Abstract
Spectrin α2 (αII-spectrin) is a scaffolding protein encoded by the Spna2 gene and constitutively expressed in most tissues. Exon trapping of Spna2 in C57BL/6 mice allowed targeted disruption of αII-spectrin. Heterozygous animals displayed no phenotype by 2 years of age. Homozygous deletion of Spna2 was embryonic lethal at embryonic day 12.5 to 16.5 with retarded intrauterine growth, and craniofacial, neural tube and cardiac anomalies. The loss of αII-spectrin did not alter the levels of αI- or βI-spectrin, or the transcriptional levels of any β-spectrin or any ankyrin, but secondarily reduced by about 80% the steady state protein levels of βII- and βIII-spectrin. Residual βII- and βIII-spectrin and ankyrins B and G were concentrated at the apical membrane of bronchial and renal epithelial cells, without impacting cell morphology. Neuroepithelial cells in the developing brain were more concentrated and more proliferative in the ventricular zone than normal; axon formation was also impaired. Embryonic fibroblasts cultured on fibronectin from E14.5 (Spna2−/−) animals displayed impaired growth and spreading, a spiky morphology, and sparse lamellipodia without cortical actin. These data indicate that the spectrin–ankyrin scaffold is crucial in vertebrates for cell spreading, tissue patterning and organ development, particularly in the developing brain and heart, but is not required for cell viability.
Key words: Ankyrin, Neuroepithelial cell, Myocardium, Axons, Ventricular zone, Actin cytoskeleton, Cell growth, Development, Exon trap
Introduction
Spectrin α2 (αII-spectrin) is a 285 kDa scaffolding protein abundant in most eukaryotic cells. Three decades of study have implicated the spectrin heterodimer formed between αII-spectrin and any of five β-spectrins in a bewildering array of cellular processes. These include a role in the formation and maintenance of specialized plasma membrane domains defining apical–basolateral and planar polarity in epithelial cells, muscle and neurons (Bennett and Baines, 2001); in the structural support of the plasma membrane and the maintenance of cell shape (Gallagher and Jarolim, 2000; Kizhatil et al., 2007); as a scaffold upon which calcium-mediated and tyrosine kinase–phosphatase signal transduction pathways converge (Nicolas et al., 2002; Nedrelow et al., 2003); as a tumor-suppressor protein involved in TGF-β–SMAD regulation (Tang et al., 2003); as a cargo selection mechanism in the secretory and endocytic pathways (De Matteis and Morrow, 2000); as a regulator of macropinocytosis (Xu et al., 2000); as a tether linking trafficking vesicles to microtubule motors (Holleran et al., 2001; Muresan et al., 2001); as a nuclear scaffold organizer (McMahon et al., 1999; Tse et al., 2001); and most recently, as a potential mechano-sensing ligand-binding switch (Stabach et al., 2009). Deletion of αII-spectrin in Drosophila melanogaster and Caenorhabditis elegans leads to late embryonic–early larval stage lethality (Moorthy et al., 2000; Dubreuil, 2006; Hammarlund et al., 2007), and recent knockdown studies of αII-spectrin in cultured cells have demonstrated growth and adhesion defects (Metral et al., 2009). However, the role of αII-spectrin in vertebrate development remains unexplored.
We have achieved targeted disruption of αII-spectrin in C57/B6 mice by the insertion of a foreign exon encoding β-galactosidase (β-gal) into the murine Spna2 gene. The resulting gene product is a short-lived and non-functional fusion protein that includes the N-terminal half of αII-spectrin fused to β-gal. Heterozygous animals (Spna2+/−) display no phenotype by 2 years of age. Homozygous animals die in utero. Embryos lacking wild-type αII-spectrin (Spna2−/−) are smaller than normal, survive to embryonic day 12.5 (E12.5), but then die due to malformations of the neural tube and cardiac systems. Thus, αII spectrin is required in vivo for late embryonic development. The cell biological consequences of its loss include instability of its cognate partners (βII- and βIII-spectrin); impaired membrane biogenesis and sorting of not only the cortical actin skeleton, but also of ankyrins B and G; and modification of pathways regulating cell spreading and growth. Conversely, spectrin loss in vivo does not affect epithelial cell shape, stability or nuclear morphology, nor is it required for cell viability. These findings have several implications for the potential role of αII-spectrin in human disease.
Results
Loss of αII-spectrin leads to a concomitant loss of two β-spectrins and selected ankyrins
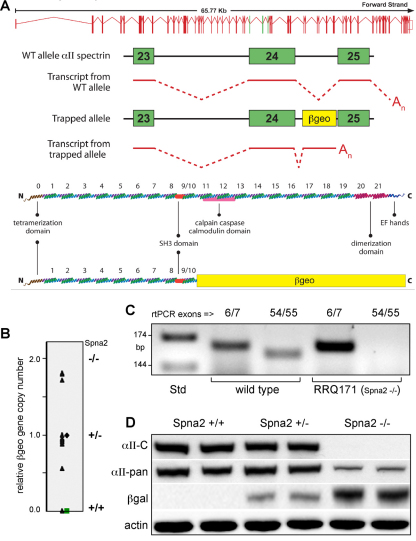
PCR analysis of murine tissues derived from ES RRQ171 identified the locus of the β-geo exon trap (encoding a β-gal–neomycin resistance fusion protein) within the intron between exons 24 and 25 of the spectrin Spna2 gene (Fig. 1A). The exon-trapped gene generates a spectrin β-gal fusion message that truncates the αII-spectrin gene product at codon 1153, corresponding to a polypeptide terminating within spectrin repeat ten, lacking the C-terminal site responsible for heterodimer formation with β-spectrin (Li et al., 2008). In tissues or cells homozygous for this insertion (Fig. 1B), mRNA encoding αII-spectrin was undetectable when probed by realtime (RT)-PCR for sequences downstream of β-geo, but not when primers upstream of exon 24 were utilized (Fig. 1C). Correspondingly, western blot analysis showed that Spna2−/− embryos did not react with αII-spectrin antibodies directed to epitopes downstream of the exon trap, whereas antibodies directed to epitopes upstream of the exon trap reacted with a band at about 260,000 relative molecular mass (Fig. 1D). This band also reacted with antibodies against β-gal, confirming its identity as a fusion product between αII-spectrin exons 1–24 and β-gal. Analysis of whole embryos for the expression of either the wild-type gene or the exon-trapped gene revealed no significant differences, with both being widely expressed and most highly expressed in the nervous system (supplementary material Fig. S1). By densitometry analysis, the ratio of the spectrin–β-gal fusion protein in Spna2−/− mice to αII-spectrin in wild-type embryos (normalized to actin) was 0.21±0.03 (±1 s.d. arbitrary units). Analysis of mRNA levels indicated that the mRNA for wild-type αII-spectrin and the mRNA for the fusion product were expressed at equal levels (Fig. 2B). Thus, the spectrin–β-gal fusion protein must be unstable relative to the wild-type protein; and the transcription level of mRNA encoding αII-spectrin is not responsive to the loss of the mature protein.
Fig. 1.
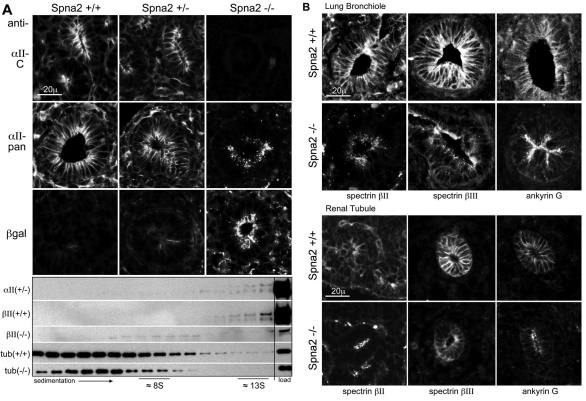
Targeted disruption of αII-spectrin. (A) An ES cell line was established using gene trap vector RRQ171 (β-geo) from Bay Genomics. Analysis by 5′ RACE identified insertion of the vector between exons 24 and 25 of the murine Spna2 gene. This created a fusion transcript with a spectrin message truncated by the addition of β-geo. A cartoon of this fusion transcript, and the anticipated fusion protein, is depicted. (B) E11.5 embryos derived from a RRQ171 heterozygous breeding pairs were genotyped by quantitative RT-PCR for β-geo. (C) RT-PCR analysis with intron-bridging primer pairs directed to upstream exons (6/7) or downstream exons (54/55) confirmed the absence of mRNA encoding full-length αII-spectrin in homozygotes. (D) Western blot analysis showed that monoclonal antibodies to αII-spectrin (αII-C) that react with peptide sequences downstream of the exon trap were negative in homozygotes. Pan-reactive anti-spectrin antibodies (αII-pan) detect the fusion protein. Antibodies to β-gal confirm the presence of the fusion protein in both the homo- and heterozygotes.
