Abstract
Background
The purpose of this study was to evaluate the long-term in-vivo hemodynamics, gas transfer and biocompatibility of an integrated artificial pump-lung (APL) developed for ambulatory respiratory support.
Methods
The study was conducted in an ovine model by surgically placing the APL between the right atrium and pulmonary artery. Nine sheep were implanted. Heparin was infused as an anticoagulant. The device flow, gas transfer and plasma free hemoglobin (PFH) were measured daily. Hematological data, platelet activation and blood biochemistry were assessed twice a week. After 30 days, the sheep were euthanized for necropsy. The explanted devices were examined for gross thrombosis.
Results
Five sheep survived for 29 to 31 days and were electively terminated. Four sheep expired or were terminated early due to mechanical failure of IV lines or device. The APL devices in the five long-term animals were capable of delivering an oxygen transfer rate of 148±18 ml/min at a flow rate of 2.99±0.46 l/min with blood oxygen saturation of 96.7±1.3%. The device flow and oxygen transfer were stable over 30 days. The animals had normal end-organ functions except for surgery-related transient alteration in kidney function, liver function, and cell and tissue injury. There was no hemolysis. The device flow path and membrane surface were free of gross thrombus.
Conclusions
The APL exhibited the capability of providing respiratory support with excellent biocompatibility, long-term reliability and the potential for bridging to lung transplant.
Keywords: Artificial organs; Device; Extracorporeal membrane oxygenation, ECMO; Lung; Transplantation, Lung
Introduction
Mechanical ventilation and extracorporeal membrane oxygenation (ECMO) are often used to provide respiratory support for patients suffering from adult respiratory distress syndrome (ARDS)/acute lung injury (ALI) and as a bridge to lung transplantation [1]. Mechanical ventilation is effective as short term support, yet the sustained and prolonged tidal volumes and airway pressures often damage the lungs via barotrauma, volutrauma, and other iatrogenic injuries [2]. ECMO is attractive since it closely simulates physiological gas exchange, but can only be used for short term support. Extended ECMO support may be possible, but in practice, these systems are limited by the complexity of their operation, increased risk of bleeding and reduced patient mobility. Patients are often bedridden, resulting in muscular atrophy and wasting syndrome that may affect patients’ survival.
Recently, ambulatory ECMO support has been implemented in a number of centers using available pumps and oxygenators [3–5], allowing patients to walk, eat and exercise. This is a significant improvement because it allows patients to be ambulatory, to keep muscles from atrophying, and to participate in physical rehabilitation that ultimately leads to a better clinical course to full recovery or better status at the time of heart/lung transplantation. The systems currently available are very bulky and multiple exchanges of oxygenators are required for long-term support.
Based on the successful utilization of paracorporeal ventricular assist devices in heart failure patients [6], we envision that a paracorporeal artificial lung device may impact quality of life for respiratory failure patients and may open options for more aggressive medical therapies, which might extend survival as well, just as with mechanical circulatory support in time. We believe that the paracorporeal artificial lung might be reasonably introduced clinically as a temporary short or long-term bridge to transplantation and as a short-term therapy for acute respiratory distress syndrome (ARDS) and related diseases. To this end, we have developed a paracorporeal artificial pump-lung (APL) for ambulatory respiratory or cardiopulmonary support. The APL is an ultracompact pump-oxygenator consisting of a uniquely designed hollow fiber membrane (HFM) bundle integrated with a magnetically levitated centrifugal impeller pump. The overall size of the APL is comparable to a 12 oz soda can. It can function either as a respiratory support device or partial cardiopulmonary support device with maximal flexibility of application in the broad spectrum of heart/lung diseases. The objective of this study was to evaluate the long-term in-vivo hemodynamics, oxygen transfer and biocompatibility performances of the APL for respiratory support in an ovine model.
Material and Methods
Device Description
The APL is an ultracompact, low prime volume, low hemolytic device for ambulatory respiratory assistance or cardiopulmonary support. It is designed to be capable of providing respiratory support (partial to full) or cardiac support for adult patients suffering from respiratory failure or cardiopulmonary collapse. The following are the APL design specifications: (1) blood flow of 3.5 L/min; (2) oxygen delivery rate of 180 ml/min; (3) pressure head of 120 mmHg; (4) 0.80 m2 surface area of membranes for gas exchange; (5) minimal RBC injury/hemolysis and platelet activation; and (6) support duration of up to 30 days and exchangeable.
The APL is suitable for either central (i.e. right atrium-to-main pulmonary artery) or peripheral cannulation (i.e. internal jugular vein-to-carotid artery or femoral vein-to-femoral artery). In both configurations the device itself, controller, oxygen source, drive line and power supply can be worn by the patients so that the patient can be ambulatory. Figure 1(a) illustrates the APL system with its surgical implantation to a human patient. Figure 1(b) shows the disposable APL device, the motor drive and controller. The APL device is 117 mm in length and 89 mm in diameter. The priming volume of the device is about 115 ml. The combined weight of the APL device and the motor/controller unit is only about 0.54 kg.
