Abstract
Both the sympathetic nervous system and the proinflammatory cytokine interleukin-18 (IL-18) play key roles in the pathophysiology of the hypertrophied failing heart. IL-18 binding protein (IL-18BP), a natural inhibitor of IL-18, counters its biological effects. β-AR stimulation induces IL-18 expression, but whether it also regulates IL-18BP is not known. Here we demonstrate that the β-AR agonist isoproterenol (ISO) increases steady state IL-18BP mRNA and protein levels in adult mouse cardiomyocytes in a β2-AR-dependent manner. We cloned mouse Il18bp 5’cis-regulatory region, and identified putative CREB and C/EBPβ transcription factor-binding sites. Forced expression of mutant CREB or C/EBPβ knockdown markedly attenuated ISO-induced Il18bp transcription and deletion or mutation of CREB and C/EBP motifs in the Il18bp promoter reduced ISO-induced promoter-reporter gene activity. ISO induced CREB and C/EBPβ activation in cardiomyocytes via PI3K/Akt and ERK1/2. Importantly, ISO-induced hypertrophy in vitro was dependent on IL-18 induction as it was blunted by IL-18 neutralizing antibodies and forced expression of IL-18BP. Moreover, ISO-induced hypertrophy was markedly attenuated in IL-18 null and IL-18BP transgenic mice. These data support the novel concept that β-AR activation, in addition to inducing cardiomyocyte hypertrophy via IL-18, concomitantly induces a countering effect by stimulating IL-18BP expression, and that ISO-induced cardiomyocyte hypertrophy may result from a net effect of IL-18 and IL-18BP induction.
Keywords: Signal transduction, transcription, cloning, molecular mechanism, adrenergic stimulation, heart failure
1. Introduction
Myocardial hypertrophy and failure are multi-factorial diseases. Sustained production of inflammatory cytokines plays a role in the initiation and progression of left ventricular hypertrophy to failure [1, 2]. Whereas low levels of cytokines may be protective for cardiomyocytes, chronically elevated levels appear to be detrimental [1–3]. Interleukin (IL)-18 is a pleiotropic cytokine belonging to the IL-1 family that is constitutively expressed as an inactive precursor in healthy cells, but during various immune, infectious, and inflammatory conditions, is converted to an active form by caspase-1 [4]. Processed (mature) IL-18 exerts its effects through the IL-18R receptor (IL-18R), a heterodimer of ligand-binding α and signal-transducing β subunits. Increased circulating IL-18 levels have been detected in patients with heart failure and a positive correlation has been demonstrated between serum IL-18 levels and the severity of myocardial dysfunction [5, 6], implying a pathophysiological role for this cytokine.
IL-18 binding protein (IL-18BP) on the other hand, is a naturally occurring, constitutively secreted inhibitor of IL-18, and a distinct gene product [7]. Differential splicing of human IL-18BP mRNA results in four isoforms: a, b, c and d. The ‘a’ isoform (IL-18BPa) exhibits the greatest affinity for IL-18 with a rapid on-rate, a slow off-rate, and a dissociation constant of 0.399 nM (versus 2.94 nM for IL-18BPc) [7], and both IL-18BPa and IL-18BPc neutralize human and mouse IL-18 by at least 95% at equimolar concentrations [7]. The b and d isoforms however, acking a complete immunoglobulin (Ig) domain, fail to bind and neutralize IL-18, indicating that IL-18BPa is the most potent isoform in man [7]. Of the four isoforms in mouse, only IL-18BPc and IL-18BPd have been shown to neutralize 95% of the IL-18 activity at equimolar concentrations [7].
IL-18BP binds IL-18 with higher affinity than IL-18 binds its receptor, and thus competitively blocks its activity [7]. The IL-18BP in the circulation of healthy humans is in at least 20-fold molar excess relative to IL-18. Thus under physiological conditions, a major portion of circulating IL-18 may be neutralized by binding to IL-18BP. In diseased states however, an active IL-18 ligand/receptor response axis may be elevated, and perhaps through more than one mechanism. For example, patients with heart failure have increased levels of IL-18 and IL-18Rα, but significantly decreased IL-18BP [5]. Thus the combination of increased IL-18 and IL-18Rα, and reduced IL-18BP may result in a positive amplification of IL-18 signaling. The therapeutic efficacy of IL-18BPa has also been demonstrated in animal models of tissue injury [8, 9].
We and others have previously reported that IL-18 is potent pro-growth factor, inducing hypertrophy of isolated cardiomyocytes and H9c2 myoblasts [10, 11]. Neutralization of TNF-α, IL-1 and IFN-γ failed to modulate IL-18-mediated cardiomyocyte hypertrophy [10], suggesting the response to IL-18 was independent of other IL-18-induced cytokines. Studies from our laboratory as well as others have also shown that chronic administration of IL-18 induces myocardial hypertrophy with increased ANF expression [12, 13], and that pressure overload (transaortic constriction; TAC)-induced hypertrophy was markedly attenuated in IL-18 null mice [14].
β-AR stimulation induces myocardial and systemic elaboration of IL-18 [15]. ISO activated both basal and inducible Il18 promoter reporter activities and stimulated IL-18 expression in cardiac microvascular endothelial cells [15]. These results indicate that ISO regulates IL-18 expression via increased gene transcription and mRNA stability, and suggest that IL-18 might contribute to chronic β-AR stimulation-induced hypertrophy and cardiac failure. However, whether β-AR stimulation also regulates IL-18BP expression is not known. Here we demonstrate for the first time that the β-AR agonist ISO upregulates IL-18BP expression in mouse cardiomyocytes in a β2-AR-dependent manner, and via PI3K/Akt and ERK1/2-dependent CREB and C/EBPβ activation. Importantly, we also report that IL-18BP blunts ISO-induced cardiomyocyte hypertrophy in vitro and myocardial hypertrophy in vivo. Using IL-18BP transgenic (IL-18BP Tg) and IL-18 null mice, we further confirmed these observations. These data suggest that strategies that raise systemic levels of IL-18BP may have therapeutic potential in the treatment of hypertrophied failing heart, a diseased state characterized by sustained β-AR activation and IL-18 signaling.
2. Materials and methods
2.1. Materials
The materials used in this report are detailed in ‘Supplementary methods’ section.
2.2. Animals
All studies were performed in compliance with the NIH Guide for the Care and Use of Laboratory Animals (DHHS Publication No. [NIH] 85-23, revised 1996), and were approved by the Institutional Animal Care and Use Committees of the University of Texas Health Science Center at San Antonio, TX and Tulane University, New Orleans, LA. Male wild type C57Bl/6 mice and homozygous IL-18−/− (IL-18 null C57Bl/6 mice) were purchased from The Jackson Laboratory (Bar Harbor, ME). IL-18 null mice have been previously described [14]. IL-18BP transgenic mice (IL-18BP Tg) that overexpress human IL-18BP isoform ‘a’ have also been previously described [16]. Non-transgenic littermates served as controls. All animals were used at ~3 months of age. Mice were subcutaneously implanted with mini-osmotic pumps (Alzet model 1007D) for continuous infusion of L-isoproterenol in 0.002% ascorbic acid at 15 mg/kg body wt/day [15]. Control mice were implanted with pumps that delivered vehicle (0.002% ascorbic acid) only. After 7 days, body weights were recorded. Blood was collected and serum separated and stored at −80°C. The heart was rapidly excised, rinsed in ice-cold physiological saline, and weighed. The atria were trimmed away, and the left (LV) and right ventricles separated. LV tissue was snap frozen in liquid nitrogen and stored at −80°C for biochemical and gene expression analysis. A portion of LV tissue was embedded in OCT for histo-morphometric analysis.
A subset of wild type male C57Bl/6 animals received the β1-AR antagonist betaxolol HCl (20 mg/kg. body wt), or the β2-AR antagonist ICI 118,551 (4 mg/kg. body wt) 1 h prior to ISO administration, and sacrificed after 3 h of ISO treatment, a time period at which maximal IL-18BP mRNA was detected. To further confirm that β2-AR stimulation induces myocardial IL-18BP expression, wild type male C57Bl/6 animals received the β2-AR agonist clenbuterol HCl (0.3 mg/kg. body wt, IP) for 3 h as previously described [15].
2.3. Adult and neonatal mouse cardiomyocytes
Calcium-tolerant adult mouse cardiomyocytes (ACM) were isolated from ~3 month-old male C57Bl/6 mice by a modified Langendorff perfusion and collagenase digestion technique, and has been previously described [17, 18]. Similarly, cardiomyocytes were isolated from IL-18 null and IL-18BP Tg mice. The yield, shape, and viability of cardiomyocytes from wild type, IL-18 null and IL-18BP Tg mice were similar (data not shown). Since ACM are extremely difficult to transfect using standard transfection protocols, we used neonatal mouse cardiomyocytes (NMCM) for promoter-reporter assays. NMCM show relatively higher transfection efficiency (~58% with only 7% cell death) as determined using the pEGFP-N1 vector. NMCM were microporated (pulse voltage: 1700 volts, pulse width: 20 ms, pulse number: 1, and the tip type: 10 µl) with 3 µg of the vector using the Neon® transfection system (MPK-5000, Invitrogen). NMCM were isolated from 1- to 3-day-old neonatal mice (C57Bl/6 background) as previously described [17, 18].
2.4. Adenoviral infection and siRNA-mediated knockdown
Cardiomyocytes were infected with adenoviruses at the indicated multiplicities of infection (MOI) (Supplementary methods). The transfection efficiency with the adenoviral vectors was near 100%, and infection with the adenoviral vectors at indicated MOI had no significant effect on cardiomyocyte shape, adherence, or viability. C/EBPβ was targeted by siRNA, and its knockdown was confirmed by immunoblotting. siRNA for GFP served as a control. Ad.CMV-IL-18BPc has been previously described [19]. Further details are provided in ‘Supplementary methods’ section.
2.5. Cell death analysis
To determine whether transduction of viral vectors, siRNA, or pharmacological inhibitors negatively affected cell viability, cell death was analyzed using the Cell Death Detection ELISAPLUS. Cell viability was also tested by trypan blue dye exclusion and microscopic visualization of shape and for cells floating in the media. Details are provided in ‘Supplementary methods’ section.
2.6. Cardiomyocyte hypertrophy in vitro and in vivo
Cardiomyocyte hypertrophy in vitro was assessed by two independent methods: increased protein, but not DNA, synthesis and cell surface area. Details are provided in ‘Supplementary methods’ section. In addition to heart wt to body weight ratios in ISO-infused animals, hypertrophy was also assessed by quantifying diameter of cardiomyocytes in the region of the cell nucleus (100 cells/heart, 4 hearts/group, and 4 groups) in H&E stained cryosections. We also analyzed total and phosphorylated forms of the protein synthesis marker ribosomal S6 protein, and ANF gene expression.