Fig. 2.
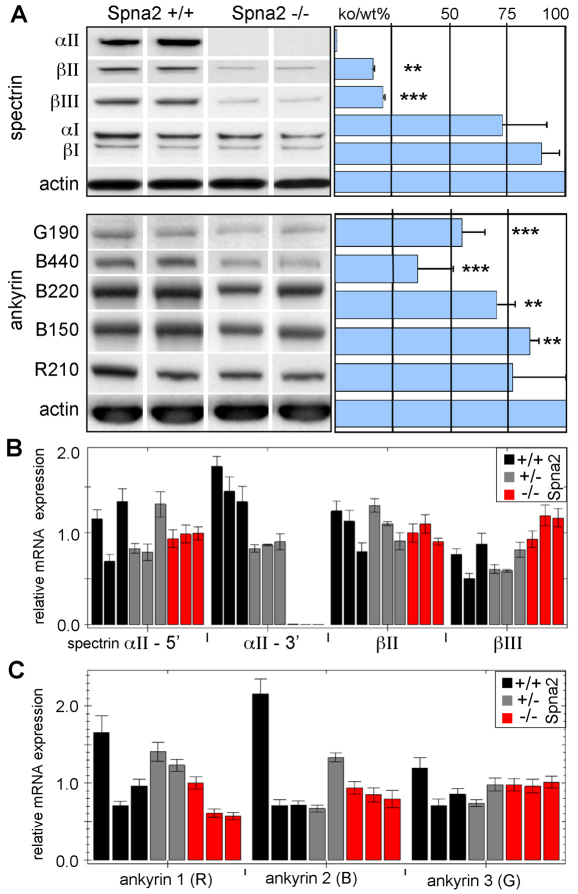
Loss of αII-spectrin destabilizes βII- and βIII-spectrin. (A) Western blot analysis of whole embryos comparing the relative steady state protein levels of several spectrins and ankyrins. Each lane represents results with a separate embryo. The loss of αII-spectrin reduced the steady state levels of βII- and βIII-spectrin to below 20% of normal; αI- and βI-spectrin were unchanged. The abundance of βIV- and βV-spectrin was too low in embryos younger than E14.5 to be reliably evaluated. Ankyrins B440 and G190 were both significantly diminished, as were ankyrins B220 and B150, albeit not to the same degree as the β-spectrins. (B,C) Quantitative RT-PCR analysis revealed that despite the change in protein levels, there were no consistent changes in the mRNA levels of any spectrin or ankyrin (except for the disrupted Spna2 gene, as measured with primers targeted to the 3′ end, αII-3′). Each analysis was performed in triplicate on two or three separate animals. Error bars show ±1 s.d. **P<0.05, ***P<0.005.
Also of interest was the fate of other proteins that are typically tightly associated with αII-spectrin, specifically spectrins βII and βIII, and ankyrins R, G, and B. Whole embryo levels of βII- and βIII-spectrin were reduced to below 20% of normal in the absence of αII-spectrin (Fig. 2A), with no change in their level of mRNA expression (Fig. 2B). This finding is consistent with earlier studies in vertebrates demonstrating that β-spectrin is degraded if not assembled with α-spectrin (Woods and Lazarides, 1985; Hanspal and Palek, 1987), and also indicates an absence of direct feedback control on the levels of β-spectrin expression. The level of spectrins αI and βI, forms predominantly (albeit not exclusively) expressed in erythrocytes, were unchanged in the Spna2−/− mice, at either the protein or mRNA level. Neither of the two remaining spectrins (βIV and βV) were expressed by E14.5 at sufficient levels to enable their reliable detection, so the impact of αII-spectrin loss on the disposition of these proteins was not evaluated.
Ankyrin expression and accumulation was less severely perturbed compared with the spectrins, but the reductions were still significant (Fig. 2A,C). The protein levels of AnkG190 (P<0.005) and AnkB440/220 (P<0.05) were slightly reduced and no significant changes in levels of mRNA encoding ankyrin were detected. The overall levels of VASP (Bournier et al., 2006), Abi (Hssh3bp1) (Ziemnicka-Kotula et al., 1998), Kap3 (Takeda et al., 2000), and 14-3-3 (Ramser et al., 2010), proteins that directly bind αIIβII-spectrin, were unchanged (supplementary material Fig. S2).
αII-spectrin deficiency is embryonic lethal, with cardiac, craniofacial and neural tube malformations
The loss of αII-spectrin is embryonic lethal. Of 18 litters generating 127 C57BL/6 mice in matings between heterozygous Spna2+/− pairs, 68 heterozygous and 27 homozygous wild-type mice were produced. No homozygous Spna2−/− mice were born. Heterozygous (Spna2+/−) mice were morphologically indistinguishable from wild-type animals. Thus, one normal Spna2 allele was sufficient and generated normal levels of αII-spectrin (Fig. 1).
Examination of the homozygous embryos at different gestational ages indicated that most (but not all) such embryos were still viable at E12.5, but that all had died by E16.5; thus the loss of αII-spectrin causes embryonic death between E12.5 and E16.5. Homozygous embryos exhibited intrauterine growth retardation; the average length of the Spna2−/− embryo at E11.5 was approximately 5.5 mm, and 7.0 mm for wild-type littermates. Whole-mount examination of heterozygous embryos revealed widespread expression of αII-spectrin throughout most tissues, but most concentrated in the heart and nervous system. The expression pattern of the mutant αII-spectrin–β-gal fusion allele was similar to that of the wild-type protein (supplementary material Fig. S1).
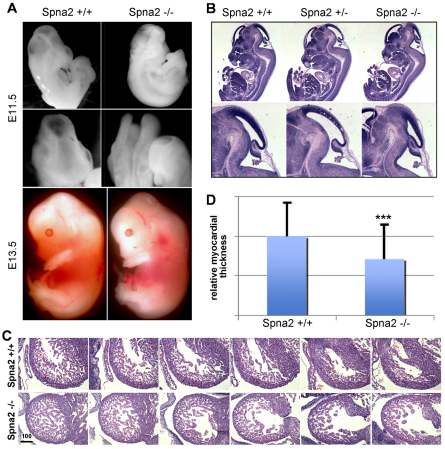
Accompanying the lethal phenotype of the Spna2−/− animals were several gross morphologic abnormalities (Fig. 3). Although not every homozygous embryo showed every abnormality, typical features associated with the Spna2−/− embryos included craniofacial abnormalities with incomplete neural tube closure; abnormal dilatation of the primary brain vesicles, constriction of the mesencephalic vesicle, along with lateral ventricle and forebrain anomalies (Fig. 3B). Outside the nervous system, αII-spectrin-deficient animals exhibited abnormal cardiac shape, cardiac dilatation, and thinning of the compact myocardium (Fig. 3C). The cardiac changes probably initiated the embryonic lethality, and were reminiscent of cardiac phenotypes observed following the disruption of TGF-β–Smad signaling (Qi et al., 2007) or following the loss of proteins that regulate actin dynamics such as Ena/VASP (Eigenthaler et al., 2003). Interestingly, both TGF-β–Smad signaling (Tang et al., 2003) and Ena/VASP (Bournier et al., 2006) interact with spectrin, although the levels of neither Ena/VASP (supplementary material Fig. S2) nor the Smad proteins (supplementary material Fig. S3) were altered in the Spna2−/− embryos (see Discussion). Two unique cardiac embryonic isoforms of αII-spectrin (αIIΣ9 and αIIΣ10) that arise by alternative splicing have also been recently reported (Zhang et al., 2010). In cell culture, these isoforms promote myocyte growth, suggesting that their loss in the Spna2−/− animals is consistent with the cardiac phenotype observed. Finally, the cardiac changes appear not to be secondary to failed hematopoiesis because the embryonic blood islands appeared normal and the embryos were pink and well oxygenated.
