Abstract
Background
Immunocompromised patients are vulnerable to severe or complicated influenza infection. Vaccination is widely recommended for this group. This systematic review and meta-analysis assesses influenza vaccination for immunocompromised patients in terms of preventing influenza-like illness and laboratory confirmed influenza, serological response and adverse events.
Methodology/Principal Findings
Electronic databases and grey literature were searched and records were screened against eligibility criteria. Data extraction and risk of bias assessments were performed in duplicate. Results were synthesised narratively and meta-analyses were conducted where feasible. Heterogeneity was assessed using I2 and publication bias was assessed using Begg's funnel plot and Egger's regression test. Many of the 209 eligible studies included an unclear or high risk of bias. Meta-analyses showed a significant effect of preventing influenza-like illness (odds ratio [OR] = 0.23; 95% confidence interval [CI] = 0.16–0.34; p<0.001) and laboratory confirmed influenza infection (OR = 0.15; 95% CI = 0.03–0.63; p = 0.01) through vaccinating immunocompromised patie nts compared to placebo or unvaccinated controls. We found no difference in the odds of influenza-like illness compared to vaccinated immunocompetent controls. The pooled odds of seroconversion were lower in vaccinated patients compared to immunocompetent controls for seasonal influenza A(H1N1), A(H3N2) and B. A similar trend was identified for seroprotection. Meta-analyses of seroconversion showed higher odds in vaccinated patients compared to placebo or unvaccinated controls, although this reached significance for influenza B only. Publication bias was not detected and narrative synthesis supported our findings. No consistent evidence of safety concerns was identified.
Conclusions/Significance
Infection prevention and control strategies should recommend vaccinating immunocompromised patients. Potential for bias and confounding and the presence of heterogeneity mean the evidence reviewed is generally weak, although the directions of effects are consistent. Areas for further research are identified.
Introduction
Respiratory disease is a leading cause of global mortality to which seasonal and pandemic influenza both make substantial contributions. For example, in the USA an estimated average 225,000 hospitalisations and 36,000 deaths per annum are attributable to seasonal influenza [1], [2]. Even the ‘mild’ 2009 influenza A(H1N1) pandemic was associated with substantial years of life lost due to mortality in younger age groups [3].
Patients with sub-optimal immune function due to disease or therapy (the immunocompromised) are recognised to be at increased risk from influenza-related complications, and are recommended for annual vaccination in many national vaccination guidelines. Concerns about influenza within immunocompromised populations include an impaired respo nse to vaccination and higher risk of complicated infection with increased mortality [4], greater and prolonged virus shedding with implications for control of transmission [5]–[8], the emergence of resistance to antiviral agents [9] and possible adverse effects of vaccination. The balance between potential benefit and harm resulting from vaccinating these groups has been hard to establish, with previous reviews finding few studies offering incontrovertible evidence of clinical protection [10]–[13]. There is uncertainty around thresholds for defining immunocompromise and the exte nt to which underlying aetiologies vary in their susceptibility to influenza and potentially their response to vaccine, with deference to clinical opinion in many cases [14]. A high burden of illness was recognised in immunocompromised patients during the 2009 influenza A(H1N1) pandemic, along with substantial nosocomial disease, proclaiming the need to re-visit the evidence base for influenza vaccination in these patients [8], [15]–[21].
We conducted a systematic review and meta-analysis to assess influenza vaccination for immunocompromised patients. We report the primary analysis and its interpretation from a public health policy perspective, to assess the overall evidence. A second manuscript will be submitted for publication which reports a secondary analysis of our data, stratified by aetiology of immunocompromise.
Methods
An abbreviated study protocol is available from the National Institute for Health Research international prospective register of systematic reviews (PROSPERO) [22], and the full protocol and PRISMA checklist are available as supporting information (see Protocol S1 and Checklist S1). Minor amendments to the original protocol were conducted to clarify the search strategy and eligibility criteria.
The study population of interest comprised all persons immunocompromised due to primary immunodeficiency (genetic defects) or secondary immunodeficiency (such as HIV infection, malignancy, or receipt of immunosuppressive drugs). Immunocompromised populations were derived from World Health Organization (WHO) and United Kingdom (UK) Department of Health immunisation policy to prevent influenza infection [14], [23]. We additionally included malnutrition and tuberculosis as conditions commonly associated with immunocompromise in developing countries. Interventions of interest comprised vaccination against seasonal influenza or 2009 influenza A(H1N1) pandemic; restricted to experimental designs for seasonal influenza but with no limitation for pandemic studies where experimental approaches would have been ethically unfeasible in most circumstances. Comparative groups included vaccinated immunocompetent controls (VICT) and immunocompromised patients given placebo or no vaccination (PNV). Outcome measures corresponded to four research questions relevant to this review: prevention of clinically diagnosed influenza or influenza-like illness (ILI) and laboratory confirmed influenza infection, serological response, and adverse events associated with vaccination. Criteria for inclusion and exclusion of studies, established in advance of executing the search str ategy, are presented in Table 1 and information sources searched to identify relevant literature are shown in Table 2.
Table 1. Study eligibility criteria.
| Inclusion criteria |
| Experimental studies or systematic reviews (± meta-analyses) reporting data on the efficacy, effectiveness, immunological response or adverse effects associated with influenza vaccination of immunocompromised patients to prevent infection from seasonal influenza or 2009 influenza A(H1N1) pandemic strain |
| Observational studies published during 2009 and 2010 reporting data on the efficacy, effectiveness, immunological response or adverse effects associated with influenza vaccination of immunocompromised patients to prevent infection from 2009 influenza A(H1N1) pandemic strain |
| Studies which recruited individuals of any age from any setting who are immunocompromised whether due to primary immunodeficiency (genetic defects) or secondary immunodeficiency (such as HIV infection, malignancy, poor nutritional status or use of immunosuppressive drugs) |
| No restriction is placed on the influenza vaccination dose, preparation, trade name, schedule or method of administration |
| Studies which report data from control or comparator treatments may include no vaccination, placebo vaccination or sham vaccination |
| Studies which have recruited immunocompromised patients and compare outcome measures with immunocompetent control study subjects |
| Studies which report data on at least one of the following outcome measures: rate of clinically diagnosed influenza or ILI/ITT patients, rate of laboratory confirmed influenza or ITTI patients, immunological response to vaccination, and adverse effects associated with vaccination |
| Full text manuscripts of studies which are publis hed in English, French, Spanish, Portuguese, Russian, or Japanese |
*Applied to respiratory and autoimmune conditions only; no specification of dosage or duration of therapy.
Table 2. Information sources.
| Category | Source |
| Databases | MEDLINE; EMBASE; CINAHL; Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; WHO Regional Indexes; J-STAGE (Japanese language); Banque de Données en Santé Publique (BDSP, French language); Index-F (Spanish language); eLIBRARY (Russian language) |
| Evidence-based reviews | Bandolier; Cochrane Library: Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), National Health Service Health Technology Assessment (NHS HTA) |
| Guidelines | NHS Evidence: NHS Clinical Knowledge Summaries, National Library of Guidelines |
| Grey literature | Web of Science; NHS Evidence; OpenSIGLE; influenza vaccine manufacturers: GlaxoSmithKline, Novaratis, Sanofi Pasteur MSD, Abbott, CSL Limited, Medimmune, Crucell, Baxter; European Vaccine Manufacturers (Brussels); International Federation of Pharmaceutical Manufacturers Associations (Geneva/Zurich); consultation with domain expert (Bram Palache, Abbott) |
| Hand searching of journals | Vaccine |
| Reference tracking | Reference lists of all included studies |
| Citation tracking | Web of Science (Science Citation Index); Google Scholar |
| Internet searching | www.google.com; www.dh.gov.uk; www.hpa.org.uk; www.who.int; www.cdc.gov; www.flu.gov |
Search strategy and study selection
Single reviewers conducted searches during January 2011, based on the term construct used for MEDLINE (see Table S1), which was subsequently adapted or translated for other information sources as appropriate. No date limit for publication was applied to studies of seasonal influenza whilst a limit of 2009–10 was applied to studies pertaining to the 2009 influenza A(H1N1) pandemic. Results were limited to human subjects and language of publication restricted to English, French, Japanese, Portuguese, Spanish and Russian.
After removal of duplicates a three-stage screening process applied the eligibility criteria to all records. Screening at title, abstract and full text was managed primarily within EndNote® ×4.0.2 (Thomson Reuters, California, USA). Records in non-compatible formats or non-English languages were manually screened. Screening was un dertaken by two reviewers in parallel, with consensus by discussion and provision for arbitration by a third reviewer. Due to insufficient resources, Spanish and Portuguese literature was screened by one reviewer.
Data collection
Data were extracted by two reviewers in parallel using a piloted template, with consensus by discussion and provision for arbitration by a third reviewer. No further data were sought from corresponding authors of eligible studies. Items extracted for study characteristics comprised country setting, objectives, design, sample size, methods of recruitment, inclusion and exclusion criteria, sequence generation, allocation, confounders and funding source. Population items comprised description of study groups, setting and stability of setting, age, sex, socioeconomic characteristics and risk factors for exposure to influenza. Intervention items comprised healthcare provider, setting in which health care delivered, description of intervention or exposure, vaccination type, route of administration, dosing schedule, and number of subjects allocated to and receiving the intervention or exposure. Outcome items comprised definition, measurement tool, timing and unit of measurements, blinding of assessors, duration of follow-up, number of measurements made (including withdrawals, exclusions and losses to follow-up), intervention and comparator results, detail of statistical analyses performed, and control for selection bias and confounding.
Extracted outcome data on immune response were classified according to Committee for Human Medicinal Products (CHMP) seroconversion and seroprotection criteria for each influenza subtype patients were vaccinated against [24]. Studies were excluded from meta-analysis if they did not provide data assessable against CHMP criteria or did not draw blood for serology at any time within 2–4 weeks post-vaccination. Geometric mean titre (GMT) and mean fold increase of haemagglutination inhibition (HI) levels pre- and post-vaccination were extracted. Adverse event data on local and systemic events were extracted according to CHMP criteria [24]. In addition, data on serious adverse events [25] and disease progression or clinical impact of immunocompromising condition were also extracted.
Risk of bias in individual studies
Risk of bias was assessed at both study and outcome level using tools produced by the Cochrane Collaboration [26] for experimental and prospective cohort designs, Downs and Black [27] for observational designs (excluding prospective cohort studies) and the US Agency for Healthcare Research Quality (AHRQ) [28] for systematic reviews. Assessments were undertaken in parallel by two reviewers reaching consensus by discussion, with provision for arbitration by a third reviewer. Abstract-only records were not subject to assessment of risk of bias due to paucity of information. Domain-based risk of bias was used to inform narrative synthesis, thus avoiding overall scores in accordance with recommendations [26], [29].
Summary measures
Descriptive statistics were calculated using Microsoft® Office Excel® 2007 version 12 (Microsoft Corporation, Richmond, USA). Where feasible, odds ratios including 95% confidence intervals and the standard error of the natural log odds ratio were calculated for input into meta-analyses.
Synthesis of results
Primary analysis was designed to synthesize appraisal of methodological quality and extracted study data by means of tabulation, narrative and meta-analysis (where appropriate). With the exception of serological outcome measures, data pertaining to the 2009 influenza A(H1N1) pandemic were pooled together with seasonal influenza data in accordance with the research aim to assess overall evidence. Meta-analysis of pooled odds ratios estimated the effect size of vaccinating immunocompromised patients versus immunocompetent controls (VICT), and of immunocompromised patients receiving vaccination versus those receiving placebo or no vaccination (PNV). Meta-analyses were conducted using Stata® version 10 (StatCorp LP, Texas, USA) initially using a random effects model. Analyses were re-executed using a fixed effects model where heterogeneity was low (I2<40%) and abandoned where heterogeneity was high (I2>85%). Statistical significanc e of pooled odds was assumed at the 5% level and assessed using the Χ 2 test. Risk of publication bias for studies subject to meta-analysis was assessed visually using Begg's funnel plots and quantified using Egger's regression test. Sub-analysis sought to utilise the UN inequality-adjusted Human Development Index 2010 (UN HDI) [30] for stratification of countries by quartile of human development to assess the strength of evidence in low resource environments.
Results
Study selection and characteristics
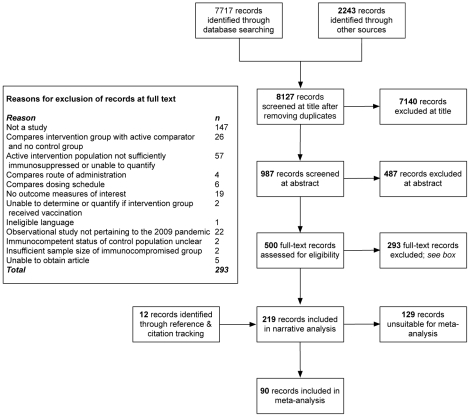
Figure 1 provides an account of the study selection process in the form of a PRISMA flow diagram [29]. The search strategy initially yielded 9,960 records (of which 1,833 were duplicates); 7,627 records were excluded as a result of screening at title and abstract stage. Reasons for exclusion of 293 records at full-text screening are shown in Figure 1. Five records were unobtainable at full text and therefore excluded [31]–[35]. Reference and citation tracking identified a further 12 eligible records, providing 219 records for narrative analysis (five in Russian, three Japanese and the remainder English). After exclusion of multiple reporting (n = 10) 209 individual studies met review eligibility criteria [11], [12], [36]–[252]; 16 pertained to vaccines against the 2009 influenza A(H1N1) pandemic virus [39], [43], [48], [55], [79], [86], [104], [170], [181], [188], [212], [223]–[225], [232], [243].
Figure 1. Summary of study selection process.
Characteristics of the eligible studies are summarised in Table 3. These data have not been presented for each individ ual study due to the volume of data extracted but are available on request. Of note is the large quantity of available data from non-randomised controlled trials (n = 137) and non-randomised clinical studies (n = 43) in addition to the limited data available from countries in medium or low categories of the UN HDI (n = 3). Sub-analysis by resource setting was therefore abandoned due to insufficient data. Immunocompromise due to human immunodeficiency syndrome (HIV), cancer and transplant were approximately equally represented with over 50 studies each, together accounting for more than three quarters (78%) of aetiological groupings.
