Abstract
Prospective memory (PM) includes the encoding and maintenance of an intention, and the retrieval and execution of this intention at the proper moment in the future. The present study expands upon previous behavioral, electrophysiological, and functional work by examining the association between grey matter volume and PM. Estimates of grey matter volume in theoretically relevant regions of interest (prefrontal, parietal, and medial temporal) were obtained in conjunction with performance on two PM tasks in a sample of 39 cognitively normal and very mildly demented older adults. The first PM task, termed focal in the literature, is supported by spontaneous retrieval of the PM intention whereas the second, termed non-focal, relies on strategic monitoring processes for successful intention retrieval. A positive relationship was observed between medial temporal volume and accuracy on the focal PM task. An examination of medial temporal lobe subregions revealed that this relationship was strongest for the hippocampus, which is considered to support spontaneous memory retrieval. There were no significant structure-behavior associations for the non-focal PM task. These novel results confirm a relationship between behavior and underlying brain structure proposed by the multiprocess theory of PM, and extend findings on cognitive correlates of medial temporal lobe integrity.
Keywords: prefrontal cortex, hippocampus, structural MRI, aging, episodic memory
1 Introduction
Prospective memory (PM) refers to the process of remembering to remember. PM requires the initial planning and formation of an intention, later recognition of a cue and recollection of its associated intention, and executing this intention in coordination with ongoing activity (Marsh et al., 2002). PM is fundamental to the performance of every-day tasks such as remembering to turn off one’s cell phone in a movie theatre or remembering to stop for groceries on the way home from work. In typical event-based PM paradigms (i.e., responding to a specific event in the future), participants engage in a primary ongoing task while simultaneously remembering to make a unique response to infrequent targets associated with a previously encoded intention (McDaniel & Einstein, 2007).
According to the multiprocess theory (McDaniel & Einstein, 2000; 2007), qualitatively different processes support the retrieval of the PM intention depending upon the context. A determining factor is the degree to which encoded features of the PM cue are extracted as part of the ongoing activity (see Einstein & McDaniel, 2005; McDaniel & Einstein, 2007). For non-focal tasks, ongoing task processing does not stimulate processing of critical PM cue features (see McDaniel & Einstein, 2007; Knight et al., 2011). For example, when the PM cue is a particular syllable (e.g., “tor”), and the ongoing activity requires a category judgment (e.g., is “tornado” a member of given category “weather”), the ongoing task emphasizes semantic features, whereas the critical recognition features for the PM cue are syllabic. This lack of overlap requires additional strategic monitoring processes for successful non-focal PM cue recognition (Einstein et al, 2005; see Shallice & Burgess, 1991, and Smith, 2003 for views of PM monitoring).
For focal tasks, information relevant to the ongoing task overlaps with encoded PM cue features. In the just mentioned category-decision activity, the whole-word target “tornado” would be a focal cue, assuming people access semantic features during intention formation and when making category decisions. From the multiprocess theory perspective, such focal cues elicit spontaneous retrieval processes to support PM (see Einstein & McDaniel, 1996; McDaniel, Robinson-Riegler, & Einstein, 1998, for initial characterizations of spontaneous PM retrieval).
The predictions of the multiprocess theory were examined in a seminal study conducted by Einstein et al. (2005; see also Scullin et al., 2010) that manipulated cue focality. Participants demonstrated significant slowing when a non-focal PM demand was embedded in an ongoing task (relative to a control condition that involved only the ongoing task), but no such costs were observed when a focal PM demand was embedded. The ongoing task costs in the non-focal condition were directly associated with PM cue detection and declined over time during the task. The authors interpreted the ongoing task costs observed in the non-focal condition, and their decline over time, as evidence for an underlying, strategic monitoring process. The lack of ongoing task costs in the focal condition, accompanied by high PM performance, suggested a more reflexive, spontaneous retrieval process supporting PM without the need for an attention-demanding monitoring process. The critical point for the present study is that the multiprocess theory anticipates engagement of two brain networks, one tied to effortful modulations of attention, and another for spontaneous retrieval. Moreover, the relative importance of these networks to PM is dependent on the relative non-focal or focal nature of the task (McDaniel & Einstein, 2007; 2011).
