Abstract
Cytotoxic T lymphocytes (CTL) play a critical role in the immunity against viruses and cancer. The antigen receptor or T-cell receptor (TCR) on CTL determines the specificity of unwanted cell targeting. The CD8 co-receptor functions in concert with the TCR to enhance TCR-mediated signaling, accounting for the remarkable sensitivity and swift signaling kinetics of the CTL response. The latter ensures efficient delivery of lytic granules to target cells, resulting in their sensitive and rapid destruction.
Introduction
Cell-mediated destruction of unwanted cells is an essential modality of the immunity against viruses and cancer. Killing its own cells is the last resort for the host to eliminate virus-infected and transformed cells. It is, therefore, essential that the killing be very specific and lead to the eradication of unwanted cells leaving normal otherwise bystander cells intact. This intricate task is exercised by cytolytic lymphocytes. They include CTL and natural killer (NK) cells that are associated with acquired or innate immune responses, respectively. Cytolytic activity of both effectors is regulated by MHC class I proteins (MHC-I) that are expressed on every cell making cytotoxic lymphocytes very well suited to recognize and, if necessary, to destroy virtually any cell in the body. MHC-I restricts presentation of antigenic peptides derived from intracellular proteins to CTL, while recognition of MHC-I by NK cells typically inhibits their cytolytic activity. CTL are equipped with clonotype specific TCR that discriminates between endogenous or self antigens and those that normally are not present in the host, i.e., foreign (1, 2). In contrast, the specificity of NK cells is assured through the balance of negative and positive signaling mediated by activating and inhibitory receptors (3). Although both CTL and NK cells are capable of killing target cells, CTL possess the unique ability to exercise highly sensitive and efficient cytolytic activity.
In this review we will focus on mechanisms controlling cytolytic activity of CTL particularly those responsible for exquisite sensitivity and rapid kinetics of target cell destruction.
Specificity
The TCR ligands are binary proteins containing an MHC moiety and a peptide antigen, termed peptide-MHC or pMHC. Each TCR recognizes various peptides bound to the same MHC. For instance, TCR of immature T cells recognize self pMHC proteins in the thymus and the strength of the TCR-pMHC interactions determines the fate of the developing T cells: they are selected positively and matured in response to weak interactions, while strong interactions result in negative selection and T cell death (4, 5). Although positively selected T cells exported to the periphery are prone to respond to peptide antigens derived from pathogens, they can still respond to self pMHC. Recognition of self pMHC ligands provides a “tonic signal” required for maintenance of peripheral T cells (6, 7) and facilitates recognition of foreign pMHC presented at a low density of target cells (8–10). In addition, T cells can recognize dissimilar MHC or allogeneic MHC (associated with self peptides) that are not present the host. Thus, the very nature of T cells assumes that the TCR should be very specific and degenerate at the same time.
How does the TCR achieve specificity for various pMHC proteins? The TCR’s ability to “read” the structure of MHC-bound peptide appears to determine the discriminatory power of the receptor. Meanwhile, the landscape of the pMHC surface recognized by the TCR is generally conserved for many pMHC combinations studied thus far (11, 12), perhaps with some exceptions (13). This suggests that the ability of the TCR to discriminate between various pMHC ligands is determined by sensing of settled differences in the structure of the pMHC contact surface. Indeed, it has been found that the presence or absence of a single hydroxyl group (replacement of Tyr by Phe) in MHC-bound peptide can have a dramatic effect on recognition of the pMHC complex by a TCR (14). On the other hand, some natural variants of the HIV Gag derived peptide, called SL9, restricted by HLA-A2 are recognized by various SL9-specific CTL despite up to 3 amino acid substitutions in the 9-amino-acid-long peptide epitope. By solving high-resolution crystal structures of 7 selected SL9 variants bound to HLA-A2, we have shown that these peptide variants bound to HLA-A2 protein that were recognized by CTL revealed remarkable similarity in their tertiary structure of the peptide backbone that was distinct from that of all other known HLA-A2-bound peptides (15). Thus, the conformation of the peptide backbone could play an important role in determining TCR specificity.
The contact surface areas of TCR-pMHC complexes are characterized by low shape complementarity values (12) leaving enough room for structural adjustment to allow recognition of various peptides bound to the same MHC. Available evidence suggests that the TCR complementarity determining region (CDR) loops possess structural plasticity allowing an adjustment of the interacting surfaces and perhaps increasing the complementarity of the TCR-pMHC interface (16–18). In addition, a recent crystal structure has shown that Vα and Vβ TCR domains can assume different orientations manifested by changes of the positioning of CDR1 and CDR 2 loops relative to CDR3 loop when the TCR bound to either pMHC class I (pMHC-I) or pMHC class II (pMHC-II) cognate ligands (19). This suggests that in addition to a TCR’s ability to discriminate between many different peptides bound to the same MHC, a single receptor can recognize peptide ligands bound to different MHC proteins maximizing diversity of available repertoire. Another possible mechanism modulating TCR specificity invokes the contribution of water molecules that are trapped in the cavities at the TCR-pMHC contact surfaces. Such water molecules could either enhance or diminish the TCR-pMHC interactions (20).
Achieving TCR specificity for a short peptide adducts bound to an MHC protein requires a very fine balance between free energy contributions derived from the TCR-peptide and TCR-MHC contacts. Because the peptide is deeply buried in the MHC binding groove, only about 20–30% of its solvent accessible area is available to form direct contacts with the TCR, while the solvent accessible area of the MHC moiety is significantly larger (12). Yet, energetic contribution of the peptide to binding energy should be at least comparable to that of the MHC to ensure TCR peptide specificity. If the MHC moiety were become the dominant contributor to the binding energy, the peptide specificity could be lost. At the same time, the MHC contribution to TCR binding ought to be sufficient to establish MHC restriction of the TCR. It is likely that to fulfill all these requirements the TCR usually assumes a conservative diagonal (canonical) orientation over pMHC (12, 21) that agrees with a sizable contribution of the peptide to binding energy and minimizes TCR interactions with MHC to preclude autoimmunity. The canonical orientation entrenches the CDR1 and CDR2 loops to be preferentially utilized to contact helices α1 and α2 of MHC, while the CDR3 loops are dedicated to recognition of the MHC-bound peptide. This nearly perfect specialization of the CDR loops in determining MHC restriction and peptide recognition suggests that some variations in the CDR3 regions should not affect the interaction between the CDR1 and CDR2 loops and the MHC moiety. The latter implies that the canonical diagonal orientation is determined by a small number of polymorphic residues at conserved positions on MHC helices. These residues are likely to form energetically dominant TCR-MHC bonds, suggesting that the TCR has an inherent predisposition to interact with MHC helices (12, 22–27). Three conserved MHC residues, two on helix α1 and one on helix α2, are thought to contribute the minimal set of interactions that determine MHC-restriction and the conserved TCR orientation over MHC-I (25). The canonical orientation also allows comparable contributions of MHC helices α1 and α2 to the interactions with CDR1 and CDR2 loops. It has to be noted that some TCRs that are available in the repertoire are not predisposed to recognize MHC proteins, but are specific for a membrane proteins of an entirely different nature and recognize the antigen in a manner similar to antibodies (28). On the other hand, we have found that the Fab fragment of an antibody recognizing peptide from ovalbumin bound to H-2Kb MHC-I protein (29) assumes the canonical (diagonal) orientation over the pMHC molecule and utilizes exactly the same strategy to recognize the MHC-bound peptide as many TCRs do (30). This suggests that TCR and antibody have a common ancestor.
