Abstract
Toxicogenomics (TGx) is a widely used technique in the preclinical stage of drug development to investigate the molecular mechanisms of toxicity. A number of candidate TGx biomarkers have now been identified and are utilized for both assessing and predicting toxicities. Further accumulation of novel TGx biomarkers will lead to more efficient, appropriate and cost effective drug risk assessment, reinforcing the paradigm of the conventional toxicology system with a more profound understanding of the molecular mechanisms of drug-induced toxicity. In this paper, we overview some practical strategies as well as obstacles for identifying and utilizing TGx biomarkers based on microarray analysis. Since clinical hepatotoxicity is one of the major causes of drug development attrition, the liver has been the best documented target organ for TGx studies to date, and we therefore focused on information from liver TGx studies. In this review, we summarize the current resources in the literature in regard to TGx studies of the liver, from which toxicologists could extract potential TGx biomarker gene sets for better hepatotoxicity risk assessment.
Keywords: toxicogenomics, biomarker, liver, microarray
Introduction
Although the term “toxicogenomics” (TGx) is relatively new, this method is now widely utilized by pharmaceutical scientists to investigate the molecular mechanisms of toxicity. Although the importance of functional genomics has been recognized since the emergence of microarray technology1,2, more attention has been focused on it since the US Food and Drug Administration (FDA) released a whitepaper3 showing that the number of new molecular entities has been decreasing since 2000, but that the costs of pharmaceutical companies for R&D of drugs have increased dramatically since 1993. One of the major attritions in the drug development process lies in unexpected adverse effects elicited in the clinical phase, and therefore the preclinical toxicological evaluation and the clinical trial steps are called ‘critical path’ of drug development in the FDA whitepaper. One estimation suggests that a 10% improvement in predicting future failure in the clinical phase would save 100 million dollars of R&D cost per drug3, and the whitepaper emphasized the importance of modernizing toxicological methodologies by applying cutting-edge techniques such as TGx and other “-omics” techniques.
One of the goals of TGx research is to identify novel biomarkers for evaluating the efficacy or toxicity in either clinical or preclinical cases, which would be as useful as such conventional biomarkers such as the blood enzyme activity of alanine aminotranferase in evaluating liver injury. The term ‘biomarker’ is defined as a characteristic that is objectively measured and evaluated as an indicator of normal biological process, pathogenic process, or pharmacologic responses to a therapeutic intervention4. In principle, any biological parameters that are objectively measurable and recordable could be potential biomarkers. One example of a ‘good biomarker’ is single nucleotide polymorphisms (SNPs) in human CYP2C9 and Vitamin K epoxide reductase genes, which are used for optimization of the dosing level of warfarin, an anticoagulant drug with a great number of serious adverse effects in the US5. Such biomarkers are not only useful for efficient drug risk management but will also lead to the establishment of promising markets for pharmaceutical companies. In TGx research, the term ‘biomarker’ does not always refer to a single gene, but may consist of sets of genes whose expression levels are closely associated with certain toxicological endpoints.
In the TGx research field, the liver has been the preferred target organ for the following reasons: i) the clinical manifestation of hepatotoxicity is one of the major causes of drug development attrition; ii) the exposure level of the liver is exceptionally high following drug treatment; and iii) it is relatively easy to collect liver samples due to its size and homogeneity. In this paper, we outline the literature resources in regard to candidate TGx biomarkers for liver toxicity and overview their significance, and advantages and major obstacles in practical use.
Microarray Technique
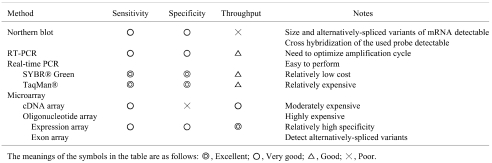
Microarray is the most mature functional genomics technique and is now utilized in various fields, including pharmacology, toxicology and nutritional science. Compared with traditional gene expression analysis techniques such as Northern blotting or RT-PCR, microarray can measure the expression levels of tens of thousands of genes simultaneously, and accordingly, the data acquisition is considerably high-throughput (Table 1). In a microarray analysis, target samples (i.e., mRNA, cRNA or cDNA) are labeled with fluorescent dyes (i.e., Cy3, Cy5, phycoerythrin, etc.) of either one or two colors. The microarray probes consist of either cDNA or oligonucleotide and are hybridized with labeled target samples which have complementary nucleotide sequences.
Table 1. Representative Analytical Methods for Gene Expression Studies.
Although microarrays can be manufactured in a lab using specific instruments, a number of microarray platforms are now commercially available, including GeneChip (Affymetrix, Inc.), Illumina (Illumina, Inc.), Codelink (GE Healthcare) and Agilent oligonucleotide arrays (Agilent Inc.). Each microarray platform has its own advantages and disadvantages. For example, in the Agilent 2-color (Cy3 and Cy5 dyes) microarray system, the Cy5 dye is extremely ozone-sensitive, and its signal is rapidly weakened under a high concentration of ozone6, which results in extremely poor data quality. On the other hand, the Affymetrix GeneChip system requires specific instruments, and therefore the initial investment is quite high, while the cost of preparing a cDNA microarray in-house is relatively low, provided the cDNA clones and spotting instrument are available. Organizing and maintaining DNA clones, however, are tedious and error-prone procedures that can easily lead to confusion, and the reliability of the obtained data may sometimes be questionable. On the other hand, commercial microarrays usually provide specified kits that contain the entire reagent necessary for all the experimental processes and, in some cases, are even equipped with specialized instruments to automate tedious work such as washing and staining the microarrays after hybridization. Therefore, commercial microarrays are generally preferred by pharmaceutical researchers because they regard these advantages to be more cost-effective in the long term.
Finding Differentially Expressed Genes
Microarray fluorescence is detected with a scanner after washing the microarray after hybridization with labeled target samples. After scanning the microarray fluorescence signals, the scanned microarray image is subjected to gridding and assignment of predefined probe information using image analysis software such as GenePix Pro (Molecular Devices). Usually, this step is performed manually, and it is therefore a tedious procedure.
In the Affymetrix GeneChip system, this process is highly automated and easy to complete. After completion of the gridding, the image data with the fluorescent signals are converted into numerical data followed by background subtraction to correct any undesirable bias of the individual data derived from the experimental conditions, sample, manufacturing variability or other factors. A set of probes comprised of two types of probe per gene are designed in the GeneChip system, namely the Perfect Match (PM) and Mismatch (MM) probes (typically 11 MM and 11 PM probes that are 25-bp nucleotides in length) per gene. The PM probe sequence is complimentary to that of the target gene, while the MM probe sequence contains one mutated sequence in the middle of the 25 bp sequence, and the MM probe is used to estimate non-specific bindings to the PM sequence. Since multiple probes are designed for one gene, one needs to evaluate the expression level of the gene by summarizing multiple probe data sets. A number of analytical algorithms have been proposed for this “summarization” of the probe level data, including MAS5, dChip, RMA and GCRMA7. MAS5 is a ‘chip-by-chip’ summarization algorithm, while dChip, RMA and GCRMA are ‘model-based’ or ‘project-based’ summarization algorithms that require relatively high performance computers to perform the calculations. In general, the project-based summarization algorithm yields better quality datasets in terms of sensitivity and reproducibility. On the other hand, MAS5 calculations are easy to compute, and there is no need to perform recalculations on whole data sets when new GeneChip data is added to a project. Thus, there is a trade-off in terms of the pros and cons of each method.
After correction of the individual data biases, the numerical data is subjected to normalization so that one can perform a comparative analysis among the microarray data sets. The easiest normalization is to adjust the global signal scale of each set of microarray data (global normalization), usually by setting it to the mean or median of the total signal data set. Another method is to use external spikes to get a standard curve, such as ‘Percellome normalization’8, in order to quantify the mRNA levels. This normalization method has been shown to be effective when the gene expression changes are extreme, such as in a uterotrophic response following activation of estrogen receptor or in an in vitro system using a primary cell culture.
After the normalization, one needs to identify the differentially expressed genes in the chemical-treated group. Since microarray analysis measures the expression levels of a large number of genes simultaneously, a straightforward pair-wise test, such as a t-test, would yield a considerable number of false-positives. (For example, if we set the significance level as P < 0.01 for Rat 230 2.0 GeneChip data consisting of > 30,000 probe sets, we may detect more than 300 positives just by chance). To prevent this multiple testing problem, P-value correction may be performed using False Discovery Rate9, or two individual filtering criterions like fold change and t-value can be used in combination. A number of filtering methods are provided in the literature, such as significance analysis of microarrays (SAM)10, and there are a great number of sophisticated algorithms available as library files on the BioConductor project website (http://www.bioconductor.org/)11 that can be implemented via the open source statistical software R (http://www.R-project.org).
Multivariate Analysis
Since microarray data consist of large amounts of numerical data, statistical knowledge, computational skills and infrastructure are required to interpret the results. Multivariate analysis methods are utilized for both data mining and pattern recognition (Table 2). “Unsupervised” multivariate analysis includes hierarchical clustering12, K-means clustering12, self-organizing map (SOM)12 and principal component analysis (PCA)13. “Supervised” multivariate analysis, or discriminant analysis, includes Support Vector Machine (SVM)14, K-Nearest Neighbors (KNN)15 and Prediction Analysis of Microarray (PAM)16. In general, each biomarker gene set requires its own specific analytical method based on the objective and manner of gene set identification.
Table 2. Representative Multivariate Analysis Methods.
Eisen et al. applied hierarchical clustering to visualize the trend of gene expression profiles17, and since then the hierarchical clustering method has been widely preferred by toxicologists when interpreting microarray data. In the case of K-means clustering and SOM, one needs to specify the number of clusters to be formed before the calculation. PCA is utilized to reduce the dimensions of the microarray data into 2 or 3; this makes it much easier to recognize the gene expression pattern.
Discriminant analysis, such as SVM, KNN and PAM, is an application of machine-learning algorithms and is frequently used for toxicity prediction based on microarray data. The sample size and appropriate selection of the training data set are crucial for establishing reliable classifiers. This type of discriminant analysis is also applied to quality control of microarray data18.
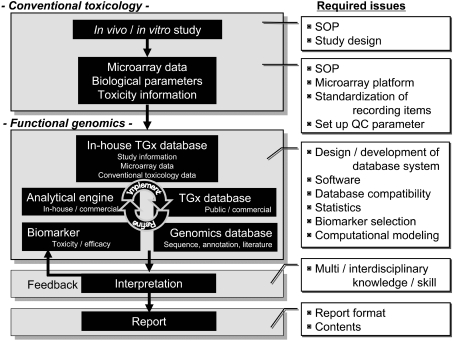
As described above, microarray analysis consists of multiple steps from in vivo / in vitro studies to microarray data interpretation (Fig. 1), and each step includes specific points to be considered in order to avoid misinterpretation of the obtained results.
Fig. 1.
General flow of a TGx study. The general flow of a TGx study is presented. Conventional toxicologic parameters, such as body / organ weights, histopathological findings, blood chemistry and toxico / pharmacokinetics, and functional genomics information, such as microarray data, are collected. The genomics data sets are huge and need to be organized into a well-designed database. Interpretation of the genomics data depends on the quality of the database, and analytical tools and an experienced researchers’ interdisciplinary knowledge and skills in biology, toxicology, statistics and computational sciences. A number of issues are yet to be determined to establish a standard operating procedure (SOP) for the public, including the content / format of the final report, recording items, statistical analysis to be performed for genomics data, etc. All the information should be appropriately recorded so that the obtained TGx data can be exchangeable across laboratories.
Literature Resources for TGx Biomarkers in Regard to Liver Toxicity
The reports in the literature related to liver toxicity-relevant gene sets obtained from TGx studies are summarized in Table 3. A great number of TGx studies of the liver have been reported using various animal models, such as rats, mice, humans, monkeys and canines, and these studies contain a number of toxicity-relevant gene sets that could be potential TGx biomarkers for assessing/predicting liver toxicity.
Table 3. TGx Biomarkers for Liver Toxicity.
Hepatotoxicity animal models using prototypical toxicants such as acetaminophen or carbon tetrachloride have been widely tested in TGx studies, and a number of gene sets associated with liver injury have been reported. Since these gene sets consist of a mixture of primary responses associated with cell death as well as secondary or more downstream responses such as inflammation caused by Kupffer cells or infiltrated lympocytes, one needs to dissect the stimulated biological pathways carefully to interpret the biological significance associated with gene expression changes.
