Abstract
Percutaneous transluminal angioplasty with stent implantation is used to dilate of arteries narrowed by atherosclerotic plaques and to revascularize coronary arteries occluded by atherothrombosis in myocardial infarction. Commonly applied drug-eluting stents release anti-proliferative or anti-inflammatory agents to reduce the incidence of in-stent stenosis. However, these stents may lead to in-stent stenosis and increase the rate late stent thrombosis, an obstacle to optimal revascularization possibly related to endothelial recovery. Here we examined the contribution of neutrophils and neutrophilic granule proteins to arterial healing after injury. We found that neutrophil-born cathelicidin (mouse CRAMP, human LL-37) promoted re-endothelization and thereby limited neointima formation after stent implantation. We then translated these findings, generating a neutrophil-instructing biofunctionalized miniaturized Nitinol stent coated with LL-37. This stent reduced in-stent stenosis in a mouse model of atherosclerosis, suggesting that LL-37 may promote vascular healing after interventional therapy.
Introduction
Progressive atherosclerosis causing luminal narrowing and obstruction require mechanical re-vascularization, which involves severe arterial damage and an inflammatory response leading to restenosis. Implantation of drug-eluting stents reduces the incidence of restenosis with a favourable safety profile and lower cost than surgical procedures (1). Clinical data have implied an increased risk of late-stent thrombosis when using drug-eluting stents as compared to bare-metal stents (2). This may partially be attributable to impaired endothelial recovery, however, the link between re-endothelialization and late-stent thrombosis is mostly based on associative evidence from autopsy and angioscopy studies. Coating with polymers e.g. poly(ethylene glycol) prevents adhesion of serum proteins and platelets to reduce in-stent thrombosis (3). Novel concepts of biofunctionalization may increase the potential to further improve treatment of in-stent restenosis and thrombosis.
The initial inflammatory response to arterial injury is characterized by early influx of neutrophils (4). These immune cells contribute to the regulation of the inflammatory cascade by discharging preformed granule proteins, e.g. azurocidin, α-defensins or LL-37, which are crucial mediators of neutrophil-dependent activities in inflammation (5,6). These secretory proteins are potent activators of antigen-presenting cells but they also activate endothelial cells (ECs) inducing permeability changes and neovascularization (7,8).
After arterial damage, re-endothelialization controls inflammation and smooth muscle cell (SMC) accumulation, thus representing a crucial mechanism to limit neointima formation. Endothelial regeneration primarily relies on migration and proliferation of ECs from adjacent vascular segments. In addition, angiogenic early outgrowth cells (EOCs) can be recruited to sites of injury for accelerating re-endothelialization (9,10). Notably, infusion of EOCs can attenuate neointimal hyperplasia after arterial injury (11,12), instigating EOC-based strategies to reduce restenosis. These include elution of cyclic RGD peptides or CXCR2 ligands, e.g. NAP-2, which can recruit EOCs to the stent surface or sites of injury (13,14).
Here we examined the involvement of neutrophil-derived granule proteins in arterial healing. We identified a prominent role for neutrophil-borne cathelicidin (mouse CRAMP, human LL-37) in promoting re-endothelialization and limiting neointima formation. Consequently, we translated these findings into a biofunctionalized stent coated with LL-37, and demonstrate that a neutrophil-instructing device can reduce in-stent stenosis in mice.
Results
Neutrophil-derived cathelicidin limits neointima formation
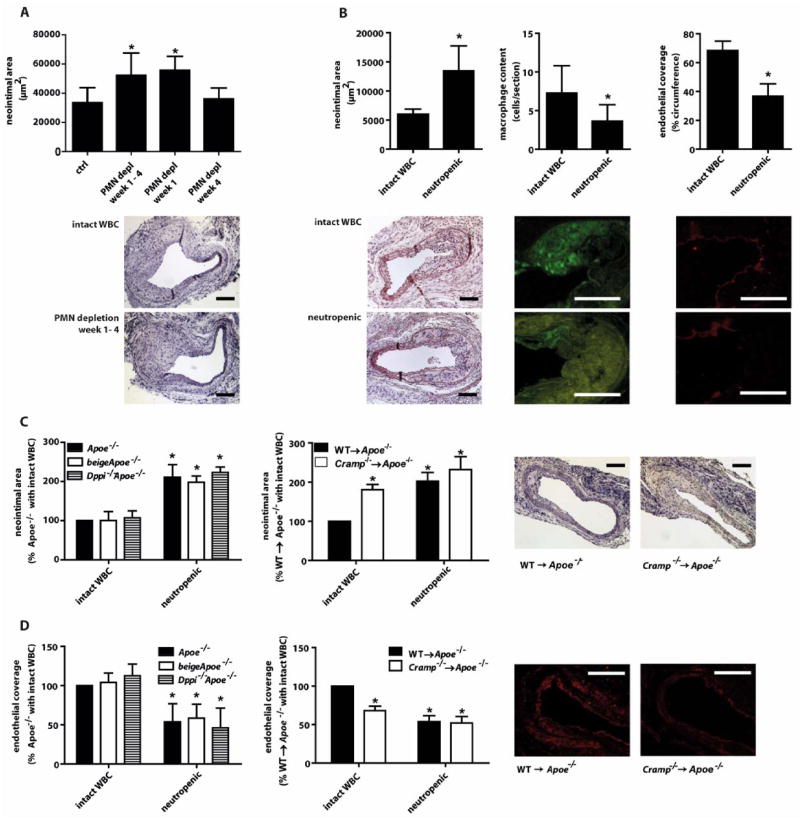