Another essential factor that determines the specificity of T cells is the strength of the TCR interaction with pMHC ligands, commonly referred to as TCR affinity. Because the TCR is analogous to the receptor on B cells (BCR), it was initially thought that the strength of TCR-pMHC interactions solely account for the specificity of T cell responses (1). In fact, initial systematic measurements of TCR affinity on the surface of live CTL indicated that the affinity of TCR for diverse pMHC ligands varies and that binding affinity is linked to the sensitivity of CTL response (31). Later, however, it has become clear that TCR affinity does not necessarily predict the T cell response (see for example (32)) and that other parameters of the TCR-pMHC interaction, i.e., life-time of the TCR engagement, could better correlate with T cell responsiveness (33, 34). In addition, comparison of pMHC binding to TCR on live CTL and in cell free systems revealed significant differences in TCR affinity values suggesting that the TCR microenvironment can influence binding parameters. In fact, it has been found that various antibodies against the CD8 co-receptor modulate the strength of cognate pMHC binding to TCR of live T cells (35). Moreover, comparison of cell free and cellular affinity of 2C TCR for diverse cognate pMHC ligands has suggested that the CD8 co-receptor could influence the TCR affinity and CTL specificity to a different extent depending on the nature of MHC and associated peptide (36). In contrast, the CD4 co-receptor that marks the CD4+ subset of T cells that are typically not cytolytic does not influence binding of soluble peptide-MHC class II (pMHC-II) proteins to TCR on live T cells to a detectable extent (37, 38).
Although in many instances affinity of pMHC ligands for TCR (either soluble or on the surface of live T cells) correlates well with the sensitivity of target cell lysis by CTL (31, 39, 40), at physiological conditions pMHC recognition by TCR occurs between the two cell membranes in 2D space. In addition, the receptor and co-receptor as well as the ligand form clusters on the cell surface (41, 42). How clustering influences parameters of the multivalent receptor-ligand interactions occurring in 2D space and the kinetics of TCR-mediated signaling are not understood. Very few approaches are currently available to address this essential issue. Measuring the affinity and kinetics of individual interactions of TCR with cognate pMHC at the interface between CD4+ T cells and supported lipid bilayers containing randomly distributed pMHC-II ligands, Mark Davis and colleagues have found that the TCR affinity for the cognate pMHC ligand in 2D space is significantly higher than that previously determined for the same TCR-pMHC interactions occurring in 3D space (43). Importantly, the increase in the affinity is due to substantially faster on-rate of the reaction, which is thought to promote pMHC rebinding to TCR facilitating triggering of TCR-mediated signaling. Unexpectedly, the lifetime of the TCR-pMHC complex was found to be shorter in 2D space. The observed changes in the TCR-pMHC binding parameters in 2D space appeared to be dependent on cytoskeleton functions as treatment of T cells with the drugs disrupting the cytoskeleton diminishes the difference in the binding measured in 2D and 3D space (43). Similar results have been produced for naïve OT-1 CTL interacting with either red blood cells or glass beads decorated by soluble pMHC ligands (44). The non-polymorphic domain of the MHC-I protein was mutated to abrogate CD8 binding in order to evaluate the kinetics of individual TCR-pMHC interactions in 2D space without the CD8 contribution. The difference in 2D kinetics of the OT-1 TCR binding to various cognate pMHC-I ligands is more pronounced compared to that measured in 3D space, showing that 2D binding parameters discriminate better between ligands with different potencies. When intact soluble cognate pMHC-I capable to bind to CD8 co-receptor has been utilized, the two-phase binding kinetic was observed (45). The first phase is attributed to the TCR-pMHC interactions, while the second phase reflects CD8-MHC-I binding that is dependent upon initiation of the proximal signaling. CD8 involvement cooperatively amplifies trimolecular interactions to enhance TCR discrimination power. In these experiments the pMHC ligands have been deliberately diluted on the surface of surrogate APC to investigate engagement of individual receptors that are not co-clustered with CD8 on naïve T cells (42). However, when pMHC are closely packed on the surface of quantum dots (QD) or assembled in oligomers such as tetramer that bind to activated CTL bearing clusters of TCR and CD8, the initial step of the binding is dominated by the CD8-MHC-I interactions that facilitate TCR engagement with cognate pMHC and the interactions are highly cooperative (9, 46). The observed distinction in the binding of pMHC ligands to naïve and activated T cells could be explained at least in part by the difference in TCR and CD8 co-clustering. This suggests that mechanisms utilized by naïve and activated CD8+ T cells to identify cognate pMHC on target cells are likely to be different.
Sensitivity
Identification of naturally processed (47–49) and optimal synthetic (e.g., (50)) peptides presented by MHC-I to CTL allowed testing the sensitivity of CTL responding to synthetic peptides and their analogs added to the extracellular medium to sensitize target cells for specific lysis by CTL in vitro (51). This approach led to identification of an optimal peptide epitope ILKEPVHGV (IV9) derived from HIV reverse transcriptase (RT) that appeared to be remarkably potent causing 50% of maximal specific lysis of HLA-A2+ target cells by cognate CTL at the concentration (SD50) of 1 pM (50). This was probably the first experimental result raising the question about the lowest limit of cognate pMHC density on target cells that is still sufficient to trigger the CTL response. From the equilibrium binding constant of IV9 to HLA-A2 on the surface of live target cells and the total number of HLA-A2 molecules per cell, as little as a single (!) IV9-HLA-A2 complex on average per target cell could be formed at 10−12 M of peptide concentration (51). From these experiments it has become evident that the number of cognate pMHC on target cells that is sufficient to elicit CTL cytolytic activity could be remarkably low. Because IV9 peptide has been biochemically isolated from the lysate of HIV-infected cells amounting to ≈12 copies per cell (49), which was sufficient to elicit a CTL response, the ability of CTL to kill infected targets presenting minute amounts of viral peptides appears to be physiologically relevant. Direct evidence of the minimal number of cognate pMHC on the target cell surface rendering the latter susceptible for specific lysis by CTL has been derived from experiments in which the amount of stoichiometrically iodinated peptide bound to MHC-I molecules on the surface of live target cells was measured at the concentration of the peptide in the extracellular medium required for half maximal target cell lysis (52). On average, 3 pMHC per cell was enough for cognate CTL to identify and to destroy target cells. Analysis of the distribution of the MHC-I proteins on killed and survived targets show that even perhaps a single cognate pMHC per target cell is still sufficient to mark it for the recognition and destruction. Initially, these data generated skepticism, particularly, because they suggested that univalent TCR engagement on target cells could elicit CTL response. While this issue is still a subject of intense debate (53, 54), the abundance of self pMHC proteins that is present on target cells along with the few cognate pMHC is thought to facilitate the recognition of rare cognate ligand by TCR on CTL. In fact, it is well documented that self pMHC are recognized by TCR on developing and peripheral T cells (see above) but usually do not induce conventional responses of T cells unless stimulatory pMHC ligands are also presented. We have shown that non-cognate (self pMHC) assembled on the surface of QD binds very well to the surface of human virus-specific CTL, but do not induce a detectable CTL response (9, 55). However, the presence of one to two cognate pMHC complexes per dot with all others being non-cognate results in a rapid and strong CTL response (9, 55). The binding of non-cognate pMHC/QD to CTL and the ability of non-cognate and cognate pMHC to cooperate in the induction of the CTL response are both CD8-dependent. The essential role of CD8 is also evident from previous findings showing that the CD8 co-receptor could enhance the sensitivity of CTL response by one million-fold or more (56). However, non-cognate pMHC assembled into tetramer did not bind to live CTL, and tetramers containing two cognate and two non-cognate pMHC did not induce measurable Ca2+ flux, an indicator of CTL response (Anikeeva and Sykulev, unpublished). We would like to propose that the high density of pMHC on the surface of QD that is similar to that found in MHC patches on live cells (41) mediates efficient CD8-MHC-I rebinding regardless of the MHC bound peptide, and that this accounts for the retention of non-cognate pMHC/QD probe at the cell surface and the cooperation of cognate and non-cognate pMHC in triggering of T cell activation. This is in accord with previous findings showing that soluble pMHC-I dimmer, in which pMHC-I molecules are linked by rigid spacers of different length, induce activation of CD8+ T cells only if the spacer is short, i.e. 5–8 nm; the same pMHC-I proteins linked by a longer spacer lose the stimulatory potency (57). Similar results have been produced with soluble pMHC-II dimers that stimulate CD4+ T cells (58). Cognate and non-cognate pMHC-II ligands in such dimers show cooperation in the induction of T cell response (8). The cooperation, however, does not depend on the CD4 co-receptor but is sensitive to the nature of non-cognate peptide bound to MHC-II. Collectively, these data suggest that cognate and non-cognate ligands, either pMHC-I or pMHC-II, cooperate in facilitating the recognition of a small number of cognate ligands. However, the mechanism of cooperation between pMHC-I and pMHC-II complexes appears to be different. CD8 co-receptor mediates engagement of MHC-I proteins, providing a basis for cooperation, while TCR recognition of non-cognate pMHC-I does not seem to significantly influence the cooperation. In contrast, recognition of non-cognate pMHC-II by TCR but not by CD4 is responsible for the cooperation.