Figure 1.
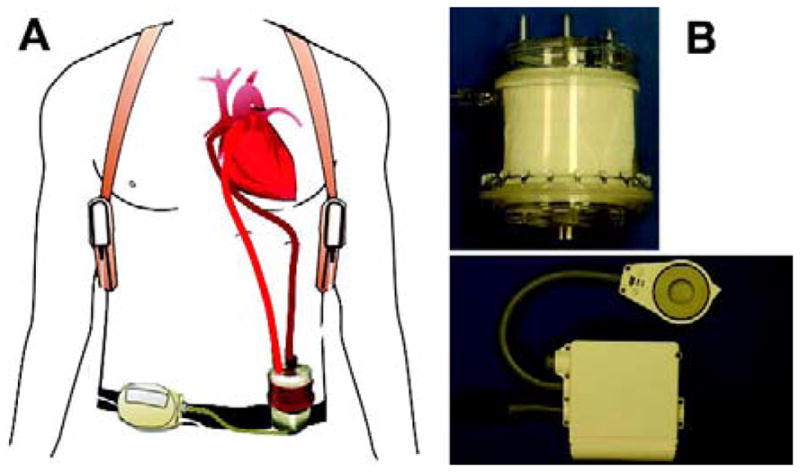
(a) The APL system: magnetically levitated pump-oxygenator and controller-motor drive assembly. (b) Typical implementation of the APL for a human patient; top: disposable device; bottom: controller and motor drive.
Figure 2 shows the sectional view of the flow path inside the APL. Venous blood is drawn from the patient into the APL pump chamber from a central cylindrical tube via a drainage cannula. Driven by a magnetically levitated rotating centrifugal pump impeller, the blood is propelled through the diffuser section and flows towards the space between the outer housing and the polymethylpentene HFM (Oxyplus, Membrana, Germany) bundle. Due to the increased hydrodynamic pressure by the impeller pump, the blood penetrates the HFM bundle in the radial direction. While the blood passes through the HFM bundle, the oxygen is transferred from the fiber lumen to the blood and the carbon dioxide is removed from the blood. The oxygenated blood is collected at the space between the HFM bundle and the center tube and returned back to the patient via the return cannula. The sweep gas enters the lumens of individual hollow fibers of the potted HFM bundle from the top and exits the device at the bottom via the channels imbedded in the diffuser fins. A vent port on the upper side of the outer housing wall (Figure 1(b)) can be used to remove the gas bubbles that may get into the device accidently. In addition, as a safety measure, an arterial filter may be added to the system to further reduce the risk of gas embolism.
Figure 2.
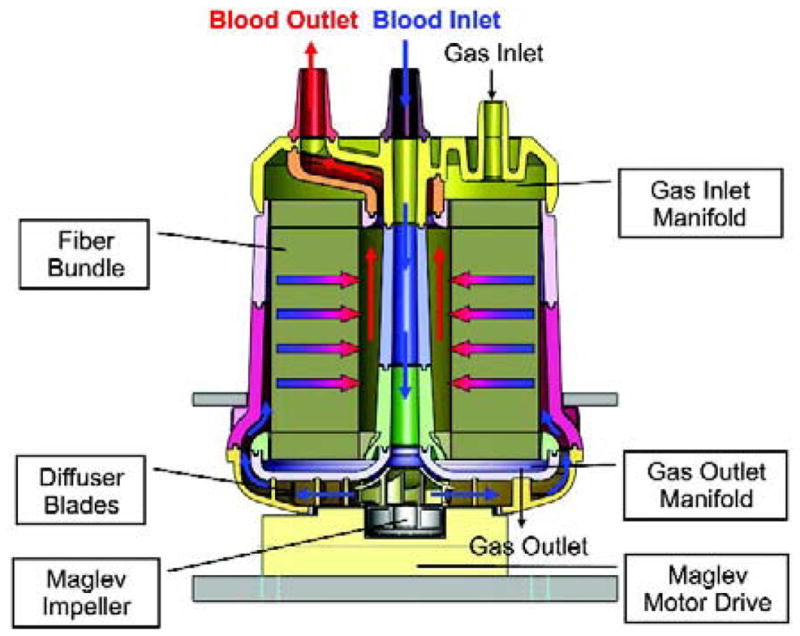
Cross-sectional view of the APL and flow path.
