Abstract
Mucosal surfaces are protected by a highly viscoelastic and adhesive mucus layer that traps most foreign particles, including conventional drug and gene carriers. Trapped particles are eliminated on the order of seconds to hours by mucus clearance mechanisms, precluding sustained and targeted drug and nucleic acid delivery to mucosal tissues. We have previously shown that polymeric coatings that minimize adhesive interactions with mucus constituents lead to particles that rapidly penetrate human mucus secretions. Nevertheless, a particular challenge in formulating drug-loaded mucus penetrating particles (MPP) is that many commonly used surfactants are either mucoadhesive, or do not facilitate efficient drug encapsulation. We tested a novel surfactant molecule for particle formulation composed of Vitamin E conjugated to 5 kDa polyethylene glycol (VP5k). We show that VP5k-coated poly(lactide-co-glycolide) (PLGA) nanoparticles rapidly penetrate human cervicovaginal mucus, whereas PLGA nanoparticles coated with polyvinyl alcohol or Vitamin E conjugated to 1 kDa PEG were trapped. Importantly, VP5k facilitated high loading of paclitaxel, a frontline chemo drug, into PLGA MPP, with controlled release for at least 4 days and negligible burst release. Our results offer a promising new method for engineering biodegradable, drug-loaded MPP for sustained and targeted delivery of therapeutics at mucosal surfaces.
Keywords: PEG, drug delivery, Vitamin E TPGS, mucin
1. Introduction
The eyes and the respiratory, gastrointestinal and cervicovaginal tracts are protected by a highly viscoelastic and adhesive mucus layer that traps most foreign particles, including conventional drug and gene nanocarriers [1–3]. Trapped particles are eliminated by different mechanisms of mucus clearance, which typically occur on the order of seconds to hours [4–5]. Thus, to achieve sustained and/or targeted drug and nucleic acid delivery to mucosal tissues, synthetic particles must rapidly penetrate mucus secretions [5–6]. Mucus penetrating particles (MPP) can be engineered by carefully tuning the surface properties of nanoparticles; in particular, a dense covalent coating of low molecular weight (MW) poly(ethylene glycol) (PEG) on polystyrene (latex) particles can effectively reduce their affinity to mucus constituents [7–8]. Reduced adhesion enables coated particles to diffuse rapidly in the interstitial fluid between mucus mesh fibers, without experiencing the bulk viscosity of mucus [9–10], at rates up to only a few-fold slower than they would diffuse through water, whereas uncoated particles are typically completely trapped [7–8, 11].
To stabilize emulsions and reduce particle aggregation during particle synthesis, storage and use, the surfaces of drug-loaded polymeric particles are usually coated with surfactants. Surfactants can influence particle size, morphology, encapsulation efficiency and drug release kinetics [12–15]. A particular challenge in formulating drug-loaded MPP is that many commonly used surfactants either yield particles that adhere to mucins (i.e. mucoadhesive particles), or do not provide efficient drug encapsulation. For example, poly(vinyl alcohol) (PVA) is one of the most widely used surfactants for nanoparticle-drug formulation, and provides high drug loading efficiency [16], but PVA-coated particles are likely mucoadhesive [17], perhaps due to hydrogen bonding between hydroxyl groups extending from the polymer backbone and mucin glycoproteins. Chitosan-coatings are also strongly mucoadhesive, perhaps due to a combination of electrostatic attraction, hydrogen bonding and hydrophobic effects [18]. One strategy to present a dense layer of low MW PEG on the surface of biodegradable MPP is to replace PVA, chitosan and other mucoadhesive surfactants commonly used in particle formulation with surfactants that contain a low MW PEG moiety. An increasingly adopted surfactant in the drug delivery community is Vitamin E-PEG1k conjugate (VP1k, commonly referred to as Vitamin E TPGS), prepared by esterifying the hydrophobic D-alpha-tocopheryl acid (i.e., Vitamin E) succinate with PEG (Figure 1) [19]. Here, we sought to test whether surfactants based on Vitamin E-PEG conjugates can facilitate the formulation of drug-loaded biodegradable MPP.
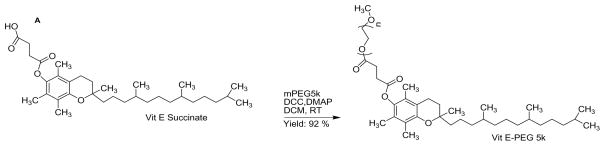
Figure 1.