The cooperation of cognate and non-cognate pMHC also occurs at the cell membrane of live target cells. One of the first pieces of evidence was derived from experiments in which the ability of a panel of H-2Kb binding peptides to influence the response of 2C CTL to T2-Kb target cells pulsed with a suboptimal dose of a strong agonist SIYRYYGL (SIY) was tested (36). A naturally occurring self peptide EQYKFYSV, termed dEV8 (59), induces positive selection of developing 2C thymocytes (60) but does not stimulate cytolytic activity of activated 2C CTL. However, dEV8 added to the extracellular medium significantly enhances specific lysis of T2-Kb target cells that have been pulsed with strong agonist SIY at a low concentration (10−13 M) (36). In another system, non-cognate peptides have been shown to enhance responses of OT-1 CTL to target cells presenting a strong agonist SIINFEKL (pOV8) (10). While introduction of non-cognate peptides clearly facilitate cytokine (INF-γ) production in this system, the effect of the same non-cognate peptides on OT-1 cytolytic activity is only marginal. This is likely due to the difference in the sensitivity of the cytolytic response and cytokine production. Consistent with this, the effect of non-stimulatory peptides is more pronounced when tested with weak agonist peptide. In addition, TAP deficient cells RMA-S that have been used as targets in these experiments still present sufficient amount endogenous peptides that could diminish the effect of exogenously added non-stimulatory peptides. In fact, comparison of RMA (TAP sufficient) and RMA-S target cells sensitized with various concentrations of cognate pOV8 peptide to stimulate OT-1 does not reveal any detectable difference in the CTL response (53). We have shown that only nine non-cognate pMHC per dot is sufficient to cooperate effectively with one to two cognate pMHC on the same dot in the binding to T cells and the induction of a CTL response (9, 55).
It has to be noted that the effect of non-cognate peptides on CD8+ T cell responses depends on the state of T cell differentiation. The enhancement is most pronounced in thymocytes, moderate in naïve T cells, and even less so in effector T cells (10). It is likely that the disparity in the extent of cooperation between cognate and non-cognate pMHC may depend on TCR and CD8 co-clustering (42) and the difference in the sensitivity of TCR-mediated signaling in the developing and mature T cells (61, 62).
While self pMHC could enhance the sensitivity of the CTL response, some peptide ligands have the opposite effect, inhibiting CTL responses and decreasing CTL sensitivity (36, 63). In some instances, it has been shown that such antagonist peptides are naturally processed and could negatively regulate the CTL response (64). Some antagonists induce altered proximal and Ca2+-mediated signaling that presumably interferes with the signaling initiated by an agonist, causing inhibition (63, 65). Other antagonists blocks the signal triggered by the agonist as evident from profound dephosphorylation of ζ chain induced by the antagonist (36). Exact mechanisms by which antagonists exercise their activities are still not understood. In particular, it is not clear whether TCR engagement with agonist and antagonist ligands should occur in close proximity on the cell membrane, which would required both ligands to be in the same cluster and to cooperate as described above for cognate and non-cognate ligands. If so, the inhibition could occur at the level of signaling microclusters that may become either activating or inhibitory, altering the signaling balance. Development of pMHC oligomers, pMHC/QD in particular, containing agonist and antagonist ligands that mimic MHC clusters promises to help in addressing this important issue.
The role of the cytolytic synapse
In recent years, it has become evident that CTL develop a highly ordered interface upon making contact with target cells or supported lipid bilayers containing adhesion molecules and pMHC ligands (66, 67). The structure of the interface features segregation of adhesion molecules and engaged TCR to resemble a bull’s eye, which has been termed the immunological synapse (IS) (68).
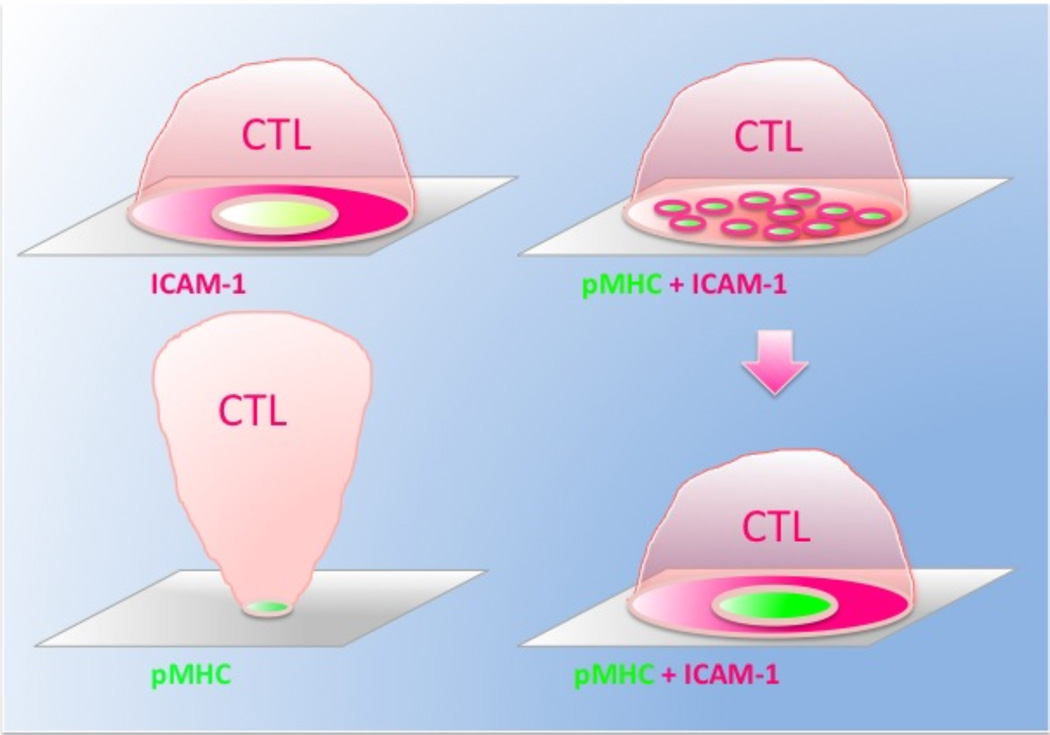
The major function of CTL is to exert cytolytic activity against target cells by direct secretion of cytolytic granules at the CTL/target cell interface. Because polarized granules are projected to the IS and are released within the central cluster of the synapse (69–72), the latter is referred to as the cytolytic synapse (CS). The TCR is concentrated in the center of the synapse that constitutes the central supramolecular activation cluster (cSMAC), while adhesion molecules segregate into the peripheral ring junction surrounding the central cluster – the peripheral supra molecular activating cluster (pSMAC)(73). A mature synapse at the contact area of CD8+ T cells (Fig. 1) is very similar to that observed for CD4+ T cells (74, 75), but it is formed more rapidly and the dynamics of the molecular segregation is CD8-dependent (70, 71, 76). In addition, the nascent synapse formed by CD8+ CTL (Fig. 1) is different from that observed for CD4+ T cells (68). Initially, CD8+ CTL accumulate pMHC proteins in a small areas surrounded by ICAM-1. These areas then rapidly coalesce into the central (cSMAC) and peripheral (pSMAC) clusters of the mature synapse. Thus, the nascent synapse of CD8+ CTL contains multiple points of segregation at the contact area and appears to be a multifocal synapse (Fig. 1). While the formation of nascent synapse was seen in experiments with supported bilayers bearing soluble analogs of MHC-I and ICAM-1 molecules, a very similar structure of the interface was also observed for CD8+ CTL interacting with live target cells (77).