Surgical Procedure
Nine Dorset hybrid sheep (45~65 kg) bred for laboratory research (Thomas Morris, Reisterstown, MD) were used. All the surgical procedures and post-operative care were carried out according to the approved protocol by the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland School of Medicine. During the course of the animal experiments, all animals received humane care in accordance with the Guide for Care and Use of Laboratory Animals (NIH publication 86–23, revised 1996).
The sheep was induced and vital signs were monitored as previously described [7]. The APL device was surgically implanted between the right atrium (RA) and pulmonary artery (PA) through a left thoracotomy. After the sheep was heparinized to achieve an activated clotting time (ACT) of 250 seconds, the Dacron graft of an arterial cannula (Abiomed, Inc., Danvers, MA) was anastomosed end-to-side to the distal PA. A 32 Fr. venous drain cannula (DLP, Medtronic, Minneapolis, MN) was placed in the RA through the right atrial appendage using the double purse-string suture technique. After the inflow and outflow cannulae were de-aired using saline, clamped and tunneled out of the chest to exit the skin on the upper left lateral chest wall, the APL device primed with heparinized saline was connected to the inflow and outflow cannulae and de-aired. The APL operation was then initiated for the in-vivo evaluation. A flow probe (Transonic System, Ithaca, NY) was placed around the outflow tubing to measure the device generated flow. The incision was closed and the animals were allowed to recover. Once the animals became conscious and could breathe on their own, the animals were extubated and transported into the specially designed cage for recovery.
Postoperative Care
The sheep were monitored daily until the study end-point. Pain was alleviated by giving Flunixin meglumin at a loading dose of 2.2-mg/kg initially and thereafter 1.1-mg/kg (IV, BID) for five days. Buprenorphine would be used (0.005–0.01 mg/kg IM or IV) to relieve pain associated with the surgical procedure if the above medicine was not sufficient. Enrofloxacin was given every 12 hours for five days. Heparin was continuously infused to maintain a targeted ACT of 150 seconds by adjusting the infused heparin rate daily. The APL was operated to produce a blood flow of ~3.0 L/min. From our early experiments, we found that the partial pressure of carbon dioxide (PCO2) of the animals’ venous blood was low (35~40 mmHg) if pure oxygen was used as the sweep gas. Thus, a mixture of oxygen (95%) and carbon dioxide (5%) was used as the sweep gas at a flow rate of ~1.0 L/min to maintain PCO2 of the animal’s venous blood within the physiological range (40 to 50 mmHg). At the conclusion of the study, each animal underwent necropsy and device explantation. A careful examination for thrombus formation, graft patency, and cannula position was performed. The internal organs and explanted device were examined grossly and microscopically for evidence of thromboembolism.
Device Function Evaluation
The device generated flow at a fixed operating speed was measured daily. The pressure drop across the fiber bundle was measured at the start and end of the study. Blood samples at the inlet and outlet of the APL were collected daily for blood gas analysis using a blood gas analyzer (Stat Profile Phox Plus L, Nova Biomedical, Waltham, MA). The oxygen transfer rate was calculated according to the published method [8]. Since 95% oxygen mixed with 5% carbon dioxide was used as the sweep gas, the carbon dioxide transfer rate could not be reliably evaluated and was not presented.
Blood Chemistry, Hematologic, and Biocompatibility Measurements
Blood samples were collected at baseline, after implantation, and twice a week for determination of complete metabolic panel (CMP), complete blood count (CBC), plasma free hemoglobin (PFH), lactic acid dehydrogenase (LDH), and platelet activation markers (percentage of P-selectin positive platelets and plasma soluble P-selectin). Collected blood samples were sent to an outside laboratory (Antech Diagnostics, Lake Success, NY) for CMP, CBC, and LDH determination. PFH was measured using a modified cyanomethemoglobin method. P-selectin (CD62p) expression on platelets was quantified with flow cytometry. Plasma soluble P-selectin levels were measured by enzyme-linked immunosorbent assay (ELISA) developed in our lab specifically for ovine [9].
Statistical Analysis
Data are presented as mean ± standard error. Data collected daily over the 30-day study period were averaged every five days. Comparisons between the measured data at baseline (pre-implant or initiation of device operation) and at subsequent times were performed using a mixed model with the sheep number as the subject variable and the data collection time as the fixed, repeated measure variable (SPSS Statistics 18, Chicago, IL). Post-hoc analysis using the LSD corrected confidence interval was used to compare all the post-operatively measured variables with the baseline data to examine the changes with time.
Results
Study Summary
The APL was successfully implanted on nine sheep. All of the animals recovered from the surgery and were able to stand, drink and eat within 24 hours. The results of the nine in-vivo animal experiments are summarized in Table 1. Five sheep survived for 29 to 31 days and were electively terminated. One sheep was terminated early when leaking from the device was observed at day 4. Three animals died due to bleeding: 1 device related and 2 non-device related. Of the 2 non-device related incidents, 1 animal chewed the heparin infusion line and the heparin infusion line used in the other animal was unexpectedly dislodged at the glued joint between the tube and luer adapter. All the devices in the animals that survived to the study end point functioned normally. The data from these animals are presented in the following sections.