2.7. Il18bp promoter-reporter assays
A 1964 bp fragment (pIl18bp-1964) of the 5'-flanking region of the mouse Il18bp gene was amplified from mouse genomic DNA using primers shown in Table 1. The sense primer contained an MluI restriction site at the 5' end, and the antisense primer contained an XhoI restriction site (lower case). The PCR product was cloned into pCR2.1-TOPO and subcloned into the pGL3-Luciferase reporter vector at the MluI/XhoI sites. The identity of the PCR product was confirmed by sequencing on both strands. In silico analysis of this region identified several putative transcription factor-binding sites (C/EBP, CREB, Sp1 and NF-κB). Accordingly, deletion constructs were created using the antisense primers shown in Table 1. NMCM were transfected with 3 µg of the reporter vectors, and cotransfected with 100 ng of the internal reference plasmid Renilla luciferase (pRL-TK) using the Neon transfection system described above. 48h after transfection, cells were treated with ISO. After 12 h cells were harvested for the dual-luciferase assay. Firefly luciferase data were normalized to that of corresponding Renilla luciferase activity. All plasmids were purified using EndoFree Plasmid Maxi kit.
Table 1.
Primers used in mouse IL-18BP analysis.
| Promoter | |
|---|---|
| Sense 1 | 5’-ggt acc CTAGGCCTAACCTTGCTTGAACA-3’ |
| Sense 2 | 5’-ggt acc CCTTCATGGAAGTGTTGACATGT-3’ |
| Sense 3 | 5’-ggt acc CCTAGGTAGGCTTGGTCATTTGT-3’ |
| Sense 4 | 5’-ggt acc CCACAACTCAGCCTGTTCTCTTA-3’ |
| Sense 5 | 5’-ggt acc CTAGAGATCCTGGATTCTGAG-3’ |
| Sense 6 | 5’-ggt acc GGCTATTTGGGGTCAGCTCTC-3’ |
| Antisense | 5’-aag ctt CCAGGCAGCTATCCTGGCTGAGCC-3’ |
| EMSA | |
| C/EBP | Sense, 5’-GAACAGGGTTGGGCAAAGCTGCG-3’ |
| Mutant C/EBP | Sense, 5’-GAACAGGGGACTAGTCAGCTGCG-3’ |
| CREB | Sense, 5’-CAGGCCTGACGTTAAGAATGGTGA-3’ |
| Mutant CREB | Sense, 5’-CAGGCCTGTGCATAAGAATGGTGA-3’ |
| ChIP assay | |
| c/EBP | Sense, 5’-CTAGGCCTAACCTTGCTTGAACA-3’ Antisense, 5’-TCTGGCACCCGTCTGAGCTCT-3’ 203nt |
| CREB | Sense, 5’-CACAGAGCAGACAGACTAATG-3’ Antisense: 5’-AAGGGGACTTCTGAGCAGGC-3’ 208nt |
| ORF | Sense, 5’-TCTGCACCTCAGACAACTGCCA-3’ Antisense, 5’-TCAATGAAGGAACCATTGCCCAG-3’ 221nt |
| Northern blot analysis (398 bp) | |
| Sense, 5’-TCTGCACCTCAGACAACTGC-3’ | |
| Antisense, 5’-TGGGCCAGAATGATGTGATA-3’ | |
2.8. Transcription factor activation
Nuclear extracts were prepared using Panomics Nuclear Extraction Kit, and is described in ‘Supplementary methods’ section. Lamin A/C and GAPDH served as loading and purity controls. CREB activation was analyzed by electrophoretic mobility shift assay (EMSA), and the oligonucleotide sequences are provided in Table 1. CREB activation was confirmed by a reporter gene assay using an adenoviral CRE-luciferase reporter vector (Ad.CRE-Luc). The empty adenoviral construct (Ad.MCS-Luc) served as a base-line control. Ad.pRL-TK served as an internal control. Firefly and Renilla luciferase activities were determined as described above. C/EBP activation was also analyzed by EMSA using gene-specific oligonucleotides (Table 1). In the gel supershift assay, the nuclear protein extract (5 µg) was preincubated for 40 minutes on ice with anti-C/EBPα or C/EBPβ antibodies (0.2 µg) before the addition of 32P-labeled C/EBP consensus double-stranded oligo as described in ‘Supplementary methods’ section. Respective normal IgG served as controls. Activation of NF-κB was analyzed by EMSA.
2.9. Chromatin immunoprecipitation (ChIP) assay
ACM were treated with ISO (10−7M) for 1 h. The ChIP assay was performed following the manufacturer’s instructions using a kit (Millipore/Upstate Biotechnology, Inc.), and is detailed in ‘Supplementary methods’ section.
2.10. mRNA expression
IL-18BP, IL-18, ANF and EMMPRIN mRNA expressions were analyzed by Northern blotting as detailed in ‘Supplementary methods’ section. 28S rRNA served as a loading control. The autoradiographic signals were semiquantified by videoimage analysis.
2.11. Protein Extraction and Western Blot Analysis
Protein extraction from cardiomyocytes and left ventricular tissue, immunoblotting, detection of the immunoreactive bands by enhanced chemiluminescence, and densitometry are all detailed in ‘Supplementary methods’ section. αTubulin and GAPDH served as loading controls. Activation of Akt , ribosomal S6 protein, and p70 S6 kinase was confirmed by immunoblotting using respective activation-specific antibodies.
2.12. Biological activity of rIL-18BPa-Fc, IL-18BPc and anti-IL-18 antibodies
The biological activity of IL-18BPc and IL-18 neutralizing antibodies on IL-18 induced NF-κB was confirmed by EMSA. ACM were infected with Ad.IL-18BPc (MOI 50 for 24 h) or pre-treated with IL-18 neutralizing antibodies (10 µg/ml for 1 h) prior to IL-18 addition (5 ng/ml for 1 h). Ad.GFP and normal goat IgG served as controls. Both Ad.IL-18BPc and anti-IL-18 antibodies significantly inhibited IL-18 induced NF-κB activation (Supplementary Figure S1-A). Secretion of IL-18BPc following Ad.IL-18BPc infection was confirmed by immunoblotting using TCA-precipitated culture supernatants (Supplementary Figure S1-B).
2.13. Statistical analysis
Comparisons between controls and various treatments were performed by analysis of variance with post hoc Dunnett's t tests. All assays were performed at least three times, and the error bars in the figures indicate the S.E. Densitometric results were shown as ratios at the bottom of the panels whenever the results are less clear.
3. Results
3.1. Isoproterenol induces IL-18BP expression via β2-AR in adult cardiomyocytes
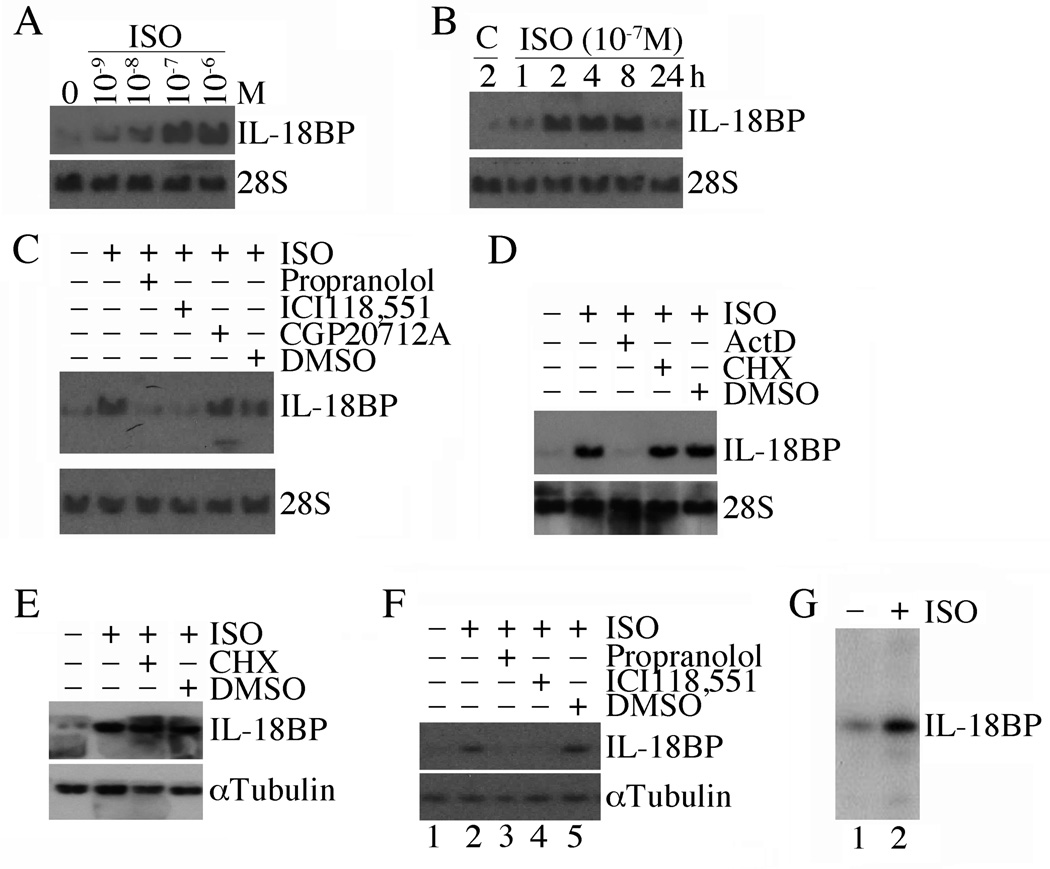
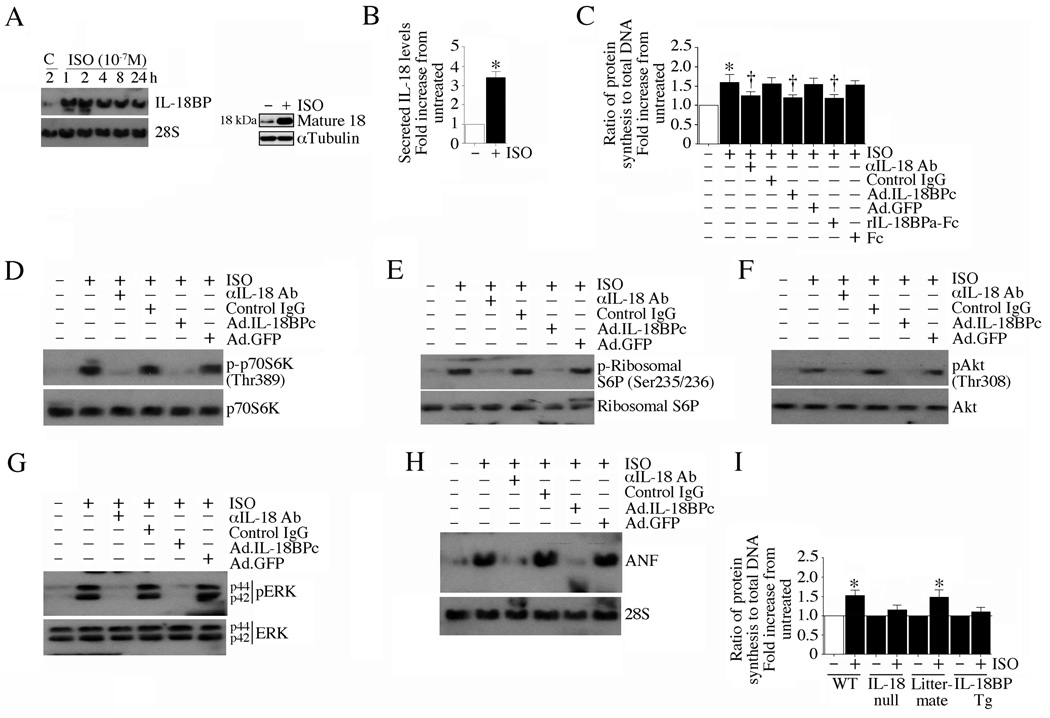
We have previously shown that ISO induces IL-18 expression both in vivo and in vitro [15]. IL-18 is powerful mediator of inflammation and its expression is tightly regulated though expression of IL-18 binding protein. Therefore we tested the hypothesis that ISO may also induce the expression of this neutralizing binding protein. Using Northern blot analysis, we demonstrate that ISO is a potent inducer of IL-18BP in adult mouse cardiomyocytes (ACM). ISO induced IL-18BP expression was both dose- (10−6 to 10−9 M; Fig. 1A) and time- (1–24 h; Fig. 1B) dependent, with peak levels detected at 10−7M and 2 h. Therefore, in all subsequent studies, ISO was used at 10−7 M. Adult cardiomyocytes express both β1 and β2 receptors under basal conditions [20]. Therefore, we next determined the β-AR specificity in mediating the response to ISO. Our results indicate that treatment with the classic non-selective β1- and β2-AR blocker propranolol HCl markedly inhibits IL-18BP mRNA expression (Fig. 1C). Moreover, while treatment with the β1-AR antagonist CGP 20712A failed to produce an effect, treatment with the β2-AR antagonist ICI 118,551 significantly attenuated ISO-induced IL-18BP expression (Fig. 1C). Since ISO induces IL-18BP mRNA expression, we next investigated whether ISO regulates its expression via increased transcription or mRNA stability. The results show that while Actinomycin D (ActD) blunts, cyclohexamide (CHX) fails to affect ISO-induced IL-18BP mRNA expression (Fig. 1D). CHX also failed to modulate IL-18BP protein levels (Fig. 1E). ISO also induced IL-18BP protein expression via β2-AR-dependent manner (Fig. 1F), and induced its secretion (Fig. 1G). These results indicate that ISO is a potent inducer of IL-18BP transcription in cardiomyocytes, and this effect is mediated by a β2-AR-dependent signaling pathway (Fig. 1).