Schematic of the conjugation of methoxy-PEG5k-OH to Vitamin E succinate to prepare Vitamin E-PEG5k conjugate (VP5k).
2. Methods
2.1. Synthesis of Vitamin E-PEG5k
PEG was conjugated to Vitamin E as described previously [20]. Briefly, Vitamin E Succinate (0.65 g, 1.0 equivalent; Sigma-Aldrich Corp., St. Louis, MO) was dissolved in DCM (dichloromethane; 20 mL) in 50 ml round type flask, and methoxy-PEG-OH (MW 5 kDa, 7.334 g, 1.2 equivalents; Sigma-Aldrich Corp., St. Louis, MO) was added afterwards. After all PEG content was dissolved, DMAP (4-dimethylaminopyridine; 15 mg, 0.1 equivalents) was added into the flask followed by addition of DCC (N,N′-dicyclohexylcarbodiimide, 0.278 g, 1.1 equivalents). The reaction mixture was stirred at room temperature overnight. Then, it was Buchner-filtered, and the filtrate was concentrated under reduced pressure to obtain crude product. To eliminate DCC and unreacted Vit E Succinate, both of which are insoluble in water, stock solution of crude product was dissolved at 5% (w/v) in ultrapure water, ultracentrifuged (75600 rcf, 20 min, 2 times; Avanti J-25 centrifuge, Beckman Coulter, Inc., Fullerton, CA), and further filtered (0.2 micron). The final yield of pure product was 92 %.
2.2. Characterization of Vitamin E-PEG5k
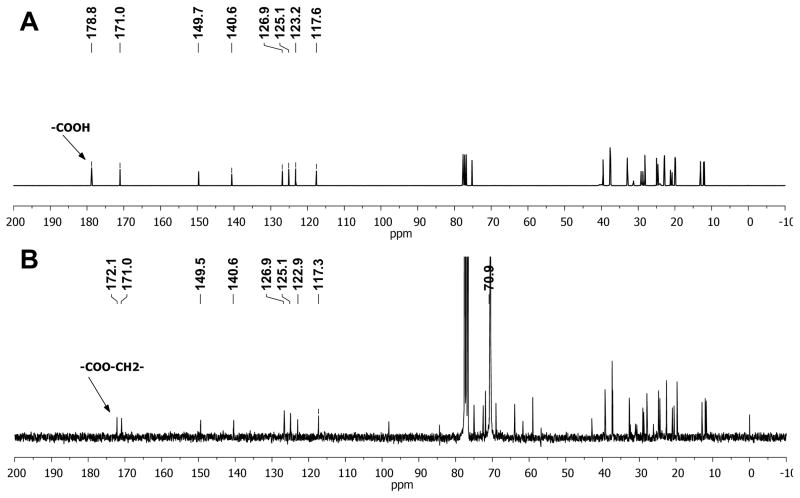
Conjugation of mPEG to Vit E Succinate was confirmed by 13-C-NMR (400 MHz, Bruker BioSpin Corporation, Billerica, MA). The carbonyl carbon of the COOH group of Vit E Succinate gave a signal at 178.8 ppm (Figure 2A), while the signal of same carbonyl carbon of Vit E-PEG5k compound shifted to 172.2 ppm due to the conjugation of mPEG to Vit E Succinate (Figure 2B). The signal of the second carbonyl carbon at 171.0 ppm, and aromatic carbons signals located between 115 ppm and 150 ppm, remained unchanged between reactant and product. Also, the -OCH2- groups of the mPEG unit in VitE-PEG5k gave a very intense signal at 70.9 ppm.
Figure 2.
13C-NMR spectrums of (A) Vitamin E Succinate and (B) VP5k confirm successful conjugation. Please refer to Methods for more details.