To address a role of neutrophils in inflammatory processes after arterial injury, we studied neointima formation in atherosclerosis-prone Apoe−/− mice with intact white blood cell count (WBC) or neutropenia (fig. 1A) 4 weeks after wire-injury. Neutrophil depletion (table S1) significantly increased neointima formation, suggesting a beneficial function of neutrophils in arterial healing. To dissect stage-dependent effects, we depleted neutrophils during the first or the fourth week after injury. Whereas early neutropenia significantly increased the neointimal area, late depletion had no effect (fig. 1A). Hence, we restricted subsequent analyses to one week post-injury (fig. 1B, fig. S1, table S2). Herein, neutropenic mice exhibited a larger neointimal area than mice with intact WBC. Paralleling a marked reduction of neutrophils in the neointima, the number of TUNEL+ cells was reduced in neutropenic mice (fig. S1). Analyzing neointimal cell composition revealed that total macrophage content was reduced in neutropenic mice (fig. 1B), in line with a role of neutrophils in inflammatory monocyte recruitment (15,16). Conversely, neutrophil depletion increased neointimal collagen content but did not significantly affect CD3+ T-lymphocyte, smooth muscle cell (SMC) content (table S2) or the number of SMCs undergoing proliferation or apoptosis (table S3), nor did LL-37 alter SMC migration, proliferation or apoptosis in vitro (fig. S2). As evident by CD31 staining, endothelial coverage was reduced by 48% in neutropenic mice, indicating diminished re-endothelialization (fig. 1B). Defective endothelial recovery in neutropenic mice was confirmed by Evans blue staining of en face prepared carotid arteries (fig. S3).
Figure 1. Neutrophil-derived cathelicidin limits neointima formation.
(A) Neointima sizes, as quantified in Movat-stained sections of carotid arteries 4 weeks after wire-injury in Apoe−/− mice depleted of neutrophils for indicated periods. Representative images show neointima formation in mice with intact white blood cell count (WBC, top) and in neutropenic mice (bottom). n=6–8. *p<0.05 versus control mice. Kruskal-Wallis-test with posthoc-Dunn-test. (B) Immunohistochemical analysis of neointima one week after wire-injury in mice with intact white blood cell count (WBC) or with neutropenia. Displayed are neointimal area (left), Mac-2+ macrophage content (middle), and luminal coverage with CD31+ endothelium (right), and representative images (lower panel). Green fluorescence stems from secondary FITC-conjugated antibody, red fluorescence derives from secondary Cy3-conjugated antibody. n=7–9. *p<0.05 versus intact WBC. Mann-Whitney test. (C,D) Neointima size (C) and endothelial coverage (D) in Apoe−/−, beigeApoe−/−, Dppi−/−Apoe−/−, WT→Apoe−/−, and Cramp−/− →Apoe−/− mice one week after wire-injury. Experiments were performed in mice with intact WBC or neutropenia. Representative images show neointima formation and CD31 staining in WT→Apoe−/−, and Cramp−/−→Apoe−/− mice one week after wire-injury. n=6–8. *p<0.05 versus Apoe−/− or WT→Apoe−/− with intact WBC. Mann-Whitney test (left), Kruskal-Wallis-test with posthoc-Dunn-test (right). Scale bars, 100 μm.
Neutrophils regulate acute inflammatory responses through discharge of preformed granule proteins (5,6). To dissect the role of distinct groups of granule proteins, we used beigeApoe−/− and Dppi−/−Apoe−/− mice, which have reduced mobilization of primary neutrophil granule contents or lack active forms of neutrophil-derived serine proteases (proteinase-3, cathepsin G, neutrophil elastase), respectively (17,18). Neointima formation and luminal CD31+ cell coverage after injury in these mice did not differ from Apoe−/− mice with intact WBC (fig. 1C,D). Neutropenic mice of all three strains behaved similarly, indicating that primary granule contents and neutrophil-derived proteases are not required for the neutrophil-mediated healing process. Hence, we examined the role of CRAMP, which attracts monocytes to promote wound healing or angiogenesis (8,18), in neointima formation. Atherosclerotic mice transplanted with Cramp−/− bone marrow (BM) (Cramp−/−→Apoe−/−) (19) displayed larger neointimal areas and reduced luminal CD31+ lining compared to mice receiving BM from wild-type mice (WT→Apoe−/−) (fig. 1C,D). Neutropenia in Cramp−/−→Apoe−/− mice did not further aggravate this phenotype, which resembled that in neutropenic WT→Apoe−/− mice.
