Abstract
Se is a potent nutritional antioxidant important for various aspects of human health. Because asthma has been demonstrated to involve increased oxidative stress, levels of Se intake have been hypothesized to play an important role in the pathogenesis of asthma. However, significant associations between Se status and prevalence or severity of asthma have not been consistently demonstrated in human studies. This highlights both the complex etiology of human asthma and the inherent problems with correlative nutritional studies. In this review, the different findings in human studies are discussed along with results from limited intervention studies. Mouse models of asthma have provided more definitive results suggesting that the benefits of Se supplementation may depend on an individual's initial Se status. This likely involves T helper cell differentiation and the mechanistic studies that have provided important insight into the effects of Se levels on immune cell function are summarized. Importantly, the benefits and adverse effects of Se supplementation must both be considered in using this nutritional supplement for treating asthma. With this in mind new approaches are discussed that may provide more safe and effective means for using Se supplementation for asthma or other disorders involving inflammation or immunity.
Keywords: selenium, selenoproteins, asthma, allergic airway inflammation, oxidative stress, immunity
1. Introduction
Selenium (Se) is an essential micronutrient that is important for various aspects of human health including proper thyroid hormone metabolism, cardiovascular health, prevention of neurodegeneration and cancer, and optimal immune responses. Most populations worldwide acquire dietary Se at levels that do not result in severe deficiency or toxicity, but there are important exceptions. For example, regions in China and New Zealand have low Se content in the soil, which may lead to insufficient Se in plants and livestock that results in low Se foods (Thomson, 2004). Other studies have shown that Se intake and serum Se concentrations in parts of Europe have recently declined, likely due to decreased use of North American grain (Rayman, 1997). This is particularly evident in the United Kingdom where there is evidence that Se intake has been declining and is now well below the levels required for optimal biological activity (Johnson et al., 2010). Thus, there is growing interest in determining whether decreases in Se intake may impact certain health conditions for these populations. The U.S. generally has high Se content in the soil and high Se intake compared to other nations, and this is reflected in relatively high average serum Se levels of 125–137 µg/L in the U.S. population (Bleys et al., 2009; Niskar et al., 2003). However, deficient Se intake may still be found within certain individuals and moderately low Se status may dramatically affect inflammation and immune responses. Also, the use of Se supplementation to increase Se status to supraphysiological levels may be exploited to modulate immune processes that drive certain health disorders, such as the T helper 2 (Th2) responses that drive allergic asthma.
Asthma is a multi-factorial inflammatory syndrome characterized by airway hyper-responsiveness, wheezing, coughing, and shortness of breath (Locksley, 2010; Miller, 2001). The complex etiology of asthma involves genetic, allergic, environmental, infectious, emotional, and nutritional factors (Maddox and Schwartz, 2002). Among these nutritional factors, Se has been hypothesized to play a particularly important role. This is largely based on the premise that oxidative stress significantly contributes to the pathogenesis of asthma and, as a potent nutritional antioxidant, dietary Se can serve to ameliorate oxidative stress and reduce asthma. Oxidative stress has indeed been detected in lower airways of asthmatic individuals and genetic polymorphisms in humans and studies in animals suggest that oxidative stress is a contributing factor in the development and severity of asthma (Riedl and Nel, 2008). Allergic asthma is characterized by a pro-oxidant pulmonary environment and allergen challenge in the lung induces rapid increases in the oxidized to reduced glutathione ratio as well as ROS levels that precede inflammatory cell infiltration (Park et al., 2009). Moreover, environmental factors such as diesel exhaust particles can have adjuvant effects that promote allergic airway inflammation in a manner that involves oxidative stress (Li et al., 2009).
Given that levels of dietary Se intake can modulate oxidative stress in various tissues including the lung, it would certainly make sense that increased intake of Se could potentially decrease asthma pathology. However, correlative or intervention studies in humans have produced conflicting data and Se supplementation is generally not recommended for asthma patients. It may be that Se levels have more influence over certain types of asthma, such as those with strong allergic components driven by Th2 immunity. This has led our laboratory to focus on the effects of dietary Se intake on the differentiation of T helper cells, and these findings are discussed in the context of other studies in the following sections. Also discussed are the various data regarding the relationship between dietary Se and asthma, potential mechanisms by which Se affects asthma, and potential uses of Se supplementation for preventing or treating asthma.
2. Metabolism of Se and biosynthesis of selenoproteins
The major form of Se ingested by humans is selenomethionine, although other forms of Se are present in foods. The biological effects of Se are mainly exerted through its incorporation into the amino acid, selenocysteine, which is co-translationally inserted into selenoproteins. The synthesis of selenoproteins requires dedicated protein factors, a specialized t-RNA (Sec-tRNASec), and mRNA cis-acting elements, and the translational processes involved in selenoprotein synthesis have been fully described elsewhere (Papp et al., 2007; Squires and Berry, 2008). Selenoprotein expression is essential for life as demonstrated by the generation of mice lacking Sec-tRNASec required for translation of all selenoproteins, which was embryonic lethal (Bosl et al., 1997). While complete dietary depletion of selenoproteins is physiologically improbable even under conditions of very low Se intake, less overt decreases in selenoprotein expression may still strongly influence inflammation and immune responses such as those involved in asthma. Also, Se supplementation may be used to elevate selenoprotein expression to above-adequate levels, which may impinge upon the immune system and this may also affect the development or severity of asthma.
There are 25 human selenoproteins, all but one of which exist as selenoproteins in rodents (Kryukov et al., 2003). A list of the selenoproteins and their functions is presented in Table 1. While broadly classified as antioxidants, selenoproteins actually exhibit a wide range of tissue distribution, cellular locations, and functions (Reeves and Hoffmann, 2009). The antioxidant properties of selenoproteins are exemplified by the glutathione peroxidase (GPx) enzymes, which utilize Se at their active sites to detoxify reactive oxygen species including hydrogen peroxide and phospholipid hydroperoxide. Thioredoxin reductase 1 and 2 (Txnrd1 and 2) perform an essential role in the regeneration of reduced thioredoxin (Trx), which provides reducing capacity for maintaining balanced redox tone within cells (Lu et al., 2009). Selenoprotein P (SelP) also has antioxidant properties, but is also crucial for the transport of Se throughout the body (Schweizer et al., 2005). Biological roles for other selenoproteins have more recently emerged, and particularly important functions for selenoproteins K and S (SelK and SelS) have been described for regulating inflammation and immunity (Curran et al., 2005; Verma et al., 2011). There is a paucity of data regarding polymorphic mutations in selenoprotein genes related to asthma, but some animal model data have provided insight on potential roles for selenoproteins in asthma as discussed in more detail below.
Table 1.
