10]. These studies have led to a much deeper understanding of miRNA biogenesis, their regulatory control on different genes, which led to an exponential increase in research for developing strategies to target them for anticancer therapy (current state of miRNA research from PubMed search is summarized in Figure 1B). Furthermore, developments in miRNA-related technologies, such as miRNA expression profiling and synthetic oligoRNA, have contributed to the identification of miRNAs involved in a number of physiological and pathological conditions.
Figure 1. MiRNA biogenesis and current status of miRNA research.
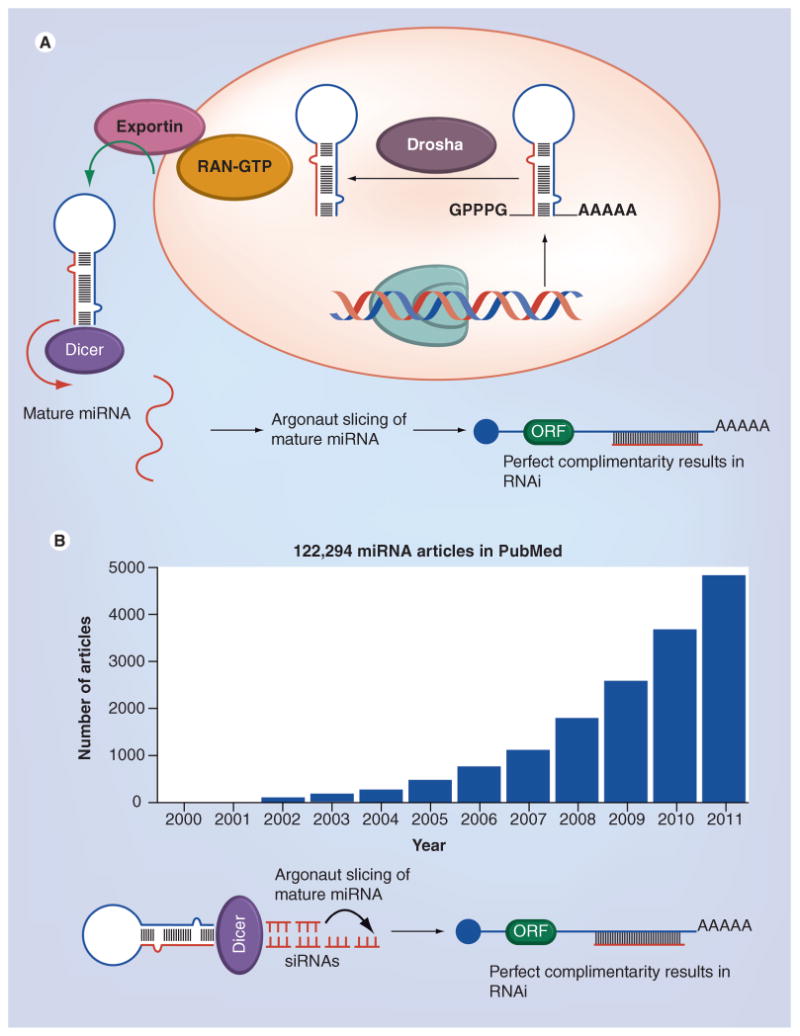
(A) miRNAs transcribed by RNA polymerase II into the primary miRNAs, having 5′ caps and poly-A tails. The primary miRNAs are processed by RNase III Drosha with its partner Pasha into the precursor miRNAs, which are then exported by the nuclear export factor Exporting 5 and its cofactor RAN-GTP into the cytoplasm. Once in the cytoplasm, the precursor miRNAs are further processed by another RNase III Dicer into the mature miRNAs. The mature miRNA is incorporated into the RISC complex and negatively regulates its target mRNA through two main mechanisms: when it binds to its target in a complementary manner, it leads to target mRNA cleavage and by binding to its target with incomplete complementary this leads to translational repression. (B) Approximate 10-year data collected from PubMed search engine under keywords ‘miRNA’ under defined limits.
In spite of different rapid advancements, some questions still remain largely unanswered, such as how miRNA expression is controlled and which genes are regulated by each miRNA. Recently, accumulating studies have shown that the majority of miRNAs are regulated epigenetically. Although epigenetics and miRNAs have been frequently described in a number of excellent reviews [12,13], few reviews have focused upon the relationship between epigenetically regulated miRNAs and their consequence on therapeutic resistance especially in complex diseases such as PC. Here, we will first illustrate the current knowledge regarding the epigenetic–miRNA regulatory networks followed by its impact on both de novo and acquired drug resistance in PC. Finally, some novel integrated concepts on identifying, understanding and targeting these deregulatory networks will be discussed in the subsequent paragraphs.
Epigenetics & miRNA regulation
Epigenetics (επι derived from the Greek meaning over, above or outer) is the study of changes produced in gene expression caused by mechanisms other than changes in the underlying DNA sequence. Examples of such changes include DNA methylation or histone deacetylation, both of which serve to suppress gene expression without altering the sequence of the target genes. Any plant, animal or human gene can undergo epigenetic regulation and these regulatory mechanisms have been thoroughly evaluated over the last 50 years. Recent studies have demonstrated that epigenetic mechanisms, including DNA methylation and histone modification are not restricted to regulating protein-encoding genes, but can also modulate the expression and function of different miRNAs [14]. Conversely, a subset of miRNAs controls the expression of important epigenetic regulators, including DNA methyltransferases, histone deacetylases and the polycomb group genes. This complicated network of feedback between miRNAs and epigenetic pathways appears to form an epigenetic–miRNA regulatory circuit (Figure 2A & B). When this regulatory circuit is disrupted, normal physiological functions are deregulated contributing to various disease processes including cancer. Even though the biogenesis of miRNA has been intensely studied and is well-described, the regulation of miRNA expression especially through epigenetic mechanisms remains largely unclear. In early studies, promoter regions had been determined for only a small subset of miRNAs, while in silico predictive studies have given blueprints of the promoter regions of miRNAs [15]. Nevertheless, most of these predicted miRNA promoters have yet to be confirmed through wet-laboratory experiments.
Figure 2. Epigenetic control of miRNAs.