Table 1.
Summary of nine in-vivo animal experiments
| Sheep No. | Weight (kg) | Duration (day) | Blood Flow (l/min) | O2 Transfer (ml/min) | Termination Summary |
|---|---|---|---|---|---|
| 1 | 47.0 | 29 | 2.3 – 3.7 | 122 – 184 | Elective |
| 2 | 45.0 | 4 | 2.5 – 3.1 | 114 – 167 | Early termination |
| 3 | 50.9 | 2 | 2.9 – 3.5 | 114 – 185 | Died due to IV line broken |
| 4 | 63.7 | 30 | 2.1 – 3.4 | 99 – 168 | Elective |
| 5 | 59.1 | 31 | 2.4 – 3.5 | 129 – 196 | Elective |
| 6 | 59.0 | 30 | 2.6 – 3.5 | 102 – 223 | Elective |
| 7 | 43.8 | 2 | 2.9 – 3.2 | 126 – 172 | Died due to device leaking |
| 8 | 51.4 | 30 | 2.3 – 3.4 | 101 – 184 | Elective |
| 9 | 57.0 | 12 | 2.7 – 3.4 | 126 – 167 | Died due to IV line broken |
Anti-Coagulation
The heparin dose and attained ACT over the 30 day period are listed in Table 2. Escalating heparin infusion dosage was required initially to maintain the targeted ACT. However the required dosage became stabilized after the second week. The similar anticoagulation requirement was observed in the juvenile sheep implanted with the child-size pediatric Jarvik 2000 heart [9].
Table 2.
Summary of Measurements during the 30-day In Vivo Study (n=5)
| Measurements | POD 0 | POD 1–5 | POD 6–10 | POD 11–15 | POD 16–20 | POD 21–25 | POD 26–30 |
|---|---|---|---|---|---|---|---|
| Heparin (U/h) | 1300.0±58.0 | 1351.3±106.0 | 1938.1±245.9 | 2566.5±317.9 | 2548.8±255.6 | 2501.0±259.2 | 2445.4±275.9 |
| Heparin (U/kg/h) | 23.7±1.1 | 24.5±1.3 | 35.1±4.1 | 47.3±7.2 | 47.0±6.0 | 46.0±5.6 | 44.9±5.7 |
| ACT (sec) | 202.6±16.2 | 157.3±6.4 | 134.6±8.2 | 151.6±8.9 | 159.2±5.4 | 167.8±8.0 | 174.8±11.2 |
|
| |||||||
| SO2 (Inlet) (%) | 70.6±4.57 | 57.2±1.9 | 56.6±1.2 | 58.8±1.7 | 60.4±1.4 | 60.8±1.7 | 61.7±3.4 |
| SO2 (outlet) (%) | 98.7±0.4 | 95.7±0.8 | 96.8±0.4 | 96.3±0.4 | 96.5±0.2 | 96.7±0.1 | 96.8±0.4 |
|
| |||||||
| Hct (%) | 32.2±3.6 | 27.3±2.1 | 24.3±1.9 | 28.8±0.8 | 34.7±0.8 | 33.2±2.8 | 35.5±3.0 |
| Platelets (×103/μl) | 631.6±103.2 | 335.0±69.0 | 667.9±35.2 | 652.1±87.9 | 481.8±127.7 | 560.8±76.9 | 602.3±54.8 |
| WBC (×103/μl) | 7.0±0.8 | 8.8±2.3 | 8.1±0.5 | 8.5±0.7 | 7.3±1.1 | 8.9±0.7 | 9.1±1.1 |
|
| |||||||
| Creatinine (mg/dl) | 0.7±0.0 | 1.7±0.6 | 1.1±0.2 | 0.8±0.0 | 0.7±0.1 | 0.8±0.1 | 0.9±0.1 |
| BUN (mg/dl) | 13.0±1.4 | 19.9±3.1 | 48.0±16.5 | 13.7±2.3 | 14.6±1.4 | 15.8±1.9 | 15.9±1.1 |
| ALT (U/L) | 16.0±1.7 | 42.1±5.2 | 18.6±4.1 | 14.0±1.8 | 9.3±1.8 | 10.8±1.5 | 12.1±2.0 |
| AST (U/L) | 96.0±4.7 | 267.4±39.9 | 207.2±115.1 | 124.3±60.7 | 47.1±12.2 | 63.2±12.1 | 92.8±21.8 |
| CPK (U/L) | 258.0±92.1 | 1553.8±391.7 | 102.2±33.2 | 96.3±21.9 | 85.0±26.7 | 100.6±33.2 | 159.4±92.4 |
| LDH (U/L) | 447.6±41.6 | 818.9±113.6 | 613.6±129.9 | 697.3±209.3 | 363.0±64.9 | 471.6±87.1 | 521.0±86.7 |
|
| |||||||
| PFH (mg/dl) | 6.8±1.5 | 6.8±0.8 | 7.0±0.5 | 9.0±0.6 | 9.0±1.2 | 7.2±0.7 | 9.5±0.9 |
| SurPsel (%) | 5.4±1.6 | 3.4±1.2 | 5.2±0.6 | 4.6±1.0 | 2.5±0.2 | 6.3±3.1 | 1.8±0.2 |