Selenoproteins and their functions
| Selenoprotein | Abbreviation | Function |
|---|---|---|
| Cytosolic glutathione peroxidase | GPx1 | Peroxide reduction in the cytoplasm |
| Gastrointestinal glutathione peroxidase | GPx2 | Peroxide reduction, mainly in the gastrointestinal tract |
| Plasma glutathione peroxidase | GPx3 | Peroxide reduction in plasma and other extracellular fluids |
| Phosholipid hydroperoxide glutathione peroxidase | GPx4 | Reduction of phospholipid hydroperoxides |
| Olfactory glutathione peroxidase | GPx6 | Peroxide reduction, found in embryos and in the olfactory epithelium |
| Thioredoxin reductase Type I | Trxrd1, TR1 | Cytoplasmic thioredoxin reductase, involved in many biological pathways |
| Thioredoxin reductase Type II | Trxrd2, TR3 | Mitocondrial thioredoxin reductase, involved in many biological pathways |
| Thioredoxin reductase Type III | Trxrd3, TR2, TGR | Thioredoxin/glutathione reductase found in mainly in testes |
| Deiodinase Type I | D1, DIO1 | Important for systemic active tyroid hormone levels. |
| Deiodinase Type II | D2, DIO2 | Important for local active tyroid hormone levels. |
| Deiodinase Type III | D3, DIO3 | Inactivates thyroid hormone |
| Selenoprotein H | SelH | Binds DNA and is involved in transcription |
| Selenoprotein I | SelI, hEPT1 | Possibly involved in phospholipid biosynthesis |
| Selenoprotein K | SelK | Transmembrane protein localized to endoplasmic reticulum, involved in calcium mobilization |
| Selenoprotein M | SelM | Possibly involved in protein-folding in the ER |
| Selenoprotein 15 | Sep15 | Possibly involved in protein-folding in the ER |
| Selenoprotein N | SelN, SEPN1, SepN | Involved in RyR-related calcium mobiliation from ER and potential role in early muscle formation |
| Selenoprotein O | SelO | Unknown |
| Selenoprotein P | SelP, SePP | Selenium transport and also functions as intracellular antioxidant in phagocytes |
| Selenoprotein R | SelR, MsrB1 | Functions as a methionine sulfoxide reductase |
| Selenoprotein S | SelS, SEPS1, SELENOS, VIMP | Transmembrane protein involved in ER stress |
| Selenoprotein T | SelT | ER protein involved in calcium mobilization |
| Selenoprotein V | SelV | Testes-specific selenoprotein of unknown function |
| Selenoprotein W | SelW, SEPW1 | Putative antioxidant role, perhaps important in muscle growth |
| Selenophosphate synthetase | SPS2 | Involved in synthesis of all selenoproteins, including itself |
3. Epidemiological and intervention studies in humans
There have been many epidemiological studies providing evidence that Se status is related to asthma, typically associating lower Se status in asthma patients compared to controls. For example, a small study involving 25 each of adult asthmatic patients and healthy subjects found that the asthma group had lower serum Se concentrations and higher indicators of oxidative stress such as thiobarbituric acid reactive substances (TBARS) (Guo et al., 2011). Also, lung function (FEV1/FVC%) was higher in subjects with higher Se status. Consistent with these findings, a number of epidemiological studies in adults have reported that asthma incidence, prevalence, or severity is associated with reduced Se status (de Luis et al., 2003; Flatt et al., 1990; Hasselmark et al., 1990; Kadrabova et al., 1996; Misso et al., 1996; Omland et al., 2002; Qujeq et al., 2003; Shaw et al., 1994; Stone et al., 1989). Studies in children have also identified associated risks with low Se status. For example, blood Se levels and GPx activity were found to be reduced in children with asthma (Kocyigit et al., 2004). In a larger study (N = 165), asthma was examined in relation to both Se and zinc (Zn) concentration in fingernails (Carneiro et al., 2011). Those children included in the highest quartile of Se and Zn concentration presented a 5-fold decrease in the prevalence ratio of asthma while children in the lowest Se range presented an almost 2.5-fold increase in the asthma prevalence ratio.
Some of the results from the above studies describe strong correlations between Se status on asthma, but several studies have failed to confirm any association (Ford et al., 2004; McKenzie et al., 1998). A large, multi-regional study conducted under the Global Allergy and Asthma European Network (GA2LEN) examined asthma prevalence/severity data from 14 centers in Europe and found no significant association between Se status and asthma levels (Burney et al., 2008). Another study even suggested that Se levels or GPx activities were positively associated with severity of bronchial responsiveness (Garcia-Larsen et al., 2007). Similarly, Se was positively correlated with lymphoproliferative response to house dust mite antigen in adult allergic asthma (Dunstan et al., 2006). Recently a systematic review and meta-analysis of nutrients related to asthma and allergy found no beneficial association between Se and disease outcome (Nurmatov et al., 2011). Altogether, these findings indicate that the relationship between dietary Se intake and asthma is not simple and approaches other than correlative investigations need to be taken.
Given the mixed results, there appears to be insufficient evidence to support the use of nutrient supplements like Se to prevent or limit asthma in children or adults. However, there is an emerging interest in the potential of dietary intervention during pregnancy to reduce the likelihood of childhood asthma. A small number of cohort studies have found associations between childhood asthma and reduced maternal intake of some nutrients (vitamin E, vitamin D, Se, Zn, and polyunsaturated fats) during pregnancy (Allan and Devereux, 2011). One large pregnancy cohort study reported that Se levels in umbilical cord were negatively associated with persistent wheeze in early children (Shaheen et al., 2004). Another birth cohort study found that maternal plasma Se concentration during early pregnancy was inversely associated with wheezing and with consulting a doctor because of wheeze in the second year of life (Devereux et al., 2007). Cord plasma Se was also inversely associated with wheezing, and consulting a doctor because of wheeze in the second year of life. The biological mechanisms by which antioxidants like Se may influence the development of childhood asthma are likely to be independent of their antioxidant properties because the associations appear limited to certain nutrients (with and without antioxidant properties) and not with all antioxidants (Allan and Devereux, 2011). Se and other nutrients affect physiological or pathophysiological conditions in addition to oxidative stress in the lung. In particular, dietary antioxidants can be very important for influencing the inflammatory conditions and immune responses underlying asthma pathology, and this has been supported by animal studies described in more detail below.
Similar to the epidemiological data described above, results from intervention studies aimed at determining the effectiveness of Se supplementation for reducing the incidence or severity of asthma have not been entirely clear or consistent. For example, one study reported significantly decreased consumption of corticosteroids after Se supplementation with 200 µg/day for 96 weeks in corticoid-dependent asthmatics (Gazdik et al., 2002). However, other studies failed to confirm any benefit from Se supplementation for asthmatic adults (Dunstan et al., 2007; Shaheen et al., 2007). Based on these findings, Se supplementation has not generally been recommended as a therapeutic modality for reducing asthma burden.