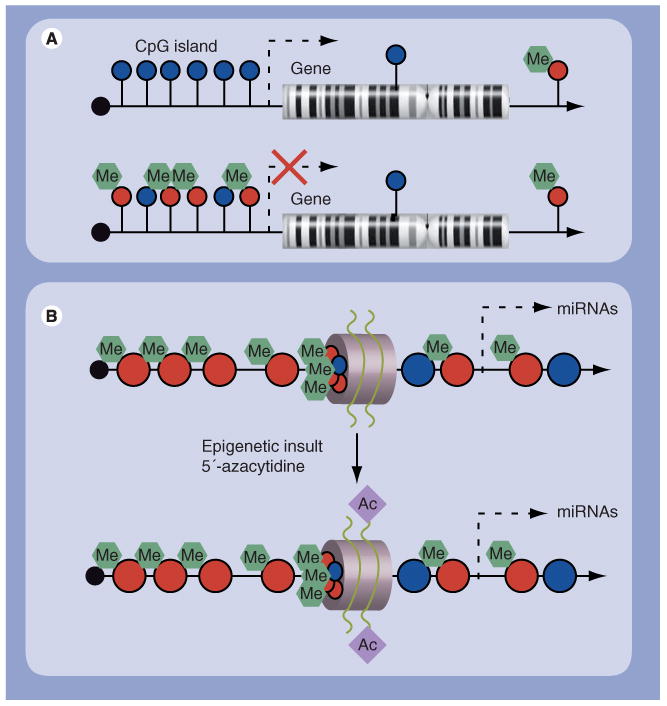
Epigenetic mechanisms such as (A) DNA methylation and (B) histone modifications contribute to the transcriptional control of miRNA expression. CpG islands can be found in the promoter regions of approximately 50% of all genes and normally remain unmethylated. Hypermethylated CpG is known for many cancers, which leads to the silencing of downstream genes. Methylation of the CpG sites and deacetylation of the histones around the promoter region contribute to its silencing in tumor cell lines. Treatment with 5-aza-2′-deoxycytidine and 4-phenylbutyric acid leads to reduced DNA methylation and increased histone acetylation, allowing the miRNA to be expressed. The gray circle depicts a nucleosome with histone tails. Blue circles on the DNA strand represent unmethylated CpG sites, and red circles methylated CpG sites. Diamonds on the histone tails represent acetyl groups.
Numerous investigations have indicated that miRNAs transcribed from CpG islands can undergo DNA methylation mediated repression in a similar fashion as any gene that is regulated through promoter methylation [16]. The earliest studies on epigenetic control of miRNAs were published in a seminal Cance Cell paper by Saito and colleagues where it was shown that the expression of miR-127 is regulated epigenetically [17]. In this study, using a bladder cancer cell model it was demonstrated that targeted demethylation resulted in the activation of certain miRNAs (miR-127). Specifically, the DNA methylation level and histone modification status at identified promoter regions of miR-127 correlated significantly with mature miR-127 expression. These findings paved the way for numerous studies documenting epigenetic modulation of miRNAs in different cancer models. In another investigation, Lujambio and colleagues have demonstrated in a colon cancer cell line model that cyclin D kinase 6 over expression is driven by epigenetic hypermethylation mediated silencing of miR-124a [18]. This particular suppressive mechanism was recapitulated in other cancers, glioblastoma multiform, gastric cancer, hematopoietic malignancies, cervical and hepatocellular cancer indicating that hypermethylation mediated epigenetic regulation of miR-124a is a global phenomena. In prostate cancer patients using tissue specimens, it has been shown that miR-145, a well-recognized tumor suppressor, was down-regulated through methylation in its promoter region along with p53 gene mutations [19]. The same study was also extended to 50 different cancer cell lines showing similar DNA hypermethylation that suppressed miR-145 expression. In an acute myeloid leukemia model, the well-recognized c-kit regulating miR-193a has been shown to be silenced by DNA hypermethylation [20]. This critical miRNA is a regulator of various important cellular processes [21]. In breast cancer metastatic models, miR-335 which is a well-recognized inhibitor of invasion of migration genes (SOX4 and extracellular metric protein tensacin C) has been shown to und ergo genetic deletion coupled with promoter methylation that renders a selective advantage by increasing its invasive and metastatic potential [22].
Aberrant miRNA expression & its consequence on PC drug resistance
Prior to examining the epigenetic regulatory mechanisms of miRNAs in PC, it is critical to evaluate how altered miRNA expression manifests their effect on drug resistance in this complex disease. With respect to PC, a number of miRNAs have been correlated to drug resistance and poor overall survival. Our laboratory was among the first to demonstrate the expression profile of miRNAs in the plasma of patients diagnosed with PC (n = 50) compared with healthy volunteers (n = 10). In this study, 37 different miRNAs were found to be down-regulated and 54 were upregulated [23] for miRNA expression in patient samples. The expression of miR-21 was significantly higher, and the expression of let-7 family (especially let-7d) and miR-146a was significantly lower in PC. Most interestingly, the expression of miR-21 was correlated with poor overall survival, and the expression of let-7 was inversely correlated with survival in this pilot study with mixed patient population. In addition, we observed that the miR-21 family was markedly overexpressed in chemoresistant PC cell line models, which was consistent with the plasma data from PC patients. This was a ‘proof-of-principle’ study, suggesting that identifying and validating the expression of miRNAs in newly diagnosed patients could possibly serve as potential biomarker for tumor aggressiveness, and such miRNAs could be useful for the screening of high-risk patients, and may also serve as targets for future drug development.
Since then a number of diffierent expression profiling studies have identified several miRNAs aberrantly expressed in PC cell lines and clinical tissue samples that were correlated with clinical outcome (Table 1) [24–26]. These studies have demonstrated that alterations in miRNA expression profiles might be useful in the prediction of the individual response to chemotherapy, and in principle, can become potential targets for therapies to increase chemosensitivity. However, only selected miRNAs have been investigated for their potential role in chemoresistance in PC. Currently, the most promising miRNA targets in PC chemoresistance against gemcitabine are miRNA-15a, miRNA-21, miRNA-34, miRNA-200b, miRNA-200c, miRNA-214, miRNA-221 and members of the let-7 family. In in vitro studies, Zhang and coworkers have demonstrated that miRNA-214 promotes survival of PC cells and is responsible for gemcitabine resistance through downregulation of the tumor suppressor gene ING4 [27].
Table 1.
Deregulated miRNAs in pancreatic ductal adenocarcinoma.