Fig. 3.
Spna2−/− embryos die at E12.5–16.5 with multiple defects. (A,B) Whole mounts depicting the gross morphological defects in head development, with frequent neural tube closure defects (E11.5 embryos shown). In cases where the neural tube closed, there remained distinct alterations in head and back curvature with craniofacial abnormalities. (C) Serial sections through the fetal heart. The Spna2−/− hearts have an irregular shaped ventricle, and a thinned compact myocardium. Scale bar: 100 μm. (D) Quantitative comparison of cardiac wall thickness. On average, the myocardium of the Spna2−/− hearts was 70.9% of the thickness of normal hearts of the same gestational age. This difference is highly significant (n=101; ***P=4.2×10−13). Error bars indicate s.d.
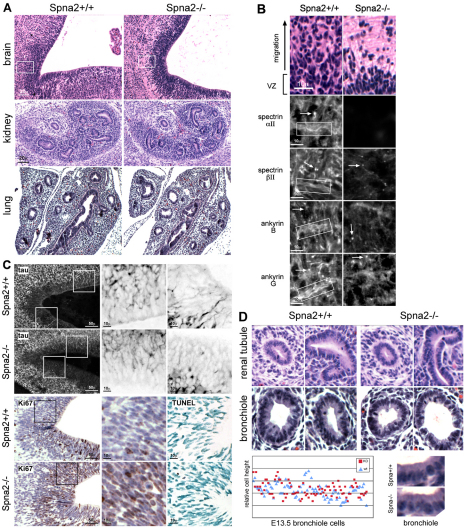
At the histological level, Spna2−/− animals displayed changes in the density of neuroepithelial cells along the ventricular zone (VZ) of the developing brain (Fig. 4A,B). This is a region of active neuronal and glial differentiation; cells from this zone normally proliferate and migrate apical to populate overlying layers (Gotz and Huttner, 2005). At about E13.5, earlier work has demonstrated that βII-spectrin and ankyrin B mark axonal sprouts in developing neuroblasts, whereas ankyrin G is a reliable marker of nascent axonal initial segments (Tang et al., 2002). These distributions can be appreciated in the Spna2+/+ animals, as shown in Fig. 4B. In the spectrin-deficient animals, these markers became restricted to an area near the soma, and the developing axonal sprouts and initial axon segments were diminished in abundance, a finding also supported by the reduced tau staining revealed in Fig. 4C. These animals also displayed enhanced nuclear density in the subventricular zone (SVZ), with more cells in the Spna2−/− animals staining positive for the proliferation marker Ki67 (34±11% vs 46±8%, P<0.006). There was no change in the level of apoptosis (as measured by TUNEL staining; Fig. 4C). Beyond greater cellular proliferation, and given the impaired cellular spreading and movement of αII-spectrin-deficient cells described below and by others (Metral et al., 2009), the enhanced cellular density apparent in the VZ and SVZ of the Spna2−/− animals could also arise from a failure of interkinetic movement of nuclei in the neuroepithelial cells of this zone, a feature related to cellular proliferation and responsible for the pseudostratified appearance of nuclei in the VZ (Gotz and Huttner, 2005). Finally, using tau as a marker of axonal processes, it is apparent that αII-spectrin deficiency impaired axon formation and/or axon guidance in the VZ (Fig. 4C). Tau-positive axons were shorter, less ordered, and often truncated at or near the soma in these animals. In this same area in the spectrin-deficient animals there was enhanced vimentin staining, a marker of glia, (supplementary material Fig. S4), suggesting that, as with the loss of βII-spectrin, αII-spectrin deficiency altered the program of neuroepithelial cell differentiation (Golestaneh et al., 2006). Earlier work has demonstrated that the micro-injection of anti-spectrin antibodies can lead to a condensation of the vimentin network (Mangeat and Burridge, 1984). It will thus be of interest in future studies to carefully examine the vimentin network in cultured spectrin-deficient cells because alterations in vimentin might contribute to some of the cell growth and phenotypic changes observed.
Fig. 4.
Histology of Spna2−/− embryos. (A) The VZ and SVZ of the developing brain display significant histological changes. Wild-type embryos at this stage of development display a thick pseudostratified VZ with active upward migration and population of overlying layers from the SVZ. In the Spna2−/− animals, the VZ was more compact, with fewer cells filling the more superficial layers (boxed area, shown enlarged in B). Other organs revealed no significant histological changes; kidney and lung are shown. In these organs, tubules and bronchioles were developing normally. (B) Enlargement of area outlined in A. In wild-type animals, αII- and βII-spectrin are rich in axon bundles (boxed) and are also present at synaptic terminals and initial axon segments (arrows). Ankyrin B is associated with unmyelinated axonal processes in the embryonic mouse (Chan et al., 1993), whereas ankyrin G is found along axons and in dendrites and concentrates in the initial axon segments (Kordeli et al., 1995). The loss of αII-spectrin disrupts the localization of these proteins, especially along the axon bundles, which no longer are marked by either spectrin or ankyrin. (C) Top two rows: Staining of sections with anti-tau demonstrates developing axon bundles in the VZ. Magnified images (3×) of the boxed areas are shown on the right with inverse contrast. Note the foreshortened, sparse and more disordered axon segments and increased concentration of tau near the soma when αII-spectrin is disrupted. Bottom two rows: Immunostaining for Ki67, a proliferation marker, reveals increased proliferation with spectrin loss. Averaged over multiple fields, and scoring any detected Ki67 staining as positive, 34±11% of cells in the wild-type animals were positive as compared with 46±8% of the cells in the spectrin-deleted animals. This represents a highly significant (P<0.006) 35% increase in proliferative activity. TUNEL staining (right) reveals no change in the level of apoptosis. (D) Higher power views of developing renal tubules and lung bronchioles indicate preservation of epithelial morphology. Comparison of epithelial cell height reveals no changes, even without laterally associated spectrin or ankyrin (see Fig. 5).
In contrast to the histological changes apparent in the brain, no histological anomalies were detected in E13.5 embryos in several other tissues. Sections of the developing kidney and lung are shown in Fig. 4A,D. Although spectrin and ankyrin presumably play a role in providing structural support to the plasma membrane, and their absence in erythrocytes (Gallagher and Jarolim, 2000) and isolated bronchial cells (Kizhatil et al., 2007) leads to profound cell-shape change, the loss of αII-spectrin (with concomitant loss of βII- and βIII-spectrin) resulted in no significant change in epithelial cell layer thickness or epithelial cell shape (Fig. 4D). Quantitative evaluation of cells with well-defined borders found no significant differences in cell height [6.46±1.1 versus 6.5±1.0 (±2 s.d. arbitrary units)] between Spna2−/− and wild-type embryos.
Spectrin loss causes a redistribution of βII- and βIII-spectrin and ankyrin
The loss of αII-spectrin perturbs the levels and distribution of its associated proteins. In the VZ at E13.5, αII- and βII-spectrin normally are found along the developing axon bundles (Fig. 4B). Ankyrins B and G display a similar distribution, while ankyrin G also concentrates in the initial axon segments. In the absence of αII-spectrin, βII-spectrin and ankyrin B are lost from the unmyelinated axonal processes and ankyrin G is no longer concentrated at initial segments.