In an attempt to reconcile the different epidemiological findings there are several potential issues to consider. First, not all of these studies took into consideration the multi-factorial etiology of this disease, particularly separating atopic vs. nonatopic asthma. Because Se levels may affect components of the immune system that drive allergic responses, it is crucial to distinguish between asthma cases involving allergic etiology from those with no allergic component. Also, the study populations involved in these different studies were quite varied and may have had important differences regarding age of allergen exposure. It is quite possible that fluctuations in Se status may have occurred in individuals over the course of disease progression and this may have contributed to disparate findings. This highlights perhaps the most important issue in using the case-control approach for nutritional studies in that it does not take into consideration potential fluctuations in nutrients like Se over time. It is impossible to determine the Se status of the individual at the time of initial exposure to allergens or asthma-triggering event, and the studies are often measuring Se status long after asthma has been established. Another major issue is the bi-directional relationship between inflammation and serum Se concentration that can greatly complicate correlative studies seeking associations between Se status and asthma. As discuss in more detail below, certain inflammatory conditions can actually lead to decreased serum Se levels. Thus, low Se status may be a result instead of a cause of airway inflammation. Overall, it is not currently possible to draw any definitive conclusions about epidemiological links between Se and asthma, and this has led us and others to utilize mouse models of allergic asthma to further study this issue.
4. Mouse models of allergic asthma
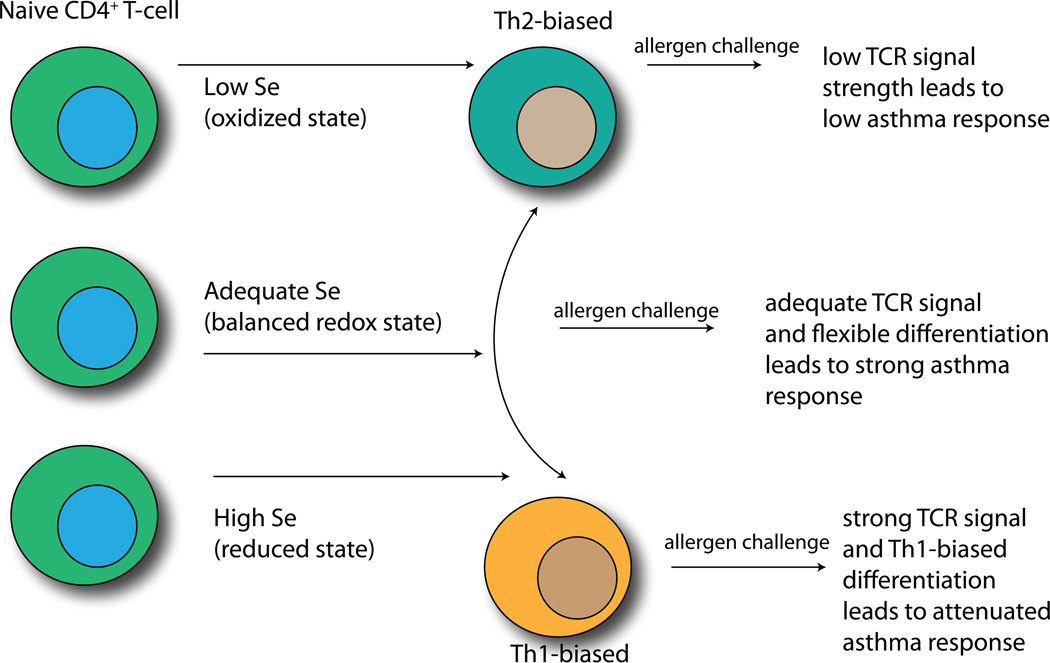
A mouse model of allergic asthma has been developed for investigating mechanisms driving the immune responses and airway inflammation associated with the human disease. This model has been well used with some variations in methodology, but the overall approach involves intraperitoneal sensitization of mice to ovalbumin (OVA) adsorbed onto aluminum hydroxide (alum), followed by intranasal challenges with OVA suspended in PBS (Grunig et al., 1998; Ikeda et al., 2003; Won et al., 2010). This process leads to airway inflammation with the hallmark features of human asthma including infiltration of inflammatory leukocytes into the lung tissue, airway hyperreactivity (AHR), epithelial damage, and tissue remodeling. Our laboratory used this model to show that dietary Se levels influenced the development of OVA-induced allergic asthma in mice (Hoffmann et al., 2007). In particular, mice were fed defined diets with low (0.08 ppm), adequate (0.25 ppm), or supplemented (1.0 or 2.7 ppm) Se that reflect moderately low, adequate, and above-adequate levels of Se intake in humans. Allergic asthma was then induced in these mice and levels of allergic airway inflammation and AHR were evaluated. Interestingly, low Se status resulted in lower asthma compared to adequate Se. The adequate Se group exhibited robust allergic asthma responses, but increasing the diets to supplemented Se levels attenuated asthma. These results may help explain some of the conflicting findings involving Se-supplementation in humans. In particular, asthma and Se intake may not be related in a simple dose-response manner and this may complicate case-control studies attempting to associate lower Se status to higher asthma prevalence. Perhaps the most interesting results from these mouse experiments were those showing that Se supplementation to induce above-adequate levels decreased Th2 cell frequency in the lung. The Th2 marker, phosphorylated-STAT6, was significantly reduced in the lung of OVA-challenged mice fed supplemented Se compared to those fed adequate levels of Se. This skewing of CD4+ T cells away from Th2-type was further supported by later studies as described in the following section.
Questions remain as to whether individual selenoproteins play a protective role in reducing oxidative stress or a more detrimental role in promoting the immune responses that drive the asthma process. Data from mouse studies have suggested that expression of certain selenoproteins may be increased during asthma. For example, lung GPx1 and liver SelP were increased in OVA-challenged mice compared with controls (Hoffmann et al., 2007). In a subsequent study, attenuation of allergen-induced eosinophilic infiltration and airway hyper-responsiveness was observed in GPx1-deficient mice compared with wild-type mice (Won et al., 2010). This suggests the upregulated expression of GPx1 in asthmatic lungs of wild-type mice described above may reflect more of a pathogenic than protective role. In another study, GPx2 expression was found to be increased in lungs of mice after induction of allergic airway disease (Dittrich et al., 2010). Furthermore, mice with targeted disruption of the GPx2 gene showed significantly enhanced airway inflammation compared to wild-type mice, suggesting its induced expression protects from disease. This is surprising, given that GPx2 expression is typically associated with the epithelium of the gastrointestinal tract (Wingler and Brigelius-Flohe, 1999). Thus, different GPx enzymes may have opposing effects on asthma (Meyer et al., 2010), and this could be due to their multiple roles in regulating both oxidative stress and immunity during the development of allergic asthma in mice. It would be of interest to include other selenoprotein knockout models to determine roles of other members of this family. While knockout models help to clarify roles for individual selenoproteins in asthma, it should be kept in mind that they do not necessarily reflect how changes in dietary Se may influence the disease.