| miRNA | Expression status | Ref. |
|---|---|---|
| miR-21 | ↑ | [23] |
| Let-7 | ↓ | [23] |
| miR-146a | ↓ | [23] |
| miR-200b,c,Let-7b-e | ↓ | [81] |
| Let-7f-1 | ↑ | [77] |
| Let-7d | ↑ | [77] |
| miR-10 | ↑ | [78] |
| miR-16b | ↑ | [79] |
| miR-16-1 | ↑ | [77] |
| miR-21 | ↑ | [77,78] |
| miR-23 | ↑ | [80] |
| miR-24 | ↑ | [81] |
| miR-31 | ↑ | [81–83] |
| miR-92 | ↑ | [77] |
| miR-95 | ↑ | [79] |
| miR-96 | ↓ | [81] |
| miR-99 | ↑ | [78] |
| miR-100 | ↑ | [77,78] |
| miR-103 | ↑ | [79–81] |
| miR-107 | ↑ | [77,78] |
| miR-125 | ↑ | [78,80,81] |
| miR-130b | ↓ | [81] |
| miR-139 | ↓ | [77] |
| miR-142-P | ↓ | [77] |
| miR-143 | ↑ | [78,81] |
| miR-145 | ↑ | [81] |
| miR-146 | ↑ | [78] |
| miR-148a | ↓ | [78,81] |
| miR-148b | ↓ | [78,81] |
| miR-155 | ↑ | [77,78,81] |
| miR-181a | ↑ | [77,78] |
| miR-181b | ↑ | [78] |
| miR-181c | ↑ | [77,78] |
| miR-181d | ↑ | [78] |
| miR-186 | ↑ | [79] |
| miR-190 | ↑ | [79] |
| miR-194 | ↑ | [80] |
| miR-196a | ↑ | [79,82] |
| miR-196b | ↑ | [82] |
| miR-199a | ↑ | [78] |
| miR-200b | ↑ | [79] |
| miR-200c | ↑ | [79,80] |
| miR-205 | ↑ | [78,81] |
| miR-210 | ↑ | [78,81] |
| miR-212 | ↑ | [77] |
| miR-213 | ↑ | [78] |
| miR-217 | ↓ | [81] |
| miR-220 | ↑ | [78] |
| miR-221 | ↑ | [77–79,81] |
| miR-222 | ↑ | [78,79,81] |
| miR-223 | ↑ | [78,81] |
| miR-301 | ↑ | [77] |
| miR-345 | ↓ | [77] |
| miR-375 | ↓ | [78,81] |
| miR-376a | ↑ | [77] |
| miR-424 | ↑ | [77] |
| miR-429 | ↑ | [82] |
On the other hand, upregulation of miRNA-15a inhibited the growth of chemoresistant PC cells by downregulating WNT3A and FGF7 in their system [28]. Additionally, another study showed a significant correlation between outcome and miRNA-21 expression in PC tissue specimens from 81 gemcitabine-treated patients [29]. Giovanetti et al. have demonstrated that patients with high miRNA-21 expression had a significantly shorter overall survival [30]. Further, they observed that transfection with precursor miRNA-21 significantly decreased antiproliferative effects and apoptosis inducing effects of gemcitabine in vitro, whereas MMP-2/MMP-9 and VEGF expression were found to be upregulated for which further mechanistic studies are warranted. Moreover, overexpression of miRNA-21 led to the downregulation of the tumor suppressor gene phosphatase and tensin homologue deleted on chromosome 10 through the activation of the PI3K/Akt-dependent pathway, rendering the cancer cells less susceptible to apoptosis. In yet another study linking miRNA as PC prognostic marker, Hwang et al. reported that lower than median miRNA-21 expression was correlated to longer overall as well as disease free survival and benefit from adjuvant gemcitabine treatment in two independent cohorts of 82 Korean and 45 Italian PC patients [29]. Furthermore, transfection with antimiRNA-21 enhanced chemosensitivity of PC cells. Giovanetti et al. demonstrated that the inhibition of miRNA-21 or miRNA-221 arrests cell cycle in the G1 phase, induces apoptosis by upregulating phosphatase and tensin homologue deleted on chromosome 10, reversion-inducing cysteine-rich protein with Kazal motifs (RECK) and p27kip1, sensitizing the effects of gemcitabine in PC cell lines [30]. Our laboratory (Ali et al.) has reported that CDF, a curcumin analogue, could sensitize PC cells to gemcitabine by inactivation of NF-κB and COX-2 and their downstream target molecules, which was in part due to downregulation of miRNA-21 and reactivation of miRNA-200b and miRNA-200c [23]. In this elegant study, inactivation of miRNA-21 led to the reactivation of phosphatase and tensin homologue deleted on chromosome 10, resulting in the inactivation of pAkt [23].
The reactivation of miR-200b and miR-200c, which may in turn, result in the reversal of epithelial-to-mesenchymal transition (EMT) phenotype, further led to sensitization of gemcitabine-resistant PC cells to gemcitabine. In a comprehensive investigation from our laboratory (Li et al.), we found that the expression of miRNA-200b, miRNA-200c, let-7b, let-7c, let-7d and let-7e was significantly down-regulated in gemcitabine-resistant cells, which showed EMT characteristics such as elongated fibroblastoid morphology, lower expression of epithelial marker E-cadherin and higher expression of mesenchymal markers such as vimentin and ZEB1 [31]. Moreover, re-expression of miRNA-200 by transfection studies or treatment of gemcitabine-resistant cells with either 3,3′-di-indolylmethabe or isofavone resulted in the downregulation of ZEB1, Slug and vimentin, which was consistent with morphologic reversal of EMT phenotype, leading to epithelial morphology. In addition, our group has also demonstrated that miRNA-146a expression was down-regulated in PC associated with aggressiveness of tumor cells [32].
The role of NF- κB in PC pathogenesis and drug response has been elucidated extensively by our group. We have elaborately published that natural agents including isoflavone and 3,3′-di-indolylmethabe can inhibit cancer cell growth and induce apoptosis through the downregulation of Notch and NF-κB signaling leading to an increased sensitivity of cancer cells to conventional chemotherapy [33,34]. Over the years, we have firmly established the critical role of NF-κB in the processes of EMT and tumor cell invasion and metastasis. In support of our findings, a thorough screening performed by Lu et al. found miRNA-301a as the most potent NF-κB activator. These studies showed that miR-301a activates NF-κB through downregulating the expression of the NF-κB repressor genes [35]. Another factor responsible for the observed resistance in PC is the subpopulation of cancer stemlike cells, which are highly resistant cells that do not respond to conventional chemotherapeutics. While the bulk of tumor cells get substantially eliminated; it remains those subpopulations of cancer stem-like cells that give rise to recurring tumors. It has been shown extensively that multiple signaling pathways, including NF-κB, a miRNA-mediated gene, contribute to the genesis and maintenance of EMT phenotypic cells and cancer stem cells or cancer stem-like cells [34–39].
Earlier studies have highlighted the global stem cell renewal regulatory network that was resulted from the dynamic interplay between chromatin remodeling, transcription factors and miRNAs [40]. Supporting these observations, in an important study, Ji et al. demonstrated that over expression of miR-34 provokes an 87% reduction of CD44+/CD133+ cancer stem cells in PC cells, and this was accompanied by a statistically significant inhibition of PC cell growth in vitro and tumor formation in vivo, as well as sensitization of chemoresistant PC cells to gemcitabine [41]. In this study, the use of miRNA-34 mimics or transfection with lentiviral miRNA-34 constructs to reactivate the tumor suppressor gene p53 in p53-deficient human PC cells was shown. The miR-34 was also found to be directly regulated by p53, modulated by its downstream target genes Bcl-2 and Notch, which are involved in the self-renewal process and survival of cancer stem cell.