Table 3. Summary of study characteristics (n = 209).
| Characteristic | Number of studies |
| Study design | |
| Systematic reviews ± meta-analyses | 3 |
| Randomised controlled trials | 23 |
| Non-randomised controlled trials | 137 |
| Non-randomised clinical studies | 43 |
| Prospective cohort studies | 1 |
| Case series | 2 |
| Setting of conduct | |
| Community or primary care | 5 |
| Outpatient department or hospital clinic | 127 |
| Other | 3 |
| Not stated | 74 |
| UN inequality-adjusted Human Development Index 2010 | |
| Very high | 186 |
| High | 16 |
| Medium | 3 |
| Low | 0 |
| No data | 4 |
| Study population (aetiology of immunocompromise) * | |
| Human immunodeficiency virus (HIV) infection | 58 |
| Cancers | 56 |
| Transpla nt recipients | 52 |
| Autoimmune diseases receiving immunosuppressive therapy | 34 |
| Respiratory diseases receiving immunosuppressive therapy | 5 |
| Other | 7 |
A median of sixty immunocompromised patients received active influenza vaccination across the 209 studies (interquartile range [IQR] 36 to 110). Studies typically administered the vaccine by intramuscular injection (n = 138) with a minority utilising intradermal (n = 20), subcutaneous (n = 2) and intranasal routes (n = 3). Forty-nine studies did not report these data (most likely intramuscular) and three studies used multiple routes of administration. The median intervention group size for included RCTs was 55 ( IQR 26 to 103) and the median placebo or no vaccination group size was 24 (IQR 17 to 56). A median of 65 vaccinated immunocompromised subjects were recruited (IQR 40 to 116) and sources of funding were declared by 114 studies.
Risk of bias within studies
Figure S1 summarises the assessment of risk of bias for 191 included experimental studies and prospective cohort designs. The majority of studies were judged at high risk of bias for sequence generation and allocation concealment domains. However, this finding is largely explained by only 23 RCTs meeting the protocol eligibility criteria. Only 10% of RCTs were at high risk of bias due to sequence generation or allocation concealment issues, although risk of bias was unclear in the majority (60% and 80% respectively). Risk of bias due to blinding of study participants, personnel and outcome assessors reduced from 22% in all studies to 5% in RCTs.
Table S2 summarises the assessment of risk of bias for two included case series [48], [232]. Bate et al (2010) scored highly within the reporting domain of the risk of bias tool whilst Vazquez-Alvarez et al (2010) scored poorly due to limited description of the study characteristics including potential confounding variables. Both studies scored poorly for external and internal validity.
Table S3 summarises the assessment of risk of bias for three included systematic reviews [11], [12], [42]. Risk of bias in all three studies was generally low, although Atashili et al (2006) did not assess quality and validity for included studies. Anema et at (2008 ) and Atashili et al (2006) conducted meta-analyses for a pooled estimate of the effectiveness of influenza vaccination in preventing ILI or laboratory confirmed infection in HIV patients, both including three prospective studies [190], [222], [247] while Atashili et al (2006) additionally included a case control study [253].
Synthesis of results
Outcomes for all 209 individual studies cannot be presented due to volume of data. We pooled studies for analyses according to review questions, irrespective of aetiology of immunocompromise (see discussion for comments on clinical heterogeneity). We identified 47 studies reporting data pertaining to the prevention of influenza-like illness, 16 on prevention of laboratory confirmed influenza, 189 on immune response to vaccination and 152 on adverse events or safety. Of these, we identified 6, 2, 12, and 11 studies respectively which reported outcomes pertaining to vaccines against the 2009 influenza A(H1N1) pandemic virus.
Influenza-like illness
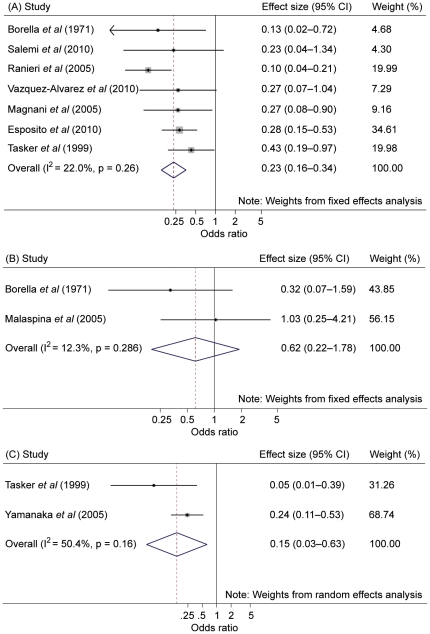
Meta-analysis pooled seven studies of ILI reported in vaccinated immunocompromised patients compared to PNV [59], [97], [159], [190], [202], [222], [232]. Figure 2 shows a pooled effect size of 0.23 (95% CI 0.16 to 0.34; p<0.001) with low statistical heterogeneity (I2 = 22.0%; p = not significant [NS]). Meta-analysis also pooled two studies of ILI reported in vaccinat ed immunocompromised patients compared to VICT [59], [160]. Figure 2 shows a pooled effect size of 0.62 (95% CI 0.22 to 1.78; p = NS) with low statistical heterogeneity (I2 = 12.3%; p = 0.286).
Figure 2. Forest plot for studies on influenza-like illness and laboratory confirmed influenza.
Legend: (A) = influenza-like illness (placebo or no vaccination comparator); (B) = influenza-like illness (vaccinated immunocompetent controls); (C) = laboratory confirmed influenza (placebo or no vaccination comparator). Note that each of the three plots shown has different scaled x-axes.
Two earlier meta-analyses considered vaccination impact on incidence of ILI in immunocompromised patients. Atashili et al (2006) pooled one RCT, two non-randomised studies and one case-control study of ILI or laboratory confirmed influenza compared to PNV, estimating a risk difference of −0.27 (95% CI −0.11 to −0.42; p = 0.004) but with significant heterogeneity (I2 = 76.8%; p = 0.003) [12]. To address methodological concerns Anema et al (2008) performed the same analysis excluding the case-control study, finding a risk ratio of 0.34 (95% CI 0.18 to 0.64; p = <0.001) again with significant heterogeneity (I2 = 73%; p = 0.02) [42].
Of those studies unsuitable for meta-analysis, we identified 22 interventional studies and one observational design where no cases of ILI were found in vaccinated immunocompromised patients (including two RCTs with a PNV comparator and 13 non-randomised studies with VICT controls). The remaining studies we identified typically showed a low incident case number of cases, with some noteworthy exceptions. Cumulative incidence of ILI in vaccinated renal transplant recipients immunosuppressed with azathioprine is reported as 5.4% and 8.3% for those on mycophenolate mofetil, compared to 8.1% in healthy controls [204]. The number of upper respiratory tract infections in vaccinated paediatr ic cancer patients completing therapy within six months of randomisation was 0.52±0.79 (mean ± standard deviation), compared to 2.73±1.49 in unvaccinated patients [97]. The inter-group difference reduced to 0.46±0.73 in patients off therapy for 6–24 months, compared to 0.69±0.73 in unvaccinated patients.
Laboratory confirmed influenza
Meta-analysis pooled two studies in vaccinated subjects with HIV compared to PNV [222], [247]. Figure 2 shows a pooled effect size of 0.15 (95% CI 0.03 to 0.63; p = 0.01) with moderate statistical heterogeneity (I2 = 50.4%; p = NS).
We found limited data from non-randomised studies showing very low numbers of incident cases of laboratory confirmed influenza post vaccination in immunocompromised patients. Nine studies reported no cases during follow-up, and two studies found a single case each. A study by Tasker and colleagues [222] reported a protective efficacy of symptomatic laboratory confirmed influenza A of 100% (95% CI 73% to 100%) in HIV patients compared to PNV controls.
Immune response to vaccination
Data on immune response to vaccination for each influenza subtype were pooled for meta-analyses based on CHMP definitions of seroconversion or seroprotection [24] and are summarised in Table 4 with the associated forest plots provided in Figure S2. Table 4 lists several highly significant pooled effects, although moderate to important levels of statistical heterogeneity were typically present. Seroconversion (SC1) with a PNV comparator group was more likely in patients receiving immunologically active vaccine, although statistically significant only for influenza B. Odds of seroconversion (SC2) following vaccination against seasonal A(H1N1) and A(H3N2) were statistically equivalent between immunocompromised patients and VICT cont rols, although the likely estimate of effect suggests an inferior response in patients. Vaccination against pandemic A/H1N1/California/7/2009 resulted in lower but non-significant odds of seroprotection compared to VICT controls although the two pooled studies gave an adjuvanted [60] and non-adjuvanted [154] vaccine in different populations, with significant statistical heterogeneity.
Table 4. Summary of meta-analyses for immune response to vaccination.
| Outcome measure | Influenza subtype | Comparator | Number of studies | Pooled ES (95% CI) | p value of ES | I2 (%) | p value of I2 |
| SC1 | A(H1N1) (S) | VICT | 50* | 0.55 (0.43 to 0.71) | <0.001 | 53.2 | <0.001 |
| SC1 | A(H3N2) | VICT | 47* | 0.55 (0.41 to 0.73) | <0.001 | 66.9 | <0.001 |
| SC1 | B | VICT | 44* | 0.48 (0.36 to 0.62) | <0.001 | 54.3 | <0.001 |
| SC1 | A(H1N1) (S) | PNV | 3 | 3.90 (0.42 to 36.64) | NS | 77.8 | 0.01 |
| SC1 | A(H3N2) | PNV | 3 | 10.93 (0.92 to 129.80) | NS | 82.5 | 0.003 |
| SC1 | B | PNV | 2 | 9.17 (1.05 to 79.97) | 0.05 | 72.7 | NS |
| SC2 | A(H1N1) (S) | VICT | 6 | 0.65 (0.39 to 1.09) | NS | 13.6 | NS |
| SC2 | A(H3N2) | VICT | 8 | 0.60 (0.25 to 1.43) | NS | 63.9 | 0.007 |
| SC2 | B | VICT | 8 | 0.42 (0.19 to 0.94) | 0.04 | 69.8 | 0.002 |
| SP | A(H1N1) (P) | VICT | 2 | 0.22 (0.02 to 2.75) | NS | 80.4 | 0.02 |
| SP | A(H1N1) (S) | VICT | 37* | 0.36 (0.26 to 0.51) | <0.001 | 56.9 | <0.001 |
| SP | A(H3N2) | VICT | 35* | 0.39 (0.26 to 0.59) | <0.001 | 64.1 | <0.001 |
| SP | B | VICT | 37* | 0.37 (0.25 to 0.53) | <0.001 | 65.1 | <0.001 |
* = some studies contributed two sets of data included in this meta-analysis; (S) = seasonal; (P) = pandemic; ES = effect size; CI = confidence interval; SC1 = seroconversion (≥4 fold rise post vaccination); SC2 = seroconversion (<1∶40 to ≥1∶40 haemagglutination inhibition titre); SP = seroprotection (≥1∶40 haemagglutination inhibition titre post vaccination); VICT = vaccinated immunocompetent controls; PNV = placebo or no vaccination; NS = not statistically significant. See Figure S2 for citation details.
Of the 85 studies unsuitable for meta-analysis reporting rates of seroconversion, seroprotection or mean geometric increase in HI titre based on serology within 2–4 weeks, many were single-arm but broadly supported the above findings. Notably, statistically equivalent rates of seroconversion (SC1) were found to influenza A(H3N2) and B in patients with primary immunodeficiency [229] and to pandemic A/H1N1/California/7/2009 in paediatric cancer patients [43], both compared to VICT controls. Similar findings were observed in studies comparing seroprotection rates [120], [158]. Further to those subject to meta-analysis only one study reporting serological data with a PNV comparator was identified, but this RCT lacked sufficient description of the control group to permit interpretation [58]. Most studies reporting GMT showed vaccinating immunocompromised patients was associated with a ≥2.5 fold rise, as per CHMP assessment criteria [254]. Immune response among cancer patients vaccinated against pandemic A/H1N1/California/07/2009 using adjuvanted [43] and unspecified [243] vaccines was statistically comparable to that of immunocompetent controls.
Adverse events and safety
Adverse event data were unsuitable for meta-analysis owing to difficulty in accurately identifying cases and denominator counts, and potential for bias due to post hoc selection of reported outcomes. Local and systemic adverse events were mapped to CHMP criteria [24] in 34 studies. These were generally self-reported, using diary cards or telephone follow-up. Where feasible median adverse event rates were calculated (see Table 5), in addition six studies each reported <3 cases of fever.
Table 5. Median adverse event rate by CHMP criteria.
| Adverse event | IC patients (%) | VICT controls (%) | PNV controls (%) |
| Local | |||
| Ecchymosis | 3.1 (2.0 to 4.2; n = 2) | 0.0 (0.0 to 0.0; n = 1) | – |
| Induration | 18.9 (10.2 to 30.0; n = 5) | 11.0 (6.3 to 15.0; n = 3) | – |
| System ic | |||
| Fever | 7.1 (0.0 to 23.3; n = 14) | 5.0 (0.0 to 16.7; n = 5) | 10.2 (10.0 to 10.3; n = 2) |
| Malaise | 23.6 (0.8 to 44.0; n = 8) | 12.0 (0.0 to 25.9; n = 5) | 22.1 (20.0 to 24.1; n = 2) |
| Shivering | 10.2 (10.2 to 10.2; n = 1) | 16.3 (16.3 to 16.3; n = 1) | – |
Values in parentheses show the reported range of adverse events and number of studies; IC = immunocompromised; VICT = vaccinated immunocompetent; PNV = placebo or no vaccination.
Eighty-seven studies reported clinical or laboratory markers of vaccination impact on the underlying immunosuppressive condition. These included CD4+ count and HIV load in HIV-positive patients, relapse and complication rate in cancer patients, allograft rejection rate in transplant patients, disease activity scores in patients with autoimmune conditions and lung function tests in respiratory patients. We did not identify consistent evidence of disease progression or worsening of clinical symptoms related to underlying immunosuppressive condition following vaccination.
Incidence of serious adverse events was reported in 21 studie s, although only 11 of these included a control group. Three hospitalisations occurred in patients with HIV [136] and one transient ischaemic attack in a separate study, which did not specify whether the case was HIV-positive or a healthy control [94]. Madan et al (2008) report biopsy-proven allograft rejection within six months of vaccination in four paediatric liver transplant recipients [158]. None of these events (nor those described in other eligible studies) was deemed due to influenza vaccination [43], [94], [136], [158]. Five of 54 paediatric cancer patients developed fever within 48 hours of receiving an adjuvanted vaccination for influenza A/H1N1/California/07/2009. However, whether this was a consequence of vaccination, underlying cancer or concomitant chemotherapy or infection was indeterminable [48].