5. T helper cell activation and differentiation
The extent and nature of naïve T cell differentiation are determined by the quantity and quality of stimulation, including antigen concentration, co-stimulatory molecules, and cytokines, as well as the frequency of responding T cells and density of antigen-presenting cells (Gett et al., 2003). Dendritic cells (DCs) provide critical signals via cell-to-cell contact and cytokines to naïve CD4+ T helper cells that influence the type of effector cells into which they develop (Kapsenberg, 2003). In this sense, factors such as oxidative stress and redox status of both the DCs and naïve CD4+ T cells may play key roles in the types of signals initiated in naïve CD4+ T cells during their activation. The number and type of T helper cells that are generated during the first encounter with antigen substantially contribute to the outcome of the immune response. In particular, CD4+ T cells become polarized during activation into Th1, Th2, Th17, Treg, or other T helper subtypes (Murphy and Reiner, 2002; Sakaguchi and Powrie, 2007; Stockinger and Veldhoen, 2007). Known transcriptional regulators of CD4+ T cell differentiation include T-bet and IL12Rβ2 (pro-Th1), GATA3 (pro-Th2), FoxP3 (pro-Treg), and RORγ(t) (pro-Th17), and signaling pathways that induce GATA3 and T-bet have been shown to be negatively regulated by each other (Hwang et al., 2005; Usui et al., 2003; Usui et al., 2006). Th2 cells produce IL-4, IL-5, and IL-13 that promote allergic asthma, so stimuli or conditions that skew naïve CD4+ T cells toward Th1 differentiation through increased T-bet would likely decrease Th2 responses through inhibition of GATA3.
As a potent antioxidant, Se has a particularly strong influence on the activation, proliferation, and differentiation of naïve T cells during the initiation of immune responses. Studies in our laboratory have shown that CD4+ T cells from mice fed the low, adequate, and supplemented Se diets described above displayed differences in T cell receptor (TCR) signaling. In particular, higher Se intake significantly increased T cell proliferative capacity, with concomitant increases in Ca2+ mobilization, oxidative burst, and translocation of nuclear factor of activated T cells (NFAT) (Hoffmann et al., 2010). This enhanced TCR signaling affected CD4+ T cell differentiation, with higher Se intake skewing differentiation toward Th1/Treg and away from Th2 phenotypes. When the CD4+ T cells from mice fed different Se diets were analyzed for ROS using fluorescent indicators such as dihydrochlorofluorescein, no differences were detected. However, increased levels of free thiols were found with increasing dietary Se, which indicated a shift in redox tone toward a reduced state. The differences in TCR-induced Ca2+ flux and proliferative capacity caused by dietary Se were eliminated when cells were treated with an exogenous source of free thiols in the form of either N-acetylcysteine (NAC) or β-mercaptoethanol, further supporting the notion that free thiols are a mechanism by which Se levels affect T cells (Hoffmann et al., 2010).
Consistent with our findings, in vivo NAC-treatment has been shown to decrease levels of allergic asthma. In particular, a mouse model involving OVA-challenges was combined with diesel exhaust particle exposure to generate allergic asthma in mice, which was inhibited by intraperitoneal injection of NAC (Li et al., 2009). Interestingly, the NAC was administered during the sensitization phase and not during the OVA-challenges in the lung. This suggests the addition of this reducing compound attenuated the initiation of Th2 responses, not the oxidative stress in the lungs. In a related study, T cells lacking selenoproteins exhibited increased levels of oxidative stress and decreased proliferative capacity, and addition of NAC restored their proliferative capacity (Shrimali et al., 2008). Studies utilizing human T cells from an individual with genetically impaired selenoprotein expression exhibited decreased proliferation when TCR-stimulated (Schoenmakers et al., 2010). The lymphocytes from this individual also had very low Txnrd activity and were unable to reduce exogenous H2O2, suggesting reduced antioxidant capacity.
T cells have a high requirement for reducing equivalents, and several lines of evidence suggest the reductive state of CD4+ T cells influences polarization during activation into different effector cell-types. For example, CD4+ T cells from mice deficient in the NADPH oxidase (NOX2−/− mice) exhibit increased Th1 cytokines upon activation compared to wild-type controls (Jackson et al., 2004). This suggests that a higher reductive state favors Th1 differentiation, which is consistent with our data involving higher Se leading to stronger Th1 differentiation. This is further supported by studies showing glutathione depletion in mice reduces Th1 responses, which also showed that antigen-presenting cells were important for this effect (Peterson et al., 1998). The reductive state of naive CD4+ T cells may affect thiol-based signals that are transmitted through redoxsensitive signaling molecules (Huang et al., 2011). There may also be effects of the redox tone on enzymes that influence the epigenetic state of the cells, which has been shown to strongly influence the polarization of T helper cells during activation and differentiation (Rothenberg and Zhang, 2011).
The poising of gene regions for rapid transcription is carried out by various epigenetic enzymes, which catalyze histone methylation, acetylation, and ADP-ribosylation, as well as DNA methylation. Importantly, the rate-limiting steps of several of these epigenetic enzymes are redox-dependent (Cyr and Domann, 2010). Some of these redox-sensitive enzymes have been shown to be affected by Se supplementation. For example, the enzyme responsible for catalyzing DNA methylation (DNA methylase) is sensitive to inhibition by Se supplementation (Cox and Goorha, 1986). Because inhibition of DNA methylation leads to a more permissive state for transcription, this suggests that increasing Se intake may lead to increased permissiveness of certain gene regions. A key selenoenzyme in mediating these effects may be Txnrd1, which produces higher levels of reduced Txn-1 in CD4+ T cells from Se supplemented mice (Hoffmann et al., 2010). Txnrd1 converts oxidized Txn-1 to reduced Txn-1 in the cytoplasm and nucleus. This is important because Txn-1 has been linked to regulation of H3K9 tri-methylation and -acetylation and to production of the cytokine, IL-2, which is involved in T cell proliferation and Th1 differentiation (Ahsan et al., 2006; Perrone et al., 2009). Thus, levels of free thiols and Txn-1 as well as other redox intermediates may represent important mechanisms by which Se supplementation affects epigenetic events in naive CD4+ T cells. Consistent with this notion, Se supplementation regulates the earliest detectable gene transcription events triggered by CD4+ T cell activation through redox intermediates (Hoffmann et al., 2010). A recent study in rats demonstrated that increasing dietary Se decreased global genomic DNA methylation in liver and colon mucosa, with specific genes particularly sensitive to this effect (Zeng and Combs, 2007). Furthermore, Se and other dietary factors have been shown to affect epigenetic mechanisms related to cancer (Barnett et al., 2010). Preliminary studies in our laboratory suggest that increasing dietary Se leads to a more permissive state in the Th1 master regulator, T-bet, but it has not yet been determined how specific this effect is for Th1 gene regions. We are currently investigating whether these epigenetic effects are a major mechanism by which dietary Se influences T cell activation and differentiation. The overall relationship between Se levels and T cell differentiation leading to different asthma outcomes is illustrated in Fig. 1.