Accumulating evidence from our work and those of others showed that the inhibition or reactivation of specific miRNAs can induce drug sensitivity by increased inhibition of cancer cell growth, invasion and metastasis. Due to their dual role in carcinogenesis, there are two direct strategies for the use of specific miRNAs as cancer therapeutics:
Antisense mediated inhibition of oncogenic miRNAs using 20-O-methyl modification of oligoribonucleotides in vitro, and the injection of a cholesterol-conjugated 20-O-methyl modified or a locked nucleic acid modified oligoribonucleotide in vivo;
Artificial expression of tumor suppressor miRNAs through either the application of synthetic miRNAs or by stable, vector-based transfection of genes coding for miRNAs [42,43].
Several publications have shown that inhibition of oncogenic miRNAs such as miRNA-21 or reactivation of tumor suppressive miRNAs such as miRNA-34 can sensitize PC cells to gemcitabine chemotherapy. These results suggest that specific targeting of miRNAs by a variety of approaches could open newer avenues for the treatment of PC by overcoming drug resistance, which will certainly improve the overall survival outcome of patients diagnosed with PC [44]. However, miRNAs are under epigenetic regulation and the effectiveness of strategies that focus on targeted inhibition and activation of miRNAs are not expected to be optimally successful unless these regulatory controls are taken into account.
Epigenetic grooming of miRNAs in PC
As described above, PC drug resistance is multifactorial that results from complex set of interacting molecular networks including the aberrant miRNAs system. As summarized in the previous paragraphs, miRNA deregulation signaling on PC drug resistance is fairly well-studied. However, there are very few studies describing epigenetic miRNA modulation in PC and their consequence on drug resistance. Nevertheless, the available literature certainly points to the existence of aberrant miRNA epigenetic modulation in PC. For example, in an interesting article by Zhang and his group showed that downregulation of miR-132 by promoter methylation results in the development of PC [45]. The authors explored the reason for the miR-132 expression differences seen between normal and tumor samples. In this study, it was found that promoter hypermethylation and reduced Sp1-binding affinity resulted in reduced expression of miR-132 in tumor tissues. As Sp1 can recruit histone deacetylase and DNA methyltransferase to repress transcription [46,47], suggesting the biological significance of epigenetic modulation of miR-132; however, it is not clear whether methylation of the miR-132 promoter is induced by Sp1 or whether hypermethylation of the promoter abrogates Sp1 binding.
In another study, miRNAs whose expressions are inactivated by hypermethylation in PC were identified [48]. The same group also determined whether this hypermethylation-mediated repression is an early event during pancreatic carcinogenesis. These investigations were focused on investigating whether the differentially methylated regions can serve as a diagnostic marker for pancreatic ductal adenocarcinoma (PC). Using microarray hybridization and reverse-transcription quantitative PCR, the level of DNA methylation was measured by bisulfite mapping and semiquantitative methylation-specific PCR. Interestingly, 29 miRNAs encoded by genes whose expression was found to be potentially inactivated by DNA hypermethylation. Focusing on miR-148a, the authors observed that its production was repressed, not only in PC samples but also in preneoplastic pancreatic intraepithelial neoplasia lesions. More importantly, it was found that hypermethylation of the DNA region encoding miR-148a was responsible for its repression, which occurs in preneoplastic pancreatic intraepithelial neoplasia preneoplastic lesions. Additionally, it was also shown that the hyper-methylated DNA region encoding miR-148a can serve as an ancillary marker for the differential diagnosis of PC and chronic pancreatitis. From these comprehensive datasets, it was concluded that the hypermethylation of the DNA region encoding miR-148a is responsible for its repression in PC precursor lesions and can be a useful tool for the differential diagnosis of PC and chronic pancreatitis.
In yet another study evaluating the epigenetic regulation of miRNAs, two human PC cell lines MiaPaCa-2 and PANC-1 were exposed to demethylating agent, 5-aza-2′-deoxycytidine or the histone deacetylase inhibitor, trichostatin A, along with their combination [49]. The miRNA expression changes in control-and drug-treated cell lines was assessed using a custom microarray platform. These studies identified 14 miRNAs that were upregulated twofold or greater in the two cell lines following exposure to both chromatin-modifying agents, including five miRNAs that were common (miR-107, miR-103, miR-29a, miR-29b and miR-320) to both MiaPaCa-2 and PANC-1 cells. Methylation-specific PCR assays for the assessment of CpG island methylation status in the 5′ promoter region of the miR-107 primary transcript demonstrated loss of methylation upon exposure to 5-aza-2′-deoxycytidine. Additional confirmation came from overexpression studies of miR-107 in MiaPaCa-2 and PANC-1 cells that resulted in in vitro growth inhibition that was linked to repression of the putative miR-107 target, cyclin-dependent kinase 6. These experiments provided a clear and strong functional basis for the epigenetic inactivation of this miRNA, and perhaps other miRNAs in PC. In another study, identification of transcriptionally silenced hypermethylated genes in PC cells was performed using microarray coupled with methyl-CpG targeted transcriptional activation array. The investigation focused on AsPC-1, MiaPaCa-2 and PANC-1 PC cell lines comparing to normal pancreatic ductal epithelial cell line. Overall, a total of 19 genes were found to be upregulated greater than twofold among the two cancer cell lines compared with normal pancreatic ductal epithelial cells. Among them certain genes (CSMD2, SLC32A1, TMEM204 and TRH) were additionally analyzed by methylation specific PCR, and all them were found to be methylated in the cell lines tested. Most significantly, CSMD2, SLC32A1 and TRH were also found to be hypermethylated in primary PC tumors as well.
In another study, hypermethylation in the well-recognized p53 locus, downstream of miR-34a, was evaluated in different cancers including PC [50]. This study was highly significant since miR-34a expression has been shown to be silenced in several types of cancer due to aberrant CpG methylation of its promoter. In this report, it was found that 19 out of 24 prostate carcinomas showed CpG methylation of the miR-34a promoter with concomitant loss of miR-34a expression. CpG methylation of the miR-34a promoter was also detected in breast, lung, colon, kidney, bladder, melanoma cell lines, primary melanoma tumor specimen and in PC cell lines. Silencing of miR-34a was dominant over its transactivation by p53 post-DNA damage. Most interestingly, re-expression of miR-34a in prostate and PC cell lines induced senescence and cell cycle arrest, at least, in part by targeting cyclin D kinase 6. These results showed that miR-34a represents a tumor suppressor gene which is inactivated epigenetically by CpG methylation and subsequent transcriptional silencing in a broad range of tumors.
The above studies clearly demonstrated the critical role of epigenetic control of miRNAs in PC and potential use of miRNAs in diagnostics and treatment of different cancers. However, prior to utilizing miRNA-based therapies against complex cancers, two important issues must be critically evaluated:
Identification of the correct miRNA and its target;
Design of therapies with maximal benefit meaning encompassing the most relevant miRNAs.