An expanding interest in PM has encouraged investigation of its neural underpinnings (e.g., Martin, et al, 2007; West, 2011; Burgess et al., 2011; McDaniel & Einstein, 2011). Using PET and fMRI, researchers have found consistent activation of several brain regions when examining event-related PM; most prominent among these is an anterior prefrontal region located approximately in Brodmann area 10 (BA 10; Burgess et al., 2001; 2011; Reynolds et al., 2009). As the vast majority of this work utilizes non-focal tasks, this region is likely an integral node in the network supporting effortful attentional processes needed for non-focal PM (Simons et al., 2006). Although much focus has been on anterior prefrontal cortex, PM success has also been linked to parietal (Burgess et al., 2001; 2011; Martin et al., 2007; Reynolds et al., 2009), and medial temporal lobe (MTL, see Burgess, Maguire, & O’Keefe, 2002 for a review) regions. Additionally, as a mainstay of cognitive control, the lateral parietal and dorsolateral prefrontal regions of the dorsal attentional network (Corbett & Shulman, 2002) are other potential loci facilitating non-focal performance.
For a network supporting spontaneous retrieval of PM intentions, there is a strong basis to examine the MTL. Functional activations in the hippocampus are tied to spatial, episodic, and recognition memory (Burgess et al, 2002; Eichenbaum et al., 2007), and even focal PM performance in a naturalistic setting (Kalpouzos et al, 2010). Similarly the volumes of MTL structures, in particular the hippocampus, have been linked to episodic (e.g., Head et al., 2008) and spatial memory (e.g., Erickson et al., 2009). The importance of the hippocampus for relational memory (Eichenbaum & Cohen, 2001; Konkel & Cohen, 2009) along with its automaticity of function (Moscovitch, 1994; Konkel & Cohen, 2009) suggest that it may be crucial for the demands of a focal PM task (see McDaniel, et al, 1999; McDaniel & Einstein, 2007). Although the hippocampus has a strong role in recollection, its surrounding structures may be integral for different aspects of memory (Ranganath et al., 2004; Aggleton & Brown, 2006). As such, the MTL subregions may be differentially important for PM.
The behavioral and functional studies to date suggest several mechanisms and brain regions important for successful performance of PM. To the authors’ knowledge, only studies of neurological patients (e.g. Groot et al., 2002; Mathias & Mansfield, 2005) have looked at the link between brain structure and performance on PM tasks, and no studies examine how these relationships differ depending on type of PM task (i.e., non-focal vs. focal). Here we examine relationships between focal and non-focal PM performance and grey matter volume in four regions-of-interest (ROIs) in a convenience sample of cognitively normal and very mildly demented older adults. We predicted that focal performance would be selectively associated with the MTL, with the strongest relationship with the hippocampus proper, whereas prefrontal and parietal region volumes would be especially associated with non-focal performance.
2 Materials and Methods
2.1 Participants
Participants were a subsample of community-dwelling older adults from a larger study examining PM performance, aging and dementia (McDaniel et al., in press). Participants were recruited from the Knight Alzheimer’s Disease Research Center at Washington University and screened for neurological illness (e.g., Parkinson’s, Huntington’s, seizures, major head injury). Participants were classified as cognitively normal (CDR=0; n=21 (16 female)) or very mildly demented (CDR=0.5; n=18 (12 female)) based on the Clinical Dementia Rating scale (CDR; Morris, 1993). A health composite score was created based on the absence or presence (coded 0 or 1) of hypertension, diabetes, history of heart problems (i.e., atrial fibrillation, angioplasty, bypass surgery, congestive heart failure, or pacemaker implantation) history of stroke or transient ischemic attack, history of depression, and mild head injury. The resulting value between 0 and 6 captures multiple health factors into a general measure of overall health, while reducing the need for multiple covariates (reducing power) in the relatively small sample. Demographics characterizing the sample are presented in Table 1.
Table 1.