Risk of bias across studies
Risk of publication bias was assessed using Begg's funnel plot and confirmed statistically where feasible using Egger's test. There was no evidence of biased reporting among studies subject to meta-analysis.
Discussion
Summary of evidence
This systematic review is the first to consider clinical and serologic outcomes following influenza vaccination in immunocompromised patients, incorporating data from the 2009 pandemic period. Our results suggest that vaccinating immunocompromised patients against influenza provides clinical protection from influenza-like illness and laboratory confirmed infection compared to placebo or no vaccination, and the rate of symptomatic disease is comparable to that observed in vaccinated healthy controls. The pooled odds of seroconversion were consistently higher in vaccinated patients compared to PNV controls, although statistical superiority was demonstrated only for influenza B. Conversely, the odds of seroconversion (SC1) and seroprotection conferred by vaccination were consistently and significantly lower in patients compared to VICT controls for seasonal influenza A(H1N1), A(H3N2) and B (see Table 4). The data reviewed offer no consistent evidence of safety concerns, disease progression or serious adverse events following influenza vaccination in immunocompromised populations. Table 5 suggests a higher median rate of malaise in vaccinated immunocompromised patients compared to VICT controls (23.6% vs 12.0%), however malaise is also elevated in PNV controls implying an association with the underlying immunocompromised state.
Limitations
Risk of bias and confounding
Many of the 209 eligible studies were at unclear or high risk of bias across most domains and the number of RCTs was relatively small (n = 23). The majority of studies (n = 137) were non-randomised trials that included a control group, but without robust randomisation selection bias between study arms cannot be excluded. Non-randomised designs may also introduce unbalanced confounding variables, and given that analyses were commonly reported unadjusted, these may reasonably influence the reported effect sizes for each pooled outcome measure. Potential confounders were anticipated and specified in the study protocol. Included cases series conducted during the 2009 influenza A(H1N1) pandemic are likewise at high risk of selection bias. Stratification of meta-analyses by risk of bias was unfeasible due to concerns with selecting a specific domain for classifying studies as ‘low’ or ‘high’. Adverse event data pr esented in Table 5 do not take account of numerous studies broadly stating absence of adverse events in vaccinated groups, potentially introducing reporting bias.
Heterogeneity
Moderate to high levels of statistical heterogeneity were present in many of the reported meta-analyses, reaching significance on numerous occasions. Even where effect sizes are consistent, clinical heterogeneity may continue to challenge the validity of meta-analysis. Potential confounders related to aetiology of immunocompromise or intervention characteristics may be responsible for such heterogeneity. This includes pooling of data arising from the 2009 influenza A(H1N1) pandemic vaccine (commonly monovalent, sometimes adjuvanted) with seasonal vaccines although <10% of studies overall involved such vaccines, only one study was included in the ILI meta-analysis (PNV comparator), and the two studies reporting data on prevention of laboratory confirmed influenza offered narrative information only. Previous exposure to influenza vaccination, timing of vaccine administration (in relation to changes in administration of immunosuppressive therapy or disease state) an d immunosenescence may also be important effect modifiers contributing to heterogeneity between the reported outcome measures. Similarly, matching between vaccine and wild type influenza strains is likely to introduce a degree of inter-seasonal variability; however, this does not affect our conclusions in terms of public health policy as these are typically designed to provide consistent advice over multiple seasons. Our analyses reported separately by aetiology of immunocompromise provide a degree of sensitivity testing for pooled results.
Other limitations
Paucity of data limited or prevented some analyses. There were insufficient data to adequately report on seroconversion or seroprotection with a PNV comparator. The planned sub-analysis of evidence from resource-poor countries was abandoned due to insufficient data arising from this setting. In addition, it is now recognised that a large proportion of the population aged ≥55 years probably had some degree of pre-existing immunity to the 2009 influenza A(H1N1) pandemic strain, adding further difficulty to the interpretation of data from the pandemic period [255]. We recognise CHMP criteria for serological response to vaccination are based on healthy volunteers aged 18 to 60 years thus may not reflect expected rates of clinical protection observed in vaccinated immunocompromised populations [256].
Implic ations for public health practice
Our data favour a policy of routinely recommending influenza vaccination to immunocompromised patient groups, who may be at higher risk of influenza and its complications [14], [257]. Many authorities, such as the UK Joint Committee on Vaccination and Immunisation and the US Advisory Committee on Immunization Practices, already recommend vaccinating immunocompromised patients and household or close contacts against influenza to minimise transmission [14], [257]. However, uptake of this intervention is currently unclear but, where data exist, these suggest sub-optimal coverage [258]. Although our findings indicate some mild and self-limiting adverse effects following vaccination, policies should acknowledge these may occur with greater frequency in certain patient groups, and make suitable provision for clinical discretion. Management of infection in immunocompromised patients can be complicated by limited effectiveness of pharmacological therapies and vaccination carries the additional benefit of mitigating emergence of resistance to antiviral agents [9].
Implications for further research
Methodological limitations affecting the current evidence base mandates new robust studies assessing the incidence of ILI and laboratory confirmed influenza in vaccinated immunocompromised patients. Similarly, robust studies are needed to inform revised CHMP seroconversion and seroprotection criteria applicable to immunocompromised patients. Further primary research is warranted to quantify factors contributing to heterogeneity, including the utility of second ‘booster’ doses, immunological adjuvants and degree of immunosuppression on rates of clinical protection and response to vaccination. Systematic reviews and meta-analyses are indicated to assess the impact of vaccinating immunocompromised patients on influenza-related morbidity and mortality. In addition, resource poor countries should be supported to conduct robust studies of influenza vaccination in their immunocompromised populations. Proportionally different comorbidities such as malnutrition or co-in fection with HIV may be encountered and response to vaccination among indigenous groups and ethnic minorities may differ in these settings compared to developed countries.
Conclusion
Our systematic review and meta-analyses suggest immunocompromised patients do manifest an immune response to vaccination that, while not as vigorous as that of healthy controls, probably confers a similar level of clinical protection against influenza and, importantly, does so without causing excess harm. Limitations including potential for bias and confounding and the presence of statistical or clinical heterogeneity mean the evidence for these assertions is generally weak, but the direction of effects are remarkably consistent. Nevertheless, our study supports national and international public health policy recommendations for the targeting of immunocompromised patients for influenza vaccination.
Supporting Information
Summary of risk of bias using the Cochrane Collaboration tool (n = 191). Legend: green = low risk of bias; yellow = unclear risk of bias; red = high risk of bias.
(PDF)
Forest plots for immune response to vaccination question. Figure S2.1. Forest plot of studies of seroconversion (≥4 fold rise in haemagglutination inhibition titre): seasonal influenza A(H1N1), vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2.2. Forest plot of studies of seroconversion (≥4 fold rise in haemagglutination inhibition titre): influenza A(H3N2), vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2.3. Forest plot of studies of seroconversion (≥4 fold rise in haemagglutination inhibition titre): seasonal influenza B, vaccinated immunocompromised patients versus vaccinated immunocompetent contr ols. Figure S2.4. Forest plot of studies of seroconversion (≥4 fold rise in haemagglutination inhibition titre): seasonal influenza A(H1N1), vaccinated immunocompromised patients versus placebo or no vaccination. Figure S2.5. Forest plot of studies of seroconversion (≥4 fold rise in haemagglutination inhibition titre): influenza A(H3N2), vaccinated immunocompromised patients versus placebo or no vaccination. Figure S2.6. Forest plot of studies of seroconversion (≥4 fold rise in haemagglutination inhibition titre): influenza B, vaccinated immunocompromised patients versus placebo or no vaccination. Figure S2.7. Forest plo t of studies of seroconversion (<1∶40 pre-vaccination to ≥1∶40 haemagglutination inhibition titre post vaccination): seasonal influenza A(H1N1), vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2.8. Forest plot of studies of seroconversion (<1∶40 pre-vaccination to ≥1∶40 haemagglutination inhibition titre post vaccination): influenza A(H3N2), vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2.9. Forest plot of studies of seroconversion (<1∶40 pre-vaccination to ≥1∶40 haemagglutination inhibition titre post vaccination): seasonal influenza B, vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2.10. Forest plot of studies of seroprotection (≥1∶40 haemagglutination inhibition titre post vaccination): seasonal influenza A(H1N1), vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2.11. Forest plot of studies of seroprotection (≥1∶40 haemagglutination inhibition titre post vaccination): influenza A(H3N2), vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2.12. Forest plot of studies of seroprotection (≥1∶40 haemagglutination inhibition titre post vaccination): seasonal influenza B, vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2< /xref>.13. Forest plot of studies of seroprotection (≥1∶40 haemagglutination inhibition titre post vaccination): pandemic influenza A(H1N1), vaccinated immunocompromised patients versus vaccinated immunocompetent controls.
(PDF)
MEDLINE search construct. Legend: PICO = research question in terms of population, intervention, comparators and outcomes. MeSH = Medical Subject Headings (US National Library of Medicine).
(PDF)
Summary of risk of bias using the Downs and Black (1998) tool (n = 2). Legend: N/A = not applicable; higher score = less risk of bias.
(PDF)
Summary of risk of bias using the US AHRQ tool (n = 3). Legend: Response indicates whether associated elements for reduction of bias have been met.
(PDF)
(PDF)
(DOC)
Acknowledgments
CRB and JSN-V-T are the primary and senior authors respectively, take responsibility for the work and act as guarantors of the data. We acknowledge and thank the following for their support and advice throughout the project: Dr Charles Penn and Sara Martins (Global Influenza Programme, World Health Organization); Dr Gayle Dolan (Health Protection Agency North East). We thank the European Vaccine Manufacturers, GlaxoSmithKline, Novartis and Sanofi Pasteur MSD for responding to our request for literature potentially relevant to this systematic review.
Footnotes
Competing Interests: The University of Nottingham Health Protection Research Group is currently in receipt of research funds from GlaxoSmithKline (GSK). The group has recently accepted an unrestricted educational grant for influenza research from F. Hoffmann-La Roche. Research on influenza funded by an unrestricted educational grant from Astra Zeneca is also underway. The aforementioned funding received from GSK, F. Hoffmann-La Roche and Astra Zeneca did not support any aspect of this study. JSN-V-T has received funding to attend influenza related meetings, lecture and consultancy fees and research funding from several influenza antiviral drug and vaccine manufacturers. All forms of personal remuneration ceased in September 2010, but influenza-related research funding from GlaxoSmithKline, F. Hoffmann-La Roche and Astra-Zeneca remains current. He is a former employee of SmithKline Beecham plc. (now GlaxoSmithKline), Roche Products Ltd, and Aventis-Pasteur MSD (now Sanofi-Pasteur MSD), al l prior to 2005, with no outstanding pecuniary interests by way of shareholdings, share options or accrued pension rights. AZ has received fees for participating in review activities from the Global Influenza Programme, World Health Organization. JE has received consultancy fees from GSK. This does not alter our adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This study was commissioned by the Global Influenza Programme, World Health Organization. The University of Nottingham Health Protection Research Group (JSN-V-T, CRB, BCM, ABH, JE, RP) is an official WHO Collaborating Centre for pandemic influenza and research. It receives limited funding from WHO in support of specific activities. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Viboud C, Miller M, Olson D, Osterholm M, Simonsen L. Preliminary estimates of mortality and years of life lost associated with the 2009 A/H1N1 pandemic in the US and comparison with past influenza seasons. PLoS Curr. 2010:RRN1153. doi: 10.1371/currents.RRN1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9:493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee N, Chan PK, Hui DS, Rainer TH, Wong E, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200:492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leekha S, Zitterkopf NL, Espy MJ, Smith TF, Thompson RL, et al. Duration of influenza A virus shedding in hospitalized patients and implications for infection control. Infect Control Hosp Epidemiol. 2007;28:1071–1076. doi: 10.1086/520101. [DOI] [PubMed] [Google Scholar]