2.3. Preparation and characterization of doxorubicin-labeled PLGA nanoparticles
To visualize particle motions in cervicovaginal mucus, poly(lactide-co-glycolide) (PLGA; MW 11 kDa, 50:50; Alkermes Inc., Cambridge, MA) was fluorescently tagged with doxorubicin (Dox; NetQem, Durham, NC) [21]. Dox-conjugated nanoparticles were formulated by a solvent diffusion/nanoprecipitation technique. Briefly, 20 mg of the polymer was dissolved in 1 mL of acetonitrile, and added dropwise into 36 mL of 1.65% Vit E-PEG5k. After the volatile organic solvent was removed by stirring for 3 hr, the particles (PLGA/VP5k) were collected by centrifugation at 12096 rcf for 20 min, washed twice and resuspended in 0.4 mL of 0.5% Pluronic F127 (BASF, Florham Park, NJ) to reduce particle aggregation and further facilitate mucus penetration. Size and ζ-potential were determined by dynamic light scattering and laser Doppler anemometry, respectively, using a Zetasizer Nano ZS90 (Malvern Instruments, Southborough, MA). Size measurements were performed at 25°C at a scattering angle of 90°. PLGA nanoparticles coated with Vit E-PEG1k (Eastman Chemical Co., Kingsport, TN) (PLGA/VP1k) were formulated by the same methodology as mentioned above.
2.4. Preparation and characterization of paclitaxel-encapsulated PLGA nanoparticles
Briefly, 20 mg of the PLGA and 4 mg paclitaxel (LC Laboratories, Woburn, MA) were dissolved in 1 mL of acetonitrile, and added dropwise into 36 mL of 1.1% Vit E-PEG5k. After the volatile organic solvent was removed by stirring for 3 hr in a chemical fume hood, the particles were collected by centrifugation at 12096 rcf for 20 min, washed twice and resuspended in 0.2 mL of ultrapure water. The particle suspension was incubated with 200 μL of 1% F127 for 30 min. We set aside 5 μL of particle suspension for the measurement of hydrodynamic size and surface charge, performed as described above. To measure drug loading, paclitaxel-loaded as well as blank particles were frozen in liquid nitrogen, lyophilized at −50 °C, dissolved in acetonitrile and characterized for paclitaxel content by HPLC equipped with a C18 reverse phase column (5 μm, 4.6×250 mm; Varian Inc., Santa Clara, CA). F127-coated paclitaxel-loaded PLGA nanoparticles were prepared similarly, with F127 used as the aqueous phase instead of VP5k, followed by washing with F127 instead water. The particle morphologies of both particle types were determined using scanning electron microscopy (JEOL JSM-6700F, Peabody, MA). To measure release kinetics, particles were suspended in phosphate buffered saline (PBS, pH 7.4) and incubated at 37 °C. Supernatant was collected at predetermined intervals, and the drug concentration measured by HPLC, as described above.
2.5. Preparation and characterization of PEG-coated latex nanoparticles
Latex beads densely coated with PEG were prepared by conjugating methoxy-PEG-amine (MW 2 kDa, Nektar Therapeutics, San Carlos, CA) to fluorescent carboxyl-modified polystyrene particles (Molecular Probes, Eugene, OR; ~200 nm) as described previously [7–8]. Size and ζ-potential were measured as described above (Table 1).
Table 1.
Characterization of nanoparticles size and ζ-potential.
| Particle | Diameter [nm] | ζ- Potential [mV] |
|---|---|---|
| PS (Uncoated) | 217 ± 5 | −59 ± 4 |
| PLGA (Uncoated) | 110 ± 4 | −50 ± 2 |
| PLGA/PVA | 141 ± 9 | −1 ± 1 |
| PLGA/VP1k | 215 ± 18 | −19 ± 3 |
| PLGA/VP5k | 271 ± 10 | −8 ± 1 |
| PS-PEG | 232 ± 7 | −2 ± 1 |
2.6. Collection of human cervicovaginal mucus (CVM)
CVM was collected as previously described [7, 22]. Briefly, undiluted cervicovaginal secretions from women with normal vaginal flora were obtained using a self-sampling menstrual collection device following a protocol approved by the Institutional Review Board of the Johns Hopkins University. The device was inserted into the vagina for ~30 s, removed, and placed into a 50 mL centrifuge tube. Samples were centrifuged at 210 rcf for 2 min to collect the mucus secretions.