Fig. 1. Isoproterenol (ISO) induces IL-18 binding protein (IL-18BP) expression in adult mouse cardiomyocytes (ACM).
A, Dose-dependent effects of ISO on IL-18BP mRNA expression. ACM were cultured for 12 h in complete medium, then in medium containing 0.5% BSA for an additional 12 h (quiescence). Quiescent ACM (2.5×105) were treated with ISO for 2 h. 20 µg of DNA-free total RNA was then analyzed for IL-18BP expression by Northern blotting. 28S rRNA served as a loading control. A representative of three independent experiments is shown. B, Time-dependent effects of ISO on IL-18BP mRNA expression. Quiescent ACM were treated with ISO (10−7M). At the indicated times, IL-18BP mRNA expression was analyzed by Northern blotting as described in A (n=3). C, ISO induces IL-18BP expression via β2-AR. Quiescent ACM were treated with the β1- and β2-AR blocker propranolol HCl (25 µM), the β1-AR antagonist CGP 20712A (300 nM in DMSO), β2-AR antagonist ICI 118,551 (300 nM in water), or DMSO for 1 h prior to ISO (10−7M for 2 h) addition. IL-18BP mRNA expression was analyzed as in A (n=3). D, ISO-induced IL-18BP is transcriptionally regulated. Quiescent ACM were treated with ISO (10−7M for 2 h) followed by the addition of the translational inhibitor cycloheximide (CHX, 20 µg/ml) or the transcriptional inhibitor actinomycin D (ActD, 5 µg/ml) for 2 h. IL-18BP mRNA expression was analyzed as in A (n=3). E, Cyclohexamide (CHX) fails to affect ISO-induced IL-18BP protein expression. ACM treated as in D were analyzed for IL-18BP expression by immunoblotting (n=3). F, ISO-induced IL-18BP protein expression is β2-AR dependent. Quiescent ACM treated as in C were analyzed for IL-18BP protein levels at 2 h by immunoblotting (n=3). G, ISO stimulates IL-18BP secretion. Culture supernatants from ACM treated with ISO (lanes 1 and 2 in F) were analyzed for IL-18BP levels by immunoblotting following TCA precipitation.
3.2. Isoproterenol induces Il18bp transcription in neonatal cardiomyocytes
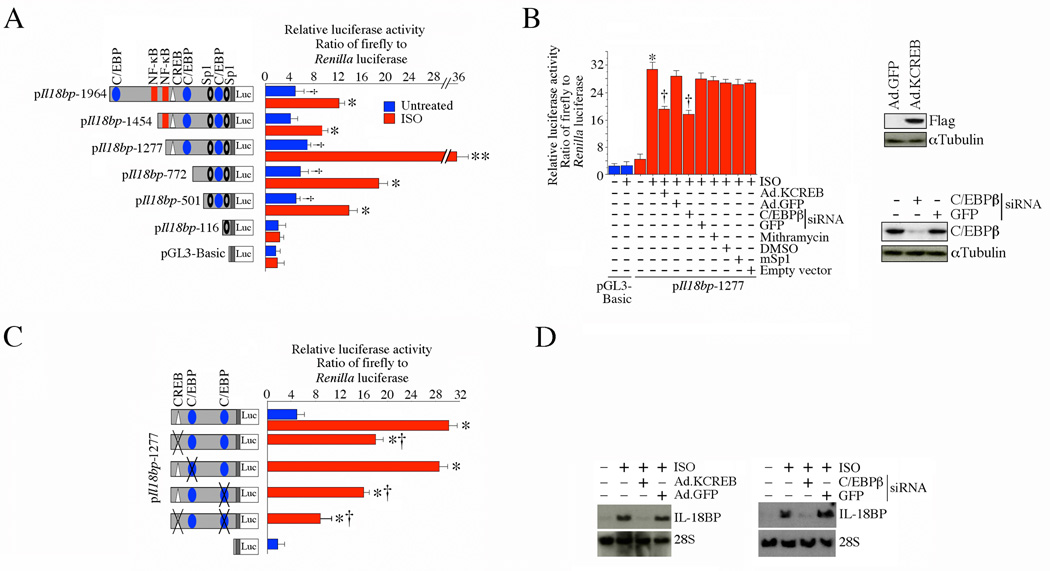
Since ISO-induced IL-18BP expression was regulated at the transcriptional level (Fig. 1D), we next cloned mouse Il18bp 5’ cis-regulatory region 1964 basepairs upstream of the transcriptional start site (Supplementary Fig. S2-A). Analysis of the promoter region by the MatInspector Professional® software identified potential binding sites for various transcription factors, including one CREB, three C/EBP, two NF-κB, and two Sp1 sites (Supplementary Fig. S2 and Fig. 2A). Since adult cardiomyocytes are difficult to transfect, we used neonatal mouse cardiomyocytes (NMCM) that show higher transfection efficiency. Transient transfection of NMCM with the full-length (1.964 kb) promoter-reporter construct showed a 2-fold increase in the reporter activity at basal conditions (versus the promoter-less pGL3-Basic), and treatment with ISO significantly increased its activity by an additional 4-fold (Fig. 2A). Progressive deletion of the promoter indicated that the construct (pIl18bp-1277) that still contained the putative CREB, C/EBP and Sp1 binding sites, showed the maximum activity following ISO treatment (Fig. 2A). Therefore, we used this construct to examine the contribution of these sites to the ISO response. Our results show that while forced expression of dnSp1 or pre-treatment with the Sp1 inhibitor mithramycin failed to modulate the response, forced expression of a Flag-tagged dominant negative mutant form CREB (Ad.KCREB; expression of Flag is confirmed by immunoblotting; upper right hand panel) and C/EBP knockdown (knockdown is shown in the lower right hand panel) blunted ISO-induced Il18bp promoter-dependent reporter gene activation (Fig. 2B). Of note, forced expression of dnp65 by adenoviral transduction failed to significantly modulate ISO-induced Il18bp promoter activity (Supplementary Fig. S2-B and C). We confirmed the role of the CREB and C/EBP sites by mutating these sites singly or together in the pIl18bp-1277 construct. While mutation of the middle C/EBP binding site had no significant effect, mutation of either the CREB or the remaining (distal) C/EBP sites markedly attenuated ISO-induced Il18bp promoter-reporter activity (Fig. 2C). These inhibitory effects were more pronounced when both the proximal C/EBP and CREB sites were mutated. Moreover, forced expression of KCREB (left hand panel) or C/EBPβ knockdown (right hand panel) inhibited ISO-mediated IL-18BP mRNA expression (Fig. 2D). Together, these results indicate that both CREB and C/EBP play critical roles in ISO-induced Il18bp transcription (Fig. 2).
Fig. 2. ISO induces Il18bp promoter-reporter activity via CREB and C/EBP.
A, Neonatal mouse cardiomyocytes (NMCM) were transfected with the indicated mouse Il18bp promoter-reporter vectors (3 µg) using the Neon® transfection system. Cells were co-transfected with the internal reference plasmid Renilla luciferase (pRL-TK; 100 ng). 48 h after transfection, cells were treated with ISO (10−7M for 12 h), and harvested for the dual-luciferase assay. †P < 0.05 versus pGL3-Basic; *P < at least 0.05, **P < 0.001 versus corresponding Untreated (n=12). B, ISO-induced Il18bp promoter-reporter activity is C/EBP and CREB-dependent. NMCM were transfected with pIl18bp-1277 promoter-reporter vector (3 µg) along with C/EBP β siRNA (60 nM), infected 24 h later with Flag-tagged mutant CREB (KCREB, MOI 50), or mutant Sp1 (MOI 10; [18]). Forty-eight hours later, cells were treated with ISO (10−7M for 12 h). pRL-TK served as transfection control as before. Expression of Flag (upper right hand panel) and knockdown of C/EBP β (lower right hand panel) were confirmed by immunoblotting. *P < 0.001 versus untreated pIl18bp-1277, †P < 0.01 versus ISO (n=12). C, Mutations in C/EBP or CREB blunt ISO-induced Il18bp promoter-reporter activity. NMCM were transfected with wild type pIl18bp-1277 or pIl18bp-1277 with mutations in C/EBP, CREB, or both as described under A. *P < 0.001 versus untreated pIl18bp-1277, †P < 0.01 versus pIl18bp-1277 + ISO (n=12). D, ISO induces IL-18BP mRNA expression via CREB and C/EBPβ. NMCM infected with Ad.KCREB (MOI 50 for 24 h) or transfected with C/EBPβ-specific siRNA prior to ISO addition (10−7M for 2 h) were analyzed for IL-18BP mRNA expression by Northern blotting (n=3).