Demographic and behavioral data.
| N=39 | Mean (SD) | Range |
|---|---|---|
| Age (years) | 78.1 (7.8) | 62-94 |
| Education (years) | 14.7 (3.0) | 10-20 |
| Health Composite* | 1.3 (1.2) | 0-4 |
| MMSE | 28.2 (1.9) | 24-30 |
| SRT Free Recall | 23.8 (10.6) | 4-10 |
| Digit Span | 11.5 (2.3) | 6-15 |
| Digit Symbol | 41.3 (12.7) | 14-67 |
| Trail Making A | 40.2 (15.4) | 19-83 |
| Trail Making B | 100.7 (42.1) | 34-180 |
| Boston Naming Test | 55.0 (5.5) | 34-60 |
| Ongoing Task RT (s) | ||
| Control Block | 1722 (453) | 994-2976 |
| Focal Block | 1737 (407) | 1165-2958 |
| Non-focal Block | 1951 (670) | 1026-3494 |
| Accuracy (%) | ||
| Ongoing Task | .95 (.02) | .90-.98 |
| Focal PM | .68 (.47) | 0-1.0 |
| Non-focal PM | .29 (.42) | 0-1.0 |
Health composite incidence rates: diabetes (5), hypertension (22), stroke (5), heart problems (14), depression (4) and mild head injury (4). Co-morbidity possible.
RT=reaction time in seconds
MMSE=Mini-Mental Status Exam (Folstein et al., 1975)
SRT=Free and Cued Selective Reminding Test (Grober, et al., 1988)
Digit Span = sum of digit span backward and forward (Wechsler, 1987)
Boston Naming Test (Mack et al., 1992)
Digit Symbol and Trail Making A and B (Wechsler, 1987)
2.2 Behavioral Task
Participants were engaged in an ongoing category-judgment task where they decided whether an exemplar word was a member of a specified category (e.g., green COLOR; see Einstein et al., 2005). The exemplar word was always presented in lowercase letters on the left, and the category was always simultaneously displayed in uppercase letters on the right. Three counterbalanced blocks of 106 word-category pairings were presented, with a category match on half of the trials. Two of these blocks had an additional embedded PM task; the third was a control block with only the ongoing category judgment task. For the focal PM block, participants were instructed to press “Q” whenever they saw a particular word (either “tortoise”, “raspberry”, or “aluminum”). For the non-focal PM block, participants were instructed to press “Q” if they ever saw a word containing a particular syllable (either ‘tor’, ‘ras’, or ‘min’). The PM targets always occurred in the exemplar rather than the category word and the PM targets always appeared on trials 31, 72, and 102 of both the focal and non-focal blocks. For each PM block, the PM cue was presented three times, increasing total trials in these blocks to 109 trials. The low number of PM trials is intended to maintain the design as a true PM task rather than creating a vigilance task, and as such is intended to capture PM processes similar to everyday life. Because the same PM target word was repeated three times in the focal condition, non-target words were also repeated to remove any distinctiveness that might arise from this repetition (cf. McDaniel & Einstein, 1993). Eleven non-targets were repeated 3 times and 9 were presented 2 times. The behavioral procedure is also described in McDaniel et al. (in press).
2.3 Imaging Protocol
The majority of images (n=30) were collected on a Siemens 1.5 Tesla Vision scanner (Erlangen, Germany). Two-to- four T1-weighted saggital MP-RAGE scans (TR=9.7ms, TE=4ms, flip angle=10°, TI=20ms, 1mm × 1mm × 1.25mm resolution) were acquired for each subject. Data for a subset of individuals (n=9) were acquired on a Siemens 3 Tesla Trio scanner. Two T1-weighted saggital MP-RAGE scans (TR=2400ms, TE=3.08ms, flip angle=8°, TI=1000ms, 1mm × 1mm × 1mm resolution) were acquired for these participants. Multiple scans for an individual were aligned using a rigid body transform and averaged together. There were on average 20.0 months (SD=18.4) between scan acquisition and behavioral testing.