Waring et al. reported that the hepatic gene expression profiles in rats following treatments with various chemicals showed clear chemical-specific patterns19. Based on this result, one can assume that such chemical-specific changes in the transcriptome profile would lead to changes in the proteome profile, the metabolome profile and eventually the histopathological phenotypes at later time points. This concept led toxicologists to expect that one might be able to utilize microarray data to predict later histopathological changes that are not detectable at earlier time points. As stated previously, such chemical-specific gene expression profiles, or ‘chemical fingerprints’, contain mixed molecular events that result from complicated interactions between biological pathways, such as xenobiotic metabolism, stress response, energy metabolism, protein synthesis / degradation, mRNA transcription / degradation, DNA repair / replication and cell growth / cell death control. By comparison with data for prototypical chemicals whose molecular mechanisms of toxicity have been well investigated, one may be able to identify the key gene sets, or TGx biomarkers whose expression levels are highly associated with specific toxicological events, by dissecting the specific molecular pathway from the mixed molecular events. These TGx biomarkers can then be utilized for the evaluation, diagnosis or prediction of toxicity based on their expression changes. For example, carcinogenicity tests in the preclinical stage of drug development require highly time- and labor-consuming tasks, and thus the identification of TGx biomarker genes for carcinogenicity prediction would dramatically reduce R&D time and costs for pharmaceutical companies.
Utilization of TGx Biomarkers
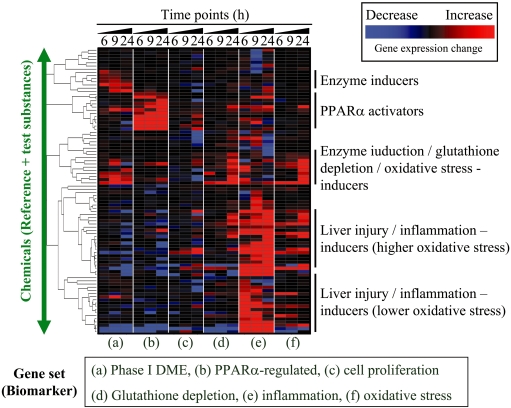
One of the practical applications of TGx biomarkers is to prioritize the drug candidates according to their toxicity profiles based on microarray data. An example is presented in Fig. 2 in which six TGx biomarkers for assessing the induction of drug metabolizing enzymes, PPARα activation, cell proliferation, glutathione depletion, inflammation or oxidative stress were used to evaluate chemical-induced toxicities in the rat liver. The general trend of the gene expression changes in each biomarker gene set was estimated using the TGP1 score174. The TGP1 score profile for each chemical is visualized by hierarchical clustering in Fig. 2, which demonstrates that each chemical shows characteristic changes in their gene expression levels that are associated with specific toxicity endpoints. Ideally, chemicals showing weaker effects in all the toxicity categories would be promising drug candidates.
Fig. 2.
Characterization of hepatic toxicity profile. An example of characterizing the hepatic toxicity profile is presented. In this figure, six TGx biomarker gene sets associated with a) phase I drug metabolizing enzyme (DME), b) PPARα-regulated genes, c) cell proliferation, d) glutathione depletion, e) inflammation and f) oxidative stress are used to assess toxicity profiles based on the microarray data for rat livers treated with one of 90 chemicals. The microarray data was retrieved from TG-GATEs, a TGx database developed by the Toxicogenomics Project in Japan (TGP), after obtaining permission. The expression changes for each biomarker set were summarized and estimated using the TGP1 score174, and the TGP1 score was subjected to hierarchical clustering. The red and blue colors indicate that the genes included in the TGx biomarker were generally up- or down-regulated, respectively, and the black color indicates that the expression level of the TGx biomarker gene sets did not show characteristic changes as a whole. Ideally, chemicals that do not affect the expression levels of genes included in the TGx biomarker would be desirable drug candidates. This strategy is applied to rank the chemicals based on the toxicity profiling.
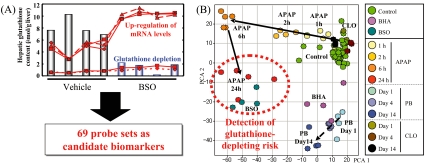
In Fig. 3, a model case is presented for identifying a candidate TGx biomarker gene set associated with glutathione depletion, which is known to play a crucial role in acetaminophen (APAP)-type liver injury175. Male F344 rats were treated with the glutathione depletor L-buthionine (S, R)-sulfoximine (BSO), and microarray analysis was conducted on the liver using RG U34A GeneChip. A total of 69 probe sets were identified with signal levels that were inversely correlated with the hepatic glutathione content (Fig. 3A). The validity of the gene set was tested using time-course microarray data for rat livers treated with APAP. As demonstrated in Fig. 3B, 69 probe sets clearly classified the animal groups following APAP treatment and showed that the 24 h APAP group was clustered together with the BSO-treated rats113; this indicates that the gene expression profiles of the APAP-treated (24 h) and BSO-treated rats are very similar and therefore that the 69 gene sets used are associated with glutathione depletion. In another experiment, more detailed TGx data were collected using another the glutathione depleting agent phorone114, and the results of that experiment showed that the ‘glutathione depletion-responsive genes’ maintain a high expression level even after the hepatic glutathione content recovered from acute glutathione depletion immediately after the phorone treatment. Accordingly, it may be better to call these genes ‘glutathione homeostasis-associated genes’ rather than ‘glutathione depletion-responsive genes’ in order to prevent misinterpretation of the microarray results.
Fig. 3.
Identification and application of TGx biomarkers for assessing glutathione depletion. A model case for identifying the candidate TGx biomarkers associated with glutathione depletion-type (acetaminophen-type) liver injury is presented. Rats were treated with a glutathione depletor L-buthionine (S, R)-sulfoximine (BSO), and GeneChip analysis was conducted on the liver. (A) A total of 69 probe sets were identified whose signal values were inversely correlated with the hepatic glutathione content. (B) The validity of the 69 probe sets as candidate TGx biomarkers for evaluation of glutathione depletion was evaluated by PCA using time-course microarray data for rat livers treated with acetaminophen. The 69 probe sets clearly classified the animal groups following acetaminophen treatment, and the acetaminophen group was clustered for 24 h together with the BSO-treated rats, suggesting that glutathione homeostasis was highly affected at this time point. Reprinted from Reference113, with permission from Elsevier.
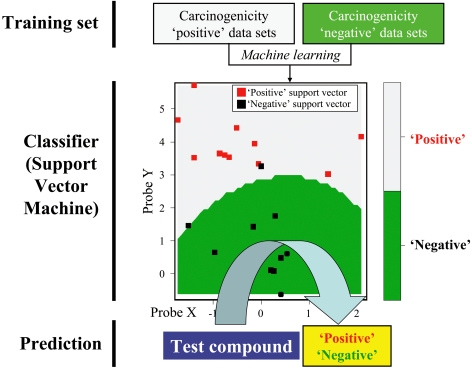
Although hierarchical clustering (Fig. 2) and PCA (Fig. 3) are easy to implement, the obtained results are sometimes not conclusive, and the interpretation of the results requires a certain level of proficiency. On the other hand, discriminant analysis, such as SVM, generates conclusive results, such as ‘toxic’ or ‘non-toxic’. The general procedure for SVM analysis is presented in Fig. 4. The first step is to prepare training data sets, such as microarray data for “carcinogenic compounds (positive)” and “non-carcinogenic compounds (negative)”. Next, one develops a ‘classifier’ with these training data sets using the machine learning algorithm of SVM. Once the classifier is developed, a positive / negative outcome can be predicted for a test compound with a known toxicological profile. Although the results produced by a discriminant analysis are conclusive, they are not reliable if the training sets are not selected properly. Furthermore, even when the cross-validation of the established classifier certifies good performance for the training data sets used, it may not work if the test compound induces a toxicity whose mechanism is rare or new and has not been considered in the training data sets. For all these reasons, the classifiers should be continuously updated to improve the classification performance.
Fig. 4.
Toxicity prediction by Support Vector Machine algorithm. Support Vector Machine is a popular discriminant analysis algorithm. The first step in this algorithm is to prepare a training data set, such as microarray data for a “carcinogenic compound (positive)” and “non-carcinogenic compound (negative)”. Next, a classifier is developed with the training data using the machine learning algorithm. By using the developed classifier, one can predict a positive / negative outcome (carcinogenic / non-carcinogenic outcome in the figure) for a test compound with an unknown toxicological profile. The accuracy of the prediction by the classifier can be estimated by cross-validation using the training data set. Gray and green indicate ‘Positive’ and ‘Negative’ classification areas, respectively. Red spots indicate the support vectors used for the classification of the test data set.
Microarray Database for TGx Research
To interpret the microarray data appropriately, it is desirable to perform comparative analysis with data obtained from prototypical toxicants. Developing a large-scale reference database, however, is not easy to accomplish, and therefore public databases, such as Gene Expression Ominibus (GEO)176, ArrayExpress177, Chemical Effects in Biological Systems (CEBS)178, Comparative Toxicogenomics Database (CTD)179 or EDGE180, can be used to obtain reference microarray data. In addition to public microarray databases, large-scale TGx databases have been developed by collaborative consortiums such as the Toxicogenomics Project in Japan (http:// wwwtgp.nibio.go.jp/index.html) and the InnoMed PredTox Consortium (http://www.innomed-predtox.com/), both of which contain microarray datasets for prototypical chemicals as well as proprietary drugs using both in vivo and in vitro systems. Animal and study information as well as microarray data can be retrieved from such databases provided that the TGx datasets were submitted with MIAME-compliant information, a guideline proposed by the Microarray and the Gene Expression Data (MGED) Society181 to facilitate microarray data sharing. Recently, a number of major scientific journals have begun to require investigators to deposit MIAME-compliant study information as well as microarray datasets at the time of or prior to the submission of manuscripts to their respective journals. This trend will continue because one cannot interpret microarray data appropriately without detailed study information.
Consistency of Microarray Data
Concerns have been raised regarding the reproducibility of microarray datasets across laboratories and microarray platforms. Some papers have reported about the inconsistency of interlaboratory / inter-platform microarray results182,183, while others have reported good concordance among laboratories184–186 or inconclusive results for this48,187. In addition to such laboratory-specific biases, a number of factors cause fluctuations in baseline animal data, such as gender, organ section, strain and fasting state before chemical dosing173. Furthermore, the vehicle substance used for animal dosing affects the baseline gene expression profile172, and therefore it is not appropriate to analyze the microarray data sets directly without consideration of the animal study conditions. In this sense, even the MIAME guidelines may not be sufficient for standardizing the TGx study conditions, and additional practical standards may be required to overcome this problem188.
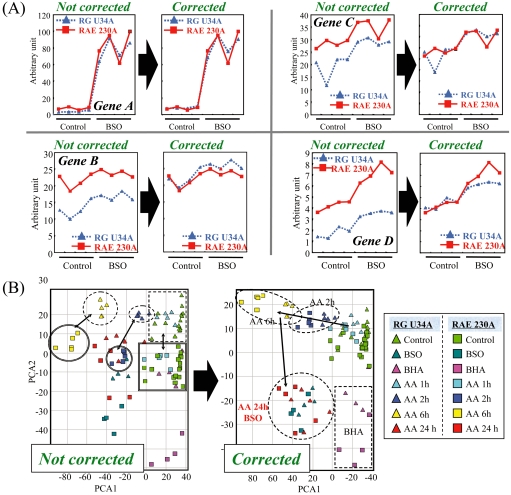
Even within the same GeneChip platform, the baseline microarray data fluctuates among laboratories. This inconsistency of microarray data is evident among the different generations of rat GeneChips, namely the RG U34A and RAE 230A arrays (Fig. 5A). Practically, we may avoid such inconsistency between two sets of array data by adjusting the median of the signal value between the two datasets (Fig. 5B)189, and ‘legacy TGx datasets’ can thereby be used together with new datasets.
Fig. 5.