3.3. Isoproterenol activates CREB in adult cardiomyocytes via cAMP, PI3K/Akt and ERK1/2
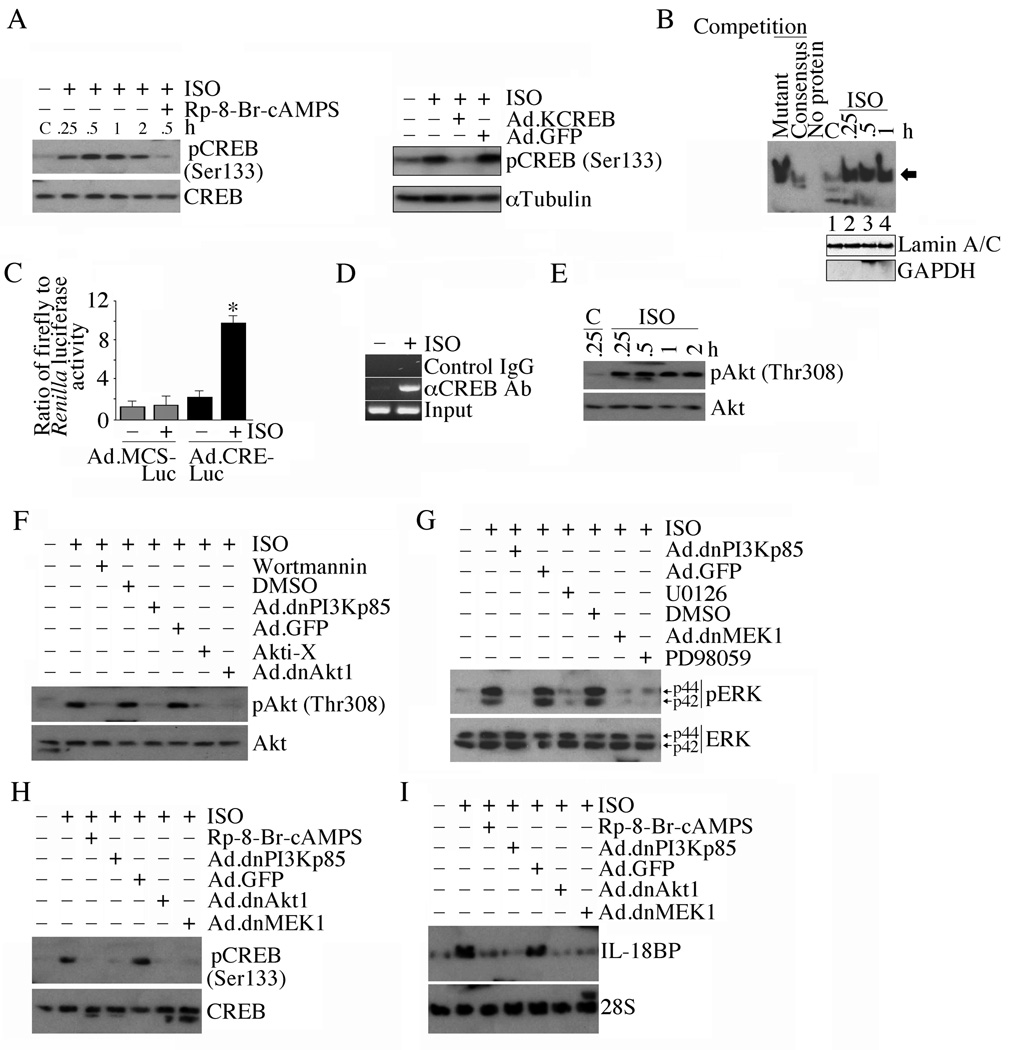
Since ISO stimulates Il18bp transcription and mRNA expression in part via CREB, we next investigated whether ISO activates CREB, and determined the underlying signal transduction pathways. Our results show that ISO induces CREB phosphorylation in a time-dependent manner, and this effect was significantly inhibited by the potent membrane permeant cAMP antagonist Rp-8-Br-cAMPS (Fig. 3A, left hand panel) and forced expression of a dominant negative mutant form of CREB (KCREB; Fig. 3A, right hand panel). Further, ISO stimulates CREB DNA binding activity (Fig. 3B) and CRE reporter gene activity (Fig. 3C), and CREB binding to the Il18bp promoter (Fig. 3D). Since CREB is a regulatory target of Akt [21], and Akt is an important intermediate in β-AR-mediated pro-growth and anti-apoptotic effects [22], we next investigated whether ISO activates Akt in cardiomyocytes. ISO induced time-dependent Akt activation (Fig. 3E), an effect that was significantly inhibited by the PI3K inhibitor wortmannin, the Akt inhibitor Akti-X, and by the adenoviral transduction of mutant PI3Kp85 (Ad.dnPI3Kp85) and Akt1 (Ad.dnAkt1) (Fig. 3F). Moreover, ISO activated ERK1/2, an effect that was markedly attenuated by wortmannin, Ad.dnPI3Kp85, U0126, Ad.dnMEK1 and PD98059 (Fig. 3G). Importantly, Rp-8-Br-cAMPS, Ad.dnPI3Kp85, Ad.dnAkt1, and Ad.dnMEK1 inhibited ISO-induced CREB activation (Fig. 3H) and IL-18BP mRNA expression (Fig. 3I). Of note, adenoviral transduction of constitutively active Akt (myr-Akt) induced CREB activation (Supplementary Fig. S3). These results indicate that ISO activates CREB in vitro, induces its binding to Il18bp promoter in vivo, and ISO-induced CREB activation and IL-18BP expression is cAMP, PI3K/Akt and ERK1/2 dependent (Fig. 3).
Fig. 3. ISO induces IL-18BP expression via CREB.
A, Time-dependent effects of ISO on CREB activation. Quiescent ACM were treated with the cAMP antagonist Rp-8-Br-cAMPS (100 µM in water for 1 h) or infected with Ad.KCREB (right hand panel) prior to ISO addition (10−7M for 30 min). Total CREB, phospho-CREB (Ser133) and αTubulin were quantified by immunoblotting (n=3). B, ISO induced CREB DNA-binding activity. ACM treated with ISO (10−7 M) for up to 1 h were analyzed for CREB DNA-binding activity by EMSA using gene-specific primers and nuclear protein extracts. Specificity of DNA binding, indicated by an arrow, was verified in competition experiments as described in ‘Materials and methods’. Unbound labeled probe at the bottom of gel is not shown. Lamin A/C (nuclear) and GAPDH (cytoplasmic) served as loading and purity controls, and are shown in the bottom panel. C, ISO-mediated CREB activation was confirmed by a reporter assay. *P < 0.001 versus Ad.MCS-Luc + IL-18. D, ISO-stimulated CREB binding to Il18bp promoter in vivo was confirmed by the ChIP assay (n = 3). E, ISO induced Akt activation. ACM treated with ISO were analyzed by immunoblotting using phospho-Akt (Thr308) and Akt antibodies (n=3). F, ISO-induced Akt activation is attenuated by PI3K and Akt inhibitors and adenoviral transduction of dnPI3Kp85 and dnAkt1 (n=3). G, ISO induces ERK1/2 activation via PI3K, Akt, and MEK1/2. ACM were pre-treated with specific inhibitors of PI3K, Akt, MEK1/2 and ERK1/2, or transduced with adenoviral mutant vectors prior to ISO addition. Total and phospho-ERK1/2 were analyzed by immunoblotting (n=3). H, ISO activates CREB via PI3K, Akt, and MEK1/2. ACM treated as in A and G were analyzed for phospho-CREB levels (n=3). I, ISO induces IL-18BP mRNA expression via PI3K, Akt, and MEK1/2. ACM treated as in A and G were analyzed for IL-18BP mRNA expression by Northern blotting (n = 3).
3.4. Isoproterenol also activates C/EBPβ in adult cardiomyocytes via PI3K/Akt and ERK1/2
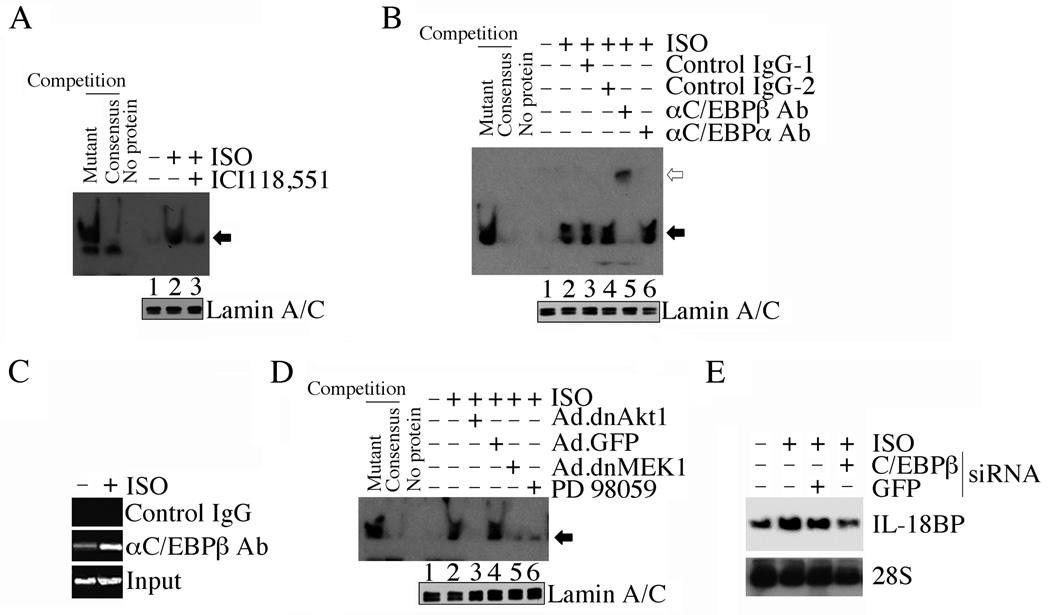
Our promoter studies also showed that the deletion construct lacking the proximal C/EBP is markedly less responsive to ISO (Fig. 2A). Mutation in the C/EBP sites also blunted Il18bp promoter-dependent reporter gene activity (Fig. 2C). Therefore, we next investigated whether ISO induces C/EBP activation, and determined the underlying signal transduction pathways. ISO potently induced C/EBP DNA binding activity in vitro (Fig. 4A), and the DNA/protein complex was supershifted by anti-C/EBPβ antibodies (Fig. 4B). Further, ISO stimulated C/EBPβ binding to Il18bp promoter in vivo (Fig. 4C). Similar to their inhibitory effects on CREB activation (Fig. 3H), Ad.dnAkt1, PD98059 and Ad.dnMEK1 all attenuated ISO-induced C/EBPβ DNA binding activity (Fig. 4D), and C/EBPβ knock down attenuated ISO-induced IL-18BP expression (Fig. 4E), indicating that ISO activates C/EBP β via PI3K/Akt and MEK/ERK1/2 (Fig. 4).
Fig. 4. ISO induces IL-18BP mRNA expression via C/EBP.