Figure 1.
Changes of cellular morphology and the structure of the synaptic interface in CTL stimulated with supported bilayers that display various combinations of cognate pMHC and ICAM-1 ligands.
Upper left: CTL exposed to ICAM-1-containing bilayers in the absence of cognate pMHC still remodel cytoskeleton as evident from the dramatic increase in area of the contact membrane and the formation of the adhesion ring junction. CD8 and TCR are accumulated within the ring junction albeit to a lesser extent. CTL adhesion is transient and individual cells undergo several cycles of detachment and subsequent adhesion to the bilayers.
Bottom left: CTL recognizing cognate pMHC at high density in the absence of LFA-1 ligand (ICAM-1) adhered to the bilayers through a very small area. There is no accumulation of TCR at the adhesion area and the CTL sporadically oscillated around this area. The CTL remained attached to the bilayers for a long time.
Upper right: After initial contact with bilayers containing cognate pMHC and ICAM-1, CTL adhere to the bilayer, showing T-cell spreading and rapid accumulation of pMHC and ICAM-1 at distinct locations over the interface (a nascent CS).
Bottom left: The foci containing aggregated pMHC and ICAM-1 then coalesced followed by large-scale molecular segregation and the formation of the cSMAC and pSMAC (a mature CS).
Adhesion molecules play a central role in the CS formation. We have shown that CTL exposure to lipid bilayers containing only ICAM-1 ligand results in changes of CTL morphology; CTL undergo cellular spreading indicative of cytoskeleton remodeling and LFA-1 segregation, evidenced by the emergence of the pSMAC-like adhesion ring junction (Fig. 1) (76). In addition, TCR and CD8 co-receptor accumulate within the ring junction though to a lesser extent compared to CTL recognizing both TCR and LFA-1 ligands on the bilayers. The behavior of CTL on the bilayers that do not contain ICAM-1 ligand but display only cognate pMHC at high density appears to be profoundly different (78). CTL still adhered to the bilayers through a very small contact area that is only slightly enriched with engaged pMHC, and the CTL do not show any signs of cellular spreading and molecular segregation (Fig. 1). Instead, the CTL exhibit a stalk-like structure connecting the contact area with the cell body oscillating around to the contact (78). Because supported lipid bilayers allow completely eliminate the contribution of adhesion molecules, these findings clearly demonstrate that the engagement of LFA-1 and perhaps other adhesion molecules but not necessarily TCR induces signaling that is critical for the cytoskeleton relaxation and remodeling; the latter is an essential prerequisite for molecular segregation and the synapse formation (79). It is notable that only a small fraction of activated CD4+ T cells are capable to form very transient antigen independent ring junctions on ICAM-1 containing supported bilayers suggesting that LFA-1 signaling in CD8+ T cells is distinct (76, 78). Neither engagement of LFA-1 nor TCR alone has been sufficient to mediate granule polarization (78).
Is there a link between mechanisms responsible for synapse formation and granule delivery to the synapse? Granule delivery to the CS is mediated by two principal movements, namely, granule recruitment to the microtubule-organizing center (MTOC) and MTOC polarization to the CTL contact interface (80, 81). Our data show that granule movement towards the microtubule organizing center (MTOC) is mediated by the kinetics of intracellular Ca2+ accumulation (71). A rise in intracellular Ca2+ is also required to regulate trafficking of other granules in the cell (82). MTOC polarization mediates delivery of the concentrated granules to the CS. Although initial MTOC translocation is thought to be Ca2+-independent and is mediated by diacylglycerol (DAG)(83), a product of PIP2 degradation by phospholipase Cγ, complete MTOC polarization to the mature synapse is associated with F-actin remodeling and segregation that are Ca2+-dependent. Segregation of F-actin generates force pulling the attached microtubules to the periphery of the synapse (84), facilitating MTOC movement to the contact area. In addition, dynein motors associated with F-actin at the periphery of the pSMAC reel in the attached microtubules, forcing the MTOC to polarize to the CS (85). Both movements require productive TCR engagement (80, 81) and are necessary to achieve complete MTOC polarization to the mature synapse, which is characterized by juxtaposition of the centrioles at the contact CTL membrane (81). The latter is likely required to bring cytolytic granules in close proximity with cell membrane to facilitate membrane fusion. Thus, both mechanisms that are involved in the development of the CS and cytolytic granule delivery to the synapse result in F-actin remodeling and actin ring formation (79) and utilize the same molecular hardware. This suggests that CS formation and CTL degranulation are linked, implicating a role of the CS in CTL cytolytic activity. This is in accord with our findings showing that the stability of the pSMAC, which is partially controlled by PKCθ, is associated with the sensitivity of target cell lysis by CTL (70). The stable pSMAC not only facilitates efficient MTOC polarization by recruitment of scaffolding protein ADAP (85) but also functions to confine the released lytic molecules between the two membranes (70). The granule confinement precludes their rapid inactivation in extracellular medium and increases the local concentration of lytic molecules at the target cell surface (86, 87) enhancing the effectiveness of target cell lysis. Thus, the formation of the pSMAC serves two different purposes, both facilitating efficiency of CTL cytolytic activity.
It has been suggested that CTL could kill target cells without mature immune synapse formation (88, 89). Meanwhile, mature synapses containing cSMAC and pSMAC are clearly evident at the CTL/target cell interface both in vitro and in vivo (69, 71, 90, 91). The formation of the bull’s-eye structure at the CTL contact surface is usually observed when the CTL contacted professional antigen presenting cells (APC), such as B cells, presenting a very high density of cognate pMHC. The APC may possess a mechanism facilitating recruitment of MHC and adhesion molecules to the contact area (92–96) that promotes the complete molecular segregation. Consistent with this, naïve T cells interacting with DC could still form antigen-independent ring junctions (97), most likely facilitated by a unique signaling promoting cytoskeleton remodeling and molecular segregation at the T cell surface.
In one study, the structure of the CTL contact area interacting with live target cells sensitized at a very low concentration of cognate peptide, which was still sufficient to elicit CTL response, has been carefully evaluated (77). Instead of a mature CS, the authors observed formation of multiple bright clusters containing pMHC and adhesion molecules that were distributed over the contact surface and resembled nascent or multifocal synapse. They have found that cognate but not non-cognate pMHC within the nascent synapse becomes immobile and have suggested that the trapping of cognate pMHC bound to the TCR significantly facilitates the sensitivity of CTL response. It has also been proposed that the transient confinement of the pMHC, which was mediated by pMHC binding to the TCR, occurs due to increased interaction with the cytoskeleton (98) or cytoskeleton-associated molecules such as ICAM-1. Consistent with this, others and we have shown that at least a fraction of MHC-I proteins on target cells is associated with ICAM-1, enhancing the sensitivity of CTL responses (94, 95). It may well be that CTL recognition of cognate pMHC at low epitope density is not sufficient to induce complete molecular segregation into the cSAMC and pSMAC, but is enough for the formation of nascent synapse that supports cytolytic activity. Because positioning of cytolytic granules and MTOC relative to the CTL/target cell interface has not been examined, the findings raise an important question about the mechanism of granule delivery to the nascent CS. The multifocal nature of the nascent CS suggests that granules may be released at many different locations at the synaptic interface, which are most likely associated with the foci containing aggregated cognate pMHC and adhesion molecules. This is consistent with the most recent observation that a fraction of cytolytic granules are paired with TCR-containing endosomes and that the pairing is mediated by SNARE protein Vti1b (99). Pairs of endosomes are enriched at the CTL contact surface suggesting that the pairing may facilitate granule transport to the synaptic interface. Inasmuch as pairing of the two endosomes increases the dwell time of lytic granules at the CS, it may be important for granule docking and initiation of membrane fusion. It has been shown that in antigen stimulated T cells TCR-containing endosomes retain activated signaling proteins facing the cytoplasm (100). Such signaling might regulate transport, docking and membrane fusion of cytolytic granules paired with TCR endosomes.