2.4 Image Analysis
Regional grey matter volume estimates were obtained using the Freesurfer image analysis suite, which implements an automated labeling procedure (Fischl et al., 2004; Desikan et al., 2006) in which each voxel in an MR image is assigned a neuroanatomical label based on probabilistic information from a manually labeled training set. This procedure is highly robust and generates anatomical labeling and regional volume estimates with a high correspondence to those obtained with manually generated labels (Fischl et al., 2004).
Regions-of-interest (ROIs) were obtained from the Desikan-Killiany atlas (Desikan et al., 2006) included as the default cortical parcellation within Freesurfer. Using the available anatomical delineations present within this atlas, ROIs were selected to approximate brain regions implicated by both neuropsychological (e.g. Groot et al., 2002; Mathias & Mansfield, 2005; McDaniel & Einstein, 2011) and neuroimaging studies of prospective memory (e.g. Reynolds et al., 2009; Burgess et al., 2011; West et al., 2011). These ROIs were anterior prefrontal cortex (APFC, within BA 10), ventral/dorsal-lateral prefrontal cortex (VL/DLPFC; combined caudal middle frontal gyrus and inferior frontal gyrus), lateral parietal cortex (combined superior and inferior parietal cortex) and medial temporal lobe (MTL; combined parahippocampal gyrus, entorhinal cortex, and hippocampus) (see FIGURE 1 and Desikan et al., 2006 for details on anatomical boundaries). Volumes were adjusted for total intracranial volume using a covariance approach (Buckner et al. 2004) and summed across hemispheres as no a priori effects of hemisphere were expected.
Figure 1.
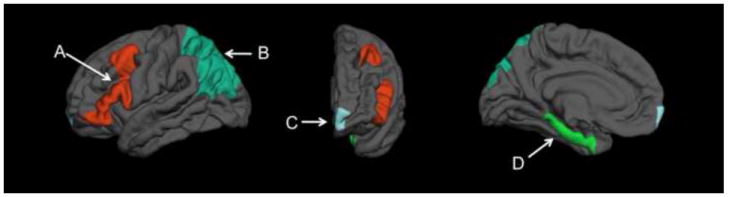
Example of ROIs displayed on the template brain from Freesurfer A) Ventral/dorso-lateral prefrontal cortex; B) Lateral parietal cortex C) Anterior prefrontal cortex; D) Medial temporal lobe
2.5 Statistical Analyses
Partial correlations were computed between each ROI (VL/DLPFC, MTL, parietal cortex, APFC) and accuracy on the focal and non-focal PM tasks. Potential confounding variables with even marginal zero-order correlations (p <.25) with behavior or brain volumes were considered as covariates. Partial correlations controlled for gender, age, CDR status, education, scanner type, and a health-composite. Because our primary interest was in behavior-structure associations, age and CDR status were treated as nuisance covariates. Additional partial correlations were conducted to examine the relationship between the volume of the MTL subregions and focal PM accuracy with the same covariates. Alpha was set at .05. As we had a priori directional hypotheses, all p-values refer to one-tailed tests.
3 Results
3.1 Behavioral Task
Behavioral data are presented in Table 1 (cf. larger sample of McDaniel et al., in press). Ongoing category-judgment accuracy was high (M=.95, SD=.02), and did not vary across the three blocks (F(2,76)=.064, p=.94). RT for the ongoing category-judgment task did significantly vary across the three blocks (F(2,72)=9.63, p<.01)1. Pairwise comparisons indicated that the category-judgment responses in the non-focal block were significantly slower than those in both the focal (p=.003) and control (p=.002) blocks, suggesting that strategic monitoring processes were supporting retrieval in the non-focal block. Most importantly, participants were significantly more accurate in remembering to respond to the appropriate cue in the focal PM (M=.65, SD=.46) than the non-focal PM (M=.29, SD=.41) task (t(40)=4.01, p<.001), replicating previous work demonstrating better performance on focal than non-focal tasks (Einstein et al., 2005; Scullin et al., 2010). Due to the low number of trials in the design and overall low accuracy, RT for PM trials cannot be reliably estimated.