Overcoming the discrepancy between old and new GeneChip data. Even within the same GeneChip platform, the inconsistency in microarray data is evident among the different generations of rat GeneChips, namely RG U34A and RAE 230A arrays, and this hinders utilization of ‘legacy TGx knowledge’ obtained from older microarrays. (A) The median signal values of the vehicle-treated rats were adjusted between the RG U34A and RAE 230A GeneChip data. The results for 4 representative genes are presented. (B) Principal component analysis using baseline-corrected RG U34A and RAE 230A GeneChip data was performed using the glutathione depletion-associated genes presented in Fig. 3. Adjustment of the baseline signal levels considerably improved the data compatibility between the RG U34A and RAE 230A GeneChip data; the spots for each treated chemical moved closer together (cf. inside area of the dashed circles). Reprinted from Reference189, with permission from Elsevier.
The MicroArray Quality Control (MAQC) Consortium performed a detailed data comparison in regard to inter / intra-platform microarrays across several laboratories and reported that microarray data shows generally high interlaboratory and inter-platform compatibility if fold-change ranking plus a less stringent statistical cutoff (such as a t-test) are used to filter the criteria, provided that the expression levels of the filtered genes are relatively high190. However, other reports have pointed out that the analytical procedure in the MAQC report was inadequate, and therefore the conclusion drawn is questionable191. In general, however, the reproducibility of interlaboratory microarray data tends to be high when the genes are filtered by fold-change values192 rather than by stringent P-values in the statistical analysis.
Species Difference Issues
Because experimental animals are used in preclinical toxicology studies, species differences are always major concerns. A number of papers have reported significant species-specific responses against chemical treatments, even among the rodents. For instance, 1,4-bis-[2-(3,5,-dichloropyridyloxy)] benzene (TCPOBOP) acts as a potent phenobarbital-type enzyme inducer in mouse liver but not in the rat or human liver. This species-specific response is associated with the substitution of Thr350 in the mouse constitutive androstane receptor (CAR), a nuclear receptor activated by TCPOBOP, with Met in rat and human CAR193–195. On the other hand, the phenobarbital-type enzyme inducer 2,4,6-triphenyldioxane-1,3 induces hepatic CYP2B in rats but not in mice196. Since CAR regulates hepatic drug metabolism enzymes and transporters197, such differential regulation may affect these dramatic species differences in drug metabolism and disposition.
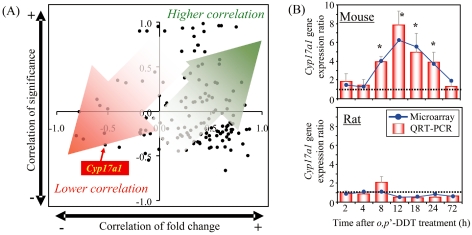
In the case of the estrogenic environmental contaminant o,p’-DDT, hepatic Cyp17a1 is preferentially upregulated in mice198 but not in rats199, even though the majority of orthologous genes exhibit similar gene expression profiles in mice and rats following o,p’-DDT treatment (Fig. 6). Since CYP17A1 is one of the key steroidogenic enzymes, the mouse-specific upregulation of Cyp17a1 may alter endocrine sex hormone homeostasis. As expected, the blood level of DHEA-S, a precursor of sex hormones produced by CYP17A1, is elevated only in mice198, and this may lead to endocrine perturbation in addition to the direct estrogenic activity of o,p’-DDT. Furthermore, the hepatic CAR mRNA level is decreased in mice but is increased in rats199, and this could result in differential xenobiotic metabolism and disposition in the liver, considering CAR’s role in regulating cassettes of hepatic drug metabolizing enzymes. Thus, marked species differences in hepatic response against chemical treatment have been observed even among rodents, and these phenomena confound the extrapolation of toxicity data from animals to humans. Nevertheless, the identification of potential modes of action as well as species-specific responses may assist in the development or selection of more appropriate models for assessing the toxicity of xenobiotics.
Fig. 6.
Species-specific regulation of the hepatic Cyp17a1 gene elicited by o,p’-DDT. Correlation analysis between mice and rats was performed using differentially expressed orthologous genes in the liver elicited by o,p’-DDT. The temporal profiles of the o,p’-DDT-treated mouse liver198 and those of the o,p’-DDT-treated rat liver199 were compared by determining the Pearson’s correlation of the temporal gene expression (fold change) and significance (p1[t] value by empirical Bayesian analysis) between orthologs, and the results of this comparison are presented as a scatter plot. Correlations of gene expression and significance approaching 1.0 indicate that the behaviors of the orthologous genes are similar and would fall within the upper right quadrant. (A) Orthologs tended to localize in the upper- or lower-right quadrants, indicating that the temporal gene expression changes for o,p’-DDT-treated mouse and rat liver are comparable. However, poor correlations between the temporal p1(t) values and gene expression fold changes would fall within the lower left quadrant. Cyp17a1, one of the poor-correlation genes, fell into this quadrant, suggesting that significant differences exist between the rat and mouse othologue expression profiles. (B) The hepatic Cyp17a1 gene expression levels following o,p’-DDT treatment were compared between rats and mice by QRT-PCR. Significant species-specific regulation of hepatic CYP17a1 gene was observed. * P < 0.05 by a two-way ANOVA followed by pairwise comparisons using Tukey’s test.
Future Perspectives
As the number of TGx biomarkers rapidly increases, some of them will be promising biomarkers that will lead to better understanding of the molecular mechanisms and prediction of toxicity in humans based on preclinical data. However, many of the candidate TGx biomarkers are applicable only to animals, and their feasibility as clinical biomarkers remains unclear. Idiosyncratic drug-induced hepatotoxicity200, which is not detectable in conventional preclinical toxicity studies, is one of the major causes of failure in drug development after the onset of clinical trials, and therefore novel TGx biomarkers which can detect signs of idiosyncratic hepatotoxicity are eagerly awaited.
Recently, seven new renal toxicity biomarkers, including Kim-1, β2-microglobulin and Cystatin C, were officially qualified for particular uses in regulatory decision-making by the US FDA and European Medicines Agency (EMEA)201. These biomarkers were submitted by the Predictive Safety Testing Consortium (PSTC) led by the non-profit Critical Path Institute (C-Path; http://www.c-path.org/). In addition to these novel renal biomarkers, TGx biomarkers for hepatotoxicity will need a similar qualification (or validation) process through collaborative research like that of C-Path.
Identification of TGx biomarkers may lead to the discovery of other biomarkers (genes, proteins or metabolites), the detection of which is easier than measuring hepatic mRNA levels. For example, renal Kim-1 gene expression is upregulated in response to renal injury202, and therefore the Kim-1 mRNA level can be a renal toxicity biomarker. However, Kim-1 protein is also detectable in urine203, and thus the urine Kim-1 protein is a much more convenient biomarker to measure compared with the renal Kim-1 mRNA level. As well, new surrogate hepatotoxicity biomarkers, which are more convenient to detect than hepatic mRNA, could be discovered through a profound understanding of the molecular mechanisms of toxicity by utilizing TGx mRNA biomarkers. ‘Ideal’ TGx biomarkers for hepatotoxicity will be those that are sensitive, specific, predictive and, above all, ‘extrapolatable’ to humans, and it is the responsibility of pharmaceutical toxicologists to discover/establish novel biomarkers to assist in the improvement of risk assessment in humans.
Acknowledgments
The authors are grateful to Dr. Kazumi Ito, Kyoko Watanabe and Noriyo Niino for their productive discussions regarding this manuscript.
References
- 1.Schena M, Heller RA, Theriault TP, Konrad K, Lachenmeier E, Davis RW. Microarrays: biotechnology’s discovery platform for functional genomics. Trends Biotechnol. 16: 301–306 1998 [DOI] [PubMed] [Google Scholar]
- 2.Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 270: 467–470 1995 [DOI] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration (FDA). Innovation or stagnation? Challenge and opportunity on the critical path to new medicinal products, 2004
- 4.Biomarkers Definitions Working GroupBiomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 69: 89–95 2001 [DOI] [PubMed] [Google Scholar]
- 5.Lesko LJ. The critical path of warfarin dosing: finding an optimal dosing strategy using pharmacogenetics. Clin Pharmacol Ther. 84: 301–303 2008 [DOI] [PubMed] [Google Scholar]
- 6.Fare TL, Coffey EM, Dai H, He YD, Kessler DA, Kilian KA, Koch JE, LeProust E, Marton MJ, Meyer MR, Stoughton RB, Tokiwa GY, Wang Y. Effects of atmospheric ozone on microarray data quality. Anal Chem. 75: 4672–4675 2003 [DOI] [PubMed] [Google Scholar]
- 7.Irizarry RA, Wu Z, Jaffee HA. Comparison of Affymetrix GeneChip expression measures. Bioinformatics. 22: 789–794 2006 [DOI] [PubMed] [Google Scholar]
- 8.Kanno J, Aisaki K, Igarashi K, Nakatsu N, Ono A, Kodama Y, Nagao T. “Per cell” normalization method for mRNA measurement by quantitative PCR and microarrays. BMC Genomics. 7: 64 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pounds SB. Estimation and control of multiple testing error rates for microarray studies. Brief Bioinform. 7: 25–36 2006 [DOI] [PubMed] [Google Scholar]
- 10.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 98: 5116–5121 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5: R80 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Draghici S. Cluster analysis. In: Data Analysis Tools for DNA Microarrays. AM Etheridge, LJ Gross, L Suzanne, PK Maini, HM Safer and EO Voit (eds). CRC Press Company, London. 276–307. 2003 [Google Scholar]
- 13.Draghici S. Analysis and visualization tools. In: Data Analysis Tools for DNA Microarrays. AM Etheridge, LJ Gross, L Suzanne, PK Maini, HM Safer and EO Voit (eds). CRC Press Company, London. 231–261. 2003 [Google Scholar]