2.7. Multiple particle tracking
Particle transport rates were measured by analyzing trajectories of fluorescent Dox-labeled PLGA nanoparticles made with VP5k, VP1k and PVA surfactants, as well as yellow-green or red fluorescent uncoated COOH-modified (PS-COOH; negative control) or PEG-coated (PS-PEG; positive control) polystyrene particles. Particle motions were recorded using a silicon-intensified target camera (VE-1000, Dage-MTI, Michigan, IN) mounted on an inverted epifluorescence microscope (Zeiss, Thornwood, NY) equipped with a 100x oil-immersion objective (N.A., 1.3) and the appropriate filters. Experiments were carried out in custom-made chamber slides, where diluted particle solutions (~1010 particles/mL) were added to 20 μL of fresh mucus to a final concentration of 3 % v/v and incubated at 37°C for 2 hr before microscopy. Trajectories of n ≥ 110 particles were analyzed for each experiment, and at least three independent experiments were performed for each condition. Movies were captured with MetaMorph software (Molecular Devices Inc., Downingtown, PA) at a temporal resolution of 66.7 ms for 20 s. The tracking resolution was 10 nm, as determined by tracking the displacements of particles immobilized with a strong adhesive [23]. The coordinates of nanoparticle centroids were transformed into time-averaged mean square displacement (MSD), calculated as <Δr2(τ)> = [x(t+τ) − x(t)]2 + [y(t+τ) − y(t)]2 [24], where x and y represent the nanoparticle coordinates at a given time and τ is the time scale or time lag. Effective diffusivity was calculated as Deff = MSD/(4τ), for 2D particle tracking. Distributions of MSDs and effective diffusivities were calculated from this data, as demonstrated previously [7–8].
3. Results & Discussion
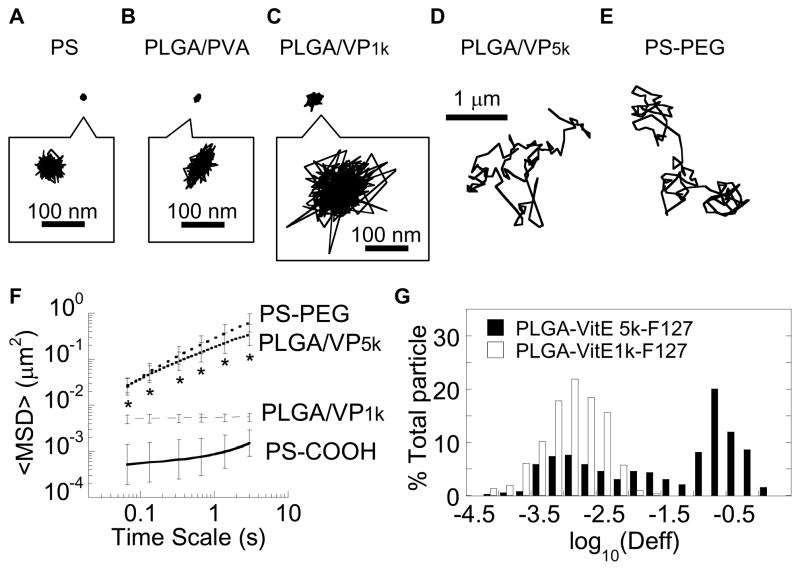
VP1k is a Generally Regarded As Safe (GRAS) excipient approved by the FDA for use in a variety of oral, topical and ophthalmic dosage forms with reduced toxicity [25–26]. It has also been increasingly used in nanoparticle formulations to provide greater colloidal stability and improved circulation time and/or bioavailability due to the presence of a surface PEG coating [19, 27–30]. We recently discovered that PEG coatings with MW as low as ~1 kDa, achieved by coating polymeric nanoparticles with Pluronic® P103, an ABA triblock copolymer with two ~1 kDa PEG chains flanking a ~3.5 kDa polypropylene oxide segment, can effectively reduce the mucoadhesion of nanoparticles [31]. To test whether coating nanoparticles with VP1k may also reduce mucoadhesion due to its comparable PEG MW, we formulated poly(lactide-co-glycolide) (PLGA) nanoparticles by nanoprecipitation with VP1k in the aqueous phase (PLGA/VP1k). The VP1k coating on the surface of the PLGA/VP1k nanoparticles was confirmed by their markedly less negative surface charge (−19 ± 3 mV) compared to the highly negative surface charge of uncoated PLGA nanoparticles (−50 ± 4 mV; Table 1). We assessed the movement of PLGA/VP1k nanoparticles in fresh human cervicovaginal mucus (CVM), collected from donors with healthy vaginal flora, using multiple particle tracking [23–24]. Despite the presence of VP1k on the particle surface, PLGA/VP1k nanoparticles were strongly trapped in human CVM to nearly the same extent as uncoated polystyrene (PS-COOH) nanoparticles and PLGA nanoparticles coated with PVA (Figure 3A–C, Supplementary Videos 1–3).