Cathelicidins are deposited at sites of endothelial injury
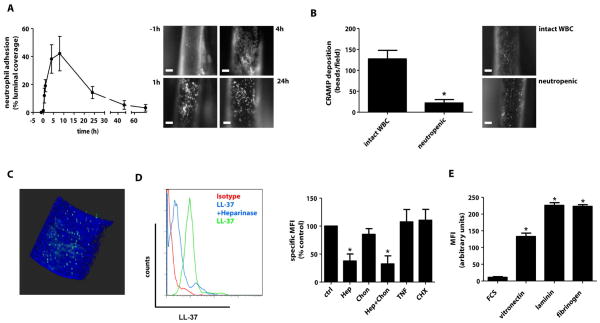
We suspected CRAMP, a highly positively charged polypeptide, to be deposited by adherent neutrophils at sites of injury. Monocyte-depleted Lysmegfp/egfpApoe−/− mice (table S4) carrying only fluorescent neutrophils (20) were used to monitor the time course of neutrophil adhesion. While neutrophil adhesion did not occur in uninjured carotid arteries, neutrophils rapidly adhered after injury peaking at 4 to 8h (fig. 2A). Hence, we studied arterial deposition of CRAMP 4h after injury. Injection of fluorescent beads conjugated to anti-CRAMP antibody allowed for in vivo detection of immobilized CRAMP (fig. 2B), which was largely abolished in neutropenic mice. Luminal location of CRAMP was corroborated by 2-photon microscopy (fig. 2C). To characterize interactions of cathelicidins with ECs, we co-incubated LL-37 with human aortic ECs (HAoECs). LL-37 binding to HAoECs was reduced by pretreatment with heparinase but not chondroitinase (fig. 2D). Activation with TNF or induction of apoptosis with cyclohexamide in HAoECs did not modulate LL-37 binding, but vitronectin, laminin and fibrinogen strongly bound LL-37, when compared to serum proteins alone (fig. 2E).
Figure 2. Neutrophils deposit cathelicidin at sites of injury.
(A) Adhesion of neutrophils to injured carotid arteries of monocyte-depleted Lysmegfp/egfpApoe−/− mice. Recordings were taken at different time points after injury and quantifications are given as percent fluorescent of total arterial area. Representative images indicate neutrophil adhesion at indicated time points. n=4–5. (B) In vivo detection of CRAMP along injured carotid arteries of mice with intact white blood cell count (WBC) or neutropenia, as detected by protein G-coupled fluorescent beads conjugated with an anti-CRAMP antibody. The number of immobilized beads was quantified. n=4. *p<0.05 versus intact WBC. Mann-Whitney-test. (C) anti-CRAMP bead complexes were detected luminally using intravital 2-photon microscopy. Blue fluorescence indicating collagen in the arterial wall originates from second harmonic generation, green fluorescence derives from beads conjugated with antibodies to CRAMP. (D) Surface binding of LL-37 to human aortic endothelial cells (HAoECs) assessed by FACS analysis. Untreated HAoECs (ctrl), HAoECs pretreated with heparinase (Hep, 50U/ml), chondroitinase (Chon, 20U/ml), or both, activated with TNF (10ng/ml, 4h) or rendered apoptotic with cyclohexamide (CHX, 500ng/ml), as indicated, were incubated with LL-37 (1 μg/ml, 15min). MFI, mean fluorescence intensity. n=7. *p<0.05 versus ctrl. Kruskal-Wallis-test with posthoc-Dunn-test. (E) Interaction of LL-37 with extracellular matrix proteins. LL-37 (1 μg/ml) was reacted with indicated extracellular matrix proteins and FCS or FCS alone immobilized on cell culture dishes and detected by immunofluorescence. n=8. *p<0.05 versus FCS alone. Kruskal-Wallis-test with posthoc-Dunn-test. Scale bars, 100 μm.
Cathelicidins mediate EOC recruitment
Neointima formation is governed by an ambiguous role of BM-derived cells. Whereas SMC progenitor cells contribute to neointimal hyperplasia, EOCs limit neointima formation (10). As neutropenia and CRAMP-deficiency affected re-endothelialization rather than SMC accumulation, we investigated their role in lesional recruitment of BM-derived EOC-like cells in Apoe−/− mice reconstituted with gfp+ BM and subjected to arterial wire-injury. After one week, gfp+ cells staining for CD31 were quantified in the neointima of mice with neutropenia or intact WBC (fig. S4). Neutrophil depletion reduced the number of BM-derived gfp+CD31+ EOC-like cells in the recovering endothelial lining.