Cells outside the nervous system also show a redistribution of the proteins normally associated with αII-spectrin, although apical–basolateral polarity is retained. Results with kidney tubules and lung bronchioles are shown (Fig. 5). As expected, in the Spna2−/− animals, there was no immunostaining of tissues with antibodies directed to the C-terminal portions of αII-spectrin (Fig. 5A). When antibodies were used that recognize the spectrin portions of the spectrin–β-gal fusion, or with β-gal antibodies, the fusion product was found concentrated along the apical membrane and submembrane in epithelial cells, a distribution unlike normal αII-spectrin (that surrounds the entire membrane). Interestingly, both distributions were apparent in heterozygous animals, suggesting that the fusion protein distributes independently of and has no effect on normal spectrin. This was also apparent when cell extracts were sedimented (Fig. 5A). In wild-type or heterozygous animals, αII-spectrin sedimented as a large multiprotein complex near 13S, as did βII-spectrin. In animals without αII-spectrin, the residual βII-spectrin sedimented near 8S, indicating that it is no longer retained in a high molecular weight multiprotein complex.
Fig. 5.
Loss of αII-spectrin leads to a redistribution of both βII- and βIII-spectrin and ankyrin. (A) In developing renal tubules, αII-spectrin is distributed uniformly over both the basolateral and apical surfaces in wild-type (Spna2+/+) embryos, as detected by both the C-terminal anti-αII-spectrin antibody (αII-C) as well as the pan-reactive spectrin antibody (αII-pan). In the Spna2−/− tubules, the residual spectrin–β-gal fusion protein is totally lost from the basolateral membrane, and concentrated in coarse apical and sub-apical pools as detected by both αII-pan and by an antibody to β-gal. In heterozygotes (Spna2+/−), the presence of the fusion protein had no detectable effect on the distribution of wild-type αII-spectrin, nor was the distribution of the fusion protein changed by the presence of αII-spectrin. Below: Sucrose density gradient analysis of proteins extracted from E13.5 embryos. In the homozygote (Spna2−/−), βII-spectrin does not sediment with the high molecular weight protein complex at 13S. (B) Immunofluorescent micrographs of bronchioles and renal tubules. Without intact αII-spectrin, βII- and βIII-spectrin and ankyrin G are largely absent from the lateral membrane of both renal and bronchiole epithelial cells. Residual βII- and βIII-spectrin and ankyrin G concentrate instead at the apical membrane in a pattern similar to that of the αII-spectrin–β-gal fusion protein.
The βII- and βIII-spectrin that remained in the renal tubules and lung bronchioles was largely redistributed to an apical submembranous compartment, coincident with the αII-spectrin–β-gal fusion protein (Fig. 5B). Ankyrin G displays the same redistribution; ankyrins B and R are only very weakly expressed in these cells at this stage of development and did not stain reliably. A consideration in these results is whether the absence of intact αII-spectrin per se is the driving force for protein redistribution, or whether it is an epiphenomenon due to a strong sorting determinant on the αII-spectrin–β-gal fusion protein present in these cells. Although we cannot formally exclude this possibility, we do not favor it, given the normal distribution of βII-spectrin and ankyrins in the heterozygote (data not shown). We also note that in Drosophila, the genetic loss of α-spectrin leads to a redistribution and mislocalization of β-spectrin (Garbe et al., 2007).
Growth and cell spreading is impaired in Spna2−/− MEFs
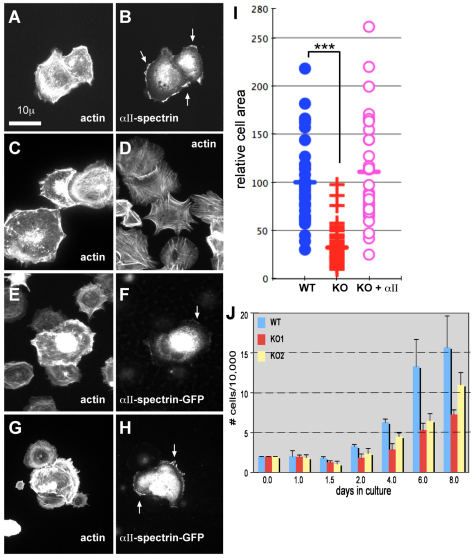
To determine the effect of spectrin loss on cellular actin and cell shape, the morphology of mouse embryonic fibroblasts (MEF) cultured from the Spna2−/− animals at E14.5 was compared with wild-type cells harvested from matched littermates. MEFs were plated on fibronectin and after 60 minutes, wild-type MEFs displayed a spread and flattened cell shape, with cortical and perinuclear actin bundles and frequent lamellipodia that were rich in both actin and αII-spectrin (Fig. 6A–C). The αII-spectrin null MEFs spread more slowly, and after 0.5–3 hours in culture, displayed many filopodia and stress fibers, but few lamellipodia, giving them a rounded and spiky appearance (Fig. 6D). These cells also lost their cortical actin ring, as well as the actin pools surrounding the nucleus. This phenotype was rescued by transfection with wild-type EYFP-tagged αII-spectrin, with restoration of a spread morphology, ruffling edge, and cortical actin (Fig. 6E–H). The expressed EYFP–αII-spectrin was apparent in the rescued cells at the ruffling edge and in lamellipodia, in a pattern similar to spectrin in wild-type cells (arrows, Fig. 6F,H). The spread area of the αII-spectrin-deficient MEFs was on average only 32.2±20% of the area of the wild-type cells (Fig. 6, scattergram). This result was highly significant (P=1.6×10−16 by ANOVA). This effect on cell spreading and size was completely rescued by transfection with EYFP–αII-spectrin.
Fig. 6.
MEFs lacking intact αII-spectrin display impaired growth and spreading, and altered morphology. (A,B) MEFs from E14.5 embryos were plated onto fibronectin, grown for up to 3 hours, and stained as indicated. The images shown here were from cells examined at 30 minutes after replating. (A) MEF from wild-type cells, stained for F-actin with rhodamine phalloidin. Note the peripheral cortical and perinuclear actin, cell spreading, and abundant ruffling edges and lamellipodia. (B) Same wild-type cells as in A, stained for αII-spectrin. Note the coincidence of spectrin with the ruffling edge (arrows). (C,D) MEFs from Spna2+/+ (C) or Spna2−/− (D) embryos under same conditions as in A, stained for actin. Note the lack of spreading and lamellipodia, and abundant filopodial projections and stress filaments in the spectrin-deficient cells. (E–H) MEFs from Spna2−/− embryos under same conditions as A, but transfected with wild-type GFP-labeled αII-spectrin. Double immunofluorescent images are shown, stained for actin or GFP. Arrows mark the accumulation of GFP–spectrin at the ruffling edge and lamellipodia. Note the recovery of cortical and perinuclear actin, ruffling, and spreading in the transfected cells expressing GFP–αII-spectrin. (I) Scatter diagram of individual cell areas of MEFs from Spna2+/+ (WT) or Spna2−/− (KO) embryos, or from Spna2−/− cells transfected with GFP–αII-spectrin (KO+αII). The KO cells are significantly less spread, with an average area just 32% of wild-type cells (***P≤1.6×10−16); there is no difference in area between the wild-type cells and the Spna2−/− cells transfected with GFP–αII-spectrin. (J) Comparison of cell growth in culture, as measured by cell count, of wild-type and two MEF lines (KO1, KO2) independently derived from separate Spna2−/− embryos.
Surprisingly given the enhanced Ki67 labeling observed in the brain of the mouse embryos, MEFs lacking αII-spectrin grew more slowly than did wild-type cells (Fig. 6, bottom). This reduction in cell proliferation was not due to increased apoptosis or oncosis because the fraction of unfixed cells permeant to propidium iodide or the level of annexin V staining was unchanged between wild-type and spectrin deficient cells (data not shown). Whereas the basis for this difference between in vivo proliferation and that observed in culture is unknown, these findings do point to a potentially significant role for spectrin in modulating the signals that control cell growth and differentiation.
Discussion
Exon trapping has been used to generate a strain of mice devoid of functional αII-spectrin. These mice display uniform embryonic lethality at E12.5 to E16.5, with gross craniofacial, neurodevelopmental and cardiac defects. Detailed analysis reveals: (1) no compensation by αI-spectrin for the loss of αII-spectrin; (2) a reduction of βII- and βIII-spectrin to about 20% of normal levels due to accelerated degradation, with no change in their mRNA levels; (3) no apparent hematological anomalies that might contribute to the cardiovascular defects; (4) preserved epithelial cell morphology in renal tubules and bronchial epithelium; (5) mis-sorting of residual βII- and βIII-spectrin and ankyrin G in epithelial cells of the kidney and lung, away from the basolateral membrane to an apical compartment; (6) abnormal cellularization of the ventricular zone of the developing brain, with impaired axon formation; and (7) impaired spreading and lamellipodia formation, and reduced proliferation, in MEF cells cultured from αII-spectrin-deficient animals.