Others and we have shown that even isolated cytolytic granules can still kill target cells, but the amount of granules needed to induce target cell lysis seems to be significantly higher than that released at the CTL contact interface (70, 86, 101). In fact, killing is still observed when the amount of released granules is below the limit of detection (70). Thus, regardless of the maturity, CS ensures directional secretion of cytolytic granules, their preservation and high local concentration at the target cell surface. Last but not the least, cytoskeleton remodeling and tight adhesion between CTL and target cell membranes is also thought to be important for subsequent rapid de-adhesion of CTL and disengagement from killed target cells (102). This is essential for CTL to rapidly exercise serial killing (103) that further increases the efficiency of the destruction of target cells.
Signaling kinetics and the efficiency of killing
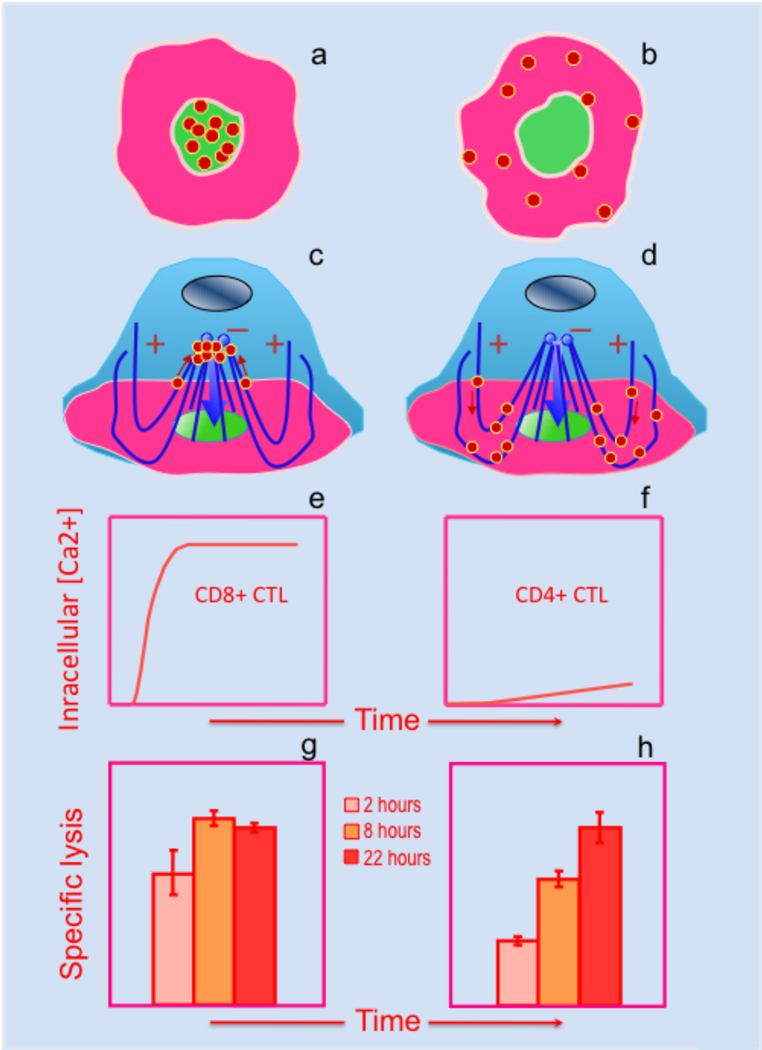
While CTL recognition of target cells bearing a low density of cognate pMHC is not suffice to induce complete molecular segregation, CTL encounters with target cells presenting cognate pMHC ligands at high density results in the formation of mature CS. This suggests that the strength of TCR engagement and the robustness of TCR-mediated signaling plays an important role in the large-scale molecular segregation of TCR and adhesion molecules. We have shown that TCR signaling may develop with different kinetics to approach its maximum at the plateau of the response and that the difference in the TCR signaling kinetics influences the efficiency of cytolytic granules delivery to the synaptic interface (71). In particular, this is evident for CTL recognizing TCR ligands delivering different stimulation strength. In these circumstances, half-maximal response of the CTL is achieved at different ligand concentration (SD50) showing greater sensitivity (lower SD50) for the stronger ligand (70). However, once the magnitude of the response approaches its maximum, i.e., plateau of the response, a further increase in the epitope density does not lead to a higher intensity of Ca2+-mediated signaling (71). Although mature CS has been evident at the plateau of the response regardless of the ligand strength, the pattern of granule polarization varies with ligand binding strength. CTL stimulation with a stronger ligand concentrates polarized granules in the cSMAC, while stimulation with a weak ligand leads to the scattering of polarized granules within the pSMAC (Fig. 2a,b) (71). The two different patterns of granule polarization suggests that cytolytic granules could be delivered to the CTL contact surface via different pathways (Fig. 2c,d) and that the choice of the pathways does not necessarily depend on the maximal signaling intensity but is governed by the time required to approach full-blown signaling (104). Indeed, comparison of the kinetics of intracellular Ca2+ accumulation in the more (CD8+) and less (CD4+) efficient CTL has shown that the TCR-mediated signaling in more efficient CTL is developed significantly faster than that in less efficient CTL (Fig. 2e,f). Importantly, stimulation of the same CTL with CD3-specific antibodies, a strong generic stimulus, showed identical responses, providing evidence that there is no intrinsic difference between the two CTL in the ability to signal swiftly (71). The faster signaling by CD8+ CTL becomes very similar to that observed for CD4+ CTL when CD8-MHC binding was abrogated (not shown). Without the contribution of CD8-MHC interactions the pattern of granule polarization in CD8+ CTL becomes very similar to that seen for CD4+ CTL demonstrating a very distinct role of CD8 co-receptor in triggering swift signaling kinetics. These findings led us to propose a model according in which the kinetics of Ca2+ mediated downstream signaling determines how rapidly granules are recruited to the MTOC (71, 104). The model proposes that the granule movement to the MTOC is regulated by the signaling kinetics, but the initial MTOC reorientation toward the CTL/target cell interface does not depend on the Ca2+ signaling. If the signaling kinetics is fast, the granules are recruited to the MTOC prior to its polarization, the concentrated granules are then delivered by subsequent MTOC polarization to the center of CS – the shortest pathway (Fig 2c). Slower Ca2+ signaling leads to MTOC polarization before the granules are recruited to the MTOC. This results in the granule rerouting to the periphery of the CS, and the granules have to cross the pSMAC to be released at the pSMAC/cSMAC border – a longer pathway (Fig 2d). The difference in signaling kinetics in CTL dictates the choice of the path of the granule delivery that is translated into a more rapid granule release (71) and more efficient destruction of target cells by CD8+ CTL (Fig. 2e,f).
Figure 2.
Two potential pathways of cytolytic granule delivery to the CS are associated with different early TCR signaling kinetics and target lysis kinetics by CTL.
Upon antigen stimulation, more (CD8+) or less (CD4+) effective CTL polarize their granules to either the center (a) or periphery (b) of the CS, respectively. We propose that cytolytic granules are rapidly concentrated around the MTOC prior to MTOC polarization in CD8+ CTL (c), but not in CD4+ CTL (d), explaining their topography within the CS in these two CTLs. Consistent with this, initial TCR engagement in more effective CD8+ CTL results in rapid and robust early TCR signaling (e) as opposed to weak early signaling induced in less effective CD4+ CTL (f). As a result, cytolytic granules are delivered and released at the CS more rapidly in more effective CTL, resulting in faster kinetics of target cell destruction by CD8+ (g), but not by CD4+ (h) CTL.
The above model also suggests that the signaling magnitude and signaling kinetics may account for different qualities of CTL cytolytic response. The magnitude is associated with the sensitivity, i.e. the ability of CTL to identify and to destroy target cells presenting very low density of cognate pMHC ligands, while the kinetics of the signal propagation determines the efficiency of the response, i.e., how rapidly CTL kill target cells. The latter seems to be a very important quality, which permits CTL to win the race against pathogen spread in most cases.
Acknowledgments
This work was supported by NIH grants to Y.S. (AI52812; CA131973). We are grateful to Larry Stern, Martin Poenie, Pierre Henkart and Philip Norris for critical reading of the manuscript and useful comments. This review is not meant to be comprehensive, but rather pursues a particular focus that reflects the views of the authors on the issues discussed.