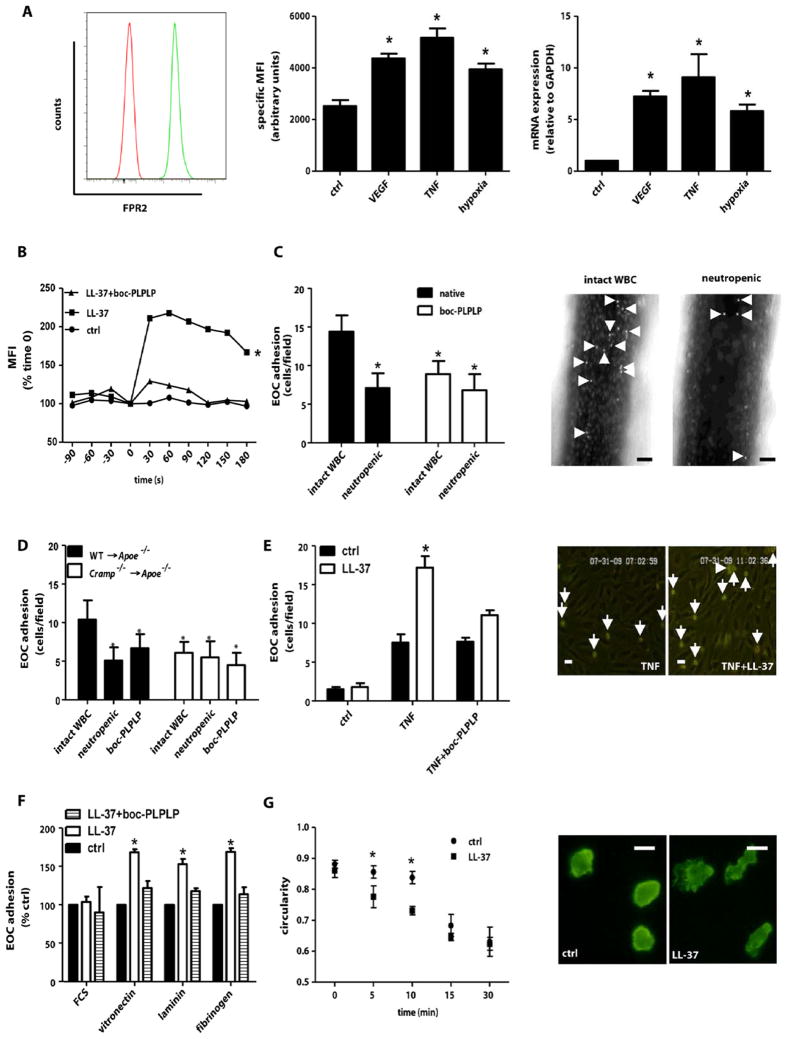
Cathelicidins exert multiple immune-regulating functions via formyl-peptide receptor 2 (FPR2) (5,21). As evidenced by flow cytometry, FPR2 was expressed on human EOCs under basal conditions and up-regulated by VEGF, TNF, or hypoxia at the transcriptional level (fig. 3A). In Ca2+-flux experiments, stimulation of EOCs with LL-37 resulted in rapid Ca2+-mobilization, a response abolished by the FPR-antagonist boc-PLPLP (fig. 3B).
Figure 3. Cathelicidin promotes EOC recruitment.
(A) Flow cytometry (left, middle) and real-time PCR (right) analyses of FPR2 expression in early outgrowth cells (EOCs). EOCs were treated with VEGF (20ng/ml), TNF (50ng/ml) or exposed to hypoxic conditions. Representative histograms for FRP2 expression (green) and isotype control (red) at baseline are shown. MFI, mean fluorescence intensity. n=4. *p<0.05 versus control. Kruskal-Wallis-test with posthoc-Dunn-test. (B) Fluorescence intensity was recorded in EOCs loaded with the Ca2+-sensitive fluo4-AM for 90s before and 180s after stimulation with LL-37 in presence or absence of boc-PLPLP (1 μM). Shown is the graph of one representative experiment. n=6. *p<0.05 versus ctrl or LL-37+boc-PLPLP. One-way analyses of variance followed by Tukey test. (C,D) Neutrophil-mediated adhesion of human EOCs to injured carotid arteries involves CRAMP-FPR. Calcein-labeled EOCs pretreated with boc-PLPLP were injected into Apoe−/− mice (C) or Apoe−/− mice transplanted with WT or Cramp−/− BM (D) with intact WBC or into neutropenic mice 4h after wire-injury. EOCs adherent to injured arteries were counted. Representative images in C show adherent EOC (arrows) in mice with intact white blood cell PLPLP were perfused over HAoEC monolayers (E) or plates coated with matrix proteins and LL-37 (F) and adherent cells were counted. Representative images in E show EOC adhesion (arrows) to TNF-activated HAoECs in presence or absence of LL-37. n=4–6. *p<0.05 versus controls and boc-PLPLP treatment. Kruskal-Wallis-test with posthoc-Dunn-test. Scale bars, 20 μm. (G) Analysis of EOC spreading. EOCs were seeded onto fibrinogen coated with or without (ctrl) LL-37. Circularity was assessed at indicated time points (right). Representative images of F-actin-stained EOCs (left). n=4. *p<0.05 versus control. Mann-Whitney test. Scale bars, 10 μm.