A, ISO induces C/EBP DNA binding activity. ACM treated with ISO and/or the β2-AR antagonist ICI 118,551 were analyzed for C/EBP DNA binding activity by EMSA using gene-specific primers and nuclear protein extracts as described under Fig. 3B (n=3). Lamin A/C served as a loading control, and is shown in the bottom panel. B, ISO-induced C/EBP DNA binding activity is super-shifted (open arrow) by anti-C/EBPβ, but not C/EBPα antibodies. The nuclear protein extracts from ISO-treated ACM were preincubated with anti-C/EBPα, C/EBPβ or respective control IgG prior to the addition of the labeled probe (n=3). Lamin A/C served as a loading control, and is shown in the bottom panel. C, ISO-stimulated C/EBPβ binding to Il18bp promoter in vivo was confirmed by the ChIP assay (n = 3). D, ISO induces C/EBP DNA binding activity via Akt, MEK1/2 and ERK1/2. ACM were infected with Ad.dnAkt1, Ad.MEK1 or pretreated with the ERK1/2 inhibitor PD98059 prior to ISO addition. C/EBP DNA binding activity was analyzed as in A (n=3). Lamin A/C served as a loading control, and is shown in the bottom panel. E, ISO induces IL-18BP mRNA expression via C/EBPβ. ACM were transfected with C/EBPβ-specific siRNA (60 nM for 48 h) prior to ISO addition (10−7M for 2 h). IL-18BP mRNA was analyzed by Northern blotting (n=3).
3.5. IL-18BP blocks isoproterenol/IL-18-mediated adult cardiomyocyte hypertrophy
IL-18 is a potent pro-hypertrophic factor, inducing cardiomyocyte hypertrophy in vitro [10] and myocardial hypertrophy in vivo [12] [13]. Since ISO induces IL-18 expression [15], we next investigated whether ISO-induced cardiomyocyte hypertrophy is IL-18 dependent. Confirming our earlier results in cardiac microvascular endothelial cells [15], ISO (10−7M) stimulated time-dependent IL-18 mRNA expression (Fig. 5A, left hand panel), intracellular IL-18 protein levels (Fig. 5A, right hand panel), and IL-18 secretion (Fig. 5B) in adult cardiomyocytes. Further, unlike the positive control doxorubicin [23], ISO failed to induce cardiomyocyte death (Supplementary Figure S5-A). At this concentration, ISO also induced the phosphorylation of Bad, a pro-apoptotic protein (Supplementary Figure S5-B), rendering it inactive. Notwithstanding this, ISO in fact induced cardiomyocyte growth; increased protein, but not DNA (Supplementary Figure S5-C) synthesis in ACM (Fig. 5C), an effect that was significantly attenuated by IL-18 neutralizing antibodies and IL-18BPc overexpression. Recombinant human IL-18BPa-Fc was equally effective in blunting ISO-induced cardiomyocyte hypertrophy (Fig. 5C). Increased cell surface area confirmed ISO’s pro-hypertrophic effects (Supplementary Figure S5-D). Of note, similar to its effects in neonatal rat cardiomyocytes [10], IL-18 induced ACM hypertrophy, and its pro-growth effects were markedly attenuated by IL-18BPc, IL-18 neutralizing antibodies and rIL-18BPa-Fc (Supplementary Fig. S5-E). IL-18 also induced several hypertrophy-associated factors in ACM (Supplementary Fig. S5-F). Similar to IL-18, ISO induced phosphorylation of the protein synthesis marker p70 S6 kinase (Thr389; Fig. 5D), phosphorylation of ribosomal S6 protein (Fig. 5E), phosphorylation of the pro-survival factors Akt (Fig. 5F) and ERK1/2 (Fig. 5G), and the mRNA expression of the pro-hypertrophic marker ANF (Fig. 5H). Importantly, these effects were markedly attenuated by the adenoviral transduction of IL-18BPc and pretreatment with IL-18 neutralizing antibodies (Fig. 5, C–H). The effect of ISO on cell growth was also carried out on cardiomyocytes from IL-18 null and IL-18BP Tg mice. Although hypertrophic effects were seen on the cardiomyocytes from wild type and littermate control mice, it was markedly attenuated in cells from IL-18 null and IL-18BP Tg mice (Fig. 5I), indicating that ISO induces cardiomyocyte hypertrophy in part via IL-18 (Fig. 5).
Fig. 5. ISO induces cardiomyocyte hypertrophy in part via IL-18.
A, B, ISO induces IL-18 expression. ACM were treated with ISO (10−7M) for up to 24 h (IL-18 mRNA, A, left hand panel), 1 h (intracellular IL-18 protein; A, right hand panel) or 24 h (secreted IL-18). IL-18 mRNA was analyzed by Northern blotting (n=3), protein by immunoblotting (n=3), and secreted IL-18 by ELISA (B). B, *P < 0.001 versus control (n=6). C, Neutralization of IL-18 blunts ISO induced cardiomyocyte hypertrophy. ACM were incubated with rIL-18BPa-Fc (10 µg/ml for 1 h), anti-IL-18 neutralizing antibodies (10 µg/ml for 1 h), or infected with Ad.IL-18BPc (MOI 50 for 24 h) prior to ISO addition (10−7M for 48 h). Hypertrophy was assessed by increased protein synthesis, but not DNA content, as described in ‘Materials and methods’. *P < 0.001 versus control, †P < 0.05 versus ISO (n=6). D–H, Neutralization of IL-18 attenuates ISO induced p70S6K (D), Ribosomal S6 protein (E), Akt (F), and ERK1/2 (G) activation and ANF mRNA expression (H). ACM treated as in C, but for 2 h (D–G) or 6 h (H) following ISO, were analyzed by immunoblotting (D–G) or Northern blotting (H) (n=3). I, ISO fails to induce hypertrophy of ACM isolated from IL-18BP Tg and IL-18 null mice. Cardiomyocytes from IL-18BP Tg and IL-18 null mice were incubated with ISO (10−7M for 48 h) and analyzed for cell growth as in C. *P < 0.001 versus control, †P < 0.05 versus ISO (n=6).
3.6. Isoproterenol-induced myocardial IL-18BP expression in vivo is β2-AR dependent
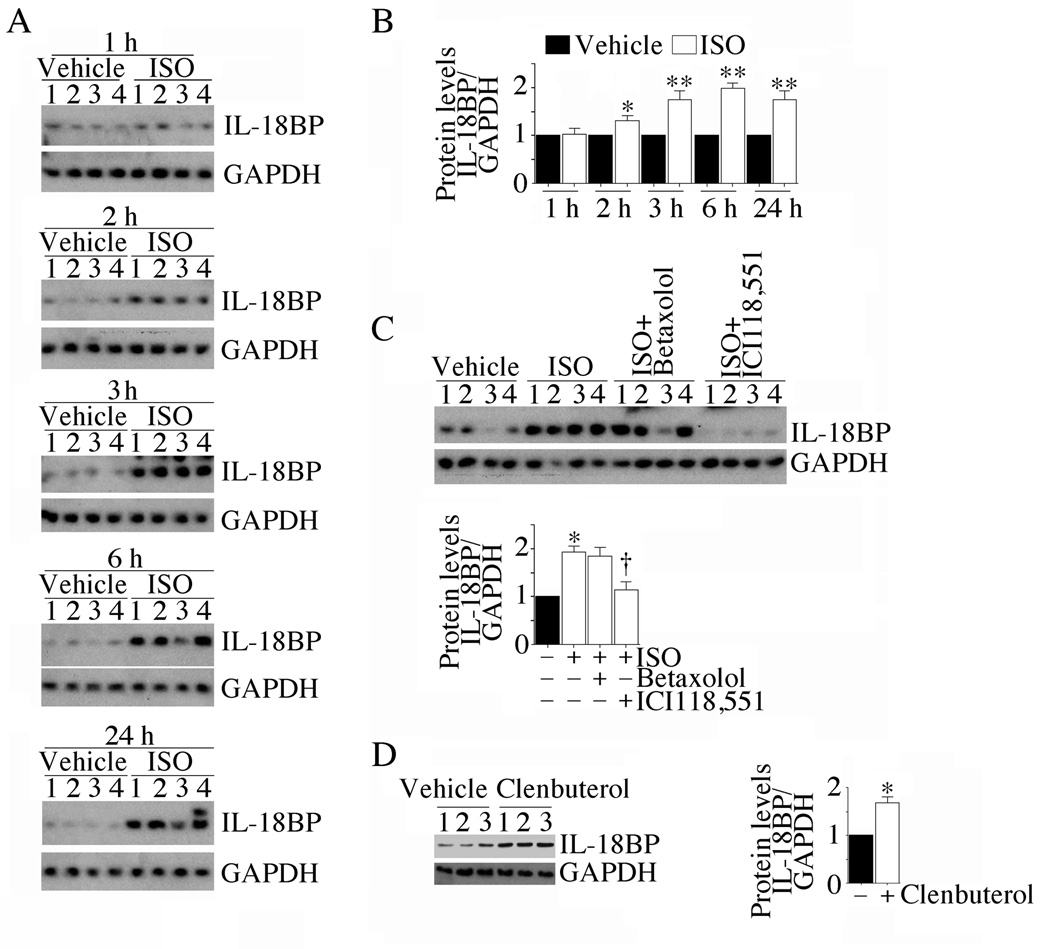
We have demonstrated that ISO is a potent inducer of IL-18BP in vitro in cardiomyocytes (Fig. 1). Therefore, we next investigated whether ISO induces IL-18BP expression in vivo in myocardium, and determined the receptor specificity. Supporting the in vitro data, ISO induced IL-18BP protein expression in myocardium in vivo in a time-dependent manner, with peak levels detected after 3 h (Fig. 6A; densitometric analysis of n=4/group is summarized in panel B), and this effect was markedly attenuated by the β2-AR antagonist ICI 118,551 (Fig. 6C; densitometric analysis of n=4/group is summarized in the lower panel). Further, clenbuterol HCl, a β2-AR agonist, induced IL-18BP protein expression in vivo (Fig. 6D; densitometric analysis of n=3/group is summarized in the right hand panel), indicating that ISO induces IL-18BP in vivo in β2-AR dependent manner (Fig. 6).
Fig. 6. ISO induces myocardial IL-18BP expression via β2-AR.
A, Time-dependent induction of IL-18BP in vivo. Male C57Bl/6 mice (n=4/group) were continuously infused with ISO (15 mg/kg body wt/day in 0.002% ascorbic acid) via miniosmotic pumps. 0.002% ascorbic acid served as a control (vehicle). At the indicated time periods, left ventricular tissue was analyzed for IL-18BP protein levels by immunoblotting. GAPDH served as a loading control. Each lane represents an individual animal (numbered at top). B, Densitometric analysis. The intensity of IL-18BP-specific band was semiquantified by densitometric analysis, and results from 4 animals at each time point were normalized to respective GAPDH levels, and presented as fold increase from vehicle-treated controls. *P < 0.05, **P < 0.01 versus control. C, ISO-induced IL-18BP expression in vivo is β2-AR dependent. Wild type mice received the β1-AR antagonist betaxolol HCl (20 mg/kg. body wt) or the β2-AR antagonist ICI 118,551 (4 mg/kg. body wt) 1 h prior to ISO administration, and sacrificed after 3 h. Densitometric analysis is shown in the lower panel. *P < 0.01 versus vehicle, †P < 0.05 versus ISO (n=4/group). D, Administration of the β2-AR agonist clenbuterol HCl induces myocardial IL-18BP mRNA expression. Wild type mice received clenbuterol HCl (0.3 mg/kg. body wt, IP) for 3 h. Myocardial IL-18BP levels were analyzed by immunoblotting. Densitometric analysis is shown in the right hand panel. *P < 0.05 versus control (n=3/group).