Figure 1.
Effects of dietary Se levels on T helper cells and asthma. Low, adequate, or high dietary Se can poise naive CD4+ T helper cells toward a Th2-bais (low Se), a flexible differentiation state (adequate Se), or a Th1-bias (high Se). Upon allergen challenge, the strongest asthma response arises in adequate Se conditions.
7. Conclusions and future directions
Does Se intake affect asthma? The studies in mice suggest that dietary Se can dramatically influence allergic asthma, but studies in humans have been inconclusive. This speaks to the complexity of asthma in humans as well as the inherent problems involved in measuring cause-and-effect relationships between bioactive nutrients and multi-factorial diseases like asthma. Based on the findings to date, Se supplementation has not generally been recommended as a preventive or therapeutic modality for reducing asthma burden. Se supplementation may be better utilized to enhance the effects of other treatment methods. For example, allergen-specific immunotherapy (IT) is the only current immune-modulating treatment for asthma. The goal of IT is to divert T helper responses away from the Th2 responses that drive the disease process and enhance Th1/Treg responses (Hawrylowicz and O'Garra, 2005). While IT has proven effective for allergic conditions such as rhinitis and conjunctivitis, the efficacy of IT for treating allergic asthma has been less impressive (Bousquet, 1999). Thus, improving the immune-modulating effects of IT for asthma may require modifying or enhancing its ability to skew responses away from Th2 and toward Th1/Treg responses. Given the effects of Se supplementation on skewed T helper responses, it may provide the ideal means to augment IT therapy.
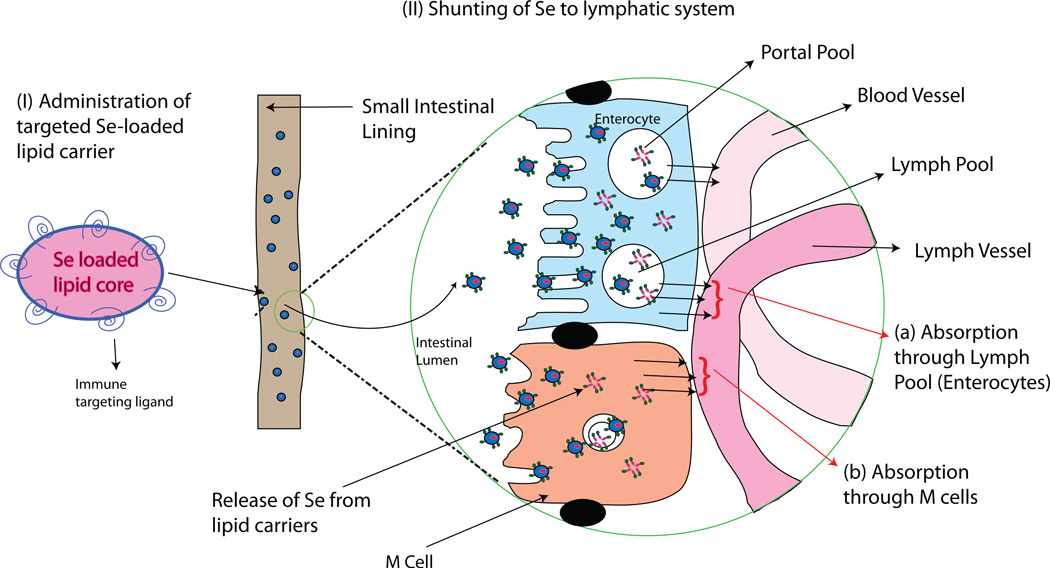
Before Se supplementation can be fully considered for treating asthma or other health disorders, issues must be addressed regarding the safety of long-term Se supplementation. Se supplementation has traditionally been carried out using oral ingestion of either sodium selenite, L-selenomethionine, or Se-enriched baker’s yeast. The form of Se that is used for supplementing human diets can be important not only for its effectiveness in enhancing Se status, but for inducing potentially adverse side-effects (Hatfield and Gladyshev, 2009). Results from the Selenium and Vitamin E Cancer Prevention Trial (SELECT) and other studies have suggested that Se supplementation may lead to increased risk for type-2 diabetes (Chen et al., 2003; Labunskyy et al., 2011; Lippman et al., 2009; McClung et al., 2004). Therefore, novel delivery systems that more selectively target the immune system could allow administration of a lower dosage of Se and decrease the associated risks. One approach may involve targeting the intestinal lymphatic regions, which have been routinely explored and used for site-specific lymphatic delivery of orally administered proteins, drugs, and vaccines (Aldwell et al., 2005; Ge et al., 2009; Xie et al., 2009). Given that the gastrointestinal tract is richly supplied with lymphoid tissues, formulations targeting these tissues may provide an effective means of delivering Se to the immune system (Fig. 2). Our laboratory is currently developing formulations to more selectively exert the immune-deviating effects of Se in order to safely reduce Th2-driven disorders like allergic asthma.
Figure 2.
Theoretical targeted delivery of Se to the immune system using novel approaches. Lipid vesicles may be constructed that contain Se in the core and immune-targeting ligands on the surface. When ingested, these lipid carriers may be taken up preferentially by M-cells and enterocytes. Once endocytosed by these cells, the Se is released from the core. Because M-cells and lymph pools lie above lymphatic vessels, Se will be shunted to lymphatic tissues and reach immune cells at higher levels compared to non-immune cells. This targeting of Se into lymphatics may allow lower overall Se concentration to be used for Se supplementation.