- 14.Brown MP, Grundy WN, Lin D, Cristianini N, Sugnet CW, Furey TS, Ares M, Haussler D. Knowledge-based analysis of microarray gene expression data by using support vector machines. Proc Natl Acad Sci USA. 97: 262–267 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pomeroy SL, Tamayo P, Gaasenbeek M, Sturla LM, Angelo M, McLaughlin ME, Kim JY, Goumnerova LC, Black PM, Lau C, Allen JC, Zagzag D, Olson JM, Curran T, Wetmore C, Biegel JA, Poggio T, Mukherjee S, Rifkin R, Califano A, Stolovitzky G, Louis DN, Mesirov JP, Lander ES, Golub TR. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 415: 436–442 2002 [DOI] [PubMed] [Google Scholar]
- 16.Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci USA. 99: 6567–6572 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 95: 14863–14868 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgoon LD, Eckel-Passow JE, Gennings C, Boverhof DR, Burt JW, Fong CJ, Zacharewski TR. Protocols for the assurance of microarray data quality and process control. Nucleic Acids Res. 33: e172 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waring JF, Jolly RA, Ciurlionis R, Lum PY, Praestgaard JT, Morfitt DC, Buratto B, Roberts C, Schadt E, Ulrich RG. Clustering of hepatotoxins based on mechanism of toxicity using gene expression profiles. Toxicol Appl Pharmacol. 175: 28–42 2001 [DOI] [PubMed] [Google Scholar]
- 20.Hamadeh HK, Bushel PR, Jayadev S, Martin K, DiSorbo O, Sieber S, Bennett L, Tennant R, Stoll R, Barrett JC, Blanchard K, Paules RS, Afshari CA. Gene expression analysis reveals chemical-specific profiles. Toxicol Sci. 67: 219–231 2002 [DOI] [PubMed] [Google Scholar]
- 21.Hamadeh HK, Bushel PR, Jayadev S, DiSorbo O, Bennett L, Li L, Tennant R, Stoll R, Barrett JC, Paules RS, Blanchard K, Afshari CA. Prediction of compound signature using high density gene expression profiling. Toxicol Sci. 67: 232–240 2002 [DOI] [PubMed] [Google Scholar]
- 22.de Longueville F, Atienzar FA, Marcq L, Dufrane S, Evrard S, Wouters L, Leroux F, Bertholet V, Gerin B, Whomsley R, Arnould T, Remacle J, Canning M. Use of a low-density microarray for studying gene expression patterns induced by hepatotoxicants on primary cultures of rat hepatocytes. Toxicol Sci. 75: 378–392 2003 [DOI] [PubMed] [Google Scholar]
- 23.Waring JF, Ciurlionis R, Jolly RA, Heindel M, Ulrich RG. Microarray analysis of hepatotoxins in vitro reveals a correlation between gene expression profiles and mechanisms of toxicity. Toxicol Lett. 120: 359–368 2001 [DOI] [PubMed] [Google Scholar]
- 24.Bulera SJ, Eddy SM, Ferguson E, Jatkoe TA, Reindel JF, Bleavins MR, De La Iglesia FA. RNA expression in the early characterization of hepatotoxicants in Wistar rats by high-density DNA microarrays. Hepatology. 33: 1239–1258 2001 [DOI] [PubMed] [Google Scholar]
- 25.Meneses-Lorente G, de Longueville F, Dos Santos-Mendes S, Bonnert TP, Jack A, Evrard S, Bertholet V, Pike A, Scott-Stevens P, Remacle J, Sohal B. An evaluation of a low-density DNA microarray using cytochrome P450 inducers. Chem Res Toxicol. 16: 1070–1077 2003 [DOI] [PubMed] [Google Scholar]
- 26.Minami K, Saito T, Narahara M, Tomita H, Kato H, Sugiyama H, Katoh M, Nakajima M, Yokoi T. Relationship between hepatic gene expression profiles and hepatotoxicity in five typical hepatotoxicant-administered rats. Toxicol Sci. 87: 296–305 2005 [DOI] [PubMed] [Google Scholar]
- 27.Ganter B, Tugendreich S, Pearson CI, Ayanoglu E, Baumhueter S, Bostian KA, Brady L, Browne LJ, Calvin JT, Day GJ, Breckenridge N, Dunlea S, Eynon BP, Furness LM, Ferng J, Fielden MR, Fujimoto SY, Gong L, Hu C, Idury R, Judo MS, Kolaja KL, Lee MD, McSorley C, Minor JM, Nair RV, Natsoulis G, Nguyen P, Nicholson SM, Pham H, Roter AH, Sun D, Tan S, Thode S, Tolley AM, Vladimirova A, Yang J, Zhou Z, Jarnagin K. Development of a large-scale chemogenomics database to improve drug candidate selection and to understand mechanisms of chemical toxicity and action. J Biotechnol. 119: 219–244 2005 [DOI] [PubMed] [Google Scholar]
- 28.Zidek N, Hellmann J, Kramer PJ, Hewitt PG. Acute hepatotoxicity: a predictive model based on focused illumina microarrays. Toxicol Sci. 99: 289–302 2007 [DOI] [PubMed] [Google Scholar]
- 29.Huang L, Heinloth AN, Zeng ZB, Paules RS, Bushel PR. Genes related to apoptosis predict necrosis of the liver as a phenotype observed in rats exposed to a compendium of hepatotoxicants. BMC Genomics. 9: 288 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eun JW, Ryu SY, Noh JH, Lee MJ, Jang JJ, Ryu JC, Jung KH, Kim JK, Bae HJ, Xie H, Kim SY, Lee SH, Park WS, Yoo NJ, Lee JY, Nam SW. Discriminating the molecular basis of hepatotoxicity using the large-scale characteristic molecular signatures of toxicants by expression profiling analysis. Toxicology. 249: 176–183 2008 [DOI] [PubMed] [Google Scholar]
- 31.Steiner G, Suter L, Boess F, Gasser R, de Vera MC, Albertini S, Ruepp S. Discriminating different classes of toxicants by transcript profiling. Environ Health Perspect. 112: 1236–1248 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Natsoulis G, Pearson CI, Gollub J. P Eynon B, Ferng J, Nair R, Idury R, Lee MD, Fielden MR, Brennan RJ, Roter AH, and Jarnagin K. The liver pharmacological and xenobiotic gene response repertoire. Mol Syst Biol. 4: 175 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slatter JG, Cheng O, Cornwell PD, de Souza A, Rockett J, Rushmore T, Hartley D, Evers R, He Y, Dai X, Hu R, Caguyong M, Roberts CJ, Castle J, Ulrich RG. Microarray-based compendium of hepatic gene expression profiles for prototypical ADME gene-inducing compounds in rats and mice in vivo. Xenobiotica. 36: 902–937 2006 [DOI] [PubMed] [Google Scholar]
- 34.Slatter JG, Templeton IE, Castle JC, Kulkarni A, Rushmore TH, Richards K, He Y, Dai X, Cheng OJ, Caguyong M, Ulrich RG. Compendium of gene expression profiles comprising a baseline model of the human liver drug metabolism transcriptome. Xenobiotica. 36: 938–962 2006 [DOI] [PubMed] [Google Scholar]
- 35.Kiyosawa N, Watanabe T, Sakuma K, Kanbori M, Niino N, Ito K, Yamoto T, Manabe S. Phylogenetic tree facilitates the understanding of gene expression data on drug metabolizing enzymes obtained by microarray analysis. Toxicol Lett. 145: 281–289 2003 [DOI] [PubMed] [Google Scholar]
- 36.Coe KJ, Nelson SD, Ulrich RG, He Y, Dai X, Cheng O, Caguyong M, Roberts CJ, Slatter JG. Profiling the hepatic effects of flutamide in rats: a microarray comparison with classical aryl hydrocarbon receptor ligands and atypical CYP1A inducers. Drug Metab Dispos. 34: 1266–1275 2006 [DOI] [PubMed] [Google Scholar]
- 37.Mori K, Blackshear PE, Lobenhofer EK, Parker JS, Orzech DP, Roycroft JH, Walker KL, Johnson KA, Marsh TA, Irwin RD, Boorman GA. Hepatic transcript levels for genes coding for enzymes associated with xenobiotic metabolism are altered with age. Toxicol Pathol. 35: 242–251 2007 [DOI] [PubMed] [Google Scholar]
- 38.Heinloth AN, Irwin RD, Boorman GA, Nettesheim P, Fannin RD, Sieber SO, Snell ML, Tucker CJ, Li L, Travlos GS, Vansant G, Blackshear PE, Tennant RW, Cunningham ML, Paules RS. Gene expression profiling of rat livers reveals indicators of potential adverse effects. Toxicol Sci. 80: 193–202 2004 [DOI] [PubMed] [Google Scholar]
- 39.Kier LD, Neft R, Tang L, Suizu R, Cook T, Onsurez K, Tiegler K, Sakai Y, Ortiz M, Nolan T, Sankar U, Li AP. Applications of microarrays with toxicologically relevant genes (tox genes) for the evaluation of chemical toxicants in Sprague Dawley rats in vivo and human hepatocytes in vitro. Mutat Res. 549: 101–113 2004 [DOI] [PubMed] [Google Scholar]
- 40.Huang Q, Jin X, Gaillard ET, Knight BL, Pack FD, Stoltz JH, Jayadev S, Blanchard KT. Gene expression profiling reveals multiple toxicity endpoints induced by hepatotoxicants. Mutat Res. 549: 147–167 2004 [DOI] [PubMed] [Google Scholar]
- 41.Hamadeh HK, Jayadev S, Gaillard ET, Huang Q, Stoll R, Blanchard K, Chou J, Tucker CJ, Collins J, Maronpot R, Bushel P, Afshari CA. Integration of clinical and gene expression endpoints to explore furan-mediated hepatotoxicity. Mutat Res. 549: 169–183 2004 [DOI] [PubMed] [Google Scholar]
- 42.Devi SS, Mehendale HM. Microarray analysis of thioacetamide-treated type 1 diabetic rats. Toxicol Appl Pharmacol. 212: 69–78 2006 [DOI] [PubMed] [Google Scholar]
- 43.Minami K, Maniratanachote R, Katoh M, Nakajima M, Yokoi T. Simultaneous measurement of gene expression for hepatotoxicity in thioacetamide-administered rats by DNA microarrays. Mutat Res. 603: 64–73 2006 [DOI] [PubMed] [Google Scholar]
- 44.Craig A, Sidaway J, Holmes E, Orton T, Jackson D, Rowlinson R, Nickson J, Tonge R, Wilson I, Nicholson J. Systems toxicology: integrated genomic, proteomic and metabonomic analysis of methapyrilene induced hepatotoxicity in the rat. J Proteome Res. 5: 1586–1601 2006 [DOI] [PubMed] [Google Scholar]
- 45.Fukushima T, Kikkawa R, Hamada Y, Horii I. Genomic cluster and network analysis for predictive screening for hepatotoxicity. J Toxicol Sci. 31: 419–432 2006 [DOI] [PubMed] [Google Scholar]
- 46.Spicker JS, Pedersen HT, Nielsen HB, Brunak S. Analysis of cell death inducing compounds. Arch Toxicol. 81: 803–811 2007 [DOI] [PubMed] [Google Scholar]
- 47.Tanaka K, Kiyosawa N, Watanabe K, Manabe S. Characterization of resistance to bromobenzene-induced hepatotoxicity by microarray. J Toxicol Sci. 32: 129–134 2007 [DOI] [PubMed] [Google Scholar]
- 48.Beyer RP, Fry RC, Lasarev MR, McConnachie LA, Meira LB, Palmer VS, Powell CL, Ross PK, Bammler TK, Bradford BU, Cranson AB, Cunningham ML, Fannin RD, Higgins GM, Hurban P, Kayton RJ, Kerr KF, Kosyk O, Lobenhofer EK, Sieber SO, Vliet PA, Weis BK, Wolfinger R, Woods CG, Freedman JH, Linney E, Kaufmann WK, Kavanagh TJ, Paules RS, Rusyn I, Samson LD, Spencer PS, Suk W, Tennant RJ, Zarbl H. Multicenter study of acetaminophen hepatotoxicity reveals the importance of biological endpoints in genomic analyses. Toxicol Sci. 99: 326–337 2007 [DOI] [PubMed] [Google Scholar]
- 49.Mei N, Guo L, Liu R, Fuscoe JC, Chen T. Gene expression changes induced by the tumorigenic pyrrolizidine alkaloid riddelliine in liver of Big Blue rats. BMC Bioinformatics. 8(Suppl 7): S4 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma MR, Polavarapu R, Roseman D, Patel V, Eaton E, Kishor PB, Nanji AA. Increased severity of alcoholic liver injury in female verses male rats: a microarray analysis. Exp Mol Pathol. 84: 46–58 2008 [DOI] [PubMed] [Google Scholar]
- 51.Elferink MG, Olinga P, Draaisma AL, Merema MT, Bauerschmidt S, Polman J, Schoonen WG, Groothuis GM. Microarray analysis in rat liver slices correctly predicts in vivo hepatotoxicity. Toxicol Appl Pharmacol. 229: 300–309 2008 [DOI] [PubMed] [Google Scholar]
- 52.Kikkawa R, Fujikawa M, Yamamoto T, Hamada Y, Yamada H, Horii I. In vivo hepatotoxicity study of rats in comparison with in vitro hepatotoxicity screening system. J Toxicol Sci. 31: 23–34 2006 [DOI] [PubMed] [Google Scholar]