Figure 3.
Effect of surfactants on the transport of PLGA particles in fresh human cervicovaginal mucus. Representative traces, for particles with an effective diffusivity within one standard error of the mean (SEM), of (A) mucoadhesive, uncoated polystyrene particles (PS-COOH; negative control), (B) PLGA particles coated with PVA (PLGA/PVA), (C) PLGA particles coated with Vitamin E TGPS (PLGA/VP1k), (D) PLGA particles coated with a novel surfactant synthesized by conjugating methoxy-PEG5k-OH to Vitamin E succinate (PLGA/VP5k), and (E) polystyrene particles densely conjugated with 2 kDa PEG (PS-PEG), known to be muco-inert (positive control). (F) Ensemble-averaged geometric mean square displacements (<MSD>) of PLGA/VP5k, PLGA/VP1k, PS-COOH and PS-PEG as a function of time scale. Error bars represent SEM. * denotes statistically significant difference for PLGA/VP5k across all time scales compared to PS-COOH and PLGA/VP1k (p < 0.01) (G) Distributions of the logarithms of individual particle effective diffusivities (Deff) at a time scale of 1 s for PLGA/VP5k and PLGA/VP1k particles.
Since we previously found PEG as low as ~1 kDa in MW can still provide an effective non-mucoadhesive coating [31], we hypothesized that the immobilization of PLGA/VP1k nanoparticles in CVM must be due to inadequate PEG shielding of the hydrophobic PLGA core by VP1k. To increase the PEG coverage, we conjugated a 5 kDa PEG to activated Vitamin E succinate (VP5k) (Figure 1). The choice of 5 kDa PEG was based on our previous finding that 2–5 kDa PEG coatings mediated rapid particle penetration in mucus, whereas 10 kDa PEG coatings did not, presumably due to interpenetration of longer PEG chains into the mucin mesh network [8]. Successful conjugation of PEG to Vitamin E was confirmed by 13C-NMR (Figure 2). We prepared VP5k-coated PLGA nanoparticles (PLGA/VP5k) using the same nanoprecipitation method as PLGA/VP1k. In comparison to PLGA/VP1k, PLGA/VP5k nanoparticles exhibited a much more neutral surface charge, suggesting the VP5k coating afforded a greater density of surface PEG coverage (Table 1).
We again performed video microscopy to record the motions of PLGA/VP5k nanoparticles in mucus, and found that they rapidly penetrated CVM, as reflected by their diffusive, Brownian (i.e., highly mobile) time-lapse traces (Figure 3D, Supplementary Video 4). The trajectories were similar to those of similarly sized, diffusive PEG-coated polystyrene (PS-PEG) particles in the same mucus samples (Figure 3E, Supplementary Video 5). In comparison, PS-COOH particles and PVA-coated PLGA particles (PLGA/PVA), which served as negative controls, were trapped. We used multiple particle tracking, a technique that allows the motions of hundreds of individual nanoparticles to be tracked, to quantify particle diffusion rates in the form of time-scale dependent ensemble mean squared displacements (<MSD>). The <MSD> of PLGA/VP5k nanoparticles in CVM was ~210 and ~33-fold higher than that for PS-COOH and PLGA/VP1k nanoparticles, respectively, at a time scale of 1s (p < 0.01; Figure 3F). The rapid transport of PLGA/VP5k nanoparticles was also reflected by the slope, α, of log-log plots of MSD versus time scale (α = 1 represents unobstructed Brownian transport, whereas increased obstruction to particle movement is reflected by a decrease in α): the average α was 0.64 for PLGA/VP5k nanoparticles compared to 0.79, 0.31 and 0.21 for PS-PEG, PS-COOH and PLGA/VP1k nanoparticles, respectively. The fastest 50% of PLGA/VP5k nanoparticles penetrated CVM with an average speed only 7-fold reduced compared to their theoretical speed in pure water, while the fastest 50% of PLGA/VP1k was slowed on average ~900-fold compared to in water (Figure 3G).