The phenotype observed in these animals is very similar, although not an exact phenocopy, to that observed in mice deficient in βII-spectrin (termed ELF in those studies) (Mishra et al., 1999; Tang et al., 2003; Golestaneh et al., 2006). The loss of βII-spectrin appears to disrupt growth and differentiation control via the TGF-β and Smad signaling pathways, and in this context βII-spectrin acts as a Smad3/4 adapter tethering these proteins to TFG-β receptors at the membrane (Tang et al., 2003). Given that the loss of αII-spectrin leads to a secondary loss of most βII- (and βIII)-spectrin, it is tempting to attribute the phenotype of the αII-spectrin null animals to a disruption of βII-spectrin. However, several features of the Spna2−/− animals indicate that their phenotype is subtly different.
The loss of TGF-β signaling in βII-spectrin null animals leads to not only cardiac and neurological defects, but also to intrahepatic biliary tree anomalies and the late onset of gastrointestinal and hepatic tumors in heterozygotes (Tang et al., 2005). The αII-spectrin mutant animals display no hepatic anomalies, at least during early embryonic development, and after 2 years the heterozygotes show no propensity to tumor formation. Similarly, both the αII- and βII-spectrin null animals display disrupted neuroepithelial cell compaction in the ventricular zone (Golestaneh et al., 2006) and both display an exaggerated proliferation of neuroepithelial precursors, suggesting that αII-spectrin-deficient animals might also harbor a defect in TGF-β-mediated cell differentiation. Although this result might be expected based on the reduced levels of βII-spectrin that follow the loss of αII-spectrin, we have so far been unable to demonstrate any changes in Smad2/3 levels or in their levels of phosphorylation in αII-spectrin-deficient animals or cells (supplementary material Fig. S3). Thus, possibly only low levels of residual βII-spectrin are sufficient to maintain TGF-β–Smad signaling pathways. This result would imply that βII-spectrin can function autonomously from αII-spectrin. There is some evidence for autonomous β-spectrin function, beginning with early observations of homopolymeric β-spectrin associated with the neuromuscular junction (Bloch and Morrow, 1989; Pumplin, 1995), and further supported by studies in Drosophila that have revealed persistent β-spectrin function in axonal pathfinding that is partially independent of α-spectrin (Garbe et al., 2007; Hulsmeier et al., 2007).
Alternatively, other changes more specific for αII-spectrin might be the genesis of the observed changes in the heart and brain. In many respects, the αII-spectrin null animals appear similar to those with deletion of n-cofilin (Bellenchi et al., 2007), which causes impaired radial migration and early compaction of the VZ due to an early exit of progenitor cells from the proliferating pool. Impaired spreading (and impaired lamellipodia formation) is a major consequence of αII-spectrin deficiency in the isolated cell culture studies reported here, as well as in earlier αII-spectrin knockdown studies (Metral et al., 2009). αII-spectrin was also found to interact via its SH3 domain with EVL/VASP, an actin regulator (Bournier et al., 2006). The importance of actin dynamics as a regulator of radial migration from the VZ is also supported by the observation that mutations in filamin A (another actin-binding protein) profoundly affect neuroepithelial polarity and migration from the VZ (Sato and Nagano, 2005). Thus, we speculate that the subtle phenotypic differences observed between the deletion of αII-spectrin and βII-spectrin reflects in part, a more widespread disruption of actin dynamics, with perhaps less contributions from impaired TGF-β–Smad signaling.
The αβ-spectrin heterotetramer also has other putative functions beyond actin binding that might contribute to the phenotype of its deficiency. The SH3 domain of αII-spectrin is involved in macropinocytosis (Xu et al., 2000) and cell signaling; it binds both non-receptor tyrosine kinases of the c-src family (Nedrelow et al., 2003) and tyrosine phosphatases (Lecomte et al., 2001). The tyrosine phosphorylation of spectrin blocks its cleavage by calpain (Nicolas et al., 2002; Nedrelow et al., 2003), an effect also modulated by Ca2+ and calmodulin (Harris and Morrow, 1990). Spectrin participates in the activation of the Rho GTPase Rac, and overexpression of the αII-spectrin SH3 domain inhibits Rac1 activation, actin filament formation, and cell spreading in cultured cells (Bialkowska et al., 2005). Interestingly, actin and Rac1 have also been implicated in the regulation of neuronal polarity and axon formation via the WAVE complex (Tahirovic et al., 2010), and the loss of Rac1 in neural crest cells leads to severe craniofacial and cardiovascular malformations (Thomas et al., 2010). We thus anticipate that a major effector pathway disrupted in the Spna2−/− animals will be Rac-mediated actin regulation.
A third aspect of spectrin's function that might contribute to the observed phenotype is its participation as a membrane stabilizer. Studies in C. elegans (Hammarlund et al., 2007) and Drosophila (Garbe et al., 2007; Hulsmeier et al., 2007) demonstrate aberrant neurite development and axon guidance, and (in the case of C. elegans) frequent axonal breaks with chaotic repair in spectrin-deficient animals (Hammarlund et al., 2007). These observations build on the classical view of the spectrin skeleton as a stabilizing and organizing infrastructure. However, the evidence for this process in vertebrate non-erythroid cells is scant. Only in mammalian erythrocytes is the spectrin–ankyrin membrane skeleton continuous and homogeneous. In other cells, including neurons, wide variations exist within the cell in terms of the membrane organization of spectrin or ankyrin, both in terms of its uniformity and composition, and in whether spectrin and/or ankyrin are even associated with the plasma membrane or localized on internal compartments (De Matteis and Morrow, 2000). When spectrin is concentrated at the membrane, it is most often at sites of specialized receptor function such as pre- and post-synaptic densities, rather than at sites that one would anticipate are subjected to high mechanical stress. However, recent data suggesting that spectrin might function as a mechanochemical signal transducer (Stabach et al., 2009) raises a speculative but interesting possibility that could reconcile the apparent fragility of spectrin-deficient axons (as observed in C. elegans) with the emerging role of spectrin as a major receptor-sorting and organizing scaffold. If stretch or bending of axons is a tropic stimulus for neuronal extension and maintenance (Van Essen, 1997), then the absence of such stimulus might activate pathways to prune unwanted axons and dendrites (Luo and O'Leary, 2005). If indeed a spectrin lattice is part of a mechanosensing pathway by which tension, stretch or bending is transduced to trophic signals, the absence of spectrin might leave neurons poised for degradation and pruning not by a mechanical instability, but rather because of an absence of maintenance signals.
Finally, it is worthwhile to consider the contributions of another major function of the spectrin–ankyrin skeleton to the pathology of the Spna2−/− animals. Based on studies in cultured cells, gene deletion studies, and linkage analysis of human diseases, it is well established that a key function of the spectrin–ankyrin skeleton is to facilitate the movement of selected membrane proteins through the secretory and endocytic pathways to points of physiologic action (reviewed in De Matteis and Morrow, 2000; Bennett and Healy, 2008). Thus, patients with mutations in ankyrin B mis-sort voltage-gated sodium channels in heart muscle suffer from long QT syndrome type 4 and sudden cardiac death. Many other proteins also depend on ankyrin for efficient sorting, including the IP3 receptor (Tuvia et al., 1999) and αI-Na,K-ATPase (Stabach et al., 2008). In lymphocytes, the trafficking of receptors CD3 and CD45 are both spectrin- and ankyrin-dependent (Pradhan and Morrow, 2002). Mutations in βIII-spectrin cause spinocerebellar ataxia type 5 in humans (Ikeda et al., 2006) (and ataxia and seizures in mice) (Perkins et al., 2010; Stankewich et al., 2010) due to impaired intracellular transport of EAAT4 and at least five other proteins associated with the synapse (Clarkson et al., 2010; Stankewich et al., 2010). Thus, given that the Spna2−/− mice are deficient in and mis-localize their ankyrins and βII- and βIII-spectrin, it is likely that in these animals there are also problems with membrane protein organization and sorting. In future studies it will be important to examine this question in detail, both in vivo and in cultured cells, to identify the full repertoire of proteins that depend on spectrin and ankyrin for their efficient sorting to the correct membrane compartment.