- 53.Morishita K, Mizukawa Y, Kasahara T, Okuyama M, Takashima K, Toritsuka N, Miyagishima T, Nagao T, Urushidani T. Gene expression profile in liver of differing ages of rats after single oral administration of acetaminophen. J Toxicol Sci. 31: 491–507 2006 [DOI] [PubMed] [Google Scholar]
- 54.Dai X, De Souza AT, Dai H, Lewis DL, Lee CK, Spencer AG, Herweijer H, Hagstrom JE, Linsley PS, Bassett DE, Ulrich RG, He YD. PPARalpha siRNA-treated expression profiles uncover the causal sufficiency network for compound-induced liver hypertrophy. PLoS Comput Biol. 3: e30 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bushel PR, Wolfinger RD, Gibson G. Simultaneous clustering of gene expression data with clinical chemistry and pathological evaluations reveals phenotypic prototypes. BMC Syst Biol. 1: 15 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Auman JT, Chou J, Gerrish K, Huang Q, Jayadev S, Blanchard K, Paules RS. Identification of genes implicated in methapyrilene-induced hepatotoxicity by comparing differential gene expression in target and nontarget tissue. Environ Health Perspect. 115: 572–578 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uehara T, Kiyosawa N, Hirode M, Omura K, Shimizu T, Ono A, Mizukawa Y, Miyagishima T, Nagao T, Urushidani T. Gene expression profiling of methapyrilene-induced hepatotoxicity in rat. J Toxicol Sci. 33: 37–50 2008 [DOI] [PubMed] [Google Scholar]
- 58.Powell CL, Kosyk O, Ross PK, Schoonhoven R, Boysen G, Swenberg JA, Heinloth AN, Boorman GA, Cunningham ML, Paules RS, Rusyn I. Phenotypic anchoring of acetaminophen-induced oxidative stress with gene expression profiles in rat liver. Toxicol Sci. 93: 213–222 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung H, Kim HJ, Jang KS, Kim M, Yang J, Kang KS, Kim HL, Yoon BI, Lee MO, Lee BH, Kim JH, Lee YS, Kong G. Comprehensive analysis of differential gene expression profiles on D-galactosamine-induced acute mouse liver injury and regeneration. Toxicology. 227: 136–144 2006 [DOI] [PubMed] [Google Scholar]
- 60.Inadera H, Tachibana S, Takasaki I, Tabuchi Y, Matsushima K, Uchida M, Shimomura A. Expression profile of liver genes in response to hepatotoxicants identified using a SAGE-based customized DNA microarray system. Toxicol Lett. 177: 20–30 2008 [DOI] [PubMed] [Google Scholar]
- 61.Konig R, Cai P, Guo X, Ansari GA. Transcriptomic analysis reveals early signs of liver toxicity in female MRL +/+ mice exposed to the acylating chemicals dichloroacetyl chloride and dichloroacetic anhydride. Chem Res Toxicol. 21: 572–582 2008 [DOI] [PubMed] [Google Scholar]
- 62.Chung H, Kim HJ, Jang KS, Kim M, Yang J, Kim JH, Lee YS, Kong G. Comprehensive analysis of differential gene expression profiles on diclofenac-induced acute mouse liver injury and recovery. Toxicol Lett. 166: 77–87 2006 [DOI] [PubMed] [Google Scholar]
- 63.Kwon SB, Park JS, Yi JY, Hwang JW, Kim M, Lee MO, Lee BH, Kim HL, Kim JH, Chung H, Kong G, Kang KS, Yoon BI. Time- and dose-based gene expression profiles produced by a bile-duct-damaging chemical, 4,4’-methylene dianiline, in mouse liver in an acute phase. Toxicol Pathol. 36: 660–673 2008 [DOI] [PubMed] [Google Scholar]
- 64.Guo L, Zhang L, Sun Y, Muskhelishvili L, Blann E, Dial S, Shi L, Schroth G, Dragan YP. Differences in hepatotoxicity and gene expression profiles by anti-diabetic PPAR gamma agonists on rat primary hepatocytes and human HepG2 cells. Mol Divers. 10: 349–360 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liguori MJ, Blomme EA, Waring JF. Trovafloxacin-induced gene expression changes in liver-derived in vitro systems: comparison of primary human hepatocytes to HepG2 cells. Drug Metab Dispos. 36: 223–233 2008 [DOI] [PubMed] [Google Scholar]
- 66.Thum T, Borlak J. Detection of early signals of hepatotoxicity by gene expression profiling studies with cultures of metabolically competent human hepatocytes. Arch Toxicol. 82: 89–101 2008 [DOI] [PubMed] [Google Scholar]
- 67.Liguori MJ, Anderson MG, Bukofzer S, McKim J, Pregenzer JF, Retief J, Spear BB, Waring JF. Microarray analysis in human hepatocytes suggests a mechanism for hepatotoxicity induced by trovafloxacin. Hepatology. 41: 177–186 2005 [DOI] [PubMed] [Google Scholar]
- 68.Ellinger-Ziegelbauer H, Gmuender H, Bandenburg A, Ahr HJ. Prediction of a carcinogenic potential of rat hepatocarcinogens using toxicogenomics analysis of short-term in vivo studies. Mutat Res. 637: 23–39 2008 [DOI] [PubMed] [Google Scholar]
- 69.Ellinger-Ziegelbauer H, Stuart B, Wahle B, Bomann W, Ahr HJ. Comparison of the expression profiles induced by genotoxic and nongenotoxic carcinogens in rat liver. Mutat Res. 575: 61–84 2005 [DOI] [PubMed] [Google Scholar]
- 70.Nakayama K, Kawano Y, Kawakami Y, Moriwaki N, Sekijima M, Otsuka M, Yakabe Y, Miyaura H, Saito K, Sumida K, Shirai T. Differences in gene expression profiles in the liver between carcinogenic and non-carcinogenic isomers of compounds given to rats in a 28-day repeat-dose toxicity study. Toxicol Appl Pharmacol. 217: 299–307 2006 [DOI] [PubMed] [Google Scholar]
- 71.Iida M, Anna CH, Holliday WM, Collins JB, Cunningham ML, Sills RC, Devereux TR. Unique patterns of gene expression changes in liver after treatment of mice for 2 weeks with different known carcinogens and non-carcinogens. Carcinogenesis. 26: 689–699 2005 [DOI] [PubMed] [Google Scholar]
- 72.Uehara T, Hirode M, Ono A, Kiyosawa N, Omura K, Shimizu T, Mizukawa Y, Miyagishima T, Nagao T, Urushidani T. A toxicogenomics approach for early assessment of potential non-genotoxic hepatocarcinogenicity of chemicals in rats. Toxicology. 250: 15–26 2008 [DOI] [PubMed] [Google Scholar]
- 73.Fielden MR, Brennan R, Gollub J. A gene expression biomarker provides early prediction and mechanistic assessment of hepatic tumor induction by nongenotoxic chemicals. Toxicol Sci. 99: 90–100 2007 [DOI] [PubMed] [Google Scholar]
- 74.Marin-Kuan M, Nestler S, Verguet C, Bezencon C, Piguet D, Mansourian R, Holzwarth J, Grigorov M, Delatour T, Mantle P, Cavin C, Schilter B. A toxicogenomics approach to identify new plausible epigenetic mechanisms of ochratoxin a carcinogenicity in rat. Toxicol Sci. 89: 120–134 2006 [DOI] [PubMed] [Google Scholar]
- 75.Tsujimura K, Asamoto M, Suzuki S, Hokaiwado N, Ogawa K, Shirai T. Prediction of carcinogenic potential by a toxicogenomic approach using rat hepatoma cells. Cancer Sci. 97: 1002–1010 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Werle-Schneider G, Wolfelschneider A, von Brevern MC, Scheel J, Storck T, Muller D, Glockner R, Bartsch H, Bartelmann M. Gene expression profiles in rat liver slices exposed to hepatocarcinogenic enzyme inducers, peroxisome proliferators, and 17alpha-ethinylestradiol. Int J Toxicol. 25: 379–395 2006 [DOI] [PubMed] [Google Scholar]
- 77.Mei N, Guo L, Zhang L, Shi L, Sun YA, Fung C, Moland CL, Dial SL, Fuscoe JC, Chen T. Analysis of gene expression changes in relation to toxicity and tumorigenesis in the livers of Big Blue transgenic rats fed comfrey (Symphytum officinale). BMC Bioinformatics. 7(Suppl 2): S16 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishimura J, Dewa Y, Muguruma M, Kuroiwa Y, Yasuno H, Shima T, Jin M, Takahashi M, Umemura T, Mitsumori K. Effect of fenofibrate on oxidative DNA damage and on gene expression related to cell proliferation and apoptosis in rats. Toxicol Sci. 97: 44–54 2007 [DOI] [PubMed] [Google Scholar]
- 79.Pogribny IP, Bagnyukova TV, Tryndyak VP, Muskhelishvili L, Rodriguez-Juarez R, Kovalchuk O, Han T, Fuscoe JC, Ross SA, Beland FA. Gene expression profiling reveals underlying molecular mechanisms of the early stages of tamoxifen-induced rat hepatocarcinogenesis. Toxicol Appl Pharmacol. 225: 61–69 2007 [DOI] [PubMed] [Google Scholar]
- 80.Sumida K, Saito K, Oeda K, Yakabe Y, Otsuka M, Matsumoto H, Sekijima M, Nakayama K, Kawano Y, Shirai T. A comparative study of gene expression profiles in rat liver after administration of alpha-hexachlorocyclohexane and lindane. J Toxicol Sci. 32: 261–288 2007 [DOI] [PubMed] [Google Scholar]
- 81.Ellinger-Ziegelbauer H, Stuart B, Wahle B, Bomann W, Ahr HJ. Characteristic expression profiles induced by genotoxic carcinogens in rat liver. Toxicol Sci. 77: 19–34 2004 [DOI] [PubMed] [Google Scholar]
- 82.Uematsu F, Takahashi M, Yoshida M, Igarashi M, Watanabe N, Suzuki N, Abe M, Rusyn I, Floyd RA, Nakae D. Distinct patterns of gene expression in hepatocellular carcinomas and adjacent non-cancerous, cirrhotic liver tissues in rats fed a choline-deficient, L-amino acid-defined diet. Cancer Sci. 96: 414–424 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iida M, Anna CH, Hartis J, Bruno M, Wetmore B, Dubin JR, Sieber S, Bennett L, Cunningham ML, Paules RS, Tomer KB, Houle CD, Merrick AB, Sills RC, Devereux TR. Changes in global gene and protein expression during early mouse liver carcinogenesis induced by non-genotoxic model carcinogens oxazepam and Wyeth-14,643. Carcinogenesis. 24: 757–770 2003 [DOI] [PubMed] [Google Scholar]
- 84.Moto M, Okamura M, Muto T, Kashida Y, Machida N, Mistumori K. Molecular pathological analysis on the mechanism of liver carcinogenesis in dicyclanil-treated mice. Toxicology. 207: 419–436 2005 [DOI] [PubMed] [Google Scholar]
- 85.Kashida Y, Takahashi A, Moto M, Okamura M, Muguruma M, Jin M, Arai K, Mitsumori K. Gene expression analysis in mice liver on hepatocarcinogenesis by flumequine. Arch Toxicol. 80: 533–539 2006 [DOI] [PubMed] [Google Scholar]
- 86.Muguruma M, Nishimura J, Jin M, Kashida Y, Moto M, Takahashi M, Yokouchi Y, Mitsumori K. Molecular pathological analysis for determining the possible mechanism of piperonyl butoxide-induced hepatocarcinogenesis in mice. Toxicology. 228: 178–187 2006 [DOI] [PubMed] [Google Scholar]
- 87.Thomas RS, O’Connell TM, Pluta L, Wolfinger RD, Yang L, Page TJ. A comparison of transcriptomic and metabonomic technologies for identifying biomarkers predictive of two-year rodent cancer bioassays. Toxicol Sci. 96: 40–46 2007 [DOI] [PubMed] [Google Scholar]
- 88.Xie Y, Liu J, Benbrahim-Tallaa L, Ward JM, Logsdon D, Diwan BA, Waalkes MP. Aberrant DNA methylation and gene expression in livers of newborn mice transplacentally exposed to a hepatocarcinogenic dose of inorganic arsenic. Toxicology. 236: 7–15 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jaworski M, Ittrich C, Hailfinger S, Bonin M, Buchmann A, Schwarz M, Kohle C. Global gene expression in Ha-ras and B-raf mutated mouse liver tumors. Int J Cancer. 121: 1382–1385 2007 [DOI] [PubMed] [Google Scholar]