3.2 Behavior-Structure Correlations
The partial correlations between structure and PM accuracy are presented in Table 2. For the non-focal PM task, there were no significant relationships between any of the ROIs and accuracy. For the focal PM task, neither parietal nor VL/DLPFC volumes were associated with accuracy. Post-hoc analyses dividing the parietal cortex into superior and inferior regions, and the VL/DLPFC into caudal middle frontal gyrus and inferior frontal gyrus did not yield any significant correlations with focal or non-focal PM accuracy. As expected, the volume of the MTL was significantly correlated with focal PM accuracy (partial r(31)=.473, p<.01). In addition, there was a significant association between APFC volume and focal PM accuracy (partial r(31)=.306, p<.05). As suggested by the scatter plot in Figure 2, the association between APFC and focal performance is highly influenced by one individual. This was confirmed by analyses of outlier statistics (e.g. Cook’s distance). Removing this individual from the analysis greatly reduced the association between focal PM accuracy and APFC (partial r=.218, p=.115). The relationship between MTL volume and focal performance cannot, however, be explained by the influence of outlier points.
Table 2.
Partial correlations between volume and behavioral performance.
| Region | Focal PM | Non-focal PM |
|---|---|---|
| Parietal | -.007 | .066 |
| VL/DLPFC | .016 | .005 |
| Anterior PFC | .306* | -.011 |
| MTL | .473** | .282+ |
p<.01;
p<.05;
p<.1
Figure 2.
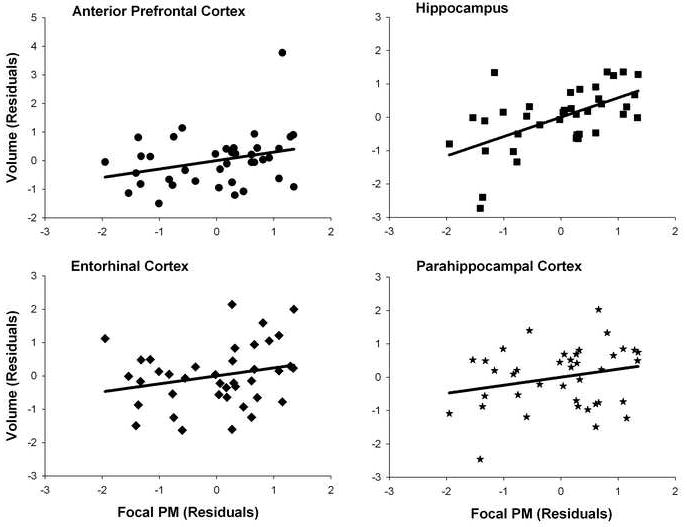
Scatter plots of associations between focal performance and regional brain volumes.
Because of the theoretical significance of particular MTL subregions to memory, we examined the associations between focal PM performance and these regions. For the focal PM task, hippocampal volume (partial r(31)=.576, p<.001) was significantly correlated with PM accuracy, but the relationship between entorhinal (partial r(31)=.230, p=.098) and parahippocampal volume (partial r(31)=.228, p=.101) and focal PM accuracy fell short of significance (FIGURE 2). Examination of the relative strength of associations between volume and PM accuracy across regions (Steiger, 1980) revealed that the a priori theorized association between hippocampal volume and focal PM accuracy was significantly stronger than that for parahippocampal (Z=2.42, p<.01), entorhinal (Z=2.34, p<.01), or APFC cortices (Z=1.72, p<.05).
4 Discussion
The goal of the current study was to examine a previously unexplored link between focal and non-focal PM performance and regional brain volume. We demonstrated a strong relationship between MTL integrity and performance on a focal PM task independent of age and cognitive status, a relationship that was not evident for non-focal PM performance. We then decomposed MTL into three subregions and examined the relationships between these regions and focal PM performance. Significant positive correlations were observed for hippocampal and parahippocampal volumes, with comparisons of correlations confirming that the strongest correlation was with hippocampus proper. These findings are theoretically significant as they support the predictions of the multiprocess theory that retrieval in a focal PM task is subserved by the hippocampus (McDaniel & Einstein, 2007).