To explore a link of the FPR2-cathelicidin axis to re-endothelialization, we compared the adhesion of human EOCs to injured carotid arteries in neutropenic mice and mice with intact WBC. Depletion of neutrophils significantly reduced adhesion of injected EOCs (fig. 3C). Similarly, pretreatment of EOCs with boc-PLPLP decreased adhesion to levels observed in neutropenic mice receiving untreated EOCs. Notably, boc-PLPLP did not further reduce adhesion of EOCs injected into neutropenic mice (fig. 3C). Moreover, platelet-depleted mice showed reduced EOC adhesion (fig. S5), likely attributable to altered EOC interactions with platelets or platelet-derived microparticles (22) or secondary effects related to lower neutrophil adhesion in thrombocytopenic mice (20). As seen for monocytes, blocking CXCR2 on EOCs reduced their adhesion (fig. S6), an effect partially independent of neutrophils, implying a non-redundant role of this putative LL-37 receptor (23). Antagonists to P2X7 or TLR4, both involved in cathelicidin-dependent cell activation, failed to alter EOC adhesion(fig. S7A).
A potential interaction of cathelicidin with FPR2 underlying neutrophil-mediated EOC adhesion was addressed in atherosclerotic mice. The adhesion of EOCs was reduced by neutrophil depletion in WT→Apoe−/− mice but did not differ between neutropenic and normal Cramp−/−→Apoe−/− mice (fig. 3D). Moreover, EOCs treated with boc-PLPLP before injection into WT→Apoe−/− or Cramp−/−→Apoe−/− mice showed similar adhesion as in neutropenic WT→Apoe−/− mice or in Cramp−/−→Apoe−/− mice with intact WBC or neutropenia, indicating that CRAMP mediates neutrophil-dependent EOC adhesion through FPRs. In-vitro flow assays confirmed that deposition of LL-37 on TNF-activated HAoECs significantly enhanced EOC arrest, which was abrogated by boc-PLPLP (fig. 3E). Moreover, LL-37 co-immobilized with vitronectin, laminin, or fibrinogen enhanced EOC adhesion, which was abolished by the FPR-antagonist (fig. 3F). Coordinated cell spreading is essential for effective adhesion, requiring cytoskeletal reorganization and actin polymerization. We therefore analyzed LL-37-dependent spreading of EOCs immobilized on fibrinogen. The presence of LL-37 rapidly decreased circularity (fig. 3G), indicating prompt cellular activation and actin reorganization.
Cathelicidins promote survival of EOCs
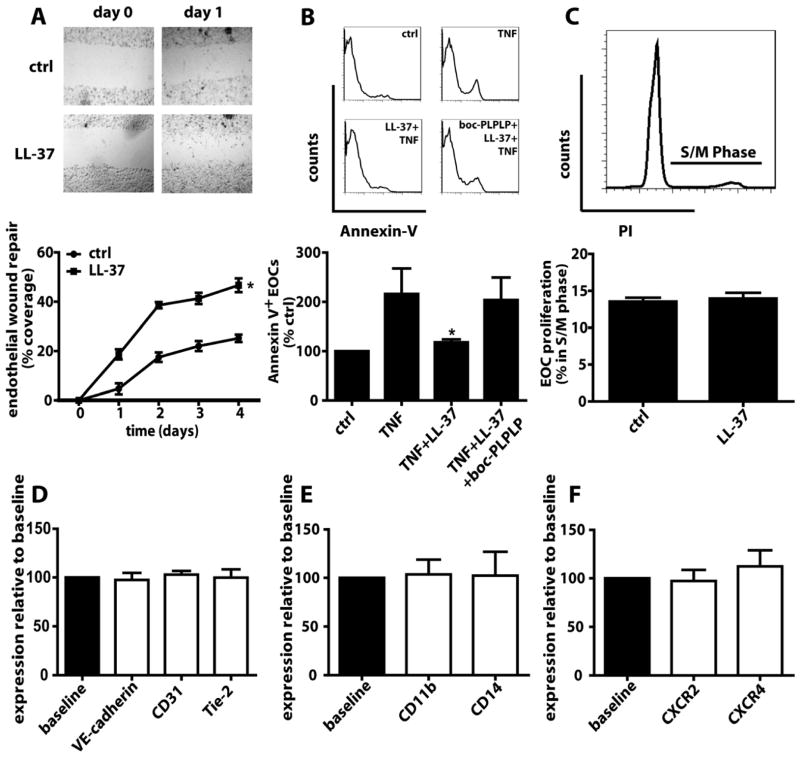