Both of these areas have benefited from integrated sciences of systems biology which are briefly described in the following sections.
Systems analysis of miRNA expression, its epigenetic regulation & consequence on gene regulation
Systems biology and molecular network modeling are important tools that are finding applications in cancer diagnosis and drug discovery [51]. Recently, we have demonstrated the utility of these technologies in discovering novel drug combinations for the treatment of PC [52–54]. Similarly, integrating computational approaches such as systems biology and molecular network modeling with high-throughput experiments have provided a long list of potential miRNA target genes, and the next critical step will be the identification of their biological functions using such knowledge. Because miRNAs have numerous target genes (ranging from few to up to 300 conserved targets), one direct approach to the analysis of miRNA functions with specific instances of biological regulation should be to map miRNA interactions to known protein–protein interactions (PPIs). Several databases of known PPIs, such as the database of interacting proteins; [55–58] and the mammalian PPI database [59,60], are good resources for these analyses. The PPI networks can be visualized together with miRNA interaction information on different platforms such as Cytoscape [61].
Another suggested approach is to map miRNA interactions to known biological pathways. The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway [61] is an excellent reference for identification of groups of miRNA target genes that may share molecular interactions and reaction networks. This and other databases provide biological pathway information, allowing researchers to determine whether groups of miRNA target genes are enriched in specific biological pathways. To determine the common functions of miRNA target genes and to connect miRNA gene regulatory pathways to PPI networks or biological pathways, functional annotation by gene ontology (GO) is commonly used [62]. GO provides a controlled language or systematic vocabulary for the description of the attributes of genes and gene products, which can be used across genomic databases. This systematic ontology acts as a key tool to annotate common functions within large clusters of genes, and can be used in predicting multivariate miRNA targets as well as pathway interactions [63,64]. A number of studies have used these integrated data mining tools to analyze the specific biological functions regulated by miRNAs [65–67].
For example, in one approach, GO analysis was used together with KEGG pathway analysis to determine the roles of 21 miRNAs shown to be expressed in hepatic stellate cells utilizing a miRNA microarray analysis [68]. These results demonstrated that apoptosis was the most enriched transduction pathway in the top 25% of the computationally predicted miRNA targets analyzed. Another example involved the analysis of the functions of the target genes of miR-24 [69]. Potential miRNA target genes were identified experimentally using microarray analysis, by detecting significantly down-regulated genes among miR-24 transfected cells with potential of miR-24 binding site. The functions of the potential miR-24 target genes were analyzed using GO analysis and the Ingenuity Pathways Analysis software. This approach suggested that miR-24 regulates cell cycle progression and DNA repair. As shown in these cases, computational prediction is a valuable tool for identifying miRNA gene networks under physiological and pathological conditions. For example, using systems biology (specifically ingenuity pathway analysis) Cloonan et al. have recently demonstrated a large network of interacting genes that are direct targets of miR-17–5p [70]. In their study, they showed that ectopic expression of miR-17–5p leads to the deregulation of cell cycle progression, resulting in enhanced proliferation in HEK293T cells. For the first time, they showed how this miRNA can drive both pro- and antiproliferative signals, allowing for the switch between oncogenic and tumor suppressor activities.
Other studies have suggested the miRNA-guided regulation of transcription factors, which underlines the key functions of miRNAs within whole gene regulatory networks [71–75]. These analyses suggest that the over representation of miRNAs within gene regulatory networks may reflect their essential roles in the mediation of feedback and feedforward regulation in cellular systems and the maintenance of cellular stability during environmental perturbation [76]. In the future, it is envisaged that different samples can be used (such as peripheral blood, stored tissue specimen and samples from fine-needle aspirates) to analyze epigenetic miRNA changes that can lead to the identification of suitable druggable nodes (Figure 3). These nodes can be targeted using either miRNAs alone or in combination with epigenetic modifying drugs such as demethylating agents and histone deacetylase inhibitors.
Figure 3. Systems biology in identifying miRNAs and their epigenetic modulation represents newer therapeutic strategies for pancreatic cancer.
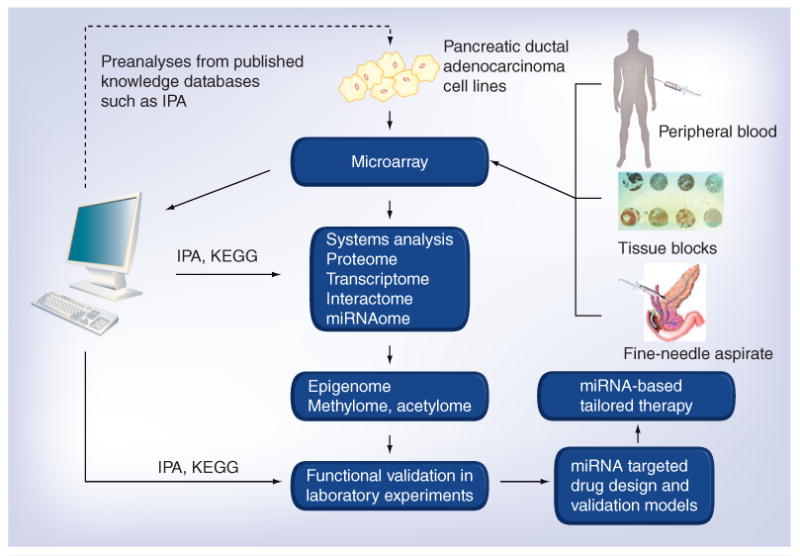
The flowchart describes the combined high-throughput experimental and computational approaches for the systems-level analysis of miRNA modulation and their targets. Preanalysis using published data can be performed computationally, followed by high-throughput (microarrays) experimental analyses on different samples from pancreatic cancer patients. The advantage of using such high-throughput technologies is also due to the fact that it requires a minimal amount of sample. Samples can be from peripheral blood during active disease or fine-needle aspirates that can be used to mine alterations in miRNAs. Additionally, the role of miRNAs and their epigenetic modulation on therapeutic response to different treatments can be evaluated in tissue block specimens obtained after chemotherapy or neoadjuvant therapies, and could be correlated with overall survival. Systems biology and network modeling could come into play as this technology can substantially reduce large microarray datasets into reduced, biologically meaningful and disease-specific information. Software such as IPA and KEGG can normalize microarray data and analyze it statistically to produce a preliminary list of gene, protein and miRNA changes with significantly up- or down-regulated expression. The technology could be beneficial for an in-depth understanding of the crosstalk within and across the proteome, transcriptome, interactome and even miRNAome because conventional molecular technology cannot produce such powerful information. The initial ‘omic information obtained from primary analysis can be utilized to investigate and analyze the epigenome, methylome and acetylome. Further validation analysis would be required to extract the biological information hidden behind the massive data. The raw and analyzed data are distributed within databases or web services, allowing other researchers to make use of this information. Finally, the obtained results can be tested using novel drug combinations using suitable test models to achieve a desirable clinical outcome for improving the overall survival of patients diagnosed with malignancies such as pancreatic cancer. IPA: Ingenuity Pathway Analysis; KEGG: Kyoto Encyclopedia of Genes and Genomes.