- 7.Giannella M, Alonso M, Garcia de Viedma D, Roa PL, Catalan P, et al. Prolonged viral shedding in pandemic influenza A(H1N1): clinical significance and viral load analysis in hospitalized patients. Clin Microbiol Infect. 2010 doi: 10.1111/j.1469-0691.2010.03399.x. doi: 10.1111/j.1469-0691.2010.03399.x. [DOI] [PubMed] [Google Scholar]
- 8.Mohty B, Thomas Y, Vukicevic M, Nagy M, Levrat E, et al. Clinical features and outcome of 2009-influenza A (H1N1) after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011 doi: 10.1038/bmt.2011.57. doi: 10.1038/bmt.2011.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meijer A, Lackenby A, Hungnes O, Lina B, van-der-Werf S, et al. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–08 season. Emerg Infect Dis. 2009;15:552–560. doi: 10.3201/eid1504.081280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ring A, Marx G, Steer C, Harper P. Influenza vaccination and chemotherapy: a shot in the dark? Support Care Cancer. 2002;10:462–465. doi: 10.1007/s00520-001-0337-9. [DOI] [PubMed] [Google Scholar]
- 11.Goossen GM, Kremer LCM, Van De Wetering MD. Influenza vaccination in children being treated with chemotherapy for cancer. Cochrane Database Syst Rev. 2009:CD006484. doi: 10.1002/14651858.CD006484.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Atashili J, Kalilani L, Adimora AA. Efficacy and clinical effectiveness of influenza vaccines in HIV-infected individuals: a meta-analysis. BMC Infect Dis. 2006;6:138. doi: 10.1186/1471-2334-6-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anema A, Mills E, Montaner J, Brownstein JS, Cooper C. Efficacy of influenza vaccination in HIV-positive patients: a systematic review and meta-analysis (Provisional abstract). HIV Medicine. 2008:57–61. doi: 10.1111/j.1468-1293.2008.00515.x. [DOI] [PubMed] [Google Scholar]
- 14.Salisbury D, Ramsay M, Noakes K, editors. Immunisation against infectious disease. London: The Stationary Office; 2006. Chapter 19 Influenza (updated January 2011). pp. 185–208. [Google Scholar]
- 15.Pebody RG, McLean E, Zhao H, Cleary P, Bracebridge S, et al. Pandemic Influenza A (H1N1) 2009 and mortality in the United Kingdom: risk factors for death, April 2009 to March 2010. Euro Surveill. 2010;15:pii = 19571. [PubMed] [Google Scholar]
- 16.Campbell CN, Mytton OT, McLean EM, Rutter PD, Pebody RG, et al. Hospitalization in two waves of pandemic influenza A(H1N1) in England. Epidemiol Infect. 2010 doi: 10.1017/S0950268810002657. doi: 10.1017/S0950268810002657. [DOI] [PubMed] [Google Scholar]
- 17.Wilking H, Buda S, von der Lippe E, Altmann D, Krause G, et al. Mortality of 2009 pandemic influenza A(H1N1) in Germany. Euro Surveill. 2010;15:pii = 19741. doi: 10.2807/ese.15.49.19741-en. [DOI] [PubMed] [Google Scholar]
- 18.Chironna M, Tafuri S, Santoro N, Prato R, Quarto M, et al. A nosocomial outbreak of 2009 pandemic influenza A(H1N1) in a paediatric oncology ward in Italy, October-November 2009. Euro Surveill. 2010;15:pii = 19454. doi: 10.2807/ese.15.01.19454-en. [DOI] [PubMed] [Google Scholar]
- 19.Lalayanni C, Sirigou A, Iskas M, Smias C, Sakellari I, et al. Outbreak of novel influenza A (H1N1) in an adult haematology department and haematopoietic cell transplantation unit: clinical presentation and outcome. J Infect. 2010;61:270–272. doi: 10.1016/j.jinf.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Kharfan-Dabaja MA, Velez A, Richards K, Greene JN, Field T, et al. Influenza A/pandemic 2009/H1N1 in the setting of allogeneic hematopoietic cell transplantation: a potentially catastrophic problem in a vulnerable population. Int J Hematol. 2010;91:124–127. doi: 10.1007/s12185-009-0464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enstone JE, Myles PR, Openshaw PJ, Gadd EM, Lim WS, et al. Nosocomial pandemic (H1N1) 2009, United Kingdom, 2009–2010. Emerg Infect Dis. 2011;17:592–598. doi: 10.3201/eid1704.101679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck C, McKenzie B, Hashim A, Nguyen-Van-Tam J. Clinical effectiveness of influenza vaccination for immunocompromised patients: a systematic review and meta-analysis. CRD Register. 2011:CRD42011001226. [Google Scholar]
- 23.World Health Organization. WHO Guidelines for Pharmacological Management of Pandemic Influenza A(H1N1) 2009 and other Influenza Viruses. Part I Recommendations. World Health Organization. 2010 [PubMed] [Google Scholar]
- 24.Committee for Proprietary Medicinal Products. Note for guidance on harmonisation of requirements for influenza vaccines. CPMP/BWP/214/96. London: European Agency for the Evaluation of Medicinal Products; 1997. [Google Scholar]
- 25.ICH Expert Working Group. ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice E6(R1). [Online]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf [Accessed 19th November 2011]
- 26.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2 [updated September 2009] ed. The Cochrane Collaboration; 2009. [Google Scholar]
- 27.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.West S, King V, Carey T, Lohr K, McKoy N, et al. Systems to rate the strength of scientific evidence. Evidence report/technology appraisal number 47. Agency for Healthcare Research and Quality. 2002 [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Human Development Report 2010 team. The Real Wealth of Nations: Pathways to Human Development. New York, USA: United Nations Development Agency; 2010. [Google Scholar]
- 31.Delafond P. Vaccins: pour ou contre? Medecine Douce. 1990:18–76. [Google Scholar]
- 32.Mesle F, Vallin J. Le rôle des vaccinations dans la baisse de la mortalité. Dossiers et Recherche. 1999:16. [Google Scholar]
- 33.Pereira G, Tondo Á. Vacunación en el paciente con virus de inmunodeficiencia humana. Arch Med Interna. 2009;31:20–22. [Google Scholar]
- 34.Falchetti, Magnani Flu vaccine in patients with orthotopic heart transplantation. Ital Heart J. 2000;1:25. [Google Scholar]
- 35.Hanania NA, Sockrider M, Wise R, Castro M, Tonascia J, et al. Immune response to influenza vaccine in patients with asthma - lack of effect of corticosteroid therapy. Am J Respir Crit Care Med. 2002:A561. [Google Scholar]
- 36.Abu-Shakra M, Press J, Sukenik S, Buskila D. Influenza virus vaccination of patients with SLE: effects on generation of autoantibodies. Clin Rheumatol. 2002;21:369–372. doi: 10.1007/s100670200099. [DOI] [PubMed] [Google Scholar]
- 37.Admon D, Engelhard D, Strauss N, Goldman N, Zakay-Rones Z. Antibody response to influenza immunization in patients after heart transplantation. Vaccine. 1997;15:1518–1522. doi: 10.1016/s0264-410x(97)00193-x. [DOI] [PubMed] [Google Scholar]
- 38.Allison JE, Glezen WP, Taber LH, Paredes A, Webster RG. Reactogenicity and immunogenicity of bivalent influenza A and monovalent influenza B virus vaccines in high-risk children. J Infect Dis. 1977;136(Suppl):S672–S676. doi: 10.1093/infdis/136.supplement_3.s672. [DOI] [PubMed] [Google Scholar]
- 39.Altamirano-Diaz L, West L, Humar A, Ely L, Urschel S, et al. Early post-transplant vaccination with pandemic influenza A/H1N1 vaccine in pediatric heart transplant recipients. Pediatr Transplant. 2011;15:172–175. doi: 10.1111/j.1399-3046.2010.01433.x. [DOI] [PubMed] [Google Scholar]
- 40.Amendola A, Boschini A, Colzani D, Anselmi G, Oltolina A, et al. Influenza vaccination of HIV-1-positive and HIV-1-negative former intravenous drug users. J Med Virol. 2001;65:644–648. doi: 10.1002/jmv.2085. [DOI] [PubMed] [Google Scholar]
- 41.Anderson H, Petrie K, Berrisford C, Charlett A, Thatcher N, et al. Seroconversion after influenza vaccination in patients with lung cancer. Br J Cancer. 1999;80:219–220. doi: 10.1038/sj.bjc.6690342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anema A, Mills E, Montaner J, Brownstein JS, Cooper C. Efficacy of influenza vaccination in HIV-positive patients: a systematic review and meta-analysis. HIV Med. 2008;9:57–61. doi: 10.1111/j.1468-1293.2008.00515.x. [DOI] [PubMed] [Google Scholar]
- 43.Antonio R, Delogu G, Sali M, Coccia P, Pierri F, et al. Efficacy of influenza A(H1N1v) vaccination in children with cancer. Pediatr Blood Cancer. 2010;55:973. [Google Scholar]
- 44.Avetisyan G, Aschan J, Hassan M, Ljungman P. Evaluation of immune responses to seasonal influenza vaccination in healthy volunteers and in patients after stem cell transplantation. Transplantation. 2008;86:257–263. doi: 10.1097/TP.0b013e3181772a75. [DOI] [PubMed] [Google Scholar]
- 45.Ballet C, Roussey-Kesler G, Aubin JT, Brouard S, Giral M, et al. Humoral and cellular responses to influenza vaccination in human recipients naturally tolerant to a kidney allograft. Am J Transplant. 2006;6:2796–2801. doi: 10.1111/j.1600-6143.2006.01533.x. [DOI] [PubMed] [Google Scholar]
- 46.Banic S, Koren S, Tomazic J, Vidmar L, Ihan A, et al. Influenza vaccination of human immunodeficiency virus 1-infected patients receiving antiretroviral therapy. Acta Virol. 2001;45:39–44. [PubMed] [Google Scholar]
- 47.Bate J, Yung C, Hoschler K, Sheasby L, Taj M, et al. Immungenicity of novel influenza A (H1N1) vaccine in UK children with cancer: a single centre study. Pediatr Blood Cancer. 2010;55:823. [Google Scholar]
- 48.Bate J, Yung CF, Hoschler K, Sheasby L, Morden J, et al. Immunogenicity of pandemic (H1N1) 2009 vaccine in children with cancer in the United Kingdom. Clin Infect Dis. 2010;51:e95–e104. doi: 10.1086/657403. [DOI] [PubMed] [Google Scholar]
- 49.Bedognetti D, Zoppoli G, Massucco C, Zupo S, Sertoli MR, et al. Impaired humoral response to influenza vaccine and prolonged B memory cell depletion as a consequence of rituximab-based immunochemotherapy in non-hodgkin lymphoma patients. J Immunother. 2009;32:992–993. [Google Scholar]
- 50.Bedognetti D, Zoppoli G, Zanardi E, Massucco C, Sertoli MR, et al. Prolonged lack of humoral response to influenza vaccine associated with a persistent depletion of B memory cells in non hodgkin's lymphoma patients treated with rituximab-containing immunochemotherapy. Clin Immunol. 2010;135:S84. doi: 10.4049/jimmunol.1004095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bektas O, Karadeniz C, Oguz A, Berberoglu S, Yilmaz N, et al. Assessment of the immune response to trivalent split influenza vaccine in children with solid tumors. Pediatr Blood Cancer. 2007;49:914–917. doi: 10.1002/pbc.21106. [DOI] [PubMed] [Google Scholar]
- 52.Bellei NCJ, Carraro E, Castelo A, Granato CFH. Risk factors for poor immune response to influenza vaccination in elderly people. Braz J Infect Dis. 2006;10:269–273. doi: 10.1590/s1413-86702006000400011. [DOI] [PubMed] [Google Scholar]
- 53.Benne CA, Kroon FP, Harmsen M, Tavares L, Kraaijeveld CA, et al. Comparison of neutralizing and hemagglutination-inhibiting antibody responses to influenza A virus vaccination of human immunodeficiency virus-infected individuals. Clin Diagn Lab Immunol. 1998;5:114–117. doi: 10.1128/cdli.5.1.114-117.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergeron A. Investigations of influenza vaccination in kidney and lung transplant populations [MSc thesis] Edmonton, Alberta: University of Alberta; 2010. 158 [Google Scholar]
- 55.Bickel M, Wieters I, Khaykin P, Nisius G, Haberl A, et al. Low rate of seroconversion after vaccination with a split virion, adjuvanted pandemic H1N1 influenza vaccine in HIV-1-infected patients. AIDS. 2010;24:F31–F35. doi: 10.1097/QAD.0b013e3283398da1. [DOI] [PubMed] [Google Scholar]
- 56.Biselli R, Fagiolo U, Nisini R, Paganelli R, Doffizi G, et al. Humoral response to influenza hemagglutinin: oligoclonal spectrotype and failure of thymopentin as immunoadjuvant. Gerontology. 1995;41:3–10. doi: 10.1159/000213656. [DOI] [PubMed] [Google Scholar]
- 57.Blumberg EA, Albano C, Pruett T, Isaacs R, Fitzpatrick J, et al. The immunogenicity of influenza virus vaccine in solid organ transplant recipients. Clin Infect Dis. 1996;22:295–302. doi: 10.1093/clinids/22.2.295. [DOI] [PubMed] [Google Scholar]
- 58.Blumberg EA, Fitzpatrick J, Stutman PC, Hayden FG, Brozena SC. Safety of influenza vaccine in heart transplant recipients. J Heart Lung Transplant. 1998;17:1075–1080. [PubMed] [Google Scholar]
- 59.Borella L, Webster RG. The immunosuppressive effects of long-term combination chemotherapy in children with acute leukemia in remission. Cancer Res. 1971;31:420–426. [PubMed] [Google Scholar]
- 60.Brakemeier S, Schweiger B, Glander P, Diekmann F, Neuma-Yer HH, et al. A single dose of an adjuvanted influenza A H1N1 vaccine (pandemrix (R)) does not provide a protective immune response in the majority of renal transplant recipients. Transpl Int. 2010;23:0050. [Google Scholar]
- 61.Briggs WA, Rozek RJ, Migdal SD, Shillis JL, Brackett RG, et al. Influenza vaccination in kidney transplant recipients: cellular and humoral immune responses. Ann Intern Med. 1980;92:471–477. doi: 10.7326/0003-4819-92-4-471. [DOI] [PubMed] [Google Scholar]
- 62.Brown AE, Steinherz PG, Miller DR, Armstrong D, Kellick MG, et al. Immunization against influenza in children with cancer: results of a three-dose trial. J Infect Dis. 1982;145:126. doi: 10.1093/infdis/145.1.126. [DOI] [PubMed] [Google Scholar]