Once immobilized, EOCs may facilitate re-endothelialization by proliferation, maturation or secretion of growth factors. We assessed the relevance of LL-37 in the repopulation of scratch wounds inflicted in EOC monolayers. Compared to controls, incubation with LL-37 promoted EOC re-growth into the wounded area (fig. 4A). Mechanistically, we investigated the effects of LL-37 on EOC apoptosis, proliferation, and maturation. LL-37 prevented TNF-induced EOC apoptosis in a FPR-dependent manner (fig. 4B) but did not affect proliferation (fig. 4C) or maturation, as evidenced by unaltered expression of endothelial (fig. 4D) and myeloid (fig. 4E) markers. Moreover, LL-37 did not modulate expression of CXCR2 and CXCR4 (fig. 4F), and serum levels of CXCL12 and mCXCL1, ligands of CXCR4 or CXCR2, respectively, were unaltered in neutropenic mice after wire-injury (table S5).
Figure 4. LL-37 promotes EOC survival.
(A) EOC monolayers were subjected to scratch injury and the recovered wound area is expressed as percentage of the initial wound area. Representative photomicrographs are displayed. n=4. *p<0.05 versus ctrl. One-way analyses of variance followed by Tukey test. (B) EOCs were treated with TNF (50ng/ml) in presence or absence of LL-37 or boc-PLPLP and reacted with Representative histograms and percentage of annexin-V+ EOCs are displayed. n=3. *p<0.05 versus TNF and TNF+LL-37+bocPLPLP. Kruskal-Wallis-test with posthoc-Dunn-test. (C) Cell cycle analysis based on propidium iodide incorporation of EOCs treated with or without LL-37. n=3. (D–F) FACS analysis of endothelial (D) and myeloid (E) markers, and chemokine receptors (F). Baseline expression was set to 100% (black bars), and expression after LL-37 stimulation (white bars) is shown relative to baseline. n=3.
Cathelicidins modulate secretory properties of EOCs
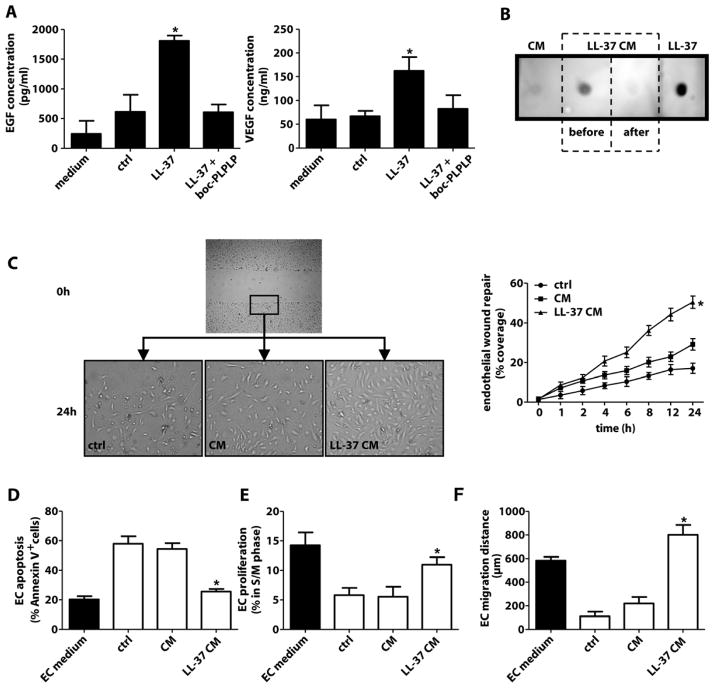
Given the low numbers of circulating EOCs and the contribution of endothelial re-growth to arterial healing, we explored whether the cathelicidin-EOC axis exerts paracrine effects on ECs. As potent sources of growth factors (22,24), EOCs exposed to LL-37 showed increased release of VEGF and EGF, which was abolished by boc-PLPLP (fig. 5A) but not antagonists to P2X7 or TLR4 (fig. S7B). To examine functional properties of EOC secretory products, confluent HAoECs were subjected to scratch injury and incubated with conditioned medium harvested from LL-37-treated EOCs (LL-37/CM), untreated EOCs (CM) or control medium with 1% FCS. To exclude effects of LL-37 in CM, LL-37 was immuno-depleted, as confirmed by dot-blot analysis (fig. 5B). Moreover, LL-37 failed to induce or enhance proliferation of HAoECs (fig. S8), indicating that direct effects on local EC proliferation are unlikely. Injured HAoEC monolayers in control medium recovered to about 20% within 24 h, and endothelial recovery was slightly improved by CM but markedly enhanced by LL-37/CM (fig. 5C). We analyzed alterations of apoptosis, proliferation or migration, possibly underlying improved recovery with LL-37/CM. Control medium and CM but not LL-37/CM induced apoptosis and reduced proliferation and migration in HAoECs, as compared to EC medium (fig. 5D–F).