The current findings extend previous research linking MTL volume to episodic (e.g., Head et al., 2008) and spatial (e.g., Erickson et al., 2009) memory. The relative contributions of the hippocampus and surrounding structures to memory, more generally, have been explained in several ways. A popular dissociation between the two is between recollection and familiarity. In numerous fMRI studies, activation in the hippocampus is associated with memories recalled by individuals, or that have a high level of specific detail. In contrast, activations in the surrounding cortices are associated with feelings of familiarity without the depth of specific details (e.g., Ranganath et al., 2004; Aggleton & Brown, 2006). The dissociations observed within the MTL in the present study could be due to a reliance on PM cue recollection rather than a signal of familiarity to support focal PM performance, although familiarity may still contribute to cue recognition in a lesser manner.
This dependence on recognition memory comes from the associative nature of PM. During intention formation, a connection is made between the PM cue and the intended response. During focal PM tasks, the ongoing activity stimulates processing of features congruent with those encoded during intention formation, triggering spontaneous retrieval of the associated response. The hippocampus has been proposed as a structure uniquely critical for such associative memory formation and retrieval (Moscovitch, 1994; Eichenbaum & Cohen, 2001; Konkel & Cohen, 2009). Therefore, it is the relational nature of the hippocampus that makes it important both for the recollection of episodic memories and thus for PM memory. Once focal PM intentions are retrieved, prefrontal executive systems might become involved in coordinating execution of the PM response alongside performance of the ongoing task (McDaniel et al., 1999; McDaniel & Einstein, 2011). Consistent with this interpretation is the observed association between focal PM and APFC volume, although the lack of robustness of this effect warrants further study.
In non-focal PM tasks, PM features are not wholly congruent with those of the ongoing activity and thus unlikely to trigger spontaneous retrieval of the associated response (cf. Moscovitch, 1994). Consequently, detecting and responding to non-focal PM cues requires additional strategic monitoring processes (McDaniel & Einstein, 2000; 2007) typically associated with prefrontal regions as seen in the fMRI work with non-focal tasks (Burgess et al 2001; 2011; Reynolds et al., 2009). Additionally, it was expected that dorsal attentional areas in parietal and VL/DLPFC regions would be integral to non-focal performance; however, no significant relationships were observed. Note that previous work has found equivalent reliability across relatively focal and non-focal PM tasks (Rose et al., 2010, albeit within another PM paradigm); accordingly, it seems unlikely that lower reliability for the non-focal task relative to the focal task was responsible for the non-focal results. It is more likely that the overall poor behavioral performance on the non-focal task limited possible detection of any relationship between volume and non-focal PM accuracy. Further, the low number of PM target trials prevented a reliable estimate of RT for use as a dependent measure. Future investigations using easier non-focal tasks with more trials would eliminate floor effects and increase the sensitivity and power of the design. Such a change would provide a more robust examination of PM performance and additionally allow investigations into structural relationships with both accuracy and reaction time.
In addition to low non-focal performance, there are other limitations of our study. The fMRI literature with PM has implicated medial temporal, parietal, and prefrontal regions of the brain (Reynolds et al., 2009; Burgess et al., 2011). These functional activations, however, do not perfectly correspond to any of the anatomical ROIs used in the current analyses with the exception of the medial temporal lobe structures. With more specific ROIs, undetected relationships between performance and structure could emerge in parietal and prefrontal areas. In future studies, functional MRI data from a PM task could be used to directly define areas of interest to maximize sensitivity when looking for relationships between structure and behavior.
Finally, the number of subjects in our sample is a limitation. As such it may be that the relationship between the MTL and focal performance is simply the strongest or most consistent effect in the data. Increasing the sample size would boost the power to detect smaller effects that could have gone undetected in the current experiment. Moreover, examining neuropsychological performance in a larger sample would be useful in assessing potential mediating factors that may be influencing the present results, such as the influence of attention and other cognitive processes. A larger sample would also allow for the analysis of potentially interesting interactions of observed relationships with age and disease status. Despite these limitations, the presented work indicates the value of examining the association between structural and behavioral measures and how this systematic examination in PM can address important and timely theoretical questions.