3.7. Isoproterenol-induced myocardial hypertrophy in wild type mice is characterized by increased IL-18BP and IL-18 expression
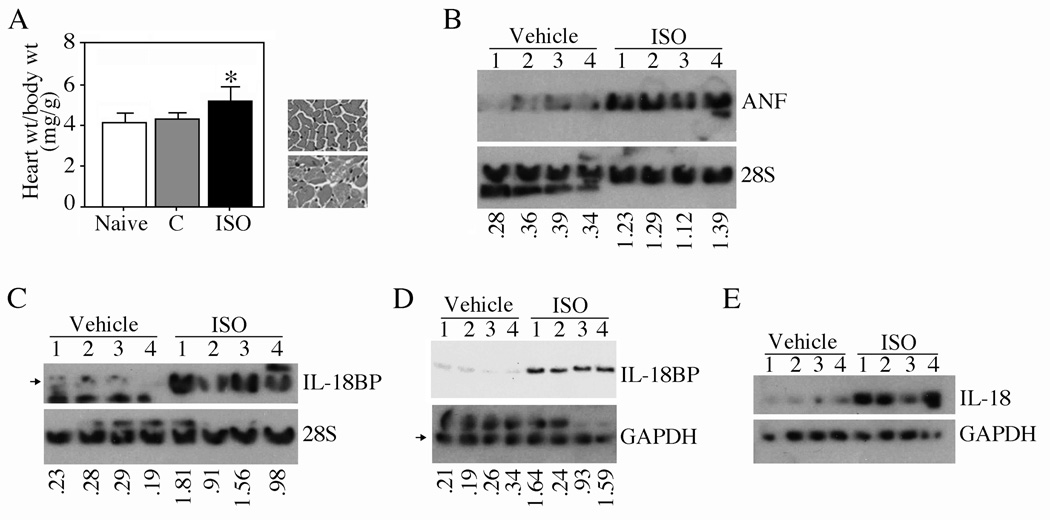
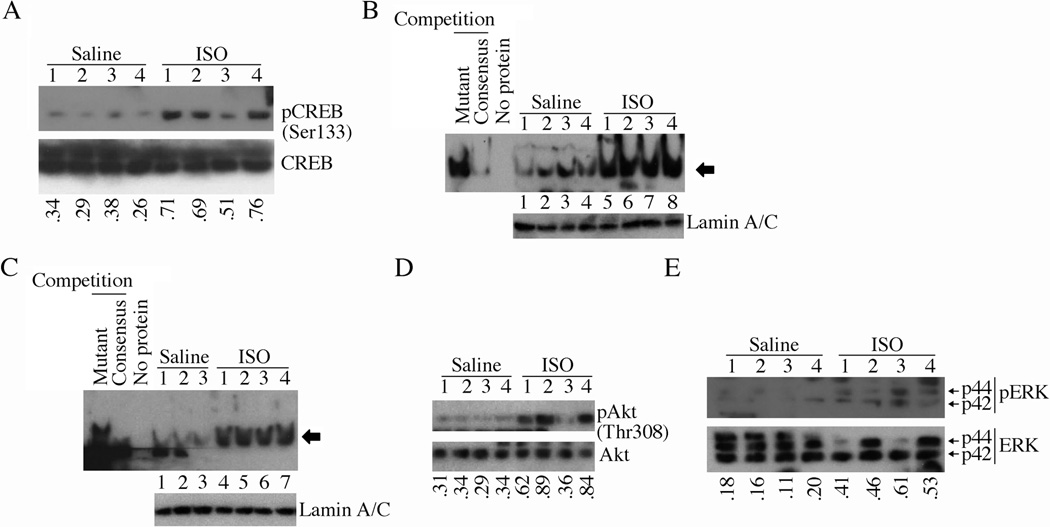
Since ISO-induced cardiomyocyte hypertrophy in vitro is characterized by enhanced expression of IL-18BP and IL-18 (Figs. 1 and 5), we next determined the expression levels IL-18BP and IL-18 in ISO-induced myocardial hypertrophy in vivo. Indeed, continuous infusion of wild type mice with ISO for one week induced myocardial hypertrophy, as evidenced by increased heart to body weight ratios (Fig. 7A; histology of heart is shown on the right) and induction of hypertrophy-associated ANF gene expression (Fig. 7B). Further, IL-18BP mRNA expression was markedly elevated (Fig. 7C), its protein levels (Fig. 7D) were modestly elevated at 7 days. ISO also upregulated IL-18 protein expression (Fig. 7E). Confirming the in vitro data, ISO induced CREB phosphorylation/activation (Fig. 8A), CREB (Fig. 8B) and C/EBP (Fig. 8C) DNA-binding activities, and Akt (Fig. 8D) and ERK1/2 (Fig. 8E) activation. Unlike the positive control doxorubicin HCl [23], ISO at the indicated concentration and duration, failed to induce cardiomyocyte death in vivo (data not shown). These results indicate that ISO-induced myocardial hypertrophy is characterized by enhanced IL-18BP and IL-18 expression (Figs. 7 and 8).
Fig. 7. ISO induces myocardial hypertrophy in vivo.
A, ISO induces myocardial hypertrophy. Male C57Bl/6 mice were continuously infused with ISO (15 mg/kg body wt/day in 0.002% ascorbic acid) via miniosmotic pumps. 0.002% ascorbic acid served as a control (C). Heart and body weights were recorded after 7 days. Histology of the heart is shown in the right panel. *P < 0.01 versus naïve or control (n=6/group). B–E, ISO induces ANF mRNA (B; ratios of ANF mRNA to respective 28S rRNA are shown at the bottom), IL-18BP mRNA (C; ratios of IL-18BP [indicated by an arrow] to respective 28S rRNA are shown at the bottom), IL-18BP protein (D; ratios of IL-18BP to respective GAPDH [indicated by an arrow] are shown at the bottom) expression and mature IL-18 protein levels (E) in left ventricular tissue from 4 animals randomly selected from vehicle and ISO-infused animals.
Fig. 8. ISO activates CREB and C/EBP in heart in vivo.
A–E, Mice administered with ISO (n=4/group) as in 6A were analyzed for CREB activation (A; ratios of pCREB to respective total CREB are shown at the bottom), CREB DNA binding activity (B; Lamin A/C served as a loading control, and is shown in the bottom panel), C/EBP DNA binding activity (C; Lamin A/C served as a loading control, and is shown in the bottom panel), activation of Akt (D; ratios of pAkt to respective total Akt is shown at the bottom) and ERK1/2 (E; ratios of pERK1/2 to respective total ERK1/2 are shown at the bottom) by immunoblotting using activation-specific antibodies (A, D, E) and EMSA using gene-specific primers and nuclear protein extracts (B, C).
3.8. Isoproterenol-induced myocardial hypertrophy is blunted in IL-18BP Tg and IL-18 null mice
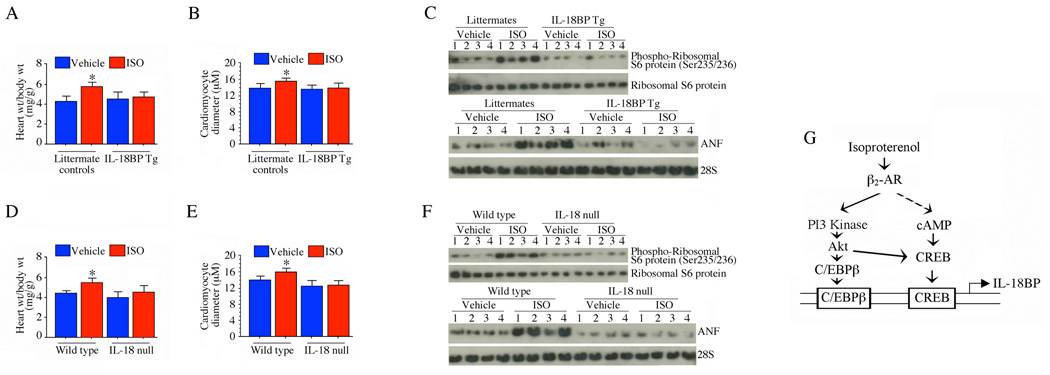
Since IL-18 and IL-18BP are both induced by ISO, but have opposite effects on myocardial hypertrophy, we further dissected their roles in ISO-induced hypertrophy using gene-altered mouse models. Infusion of ISO into the overexpressing IL-18BP Tg mice resulted in significantly less hypertrophy compared to littermate, non-Tg controls (Fig. 9A). Further, cardiomyocyte diameter (Fig. 9B), protein levels of phospho-ribosomal S6 protein (Fig. 9C, upper panel) and ANF mRNA expression (Fig. 9C, lower panel) were markedly attenuated in ISO-infused IL-18BP Tg mice. This is consistent with our in vitro studies that ISO-induced IL-18 contributes to cardiomyocyte hypertrophy and this is blocked by IL-18BP (Fig. 5A–C). Furthermore, it would be expected that in IL-18 null mice, ISO-induced myocardial hypertrophy and hypertrophy-associated markers should also be reduced compared to matched controls, and this was indeed our observation (Fig. 9D–F). Our studies indicate that β-AR stimulation induces IL-18 expression, which is pro-hypertrophic, but also induces IL-18BP, which counters the IL-18 effects. As ISO ultimately promotes cardiomyocyte and myocardial hypertrophy, the concomitant induction of IL-18BP may act as a natural negative regulatory mechanism to limit the effects of IL-18 on this process (Fig. 9).
Fig. 9. ISO-induced myocardial hypertrophy is markedly attenuated in IL-18BP transgenic (Tg) and IL-18 null mice.
A, Three month-old male IL-18BP Tg and IL-18 null mice were continuously infused with ISO (15 mg/kg body wt.) via subcutaneously implanted osmotic minipumps. Heart and body weights were measured after 7 days. *P < 0.05 versus corresponding vehicle-administered animals (n=6/group). B, Hypertrophy was also assessed by quantifying diameter of cardiomyocytes from IL-18BP Tg and littermates in the region of the cell nucleus in H&E stained cryosections’. *P < 0.01 versus corresponding vehicle-administered animals (100 cells/heart, 4 hearts/group). C, The pro-hypertrophic markers p70 S6 kinase and ANF mRNA expression were markedly attenuated in IL-18BP Tg mice. LV tissue from 7 day ISO-infused IL-18BP Tg and littermates (n=4/group) were analyzed for phospho-p70S6K by immunoblotting (upper panel) and ANF mRNA expression by Northern blotting (lower panel). D, ISO-induced myocardial hypertrophy is attenuated in IL-18 null mice. Wild type and IL-18 null mice treated with ISO were analyzed for myocardial hypertrophy as in A. E, Hypertrophy was also assessed by quantifying diameter of cardiomyocytes from wild type and IL-18 null mice as in B. *P < 0.01 versus corresponding vehicle-administered animals (100 cells/heart, 4 hearts/group). F, The pro-hypertrophic markers p70 S6 kinase ANF mRNA expression were markedly attenuated in IL-18 null mice. LV tissue from 7 day ISO-infused wild type and IL-18 null (n=4/group) were analyzed for phospho-p70S6K by immunoblotting (upper panel) and ANF mRNA expression by Northern blotting (lower panel). G, Schema showing the signal transduction pathways involved in ISO induced IL-18BP expression. The β-AR agonist ISO induces IL-18BP expression via β2-AR-dependent PI3K/Akt-mediated C/EBPβ and CREB activation.