- 90.Takashima K, Ito Y, Gonzalez FJ, Nakajima T. Different mechanisms of DEHP-induced hepatocellular adenoma tumorigenesis in wild-type and Ppar alpha-null mice. J Occup Health. 50: 169–180 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu J, Xie Y, Ducharme DM, Shen J, Diwan BA, Merrick BA, Grissom SF, Tucker CJ, Paules RS, Tennant R, Waalkes MP. Global gene expression associated with hepatocarcinogenesis in adult male mice induced by in utero arsenic exposure. Environ Health Perspect. 114: 404–411 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coulouarn C, Gomez-Quiroz LE, Lee JS, Kaposi-Novak P, Conner EA, Goldina TA, Onishchenko GE, Factor VM, Thorgeirsson SS. Oncogene-specific gene expression signatures at preneoplastic stage in mice define distinct mechanisms of hepatocarcinogenesis. Hepatology. 44: 1003–1011 2006 [DOI] [PubMed] [Google Scholar]
- 93.Harris AJ, Dial SL, Casciano DA. Comparison of basal gene expression profiles and effects of hepatocarcinogens on gene expression in cultured primary human hepatocytes and HepG2 cells. Mutat Res. 549: 79–99 2004 [DOI] [PubMed] [Google Scholar]
- 94.van Delft JH, van Agen E, van Breda SG, Herwijnen MH, Staal YC, Kleinjans JC. Comparison of supervised clustering methods to discriminate genotoxic from non-genotoxic carcinogens by gene expression profiling. Mutat Res. 575: 17–33 2005 [DOI] [PubMed] [Google Scholar]
- 95.Jolly RA, Ciurlionis R, Morfitt D, Helgren M, Patterson R, Ulrich RG, Waring JF. Microvesicular steatosis induced by a short chain fatty acid: effects on mitochondrial function and correlation with gene expression. Toxicol Pathol. 32(Suppl 2): 19–25 2004 [DOI] [PubMed] [Google Scholar]
- 96.Chung H, Hong DP, Kim HJ, Jang KS, Shin DM, Ahn JI, Lee YS, Kong G. Differential gene expression profiles in the steatosis/fibrosis model of rat liver by chronic administration of carbon tetrachloride. Toxicol Appl Pharmacol. 208: 242–254 2005 [DOI] [PubMed] [Google Scholar]
- 97.Fletcher N, Wahlstrom D, Lundberg R, Nilsson CB, Nilsson KC, Stockling K, Hellmold H, Hakansson H. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters the mRNA expression of critical genes associated with cholesterol metabolism, bile acid biosynthesis, and bile transport in rat liver: a microarray study. Toxicol Appl Pharmacol. 207: 1–24 2005 [DOI] [PubMed] [Google Scholar]
- 98.Lee MH, Hong I, Kim M, Lee BH, Kim JH, Kang KS, Kim HL, Yoon BI, Chung H, Kong G, Lee MO. Gene expression profiles of murine fatty liver induced by the administration of valproic acid. Toxicol Appl Pharmacol. 220: 45–59 2007 [DOI] [PubMed] [Google Scholar]
- 99.Yin HQ, Kim M, Kim JH, Kong G, Kang KS, Kim HL, Yoon BI, Lee MO, Lee BH. Differential gene expression and lipid metabolism in fatty liver induced by acute ethanol treatment in mice. Toxicol Appl Pharmacol. 223: 225–233 2007 [DOI] [PubMed] [Google Scholar]
- 100.Lee MH, Kim M, Lee BH, Kim JH, Kang KS, Kim HL, Yoon BI, Chung H, Kong G, Lee MO. Subchronic effects of valproic acid on gene expression profiles for lipid metabolism in mouse liver. Toxicol Appl Pharmacol. 226: 271–284 2008 [DOI] [PubMed] [Google Scholar]
- 101.Lee MH, Hong I, Kim M, Lee BH, Kim JH, Kang KS, Kim HL, Yoon BI, Chung H, Kong G, Lee MO. Gene expression profiles of murine fatty liver induced by the administration of methotrexate. Toxicology. 249: 75–84 2008 [DOI] [PubMed] [Google Scholar]
- 102.De Gottardi A, Vinciguerra M, Sgroi A, Moukil M. Ravier-Dall’Antonia F, Pazienza V, Pugnale P, Foti M, and Hadengue A. Microarray analyses and molecular profiling of steatosis induction in immortalized human hepatocytes. Lab Invest. 87: 792–806 2007 [DOI] [PubMed] [Google Scholar]
- 103.Chiappini F, Barrier A, Saffroy R, Domart MC, Dagues N, Azoulay D, Sebagh M, Franc B, Chevalier S, Debuire B, Dudoit S, Lemoine A. Exploration of global gene expression in human liver steatosis by high-density oligonucleotide microarray. Lab Invest. 86: 154–165 2006 [DOI] [PubMed] [Google Scholar]
- 104.McMillian M, Nie AY, Parker JB, Leone A, Bryant S, Kemmerer M, Herlich J, Liu Y, Yieh L, Bittner A, Liu X, Wan J, Johnson MD. A gene expression signature for oxidant stress/reactive metabolites in rat liver. Biochem Pharmacol. 68: 2249–2261 2004 [DOI] [PubMed] [Google Scholar]
- 105.Wu Y, Zhang X, Bardag-Gorce F, Robel RC, Aguilo J, Chen L, Zeng Y, Hwang K, French SW, Lu SC, Wan YJ. Retinoid X receptor alpha regulates glutathione homeostasis and xenobiotic detoxification processes in mouse liver. Mol Pharmacol. 65: 550–557 2004 [DOI] [PubMed] [Google Scholar]
- 106.Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 339: 79–88 2006 [DOI] [PubMed] [Google Scholar]
- 107.Yates MS, Kwak MK, Egner PA, Groopman JD, Bodreddigari S, Sutter TR, Baumgartner KJ, Roebuck BD, Liby KT, Yore MM, Honda T, Gribble GW, Sporn MB, Kensler TW. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res. 66: 2488–2494 2006 [DOI] [PubMed] [Google Scholar]
- 108.Dostalek M, Hardy KD, Milne GL, Morrow JD, Chen C, Gonzalez FJ, Gu J, Ding X, Johnson DA, Johnson JA, Martin MV, Guengerich FP. Development of oxidative stress by cytochrome P450 induction in rodents is selective for barbiturates and related to loss of pyridine nucleotide-dependent protective systems. J Biol Chem. 283: 17147–17157 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Merrill CL, Ni H, Yoon LW, Tirmenstein MA, Narayanan P, Benavides GR, Easton MJ, Creech DR, Hu CX, McFarland DC, Hahn LM, Thomas HC, Morgan KT. Etomoxir-induced oxidative stress in HepG2 cells detected by differential gene expression is confirmed biochemically. Toxicol Sci. 68: 93–101 2002 [DOI] [PubMed] [Google Scholar]
- 110.Hirode M, Ono A, Miyagishima T, Nagao T, Ohno Y, Urushidani T. Gene expression profiling in rat liver treated with compounds inducing phospholipidosis. Toxicol Appl Pharmacol. 229: 290–299 2008 [DOI] [PubMed] [Google Scholar]
- 111.Sawada H, Takami K, Asahi S. A toxicogenomic approach to drug-induced phospholipidosis: analysis of its induction mechanism and establishment of a novel in vitro screening system. Toxicol Sci. 83: 282–292 2005 [DOI] [PubMed] [Google Scholar]
- 112.Sawada H, Taniguchi K, Takami K. Improved toxicogenomic screening for drug-induced phospholipidosis using a multiplexed quantitative gene expression ArrayPlate assay. Toxicol In Vitro. 20: 1506–1513 2006 [DOI] [PubMed] [Google Scholar]
- 113.Kiyosawa N, Ito K, Sakuma K, Niino N, Kanbori M, Yamoto T, Manabe S, Matsunuma N. Evaluation of glutathione deficiency in rat livers by microarray analysis. Biochem Pharmacol. 68: 1465–1475 2004 [DOI] [PubMed] [Google Scholar]
- 114.Kiyosawa N, Uehara T, Gao W, Omura K, Hirode M, Shimizu T, Mizukawa Y, Ono A, Miyagishima T, Nagao T, Urushidani T. Identification of glutathione depletion-responsive genes using phorone-treated rat liver. J Toxicol Sci. 32: 469–486 2007 [DOI] [PubMed] [Google Scholar]
- 115.Mattes WB, Daniels KK, Summan M, Xu ZA, Mendrick DL. Tissue and species distribution of the glutathione pathway transcriptome. Xenobiotica. 36: 1081–1121 2006 [DOI] [PubMed] [Google Scholar]
- 116.Pan Q, Zhang ZB, Zhang X, Shi J, Chen YX, Han ZG, Xie WF. Gene expression profile analysis of the spontaneous reversal of rat hepatic fibrosis by cDNA microarray. Dig Dis Sci. 52: 2591–2600 2007 [DOI] [PubMed] [Google Scholar]
- 117.Takahara Y, Takahashi M, Wagatsuma H, Yokoya F, Zhang QW, Yamaguchi M, Aburatani H, Kawada N. Gene expression profiles of hepatic cell-type specific marker genes in progression of liver fibrosis. World J Gastroenterol. 12: 6473–6499 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jiroutova A, Slavkovsky R, Cermakova M, Majdiakova L, Hanovcova I, Bolehovska R, Hajzlerova M, Radilova H, Ruszova E, Kanta J. Expression of mRNAs related to connective tissue metabolism in rat hepatic stellate cells and myofibroblasts. Exp Toxicol Pathol. 58: 263–273 2007 [DOI] [PubMed] [Google Scholar]
- 119.Qiang H, Lin Y, Zhang X, Zeng X, Shi J, Chen YX, Yang MF, Han ZG, Xie WF. Differential expression genes analyzed by cDNA array in the regulation of rat hepatic fibrogenesis. Liver Int. 26: 1126–1137 2006 [DOI] [PubMed] [Google Scholar]
- 120.Tugues S, Morales-Ruiz M, Fernandez-Varo G, Ros J, Arteta D, Munoz-Luque J, Arroyo V, Rodes J, Jimenez W. Microarray analysis of endothelial differentially expressed genes in liver of cirrhotic rats. Gastroenterology. 129: 1686–1695 2005 [DOI] [PubMed] [Google Scholar]
- 121.Vickers AE, Saulnier M, Cruz E, Merema MT, Rose K, Bentley P, Olinga P. Organ slice viability extended for pathway characterization: an in vitro model to investigate fibrosis. Toxicol Sci. 82: 534–544 2004 [DOI] [PubMed] [Google Scholar]
- 122.Utsunomiya T, Okamoto M, Hashimoto M, Yoshinaga K, Shiraishi T, Tanaka F, Mimori K, Inoue H, Watanabe G, Barnard GF, Mori M. A gene-expression signature can quantify the degree of hepatic fibrosis in the rat. J Hepatol. 41: 399–406 2004 [DOI] [PubMed] [Google Scholar]
- 123.De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 132: 1937–1946 2007 [DOI] [PubMed] [Google Scholar]
- 124.Liu XJ, Yang L, Luo FM, Wu HB, Qiang Q. Association of differentially expressed genes with activation of mouse hepatic stellate cells by high-density cDNA microarray. World J Gastroenterol. 10: 1600–1607 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jiang Y, Liu J, Waalkes M, Kang YJ. Changes in the gene expression associated with carbon tetrachloride-induced liver fibrosis persist after cessation of dosing in mice. Toxicol Sci. 79: 404–410 2004 [DOI] [PubMed] [Google Scholar]
- 126.Zindy PJ, L’Helgoualc’h A, Bonnier D, Le Bechec A, Bourd-Boitin K, Zhang CX, Musso O, Glaise D, Troadec MB, Loreal O, Turlin B, Leger J, Clement B, Theret N. Upregulation of the tumor suppressor gene menin in hepatocellular carcinomas and its significance in fibrogenesis. Hepatology. 44: 1296–1307 2006 [DOI] [PubMed] [Google Scholar]
- 127.Kim S, Park YM. Specific gene expression patterns in liver cirrhosis. Biochem Biophys Res Commun. 334: 681–688 2005 [DOI] [PubMed] [Google Scholar]
- 128.Sancho-Bru P, Bataller R, Gasull X, Colmenero J, Khurdayan V, Gual A, Nicolas JM, Arroyo V, Gines P. Genomic and functional characterization of stellate cells isolated from human cirrhotic livers. J Hepatol. 43: 272–282 2005 [DOI] [PubMed] [Google Scholar]
- 129.Kannangai R, Diehl AM, Sicklick J, Rojkind M, Thomas D, Torbenson M. Hepatic angiomyolipoma and hepatic stellate cells share a similar gene expression profile. Hum Pathol. 36: 341–347 2005 [DOI] [PubMed] [Google Scholar]