| SoPsel (ng/μl) | 0.4±0.1 | 0.9±0.4 | 1.4±0.4 | 1.4±0.2 | 0.9±0.2 | 1.7±0.4 | 1.8±0.4 |
ACT: activated clotting time; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BUN: blood urea nitrogen; CPK: Creatine phosphokinase; Hct: Hematocrit; LDH: lactate dehydrogenase; PFH: plasma free hemoglobin; POD: post-operative day; SO2: oxygen saturation; SoPsel: plasma soluble P-selectin; SurPsel: surface expressed P-selectin; WBC: white blood cells
Device Pumping Function and Oxygen Transfer Performance
The device flow and oxygen transfer performance of the APL in the five animals over the 30 day period are shown in Figure 3. Due to the slight differences in each sheep’s hemodynamic responses to the surgery, cannula position and the device placement, the operating speed of the APL was adjusted between 5000 rpm and 6000 rpm so that a device flow of ~3.25 l/min was generated at the beginning. Thereafter, the operation speed remained the same. The device flow decreased slightly in the first few days, remained stable in the first two weeks and gradually decreased after the first two weeks. The change in the flow rate after the first two weeks reached statistical significance (p<0.05). After an examination of the factors affecting the device pumping function, it was noticed that this change corresponded well to the increase in hematocrit (See Table 2). Since the blood viscosity increases with hematocrit, the increase would cause the reduction of the flow rate under the same hemodynamic pressure head. It is expected that the flow generated by the APL would decrease due to the increase in the hematocrit even without any device performance deterioration. The device flow was reanalyzed by factoring the effect of hematocrit (viscosity) of the blood at all the time points. There is no statistical difference in the hematocrit adjusted device flow from the baseline over the 30-day duration (p>0.25, baseline vs. all time points).
Figure 3.
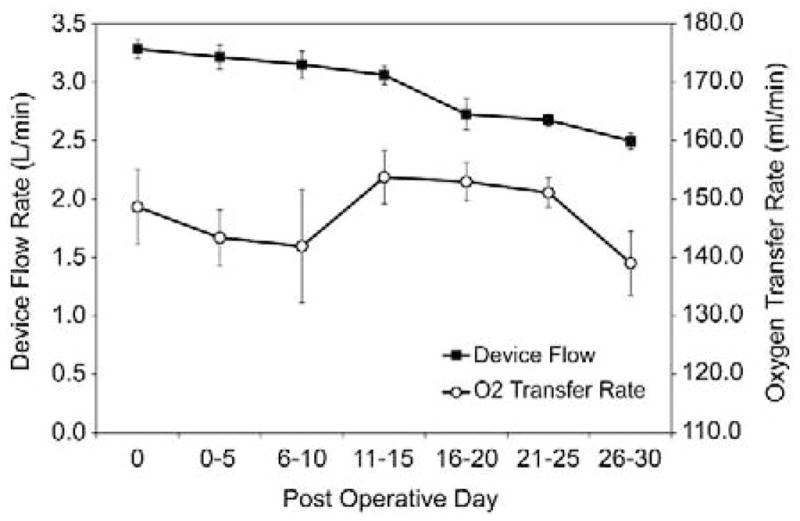
The device generated blood flow and oxygen transfer rate over the 30-day study period. Data were averaged every five days.
The oxygen transfer rate in individual animals varied depending on the animals’ hematological conditions and device flows. The highest oxygen transfer rate reached 222.5 ml/min at a device flow of 3.1 L/min at one time point in one animal while the animal had a very low oxygen partial pressure (PO2) in the venous blood (PO2: 22.3 mmHg, SO2: 43.2%, Hematocrit: 32%). The overall oxygen transfer rate remained stable over the 30-day period. There was no statistical difference between the oxygen transfer rates at the baseline and at the subsequent time points over the 30-day period (p>0.29). In spite of the variation of the animals’ venous blood, the oxygen saturation at the APL outlet was stable over the 30-day period and the normal physiological arterial blood condition (>95%) was achieved (see Table 2).