- 63.Brunell PA. Immunologic response of immunosuppressed children to influenza vaccine. MMWR Recomm Rep. 1977;26:54. [Google Scholar]
- 64.Brydak LB, Calbecka M. Immunogenicity of influenza vaccine in patients with hemato-oncological disorders. Leuk Lymphoma. 1999;32:369–374. doi: 10.3109/10428199909167399. [DOI] [PubMed] [Google Scholar]
- 65.Brydak LB, Guzy J, Starzyk J, Machala M, Gozdz SS. Humoral immune response after vaccination against influenza in patients with breast cancer. Support Care Cancer. 2000;9:65–68. doi: 10.1007/s005200000186. [DOI] [PubMed] [Google Scholar]
- 66.Brydak LB, Hryniewicz HJ, Machala M, Horban A. Humoral response to influenza vaccination in HIV-infected patients. Clin Drug Invest. 1999;17:441–449. [Google Scholar]
- 67.Brydak LB, Machala M, Centkowski P, Warzocha K, Bilinski P. Humoral response to hemagglutinin components of influenza vaccine in patients with non-Hodgkin malignant lymphoma. Vaccine. 2006;24:6620–6623. doi: 10.1016/j.vaccine.2006.05.100. [DOI] [PubMed] [Google Scholar]
- 68.Brydak LB, Machala M, Laguna P, Rokicka-Milewska R. Antibody response to influenza vaccination in splenectomized patients in Poland. J Clin Immunol. 2004;24:225–236. doi: 10.1023/B:JOCI.0000025444.24160.d5. [DOI] [PubMed] [Google Scholar]
- 69.Brydak LB, Rokicka-Milewska R, Machala M, Jackowska T. Studies on the humoral immune response to hemagglutinin of influenza vaccine in children with acute lymphoblastic leukemia after chemotherapy treatment. Int J Pediatr Hematol Oncol. 2000;7:29–40. [Google Scholar]
- 70.Brydak LB, Rokicka-Milewska R, Machala M, Jackowska T, Sikorska-Fic B. Immunogenicity of subunit trivalent influenza vaccine in children with acute lymphoblastic leukemia. Pediatr Infect Dis J. 1998;17:125–129. doi: 10.1097/00006454-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 71.Bucalossi A, Marotta G, Galieni P, Bigazzi C, Valenzin PE, et al. Immunological response to influenza virus vaccine in B-cell chronic lymphocytic leukaemia patients. Acta Haematol. 1995;93:56. doi: 10.1159/000204095. [DOI] [PubMed] [Google Scholar]
- 72.Burbach G, Bienzle U, Stark K, Rayes N, Neuhaus R, et al. Influenza vaccination in liver transplant recipients. Transplantation. 1999;67:753–755. doi: 10.1097/00007890-199903150-00019. [DOI] [PubMed] [Google Scholar]
- 73.Candon S, Thervet E, Lebon P, Suberbielle C, Lima C, et al. Humoral and Cellular Immune Responses after Influenza Vaccination in Kidney Transplant Recipients. Am J Transplant. 2009;9:658. doi: 10.1111/j.1600-6143.2009.02787.x. [DOI] [PubMed] [Google Scholar]
- 74.Candon S, Thervet E, Lebon P, Suberbielle C, Zuber J, et al. Humoral and cellular immune responses after influenza vaccination in kidney transplant recipients. Am J Transplant. 2009;9:2346–2354. doi: 10.1111/j.1600-6143.2009.02787.x. [DOI] [PubMed] [Google Scholar]
- 75.Canestri A, Krivine A, Assoumou L, Le Corre M, Rozenberg F, et al. Maraviroc does not affect humoral response to the pandemic influenza A-H1N1v 2009 adjuvanted vaccine in HIV-1-infected patients. AIDS. 2010;24:2887–2889. doi: 10.1097/QAD.0b013e3283402bc1. [DOI] [PubMed] [Google Scholar]
- 76.Carroll RN, Marsh SD, O'Donoghue EP, Breeze DC, Shackman R. Response to influenza vaccine by renal transplant patients. BMJ. 1974;2:701–703. doi: 10.1136/bmj.2.5921.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cavdar C, Sayan M, Sifil A, Artuk C, Yilmaz N, et al. The comparison of antibody response to influenza vaccination in continuous ambulatory peritoneal dialysis, hemodialysis and renal transplantation patients. Scand J Urol Nephrol. 2003;37:71–76. doi: 10.1080/00365590310008749. [DOI] [PubMed] [Google Scholar]
- 78.Centkowski P, Brydak L, Machala M, Kalinka-Warzocha E, Blasinska-Morawiec M, et al. Immunogenicity of influenza vaccination in patients with non-Hodgkin lymphoma. J Clin Immunol. 2007;27:339–346. doi: 10.1007/s10875-007-9073-3. [DOI] [PubMed] [Google Scholar]
- 79.Chadha MK, Fakih MG, Tian L, Mashtare T, Nesline M, et al. Effect of 25 hydroxy vitamin D status on serological response to influenza vaccine in cancer patients. J Clin Oncol. 2009;27:e20575. [Google Scholar]
- 80.Chadwick EG, Chang G, Decker MD, Yogev R, Dimichele D, et al. Serologic response to standard inactivated influenza vaccine in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1994;13:206–211. doi: 10.1097/00006454-199403000-00008. [DOI] [PubMed] [Google Scholar]
- 81.Chalmers A, Scheifele D, Patterson C, Williams D, Weber J, et al. Immunization of patients with rheumatoid arthritis against influenza: a study of vaccine safety and immunogenicity. J Rheumatol. 1994;21:1203–1206. [PubMed] [Google Scholar]
- 82.Chebotareva TA, Mazankova LN, Malinovskaya VV, Kariaeva SK, Lazarev VV. Clinical effectiveness of prevention of influenza and ARVI among children living in ecologically disadvantaged regions. Pediatrija. 2009;88:104–111. [Google Scholar]
- 83.Chisholm J, Howe K, Taj M, Zambon M. Influenza immunisation in children with solid tumours. Eur J Cancer. 2005;41:2280–2287. doi: 10.1016/j.ejca.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 84.Chisholm JC, Devine T, Charlett A, Pinkerton CR, Zambon M. Response to influenza immunisation during treatment for cancer. Arch Dis Child. 2001;84:496–500. doi: 10.1136/adc.84.6.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Danziger-Isakov L, Cherkassky L, Siegel H, McManamon M, Kramer K, et al. Effects of influenza immunization on humoral and cellular alloreactivity in humans. Transplantation. 2010;89:838–844. doi: 10.1097/TP.0b013e3181ca56f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Lavallade H, Garland P, Sekine T, Hoschler K, Marin D, et al. Repeated vaccination is required to optimize seroprotection against H1N1 in the immunocompromised host. Haematologica. 2011;96:307–314. doi: 10.3324/haematol.2010.032664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Roux A, Marx A, Burkhardt O, Schweiger B, Borkowski A, et al. Impact of corticosteroids on the immune response to a MF59-adjuvanted influenza vaccine in elderly COPD-patients. Vaccine. 2006;24:1537–1542. doi: 10.1016/j.vaccine.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 88.DeBruyn JCC. Immunogenicity and safety of influenza vaccination in children with inflammatory bowel disease [MSc thesis] Calgary, Alberta: University of Calgary; 2010. 131. [DOI] [PubMed] [Google Scholar]
- 89.Del Porto F, Lagana B, Biselli R, Donatelli I, Campitelli L, et al. Influenza vaccine administration in patients with systemic lupus erythematosus and rheumatoid arthritis. Safety and immunogenicity. Vaccine. 2006;24:3217–3223. doi: 10.1016/j.vaccine.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 90.Dengler TJ, Strnad N, Buhring I, Zimmermann R, Girgsdies O, et al. Differential immune response to influenza and pneumococcal vaccination in immunosuppressed patients after heart transplantation. Transplantation. 1998;66:1340–1347. doi: 10.1097/00007890-199811270-00014. [DOI] [PubMed] [Google Scholar]
- 91.Dopp JM, Wiegert NA, Moran JJ, Francois ML, Radford KL, et al. Effect of annual influenza immunization on antibody response in lung transplant patients. Prog Transplant. 2009;19:153–159. doi: 10.1177/152692480901900209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dorrell L, Hassan I, Marshall S, Chakraverty P, Ong E. Clinical and serological responses to an inactivated influenza vaccine in adults with HIV infection, diabetes, obstructive airways disease, elderly adults and healthy volunteers. Int J STD AIDS. 1997;8:776–779. doi: 10.1258/0956462971919264. [DOI] [PubMed] [Google Scholar]
- 93.Duchini A, Hendry RM, Nyberg LM, Viernes ME, Pockros PJ. Immune response to influenza vaccine in adult liver transplant recipients. Liver Transpl. 2001;7:311–313. doi: 10.1053/jlts.2001.23010. [DOI] [PubMed] [Google Scholar]
- 94.Durando P, Fenoglio D, Boschini A, Ansaldi F, Icardi G, et al. Safety and immunogenicity of two influenza virus subunit vaccines, with or without MF59 adjuvant, administered to human immunodeficiency virus type 1-seropositive and -seronegative adults. Clin Vaccine Immunol. 2008;15:253–259. doi: 10.1128/CVI.00316-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Edvardsson VO, Flynn JT, Deforest A, Kaiser BA, Schulman SL, et al. Effective immunization against influenza in pediatric renal transplant recipients. Clin Transplant. 1996;10:556–560. [PubMed] [Google Scholar]
- 96.Engelhard D, Nagler A, Hardan I, Morag A, Aker M, et al. Antibody response to a two-dose regimen of influenza vaccine in allogeneic T cell-depleted and autologous BMT recipients. Bone Marrow Transplant. 1993;11:1–5. [PubMed] [Google Scholar]
- 97.Esposito S, Cecinati V, Scicchitano B, Delvecchio GC, Santoro N, et al. Impact of influenza-like illness and effectiveness of influenza vaccination in oncohematological children who have completed cancer therapy. Vaccine. 2010;28:1558–1565. doi: 10.1016/j.vaccine.2009.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feery BJ, Sullivan JR, Hurley TH, Evered MG. Immunization with influenza vaccine in patients with haematological malignant disease. Med J Aust. 1977;1:292–294. doi: 10.5694/j.1326-5377.1977.tb130704.x. [DOI] [PubMed] [Google Scholar]
- 99.Fomin I, Caspi D, Levy V, Varsano N, Shalev Y, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis. 2006;65:191–194. doi: 10.1136/ard.2005.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fowke KR, Damico R, Chernoff DN, Pottage JC, Benson CA, et al. Immunologic and virologic evaluation after influenza vaccination of HIV-1-infected patients. AIDS. 1997;11:1013–1021. doi: 10.1097/00002030-199708000-00010. [DOI] [PubMed] [Google Scholar]
- 101.Fraund S, Wagner D, Pethig K, Drescher J, Girgsdies OE, et al. Influenza vaccination in heart transplant recipients. J Heart Lung Transplant. 1999;18:220–225. doi: 10.1016/s1053-2498(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 102.Fuller JD, Craven DE, Steger KA, Cox N, Heeren TC, et al. Influenza vaccination of human immunodeficiency virus (HIV)-infected adults: impact on plasma levels of HIV type 1 RNA and determinants of antibody response. Clin Infect Dis. 1999;28:541–547. doi: 10.1086/515170. [DOI] [PubMed] [Google Scholar]
- 103.Furth SL, Neu AM, McColley SA, Case B, Steinhoff M, et al. Immune response to influenza vaccination in children with renal disease. Pediatr Nephrol. 1995;9:566–568. doi: 10.1007/BF00860934. [DOI] [PubMed] [Google Scholar]
- 104.Gabay C, Meier S, Gascon D, Posfay-Barbe K, Combescure C, et al. The influence of immunosuppressive therapy and underlying diseases on vaccine responses to influenza A H1N1/09 vaccines in inflammatory rheumatic diseases. Swiss Med Wkly. 2010;140:7S. [Google Scholar]
- 105.Gandhi MK, Egner W, Sizer L, Inman I, Zambon M, et al. Antibody responses to vaccinations given within the first two years after transplant are similar between autologous peripheral blood stem cell and bone marrow transplant recipients. Bone Marrow Transplant. 2001;28:775–781. doi: 10.1038/sj.bmt.1703239. [DOI] [PubMed] [Google Scholar]
- 106.Ganz PA, Shanley JD, Cherry JD. Responses of patients with neoplastic diseases to influenza virus vaccine. Cancer. 1978;42:2244–2247. doi: 10.1002/1097-0142(197811)42:5<2244::aid-cncr2820420523>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 107.Gelinck LB, Teng YK, Rimmelzwaan GF, van den Bemt BJ, Kroon FP, et al. Poor serological responses upon influenza vaccination in patients with rheumatoid arthritis treated with rituximab. Ann Rheum Dis. 2007;66:1402–1403. doi: 10.1136/ard.2007.071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gelinck LB, van der Bijl AE, Beyer WE, Visser LG, Huizinga TW, et al. The effect of anti-tumour necrosis factor alpha treatment on the antibody response to influenza vaccination. Ann Rheum Dis. 2008;67:713–716. doi: 10.1136/ard.2007.077552. [DOI] [PubMed] [Google Scholar]
- 109.Glesby MJ, Hoover DR, Farzadegan H, Margolick JB, Saah AJ. The effect of influenza vaccination on human immunodeficiency virus type 1 load: a randomized, double-blind, placebo-controlled study. J Infect Dis. 1996;174:1332–1336. doi: 10.1093/infdis/174.6.1332. [DOI] [PubMed] [Google Scholar]
- 110.Grekas D, Alivanis P, Kiriazopoulou V, Dioudis C, Sioulis A, et al. Influenza vaccination on renal transplant patients is safe and serologically effective. Int J Clin Pharmacol Ther Toxicol. 1993;31:553–556. [PubMed] [Google Scholar]
- 111.Gribabis DA, Panayiotidis P, Boussiotis VA, Hannoun C, Pangalis GA. Influenza virus vaccine in B-cell chronic lymphocytic leukaemia patients. Acta Haematol. 1994;91:115–118. doi: 10.1159/000204315. [DOI] [PubMed] [Google Scholar]
- 112.Gross PA, Lee H, Wolff JA, Hall CB, Minnefore AB, et al. Influenza immunization in immunosuppressed children. J Pediatr. 1978;92:30–35. doi: 10.1016/s0022-3476(78)80065-1. [DOI] [PubMed] [Google Scholar]
- 113.Gunthard HF, Wong JK, Spina CA, Ignacio C, Kwok S, et al. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent antiretroviral therapy. J Infect Dis. 2000;181:522–531. doi: 10.1086/315260. [DOI] [PubMed] [Google Scholar]
- 114.Hajiabdolbaghi M, Jam S, SeyedAlinaghi S, Jafari S, Badie BM, et al. Adverse reactions of trivalent influenza vaccine in HIV-infected individuals. Acta Medica Iranica. 2010;48:95–100. [PubMed] [Google Scholar]
- 115.Halasa N, Englund J, Nachman S, Weinberg G, Huber V, et al. Safety of live attenuated of live attenuated influenza vaccine in mild to moderately immunocompromised children with children. Acta Paediatr. 2009;98:151–152. doi: 10.1016/j.vaccine.2011.03.097. [DOI] [PubMed] [Google Scholar]
- 116.Hanania NA, Sockrider M, Castro M, Holbrook JT, Tonascia J, et al. Immune response to influenza vaccination in children and adults with asthma: effect of corticosteroid therapy. J Allergy Clin Immunol. 2004;113:717–724. doi: 10.1016/j.jaci.2003.12.584. [DOI] [PubMed] [Google Scholar]