References
- 1.Eisen HN, Sykulev Y, Tsomides TJ. The antigen-specific T-cell receptor and its reactions with peptide-MHC complexes. In: Haber E, editor. Antigen-Binding Molecules: Antibodies and T-cell Receptors. San Diego: Academic Press; 1997. pp. 1–56. [Google Scholar]
- 2.Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 3.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashton-Rickardt PG, Tonegawa S. A differential-avidity model for T-cell selection. Immunol Today. 1994 Aug;15(8):362–366. doi: 10.1016/0167-5699(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 5.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 6.Germain RN, Stefanova I, Dorfman J. Self-recognition and the regulation of CD4+ T cell survival. Adv Exp Med Biol. 2002;512:97–105. doi: 10.1007/978-1-4615-0757-4_13. [DOI] [PubMed] [Google Scholar]
- 7.Takada K, Jameson SC. Self-class I MHC molecules support survival of naive CD8 T cells, but depress their functional sensitivity through regulation of CD8 expression levels. J Exp Med. 2009;206:2253–2269. doi: 10.1084/jem.20082553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 9.Anikeeva N, Lebedeva T, Clapp AR, Goldman ER, Dustin ML, Mattoussi H, Sykulev Y. Quantum dot/peptide-MHC biosensors reveal strong CD8-dependent cooperation between self and viral antigens that augment the T cell response. Proc Natl Acad Sci U S A. 2006;103:16846–16851. doi: 10.1073/pnas.0607771103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yachi PP, Lotz C, Ampudia J, Gascoigne NR. T cell activation enhancement by endogenous pMHC acts for both weak and strong agonists but varies with differentiation state. J Exp Med. 2007;204:2747–2757. doi: 10.1084/jem.20062610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madden DR. The three-dimensional structure of peptide-MHC complexes. Annu Rev Immunol. 1995;13:587–622. doi: 10.1146/annurev.iy.13.040195.003103. [DOI] [PubMed] [Google Scholar]
- 12.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 13.Burrows SR, Rossjohn J, McCluskey J. Have we cut ourselves too short in mapping CTL epitopes? Trends Immunol. 2006;27:11–16. doi: 10.1016/j.it.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Wu MX, Tsomides TJ, Eisen HN. Tissue distribution of natural peptides derived from a ubiquitous dehydrogenase, including a novel liver-specific peptide that demonstrates the pronounced specificity of low affinity T cell reactions. J Immunol. 1995;154:4495–4502. [PubMed] [Google Scholar]
- 15.Martinez-Hackert E, Anikeeva N, Kalams SA, Walker BD, Hendrickson WA, Sykulev Y. Structural basis for degenerate recognition of natural HIV peptide variants by cytotoxic lymphocytes. J Biol Chem. 2006;281:20205–20212. doi: 10.1074/jbc.M601934200. [DOI] [PubMed] [Google Scholar]
- 16.Boniface JJ, Reich Z, Lyons DS, Davis MM. Thermodynamics of T cell receptor binding to peptide-MHC: evidence for a general mechanism of molecular scanning. Proc Natl Acad Sci U S A. 1999;96:11446–11451. doi: 10.1073/pnas.96.20.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia K, Degano M, Pease L, Huang M, Peterson P, Teyton L, Wilson I. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science. 1998;279:1166–1172. doi: 10.1126/science.279.5354.1166. [DOI] [PubMed] [Google Scholar]
- 18.Willcox B, Gao G, Wyer J, Ladbury J, Bell J, Jakobsen B, van der Merwe P. TCR binding to peptide-MHC stabilizes a flexible recognition interface. Immunity. 1999;10:357–365. doi: 10.1016/s1074-7613(00)80035-7. [DOI] [PubMed] [Google Scholar]
- 19.Yin L, Huseby E, Scott-Browne J, Rubtsova K, Pinilla C, Crawford F, Marrack P, Dai S, Kappler JW. A Single T Cell Receptor Bound to Major Histocompatibility Complex Class I and Class II Glycoproteins Reveals Switchable TCR Conformers. Immunity. 2011;35(1):23–33. doi: 10.1016/j.immuni.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anikeeva N, Lebedeva T, Krogsgaard M, Tetin SY, Martinez-Hackert E, Kalams SA, Davis MM, Sykulev Y. Distinct molecular mechanisms account for the specificity of two different T-cell receptors. Biochemistry. 2003a;42:4709–4716. doi: 10.1021/bi026864+. [DOI] [PubMed] [Google Scholar]
- 21.Rudolph MG, Wilson IA. The specificity of TCR/pMHC interaction. Curr Opin Immunol. 2002;14:52–65. doi: 10.1016/s0952-7915(01)00298-9. [DOI] [PubMed] [Google Scholar]
- 22.Feng D, Bond CJ, Ely LK, Maynard J, Garcia KC. Structural evidence for a germline-encoded T cell receptor-major histocompatibility complex interaction 'codon'. Nat Immunol. 2007;8:975–983. doi: 10.1038/ni1502. [DOI] [PubMed] [Google Scholar]
- 23.Mazza C, Auphan-Anezin N, Gregoire C, Guimezanes A, Kellenberger C, Roussel A, Kearney A, van der Merwe PA, Schmitt-Verhulst AM, Malissen B. How much can a T-cell antigen receptor adapt to structurally distinct antigenic peptides? Embo J. 2007;26:1972–1983. doi: 10.1038/sj.emboj.7601605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huseby ES, White J, Crawford F, Vass T, Becker D, Pinilla C, Marrack P, Kappler JW. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Tynan FE, Burrows SR, Buckle AM, Clements CS, Borg NA, Miles JJ, Beddoe T, Whisstock JC, Wilce MC, Silins SL, Burrows JM, Kjer-Nielsen L, Kostenko L, Purcell AW, McCluskey J, Rossjohn J. T cell receptor recognition of a 'super-bulged' major histocompatibility complex class I-bound peptide. Nat Immunol. 2005;6:1114–1122. doi: 10.1038/ni1257. [DOI] [PubMed] [Google Scholar]
- 26.Colf LA, Bankovich AJ, Hanick NA, Bowerman NA, Jones LL, Kranz DM, Garcia KC. How a single T cell receptor recognizes both self and foreign MHC. Cell. 2007;129:135–146. doi: 10.1016/j.cell.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 27.Marrack P, Scott-Browne JP, Dai S, Gapin L, Kappler JW. Evolutionarily conserved amino acids that control TCR-MHC interaction. Annu Rev Immunol. 2008;26:171–203. doi: 10.1146/annurev.immunol.26.021607.090421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Laethem F, Sarafova SD, Park JH, Tai X, Pobezinsky L, Guinter TI, Adoro S, Adams A, Sharrow SO, Feigenbaum L, Singer A. Deletion of CD4 and CD8 coreceptors permits generation of alphabetaT cells that recognize antigens independently of the MHC. Immunity. 2007;27:735–750. doi: 10.1016/j.immuni.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 30.Mareeva T, Martinez-Hackert E, Sykulev Y. How a T cell receptor-like antibody recognizes major histocompatibility complex-bound peptide. J Biol Chem. 2008;283:29053–29059. doi: 10.1074/jbc.M804996200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sykulev Y, Brunmark A, Jackson M, Cohen RJ, Peterson PA, Eisen HN. Kinetics and affinity of reactions between an antigen-specific T-cell receptor and peptide-MHC complexes. Immunity. 1994a;1:15–22. doi: 10.1016/1074-7613(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 32.Al-Ramadi BK, Jelonek MT, Boyd LF, Margulies DH, Bothwell AL. Lack of strick correlation of functional sensitization with the apparent affinity of MHC/peptide complexes for the TCR. J Immunol. 1995;155:662–673. [PubMed] [Google Scholar]
- 33.Lyons DS, Lieberman SA, Hampl J, Boniface JJ, Chien Y-h, Davis MM. A TCR binds to antagonist ligands with lower affinities and faster dissociation rates than to agonists. Immunity. 1996;5:53–61. doi: 10.1016/s1074-7613(00)80309-x. [DOI] [PubMed] [Google Scholar]
- 34.Kersh GJ, Kersh EN, Fremont DH, Allen PM. High- and low-potency ligands with similar affinities for the TCR: the importance of kinetics in TCR signaling. Immunity. 1998;9:817–826. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 35.Luescher IF, Vivier E, Layer A, Mahiou J, Godeau F, Malissen B, Romero P. CD8 modulation of T-cell antigen receptor-ligand interactions on living cytotoxic T lymphocytes. Nature. 1995;373:353–356. doi: 10.1038/373353a0. [DOI] [PubMed] [Google Scholar]