Conclusion & future perspective
PC is considered a death sentence and it is a disease that has been well known to be refractory to conventional chemotherapeutics. Over the years, large scale molecular, genomic and proteomic studies have revealed that PC is a highly complex disease with multiple deregulatory signaling co-operating to render PC highly resistant to different treatment modalities. Recent evidence has also indicated that PC has a deregulated miRNA system as well. Like any other genes, miRNAs are also susceptible to epigenetic modulation and this adds to the complexity in the design of miRNA targeted or other therapies that alter the miRNA status. Even though some studies do prove nongenetic modulation of miRNAs; however, our knowledge of PC epigenetic modulation of miRNAs is still in its infancy. Using high-throughput experiments, researchers have retrieved large amounts of data containing a variety of information on epigenetic regulation of miRNAs and their regulatory control over various pathways. These studies have allowed for the identification of the entire miRNAome in a systematic way under different biological conditions. Additionally, such integrated approaches have provided information on the interactive nature of miRNA systems spanning various signaling pathways in cells or tissues, at different stages of development, and in physiological or pathological stages of complex malignancies such as PC. Even though the information is enormous it is still error prone (carrying false-positives). Nevertheless, the initial version of miRNAome is of sufficient quality to pin-point key deregulatory miRNAs which could help in the rational design of novel targeted regimens.
Such computational approaches have played a key role in extracting lists of miRNAs that warrant further analysis. These lists can be compared with one another, combined together to identify tendencies, incorporated with other information obtained from public databases, and so on. Computational approaches would allow interrogating a variety of biological knowledge that can be extracted from an overview of these phenomena. Integrated analysis of expression patterns comes in handy when the molecules under study have complex biological functions, such as those of miRNA. Since the target mRNAs are regulated in a ‘one-to-many’ and a ‘many-to-one’ manner and the degree of regulation varies case-by-case or becomes context dependent. When accumulated miRNA–mRNA interactions identify biological functions, it will be necessary to look at those interactions comprehensively and recognize them as part of a gene regulatory network. Therefore, we suggest that further weight should be given to high-throughput analyses combined with computational approaches, as an effective methodology to achieve a systems-level understanding of complex biological functions of epigenetically groomed miRNAs. This will aid in the design of newer strategies that could regroom the miRNAs for therapeutic benefit in to realizing the dream of better and improved outcome of patients diagnosed with deadly diseases such as PC.
Executive summary.
Pancreatic cancer is resistant to conventional therapeutics & carries a complex molecular make-up
Pancreatic cancer is by far an incurable disease that is refractory to different chemotherapies and targeted therapies. The failure of different treatment regimens is due to the fact that this disease harbors a complex set of deregulatory signaling mechanisms.
miRNA deregulation & pancreatic cancer drug resistance
Among the key altered pathways in pancreatic cancer is the aberrant expression of short nc miRNAs that regulate different signaling pathways leading to drug resistance. Additionally, miRNAs are themselves under a multitier and complex cellular regulatory control including epigenetic control.
Epigenetic regulation of miRNAs
Emerging evidence suggests that miRNAs are under epigenetic regulation (a regulation that does not involve actual coding sequence).
Identification and characterization of the epigenetic regulation of miRNAs need to be mechanistically investigated in order to achieve therapeutically beneficial outcome of novel treatment regimens.
Systems biology in understanding miRNAs' epigenetic regulation
Newer integrated systems technology can help to identify these systems levels changes and are being applied for the identification of epigenetically modulated miRNAs.
Through this article, we provide comprehensive information on the existing knowledge of epigenetics and its relations to drug resistance and further introduced some novel integrate strategies to overcome drug resistance in PC.
Footnotes
Financial & competing interests disclosure: RM Mohammad and FH Sarkar acknowledge NIH funding, grant numbers R01CA109389 and 5R01CA101870, respectively. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. Erratum in: CA Cancer J. Clin. 61(2), 134 (2011) [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for gallbladder cancer across the world. HPB (Oxford) 2008;10(5):327–331. doi: 10.1080/13651820802007464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL, et al. Expression profling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120(5):1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Lee CT, Risom T, Strauss WM. Evolutionary conservation of microRNA regulatory circuits: an examination of microRNA gene complexity and conserved microRNA-target interactions through metazoan phylogeny. DNA Cell Biol. 2007;26(4):209–218. doi: 10.1089/dna.2006.0545. [DOI] [PubMed] [Google Scholar]
- 8.Lagos-Quintana M, Rauhut R, Meyer J, Borkhardt A, Tuschl T. New microRNAs from mouse and human. RNA. 2003;9(2):175–179. doi: 10.1261/rna.2146903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12(9):735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 10.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 11.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39(Database issue):D152–D157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12■.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455(7209):58–63. doi: 10.1038/nature07228. An exhaustive review that covers the topic of miRNA-protein regulation. It details the widespread changes in protein systhesis by miRNAs. [DOI] [PubMed] [Google Scholar]
- 13■.Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8(2):93–103. doi: 10.1038/nrg1990. Comprehensive information on epigenetic mechanisms. [DOI] [PubMed] [Google Scholar]
- 14.Sato F, Tsuchiya S, Meltzer SJ, Shimizu K. MicroRNAs and epigenetics. FEBS J. 2011;278(10):1598–1609. doi: 10.1111/j.1742-4658.2011.08089.x. [DOI] [PubMed] [Google Scholar]
- 15.Corcoran DL, Pandit KV, Gordon B, Bhattacharjee A, Kaminski N, Benos PV. Features of mammalian microRNA promoters emerge from polymerase II chromatin immunoprecipitation data. PLoS ONE. 2009;4(4):e5279. doi: 10.1371/journal.pone.0005279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urdinguio RG, Sanchez-Mut JV, Esteller M. Epigenetic mechanisms in neurological diseases: genes, syndromes, and therapies. Lancet Neurol. 2009;8(11):1056–1072. doi: 10.1016/S1474-4422(09)70262-5. [DOI] [PubMed] [Google Scholar]