- 130.Shao RX, Hoshida Y, Otsuka M, Kato N, Tateishi R, Teratani T, Shiina S, Taniguchi H, Moriyama M, Kawabe T, Omata M. Hepatic gene expression profiles associated with fibrosis progression and hepatocarcinogenesis in hepatitis C patients. World J Gastroenterol. 11: 1995–1999 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zindy P, Andrieux L, Bonnier D, Musso O, Langouet S, Campion JP, Turlin B, Clement B, Theret N. Upregulation of DNA repair genes in active cirrhosis associated with hepatocellular carcinoma. FEBS Lett. 579: 95–99 2005 [DOI] [PubMed] [Google Scholar]
- 132.Chen L, Goryachev A, Sun J, Kim P, Zhang H, Phillips MJ, Macgregor P, Lebel S, Edwards AM, Cao Q, Furuya KN. Altered expression of genes involved in hepatic morphogenesis and fibrogenesis are identified by cDNA microarray analysis in biliary atresia. Hepatology. 38: 567–576 2003 [DOI] [PubMed] [Google Scholar]
- 133.Ito K, Kiyosawa N, Kumagai K, Manabe S, Matsunuma N, Yamoto T. Molecular mechanism investigation of cycloheximide-induced hepatocyte apoptosis in rat livers by morphological and microarray analysis. Toxicology. 219: 175–186 2006 [DOI] [PubMed] [Google Scholar]
- 134.Nair S, Xu C, Shen G, Hebbar V, Gopalakrishnan A, Hu R, Jain MR, Liew C, Chan JY, Kong AN. Toxicogenomics of endoplasmic reticulum stress inducer tunicamycin in the small intestine and liver of Nrf2 knockout and C57BL/6J mice. Toxicol Lett. 168: 21–39 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Flowers MT, Keller MP, Choi Y, Lan H, Kendziorski C, Ntambi JM, Attie AD. Liver gene expression analysis reveals endoplasmic reticulum stress and metabolic dysfunction in SCD1-deficient mice fed a very low-fat diet. Physiol Genomics. 33: 361–372 2008 [DOI] [PubMed] [Google Scholar]
- 136.Szczesna-Skorupa E, Chen CD, Liu H, Kemper B. Gene expression changes associated with the endoplasmic reticulum stress response induced by microsomal cytochrome p450 overproduction. J Biol Chem. 279: 13953–13961 2004 [DOI] [PubMed] [Google Scholar]
- 137.Igoudjil A, Massart J, Begriche K, Descatoire V, Robin MA, Fromenty B. High concentrations of stavudine impair fatty acid oxidation without depleting mitochondrial DNA in cultured rat hepatocytes. Toxicol In Vitro. 22: 887–898 2008 [DOI] [PubMed] [Google Scholar]
- 138.Coe KJ, Jia Y, Ho HK, Rademacher P, Bammler TK, Beyer RP, Farin FM, Woodke L, Plymate SR, Fausto N, Nelson SD. Comparison of the cytotoxicity of the nitroaromatic drug flutamide to its cyano analogue in the hepatocyte cell line TAMH: evidence for complex I inhibition and mitochondrial dysfunction using toxicogenomic screening. Chem Res Toxicol. 20: 1277–1290 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Desai VG, Lee T, Delongchamp RR, Leakey JE, Lewis SM, Lee F, Moland CL, Branham WS, Fuscoe JC. Nucleoside reverse transcriptase inhibitors (NRTIs)-induced expression profile of mitochondria-related genes in the mouse liver. Mitochondrion. 8: 181–195 2008 [DOI] [PubMed] [Google Scholar]
- 140.Desai VG, Lee T, Delongchamp RR, Moland CL, Branham WS, Fuscoe JC, Leakey JE. Development of mitochondria-specific mouse oligonucleotide microarray and validation of data by real-time PCR. Mitochondrion. 7: 322–329 2007 [DOI] [PubMed] [Google Scholar]
- 141.Currie RA, Bombail V, Oliver JD, Moore DJ, Lim FL, Gwilliam V, Kimber I, Chipman K, Moggs JG, Orphanides G. Gene ontology mapping as an unbiased method for identifying molecular pathways and processes affected by toxicant exposure: application to acute effects caused by the rodent non-genotoxic carcinogen diethylhexylphthalate. Toxicol Sci. 86: 453–469 2005 [DOI] [PubMed] [Google Scholar]
- 142.Tamura K, Ono A, Miyagishima T, Nagao T, Urushidani T. Profiling of gene expression in rat liver and rat primary cultured hepatocytes treated with peroxisome proliferators. J Toxicol Sci. 31: 471–490 2006 [DOI] [PubMed] [Google Scholar]
- 143.Omura K, Kiyosawa N, Uehara T, Hirode M, Shimizu T, Miyagishima T, Ono A, Nagao T, Urushidani T. Gene expression profiling of rat liver treated with serum triglyceride-decreasing compounds. J Toxicol Sci. 32: 387–399 2007 [DOI] [PubMed] [Google Scholar]
- 144.Guo Y, Jolly RA, Halstead BW, Baker TK, Stutz JP, Huffman M, Calley JN, West A, Gao H, Searfoss GH, Li S, Irizarry AR, Qian HR, Stevens JL, Ryan TP. Underlying mechanisms of pharmacology and toxicity of a novel PPAR agonist revealed using rodent and canine hepatocytes. Toxicol Sci. 96: 294–309 2007 [DOI] [PubMed] [Google Scholar]
- 145.Yadetie F, Laegreid A, Bakke I, Kusnierczyk W, Komorowski J, Waldum HL, Sandvik AK. Liver gene expression in rats in response to the peroxisome proliferator-activated receptor-alpha agonist ciprofibrate. Physiol Genomics. 15: 9–19 2003 [DOI] [PubMed] [Google Scholar]
- 146.Yamazaki K, Kuromitsu J, Tanaka I. Microarray analysis of gene expression changes in mouse liver induced by peroxisome proliferator- activated receptor alpha agonists. Biochem Biophys Res Commun. 290: 1114–1122 2002 [DOI] [PubMed] [Google Scholar]
- 147.Patsouris D, Reddy JK, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology. 147: 1508–1516 2006 [DOI] [PubMed] [Google Scholar]
- 148.Woods CG, Kosyk O, Bradford BU, Ross PK, Burns AM, Cunningham ML, Qu P, Ibrahim JG, Rusyn I. Time course investigation of PPARalpha- and Kupffer cell-dependent effects of WY-14,643 in mouse liver using microarray gene expression. Toxicol Appl Pharmacol. 225: 267–277 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kato N, Shibutani M, Takagi H, Uneyama C, Lee KY, Takigami S, Mashima K, Hirose M. Gene expression profile in the livers of rats orally administered ethinylestradiol for 28 days using a microarray technique. Toxicology. 200: 179–192 2004 [DOI] [PubMed] [Google Scholar]
- 150.Henriquez-Hernandez LA, Flores-Morales A, Santana-Farre R, Axelson M, Nilsson P, Norstedt G, Fernandez-Perez L. Role of pituitary hormones on 17alpha-ethinylestradiol-induced cholestasis in rat. J Pharmacol Exp Ther. 320: 695–705 2007 [DOI] [PubMed] [Google Scholar]
- 151.Stahlberg N, Merino R, Hernandez LH, Fernandez-Perez L, Sandelin A, Engstrom P, Tollet-Egnell P, Lenhard B, Flores-Morales A. Exploring hepatic hormone actions using a compilation of gene expression profiles. BMC Physiol. 5: 8 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol. 17: 203–208 2003 [DOI] [PubMed] [Google Scholar]
- 153.Vezina CM, Walker NJ, Olson JR. Subchronic exposure to TCDD, PeCDF, PCB126, and PCB153: effect on hepatic gene expression. Environ Health Perspect. 112: 1636–1644 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kondraganti SR, Muthiah K, Jiang W, Barrios R, Moorthy B. Effects of 3-methylcholanthrene on gene expression profiling in the rat using cDNA microarray analyses. Chem Res Toxicol. 18: 1634–1641 2005 [DOI] [PubMed] [Google Scholar]
- 155.Pastorelli R, Carpi D, Campagna R, Airoldi L, Pohjanvirta R, Viluksela M, Hakansson H, Boutros PC, Moffat ID, Okey AB, Fanelli R. Differential expression profiling of the hepatic proteome in a rat model of dioxin resistance: correlation with genomic and transcriptomic analyses. Mol Cell Proteomics. 5: 882–894 2006 [DOI] [PubMed] [Google Scholar]
- 156.Boverhof DR, Burgoon LD, Tashiro C, Sharratt B, Chittim B, Harkema JR, Mendrick DL, Zacharewski TR. Comparative toxicogenomic analysis of the hepatotoxic effects of TCDD in Sprague Dawley rats and C57BL/6 mice. Toxicol Sci. 94: 398–416 2006 [DOI] [PubMed] [Google Scholar]
- 157.Silkworth JB, Carlson EA, McCulloch C, Illouz K, Goodwin S, Sutter TR. Toxicogenomic analysis of gender, chemical, and dose effects in livers of TCDD- or aroclor 1254-exposed rats using a multifactor linear model. Toxicol Sci. 102: 291–309 2008 [DOI] [PubMed] [Google Scholar]
- 158.Boverhof DR, Burgoon LD, Tashiro C, Chittim B, Harkema JR, Jump DB, Zacharewski TR. Temporal and dose-dependent hepatic gene expression patterns in mice provide new insights into TCDD-Mediated hepatotoxicity. Toxicol Sci. 85: 1048–1063 2005 [DOI] [PubMed] [Google Scholar]
- 159.Yoon CY, Park M, Kim BH, Park JY, Park MS, Jeong YK, Kwon H, Jung HK, Kang H, Lee YS, Lee BJ. Gene expression profile by 2,3,7,8-tetrachlorodibenzo-p-dioxin in the liver of wild-type (AhR+/+) and aryl hydrocarbon receptor-deficient (AhR-/-) mice. J Vet Med Sci. 68: 663–668 2006 [DOI] [PubMed] [Google Scholar]
- 160.Hayes KR, Zastrow GM, Nukaya M, Pande K, Glover E, Maufort JP, Liss AL, Liu Y, Moran SM, Vollrath AL, Bradfield CA. Hepatic transcriptional networks induced by exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Chem Res Toxicol. 20: 1573–1581 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sato S, Shirakawa H, Tomita S, Ohsaki Y, Haketa K, Tooi O, Santo N, Tohkin M, Furukawa Y, Gonzalez FJ, Komai M. Low-dose dioxins alter gene expression related to cholesterol biosynthesis, lipogenesis, and glucose metabolism through the aryl hydrocarbon receptor-mediated pathway in mouse liver. Toxicol Appl Pharmacol. 229: 10–19 2008 [DOI] [PubMed] [Google Scholar]
- 162.N’Jai A, Boverhof DR, Dere E, Burgoon LD, Tan YS, Rowlands JC, Budinsky RA, Stebbins KE, Zacharewski TR. Comparative temporal toxicogenomic analysis of TCDD- and TCDF-mediated hepatic effects in immature female C57BL/6 mice. Toxicol Sci. 103: 285–297 2008 [DOI] [PubMed] [Google Scholar]
- 163.Kim WK, In YJ, Kim JH, Cho HJ, Kang S, Lee CY, Lee SC. Quantitative relationship of dioxin-responsive gene expression to dioxin response element in Hep3B and HepG2 human hepatocarcinoma cell lines. Toxicol Lett. 165: 174–181 2006 [DOI] [PubMed] [Google Scholar]
- 164.Waring JF, Liguori MJ, Luyendyk JP, Maddox JF, Ganey PE, Stachlewitz RF, North C, Blomme EA, Roth RA. Microarray analysis of lipopolysaccharide potentiation of trovafloxacin-induced liver injury in rats suggests a role for proinflammatory chemokines and neutrophils. J Pharmacol Exp Ther. 316: 1080–1087 2006 [DOI] [PubMed] [Google Scholar]
- 165.McMillian M, Nie AY, Parker JB, Leone A, Kemmerer M, Bryant S, Herlich J, Yieh L, Bittner A, Liu X, Wan J, Johnson MD. Inverse gene expression patterns for macrophage activating hepatotoxicants and peroxisome proliferators in rat liver. Biochem Pharmacol. 67: 2141–2165 2004 [DOI] [PubMed] [Google Scholar]
- 166.Liu J, Saavedra JE, Lu T, Song JG, Clark J, Waalkes MP, Keefer LKO. (2)-Vinyl 1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate protection against D-galactosamine/ endotoxin-induced hepatotoxicity in mice: genomic analysis using microarrays. J Pharmacol Exp Ther. 300: 18–25 2002 [DOI] [PubMed] [Google Scholar]