- 36.Sykulev Y, Vugmeyster Y, Brunmark A, Ploegh H, Eisen H. Peptide antagonism and T cell receptor interactions with peptide-MHC complexes. Immunity. 1998;9(4):475–483. doi: 10.1016/s1074-7613(00)80631-7. [DOI] [PubMed] [Google Scholar]
- 37.Matsui K, Boniface JJ, Reay PA, Schild H, Fazekas de St. Groth B, Davis MM. Low affinity interaction of peptide-MHC complexes with T cell receptors. Science. 1991;254:1788–1791. doi: 10.1126/science.1763329. [DOI] [PubMed] [Google Scholar]
- 38.Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 39.Sykulev Y, Cohen RJ, Eisen HN. The law of mass action governs antigen-stimulated cytolytic activity of CD8+ cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1995;92:11990–11992. doi: 10.1073/pnas.92.26.11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian S, Maile R, Collins EJ, Frelinger JA. CD8+ T cell activation is governed by TCR-peptide/MHC affinity, not dissociation rate. J Immunol. 2007;179:2952–2960. doi: 10.4049/jimmunol.179.5.2952. [DOI] [PubMed] [Google Scholar]
- 41.Hwang J, Gheber LA, Margolis L, Edidin M. Domains in cell plasma membranes investigated by near-field scanning optical microscopy. Biophys J. 1998;74:2184–2190. doi: 10.1016/S0006-3495(98)77927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong L, Zeng G, Lu X, Wang RC, Gong G, Yan L, Huang D, Chen ZW. NSOM/QD-based direct visualization of CD3-induced and CD28-enhanced nanospatial coclustering of TCR and coreceptor in nanodomains in T cell activation. PLoS One. 2009;4:e5945. doi: 10.1371/journal.pone.0005945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huppa JB, Axmann M, Mortelmaier MA, Lillemeier BF, Newell EW, Brameshuber M, Klein LO, Schutz GJ, Davis MM. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 2010;463:963–967. doi: 10.1038/nature08746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J, Zarnitsyna VI, Liu B, Edwards LJ, Jiang N, Evavold BD, Zhu C. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 2010;464:932–936. doi: 10.1038/nature08944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang N, Huang J, Edwards LJ, Liu B, Zhang Y, Beal CD, Evavold BD, Zhu C. Two-stage cooperative T cell receptor-peptide major histocompatibility complex-CD8 trimolecular interactions amplify antigen discrimination. Immunity. 2011;34:13–23. doi: 10.1016/j.immuni.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gakamsky DM, Luescher IF, Pramanik A, Kopito RB, Lemonnier F, Vogel H, Rigler R, Pecht I. CD8 kinetically promotes ligand binding to the T-cell antigen receptor. Biophys J. 2005;89:2121–2133. doi: 10.1529/biophysj.105.061671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Bleek GM, Nathenson SG. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990;348:213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- 48.Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL., Jr Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 49.Tsomides TJ, Aldovini A, Johnson RP, Walker BD, Young RA, Eisen HN. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J Exp Med. 1994;180:1283–1293. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsomides TJ, Walker BD, Eisen HN. An optimal viral peptide recognized by CD8+ T cells binds very tightly to the restricting class I major histocompatibility complex protein on intact cells but not to the purified class I protein. Proc Natl Acad Sci USA. 1991;88:11276–11280. doi: 10.1073/pnas.88.24.11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kageyama S, Tsomides TJ, Sykulev Y, Eisen HN. Variations in the number of peptide-MHC class I complexes required to activate cytotoxic T cell responses. J Immunol. 1995;154:567–576. [PubMed] [Google Scholar]
- 52.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 53.Sporri R, Reis e Sousa C. Self peptide/MHC class I complexes have a negligible effect on the response of some CD8+ T cells to foreign antigen. Eur J Immunol. 2002;32:3161–3170. doi: 10.1002/1521-4141(200211)32:11<3161::AID-IMMU3161>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 54.Ma Z, Sharp KA, Janmey PA, Finkel TH. Surface-anchored monomeric agonist pMHCs alone trigger TCR with high sensitivity. PLoS Biol. 2008;6:e43. doi: 10.1371/journal.pbio.0060043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anikeeva N, Gakamsky D, Sykulev Y, editors. Quantum Dots as a Unique Nanoscaffold to Mimic Membrane Receptor Clustering. Nanotech World Conference; NSTI Nanotech; Boston. 2011. [Google Scholar]
- 56.Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18:255–264. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 57.Cebecauer M, Guillaume P, Mark S, Michielin O, Boucheron N, Bezard M, Meyer BH, Segura JM, Vogel H, Luescher IF. CD8+ Cytotoxic T Lymphocyte Activation by Soluble Major Histocompatibility Complex-Peptide Dimers. J Biol Chem. 2005;280:23820–23828. doi: 10.1074/jbc.M500654200. [DOI] [PubMed] [Google Scholar]
- 58.Cochran JR, Cameron TO, Stone JD, Lubetsky JB, Stern LJ. Receptor proximity, not intermolecular orientation, is critical for triggering T-cell activation. J Biol Chem. 2001;276:28068–28074. doi: 10.1074/jbc.M103280200. [DOI] [PubMed] [Google Scholar]
- 59.Tallquist MD, Yun TJ, Pease LR. A single T cell receptor recognizes structurally distinct MHC/peptide complexes with high specificity. J Exp Med. 1996;184:1017–1026. doi: 10.1084/jem.184.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delaney JR, Sykulev Y, Eisen HN, Tonegawa S. Differences in the level of expression of class I major histocompatibility complex proteins on thymic epithelial and dendritic cells influence the decision of immature thymocytes between positive and negative selection. Proc Natl Acad Sci USA. 1998;95:5235–5240. doi: 10.1073/pnas.95.9.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, Klein LO, Davis MM, Chen CZ. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 62.Adachi K, Davis MM. T-cell receptor ligation induces distinct signaling pathways in naive vs. antigen-experienced T cells. Proc Natl Acad Sci U S A. 2011;108:1549–1554. doi: 10.1073/pnas.1017340108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jameson SC, Carbone FR, Bevan MJ. Clone-specific T cell receptor antagonists of major histocompatibility complex class I-restricted cytotoxic T cells. J Exp Med. 1993;177:1541–1550. doi: 10.1084/jem.177.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klenerman P, S R-J, McAdam S, Edwards J, Daenke S, Lalloo D, Köppe B, Rosenberg W, Boyd D, Edwards A, Giangrande P, Phillips RE, McMichael AJ. Cytotoxic T-cell activity antagonized by naturally occurring HIV-1 gag variants. Nature. 1994;369:403–407. doi: 10.1038/369403a0. [DOI] [PubMed] [Google Scholar]