Figure 5. LL-37-treated EOCs exert paracrine proendothelial effects.
(A) EOCs were treated with medium (ctrl) or LL-37 (1 μg/ml) in presence or absence of boc-PLPLP. EGF and VEGF were measured in medium or supernatant of non-activated EOCs (ctrl) or LL-37-treated EOCs. n=4. *p<0.05 versus all bars. Kruskal-Wallis-test with posthoc-Dunn-test. (B) LL-37 was detected in conditioned medium harvested from untreated EOCs (CM), LL-37-treated EOCs before (LL-37 CM, before) or after (LL-37 CM, after) immuno-depletion of LL-37, or synthetic LL-37 by dot blot. (C) Human aortic endothelial cell (HAoEC) monolayers were wounded linearly and the recovered area is expressed as percentage of the initial wound area. Monolayers were treated with medium containing 1% FCS (ctrl), CM from untreated EOCs or LL-37-immuno-depleted CM from LL-37-treated EOCs (LL-37 CM). n=3. *p<0.05 versus other groups. One-way analyses of variance followed by Tukey test. (D–F) HAoEC apoptosis (D), proliferation (E), and migration (F) is promoted by supernatant of LL-37-treated EOCs. Apoptosis is expressed as percentage of annexin-V+ HAoECs. Proliferation was assessed by cell cycle analysis using propidium iodide. The migration distance was measured after time-lapse tracking of HAoEC movement. HAoECs were treated as in (C). EC growth medium (solid bar) served as control. n=5. *p<0.05 versus ctrl and CM-treatment. Kruskal-Wallis-test with posthoc-Dunn-test.
Reduced stenosis in cathelicidin-biofunctionalized stents
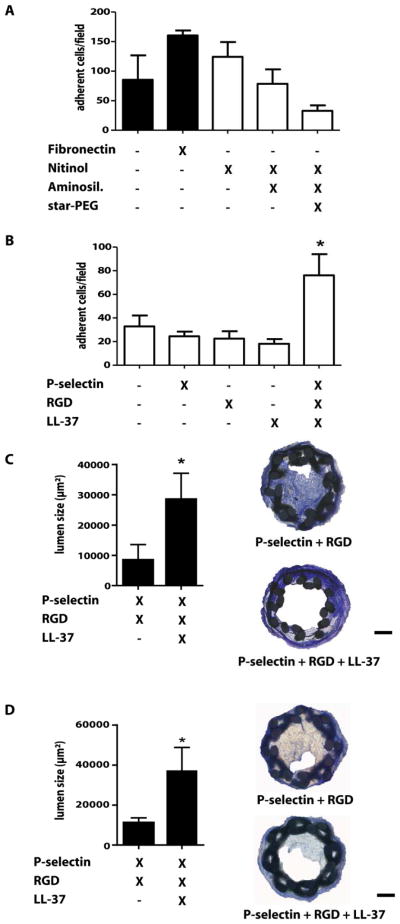
Due to its biocompatibility, corrosion resistance and elasticity, Nitinol is frequently used for stents. The role of the LL-37-EOC axis in protecting against neointima formation prompted us to develop biofunctionalized stents exploiting this principle. We assessed the biofunctional properties of surface-modified Nitinol in EOC adhesion assays. First, Nitinol foils were aminosilanized (AS) with N-[3-trimethoxysilyl-propyl]ethylenediamine and coated with six-arm star-shaped polyethylene glycol (star-PEG) creating an inert and anti-adhesive surface, as evidenced by minimal unspecific background adhesion of EOCs, when compared to uncoated or fibronectin-coated plastic surfaces or untreated Nitinol foils (fig. 6A). The aminosilanized, star-PEG-coated Nitinol foils were biofunctionalized by dip-coating with recombinant P-selectin, RGD, LL-37 individually or in combination. The adhesion of EOCs to Nitinol foils was unaffected by coating with any proteins alone but markedly enhanced by the combination of P-selectin, RGD, and LL-37 (fig. 6B). This prompted us to explore the effectiveness of LL-37-coated stents in mice. Miniaturized stents with shape-memory were braided from Nitinol wire, aminosilanized and coated with star-PEG, and biofunctionalized by covalently binding P-selectin/RGD without or with LL-37, which was well retained on stent surfaces, as shown by 2-photon microscopy and fluorescent LL-37 (fig. S9).