4.0 Discussion
Myocardial hypertrophy and heart failure are characterized by sustained β-AR activation and chronically elevated expression of proinflammatory cytokines [1, 2]. IL-18 is a proinflammatory cytokine that modulates cardiomyocyte growth in vitro and myocardial hypertrophy in vivo [10, 13]. Increased levels of IL-18 are associated with hypertrophy and failure in humans.
We have previously demonstrated that the β-AR agonist ISO is a potent inducer of IL-18 expression in the mouse heart in vivo [15]. Here we show that ISO is also a potent inducer of IL-18 in cardiomyocytes in vitro. Importantly, we also demonstrated for the first time that ISO induces the expression of IL-18BP, a neutralizing IL-18 binding protein, via a β2-AR-dependent signaling. ISO activates CREB and C/EBPβ in a PI3K/Akt and ERK1/2-dependent manner, and induces Il18bp transcription in part via CREB and C/EBPβ (Fig. 9G). Moreover, continuous infusion of ISO induces myocardial hypertrophy, and is associated with enhanced CREB and C/EBPβ DNA-binding activity, Akt and ERK1/2 activation, and IL-18BP expression, indicating that ISO-induced myocardial hypertrophy is paradoxically accompanied by increased IL-18BP expression in vivo. Importantly, our data suggest that ISO-induced IL-18BP expression, at least initially, provides a negative regulatory mechanism to attenuate IL-18’s pro-hypertrophic effects both in vivo and in vitro. Interestingly, however, while ISO-induced IL-18BP mRNA expression in cardiomyocytes reached near basal levels by 24 h, induction of IL-18 was rapid, and persisted at high levels throughout the 24 h study period, suggesting that down-regulation of IL-18BP and sustained IL-18 expression due to impaired βAR signaling may contribute to myocardial hypertrophy in chronically stressed heart.
Myocardial hypertrophy and failure are characterized by sustained activation of β-AR stimulation [24]. β2-AR is a G protein-coupled receptor, and signals via both stimulatory (Gs) and inhibitory (Gi) G proteins [25]. Stimulation of Gs signaling activates adenylyl cyclase (AC), cAMP generation, and activation of protein kinase A (PKA). In contrast, Gi signaling attenuates AC, inhibits cAMP synthesis and PKA activation [25]. Intriguingly, our results showed that ISO-induced IL-18BP expression is both CREB and C/EBPβ-dependent, and treatment with pertussis toxin, a Gi inhibitor, while enhancing CREB activation, moderately inhibited IL-18BP expression (data not shown), suggesting the critical role of C/EBP, and that ISO-induces IL-18BP expression via both Gi-dependent and independent mechanisms. Of note, chronic ISO treatment can lead to desensitization of β-AR signaling, a Gi-mediated function [26–28]. Therefore, it is possible that low levels of IL-18BP detected in cardiomyocytes at 24 h following ISO treatment may be due to desensitization of the β2-AR signaling.
Our results demonstrate that CREB and C/EBPβ are both critical to ISO-mediated Il18bp transcription in mouse cardiomyocytes. Of note, human Il18bp has also been shown to be a C/EBPβ-responsive gene [29]. Interestingly, C/EBPβ itself is a CREB-responsive gene. The C/EBP promoter contains two CRE sites, and activated CREB induces C/EBPβ transcription [30], suggesting that ISO-induced CREB not only induces Il18bp transcription, but will positively regulate C/EBP, thus potentiating its effects on IL-18BP expression. Interestingly, CREB has also been shown to play a role in the induction of various genes associated with myocardial hypertrophy and failure. We also found that ISO upregulates ANF, and ANF is CREB responsive [31]. Significantly, CREB has been shown to positively regulate ADRB2 (adrenergic, beta-2-, receptor, surface; β2-AR) transcription [32], suggesting that ISO-mediated CREB activation upregulates not only Il18bp, but also ADRB2 and various other hypertrophy associated genes.
In addition to CREB and C/EBPβ, we also identified potential binding sites for NF-κB and Sp1 in the mouse Il18bp promoter. However, despite the fact that ISO activated NF-κB and induced IL-18 expression via NF-κB (Supplementary Fig. S2-B), overexpression of dnp65 mutant failed to significantly modulate ISO-mediated Il18bp transcription in cardiomyocytes (Supplementary Fig. S2-B), suggesting that mouse Il18bp may not be a κB-responsive gene in cardiomyocytes treated with ISO. Previous reports suggest that following activation, phospho-CREB competes with p65 for the limited amounts of the transcriptional cofactor CREB-binding protein (CBP)/p300 available [33]. In contrast, since the catalytic subunit of protein kinase A (PKAc) phosphorylates p65 [34], the possibility exists that phospho-p65 and CBP interaction may potentiate ISO-induced CREB activation and Il18bp transcription. In fact, in a recent report the antioxidant PDTC was shown to inhibit TNF-α-induced IL-18BPa expression in human rheumatoid arthritis synovial fibroblasts [35], implying a role for NF-κB in human IL-18BPa induction. Conceivably, the regulation of Il18bp transcription is not only stimulus-specific, but also species and cell type dependent. Alternatively, NF-κB may associate with C/EBPβ, and their cross coupling might synergistically induce IL-18BP expression. In support of this hypothesis, it has been previously reported that NF-κB and C/EBPβ physically associate with each other via the Rel homology domain of NF-κB and the bZIP region of C/EBPβ, and this cross coupling results in inhibition of promoters with κB enhancer motifs and in the synergistic stimulation of promoters with C/EBP binding sites [36]. Of note, crosstalk between NF-κBp65 and C/EBPβ has also been described in the induction of several inflammatory genes, including IL-6 and IL-8 [37, 38]. Whether such an interaction plays a role in IL-18BP expression in mouse cardiomyocytes is not known.
Although Sp1 is a transcriptional regulator of several cardiac genes and genes involved in cell growth [39], in our studies pretreatment with the Sp1 inhibitor mithramycin failed to affect Il18bp transcription. However, confirming our earlier results [18], the Sp1 inhibitor mithramycin inhibited IL-18-induced EMMPRIN expression (Supplementary Figure S2-C). Since cAMP deglycosylates Sp1, and targets the hypoglycosylated Sp1 for rapid proteolytic degradation [40], it needs to be investigated whether ISO-treatment results in Sp1 deglycosylation and whether overexpression of wild type Sp1 by itself induces Il18bp transcription.
Myocardial hypertrophy and heart failure are complex disease processes [2]. It is known that a number of proinflammatory cytokines play critical roles in the initiation and progression of myocardial hypertrophy and transition to heart failure [1, 2]. Previously we demonstrated that ISO upregulates cardiac IL-1β, IL-6 and TNF-α [41, 42]. However, it appears to do so without significantly altering their systemic levels [41, 42], suggesting that it is the local expression of these cytokines that promotes the underlying processes responsible for cardiac hypertrophy following β-AR stimulation. IL-18 is a proinflammatory cytokine that is expressed by all myocardial constituent cells, and its induction is characterized by increases in both local and systemic levels. Since the locally generated and the systemic IL-18 can further amplify inflammation via induction of other cytokines and chemokines, our studies identify it as a critical mediator, and thus a potential therapeutic target, of cardiac hypertrophy. Here we show that IL-18BP is also induced in cardiomyoctes by ISO treatment, and that both in vitro and in vivo it acts to limit the prohypertrophic effects of IL-18. Therefore IL-18BP may have potential use in the treatment of the hypertrophied failing heart.
Research highlights.
𠖺The β-AR agonist isoproterenol induces cardiomyocyte hypertrophy in vitro and in vivo via IL-18. 𠖺 Isoproterenol induces the expression of IL-18BP, a neutralizing IL-18 binding protein via β2-AR. 𠖺 Isoproterenol induces IL-18BP expression via PI3K/Akt and ERK1/2-dependent CREB and C/EBPβ activation. 𠖺 Isoproterenol induced cardiomyocyte hypertrophy is blunted in IL-18BP Tg and IL-18 null mice. 𠖺 IL-18BP may have therapeutic potential in the treatment of hypertrophied failing heart.
Supplementary Material
Acknowledgements
This work was supported by the Veterans Affairs Office of Research and Development-Biomedical Laboratory Research and Development Service Award 1IO1BX000246 and the NHLBI Grant HL-86787 (to BC). SM is supported by I01BX007080 and AI043279. PD is a supported by HL-70241 and HL-80682. CAD is supported by AI15614. The contents of this report do not represent the views of the Department of Veterans Affairs or the United States Government.