Hematology and End-Organ Function
Hematological data (hematocrit, platelet and white blood cell (WBC) counts) are summarized in Table 2. There was a drop in hematocrit from the pre-surgical value of 32.3% to 27% after the surgery. Post-operative hemodilution could be attributed to blood loss and fluid infusion during the surgery. Two weeks after the surgery, the hematocrit returned to the baseline level and gradually increased to 35.5% at the study end point. In one sheep, there was an unexplained drop in hematocrit on day 5 although there was no hemolysis or other abnormalities. 900 ml of blood was transfused to this sheep on day 6. The sudden drop of hematocrit in this particular sheep resulted in the lowest hematocrit at day 6 for the whole group. Although there is no statistical difference between the baseline hematocrit and the values at the subsequent time points (p> 0.10), the difference between the hematocrits at day 6–10 and days 15 to 30 were significantly different (p<0.036).
The platelet count also had a similar initial drop from its respective pre-surgical value after the surgery. The decrease in the platelet count was significant compared to the pre-surgical baseline value (p<0.05). However, the platelet count increased thereafter, and returned to the normal range after the first week. The WBC count varied slightly, but remained in the normal range. The variations in the WBC count at the post-operative time points did not reach statistical significance compared to their pre-surgical baseline value (p>0.19).
Selected laboratory tests for kidney function (creatinine, blood urea nitrogen (BUN)), liver function (alanine transaminase (ALT), aspartate aminotransferase (AST)), and cell and tissue injury (creatine phosphokinase (CPK), LDH) are presented in Table 2. Among the five sheep, the creatinine and BUN values in three sheep remained in the normal range throughout the study. The creatinine and BUN values in the other two sheep had an acute elevation at days 1 to 6 and returned to the normal range at day 9 and remained stable thereafter. This unusual elevation in two sheep may be attributed to the transient acute kidney insufficiency probably induced by low flow state and/or blood loss during the surgery. The device wasn’t the cause of this problem since the kidneys from these two sheep were found to be normal without infarcts during the necropsy and post mortem examination. ALT, AST, CPK and LDH were all significantly elevated in the first week and returned to the normal ranges at days 6 to 15 and remained stable throughout the study. Similar trends for the respective laboratory tests were reported by others [10].
Biocompatibility
The PFH and platelet activation markers over the 30 day period are shown in Table 2. The PFH fluctuated daily but remained in the normal range. The percentage of CD62p positive platelets was below 10%. There was no statistical difference between the percentages of CD62p positive platelets at the post-operative time points and the baseline level. The soluble P-selectin concentration initially peaked significantly at 6 hours immediately after the surgery, indicating a significant rise in platelet activation state at this time (0.42±0.09 vs. 3.5±0.5 ng/ml, p<0.05). After peaking during the first 24 hours, the soluble P-selectin concentration decreased from the peak, but remained slightly elevated compared to the baseline concentration.
Necropsy Finding and Examination of Explanted Devices
Both the left thoracotomy incision and cannula exit sites healed well without any evidence of infection. The hearts from the animals were normal in size and shape without evidence of infarction, thrombi formation or infective vegetations (Figure 4(a)). Localized endocardial bruising to the right atrium by the inflow cannula tip was found in one animal. The left lungs were normal at the gross appearance, with a limited area of atelectasis. The right lungs were fully expanded and of normal appearance. At gross examination, no lesion was found and no clots were present in the major vessels and branches of the lungs (Figure 4(a)). The left kidney from one animal had a 4 mm × 4 mm infarct while the right kidney was free of infarcts. The kidney function of this animal was completely normal over the course of the study. The kidneys from the other four animals were normal and free of any infarct. One of the four animals with the kidneys of normal appearance had elevated creatinine and BUN laboratory test values in days 1 to 6. The other organs (liver, spleen, gastrointestinal tract, brain) were of normal shape and appearance.
Figure 4.
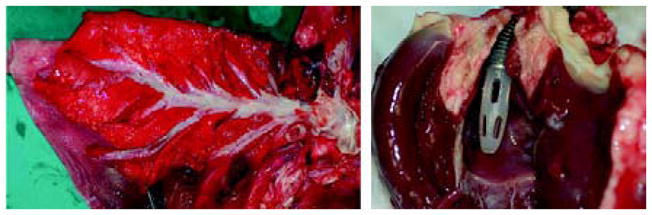
(a) Photographs of dissected pulmonary artery and braches of one explanted lung and inflow cannula tip in the right atrium of the heart. (b) Photographs of two explanted APL devices. (c) Photographs of disassembled hollow fiber membrane sheets from one explanted APL device.
The inflow and outflow cannulae were free of thrombus. There was a thin layer of intima inside the Dacron graft section of the outflow cannula. The graft section in one animal was slightly kinked at the fused interface between the Dacron graft and polyurethane tube. There was an adherent clot in the kinked site. Rings of thrombus were also found at some of the transition steps between the cannula and connectors.