Based on the comparable speeds in CVM of PLGA/VP5k nanoparticles and PS-PEG nanoparticles, the surface-adsorbed, non-covalent VP5k coating appeared to resist mucoadhesion to the same extent as PEG coatings generated by covalent conjugation under harsh conditions (vortex and sonication) for prolonged durations (overnight). In contrast, neither co-precipitation of PLGA and PEG nor incubation of PLGA particles with PEG produced mobile particles in several independent mucus samples (data not shown). Our findings highlight a number of potential advantages of using VP5k-coated particles for drug delivery applications. An important criterion for a suitable surfactant for the formulation of biodegradable MPP drug carriers is efficient encapsulation of therapeutics. The elimination of post-formulation PEGylation is expected to markedly improve the drug loading and encapsulation efficiency of cargo therapeutics into biodegradable MPP drug carriers. In addition, the non-covalent adsorption of VP5k onto particle surfaces can likely be used to coat a wide range of polymeric core materials; materials with optimal degradation kinetics and polymer-drug affinity can therefore be chosen to achieve tailored release profiles and high encapsulation efficiencies for a wide array of cargo therapeutics. Although we are not aware of safety studies of VP1k in the human lung or vagina, VP1k has been incorporated into various oral, topical and ophthalmic dosage forms with no signs of acute toxicity; we thus expect VP5k to likely be safe for mucosal drug delivery. Finally, our method involves only incorporation of VP5k in the aqueous phase of the typical solvent diffusion formulation method, a simple process that may accelerate economical and scalable translational development of the MPP technology.
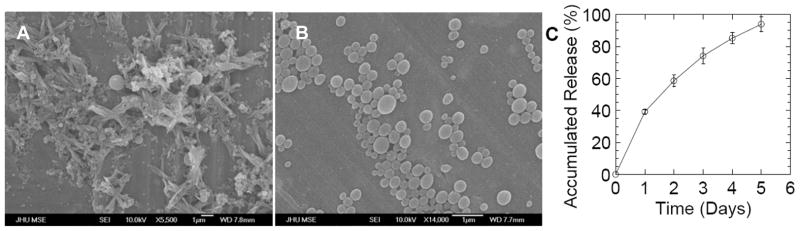
As a proof of concept to demonstrate the PLGA/VP5k nanoparticle platform can facilitate high drug loading levels, we encapsulated paclitaxel, a widely used anti-neoplastic agent that stabilizes microtubules and arrests tumor cells in the G2/M cell cycle phase [32]. We first prepared pactlitaxel-loaded particles by co-precipitation of paclitaxel and PLGA using 1% Pluronic F127 as the sole surfactant in the aqueous phase (PLGA/Paclitaxel/F127), a process which we have shown to generate MPP [31]. Electron micrographs of PLGA/Paclitaxel/F127 particles showed extensive formation of crystalline structures on the exterior of the spherical particles (presumably paclitaxel crystals formed due to low water solubility [33]), indicating poor encapsulation (Figure 4A). Particles prepared without surfactants also exhibited similar crystalline structures outside of particles (data not shown). In contrast, paclitaxel-loaded particles prepared with VP5k (PLGA/Paclitaxel/VP5k) were free of any visible crystals and exhibited uniform, smooth and nonporous surfaces (Figure 4B). The paclitaxel loading was 7.9 ± 0.5% (weight of paclitaxel to weight of polymer/surfactant) into particles with an average diameter of 245 ± 14 nm. These particles exhibited minimal burst effect, followed by sustained release for ~5 days (Figure 4C).
Figure 4.
Characterization of paclitaxel-encapsulated polymeric particles. (A) SEM images of PLGA particles prepared with a commonly used surfactant (Pluronic F127) show extensive paclitaxel crystal formation due to poor encapsulation of paclitaxel into the particles. PLGA particles prepared without surfactants exhibit similar drug crystals (data not shown). (B) SEM images of PLGA particles prepared with VP5k surfactant show no trace of paclitaxel crystals in solution. (C) Release of paclitaxel from PLGA/VP5k particles at 37°C in PBS.