Acknowledgements
This work was supported by NIH grants R21AT004844 and G12RR003061. Thanks to Dr. Mahavir Chougule for his contributions to our collaborative work on selenium nanoparticles.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahsan MK, Masutani H, Yamaguchi Y, Kim YC, Nosaka K, Matsuoka M, Nishinaka Y, Maeda M, Yodoi J. Loss of interleukin-2-dependency in HTLV-I-infected T cells on gene silencing of thioredoxin-binding protein-2. Oncogene. 2006;25(15):2181–2191. doi: 10.1038/sj.onc.1209256. [DOI] [PubMed] [Google Scholar]
- Aldwell FE, Baird MA, Fitzpatrick CE, McLellan AD, Cross ML, Lambeth MR, Buchan GS. Oral vaccination of mice with lipid-encapsulated Mycobacterium bovis BCG: anatomical sites of bacterial replication and immune activity. Immunol Cell Biol. 2005;83(5):549–553. doi: 10.1111/j.1440-1711.2005.01369.x. [DOI] [PubMed] [Google Scholar]
- Allan K, Devereux G. Diet and asthma: nutrition implications from prevention to treatment. J Am Diet Assoc. 2011;111(2):258–268. doi: 10.1016/j.jada.2010.10.048. [DOI] [PubMed] [Google Scholar]
- Barnett M, Bermingham E, McNabb W, Bassett S, Armstrong K, Rounce J, Roy N. Investigating micronutrients and epigenetic mechanisms in relation to inflammatory bowel disease. Mutat Res. 2010;690(1-2):71–80. doi: 10.1016/j.mrfmmm.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Bleys J, Navas-Acien A, Laclaustra M, Pastor-Barriuso R, Menke A, Ordovas J, Stranges S, Guallar E. Serum selenium and peripheral arterial disease: results from the national health and nutrition examination survey 2003–2004. Am J Epidemiol. 2009;169(8):996–1003. doi: 10.1093/aje/kwn414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosl MR, Takaku K, Oshima M, Nishimura S, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp) Proc Natl Acad Sci U S A. 1997;94(11):5531–5534. doi: 10.1073/pnas.94.11.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet J. Specific immunotherapy in asthma. Allergy. 1999;54(Suppl 56):37–38. doi: 10.1111/j.1398-9995.1999.tb04438.x. [DOI] [PubMed] [Google Scholar]
- Burney P, Potts J, Makowska J, Kowalski M, Phillips J, Gnatiuc L, Shaheen S, Joos G, Van Cauwenberge P, van Zele T, Verbruggen K, van Durme Y, Derudder I, Wohrl S, Godnic-Cvar J, Salameh B, Skadhauge L, Thomsen G, Zuberbier T, Bergmann KC, Heinzerling L, Renz H, Al-Fakhri N, Kosche B, Hildenberg A, Papadopoulos NG, Xepapadaki P, Zannikos K, Gjomarkaj M, Bruno A, Pace E, Bonini S, Bresciani M, Gramiccioni C, Fokkens W, Weersink EJ, Carlsen KH, Bakkeheim E, Loureiro C, Villanueva CM, Sanjuas C, Zock JP, Lundback B, Janson C. A case-control study of the relation between plasma selenium and asthma in European populations: a GAL2EN project. Allergy. 2008;63(7):865–871. doi: 10.1111/j.1398-9995.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- Carneiro MF, Rhoden CR, Amantea SL, Barbosa F., Jr Low Concentrations of Selenium and Zinc in Nails are Associated with Childhood Asthma. Biol Trace Elem Res. 2011 May 24; doi: 10.1007/s12011-011-9080-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Chen X, Scholl TO, Leskiw MJ, Donaldson MR, Stein TP. Association of glutathione peroxidase activity with insulin resistance and dietary fat intake during normal pregnancy. J Clin Endocrinol Metab. 2003;88(12):5963–5968. doi: 10.1210/jc.2003-030544. [DOI] [PubMed] [Google Scholar]
- Cox R, Goorha S. A study of the mechanism of selenite-induced hypomethylated DNA and differentiation of Friend erythroleukemic cells. Carcinogenesis. 1986;7(12):2015–2018. doi: 10.1093/carcin/7.12.2015. [DOI] [PubMed] [Google Scholar]
- Curran JE, Jowett JB, Elliott KS, Gao Y, Gluschenko K, Wang J, Abel Azim DM, Cai G, Mahaney MC, Comuzzie AG, Dyer TD, Walder KR, Zimmet P, MacCluer JW, Collier GR, Kissebah AH, Blangero J. Genetic variation in selenoprotein S influences inflammatory response. Nat Genet. 2005;37(11):1234–1241. doi: 10.1038/ng1655. [DOI] [PubMed] [Google Scholar]
- Cyr AR, Domann FE. The Redox Basis of Epigenetic Modifications: From Mechanisms to Functional Consequences. Antioxid Redox Signal. 2011;15(2):551–589. doi: 10.1089/ars.2010.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Luis DA, Izaola O, Aller R, Armentia A, Cuellar L. [Antioxidant and fat intake in patients with polinic asthma] Med Clin (Barc) 2003;121(17):653–654. [PubMed] [Google Scholar]
- Devereux G, McNeill G, Newman G, Turner S, Craig L, Martindale S, Helms P, Seaton A. Early childhood wheezing symptoms in relation to plasma selenium in pregnant mothers and neonates. Clin Exp Allergy. 2007;37(7):1000–1008. doi: 10.1111/j.1365-2222.2007.02757.x. [DOI] [PubMed] [Google Scholar]
- Dittrich AM, Meyer HA, Krokowski M, Quarcoo D, Ahrens B, Kube SM, Witzenrath M, Esworthy RS, Chu FF, Hamelmann E. Glutathione peroxidase-2 protects from allergen-induced airway inflammation in mice. Eur Respir J. 2010;35(5):1148–1154. doi: 10.1183/09031936.00026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan JA, Breckler L, Hale J, Lehmann H, Franklin P, Lyons G, Ching SY, Mori TA, Barden A, Prescott SL. Associations between antioxidant status, markers of oxidative stress and immune responses in allergic adults. Clin Exp Allergy. 2006;36(8):993–1000. doi: 10.1111/j.1365-2222.2006.02539.x. [DOI] [PubMed] [Google Scholar]
- Dunstan JA, Breckler L, Hale J, Lehmann H, Franklin P, Lyons G, Ching SY, Mori TA, Barden A, Prescott SL. Supplementation with vitamins C, E, beta-carotene and selenium has no effect on anti-oxidant status and immune responses in allergic adults: a randomized controlled trial. Clin Exp Allergy. 2007;37(2):180–187. doi: 10.1111/j.1365-2222.2007.02657.x. [DOI] [PubMed] [Google Scholar]
- Flatt A, Pearce N, Thomson CD, Sears MR, Robinson MF, Beasley R. Reduced selenium in asthmatic subjects in New Zealand. Thorax. 1990;45(2):95–99. doi: 10.1136/thx.45.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES, Mannino DM, Redd SC. Serum antioxidant concentrations among US adults with self-reported asthma. J Asthma. 2004;41(2):179–187. doi: 10.1081/jas-120026075. [DOI] [PubMed] [Google Scholar]
- Garcia-Larsen V, Chinn S, Arts IC, Amigo H, Rona RJ. Atopy, wheeze and bronchial responsiveness in young Chilean adults. Do dietary antioxidants matter? Allergy. 2007;62(6):714–715. doi: 10.1111/j.1398-9995.2007.01357.x. [DOI] [PubMed] [Google Scholar]
- Gazdik F, Kadrabova J, Gazdikova K. Decreased consumption of corticosteroids after selenium supplementation in corticoid-dependent asthmatics. Bratisl Lek Listy. 2002;103(1):22–25. [PubMed] [Google Scholar]