To the authors’ knowledge, the work presented here is the first examination into the associations between regional volume and PM outside of neurological populations. As predicted by previous work (McDaniel & Einstein, 2011), the strongest behavior-structure relationship was between MTL volume, in particular the hippocampus, and focal PM accuracy. This relationship suggests an important role for the hippocampus in focal PM tasks. The novel results described here illustrate the beneficial aspects of examining anatomical and behavioral information in parallel and provide support for the neuropsychological implications of the multiprocess theory of PM (McDaniel & Einstein, 2011),
Table 3.
Distribution of Prospective Memory Behavioral Data
| Task | 0 correct | 1 correct | 2 correct | 3 correct |
|---|---|---|---|---|
| Focal | 30.8% | 2.6% | 7.7% | 59.0% |
| Non-Focal | 61.5% | 10.3% | 7.7% | 20.5% |
Highlights.
Associations between regional brain volumes and prospective memory (PM) examined.
Medial temporal lobe volume positively correlated with focal PM performance.
Effect was strongest for hippocampus.
No regions significantly correlated with performance on the non-focal PM task.
Acknowledgments
We thank the Clinical Core of the Washington University Knight Alzheimer’s Disease Research Center for participant assessments and the Imaging Core for structural MRI data. This work was supported by the National Institutes of Health (P50 AG05861, P01 AG03991 and P01 AG026276). Brian A Gordon, Jill T Shelton, and Julie M. Bugg were supported by the National Institute of Aging (5T32AG00030).
Footnotes
Two subjects were eliminated from the RT analysis, one for measurement error and one determined to be an outlier as it was more than 3 standard devaitions away from the group mean
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Brown MW. Interleaving brain systems for episodic and recognition memory. Trends in Cognitive Sciences. 2006;10(10):455–463. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Gonen-Yaacovi G, Volle E. Functional neuroimaging studies of prospective memory: What have we learnt so far? Neuropsychologia. 2011;49(8):2246–2257. doi: 10.1016/j.neuropsychologia.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39(6):545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. New York: Oxford University Press; 2001. [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Retrieval processes in prospective memory: Theoretical approaches and some new empirical findings. In: Brandimonte M, Einstein GO, McDaniel MA, editors. Prospective memory: Theory and applications. Mahwah, NJ: Erlbaum; 1996. pp. 115–141. [Google Scholar]
- Einstein GO, McDaniel MA, Thomas R, Mayfield S, Shank H, Morrisette N, et al. Multiple processes in prospective memory retrieval: factors determining monitoring versus spontaneous retrieval. Journal of Experimental Psychology General. 2005;134(3):327–342. doi: 10.1037/0096-3445.134.3.327. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19(10):1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;3:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- Groot CT, Wilson BA, Evans J, Watson P. Prospective memory functioning in people with and without brain injury. Journal of the International Neuropsychological Society. 2002;8:645–654. doi: 10.1017/s1355617702801321. [DOI] [PubMed] [Google Scholar]
- Head D, Rodrigue KM, Kennedy KM, Raz N. Neuroanatomical and cognitive mediators of age-related differences in episodic memory. Neuropsychology. 2008;22(4):491–507. doi: 10.1037/0894-4105.22.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpouzos G, Eriksson J, Sjolie D, Molin J, Nyberg L. Neurocognitive systems related to real-world prospective memory. PloS One. 2010;5(10):e13304. doi: 10.1371/journal.pone.0013304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JB, Meeks JT, Marsh RL, Cook GI, Brewer GA, Hicks JL. An observation on the spontaneous noticing of prospective memory event-based cues. Journal of Experimental Psychology Learning, Memory, and Cognition. 2011;37(2):298–307. doi: 10.1037/a0021969. [DOI] [PubMed] [Google Scholar]
- Konkel A, Cohen NJ. Relational memory and the hippocampus: representations and methods. Frontiers in Neuroscience. 2009;3(2):166–174. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack WJ, Freed DM, Williams BW, Henderson VW. Boston Naming Test: shortened versions for use in Alzheimer’s disease. Journal of Gerontology: Psychological Sciences. 1992;45:154–158. doi: 10.1093/geronj/47.3.p154. [DOI] [PubMed] [Google Scholar]