- 117.Hayney MS, Moran J, Wiegert NA, Burlingham WJ. Lung transplant patients' T cell responses to influenza vaccine viruses between seasons. Vaccine. 2008;26:2596–2600. doi: 10.1016/j.vaccine.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 118.Hayney MS, Welter DL, Francois M, Reynolds AM, Love RB. Influenza vaccine antibody responses in lung transplant recipients. Prog Transplant. 2004;14:346–351. doi: 10.1177/152692480401400410. [DOI] [PubMed] [Google Scholar]
- 119.Hodges GR, Davis JW, Lewis HD, Jr, Whittier FC, Jr, Siegel CD, et al. Response to influenza A vaccine among high-risk patients. South Med J. 1979;72:29–32. doi: 10.1097/00007611-197901000-00010. [DOI] [PubMed] [Google Scholar]
- 120.Holvast A, van Assen S, de Haan A, Huckriede A, Benne CA, et al. Effect of a second, booster, influenza vaccination on antibody responses in quiescent systemic lupus erythematosus: an open, prospective, controlled study. Rheumatology. 2009;48:1294–1299. doi: 10.1093/rheumatology/kep200. [DOI] [PubMed] [Google Scholar]
- 121.Holvast A, van Assen S, de Haan A, Huckriede A, Benne CA, et al. Studies of cell-mediated immune responses to influenza vaccination in systemic lupus erythematosus. Arthritis Rheum. 2009;60:2438–2447. doi: 10.1002/art.24679. [DOI] [PubMed] [Google Scholar]
- 122.Hsieh YC, Lu MY, Kao CL, Chiang BL, Lin DT, et al. Response to influenza vaccine in children with leukemia undergoing chemotherapy. J Formos Med Assoc. 2002;101:700–704. [PubMed] [Google Scholar]
- 123.Huang KL, Armstrong JA, Ho M. Antibody response after influenza immunization in renal transplant patients receiving cyclosporin A or azathioprine. Infect Immun. 1983;40:421–424. doi: 10.1128/iai.40.1.421-424.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang KL, Ruben FL, Rinaldo CR, Jr, Kingsley L, Lyter DW, et al. Antibody responses after influenza and pneumococcal immunization in HIV-infected homosexual men. JAMA. 1987;257:2047–2050. [PubMed] [Google Scholar]
- 125.Huengsberg M, Chakraverty MP, Cooper G, Shahmanesh M. Response to influenza immunisation in asymptomatic HIV infected men. Genitourin Med. 1995;71:355–357. doi: 10.1136/sti.71.6.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ioniţă E, Gherghina I, Lupulescu E, Alexandrescu V, Tabra ME. The response in hemagglutinoinhibiting antibodies following influenza vaccination of HIV-infected children. Roum Arch Microbiol Immunol. 1998;57:53–57. [PubMed] [Google Scholar]
- 127.Iorio AM, Alatri A, Francisci D, Preziosi R, Neri M, et al. Immunogenicity of influenza vaccine (1993–94 winter season) in HIV-seropositive and -seronegative ex-intravenous drug users. Vaccine. 1997;15:97–102. doi: 10.1016/s0264-410x(96)00057-6. [DOI] [PubMed] [Google Scholar]
- 128.Isbel NM, Smith KGC, Leydon JA, Walker RG, Becker GJ. Mycophenolate mofetil suppresses the humoral response to influenza vaccination in renal transplant recipients [abstract]. Nephrology. 1997;3:S327. [Google Scholar]
- 129.Issa NC, Marty FM, Gagne LS, Koo S, Verrill KA, et al. Seroprotective titers against 2009 H1N1 influenza A virus after vaccination in allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2011;17:434–438. doi: 10.1016/j.bbmt.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaine JL, Kivitz AJ, Birbara C, Luo AY. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34:272–279. [PubMed] [Google Scholar]
- 131.Kanakoudi-Tsakalidou F, Trachana M, Pratsidou-Gertsi P, Tsitsami E, Kyriazopoulou-Dalaina V. Influenza vaccination in children with chronic rheumatic diseases and long-term immunosuppressive therapy. Clin Exp Rheumatol. 2001;19:589–594. [PubMed] [Google Scholar]
- 132.Kapetanovic MC, Saxne T, Nilsson JA, Geborek P. Influenza vaccination as model for testing immune modulation induced by anti-TNF and methotrexate therapy in rheumatoid arthritis patients. Rheumatology. 2007;46:608–611. doi: 10.1093/rheumatology/kel366. [DOI] [PubMed] [Google Scholar]
- 133.Keshtkar-Jahromi M, Argani H, Rahnavardi M, Mirchi E, Atabak S, et al. Antibody response to influenza immunization in kidney transplant recipients receiving either azathioprine or mycophenolate: a controlled trial. Am J Nephrol. 2008;28:654–660. doi: 10.1159/000119742. [DOI] [PubMed] [Google Scholar]
- 134.Kimball P, Verbeke S, Flattery M, Rhodes C, Tolman D. Influenza vaccination does not promote cellular or humoral activation among heart transplant recipients. Transplantation. 2000;69:2449–2451. doi: 10.1097/00007890-200006150-00042. [DOI] [PubMed] [Google Scholar]
- 135.Kimball P, Verbeke S, Tolman D. Influenza vaccination among heart transplant recipients. Transplant Proc. 2001;33:1785–1786. doi: 10.1016/s0041-1345(00)02679-8. [DOI] [PubMed] [Google Scholar]
- 136.King JC, Jr, Fast PE, Zangwill KM, Weinberg GA, Wolff M, et al. Safety, vaccine virus shedding and immunogenicity of trivalent, cold-adapted, live attenuated influenza vaccine administered to human immunodeficiency virus-infected and noninfected children. Pediatr Infect Dis J. 2001;20:1124–1131. doi: 10.1097/00006454-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 137.King JC, Jr, Treanor J, Fast PE, Wolff M, Yan L, et al. Comparison of the safety, vaccine virus shedding, and immunogenicity of influenza virus vaccine, trivalent, types A and B, live cold-adapted, administered to human immunodeficiency virus (HIV)-infected and non-HIV-infected adults. J Infect Dis. 2000;181:725–728. doi: 10.1086/315246. [DOI] [PubMed] [Google Scholar]
- 138.Knysz B, Brydak LB, Inglot M, Gladysz A. The response to vaccination against influenza in the season 1991/92 in HIV-1 infected persons. Problemy HIV i AIDS. 2001;7:29–34. [Google Scholar]
- 139.Kosalaraksa P, Srirompotong U, Newman R, Lumbiganon P, Wood JM. Serological response to influenza vaccine in HIV-infected children in orphanages. 2nd Thailand Human Influenza Research Meeting, 21–22 October 2009, Bangkok, Thailand.
- 140.Kostyanaya IE, Meisner AF, Aksenova VA, Baturo AP. Experience in administration of the vaccines “Pneumo 23” and “VAXIGRIP” among children at risk infected with Mycobacterium tuberculosis. Bulleten Vakzinazija 1: [epub] 2002 [Google Scholar]
- 141.Kroon FP, Rimmelzwaan GF, Roos MT, Osterhaus AD, Hamann D, et al. Restored humoral immune response to influenza vaccination in HIV-infected adults treated with highly active antiretroviral therapy. AIDS. 1998;12:F217–F223. doi: 10.1097/00002030-199817000-00002. [DOI] [PubMed] [Google Scholar]
- 142.Kroon FP, van Dissel JT, de Jong JC, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS. 1994;8:469–476. doi: 10.1097/00002030-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 143.Kroon FP, van Dissel JT, de Jong JC, Zwinderman K, van Furth R. Antibody response after influenza vaccination in HIV-infected individuals: a consecutive 3-year study. Vaccine. 2000;18:3040–3049. doi: 10.1016/s0264-410x(00)00079-7. [DOI] [PubMed] [Google Scholar]
- 144.Kubiet MA, Gonzalez-Rothi RJ, Cottey R, Bender BS. Serum antibody response to influenza vaccine in pulmonary patients receiving corticosteroids. Chest. 1996;110:367–370. doi: 10.1378/chest.110.2.367. [DOI] [PubMed] [Google Scholar]
- 145.Kubota T, Nii T, Nanki T, Kohsaka H, Harigai M, et al. Anti-tumor necrosis factor therapy does not diminish the immune response to influenza vaccine in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2007;17:531–533. doi: 10.1007/s10165-007-0632-5. [DOI] [PubMed] [Google Scholar]
- 146.Kudo M, Onuma S, Kamio T, Endo Y, Tanaka H, et al. Efficacy of influenza vaccination in children with renal diseases undergone immunosuppressive therapy. Nihon Shoni Jinzobyo Gakkai Zasshi. 2005;18:21–25. [Google Scholar]
- 147.Kumar D, Bergeron A, Hidalgo L, Campbell P, Wasilenko S, et al. Cell-Mediated Immune Responses to Influenza Vaccination in Renal Transplant Recipients. Am J Transplant. 2010;10:207–207. [Google Scholar]
- 148.Kumar D, Bergeron A, Humar A, Hidalgo L, Manuel O, et al. Does influenza vaccination induce de novo HLA alloantibody formation in transplant recipients? Am J Transplant. 2010;10:207–208. [Google Scholar]
- 149.Kumar SS, Ventura AK, VanderWerf B. Influenza vaccination in renal transplant recipients. JAMA. 1978;239:840–842. [PubMed] [Google Scholar]
- 150.Lange B, Shapiro SA, Waldman MT, Proctor E, Arbeter A. Antibody responses to influenza immunization of children with acute lymphoblastic leukemia. J Infect Dis. 1979;140:402–406. doi: 10.1093/infdis/140.3.402. [DOI] [PubMed] [Google Scholar]
- 151.Lawal A, Basler C, Branch A, Gutierrez J, Schwartz M, et al. Influenza vaccination in orthotopic liver transplant recipients: absence of post administration ALT elevation. Am J Transplant. 2004;4:1805–1809. doi: 10.1111/j.1600-6143.2004.00564.x. [DOI] [PubMed] [Google Scholar]
- 152.Lo W, Whimbey E, Elting L, Couch R, Cabanillas F, et al. Antibody response to a two-dose influenza vaccine regimen in adult lymphoma patients on chemotherapy. Eur J Clin Microbiol Infect Dis. 1993;12:778–782. doi: 10.1007/BF02098469. [DOI] [PubMed] [Google Scholar]
- 153.Louie JS, Nies KM, Shoji KT, Fraback RC, Abrass C, et al. Clinical and antibody responses after influenza immunization in systemic lupus erythematosus. Ann Intern Med. 1978;88:790–792. doi: 10.7326/0003-4819-88-6-790. [DOI] [PubMed] [Google Scholar]
- 154.Lu CC, Wang YC, Lai JH, Lee TSH, Lin HT, et al. A/H1N1 influenza vaccination in patients with systemic lupus erythematosus: Safety and immunity. Vaccine. 2011;29:444–450. doi: 10.1016/j.vaccine.2010.10.081. [DOI] [PubMed] [Google Scholar]
- 155.Lu Y, Jacobson DL, Ashworth LA, Grand RJ, Meyer AL, et al. Immune response to influenza vaccine in children with inflammatory bowel disease. Am J Gastroenterol. 2009;104:444–453. doi: 10.1038/ajg.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lyall EG, Charlett A, Watkins P, Zambon M. Response to influenza virus vaccination in vertical HIV infection. Arch Dis Child. 1997;76:215–218. doi: 10.1136/adc.76.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Macias J, Pineda JA, Leal M, Abad MA, Delgado J, et al. HIV-1 plasma viremia not increased in patients receiving highly active antiretroviral therapy after influenza vaccination. Eur J Clin Microbiol Infect Dis. 2001;20:46–48. doi: 10.1007/s100960000421. [DOI] [PubMed] [Google Scholar]
- 158.Madan RP, Tan M, Fernandez-Sesma A, Moran TM, Emre S, et al. A prospective, comparative study of the immune response to inactivated influenza vaccine in pediatric liver transplant recipients and their healthy siblings. Clin Infect Dis. 2008;46:712–718. doi: 10.1086/527391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Magnani G, Falchetti E, Pollini G, Bacchi-Reggiani L, Grigioni F, et al. Safety and efficacy of two types of influenza vaccination in heart transplant recipients: A prospective randomised controlled study. J Heart Lung Transplant. 2005;24:588–592. doi: 10.1016/j.healun.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 160.Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, et al. Compromised B cell responses in influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191:1442–1450. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 161.Malleson PN, Tekano JL, Scheifele DW, Weber JM. Influenza immunization in children with chronic arthritis: A prospective study. J Rheumatol. 1993;20:1769–1773. [PubMed] [Google Scholar]
- 162.Mamula P, Markowitz JE, Piccoli DA, Klimov A, Cohen L, et al. Immune response to influenza vaccine in pediatric patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:851–856. doi: 10.1016/j.cgh.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 163.Marczynska M, Brydak LB, Machala M, Oldakowska A, Zegadlo M. Influenza vaccination in HIV-infected children. Acta Paediatr. 2001;90:466–467. [PubMed] [Google Scholar]
- 164.Matsuzaki A, Suminoe A, Koga Y, Kinukawa N, Kusuhara K, et al. Immune response after influenza vaccination in children with cancer. Pediatr Blood Cancer. 2005;45:831–837. doi: 10.1002/pbc.20470. [DOI] [PubMed] [Google Scholar]
- 165.Mauch TJ, Crouch NA, Freese DK, Braunlin EA, Dunn DL, et al. Antibody response of pediatric solid organ transplant recipients to immunization against influenza virus. J Pediatr. 1995;127:957–960. doi: 10.1016/s0022-3476(95)70037-4. [DOI] [PubMed] [Google Scholar]
- 166.Mazza JJ, Yale SH, Arrowood JR, Reynolds CE, Glurich I, et al. Efficacy of the influenza vaccine in patients with malignant lymphoma. Clin Med Res. 2005;3:214–220. doi: 10.3121/cmr.3.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mazzone PJ, Mossad SB, Mawhorter SD, Mehta AC, Schilz RJ, et al. The humoral immune response to influenza vaccination in lung transplant patients. Eur Respir J. 2001;18:971–976. doi: 10.1183/09031936.01.00215201. [DOI] [PubMed] [Google Scholar]
- 168.Mercado U. Antibody response to influenza immunization in patients with systemic lupus erythematosus. J Rheumatol. 2003;30: 2295; author reply:2295–2296. [PubMed] [Google Scholar]
- 169.Mercado U, Acosta H, Avendano L. Influenza vaccination of patients with systemic lupus erythematosus. Rev Invest Clin. 2004;56:16–20. [PubMed] [Google Scholar]
- 170.Meyer S, Adam M, Ilchmann C, Eulenburg C, Sattinger E, et al. Antibody response after a single dose of an AS03-adjuvanted split-virion influenza A (H1N1) vaccine in heart transplant recipients. Transpl Int. 2010;23:0028. doi: 10.1097/TP.0b013e3182115be0. [DOI] [PubMed] [Google Scholar]
- 171.Miotti PG, Nelson KE, Dallabetta GA, Farzadegan H, Margolick J, et al. The influence of HIV infection on antibody responses to a two-dose regimen of influenza vaccine. JAMA. 1989;262:779–783. [PubMed] [Google Scholar]
- 172.Miyamae T, Iwata N, Imagawa T, Mori M, Mitsuda T, et al. Efficacy and side effects of influenza vaccine for patients with pediatric rheumatic diseases under steroid and/or immunosuppressive therapy. J Pediatr Infect Dis Immunol. 2002;14:121–124. [Google Scholar]