- 17.Saito Y, Liang G, Egger G, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9(6):435–443. doi: 10.1016/j.ccr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Lujambio A, Esteller M. CpG island hypermethylation of tumor suppressor microRNAs in human cancer. Cell Cycle. 2007;6(12):1455–1459. [PubMed] [Google Scholar]
- 19.Suh SO, Chen Y, Zaman MS, et al. MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis. 2011;32(5):772–778. doi: 10.1093/carcin/bgr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao XN, Lin J, Li YH, et al. MicroRNA-193a represses c-kit expression and functions as a methylation-silenced tumor suppressor in acute myeloid leukemia. Oncogene. 2011;30(31):3416–3428. doi: 10.1038/onc.2011.62. [DOI] [PubMed] [Google Scholar]
- 21.Gao XN, Lin J, Gao L, Li YH, Wang LL, Yu L. MicroRNA-193b regulates c-kit proto-oncogene and represses cell proliferation in acute myeloid leukemia. Leuk Res. 2011;35(9):1226–1232. doi: 10.1016/j.leukres.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Png KJ, Yoshida M, Zhang XH, Shu W, Lee H, Rimner A, et al. MicroRNA-335 inhibits tumor reinitiation and is silenced through genetic and epigenetic mechanisms in human breast cancer. Genes Dev. 2011;25(3):226–231. doi: 10.1101/gad.1974211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali S, Ahmad A, Banerjee S, et al. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;70(9):3606–3617. doi: 10.1158/0008-5472.CAN-09-4598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Mees ST, Schleicher C, Mardin WA, Senninger N, Colombo-Benkmann M, Haier J. Analyzing miRNAs in ductal adenocarcinomas of the pancreas. J Surg Res. 2011;169(2):241–246. doi: 10.1016/j.jss.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Dhayat S, Mardin WA, Mees ST, Haier J. Epigenetic markers for chemosensitivity and chemoresistance in pancreatic cancer: a review. Int J Cancer. 2011;129(5):1031–1041. doi: 10.1002/ijc.26078. [DOI] [PubMed] [Google Scholar]
- 26■.Mardin WA, Mees ST. MicroRNAs: novel diagnostic and therapeutic tools for pancreatic ductal adenocarcinoma? Ann Surg Oncol. 2009;16(11):3183–3189. doi: 10.1245/s10434-009-0623-1. Provides insight into the different newer technologies in the area of pancreatic cancer diagnosis and therapeutics. The reader can gain insight into the various challenges facing pancreatic cancer research and how newly discovered tools can help in overcoming these problems. [DOI] [PubMed] [Google Scholar]
- 27.Zhang XJ, Ye H, Zeng CW, He B, Zhang H, Chen YQ. Dysregulation of miR-15a and miR-214 in human pancreatic cancer. J Hematol Oncol. 2010;3:46. doi: 10.1186/1756-8722-3-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giovannetti E, Erozenci A, Smit J, Danesi R, Peters GJ. Molecular mechanisms underlying the role of microRNAs (miRNAs) in anticancer drug resistance and implications for clinical practice. Crit Rev Oncol Hematol. 2011 doi: 10.1016/j.critrevonc.2011.03.010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Hwang JH, Voortman J, Giovannetti E, et al. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS ONE. 2010;5(5):e10630. doi: 10.1371/journal.pone.0010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giovannetti E, Funel N, Peters GJ, et al. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 2010;70(11):4528–4538. doi: 10.1158/0008-5472.CAN-09-4467. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Vandenboom TG, Kong D, Wang Z, Ali S, Philip PA, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69(16):6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Vandenboom TG, Wang Z, et al. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70(4):1486–1495. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarkar FH, Li Y. Cell signaling pathways altered by natural chemopreventive agents. Mutat Res. 2004;555(1–2):53–64. doi: 10.1016/j.mrfmmm.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Sarkar FH, Li Y, Wang Z, Kong D. Cellular signaling perturbation by natural products. Cell Signal. 2009;21(11):1541–1547. doi: 10.1016/j.cellsig.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Z, Li Y, Takwi A, et al. miR-301a as an NF-κB activator in pancreatic cancer cells. EMBO J. 2011;30(1):57–67. doi: 10.1038/emboj.2010.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregory PA, Bracken CP, Bert AG, Goodall GJ. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7(20):3112–3118. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- 37.Dirks PB. MicroRNAs and parallel stem cell lives. Cell. 2009;138(3):423–424. doi: 10.1016/j.cell.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 38.Gibbons DL, Lin W, Creighton CJ, et al. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev. 2009;23(18):2140–2151. doi: 10.1101/gad.1820209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng LC, Tavazoie M, Doetsch F. Stem cells: from epigenetics to microRNAs. Neuron. 2005;46(3):363–367. doi: 10.1016/j.neuron.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 40.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10(5):593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 41.Ji Q, Hao X, Zhang M, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS ONE. 2009;4(8):e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson CD, Esquela-Kerscher A, Stefani G, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67(16):7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 43.Kota J, Chivukula RR, O'Donnell KA, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137(6):1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seto AG. The road toward microRNA therapeutics. Int J Biochem Cell Biol. 2010;42(8):1298–1305. doi: 10.1016/j.biocel.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Zhang S, Hao J, Xie F, et al. Downregulation of miR-132 by promoter methylation contributes to pancreatic cancer development. Carcinogenesis. 2011;32(8):1183–1189. doi: 10.1093/carcin/bgr105. [DOI] [PubMed] [Google Scholar]
- 46.Won J, Yim J, Kim TK. Opposing regulatory roles of E2F in human telomerase reverse transcriptase (hTERT) gene expression in human tumor and normal somatic cells. FASEB J. 2002;16(14):1943–1945. doi: 10.1096/fj.02-0311fje. [DOI] [PubMed] [Google Scholar]
- 47.Esteve PO, Chin HG, Pradhan S. Molecular mechanisms of transactivation and doxorubicin-mediated repression of survivin gene in cancer cells. J Biol Chem. 2007;282(4):2615–2625. doi: 10.1074/jbc.M606203200. [DOI] [PubMed] [Google Scholar]
- 48.Hanoun N, Delpu Y, Suriawinata AA, et al. The silencing of microRNA-148a production by DNA hypermethylation is an early event in pancreatic carcinogenesis. Clin Chem. 2010;56(7):1107–1118. doi: 10.1373/clinchem.2010.144709. [DOI] [PubMed] [Google Scholar]
- 49.Lee KH, Lotterman C, Karikari C, et al. Epigenetic silencing of MicroRNA miR-107 regulates cyclin-dependent kinase 6 expression in pancreatic cancer. Pancreatology. 2009;9(3):293–301. doi: 10.1159/000186051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lodygin D, Tarasov V, Epanchintsev A, et al. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7(16):2591–2600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 51■■.Pujol A, Mosca R, Farres J, Aloy P. Unveiling the role of network and systems biology in drug discovery. Trends Pharmacol Sci. 2010;31(3):115–123. doi: 10.1016/j.tips.2009.11.006. Seminal article that gives a simplistic overview on systems biology and its implication in cancer drug discovery. Helpful for readers who are new to the feld to understand the power of systems biology in the area of oncology. [DOI] [PubMed] [Google Scholar]