All of the APL devices explanted from the five sheep were clean and without structural failure (Figure 4(b)). There were no massive occlusive clots or deposited materials in the fiber bundle as commonly observed in the explanted oxygenators used in cardiopulmonary bypass or respiratory support [10–12]. Three of the five explanted devices were free of gross thrombotic formation in the fiber bundle and flow path. The other two had isolated clots in the space between the outer housing and the outer layer of the fiber bundle. There were scattered micro thrombi (<1 mm) in some areas of the fiber bundle and flow path (Figure 4(c)). The microscopic examination showed that these micro thrombi mainly formed on the wefts of the fiber sheets and at the crevices of the blood contacting surfaces.
Comment
Various concepts for integrated pump-oxygenator devices have been presented before. The pumping function can be achieved by mating a blood pump to an oxygenator or rotating either an impeller within a fiber bundle or rotating the fiber bundle itself. The integrated pump-oxygenator in the demised Cardiovention CORx system represents the strategy of serially connected pump-oxygenator. The CORx system has only been used for cardiopulmonary bypass during cardiac surgical procedures, and not as an ambulatory respiratory system. The rotational component in these devices is usually supported by a mechanical shaft (with or without seal) and bearing, whereas a lot of problems may arise, such as leaking, part failure, heat generation, hemolysis, platelet activation and thrombosis formation. In our new design, the utilization of the magnetically levitated rotor/impeller with a uniquely configured flow path across the fiber bundle helped mitigate the thromboembolic phenomenon and biocompatibility problem. The only moving component within the APL is a magnetically levitated rotor/impeller. This feature greatly reduces the risk of hemolysis, thrombus formation and mechanical failure. The unique uniform radial flow path across the HFM bundle eliminates the flow stagnation. This feature ensures that the fiber membranes are effectively and uniformly perfused to achieve maximum gas exchange efficiency and there are no deleterious stagnant flow or high shear stress areas.
The early transient changes in hematocrit, CBC, platelet activation, CPK, LDH and liver transaminases were most likely to be surgery related since they all returned to their respective normal ranges after two weeks. Thus, it is reasonable to make the assumption that the blood chemistry data collected after recovery from the surgical trauma should be true indicators of biocompatibility. If an implanted device causes blood cell damage, end-organ dysfunction and detrimental biologic responses, laboratory tests for these indications or functions should remain at abnormal levels. Both the surface-expressed p-selectin and soluble p-selectin also exhibited a similar trend. These observations indicate that the newly developed APL is biocompatible.
Although isolated clots were observed in some of the devices, they did not impair the device functionality. During the experiments, both the pump function and oxygen transfer rate over the 30-day period were stable. It is interesting to notice that the clots (~1 mm) were only observed in the flow path before fiber bundle. Since we observed the rings of thrombus in the transition steps between the tubing and connectors, some of them might have embolized and were entrapped by the fiber bundle. The fibers might have acted as a clot filter if clots had formed upstream.
The existence of the kidney infarct in one of the study animals might not be related to the thrombosis in the implanted APL. If there were clots dislodged from the APL, the clots would have been trapped in the natural lungs and could not reach the kidneys since the APL was implanted in the right side of the heart. We have seen kidney infarcts in sheep without any surgical intervention. Therefore we believe this infarct may have been a pre-existing condition.
The newly developed APL is an integrated pump oxygenator system that can provide both respiratory and cardiopulmonary support. Overall, the results from the animal studies are excellent, showing paracorporeal placement, partial to complete respiratory support, excellent gas transfer efficiency, excellent biocompatibility and long-term reliability. The performance was stable for at least 30 days during the five long-term animal experiments. Compared to the conventional system, the prime volume and foreign surface area of the system are greatly reduced therefore the risks of systemic inflammatory reaction and hemodilution can be minimized. The new compact system reduces the complexity of operation and is suitable for ambulatory respiratory support. The extracorporeal placement enables easy exchange and permits sustained long-term (several months) respiratory support for bridge to lung transplant.