- 173.Montoya CJ, Toro MF, Aguirre C, Bustamante A, Hernandez M, et al. Abnormal humoral immune response to influenza vaccination in pediatric type-1 human immunodeficiency virus infected patients receiving highly active antiretroviral therapy. Mem Inst Oswaldo Cruz. 2007;102:501–508. doi: 10.1590/s0074-02762007005000055. [DOI] [PubMed] [Google Scholar]
- 174.Mulder SF, Jacobs JFM, Olde Nordkamp MAM, Kremer IK, Mulders PFA, et al. Immunological response to influenza vaccine in cancer patients undergoing treatment with sunitinib or sorafenib. Eur J Cancer. 2009;7(Suppl):116. [Google Scholar]
- 175.Nailescu C, Xu X, Zhou H, Hall H, Wilson AC, et al. Influenza vaccine after pediatric kidney transplant: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol. 2011;26:459–467. doi: 10.1007/s00467-010-1729-1. [DOI] [PubMed] [Google Scholar]
- 176.Nelson KE, Clements ML, Miotti P, Cohn S, Polk BF. The influence of human immunodeficiency virus (HIV) infection on antibody responses to influenza vaccines. Ann Intern Med. 1988;109:383–388. doi: 10.7326/0003-4819-109-5-383. [DOI] [PubMed] [Google Scholar]
- 177.Nicholson K, Nguyen-Van-Tam J, Ahmed A, Wiselka M, Leese J, et al. Randomised placebo-controlled crossover trial on effect of inactivated influenza vaccine on pulmonary function in asthma. Lancet. 1998;351:326–331. doi: 10.1016/S0140-6736(97)07468-0. [DOI] [PubMed] [Google Scholar]
- 178.Nordoy T, Aaberge IS, Husebekk A, Samdal HH, Steinert S, et al. Cancer patients undergoing chemotherapy show adequate serological response to vaccinations against influenza virus and Streptococcus pneumoniae. Med Oncol. 2002;19:71–78. doi: 10.1385/MO:19:2:71. [DOI] [PubMed] [Google Scholar]
- 179.O'Brien WA, Grovit-Ferbas K, Namazi A, Ovcak-Derzic S, Wang HJ, et al. Human immunodeficiency virus-type 1 replication can be increased in peripheral blood of seropositive patients after influenza vaccination. Blood. 1995;86:1082–1089. [PubMed] [Google Scholar]
- 180.Oren S, Mandelboim M, Braun-Moscovici Y, Paran D, Ablin J, et al. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann Rheum Dis. 2008;67:937–941. doi: 10.1136/ard.2007.077461. [DOI] [PubMed] [Google Scholar]
- 181.Orlando G, Pariani E, Mazza F, Tanzi E, Meraviglia P, et al. Pandemic influenza vaccine in adult HIV-1-infected patients. AIDS. 2010;24:2142–2143. doi: 10.1097/QAD.0b013e32833cfcb0. [DOI] [PubMed] [Google Scholar]
- 182.Ortbals DW, Liebhaber H, Presant CA, Van Amburg AL, 3rd, Lee JY. Influenza immunization of adult patients with malignant diseases. Ann Intern Med. 1977;87:552–557. doi: 10.7326/0003-4819-87-5-552. [DOI] [PubMed] [Google Scholar]
- 183.Pabico RC, Douglas RG, Betts RF, McKenna BA, Freeman RB. Antibody response to influenza vaccination in renal transplant patients: correlation with allograft function. Ann Intern Med. 1976;85:431–436. doi: 10.7326/0003-4819-85-4-431. [DOI] [PubMed] [Google Scholar]
- 184.Park CL, Frank AL, Sullivan M, Jindal P, Baxter BD. Influenza vaccination of children during acute asthma exacerbation and concurrent prednisone therapy. Pediatrics. 1996;98:196–200. [PubMed] [Google Scholar]
- 185.Pinto LA, Blazevic V, Anderson SA, Venzon DJ, Trubey CM, et al. Influenza virus-stimulated generation of anti-human immunodeficiency virus (HIV) activity after influenza vaccination in HIV-infected individuals and healthy control subjects. J Infect Dis. 2001;183:1000–1008. doi: 10.1086/319277. [DOI] [PubMed] [Google Scholar]
- 186.Porter CC, Edwards KM, Zhu Y, Frangoul H. Immune responses to influenza immunization in children receiving maintenance chemotherapy for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2004;42:36–40. doi: 10.1002/pbc.10459. [DOI] [PubMed] [Google Scholar]
- 187.Ragni MV, Ruben FL, Winkelstein A, Spero JA, Bontempo FA, et al. Antibody responses to immunization of patients with hemophilia with and without evidence of human immunodeficiency virus (human T-lymphotropic virus type III) infection. J Lab Clin Med. 1987;109:545–549. [PubMed] [Google Scholar]
- 188.Rambal V, Mueller K, Nickel P, Horstrup J, Friedrich P, et al. Evaluation of Safety and Efficacy of H1N1 Swine-Origin Influenza A Vaccination in Renal Transplant Patients. Am J Transplant. 2010;10:564–565. [Google Scholar]
- 189.Ramilo O, Hicks PJ, Borvak J, Gross LM, Zhong D, et al. T cell activation and human immunodeficiency virus replication after influenza immunization of infected children. Pediatr Infect Dis J. 1996;15:197–203. doi: 10.1097/00006454-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 190.Ranieri R, Veronelli A, Santambrogio C, Pontiroli AE. Impact of influenza vaccine on response to vaccination with pneumococcal vaccine in HIV patients. AIDS Res Human Retroviruses. 2005;21:407–409. doi: 10.1089/aid.2005.21.407. [DOI] [PubMed] [Google Scholar]
- 191.Rapezzi D, Sticchi L, Racchi O, Mangerini R, Ferraris AM, et al. Influenza vaccine in chronic lymphoproliferative disorders and multiple myeloma. Eur J Haematol. 2003;70:225–230. doi: 10.1034/j.1600-0609.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 192.Reboli AC, Palmer A, Byrne B, McCoy P, Chernoff D, et al. Influenza vaccination has no effect on viral load in HIV-infected patients with higher CD4 counts. Clin Infect Dis. 1996;23:907. [Google Scholar]
- 193.Reid DW, Bromly CL, Stenton SC, Hendrick DJ, Bourke SJ. A double-blind placebo-controlled study of the effect of influenza vaccination on airway responsiveness in asthma. Respir Med. 1998;92:1010–1011. doi: 10.1016/s0954-6111(98)90346-8. [DOI] [PubMed] [Google Scholar]
- 194.Reilly A, Kersun LS, McDonald K, Weinberg A, Jawad AF, et al. The efficacy of influenza vaccination in a pediatric oncology population. J Pediatr Hematol Oncol. 2010;32:e177–181. doi: 10.1097/MPH.0b013e3181d869f3. [DOI] [PubMed] [Google Scholar]
- 195.Ristow SC, Douglas RG, Jr, Condemi JJ. Influenza vaccination of patients with systemic lupus erythematosus. Ann Intern Med. 1978;88:786–789. doi: 10.7326/0003-4819-88-6-786. [DOI] [PubMed] [Google Scholar]
- 196.Robertson JD, Nagesh K, Jowitt SN, Dougal M, Anderson H, et al. Immunogenicity of vaccination against influenza, Streptococcus pneumoniae and Haemophilus influenzae type B in patients with multiple myeloma. Br J Cancer. 2000;82:1261–1265. doi: 10.1054/bjoc.1999.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Romanowska M, Banaszkiewicz A, Nowak I, Radzikowski A, Brydak LB. Immunization against influenza during the 2005/2006 epidemic season and the humoral response in children with diagnosed inflammatory bowel disease (IBD). Med Sci Monit. 2010;16:CR433–439. [PubMed] [Google Scholar]
- 198.Rosok B, Voltersvik P, Bjerknes R, Axelsson M, Haaheim LR, et al. Dynamics of HIV-1 replication following influenza vaccination of HIV+ individuals. Clin Exp Immunol. 1996;104:203–207. doi: 10.1046/j.1365-2249.1996.25732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rucker RP, Day NK, Good RA, Kamchaisatian W, Emmanuel P, et al. Effect of influenza virus vaccine on the expression of human immunodeficiency virus co-receptor CCR5. Ann Allergy Asthma Immunol. 2004;93:272–276. doi: 10.1016/S1081-1206(10)61500-1. [DOI] [PubMed] [Google Scholar]
- 200.Rytel MW, Niebojewski RA, Rosenkranz MA, Sedmak G. Humoral and cell mediated immune response to bivalent influenza A/NJ/76 and A/Vict/75 vaccine in renal allograft recipients. Dev Biol Stand. 1977;39:225–230. [PubMed] [Google Scholar]
- 201.Salassa B, Zucco S, Macor A, Sciullo D, Ruffatto R, et al. Influenza vaccine response in HIV patients. Int Conf AIDS. 1996;11:121. [Google Scholar]
- 202.Salemi S, Picchianti-Diamanti A, Germano V, Donatelli I, Di Martino A, et al. Influenza vaccine administration in rheumatoid arthritis patients under treatment with TNFalpha blockers: safety and immunogenicity. Clin Immunol. 2010;134:113–120. doi: 10.1016/j.clim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 203.Salles MJ, Sens YA, Boas LS, Machado CM. Influenza virus vaccination in kidney transplant recipients: serum antibody response to different immunosuppressive drugs. Clin Transplant. 2010;24:E17–E23. doi: 10.1111/j.1399-0012.2009.01095.x. [DOI] [PubMed] [Google Scholar]
- 204.Sanchez-Fructuoso AI, Prats D, Naranjo P, Fernandez-Perez C, Gonzalez Ma J, et al. Influenza virus immunization effectivity in kidney transplant patients subjected to two different triple-drug therapy immunosuppression protocols: Mycophenolate versus azathioprine. Transplantation. 2000;69:436–439. doi: 10.1097/00007890-200002150-00023. [DOI] [PubMed] [Google Scholar]
- 205.Schafer AI, Churchill WH, Ames P, Weinstein L. The influence of chemotherapy on response of patients with hematologic malignancies to influenza vaccine. Cancer. 1979;43:25–30. doi: 10.1002/1097-0142(197901)43:1<25::aid-cncr2820430103>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 206.Scharpe J, Evenepoel P, Maes B, Bammens B, Claes K, et al. Influenza vaccination is efficacious and safe in renal transplant recipients. Am J Transplant. 2008;8:332–337. doi: 10.1111/j.1600-6143.2007.02066.x. [DOI] [PubMed] [Google Scholar]
- 207.Schneider MM, Sprenger MJ, Hoepelman IM, van der Graaf Y, Borleffs JC. Antibody response to tetravalent influenza subunit vaccine in patients infected with human immunodeficiency virus type 1. Int J Antimicrob Agents. 1996;6:195–200. doi: 10.1016/0924-8579(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 208.Shahgholi E, Ehsani MA, Salamati P, Maysamie A, Sotoudeh K, et al. Immunogenicity of trivalent influenza vaccine in children with acute lymphoblastic leukemia during maintenance therapy. Pediatr Blood Cancer. 2010;54:716–720. doi: 10.1002/pbc.22421. [DOI] [PubMed] [Google Scholar]
- 209.Shildt RA, Luedke DW, Kasai G, El-Beheri S, Laham MN. Antibody response to influenza immunization in adult patients with malignant disease. Cancer. 1979;44:1629–1635. doi: 10.1002/1097-0142(197911)44:5<1629::aid-cncr2820440514>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 210.Soesman NM, Rimmelzwaan GF, Nieuwkoop NJ, Beyer WE, Tilanus HW, et al. Efficacy of influenza vaccination in adult liver transplant recipients. J Med Virol. 2000;61:85–93. [PubMed] [Google Scholar]
- 211.Spies CD, Kip M, Lau A, Sander M, Breuer JP, et al. Influence of vaccination and surgery on HLA-DR expression in patients with upper aerodigestive tract cancer. J Int Med Res. 2008;36:296–307. doi: 10.1177/147323000803600212. [DOI] [PubMed] [Google Scholar]
- 212.Spitaleri G, Delmonte A, Toffalorio F, De Pas TM, Gregorc V. Safety of concomitant administration of seasonal and/or H1N1 flu vaccination in patients receiving erlotinib for advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:752–754. doi: 10.1097/JTO.0b013e3181d6b8a1. [DOI] [PubMed] [Google Scholar]
- 213.Staprans SI, Hamilton BL, Follansbee SE, Elbeik T, Barbosa P, et al. Activation of virus replication after vaccination of HIV-1-infected individuals. J Exp Med. 1995;182:1727–1737. doi: 10.1084/jem.182.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Steinherz PG, Brown AE, Gross PA, Braun D, Ghavimi F, et al. Influenza immunization of children with neoplastic diseases. Cancer. 1980;45:750–756. doi: 10.1002/1097-0142(19800215)45:4<750::aid-cncr2820450423>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 215.Stiver HG, Graves P, Meiklejohn G, Schroter G, Eickhoff TC. Impaired serum antibody response to inactivated influenza A and B vaccine in renal transplant recipients. Infect Immun. 1977;16:738–741. doi: 10.1128/iai.16.3.738-741.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Stiver HG, Weinerman BH. Impaired serum antibody response to inactivated influenza A and B vaccine in cancer patients. Can Med Assoc J. 1978;119:733–735, 738. [PMC free article] [PubMed] [Google Scholar]
- 217.Sumaya CV, Williams TE, Brunell PA. Bivalent influenza vaccine in children with cancer. J Infect Dis. 1977;136(Suppl):S656–660. doi: 10.1093/infdis/136.supplement_3.s656. [DOI] [PubMed] [Google Scholar]
- 218.Takata T, Suzumiya J, Ishikawa T, Takamatsu Y, Ikematsu H, et al. Attenuated antibody reaction for the primary antigen but not for the recall antigen of influenza vaccination in patients with non-Hodgkin B-cell lymphoma after the administration of rituximab-CHOP. J Clin Exp Hematop. 2009;49:9–13. doi: 10.3960/jslrt.49.9. [DOI] [PubMed] [Google Scholar]
- 219.Tanzi E, Esposito S, Bojanin J, Amendola A, Trabattoni D, et al. Immunogenicity and effect of a virosomal influenza vaccine on viral replication and T-cell activation in HIV-infected children receiving highly active antiretroviral therapy. J Med Virol. 2006;78:440–445. doi: 10.1002/jmv.20559. [DOI] [PubMed] [Google Scholar]
- 220.Tarasova AA. Effectiveness of influenza vaccination of children with rheumatic diseases. Detskie Infekzii. 2006;3:43–46. [Google Scholar]
- 221.Tasker SA, O'Brien WA, Treanor JJ, Weiss PJ, Olson PE, et al. Effects of influenza vaccination in HIV-infected adults: a double-blind, placebo-controlled trial. Vaccine. 1998;16:1039–1042. doi: 10.1016/s0264-410x(97)00275-2. [DOI] [PubMed] [Google Scholar]
- 222.Tasker SA, Treanor JJ, Paxton WB, Wallace MR. Efficacy of influenza vaccination in HIV-infected persons - a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;131:430–433. doi: 10.7326/0003-4819-131-6-199909210-00006. [DOI] [PubMed] [Google Scholar]
- 223.Tebas P, Frank I, Lewis M, Quinn J, Zifchak L, et al. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS. 2010;24:2187–2192. doi: 10.1097/QAD.0b013e32833c6d5c. [DOI] [PubMed] [Google Scholar]
- 224.Thibault F, Noble CP, Morelon E, Daoud S, Cahen R, et al. Safety and efficacy of an inactivated, unadjuvanted vaccine adainst the novel influenza A variant (H1N1v) in renal transplant recipients (transfluvac study). 2010. P573 Options for the Control of Influenza VII, 3–7 September 2010, Hong Kong, SAR, China.