Materials and Methods
Generation of C57BL/6 (Spna2−/−) mice
The embryonic stem (ES) cell line RRQ171 (BayGenomics, http://www.genetrap.org) was generated by gene-trap protocol with pGTOLxf, containing a splice-acceptor sequence subcloned 5′ end of a β-geo reporter cassette encoding a β-gal–neomycin resistance fusion protein. ES cells heterozygous for the targeted mutation were microinjected into C57BL/6 blastocysts and implanted into pseudo-pregnant foster mothers. Male chimeras were mated with C57BL/6 females. Ten backcrosses established the gene-trapped allele on a C57BL/6 background. Mice were genotyped by quantitative RT-PCR using primers bridging the β-geo insertion and wild-type Spna2 sequence. The PCR primer sets used for detection of the exon trap were: 5′-CAAATGGCGATTACCGTTGA-3′ and 3′-GACAGTATCGGCCTCAGGAAGATCG-5′. Spectrin primers used were exon 6 sense; exon 7 reverse; exon 54 sense and exon 55 reverse. All animal experiments were performed according to the relevant regulatory standards.
Analysis of mRNA and protein expression
Embryos were harvested from pregnant heterozygous mice typically at E13.5 to E14.5. The levels of αII, βII, βIII, βIV, and βV–spectrin mRNA expression were measured by real-time PCR, using primers summarized in supplementary material Table S1. For western blot analysis, embryos were homogenized in 3.0 ml extract buffer (20 mM HEPES pH 7.4, 120 mM NaCl, 25 mM KCl, 2 mM EDTA, 1 mM EGTA, 1% Triton X-100) supplemented with Protease Arrest (Calbiochem) (1:200). Supernatants were cleared at 28,000 g for 10 minutes at 4°C, analyzed by SDS-PAGE (NUPAGE Gel System, Invitrogen). Antibodies were mouse monoclonal antibodies to αII-spectrin (Chemicon-clone 1622, and Santa Cruz Biotechnology clone C-11), βII-spectrin (Pharmingen), β-actin clone AC-74 (Sigma), and β-galactosidase (Developmental Studies Hybridoma Bank). Rabbit polyclonals were: αII-spectrin (Raf-A) and αI/βI-spectrin (Ras-C) (Harris et al., 1985), ankyrin R (Cianci et al., 1988), βIII-spectrin (Stankewich et al., 1998), GFP (Clontech), βIV-spectrin (a gift from Michele Solimena, Technical University, Dresden, Germany). Other antibodies included anti-ankyrins B and G, rabbit anti-VASP (sc-13975); mouse anti-pan14-3-3 (sc-1657); rabbit anti-Abi (sc-30038), all from Santa Cruz Biotechnology. Transduction Labs provided mouse anti-KAP3 (K55520) and Cell Signaling provided mouse anti-Tau (4019). All antibodies were used at 1:1000 dilutions for western blot analysis except that actin Mab was used at 1:20,000. For western blot analysis, protein loadings were calculated so as to equalize the total protein load for each sample. This generally gave comparable levels of actin loading, which served as an internal marker. Each lane of each western blot represents a replicate sample from a different embryo.
Gradient sedimentation
Whole fresh E11.5 embryos were homogenized at 4°C in 1 ml of homogenization buffer (20 mM HEPES pH 7.4, 120 mM NaCl, 25 mM KCl, 2 mM EDTA, 1 mM EGTA, 0.5% Triton X-100) supplemented with Protease Arrest (Calbiochem). After centrifugation (1000 g for 10 minutes) to remove tissue debris, homogenates were applied to a 5–20%, 13.5 ml continuous sucrose gradient and spun in a SW-40 rotor (Beckman) at 40,000 rpm for 22.5 hours. Aliquots of 0.5 ml were decanted from the top of the gradient mechanically using a gradient fractionator (Labconco). The refractive index, which correlates with density, of all fractions was analyzed with a refractometer.
Histology and immunolabeling
Immunoperoxidase staining of whole-mount embryos in paraffin sections was carried out as before (Stankewich et al., 2006). Primary antibodies were used at a 1:500 dilution. For immunofluorescence, after the application of the primary antibody, sections were incubated with either goat anti-mouse or anti-rabbit secondary antibodies conjugated to Alexa Fluor dyes (Invitrogen) diluted 1:1000 for 1 hour at room temperature. To stain nuclei, slides were incubated in Hoechst dye 33342 for 10 minutes. For visualization by alkaline phosphatase, the EnVision G|2 reagent kit (Dako) was used in combination with Ferangi Blue (Biocare Medical). Tissues were counterstained with Nuclear Fast Red (PolyScientific). Slides were viewed by bright-field or fluorescent microscopy using an Olympus AX70 microscope; image acquisition was processed using OpenLab software (Improvision, Lexington, MA).
Analysis of primary mouse skin embryonic fibroblasts
Wild-type and Spna2−/− MEFs were harvested at E14.5 from embryos of heterozygous crosses, and established in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). All experiments were duplicated in at least three independently established lines at passage numbers 3–9. All cell lines were individually genotyped. For cell growth assays, 2×104 MEF cells were plated into each well of a 24-well Falcon tissue culture plate. Cells were grown at 37°C; at each time point cells were suspended in 0.05% trypsin–EDTA and counted on an Improved Neubauer hemocytometer (each data point represents an average of six samples). To test TGF-β1 stimulation, NIH3T3 cells and MEF cells were grown and maintained in DMEM with 10% FBS (complete DMEM). Cells were plated at 70–80% confluence in complete DMEM. After 24 hours, culture media was exchanged with DMEM supplemented with only 1% FBS for 6 hours, then mouse TGF-β1 (Cell Signaling 5231) was added at 0, 2.5 or 10 ng/ml for 24 and 48 hours. Cells were subsequently solubilized in gel loading buffer and proteins analyzed by western blot analysis. Antibodies used were rabbit anti-Smad2/3 and phospho-Smad2 (S465/467) (Cell Signaling s3102 and138D4, respectively); mouse monoclonal anti-Smad3 (Santa Cruz Biotechnology sc-101154); mouse monoclonal anti-Smad4 (Santa Cruz Biotechnology sc-7966); and anti-Smad7, a gift from Mark Kidd (Yale University, New Haven, CT).
Cell spreading
MEFs were plated onto cover slips coated with 10 μg/ml fibronectin (Santa Cruz sc-29011). After 1–3 hours, cells were fixed with 3.0% formaldehyde and stained with Alexa Fluor 488–phallodin (Invitrogen). Images were captured and processed as above. From each analysis, 500 cells were randomly selected for evaluation. The relative area of the spread cells in both control and Spna2−/− MEFs was measured using the Photoshop CS magic wand tool to score pixel number within their well-defined cell borders. For recovery experiments, MEFs were transiently transfected with Lipofectamine 2000 mixed with plasmid encoding fluorescently tagged full-length αII-spectrin according to the manufacturer's instructions (Invitrogen). The pEYFP–αII-spectrin was prepared by subcloning a complementary DNA (cDNA)-encoding full-length human αII-spectrin (GenBank U83867) into the pEYFP-C1 vector (Clontech), and was then shuttled into the pPRIPu vector (Street et al., 2006).
Supplementary Material
Acknowledgements
We thank Michele Solimena for the βIV-spectrin antibody.