- Ge W, Hu PZ, Huang Y, Wang XM, Zhang XM, Sun YJ, Li ZS, Si SY, Sui YF. The antitumor immune responses induced by nanoemulsion-encapsulated MAGE1-HSP70/SEA complex protein vaccine following different administration routes. Oncol Rep. 2009;22(4):915–920. doi: 10.3892/or_00000517. [DOI] [PubMed] [Google Scholar]
- Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4(4):355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282(5397):2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CH, Liu PJ, Hsia S, Chuang CJ, Chen PC. Role of certain trace minerals in oxidative stress, inflammation, CD4/CD8 lymphocyte ratios and lung function in asthmatic patients. Ann Clin Biochem. 2011;48(Pt 4):344–351. doi: 10.1258/acb.2011.010266. [DOI] [PubMed] [Google Scholar]
- Hasselmark L, Malmgren R, Unge G, Zetterstrom O. Lowered platelet glutathione peroxidase activity in patients with intrinsic asthma. Allergy. 1990;45(7):523–527. doi: 10.1111/j.1398-9995.1990.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Hatfield DL, Gladyshev VN. The Outcome of Selenium and Vitamin E Cancer Prevention Trial (SELECT) reveals the need for better understanding of selenium biology. Mol Interv. 2009;9(1):18–21. doi: 10.1124/mi.9.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5(4):271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- Hoffmann FW, Hashimoto AC, Shafer LA, Dow S, Berry MJ, Hoffmann PR. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J Nutr. 2010;140(6):1155–1161. doi: 10.3945/jn.109.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann PR, Jourdan-Le Saux C, Hoffmann FW, Chang PS, Bollt O, He Q, Tam EK, Berry MJ. A role for dietary selenium and selenoproteins in allergic airway inflammation. J Immunol. 2007;179(5):3258–3267. doi: 10.4049/jimmunol.179.5.3258. [DOI] [PubMed] [Google Scholar]
- Huang Z, Rose AH, Hoffmann PR. The Role of Selenium in Inflammation and Immunity: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid Redox Signal. 2011 Sep 28; doi: 10.1089/ars.2011.4145. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307(5708):430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- Ikeda RK, Miller M, Nayar J, Walker L, Cho JY, McElwain K, McElwain S, Raz E, Broide DH. Accumulation of peribronchial mast cells in a mouse model of ovalbumin allergen induced chronic airway inflammation: modulation by immunostimulatory DNA sequences. J Immunol. 2003;171(9):4860–4867. doi: 10.4049/jimmunol.171.9.4860. [DOI] [PubMed] [Google Scholar]
- Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5(8):818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- Johnson CC, Fordyce FM, Rayman MP. Symposium on 'Geographical and geological influences on nutrition': Factors controlling the distribution of selenium in the environment and their impact on health and nutrition. Proc Nutr Soc. 2010;69(1):119–132. doi: 10.1017/S0029665109991807. [DOI] [PubMed] [Google Scholar]
- Kadrabova J, Mad'aric A, Kovacikova Z, Podivinsky F, Ginter E, Gazdik F. Selenium status is decreased in patients with intrinsic asthma. Biol Trace Elem Res. 1996;52(3):241–248. doi: 10.1007/BF02789165. [DOI] [PubMed] [Google Scholar]
- Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3(12):984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- Kocyigit A, Armutcu F, Gurel A, Ermis B. Alterations in plasma essential trace elements selenium, manganese, zinc, copper, and iron concentrations and the possible role of these elements on oxidative status in patients with childhood asthma. Biol Trace Elem Res. 2004;97(1):31–41. doi: 10.1385/BTER:97:1:31. [DOI] [PubMed] [Google Scholar]
- Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300(5624):1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- Labunskyy VM, Lee BC, Handy DE, Loscalzo J, Hatfield DL, Gladyshev VN. Both Maximal Expression of Selenoproteins and Selenoprotein Deficiency Can Promote Development of Type 2 Diabetes-Like Phenotype in Mice. Antioxid Redox Signal. 2011;4(12):2327–2336. doi: 10.1089/ars.2010.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Takizawa H, Azuma A, Kohyama T, Yamauchi Y, Kawada T, Kudoh S, Sugawara I. The effects of oxidative stress induced by prolonged low-dose diesel exhaust particle exposure on the generation of allergic airway inflammation differ between BALB/c and C57BL/6 mice. Immunopharmacol Immunotoxicol. 2009;31(2):230–237. doi: 10.1080/08923970802383316. [DOI] [PubMed] [Google Scholar]
- Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr, Baker LH, Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM. Asthma and allergic inflammation. Cell. 2010;140(6):777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Berndt C, Holmgren A. Metabolism of selenium compounds catalyzed by the mammalian selenoprotein thioredoxin reductase. Biochim Biophys Acta. 2009;1790(11):1513–1519. doi: 10.1016/j.bbagen.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Maddox L, Schwartz DA. The pathophysiology of asthma. Annu Rev Med. 2002;53:477–498. doi: 10.1146/annurev.med.53.082901.103921. [DOI] [PubMed] [Google Scholar]
- McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A. 2004;101(24):8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie RC, Rafferty TS, Beckett GJ. Selenium: an essential element for immune function. Immunol Today. 1998;19(8):342–345. doi: 10.1016/s0167-5699(98)01294-8. [DOI] [PubMed] [Google Scholar]
- Meyer HA, Dittrich AM, Hamelmann E. Different isoforms of glutathione peroxidase cause opposing effects during the development of allergic asthma in mice. Antioxid Redox Signal. 2010;14(1):169–170. doi: 10.1089/ars.2010.3591. [DOI] [PubMed] [Google Scholar]
- Miller AL. The etiologies, pathophysiology, and alternative/complementary treatment of asthma. Altern Med Rev. 2001;6(1):20–47. [PubMed] [Google Scholar]
- Misso NL, Powers KA, Gillon RL, Stewart GA, Thompson PJ. Reduced platelet glutathione peroxidase activity and serum selenium concentration in atopic asthmatic patients. Clin Exp Allergy. 1996;26(7):838–847. [PubMed] [Google Scholar]
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2(12):933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Niskar AS, Paschal DC, Kieszak SM, Flegal KM, Bowman B, Gunter EW, Pirkle JL, Rubin C, Sampson EJ, McGeehin M. Serum selenium levels in the US population: Third National Health and Nutrition Examination Survey 1988–1994. Biol Trace Elem Res. 2003;91(1):1–10. doi: 10.1385/BTER:91:1:1. [DOI] [PubMed] [Google Scholar]
- Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta-analysis. J Allergy Clin Immunol. 2011;127(3):724–733. doi: 10.1016/j.jaci.2010.11.001. e721-730. [DOI] [PubMed] [Google Scholar]
- Omland O, Deguchi Y, Sigsgaard T, Hansen JC. Selenium serum and urine is associated to mild asthma and atopy. The SUS study. J Trace Elem Med Biol. 2002;16(2):123–127. doi: 10.1016/S0946-672X(02)80039-6. [DOI] [PubMed] [Google Scholar]
- Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9(7):775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- Park CS, Kim TB, Lee KY, Moon KA, Bae YJ, Jang MK, Cho YS, Moon HB. Increased oxidative stress in the airway and development of allergic inflammation in a mouse model of asthma. Ann Allergy Asthma Immunol. 2009;103(3):238–247. doi: 10.1016/S1081-1206(10)60188-3. [DOI] [PubMed] [Google Scholar]
- Perrone L, Devi TS, Hosoya K, Terasaki T, Singh LP. Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J Cell Physiol. 2009;221(1):262–272. doi: 10.1002/jcp.21852. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci U S A. 1998;95(6):3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qujeq D, Hidari B, Bijani K, Shirdel H. Glutathione peroxidase activity and serum selenium concentration in intrinsic asthmatic patients. Clin Chem Lab Med. 2003;41(2):200–202. doi: 10.1515/CCLM.2003.032. [DOI] [PubMed] [Google Scholar]
- Rayman MP. Dietary selenium: time to act. BMJ. 1997;314(7078):387–388. doi: 10.1136/bmj.314.7078.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MA, Hoffmann PR. The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci. 2009;66(15):2457–2478. doi: 10.1007/s00018-009-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl MA, Nel AE. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr Opin Allergy Clin Immunol. 2008;8(1):49–56. doi: 10.1097/ACI.0b013e3282f3d913. [DOI] [PubMed] [Google Scholar]
- Rothenberg EV, Zhang JA. T-Cell Identity and Epigenetic Memory. Curr Top Microbiol Immunol. 2011 Aug 10; doi: 10.1007/82_2011_168. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Powrie F. Emerging challenges in regulatory T cell function and biology. Science. 2007;317(5838):627–629. doi: 10.1126/science.1142331. [DOI] [PubMed] [Google Scholar]
- Schoenmakers E, Agostini M, Mitchell C, Schoenmakers N, Papp L, Rajanayagam O, Padidela R, Ceron-Gutierrez L, Doffinger R, Prevosto C, Luan J, Montano S, Lu J, Castanet M, Clemons N, Groeneveld M, Castets P, Karbaschi M, Aitken S, Dixon A, Williams J, Campi I, Blount M, Burton H, Muntoni F, O'Donovan D, Dean A, Warren A, Brierley C, Baguley D, Guicheney P, Fitzgerald R, Coles A, Gaston H, Todd P, Holmgren A, Khanna KK, Cooke M, Semple R, Halsall D, Wareham N, Schwabe J, Grasso L, Beck-Peccoz P, Ogunko A, Dattani M, Gurnell M, Chatterjee K. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J Clin Invest. 2010;120(12):4220–4235. doi: 10.1172/JCI43653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer U, Streckfuss F, Pelt P, Carlson BA, Hatfield DL, Kohrle J, Schomburg L. Hepatically derived selenoprotein P is a key factor for kidney but not for brain selenium supply. Biochem J. 2005;386(Pt 2):221–226. doi: 10.1042/BJ20041973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen SO, Newson RB, Henderson AJ, Emmett PM, Sherriff A, Cooke M. Umbilical cord trace elements and minerals and risk of early childhood wheezing and eczema. Eur Respir J. 2004;24(2):292–297. doi: 10.1183/09031936.04.00117803. [DOI] [PubMed] [Google Scholar]
- Shaheen SO, Newson RB, Rayman MP, Wong AP, Tumilty MK, Phillips JM, Potts JF, Kelly FJ, White PT, Burney PG. Randomised, double blind, placebo-controlled trial of selenium supplementation in adult asthma. Thorax. 2007;62(6):483–490. doi: 10.1136/thx.2006.071563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R, Woodman K, Crane J, Moyes C, Kennedy J, Pearce N. Risk factors for asthma symptoms in Kawerau children. N Z Med J. 1994;107(987):387–391. [PubMed] [Google Scholar]
- Shrimali RK, Irons RD, Carlson BA, Sano Y, Gladyshev VN, Park JM, Hatfield DL. Selenoproteins mediate T cell immunity through an antioxidant mechanism. J Biol Chem. 2008;283(29):20181–20185. doi: 10.1074/jbc.M802559200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires JE, Berry MJ. Eukaryotic selenoprotein synthesis: mechanistic insight incorporating new factors and new functions for old factors. IUBMB Life. 2008;60(4):232–235. doi: 10.1002/iub.38. [DOI] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19(3):281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Stone J, Hinks LJ, Beasley R, Holgate ST, Clayton BA. Reduced selenium status of patients with asthma. Clin Sci (Lond) 1989;77(5):495–500. doi: 10.1042/cs0770495. [DOI] [PubMed] [Google Scholar]
- Thomson CD. Assessment of requirements for selenium and adequacy of selenium status: a review. Eur J Clin Nutr. 2004;58(3):391–402. doi: 10.1038/sj.ejcn.1601800. [DOI] [PubMed] [Google Scholar]
- Usui T, Nishikomori R, Kitani A, Strober W. GATA-3 suppresses Th1 development by downregulation of Stat4 and not through effects on IL-12Rbeta2 chain or T-bet. Immunity. 2003;18(3):415–428. doi: 10.1016/s1074-7613(03)00057-8. [DOI] [PubMed] [Google Scholar]
- Usui T, Preiss JC, Kanno Y, Yao ZJ, Bream JH, O'Shea JJ, Strober W. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med. 2006;203(3):755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Hoffmann FW, Kumar M, Huang Z, Roe K, Nguyen-Wu E, Hashimoto AS, Hoffmann PR. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J Immunol. 2011:186. doi: 10.4049/jimmunol.1002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler K, Brigelius-Flohe R. Gastrointestinal glutathione peroxidase. Biofactors. 1999;10(2–3):245–249. doi: 10.1002/biof.5520100223. [DOI] [PubMed] [Google Scholar]
- Won HY, Sohn JH, Min HJ, Lee K, Woo HA, Ho YS, Park JW, Rhee SG, Hwang ES. Glutathione peroxidase 1 deficiency attenuates allergen-induced airway inflammation by suppressing Th2 and Th17 cell development. Antioxid Redox Signal. 2010;13(5):575–587. doi: 10.1089/ars.2009.2989. [DOI] [PubMed] [Google Scholar]
- Xie Y, Bagby TR, Cohen MS, Forrest ML. Drug delivery to the lymphatic system: importance in future cancer diagnosis and therapies. Expert Opin Drug Deliv. 2009;6(8):785–792. doi: 10.1517/17425240903085128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Combs GF., Jr Selenium as an anticancer nutrient: roles in cell proliferation and tumor cell invasion. J Nutr Biochem. 2008;19(1):1–7. doi: 10.1016/j.jnutbio.2007.02.005. [DOI] [PubMed] [Google Scholar]