- 52.Azmi AS, Banerjee S, Ali S, et al. Network modeling of MDM2 inhibitor-oxaliplatin combination reveals biological synergy in wt-p53 solid tumors. Oncotarget. 2011;2(5):378–392. doi: 10.18632/oncotarget.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azmi AS, Beck FW, Sarkar FH, Mohammad RM. Network perspectives on HDM2 inhibitor chemotherapy combinations. Curr Pharm Des. 2011;17(6):640–652. doi: 10.2174/138161211795222612. [DOI] [PubMed] [Google Scholar]
- 54■■.Azmi AS, Wang Z, Philip PA, Mohammad RM, Sarkar FH. Proof of concept: network and systems biology approaches aid in the discovery of potent anticancer drug combinations. Mol Cancer Ther. 2010;9(12):3137–3144. doi: 10.1158/1535-7163.MCT-10-0642. This Faculty of 1000 evaluated proof-of-concept review gives an overview on the use of integrated technologies in pancreatic cancer combination drug design and biomarker discovery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xenarios I, Rice DW, Salwinski L, Baron MK, Marcotte EM, Eisenberg D. DIP: the database of interacting proteins. Nucleic Acids Res. 2000;28(1):289–291. doi: 10.1093/nar/28.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xenarios I, Eisenberg D. Protein interaction databases. Curr Opin Biotechnol. 2001;12(4):334–339. doi: 10.1016/s0958-1669(00)00224-x. [DOI] [PubMed] [Google Scholar]
- 57.Xenarios I, Fernandez E, Salwinski L, Duan, et al. DIP: the database of interacting proteins: 2001 update. Nucleic Acids Res. 2001;29(1):239–241. doi: 10.1093/nar/29.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xenarios I, Salwinski L, Duan XJ, Higney P, Kim SM, Eisenberg D. DIP, the database of interacting proteins: a research tool for studying cellular networks of protein interactions. Nucleic Acids Res. 2002;30(1):303–305. doi: 10.1093/nar/30.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mewes HW, Elzanowski A, George DG. Protein sequence databases: database management, data structures and data access. Biochem Soc Trans. 1989;17(5):843–845. doi: 10.1042/bst0170843. [DOI] [PubMed] [Google Scholar]
- 60.Mewes HW, Dietmann S, Frishman D, et al. MIPS: analysis and annotation of genome information in 2007. Nucleic Acids Res. 2008;36(Database issue):D196–D201. doi: 10.1093/nar/gkm980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cline MS, Smoot M, Cerami E, et al. Integration of biological networks and gene expression data using Cytoscape. Nat Protoc. 2007;2(10):2366–2382. doi: 10.1038/nprot.2007.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unifcation of biology The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol. 2003;5(1):R1. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shalgi R, Lieber D, Oren M, Pilpel Y. Global and local architecture of the mammalian microRNA-transcription factor regulatory network. PLoS Comput Biol. 2007;3(7):e131. doi: 10.1371/journal.pcbi.0030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim LP, Lau NC, Garrett-Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433(7027):769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 66.Neilson JR, Zheng GX, Burge CB, Sharp PA. Dynamic regulation of miRNA expression in ordered stages of cellular development. Genes Dev. 2007;21(5):578–589. doi: 10.1101/gad.1522907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bonci D, Coppola V, Musumeci M, et al. The miR-15a-miR-16–11 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med. 2008;14(11):1271–1277. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- 68.Guo CJ, Pan Q, Jiang B, Chen GY, Li DG. Effects of upregulated expression of microRNA-16 on biological properties of culture-activated hepatic stellate cells. Apoptosis. 2009;14(11):1331–1340. doi: 10.1007/s10495-009-0401-3. [DOI] [PubMed] [Google Scholar]
- 69.Lal A, Navarro F, Maher CA, et al. miR-24 inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to ‘seedless’ 3′UTR microRNA recognition elements. Mol Cell. 2009;35(5):610–625. doi: 10.1016/j.molcel.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cloonan N, Brown MK, Steptoe AL, et al. The miR-17-5p microRNA is a key regulator of the G1/S phase cell cycle transition. Genome Biol. 2008;9(8):R127. doi: 10.1186/gb-2008-9-8-r127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marson A, Levine SS, Cole MF, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134(3):521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ragusa M, Majorana A, Statello L, et al. Specifc alterations of microRNA transcriptome and global network structure in colorectal carcinoma after cetuximab treatment. Mol Cancer Ther. 2010;9(12):3396–3409. doi: 10.1158/1535-7163.MCT-10-0137. [DOI] [PubMed] [Google Scholar]
- 73.Osella M, Bosia C, Cora D, Caselle M. The role of incoherent microRNA-mediated feedforward loops in noise buffering. PLoS Comput Biol. 2011;7(3):e1001101. doi: 10.1371/journal.pcbi.1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat Med. 2011;17(1):64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schlesinger J, Schueler M, Grunert M, et al. The cardiac transcription network modulated by Gata4, Mef2a, Nκx2.5, Srf, histone modifications, and microRNAs. PLoS Genet. 2011;7(2):e1001313. doi: 10.1371/journal.pgen.1001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herranz H, Cohen SM. MicroRNAs and gene regulatory networks: managing the impact of noise in biological systems. Genes Dev. 2010;24(13):1339–1344. doi: 10.1101/gad.1937010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee EJ, Gusset Y, Jiang J, et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120(5):1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297(17):1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, Li M, Wang H, et al. Profiling of 95 microRNAs in pancreatic cancer cell lines and surgical specimens by real-time PCR analysis. World J Surg. 2009;33(4):698–709. doi: 10.1007/s00268-008-9833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mees ST, Schleicher C, Mardin WA, Senninger N, Colombo-Benkmann M, Haier J. Analyzing miRNAs in ductal adenocarcinomas of the pancreas. J Surg Res. 2011;169(2):241–246. doi: 10.1016/j.jss.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 81.Szafranska AE, Davison TS, John J, et al. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene. 2007;26(30):4442–4452. doi: 10.1038/sj.onc.1210228. [DOI] [PubMed] [Google Scholar]
- 82.Szafranska AE, Doleshal M, Edmunds HS, et al. Analysis of microRNAs in pancreatic fne-needle aspirates can classify benign and malignant tissues. Clin Chem. 2008;54(10):1716–1724. doi: 10.1373/clinchem.2008.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szafranska AE, Davison TS, Shingara J, et al. Accurate molecular characterization of formalin-fixed, paraffin-embedded tissues by microRNA expression profiling. J Mol Diagn. 2008;10(5):415–423. doi: 10.2353/jmoldx.2008.080018. [DOI] [PMC free article] [PubMed] [Google Scholar]