Footnotes
Funding
This study was supported in part by grants from the National Institutes of Health [grant numbers R01-HL28560 and R01-DK DK43812] to J.S.M. and by a grant from the National Ataxia Foundation to M.C.S. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.080374/-/DC1
References
- Bellenchi G. C., Gurniak C. B., Perlas E., Middei S., Ammassari-Teule M., Witke W. (2007). N-cofilin is associated with neuronal migration disorders and cell cycle control in the cerebral cortex. Genes. Dev. 21, 2347-2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V., Baines A. J. (2001). Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 81, 1353-1392 [DOI] [PubMed] [Google Scholar]
- Bennett V., Healy J. (2008). Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol. Med. 14, 28-36 [DOI] [PubMed] [Google Scholar]
- Bialkowska K., Saido T. C., Fox J. E. (2005). SH3 domain of spectrin participates in the activation of Rac in specialized calpain-induced integrin signaling complexes. J. Cell Sci. 118, 381-395 [DOI] [PubMed] [Google Scholar]
- Bloch R. J., Morrow J. S. (1989). An unusual β-spectrin associated with clustered acetylcholine receptors. J. Cell Biol. 108, 481-493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bournier O., Kroviarski Y., Rotter B., Nicolas G., Lecomte M. C., Dhermy D. (2006). Spectrin interacts with EVL (Enabled/vasodilator-stimulated phosphoprotein-like protein), a protein involved in actin polymerization. Biol. Cell. 98, 279-293 [DOI] [PubMed] [Google Scholar]
- Chan W., Kordeli E., Bennett V. (1993). 440-kD ankyrinB: structure of the major developmentally regulated domain and selective localization in unmyelinated axons. J. Cell Biol. 123, 1463-1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianci C. D., Giorgi M., Morrow J. S. (1988). Phosphorylation of ankyrin down-regulates its cooperative interaction with spectrin and protein 3. J. Cell Biochem. 37, 301-315 [DOI] [PubMed] [Google Scholar]
- Clarkson Y. L., Gillespie T., Perkins E. M., Lyndon A. R., Jackson M. (2010). {beta}-III spectrin mutation L253P associated with spinocerebellar ataxia type 5 interferes with binding to Arp1 and protein trafficking from the Golgi. Hum. Mol. Genet. 19, 3634-3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis M. A., Morrow J. S. (2000). Spectrin tethers and mesh in the biosynthetic pathway. J. Cell Sci. 113, 2331-2343 [DOI] [PubMed] [Google Scholar]
- Dubreuil R. R. (2006). Functional links between membrane transport and the spectrin cytoskeleton. J. Membr. Biol. 211, 151-161 [DOI] [PubMed] [Google Scholar]
- Eigenthaler M., Engelhardt S., Schinke B., Kobsar A., Schmitteckert E., Gambaryan S., Engelhardt C. M., Krenn V., Eliava M., Jarchau T., et al. (2003). Disruption of cardiac Ena-VASP protein localization in intercalated disks causes dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 285, H2471-H2481 [DOI] [PubMed] [Google Scholar]
- Gallagher P. G., Jarolim P. (2000). red cell membrane disorders. In Hematology: Basic Principles and Practice, 5th edn (eds Hoffman R., Benz E. J., Jr, Shattil S. J., Furie B., Cohen H. J., Silberstein L. E., McGlave P.), pp. 576-610 New York: Churchill Livingstone; [Google Scholar]
- Garbe D. S., Das A., Dubreuil R. R., Bashaw G. J. (2007). beta-Spectrin functions independently of Ankyrin to regulate the establishment and maintenance of axon connections in the Drosophila embryonic CNS. Development 134, 273-284 [DOI] [PubMed] [Google Scholar]
- Golestaneh N., Tang Y., Katuri V., Jogunoori W., Mishra L., Mishra B. (2006). Cell cycle deregulation and loss of stem cell phenotype in the subventricular zone of TGF-beta adaptor elf−/− mouse brain. Brain Res. 1108, 45-53 [DOI] [PubMed] [Google Scholar]
- Gotz M., Huttner W. B. (2005). The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6, 777-788 [DOI] [PubMed] [Google Scholar]
- Hammarlund M., Jorgensen E. M., Bastiani M. J. (2007). Axons break in animals lacking beta-spectrin. J. Cell Biol. 176, 269-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanspal M., Palek J. (1987). Synthesis and assembly of membrane skeletal proteins in mammalian red cell precursors. J. Cell Biol. 105, 1417-1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. S., Morrow J. S. (1990). Calmodulin and calcium-dependent protease I coordinately regulate the interaction of fodrin with actin. Proc. Natl. Acad. Sci USA 87, 3009-3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. S., Green L. A., Ainger K. J., Morrow J. S. (1985). Mechanism of cytoskeletal regulation (I): functional differences correlate with antigenic dissimilarity in human brain and erythrocyte spectrin. Biochim. Biophys. Acta 830, 147-158 [DOI] [PubMed] [Google Scholar]
- Holleran E. A., Ligon L. A., Tokito M., Stankewich M. C., Morrow J. S., Holzbaur E. L. (2001). beta III spectrin binds to the Arp1 subunit of dynactin. J. Biol. Chem. 276, 36598-36605 [DOI] [PubMed] [Google Scholar]
- Hulsmeier J., Pielage J., Rickert C., Technau G. M., Klambt C., Stork T. (2007). Distinct functions of alpha-Spectrin and beta-Spectrin during axonal pathfinding. Development 134, 713-722 [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Dick K. A., Weatherspoon M. R., Gincel D., Armbrust K. R., Dalton J. C., Stevanin G., Durr A., Zuhlke C., Burk K., et al. (2006). Spectrin mutations cause spinocerebellar ataxia type 5. Nat. Genet. 38, 184-190 [DOI] [PubMed] [Google Scholar]
- Kizhatil K., Yoon W., Mohler P. J., Davis L. H., Hoffman J. A., Bennett V. (2007). Ankyrin-G and beta2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. J. Biol. Chem. 282, 2029-2037 [DOI] [PubMed] [Google Scholar]
- Kordeli E., Lambert S., Bennett V. (1995). AnkyrinG. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J. Biol. Chem. 270, 2352-2359 [DOI] [PubMed] [Google Scholar]
- Lecomte M. C., Nicolas G., Fournier C., Galand C., Malbert-Colas L., Bournier O., Dhermy D., Grandchamps B. (2001). Interaction of spectrin with the low molecular weight phosphotyrosine phosphatese (LMW-PTP). Cell Mol. Biol. Lett. 6, 216 [PubMed] [Google Scholar]
- Li D., Tang H. Y., Speicher D. W. (2008). A structural model of the erythrocyte spectrin heterodimer initiation site determined using homology modeling and chemical cross-linking. J. Biol. Chem. 283, 1553-1562 [DOI] [PubMed] [Google Scholar]
- Luo L., O'Leary D. D. (2005). Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 28, 127-156 [DOI] [PubMed] [Google Scholar]
- Mangeat P. H., Burridge K. (1984). Immunoprecipitation of nonerythrocyte spectrin within live cells following microinjection of specific antibodies: relation to cytoskeletal structures. J. Cell Biol. 98, 1363-1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon L. W., Walsh C. E., Lambert M. W. (1999). Human alpha spectrin II and the fanconi anemia proteins FANCA and FANCC interact to form a nuclear complex. J. Biol. Chem. 274, 32904-32908 [DOI] [PubMed] [Google Scholar]
- Metral S., Machnicka B., Bigot S., Colin Y., Dhermy D., Lecomte M. C. (2009). AlphaII-spectrin is critical for cell adhesion and cell cycle. J. Biol. Chem. 284, 2409-2418 [DOI] [PubMed] [Google Scholar]
- Mishra L., Cai T., Yu P., Monga S. P., Mishra B. (1999). Elf3 encodes a novel 200-kD beta-spectrin: role in liver development. Oncogene 18, 353-364 [DOI] [PubMed] [Google Scholar]