- 167.Ding H, Shi GG, Yu X, Yu JP, Huang JA. Modulation of GdCl3 and Angelica sinensis polysaccharides on differentially expressed genes in liver of hepatic immunological injury mice by cDNA microarray. World J Gastroenterol. 9: 1072–1076 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sunil VR, Patel KJ, Nilsen-Hamilton M, Heck DE, Laskin JD, Laskin DL. Acute endotoxemia is associated with upregulation of lipocalin 24p3/Lcn2 in lung and liver. Exp Mol Pathol. 83: 177–187 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Higgins MA, Berridge BR, Mills BJ, Schultze AE, Gao H, Searfoss GH, Baker TK, Ryan TP. Gene expression analysis of the acute phase response using a canine microarray. Toxicol Sci. 74: 470–484 2003 [DOI] [PubMed] [Google Scholar]
- 170.Rokushima M, Omi K, Araki A, Kyokawa Y, Furukawa N, Itoh F, Imura K, Takeuchi K, Okada M, Kato I, Ishizaki J. A toxicogenomic approach revealed hepatic gene expression changes mechanistically linked to drug-induced hemolytic anemia. Toxicol Sci. 95: 474–484 2007 [DOI] [PubMed] [Google Scholar]
- 171.Bleasby K, Castle JC, Roberts CJ, Cheng C, Bailey WJ, Sina JF, Kulkarni AV, Hafey MJ, Evers R, Johnson JM, Ulrich RG, Slatter JG. Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug disposition. Xenobiotica. 36: 963–988 2006 [DOI] [PubMed] [Google Scholar]
- 172.Takashima K, Mizukawa Y, Morishita K, Okuyama M, Kasahara T, Toritsuka N, Miyagishima T, Nagao T, Urushidani T. Effect of the difference in vehicles on gene expression in the rat liver–analysis of the control data in the Toxicogenomics Project Database. Life Sci. 78: 2787–2796 2006 [DOI] [PubMed] [Google Scholar]
- 173.Boedigheimer MJ, Wolfinger RD, Bass MB, Bushel PR, Chou JW, Cooper M, Corton JC, Fostel J, Hester S, Lee JS, Liu F, Liu J, Qian HR, Quackenbush J, Pettit S, Thompson KL. Sources of variation in baseline gene expression levels from toxicogenomics study control animals across multiple laboratories. BMC Genomics. 9: 285 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kiyosawa N, Shiwaku K, Hirode M, Omura K, Uehara T, Shimizu T, Mizukawa Y, Miyagishima T, Ono A, Nagao T, Urushidani T. Utilization of a one-dimensional score for surveying chemical-induced changes in expression levels of multiple biomarker gene sets using a large-scale toxicogenomics database. J Toxicol Sci. 31: 433–448 2006 [DOI] [PubMed] [Google Scholar]
- 175.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 31: 1499–1506 2003 [DOI] [PubMed] [Google Scholar]
- 176.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30: 207–210 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Brazma A, Parkinson H, Sarkans U, Shojatalab M, Vilo J, Abeygunawardena N, Holloway E, Kapushesky M, Kemmeren P, Lara GG, Oezcimen A, Rocca-Serra P, Sansone SA. ArrayExpress–a public repository for microarray gene expression data at the EBI. Nucleic Acids Res. 31: 68–71 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Waters M, Stasiewicz S, Merrick BA, Tomer K, Bushel P, Paules R, Stegman N, Nehls G, Yost KJ, Johnson CH, Gustafson SF, Xirasagar S, Xiao N, Huang CC, Boyer P, Chan DD, Pan Q, Gong H, Taylor J, Choi D, Rashid A, Ahmed A, Howle R, Selkirk J, Tennant R, Fostel J. CEBS–chemical effects in biological systems: a public data repository integrating study design and toxicity data with microarray and proteomics data. Nucleic Acids Res. 36: D892–D900 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mattingly CJ, Rosenstein MC, Davis AP, Colby GT, Forrest JN, Boyer JL. The comparative toxicogenomics database: a cross-species resource for building chemical-gene interaction networks. Toxicol Sci. 92: 587–595 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hayes KR, Vollrath AL, Zastrow GM, McMillan BJ, Craven M, Jovanovich S, Rank DR, Penn S, Walisser JA, Reddy JK, Thomas RS, Bradfield CA. EDGE: a centralized resource for the comparison, analysis, and distribution of toxicogenomic information. Mol Pharmacol. 67: 1360–1368 2005 [DOI] [PubMed] [Google Scholar]
- 181.Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, Gaasterland T, Glenisson P, Holstege FC, Kim IF, Markowitz V, Matese JC, Parkinson H, Robinson A, Sarkans U, Schulze-Kremer S, Stewart J, Taylor R, Vilo J, Vingron M. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet. 29: 365–371 2001 [DOI] [PubMed] [Google Scholar]
- 182.Mah N, Thelin A, Lu T, Nikolaus S, Kuhbacher T, Gurbuz Y, Eickhoff H, Kloppel G, Lehrach H, Mellgard B, Costello CM, Schreiber S. A comparison of oligonucleotide and cDNA-based microarray systems. Physiol Genomics. 16: 361–370 2004 [DOI] [PubMed] [Google Scholar]
- 183.Severgnini M, Bicciato S, Mangano E, Scarlatti F, Mezzelani A, Mattioli M, Ghidoni R, Peano C, Bonnal R, Viti F, Milanesi L, De Bellis G, Battaglia C. Strategies for comparing gene expression profiles from different microarray platforms: application to a case-control experiment. Anal Biochem. 353: 43–56 2006 [DOI] [PubMed] [Google Scholar]
- 184.Waring JF, Ulrich RG, Flint N, Morfitt D, Kalkuhl A, Staedtler F, Lawton M, Beekman JM, Suter L. Interlaboratory evaluation of rat hepatic gene expression changes induced by methapyrilene. Environ Health Perspect. 112: 439–448 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chu TM, Deng S, Wolfinger R, Paules RS, Hamadeh HK. Cross-site comparison of gene expression data reveals high similarity. Environ Health Perspect. 112: 449–455 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fielden MR, Nie A, McMillian M, Elangbam CS, Trela BA, Yang Y, Dunn RT, Dragan Y, Fransson-Stehen R, Bogdanffy M, Adams SP, Foster WR, Chen SJ, Rossi P, Kasper P, Jacobson-Kram D, Tatsuoka KS, Wier PJ, Gollub J, Halbert DN, Roter A, Young JK, Sina JF, Marlowe J, Martus HJ, Aubrecht J, Olaharski AJ, Roome N, Nioi P, Pardo I, Snyder R, Perry R, Lord P, Mattes W, Car BD. Interlaboratory evaluation of genomic signatures for predicting carcinogenicity in the rat. Toxicol Sci. 103: 28–34 2008 [DOI] [PubMed] [Google Scholar]
- 187.Baker VA, Harries HM, Waring JF, Duggan CM, Ni HA, Jolly RA, Yoon LW, De Souza AT, Schmid JE, Brown RH, Ulrich RG, Rockett JC. Clofibrate-induced gene expression changes in rat liver: a cross-laboratory analysis using membrane cDNA arrays. Environ Health Perspect. 112: 428–438 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Burgoon LD. The need for standards, not guidelines, in biological data reporting and sharing. Nat Biotechnol. 24: 1369–1373 2006 [DOI] [PubMed] [Google Scholar]
- 189.Kiyosawa N, Ito K, Watanabe K, Kanbori M, Niino N, Manabe S, Yamoto T. Utilization of a toxicogenomic biomarker for evaluation of chemical-induced glutathione deficiency in rat livers across the GeneChip data of different generations. Toxicol Lett. 163: 161–169 2006 [DOI] [PubMed] [Google Scholar]
- 190.Shi L, Reid LH, Jones WD, Shippy R, Warrington JA, Baker SC, Collins PJ, de Longueville F, Kawasaki ES, Lee KY, Luo Y, Sun YA, Willey JC, Setterquist RA, Fischer GM, Tong W, Dragan YP, Dix DJ, Frueh FW, Goodsaid FM, Herman D, Jensen RV, Johnson CD, Lobenhofer EK, Puri RK, Schrf U, Thierry-Mieg J, Wang C, Wilson M, Wolber PK, Zhang L, Amur S, Bao W, Barbacioru CC, Lucas AB, Bertholet V, Boysen C, Bromley B, Brown D, Brunner A, Canales R, Cao XM, Cebula TA, Chen JJ, Cheng J, Chu TM, Chudin E, Corson J, Corton JC, Croner LJ, Davies C, Davison TS, Delenstarr G, Deng X, Dorris D, Eklund AC, Fan XH, Fang H, Fulmer-Smentek S, Fuscoe JC, Gallagher K, Ge W, Guo L, Guo X, Hager J, Haje PK, Han J, Han T, Harbottle HC, Harris SC, Hatchwell E, Hauser CA, Hester S, Hong H, Hurban P, Jackson SA, Ji H, Knight CR, Kuo WP, LeClerc JE, Levy S, Li QZ, Liu C, Liu Y, Lombardi MJ, Ma Y, Magnuson SR, Maqsodi B, McDaniel T, Mei N, Myklebost O, Ning B, Novoradovskaya N, Orr MS, Osborn TW, Papallo A, Patterson TA, Perkins RG, Peters EH, Peterson R, Philips KL, Pine PS, Pusztai L, Qian F, Ren H, Rosen M, Rosenzweig BA, Samaha RR, Schena M, Schroth GP, Shchegrova S, Smith DD, Staedtler F, Su Z, Sun H, Szallasi Z, Tezak Z, Thierry-Mieg D, Thompson KL, Tikhonova I, Turpaz Y, Vallanat B, Van C, Walker SJ, Wang SJ, Wang Y, Wolfinger R, Wong A, Wu J, Xiao C, Xie Q, Xu J, Yang W, Zhong S, Zong Y, Slikker W, Jr. . The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol. 24: 1151–1161 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen JJ, Hsueh HM, Delongchamp RR, Lin CJ, Tsai CA. Reproducibility of microarray data: a further analysis of microarray quality control (MAQC) data. BMC Bioinformatics. 8: 412 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Guo L, Lobenhofer EK, Wang C, Shippy R, Harris SC, Zhang L, Mei N, Chen T, Herman D, Goodsaid FM, Hurban P, Phillips KL, Xu J, Deng X, Sun YA, Tong W, Dragan YP, Shi L. Rat toxicogenomic study reveals analytical consistency across microarray platforms. Nat Biotechnol. 24: 1162–1169 2006 [DOI] [PubMed] [Google Scholar]
- 193.Poland A, Mak I, Glover E. Species differences in responsiveness to 1,4-bis[2-(3,5-dichloropyridyloxy)]-benzene, a potent phenobarbital-like inducer of microsomal monooxygenase activity. Mol Pharmacol. 20: 442–450 1981 [PubMed] [Google Scholar]
- 194.Pustylnyak VO, Gulyaeva LF, Lyakhovich VV. Induction of cytochrome P4502B: role of regulatory elements and nuclear receptors. Biochemistry (Mosc). 72: 608–617 2007 [DOI] [PubMed] [Google Scholar]
- 195.Suino K, Peng L, Reynolds R, Li Y, Cha JY, Repa JJ, Kliewer SA, Xu HE. The nuclear xenobiotic receptor CAR: structural determinants of constitutive activation and heterodimerization. Mol Cell. 16: 893–905 2004 [DOI] [PubMed] [Google Scholar]
- 196.Pustylnyak VO, Lebedev AN, Gulyaeva LF, Lyakhovich VV, Slynko NM. Comparative study of CYP2B induction in the liver of rats and mice by different compounds. Life Sci. 80: 324–328 2007 [DOI] [PubMed] [Google Scholar]
- 197.Wang H, LeCluyse EL. Role of orphan nuclear receptors in the regulation of drug-metabolising enzymes. Clin Pharmacokinet. 42: 1331–1357 2003 [DOI] [PubMed] [Google Scholar]
- 198.Kiyosawa N, Kwekel JC, Burgoon LD, Dere E, Williams KJ, Tashiro C, Chittim B, Zacharewski TR. Species-specific regulation of PXR/CAR/ER-target genes in the mouse and rat liver elicited by o,p’-DDT. BMC. Genomics. 9: 487 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kiyosawa N, Kwekel JC, Burgoon LD, Williams KJ, Tashiro C, Chittim B, Zacharewski TR. o,p’-DDT elicits PXR/CAR-, not ER-, mediated responses in the immature ovariectomized rat liver. Toxicol Sci. 101: 350–363 2008 [DOI] [PubMed] [Google Scholar]
- 200.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 4: 489–499 2005 [DOI] [PubMed] [Google Scholar]
- 201.FDA FDA, European medicines agency to consider additional test results when assessing new drug safety 2008, 2008
- 202.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 273: 4135–4142 1998 [DOI] [PubMed] [Google Scholar]
- 203.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 286: F552–F563 2004 [DOI] [PubMed] [Google Scholar]