In summary, VP5k is a novel surfactant for polymer formulation that simultaneously enables several highly desirable features for a biodegradable MPP drug delivery platform: (1) rapid penetration of undiluted human mucus; (2) good nanoparticle dispersity, low porosity and a smooth surface; (3) high loading of a small molecule drug (paclitaxel); and (4) sustained release of the drug over several days with minimal burst. The same features cannot easily be simultaneously achieved using conventional surfactants, such as PVA or VP1k. Given that human CVM possesses biochemical and rheological properties similar to those of mucus fluids derived from the eyes, nose, lungs, gastrointestinal tract and more [1–2, 5], we expect the VP5k coating to also facilitate rapid particle penetration at other mucosal surfaces. Additional surfactants with functional characteristics similar to VP5k may be generated by conjugating PEG or other non-mucoadhesive polymers of an appropriate MW to hydrophobic or charged molecules. We anticipate the use of VP5k to extend beyond mucosal drug delivery applications, since denser PEG coatings are expected to further improve colloidal stability in other biological fluids, as well as prolong the circulation time of nanoparticles administered intravenously.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grants (R21HL089816, R01CA140746 and R21AI079740), the TUBITAK 2214 Program and OYP (O.M.), a Croucher Foundation Fellowship (S.K.L.), and NSF Graduate Research Fellowships (L.E. and Y.Y.W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Conflict of Interest. The mucus penetrating particle technology described in this publication is being developed by Kala Pharmaceuticals. Dr. Hanes is co-founder and serves on the Board of Directors of Kala. Dr. Hanes owns company stock, which is subject to certain restrictions under University policy. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. Dr. Lai serves as an advisor of Kala and owns company stock, which is subject to restrictions managed by the University of North Carolina in accordance with its conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lai SK, Wang YY, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv Drug Deliv Rev. 2009;61:86–100. doi: 10.1016/j.addr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cone R. Barrier properties of mucus. Adv Drug Deliv Rev. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Csaba N, Garcia-Fuentes M, Alonso MJ. Nanoparticles for nasal vaccination. Adv Drug Deliv Rev. 2009;61:140–157. doi: 10.1016/j.addr.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cu Y, Saltzman WM. Drug delivery: Stealth particles give mucus the slip. Nat Mater. 2009;8:11–13. doi: 10.1038/nmat2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci USA. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang YY, Lai SK, Suk JS, Pace A, Cone R, Hanes J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew Chem Int Ed Engl. 2008;47:9726–9729. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai SK, Wang YY, Hida K, Cone R, Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc Natl Acad Sci U S A. 107:598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cu Y, Saltzman WM. Controlled Surface Modification with Poly(ethylene)glycol Enhances Diffusion of PLGA Nanoparticles in Human Cervical Mucus. Mol Pharm. 2008;6:173–181. doi: 10.1021/mp8001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang BC, Dawson M, La SK, Wang YY, Suk JS, Yang M, Zeitlin P, Boyle MP, Fu J, Hanes J. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc Natl Acad Sci USA. 2009;106:19268–19273. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manchanda R, Fernandez-Fernandez A, Nagesetti A, McGoron AJ. Preparation and characterization of a polymeric (PLGA) nanoparticulate drug delivery system with simultaneous incorporation of chemotherapeutic and thermo-optical agents. Colloid Surface B. 2010;75:260–267. doi: 10.1016/j.colsurfb.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 13.Mitra A, Lin S. Effect of surfactant on fabrication and characterization of paclitaxel-loaded polybutylcyanoacrylate nanoparticulate delivery systems. J Pharm Pharmacol. 2003;55:895–902. doi: 10.1211/0022357021341. [DOI] [PubMed] [Google Scholar]
- 14.Coombes AG, Yeh MK, Lavelle EC, Davis SS. The control of protein release from poly(DL-lactide co-glycolide) microparticles by variation of the external aqueous phase surfactant in the water-in oil-in water method. J Control Release. 1998;52:311–320. doi: 10.1016/s0168-3659(98)00006-6. [DOI] [PubMed] [Google Scholar]