- Moorthy S., Chen L., Bennett V. (2000). Caenorhabditis elegans beta-G spectrin is dispensable for establishment of epithelial polarity, but essential for muscular and neuronal function. J. Cell Biol. 149, 915-930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muresan V., Stankewich M. C., Steffen W., Morrow J. S., Holzbaur E. L., Schnapp B. J. (2001). Dynactin-dependent, dynein-driven vesicle transport in the absence of membrane proteins: a role for spectrin and acidic phospholipids. Mol. Cell 7, 173-183 [DOI] [PubMed] [Google Scholar]
- Nedrelow J. H., Cianci C. D., Morrow J. S. (2003). c-Src binds alpha II spectrin's Src homology 3 (SH3) domain and blocks calpain susceptibility by phosphorylating Tyr1176. J. Biol. Chem. 278, 7735-7741 [DOI] [PubMed] [Google Scholar]
- Nicolas G., Fournier C. M., Galand C., Malbert-Colas L., Bournier O., Kroviarski Y., Bourgeois M., Camonis J. H., Dhermy D., Grandchamp B., et al. (2002). Tyrosine phosphorylation regulates alpha II spectrin cleavage by calpain. Mol. Cell Biol. 22, 3527-3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins E. M., Clarkson Y. L., Sabatier N., Longhurst D. M., Millward C. P., Jack J., Toraiwa J., Watanabe M., Rothstein J. D., Lyndon A. R., et al. (2010). Loss of beta-III spectrin leads to Purkinje cell dysfunction recapitulating the behavior and neuropathology of spinocerebellar ataxia type 5 in humans. J. Neurosci. 30, 4857-4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan D., Morrow J. (2002). The spectrin-ankyrin skeleton controls CD45 surface display and interleukin-2 production. Immunity 17, 303-315 [DOI] [PubMed] [Google Scholar]
- Pumplin D. W. (1995). The membrane skeleton of acetylcholine receptor domains in rat myotubes contains antiparallel homodimers of β-spectrin in filaments quantitatively resembling those of erythrocytes. J. Cell Sci. 108, 3145-3154 [DOI] [PubMed] [Google Scholar]
- Qi X., Yang G., Yang L., Lan Y., Weng T., Wang J., Wu Z., Xu J., Gao X., Yang X. (2007). Essential role of Smad4 in maintaining cardiomyocyte proliferation during murine embryonic heart development. Dev. Biol. 311, 136-146 [DOI] [PubMed] [Google Scholar]
- Ramser E. M., Buck F., Schachner M., Tilling T. (2010). Binding of alphaII spectrin to 14-3-3beta is involved in NCAM-dependent neurite outgrowth. Mol. Cell Neurosci. 45, 66-74 [DOI] [PubMed] [Google Scholar]
- Sato M., Nagano T. (2005). Involvement of filamin A and filamin A-interacting protein (FILIP) in controlling the start and cell shape of radially migrating cortical neurons. Anat. Sci. Int. 80, 19-29 [DOI] [PubMed] [Google Scholar]
- Stabach P. R., Devarajan P., Stankewich M. C., Bannykh S., Morrow J. S. (2008). Ankyrin facilitates intracellular trafficking of alpha1-Na+-K+-ATPase in polarized cells. Am. J. Physiol. Cell Physiol. 295, C1202-C1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabach P. R., Simonovic I., Ranieri M. A., Aboodi M. S., Steitz T. A., Simonovic M., Morrow J. S. (2009). The structure of the ankyrin-binding site of beta-spectrin reveals how tandem spectrin-repeats generate unique ligand-binding properties. Blood 113, 5377-5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankewich M. C., Stabach P. R., Morrow J. S. (2006). Human Sec31B: a family of new mammalian orthologues of yeast Sec31p that associate with the COPII coat. J. Cell Sci. 119, 958-969 [DOI] [PubMed] [Google Scholar]
- Stankewich M. C., Gwynn B., Ardito T., Ji L., Kim J., Robledo R. F., Lux S. E., Peters L. L., Morrow J. S. (2010). Targeted deletion of betaIII spectrin impairs synaptogenesis and generates ataxic and seizure phenotypes. Proc. Natl. Acad. Sci. USA 107, 6022-6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankewich M. C., Tse W. T., Peters L. L., Ch'ng Y., John K. M., Stabach P. R., Devarajan P., Morrow J. S., Lux S. E. (1998). A widely expressed betaIII spectrin associated with Golgi and cytoplasmic vesicles. Proc. Natl. Acad. Sci. USA 95, 14158-14163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street M., Marsh S. J., Stabach P. R., Morrow J. S., Brown D. A., Buckley N. J. (2006). Stimulation of Galphaq-coupled M1 muscarinic receptor causes reversible spectrin redistribution mediated by PLC, PKC and ROCK. J. Cell Sci. 119, 1528-1536 [DOI] [PubMed] [Google Scholar]
- Tahirovic S., Hellal F., Neukirchen D., Hindges R., Garvalov B. K., Flynn K. C., Stradal T. E., Chrostek-Grashoff A., Brakebusch C., Bradke F. (2010). Rac1 regulates neuronal polarization through the WAVE complex. J. Neurosci. 30, 6930-6943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Yamazaki H., Seog D. H., Kanai Y., Terada S., Hirokawa N. (2000). Kinesin superfamily protein 3 (KIF3) motor transports fodrin-associating vesicles important for neurite building. J. Cell Biol. 148, 1255-1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Katuri V., Iqbal S., Narayan T., Wang Z., Lu R. S., Mishra L., Mishra B. (2002). ELF a beta-spectrin is a neuronal precursor cell marker in developing mammalian brain; structure and organization of the elf/beta-G spectrin gene. Oncogene 21, 5255-5267 [DOI] [PubMed] [Google Scholar]
- Tang Y., Katuri V., Dillner A., Mishra B., Deng C. X., Mishra L. (2003). Disruption of transforming growth factor-beta signaling in ELF beta-spectrin-deficient mice. Science 299, 574-577 [DOI] [PubMed] [Google Scholar]
- Tang Y., Katuri V., Srinivasan R., Fogt F., Redman R., Anand G., Said A., Fishbein T., Zasloff M., Reddy E. P., et al. (2005). Transforming growth factor-beta suppresses nonmetastatic colon cancer through Smad4 and adaptor protein ELF at an early stage of tumorigenesis. Cancer Res. 65, 4228-4237 [DOI] [PubMed] [Google Scholar]
- Thomas P. S., Kim J., Nunez S., Glogauer M., Kaartinen V. (2010). Neural crest cell-specific deletion of Rac1 results in defective cell-matrix interactions and severe craniofacial and cardiovascular malformations. Dev. Biol. 340, 613-625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse W. T., Tang J., Jin O., Korsgren C., John K. M., Kung A. L., Gwynn B., Peters L. L., Lux S. E. (2001). A new spectrin, beta IV, has a major truncated isoform that associates with promyelocytic leukemia protein nuclear bodies and the nuclear matrix. J. Biol. Chem. 276, 23974-23985 [DOI] [PubMed] [Google Scholar]
- Tuvia S., Buhusi M., Davis L., Reedy M., Bennett V. (1999). Ankyrin-B is required for intracellular sorting of structurally diverse Ca2+ homeostasis proteins. J. Cell Biol. 147, 995-1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D. C. (1997). A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385, 313-318 [DOI] [PubMed] [Google Scholar]
- Woods C. M., Lazarides E. (1985). Degradation of unassembled alpha- and beta-spectrin by distinct intracellular pathways: regulation of spectrin topogenesis by beta-spectrin degradation. Cell 40, 959-969 [DOI] [PubMed] [Google Scholar]
- Xu J., Ziemnicka D., Merz G. S., Kotula L. (2000). Human spectrin Src homology 3 domain binding protein 1 regulates macropinocytosis in NIH 3T3 cells. J. Cell Sci. 113 Pt 21, 3805-3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Resneck W. G., Lee P. C., Randall W. R., Bloch R. J., Ursitti J. A. (2010). Characterization and expression of a heart-selective alternatively spliced variant of alpha II-spectrin, cardi+, during development in the rat. J. Mol. Cell Cardiol. 48, 1050-1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemnicka-Kotula D., Xu J., Gu H., Potempska A., Kim K. S., Jenkins E. C., Trenkner E., Kotula L. (1998). Identification of a candidate human spectrin Src homology 3 domain-binding protein suggests a general mechanism of association of tyrosine kinases with the spectrin-based membrane skeleton. J. Biol. Chem. 273, 13681-13692 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.