- 65.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-z and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 66.Dustin ML, Long EO. Cytotoxic immunological synapses. Immunol Rev. 235:24–34. doi: 10.1111/j.0105-2896.2010.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jenkins MR, Griffiths GM. The synapse and cytolytic machinery of cytotoxic T cells. Curr Opin Immunol. 2010;22:308–313. doi: 10.1016/j.coi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 69.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The Immunological Synapse of CTL Contains a Secretory Domain and Membrane Bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 70.Beal AM, Anikeeva N, Varma R, Cameron TO, Norris PJ, Dustin ML, Sykulev Y. Protein kinase Ctheta regulates stability of the peripheral adhesion ring junction and contributes to the sensitivity of target cell lysis by CTL. J Immunol. 2008;181:4815–4824. doi: 10.4049/jimmunol.181.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beal AM, Anikeeva N, Varma R, Cameron TO, Vasiliver-Shamis G, Norris PJ, Dustin ML, Sykulev Y. Kinetics of early T cell receptor signaling regulate the pathway of lytic granule delivery to the secretory domain. Immunity. 2009;31:632–642. doi: 10.1016/j.immuni.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jenkins MR, Tsun A, Stinchcombe JC, Griffiths GM. The strength of T cell receptor signal controls the polarization of cytotoxic machinery to the immunological synapse. Immunity. 2009;31:621–631. doi: 10.1016/j.immuni.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monks C, Freiberg B, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 74.Dustin ML, Chakraborty AK, Shaw AS. Understanding the structure and function of the immunological synapse. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a002311. a002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thauland TJ, Parker DC. Diversity in immunological synapse structure. Immunology. 2010;131:466–472. doi: 10.1111/j.1365-2567.2010.03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Somersalo K, Anikeeva N, Sims TN, Thomas VK, Strong RK, Spies T, Lebedeva T, Sykulev Y, Dustin ML. Cytotoxic T lymphocytes form an antigen-independent ring junction. J Clin Invest. 2004;113:49–57. doi: 10.1172/JCI200419337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Segura JM, Guillaume P, Mark S, Dojcinovic D, Johannsen A, Bosshard G, Angelov G, Legler DF, Vogel H, Luescher IF. Increased mobility of major histocompatibility complex I-peptide complexes decreases the sensitivity of antigen recognition. J Biol Chem. 2008;283:24254–24263. doi: 10.1074/jbc.M803549200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anikeeva N, Somersalo K, Sims TN, Thomas VK, Dustin ML, Sykulev Y. Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 2005;102:6437–6442. doi: 10.1073/pnas.0502467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suzuki J, Yamasaki S, Wu J, Koretzky GA, Saito T. The actin cloud induced by LFA-1-mediated outside-in signals lowers the threshold for T-cell activation. Blood. 2007;109:168–175. doi: 10.1182/blood-2005-12-020164. [DOI] [PubMed] [Google Scholar]
- 80.Poenie M, Kuhn J, Combs J. Real-time visualization of the cytoskeleton and effector functions in T cells. Curr Opin Immunol. 2004;16:428–438. doi: 10.1016/j.coi.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 81.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 82.Ribeiro M, McNamara JC. Calcium movements during pigment aggregation in freshwater shrimp chromatophores. Pigment Cell Res. 2007;20:70–77. doi: 10.1111/j.1600-0749.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 83.Quann EJ, Merino E, Furuta T, Huse M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009;10:627–635. doi: 10.1038/ni.1734. [DOI] [PubMed] [Google Scholar]
- 84.Stinchcombe JC, Griffiths GM. Secretory mechanisms in cell-mediated cytotoxicity. Annu Rev Cell Dev Biol. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- 85.Combs J, Kim SJ, Tan S, Ligon LA, Holzbaur EL, Kuhn J, Poenie M. Recruitment of dynein to the Jurkat immunological synapse. Proc Natl Acad Sci U S A. 2006;103:14883–14888. doi: 10.1073/pnas.0600914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Henkart PA, Millard PJ, Reynolds CW, Henkart MP. Cytolytic activity of purified cytoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J Exp Med. 1984;160:75–93. doi: 10.1084/jem.160.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Saint Basile G, Menasche G, Fischer A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat Rev Immunol. 2010;10:568–579. doi: 10.1038/nri2803. [DOI] [PubMed] [Google Scholar]
- 88.Faroudi M, Utzny C, Salio M, Cerundolo V, Guiraud M, Muller S, Valitutti S. Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: manifestation of a dual activation threshold. Proc Natl Acad Sci U S A. 2003;100:14145–14150. doi: 10.1073/pnas.2334336100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 90.Potter TA, Grebe K, Freiberg B, Kupfer A. Formation of supramolecular activation clusters on fresh ex vivo CD8+ T cells after engagement of the T cell antigen receptor and CD8 by antigen-presenting cells. Proc Natl Acad Sci U S A. 2001;98:12624–12629. doi: 10.1073/pnas.221458898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barcia C, Thomas CE, Curtin JF, King GD, Wawrowsky K, Candolfi M, Xiong WD, Liu C, Kroeger K, Boyer O, Kupiec-Weglinski J, Klatzmann D, Castro MG, Lowenstein PR. In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain. J Exp Med. 2006;203:2095–2107. doi: 10.1084/jem.20060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chow A, Toomre D, Garrett W, Mellman I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature. 2002;418:988–994. doi: 10.1038/nature01006. [DOI] [PubMed] [Google Scholar]
- 93.Boes M, Cerny J, Massol R, Op den Brouw M, Kirchhausen T, Chen J, Ploegh HL. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature. 2002;418:983–988. doi: 10.1038/nature01004. [DOI] [PubMed] [Google Scholar]
- 94.Bacso Z, Bene L, Damjanovich L, Damjanovich S. INF-gamma rearranges membrane topography of MHC-I and ICAM-1 in colon carcinoma cells. Biochem Biophys Res Commun. 2002;290:635–640. doi: 10.1006/bbrc.2001.6246. [DOI] [PubMed] [Google Scholar]
- 95.Lebedeva T, Anikeeva N, Kalams SA, Walker BD, Gaidarov I, Keen JH, Sykulev Y. Major histocompatibility complex class I-intercellular adhesion molecule-1 association on the surface of target cells: implications for antigen presentation to cytotoxic T lymphocytes. Immunology. 2004;113:460–471. doi: 10.1111/j.1365-2567.2004.01985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lebedeva T, Dustin ML, Sykulev Y. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr Opin Immunol. 2005;17:251–258. doi: 10.1016/j.coi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 97.Revy P, Sospedra M, Barbour B, Trautmann A. Functional antigen-independent synapses formed between T cells and dendritic cells. Nat Immunol. 2001;2:925–931. doi: 10.1038/ni713. [DOI] [PubMed] [Google Scholar]
- 98.Carpen O, Pallai P, Staunton DE, Springer TA. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and alpha-actinin. J Cell Biol. 1992;118:1223–1234. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qu B, Pattu V, Junker C, Schwarz EC, Bhat SS, Kummerow C, Marshall M, Matti U, Neumann F, Pfreundschuh M, Becherer U, Rieger H, Rettig J, Hoth M. Docking of lytic granules at the immunological synapse in human CTL requires Vti1b-dependent pairing with CD3 endosomes. J Immunol. 2011;186:6894–6904. doi: 10.4049/jimmunol.1003471. [DOI] [PubMed] [Google Scholar]
- 100.Yudushkin IA, Vale RD. Imaging T-cell receptor activation reveals accumulation of tyrosine-phosphorylated CD3zeta in the endosomal compartment. Proc Natl Acad Sci U S A. 2010;107:22128–22133. doi: 10.1073/pnas.1016388108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poenie M, Tsien RY, Schmitt-Verhulst A-M. Sequential activation and lethal hit measured by [Ca2+] in individual cytolytic T cells and targets. EMBO J. 1987;6:2223–2232. doi: 10.1002/j.1460-2075.1987.tb02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Semmrich M, Smith A, Feterowski C, Beer S, Engelhardt B, Busch DH, Bartsch B, Laschinger M, Hogg N, Pfeffer K, Holzmann B. Importance of integrin LFA-1 deactivation for the generation of immune responses. J Exp Med. 2005;201:1987–1998. doi: 10.1084/jem.20041850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wiedemann A, Depoil D, Faroudi M, Valitutti S. Cytotoxic T lymphocytes kill multiple targets simultaneously via spatiotemporal uncoupling of lytic and stimulatory synapses. Proc Natl Acad Sci U S A. 2006;103:10985–10990. doi: 10.1073/pnas.0600651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sykulev Y. T cell receptor signaling kinetics takes the stage. Sci Signal. 2010;3:pe50. doi: 10.1126/scisignal.3153pe50. [DOI] [PMC free article] [PubMed] [Google Scholar]