Abbreviations
- ACM
adult mouse cardiomyocytes
- ADRB2
adrenergic, beta-2-, receptor, surface
- ANF
atrial natriuretic factor
- β-AR
beta adrenergic receptor
- bZIP
the basic leucine zipper domain
- cAMP
cyclic adenosine monophosphate
- CBP
CREB binding protein
- C/EBP
CCAAT/enhancer-binding protein
- CMV
cytomegalo virus
- CRE
cAMP-response element
- CREB
CRE-binding protein
- db-cAMP
dibutyryl-cAMP
- DMSO
dimethylsulfoxide
- dn
dominant negative
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal-regulated protein kinase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GFP
green fluorescent protein
- Gi
inhibitory G proteins
- IL
Interleukin
- IL-18BP
IL-18 binding protein
- ISO
isoproterenol
- Luc
luciferase
- KCREB
dominant negative mutant form CREB
- MEK
mitogen-activated protein kinase kinase
- NF-κB
nuclear factor κB
- NMCM
neonatal mouse cardiomyocytes
- nt
nucleotide
- null
homozygous knockout
- PDTC
pyrrolidine dithiocarbamate
- PI3K
phosphatidylinositol-3 kinase
- PKA
protein kinase A
- PTx
pertussis toxin
- siRNA
small interference RNA
- Sp1
specificity protein 1
- TAC
transverse aortic constriction
- TNF
tumor necrosis factor
- Tg
transgenic
- wt
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None declared
References
- 1.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002 Nov 29;91(11):988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 2.Mann DL. Targeted anticytokine therapy and the failing heart. Am J Cardiol. 2005 Jun 6;95(11A):9C–16C. doi: 10.1016/j.amjcard.2005.03.007. discussion 38C–40C. [DOI] [PubMed] [Google Scholar]
- 3.Nakano M, Knowlton AA, Dibbs Z, Mann DL. Tumor necrosis factor-alpha confers resistance to hypoxic injury in the adult mammalian cardiac myocyte. Circulation. 1998 Apr 14;97(14):1392–1400. doi: 10.1161/01.cir.97.14.1392. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA, Novick D, Puren AJ, Fantuzzi G, Shapiro L, Muhl H, et al. Overview of interleukin-18: more than an interferon-gamma inducing factor. J Leukoc Biol. 1998 Jun;63(6):658–664. [PubMed] [Google Scholar]
- 5.Mallat Z, Heymes C, Corbaz A, Logeart D, Alouani S, Cohen-Solal A, et al. Evidence for altered interleukin 18 (IL)-18 pathway in human heart failure. FASEB J. 2004 Nov;18(14):1752–1754. doi: 10.1096/fj.04-2426fje. [DOI] [PubMed] [Google Scholar]
- 6.Seta Y, Kanda T, Tanaka T, Arai M, Sekiguchi K, Yokoyama T, et al. Interleukin-18 in patients with congestive heart failure: induction of atrial natriuretic peptide gene expression. Res Commun Mol Pathol Pharmacol. 2000 Jul–Aug;108(1–2):87–95. [PubMed] [Google Scholar]
- 7.Kim SH, Eisenstein M, Reznikov L, Fantuzzi G, Novick D, Rubinstein M, et al. Structural requirements of six naturally occurring isoforms of the IL-18 binding protein to inhibit IL-18. Proc Natl Acad Sci U S A. 2000 Feb 1;97(3):1190–1195. doi: 10.1073/pnas.97.3.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ten Hove T, Corbaz A, Amitai H, Aloni S, Belzer I, Graber P, et al. Blockade of endogenous IL-18 ameliorates TNBS-induced colitis by decreasing local TNF-alpha production in mice. Gastroenterology. 2001 Dec;121(6):1372–1379. doi: 10.1053/gast.2001.29579. [DOI] [PubMed] [Google Scholar]
- 9.Faggioni R, Cattley RC, Guo J, Flores S, Brown H, Qi M, et al. IL-18-binding protein protects against lipopolysaccharide- induced lethality and prevents the development of Fas/Fas ligand-mediated models of liver disease in mice. J Immunol. 2001 Nov 15;167(10):5913–5920. doi: 10.4049/jimmunol.167.10.5913. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekar B, Mummidi S, Claycomb WC, Mestril R, Nemer M. Interleukin-18 is a pro-hypertrophic cytokine that acts through a phosphatidylinositol 3-kinase-phosphoinositi-dedependent kinase-1-Akt-GATA4 signaling pathway in cardiomyocytes. J Biol Chem. 2005 Feb 11;280(6):4553–4567. doi: 10.1074/jbc.M411787200. [DOI] [PubMed] [Google Scholar]
- 11.Majumdar G, Johnson IM, Kale S, Raghow R. Epigenetic regulation of cardiac muscle-specific genes in H9c2 cells by Interleukin-18 and histone deacetylase inhibitor m-carboxycinnamic acid bis-hydroxamide. Mol Cell Biochem. 2008 May;312((1–2):47–60. doi: 10.1007/s11010-008-9720-x. [DOI] [PubMed] [Google Scholar]
- 12.Platis A, Yu Q, Moore D, Khojeini E, Tsau P, Larson D. The effect of daily administration of IL-18 on cardiac structure and function. Perfusion. 2008 Jul;23(4):237–242. doi: 10.1177/0267659108101511. [DOI] [PubMed] [Google Scholar]
- 13.Woldbaek PR, Sande JB, Stromme TA, Lunde PK, Djurovic S, Lyberg T, et al. Daily administration of interleukin-18 causes myocardial dysfunction in healthy mice. Am J Physiol Heart Circ Physiol. 2005 Aug;289(2):H708–H714. doi: 10.1152/ajpheart.01179.2004. [DOI] [PubMed] [Google Scholar]
- 14.Colston JT, Boylston WH, Feldman MD, Jenkinson CP, de la Rosa SD, Barton A, et al. Interleukin-18 knockout mice display maladaptive cardiac hypertrophy in response to pressure overload. Biochem Biophys Res Commun. 2007 Mar 9;354(2):552–558. doi: 10.1016/j.bbrc.2007.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandrasekar B, Marelli-Berg FM, Tone M, Bysani S, Prabhu SD, Murray DR. Beta-adrenergic stimulation induces interleukin-18 expression via beta2-AR, PI3K, Akt, IKK, and NF-kappaB. Biochem Biophys Res Commun. 2004 Jun 25;319(2):304–311. doi: 10.1016/j.bbrc.2004.04.185. [DOI] [PubMed] [Google Scholar]
- 16.Fantuzzi G, Banda NK, Guthridge C, Vondracek A, Kim SH, Siegmund B, et al. Generation and characterization of mice transgenic for human IL-18-binding protein isoform a. J Leukoc Biol. 2003 Nov;74(5):889–896. doi: 10.1189/jlb.0503230. [DOI] [PubMed] [Google Scholar]
- 17.Shanmugam P, Valente AJ, Prabhu SD, Venkatesan B, Yoshida T, Delafontaine P, et al. Angiotensin-II type 1 receptor and NOX2 mediate TCF/LEF and CREB dependent WISP1 induction and cardiomyocyte hypertrophy. J Mol Cell Cardiol. Jun;50(6):928–938. doi: 10.1016/j.yjmcc.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy VS, Prabhu SD, Mummidi S, Valente AJ, Venkatesan B, Shanmugam P, et al. Interleukin-18 induces EMMPRIN expression in primary cardiomyocytes via JNK/Sp1 signaling and MMP-9 in part via EMMPRIN and through AP-1 and NF-kappaB activation. Am J Physiol Heart Circ Physiol. Oct;299(4):H1242–H1254. doi: 10.1152/ajpheart.00451.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smeets RL, van de Loo FA, Arntz OJ, Bennink MB, Joosten LA, van den Berg WB. Adenoviral delivery of IL-18 binding protein C ameliorates collagen-induced arthritis in mice. Gene Ther. 2003 Jun;10(12):1004–1011. doi: 10.1038/sj.gt.3301986. [DOI] [PubMed] [Google Scholar]
- 20.Morisco C, Zebrowski DC, Vatner DE, Vatner SF, Sadoshima J. Beta-adrenergic cardiac hypertrophy is mediated primarily by the beta(1)-subtype in the rat heart. J Mol Cell Cardiol. 2001 Mar;33(3):561–573. doi: 10.1006/jmcc.2000.1332. [DOI] [PubMed] [Google Scholar]
- 21.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998 Dec 4;273(49):32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 22.Morisco C, Zebrowski D, Condorelli G, Tsichlis P, Vatner SF, Sadoshima J. The Akt-glycogen synthase kinase 3beta pathway regulates transcription of atrial natriuretic factor induced by beta-adrenergic receptor stimulation in cardiac myocytes. J Biol Chem. 2000 May 12;275(19):14466–14475. doi: 10.1074/jbc.275.19.14466. [DOI] [PubMed] [Google Scholar]
- 23.Venkatesan B, Prabhu SD, Venkatachalam K, Mummidi S, Valente AJ, Clark RA, et al. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal. May;22(5):809–820. doi: 10.1016/j.cellsig.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bristow MR. beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000 Feb 8;101(5):558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 25.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997 Nov 6;390(6655):88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 26.Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, et al. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002 Oct 25;298(5594):834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- 27.Mialet-Perez J, Green SA, Miller WE, Liggett SB. A primate-dominant third glycosylation site of the beta2-adrenergic receptor routes receptors to degradation during agonist regulation. J Biol Chem. 2004 Sep 10;279(37):38603–38607. doi: 10.1074/jbc.M403708200. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001 Mar;53(1):1–24. [PubMed] [Google Scholar]
- 29.Hurgin V, Novick D, Rubinstein M. The promoter of IL-18 binding protein: activation by an IFN-gamma -induced complex of IFN regulatory factor 1 and CCAAT/enhancer binding protein beta. Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):16957–16962. doi: 10.1073/pnas.262663399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niehof M, Manns MP, Trautwein C. CREB controls LAP/C/EBP beta transcription. Mol Cell Biol. 1997 Jul;17(7):3600–3613. doi: 10.1128/mcb.17.7.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornelius T, Holmer SR, Muller FU, Riegger GA, Schunkert H. Regulation of the rat atrial natriuretic peptide gene after acute imposition of left ventricular pressure overload. Hypertension. 1997 Dec;30(6):1348–1355. doi: 10.1161/01.hyp.30.6.1348. [DOI] [PubMed] [Google Scholar]
- 32.Collins S, Altschmied J, Herbsman O, Caron MG, Mellon PL, Lefkowitz RJ. A cAMP response element in the beta 2-adrenergic receptor gene confers transcriptional autoregulation by cAMP. J Biol Chem. 1990 Nov 5;265(31):19330–19335. [PubMed] [Google Scholar]
- 33.Parry GC, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J Immunol. 1997 Dec 1;159(11):5450–5456. [PubMed] [Google Scholar]
- 34.Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998 Apr;1(5):661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 35.Marotte H, Ahmed S, Ruth JH, Koch AE. Blocking ERK-1/2 reduces tumor necrosis factor alpha-induced interleukin-18 bioactivity in rheumatoid arthritis synovial fibroblasts by induction of interleukin-18 binding protein A. Arthritis Rheum. Mar;62(3):722–731. doi: 10.1002/art.27269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein B, Cogswell PC, Baldwin AS., Jr Functional and physical associations between NF-kappa B and C/EBP family members: a Rel domain-bZIP interaction. Mol Cell Biol. 1993 Jul;13(7):3964–3974. doi: 10.1128/mcb.13.7.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, et al. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunsch C, Lang RK, Rosen CA, Shannon MF. Synergistic transcriptional activation of the IL-8 gene by NF-kappa B p65 (RelA) and NF-IL-6. J Immunol. 1994 Jul 1;153(1):153–164. [PubMed] [Google Scholar]
- 39.Flesch M. On the trail of cardiac specific transcription factors. Cardiovasc Res. 2001 Apr;50(1):3–6. doi: 10.1016/s0008-6363(01)00218-8. [DOI] [PubMed] [Google Scholar]
- 40.Han I, Kudlow JE. Reduced O glycosylation of Sp1 is associated with increased proteasome susceptibility. Mol Cell Biol. 1997 May;17(5):2550–2558. doi: 10.1128/mcb.17.5.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray DR, Prabhu SD, Chandrasekar B. Chronic beta-adrenergic stimulation induces myocardial proinflammatory cytokine expression. Circulation. 2000 May 23;101(20):2338–2341. doi: 10.1161/01.cir.101.20.2338. [DOI] [PubMed] [Google Scholar]
- 42.Prabhu SD, Chandrasekar B, Murray DR, Freeman GL. beta-adrenergic blockade in developing heart failure: effects on myocardial inflammatory cytokines, nitric oxide, and remodeling. Circulation. 2000 May 2;101(17):2103–2109. doi: 10.1161/01.cir.101.17.2103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.