- 225.Tremblay CL, Rouleau D, Fortin C, Toma E, Sylla M, et al. Immunogenicity and tolerability of an inactivated and adjuvanted pandemic H1N1 influenza vaccine, in HIV-1-infected patients. Vaccine. 2011;29:1359–1363. doi: 10.1016/j.vaccine.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 226.Tsuru T, Suzaki M, Yoshio N, Mima T, Nakashima H, et al. Immune response against influenza vaccination in patients with rheumatoid arthritis undergoing IL-6 inhibition therapy (tocilizumab) compared with DMARDs. Nihon Rinsho Men'eki Gakkai Sokai Shorokushu. 2008;36:124. [Google Scholar]
- 227.van Assen S, Holvast A, Benne CA, Posthumus MD, van Leeuwen MA, et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010;62:75–81. doi: 10.1002/art.25033. [DOI] [PubMed] [Google Scholar]
- 228.van Assen S, Holvast A, Telgt DS, Benne CA, de Haan A, et al. Serological responses after vaccination for influenza in patients with primary humoral immunodeficiency. Clin Exp Immunol. 2008;154:117. [Google Scholar]
- 229.van Assen S, Holvast A, Telgt DS, Benne CA, de Haan A, et al. Patients with humoral primary immunodeficiency do not develop protective anti-influenza antibody titers after vaccination with trivalent subunit influenza vaccine. Clin Immunol. 2010;136:228–235. doi: 10.1016/j.clim.2010.03.430. [DOI] [PubMed] [Google Scholar]
- 230.van der Velden AM, Mulder AH, Hartkamp A, Diepersloot RJ, van Velzen-Blad H, et al. Influenza virus vaccination and booster in B-cell chronic lymphocytic leukaemia patients. Eur J Intern Med. 2001;12:420–424. doi: 10.1016/s0953-6205(01)00149-2. [DOI] [PubMed] [Google Scholar]
- 231.Vaziri S, Azad TM, Najafi F, Janbakhsh A, Sayyad B, et al. Surveying immunogenicity and safety of influenza vaccination in health care workers and HIV-infected individuals. Iran J Clin Infect Dis. 2009;4:19–23. [Google Scholar]
- 232.Vazquez-Alvarez MD, Medrano-Lopez C, Camino-Lopez M. H1N1 influenza vaccination and infection in pediatric heart transplants. J Heart Lung Transplant. 2010;29:1318. doi: 10.1016/j.healun.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 233.Versluis DJ, Beyer WE, Masurel N, Weimar W. Influenza vaccination in dialysis and transplant patients. Antiviral Res. 1985;(Suppl 1):289–292. doi: 10.1016/s0166-3542(85)80040-1. [DOI] [PubMed] [Google Scholar]
- 234.Versluis DJ, Beyer WE, Masurel N, Wenting GJ, Weimar W. Impairment of the immune response to influenza vaccination in renal transplant recipients by cyclosporine, but not azathioprine. Transplantation. 1986;42:376–379. doi: 10.1097/00007890-198610000-00009. [DOI] [PubMed] [Google Scholar]
- 235.Versluis DJ, Wenting GJ, Beyer WEP, et al. Cyclosporine A impairs the humoral immune response after influenza vaccination in renal transplant recipients. Transplant Proc. 1986;18:1348–1349. doi: 10.1097/00007890-198610000-00009. [DOI] [PubMed] [Google Scholar]
- 236.Vigano A, Bricalli D, Trabattoni D, Salvaggio A, Ruzzante S, et al. Immunization with both T cell-dependent and T cell-independent vaccines augments HIV viral load secondarily to stimulation of tumor necrosis factor alpha. AIDS Res Human Retroviruses. 1998;14:727–734. doi: 10.1089/aid.1998.14.727. [DOI] [PubMed] [Google Scholar]
- 237.Vigano A, Zuccotti GV, Pacei M, Erba P, Castelletti E, et al. Humoral and cellular response to influenza vaccine in HIV-infected children with full viroimmunologic response to antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;48:289–296. doi: 10.1097/QAI.0b013e3181632cda. [DOI] [PubMed] [Google Scholar]
- 238.Weinberg GA, King JC, Jr, Zangwill K, Fast PE, Wolff M, et al. Safety and immunogenicity of cold-adapted, live attenuated, influenza vaccine, trivalent (LAIV) in HIV-infected children. Pediatr Res. 2001;49:242a. doi: 10.1097/00006454-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 239.Wiesik-Szewczyk E, Romanowska M, Mielnik P, Chwalinska-Sadowska H, Brydak LB, et al. Anti-influenza vaccination in systemic lupus erythematosus patients: an analysis of specific humoral response and vaccination safety. Clin Rheumatol. 2010;29:605–613. doi: 10.1007/s10067-010-1373-y. [DOI] [PubMed] [Google Scholar]
- 240.Willcocks LC, Chaudhry AN, Smith JC, Ojha S, Doffinger R, et al. The effect of sirolimus therapy on vaccine responses in transplant recipients. Am J Transplant. 2007;7:2006–2011. doi: 10.1111/j.1600-6143.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- 241.Winer RL, Ellis MH, Cesario TC, Mirahmadi KS, Tilles JG. Swine influenza vaccination in transplant and dialysis patients. Dial Transplant. 1978;7:207–210. [Google Scholar]
- 242.Wyzgal J, Brydak LB, Zygier D, Paczek L, Rowinski W, et al. Study on efficacy of influenza vaccination in renal allograft recipients. Transplant Proc. 2002;34:572–575. doi: 10.1016/s0041-1345(01)02849-4. [DOI] [PubMed] [Google Scholar]
- 243.Xu Y, Methuku N, Coimbatore P, Fitzgerald T, Huang Y, et al. A prospective study of the immunogenicity of inactivated influenza A (H1N1) 2009 monovalent vaccine in patients who have solid or hematologic malignancies. 2009. 48th Annual Meeting of the Infectious Diseases Society of America, 21–24 October 2010, Vancouver, Canada. pp. Abstract 5075, poster LB-5013.
- 244.Yalcin S, Kondolot M, Albayrak N, Altas B, Karacan Y, et al. Serological response to influenza vaccine haematopoetic stem cell transplantation patients. Bone Marrow Transplant. 2010;45:S204. doi: 10.1007/s00277-009-0897-1. [DOI] [PubMed] [Google Scholar]
- 245.Yalçin SS, Kondolot M, Albayrak N, Altas AB, Karacan Y, et al. Serological response to influenza vaccine after hematopoetic stem cell transplantation. Ann Hematol. 2010;89:913–918. doi: 10.1007/s00277-009-0897-1. [DOI] [PubMed] [Google Scholar]
- 246.Yamada A, Yamawaki H, Tsuda N, Baba K, Yabuuchi H, et al. Trial of split-product trivalent influenza vaccine in high-risk children. Biken J. 1982;25:89–95. [PubMed] [Google Scholar]
- 247.Yamanaka H, Teruya K, Tanaka M, Kikuchi Y, Takahashi T, et al. Efficacy and immunologic responses to influenza vaccine in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005;39:167–173. [PubMed] [Google Scholar]
- 248.Yerly S, Wunderli W, Wyler CA, Kaiser L, Hirschel B, et al. Influenza immunization of HIV-1-infected patients does not increase HIV-1 viral load. AIDS. 1994;8:1503–1504. doi: 10.1097/00002030-199410000-00022. [DOI] [PubMed] [Google Scholar]
- 249.Zuccotti GV, Cucchi C, Sala D, Giovannini M. Immunogenicity and safety of a virosomal influenza vaccine in HIV-infected children. Acta Paediatr. 2002;91: 486; author reply:487. doi: 10.1111/j.1651-2227.2002.tb01676.x. [DOI] [PubMed] [Google Scholar]
- 250.Zuccotti GV, Zenga A, Durando P, Massone L, Bruzzone B, et al. Immunogenicity and tolerability of a trivalent virosomal influenza vaccine in a cohort of HIV-infected children. J Int Med Res. 2004;32:492–499. doi: 10.1177/147323000403200506. [DOI] [PubMed] [Google Scholar]
- 251.Zykov MP, Rafaelskaya TI, Volkon VM, Knoring BE, Shenderova RI, et al. Influenza virus vaccination of patients with pulmonary tuberculosis. Problemy Tuberkuleza. 1980;58:3–5. [Google Scholar]
- 252.Zykov MP, Subbotina TI, Vishnevsky BI, Shenderova RI, Knoring BE, et al. Vaccination against influenza in patients with pulmonary tuberculosis in tuberculosis dispensaries. Problemy Tuberkuleza. 1983;61:17–21. [PubMed] [Google Scholar]
- 253.Fine AD, Bridges CB, De Guzman AM, Glover L, Zeller B, et al. Influenza A among patients with human immunodeficiency virus: an outbreak of infection at a residential facility in New York City. Clin Infect Dis. 2001;32:1784–1791. doi: 10.1086/320747. [DOI] [PubMed] [Google Scholar]
- 254.Committee for Human Medicinal Products. Guideline on influenza vaccines prepared from viruses with the potential to cause a pandemic and intended for use outside of the core dossier context. EMEA/CHMP/VWP/263499/2006. London: European Medicines Agency; 2007. [Google Scholar]
- 255.Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, et al. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375:1100–1108. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 256.Montomoli E. Correlates of protection. In: Rappuoli R, Del Giudice G, editors. Influenza vaccines for the future. Basel: Berkhauser Verlag; 2008. pp. 139–158. [Google Scholar]
- 257.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 258.Haroon M, Adeeb F, Eltahir A, Harney S. The uptake of influenza and pneumococcal vaccination among immunocompromised patients attending rheumatology outpatient clinics. Joint Bone Spine: [Epub ahead of print] 2010 doi: 10.1016/j.jbspin.2010.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of risk of bias using the Cochrane Collaboration tool (n = 191). Legend: green = low risk of bias; yellow = unclear risk of bias; red = high risk of bias.
(PDF)
Forest plots for immune response to vaccination question. Figure S2.1. Forest plot of studies of seroconversion (≥4 fold rise in haemagglutination inhibition titre): seasonal influenza A(H1N1), vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2.2. Forest plot of studies of seroconversion (≥4 fold rise in haemagglutination inhibition titre): influenza A(H3N2), vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2.3. Forest plot of studies of seroconversion (≥4 fold rise in haemagglutination inhibition titre): seasonal influenza B, vaccinated immunocompromised patients versus vaccinated immunocompetent contr ols. Figure S2.4. Forest plot of studies of seroconversion (≥4 fold rise in haemagglutination inhibition titre): seasonal influenza A(H1N1), vaccinated immunocompromised patients versus placebo or no vaccination. Figure S2.5. Forest plot of studies of seroconversion (≥4 fold rise in haemagglutination inhibition titre): influenza A(H3N2), vaccinated immunocompromised patients versus placebo or no vaccination. Figure S2.6. Forest plot of studies of seroconversion (≥4 fold rise in haemagglutination inhibition titre): influenza B, vaccinated immunocompromised patients versus placebo or no vaccination. Figure S2.7. Forest plo t of studies of seroconversion (<1∶40 pre-vaccination to ≥1∶40 haemagglutination inhibition titre post vaccination): seasonal influenza A(H1N1), vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2.8. Forest plot of studies of seroconversion (<1∶40 pre-vaccination to ≥1∶40 haemagglutination inhibition titre post vaccination): influenza A(H3N2), vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2.9. Forest plot of studies of seroconversion (<1∶40 pre-vaccination to ≥1∶40 haemagglutination inhibition titre post vaccination): seasonal influenza B, vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2.10. Forest plot of studies of seroprotection (≥1∶40 haemagglutination inhibition titre post vaccination): seasonal influenza A(H1N1), vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2.11. Forest plot of studies of seroprotection (≥1∶40 haemagglutination inhibition titre post vaccination): influenza A(H3N2), vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2.12. Forest plot of studies of seroprotection (≥1∶40 haemagglutination inhibition titre post vaccination): seasonal influenza B, vaccinated immunocompromised patients versus vaccinated immunocompetent controls. Figure S2< /xref>.13. Forest plot of studies of seroprotection (≥1∶40 haemagglutination inhibition titre post vaccination): pandemic influenza A(H1N1), vaccinated immunocompromised patients versus vaccinated immunocompetent controls.
(PDF)
MEDLINE search construct. Legend: PICO = research question in terms of population, intervention, comparators and outcomes. MeSH = Medical Subject Headings (US National Library of Medicine).
(PDF)
Summary of risk of bias using the Downs and Black (1998) tool (n = 2). Legend: N/A = not applicable; higher score = less risk of bias.
(PDF)
Summary of risk of bias using the US AHRQ tool (n = 3). Legend: Response indicates whether associated elements for reduction of bias have been met.
(PDF)
(PDF)
(DOC)