Acknowledgments
The authors thank Ms. Yanan Ding and Dr. Jingping Hu for assistance with the hematological measurements of blood samples. This work was supported by the National Institutes of Health grants (R01HL082631, R42 HL084807, and R01HL088100).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bartlett RH. Extracorporeal life support: history and new directions. Semin Perinatol. 2005;29(1):2–7. doi: 10.1053/j.semperi.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Weinacker AB, Vaszar LT. Acute respiratory distress syndrome: physiology and new management strategies. Annu Rev Med. 2001;52:221–37. doi: 10.1146/annurev.med.52.1.221. [DOI] [PubMed] [Google Scholar]
- 3.Mangi AA, Mason DP, Yun JJ, Murthy SC, Pettersson GB. Bridge to lung transplantation using short-term ambulatory extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2010;140(3):713–5. doi: 10.1016/j.jtcvs.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Garcia JP, Iacono A, Kon ZN, Griffith BP. Ambulatory extracorporeal membrane oxygenation: a new approach for bridge-to-lung transplantation. J Thorac Cardiovasc Surg. 2010;139(6):e137–9. doi: 10.1016/j.jtcvs.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Broomé M, Palmér K, Scherstén H, Frenckner B, Nilsson F. Prolonged extracorporeal membrane oxygenation and circulatory support as bridge to lung transplant. Ann Thorac Surg. 2008;86(4):1357–60. doi: 10.1016/j.athoracsur.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 6.Potapov EV, Loforte A, Weng Y, et al. Experience with over 1000 implanted ventricular assist devices. J Card Surg. 2008;23(3):185–94. doi: 10.1111/j.1540-8191.2008.00606.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang T, Cheng G, Koert A, et al. Functional and biocompatibility performances of an integrated Maglev pump-oxygenator. Artif Organs. 2009;33(1):36–45. doi: 10.1111/j.1525-1594.2008.00672.x. [DOI] [PubMed] [Google Scholar]
- 8.Dierickx PW, De Wachter DS, De Somer F, Van Nooten G, Verdonck PR. Mass transfer characteristics of artificial lungs. ASAIO J. 2001;47(6):628–33. doi: 10.1097/00002480-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Gibber M, Wu ZJ, Chang WB, et al. In vivo experience of the child-size pediatric Jarvik 2000 heart: update. ASAIO J. 2010;56(4):369–76. doi: 10.1097/MAT.0b013e3181dbe55e. [DOI] [PubMed] [Google Scholar]
- 10.Sato H, Hall CM, Lafayette NG, et al. Thirty-day in-parallel artificial lung testing in sheep. Ann Thorac Surg. 2007;84(4):1136–43. doi: 10.1016/j.athoracsur.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 11.Snyder TA, Eash HJ, Litwak KN, et al. Blood biocompatibility assessment of an intravenous gas exchange device. Artif Organs. 2006;30(9):657–64. doi: 10.1111/j.1525-1594.2006.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehle K, Philipp A, Gleich O, et al. Efficiency in extracorporeal membrane oxygenation-cellular deposits on polymethylpentene membranes increase resistance to blood flow and reduce gas exchange capacity. ASAIO J. 2008;54(6):612–7. doi: 10.1097/MAT.0b013e318186a807. [DOI] [PubMed] [Google Scholar]
- 13.Wu ZJ, Gartner M, Litwak KN, Griffith BP. Progress toward an ambulatory pump-lung. J Thorac Cardiovasc Surg. 2005;130(4):973–8. doi: 10.1016/j.jtcvs.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 14.Svitek RG, Frankowski BJ, Federspiel WJ. Evaluation of a pumping assist lung that uses a rotating fiber bundle. ASAIO J. 2005;51(6):773–80. doi: 10.1097/01.mat.0000178970.00971.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tatsumi E, Takano H, Taenaka Y, et al. Development of an ultracompact integrated heart-lung assist device. Artif Organs. 1999;23(6):518–23. doi: 10.1046/j.1525-1594.1999.06394.x. [DOI] [PubMed] [Google Scholar]
- 16.von Segesser LK, Tozzi P, Mallbiabrrena I, Jegger D, Horisberger J, Corno A. Miniaturization in cardiopulmonary bypass. Perfusion. 2003;18(4):219–24. doi: 10.1191/0267659103pf676oa. [DOI] [PubMed] [Google Scholar]
- 17.Arens J, Schnoering H, Pfennig M, et al. The Aachen MiniHLM--a miniaturized heartlung machine for neonates with an integrated rotary blood pump. Artif Organs. 2010;34(9):707–13. doi: 10.1111/j.1525-1594.2010.01082.x. [DOI] [PubMed] [Google Scholar]
- 18.Pantalos GM, Horrell T, Merkley T, et al. In vitro characterization and performance testing of the ension pediatric cardiopulmonary assist system. ASAIO J. 2009;55(3):282–6. doi: 10.1097/MAT.0b013e3181909d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopp R, Bensberg R, Arens J, et al. A miniaturized extracorporeal membrane oxygenator with integrated rotary blood pump: preclinical in vivo testing. ASAIO J. 2011;57(3):158–63. doi: 10.1097/MAT.0b013e31820bffa9. [DOI] [PubMed] [Google Scholar]
- 20.Asakawa Y, Funakubo A, Fukunaga K, et al. Development of an implantable oxygenator with cross-flow pump. ASAIO J. 2006;52:291–5. doi: 10.1097/01.mat.0000216165.21432.ee. [DOI] [PubMed] [Google Scholar]