- Marsh RL, Hicks JL, Watson V. The dynamics of intention retrieval and coordination of action in event-based prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2002;28:652–659. doi: 10.1037//0278-7393.28.4.652. [DOI] [PubMed] [Google Scholar]
- Martin T, McDaniel MA, Guynn MJ, Houck JM, Woodruff CC, Bish JP, et al. Brain regions and their dynamics in prospective memory retrieval: a MEG study. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology. 2007;64(3):247–258. doi: 10.1016/j.ijpsycho.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Mathias JL, Mansfield KM. Prospective and declarative memory problems following moderate and severe traumatic brain injury. Brain Injury. 2005;19(4):271–282. doi: 10.1080/02699050400005028. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. The importance of cue familiarity and distinctiveness in prospective memory. Memory. 1993;1:23–41. doi: 10.1080/09658219308258223. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Strategic and automatic processes in prospective memory retrieval: A multiprocess framework. Applied Cognitive Psychology. 2000;14:S127–S144. [Google Scholar]
- McDaniel MA, Einstein GO. Prospective memory: An overview and synthesis of an emerging field. Thousand Oaks, CA: Sage; 2007. [Google Scholar]
- McDaniel MA, Einstein GO. The neuropsychology of prospective memory in normal aging: a componential approach. Neuropsychologia. 2011;49(8):2147–2155. doi: 10.1016/j.neuropsychologia.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA, Glisky EL, Rubin SR, Guynn MJ, Routhieaux BC. Prospective memory: a neuropsychological study. Neuropsychology. 1999;13(1):103–110. doi: 10.1037//0894-4105.13.1.103. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Robinson-Riegler B, Einstein GO. Prospective remembering: Perceptually-driven or conceptually-driven processes? Memory & Cognition. 1998;26:121–134. doi: 10.3758/bf03211375. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Shelton JT, Breneiser JE, Moynan S, Balota DA. Focal and non-focal prospective memory performance in very mild dementia: a signature decline. Neuropsychology. doi: 10.1037/a0021682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working with memory: Evaluation of a component process model and comparisons with other models. In: Shacter DL, Tulving E, editors. Memory Systems. Cambridge: MT Press; 1994. pp. 269–310. [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42(1):2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, West R, Braver T. Distinct neural circuits support transient and sustained processes in prospective memory and working memory. Cerebral Cortex (New York, N Y : 1991) 2009;19(5):1208–1221. doi: 10.1093/cercor/bhn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose N, Rendell PG, McDaniel MA, Aberle I, Kliegel M. Age and Individual Differences in Prospective Memory during a “Virtual Week”: The Roles of Working Memory, Vigilance, Task Regularity, and Cue Focality. Psychology and Aging. 2010;25:595–605. doi: 10.1037/a0019771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK, McDaniel MA, Shelton JT, Lee JH. Focal/nonfocal cue effects in prospective memory: monitoring difficulty or different retrieval processes? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36(3):736–749. doi: 10.1037/a0018971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Burgess P. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Simons JS, Scholvinck ML, Gilbert SJ, Frith CD, Burgess PW. Differential components of prospective memory? Evidence from fMRI. Neuropsychologia. 2006;44(8):1388–1397. doi: 10.1016/j.neuropsychologia.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Smith RE. The cost of remembering to remember in event-based prospective memory: Investigating the capacity demands of delayed intention performance. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:347–361. doi: 10.1037/0278-7393.29.3.347. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251. [Google Scholar]
- Wechsler D. Manual: Wechsler Memory Scale-Revised. San Antonio, Texas: Psychological Corporation; 1987. [Google Scholar]
- West R. The temporal dynamics of prospective memory: A review of the ERP and prospective memory literature. Neuropsychologia. 2011;49(8):2233–2245. doi: 10.1016/j.neuropsychologia.2010.12.028. [DOI] [PubMed] [Google Scholar]