- 15.Rosa GD, Iommelli R, La Rotonda MI, Miro A, Quaglia F. Influence of the co-encapsulation of different non-ionic surfactants on the properties of PLGA insulin-loaded microspheres. J Control Release. 2000;69:283–295. doi: 10.1016/s0168-3659(00)00315-1. [DOI] [PubMed] [Google Scholar]
- 16.Shakesheff KM, Evora C, Soriano II, Langer R. The Adsorption of Poly(vinyl alcohol) to Biodegradable Microparticles Studied by X-Ray Photoelectron Spectroscopy (XPS) J Colloid Interface Sci. 1997;185:538–547. doi: 10.1006/jcis.1996.4637. [DOI] [PubMed] [Google Scholar]
- 17.Peppas NA, Mongia NK. Ultrapure poly(vinyl alcohol) hydrogels with mucoadhesive drug delivery characteristics. European Journal of Pharmaceutics and Biopharmaceutics. 1997;43:51–58. [Google Scholar]
- 18.Prego C, Torres D, Alonso MJ. The potential of chitosan for the oral administration of peptides. Exp Opin Drug Deliv. 2005;2:843–854. doi: 10.1517/17425247.2.5.843. [DOI] [PubMed] [Google Scholar]
- 19.Mu L, Feng SS. Vitamin E TPGS used as emulsifier in the solvent evaporation/extraction technique for fabrication of polymeric nanospheres for controlled release of paclitaxel (Taxol (R)) J Control Release. 2002;80:129–144. doi: 10.1016/s0168-3659(02)00025-1. [DOI] [PubMed] [Google Scholar]
- 20.Collnot EM, Baldes C, Wempe MF, Hyatt J, Navarro L, Edgar KJ, Schaefer UF, Lehr CM. Influence of vitamin E TPGS poly(ethylene glycol) chain length on apical efflux transporters in Caco-2 cell monolayers. J Control Release. 2006;111:35–40. doi: 10.1016/j.jconrel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Yoo HS, Oh JE, Lee KH, Park TG. Biodegradable nanoparticles containing doxorubicin-PLGA conjugate for sustained release. Pharm Res. 1999;16:1114–1118. doi: 10.1023/a:1018908421434. [DOI] [PubMed] [Google Scholar]
- 22.Boskey ER, Moench TR, Hees PS, Cone RA. A self-sampling method to obtain large volumes of undiluted cervicovaginal secretions. Sex Transm Dis. 2003;30:107–109. doi: 10.1097/00007435-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Apgar J, Tseng Y, Fedorov E, Herwig MB, Almo SC, Wirtz D. Multiple-particle tracking measurements of heterogeneities in solutions of actin filaments and actin bundles. Biophys J. 2000;79:1095–1106. doi: 10.1016/S0006-3495(00)76363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suh J, Dawson M, Hanes J. Real-time multiple-particle tracking: applications to drug and gene delivery. Adv Drug Deliv Rev. 2005;57:63–78. doi: 10.1016/j.addr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Inactive ingredient search for approved drug products, “tocophersolan”. U.S. Food and Drug Administration; 2011. [Google Scholar]
- 26.Constantinides PP, Han J, Davis SS. Advances in the use of tocols as drug delivery vehicles. Pharm Res. 2006;23:243–255. doi: 10.1007/s11095-005-9262-9. [DOI] [PubMed] [Google Scholar]
- 27.Dong X, Mattingly CA, Tseng M, Cho M, Adams VR, Mumper RJ. Development of new lipid-based paclitaxel nanoparticles using sequential simplex optimization. Eur J Pharm Biopharm. 2009;72:9–17. doi: 10.1016/j.ejpb.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mu L, Feng SS. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin E TPGS. J Control Release. 2003;86:33–48. doi: 10.1016/s0168-3659(02)00320-6. [DOI] [PubMed] [Google Scholar]
- 29.Zhao L, Feng SS. Enhanced oral bioavailability of paclitaxel formulated in vitamin E-TPGS emulsified nanoparticles of biodegradable polymers: in vitro and in vivo studies. J Pharm Sci. 2010;99:3552–3560. doi: 10.1002/jps.22113. [DOI] [PubMed] [Google Scholar]
- 30.Ke WT, Lin SY, Ho HO, Sheu MT. Physical characterizations of microemulsion systems using tocopheryl polyethylene glycol 1000 succinate (TPGS) as a surfactant for the oral delivery of protein drugs. J Control Release. 2005;102:489–507. doi: 10.1016/j.jconrel.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 31.Yang M, Lai SK, Wang YY, Zhong W, Happe C, Zhang M, Fu J, Hanes J. Biodegradable Nanoparticles Composed Entirely of Safe Materials that Rapidly Penetrate Human Mucus. Angew Chem Int Ed Engl. 2011;50:2597–2600. doi: 10.1002/anie.201006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhalla KN. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–9086. doi: 10.1038/sj.onc.1207233. [DOI] [PubMed] [Google Scholar]
- 33.Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235:179–192. doi: 10.1016/s0378-5173(01)00986-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.