Figure 6. LL-37 coating reduces in-stent stenosis.
(A,B) EOCs were seeded onto Nitinol foils coated as indicated for 15min and the number of adherent EOCs was quantified. *p<0.05 versus aminosilanized, star-PEG-coated foils. n=4. Kruskal-Wallis-test with posthoc-Dunn-test. (C,D) RGD/P-selectin- or RGD/P-selectin/LL-37-coated stents were implanted into Apoe−/− mice and after 1 (C) or 4 weeks (D) luminal areas were analyzed by Giemsa staining. Representative images are displayed. Scale bars, 100 μm. n=9. *p<0.05. Mann-Whitney test.
Stents were implanted into the left carotid artery of Apoe−/− mice through insertion into a silicon tube, careful forward feeding of the stent whilst retracting the tube to allow for shape memory-based expansion (fig. S9). After one week and 4 weeks, we performed histological analyses to assess lumen and neointima by Giemsa staining. Whereas neointima size was only reduced in mice receiving LL-37-coated stents compared to bare-metal stents, aminosilanized, star-PEG- and P-selectin/RGD-coated stents without LL-37 (fig. S10), the luminal area was markedly increased in LL-37-coated stents (fig. 6C,D), revealing protective effects against in-stent stenosis. Improved endothelial coverage of the luminal lining with CD31+ endothelial cells was observed by immunofluoresence staining in carotid arteries that had received LL-37-coated stents, as compared to control stents, and an abundance of CD31+ endothelial cells within the stented area but no significant thrombosis was detected in carotid arteries with LL-37-coated stents (fig. S11). As seen in other models of arterial injury, e.g. wire-induced denudation, in mice a period of 4 weeks is commonly sufficient to obtain a maximal degree of neointimal hyperplasia. Hence, it is conceivable that long-term efficiency and patency of LL37-coated stents would persist beyond 4 weeks. To corroborate that CRAMP limits in-stent stenosis, we implanted uncoated Nitinol stents in Apoe−/− mice reconstituted with WT or Cramp−/− BM. Mice lacking leukocyte CRAMP displayed larger neointima but reduced in-stent lumen size (fig. S12).
Discussion
Neutrophils are the first-line of immune cells recruited to sites of injury after percutaneous transluminal angioplasty, and the rapid release of their granule proteins critically shapes the vascular inflammatory response. Here we identify cathelicidins as important mediators of neutrophil-dependent repair after arterial injury. The activity of these effectors is based on their EOC-activating capacities enhancing EOC recruitment and release of regenerative growth factors. Both mechanisms cooperate to promote re-endothelialization and to reduce the extent of neointima formation. We adopted this concept in a translational approach to design a LL-37-coated stent, which is effective in limiting in-stent stenosis.
Neutrophils have been underestimated players in arterial disease. Recent studies reveal an exacerbating effect of neutrophils in atherosclerosis, which is promoted by neutrophilia but attenuated in neutropenic mice (20). In contrast, we show that neutrophils exert protective effects during acute arterial injury. Previous studies revealed that a lack of β2-integrins (25,26) or P-selectin (27), both expressed by neutrophils, results in smaller neointima size. As these molecules are not specific for neutrophils and also involved in recruiting monocytes, platelets and progenitor cells, we however believe that these studies allow for identification of molecular rather than cellular targets. Moreover, the contribution of P-selectin to neointima formation seems to be restricted to platelets (28). In contrast, blocking mCXCL1, which can target neutrophil recruitment, increases neointima formation, while decreasing endothelial recovery (29). Conversely, elevated neutrophil counts after G-CSF application were found to reduce neointima size and promote re-endothelialization (30). Hence, it seems justified to conclude that neutrophils can exert protective effects during early phases of arterial injury.
Current concepts hold that the healing after vascular injury is differentially affected by recruitment of BM-derived progenitor cells: whereas EOCs are thought to limit neointima formation by accelerating re-endothelialization, SMC progenitor cells contribute to neointimal hyperplasia (10). Our analysis unveiled that neutropenia affects endothelial recovery but not SMC content in neointimal lesions. Thus, we focused on the interrelation between neutrophils and EOCs. Two major mechanisms are thought to account for beneficial effects of EOCs on endothelial recovery: recruitment and release of growth factors promoting EC re-growth (31).
The CXCR4-CXCL12 axis is a major determinant of EOC mobilization and homing to sites of arterial injury. Platelet-derived microparticles amplify vasoregenerative functions of EOCs by transferring CXCR4 (22). Moreover, the CXCR2-CXCL1 axis plays a pivotal role in EOC recruitment and angiogenesis, as evidenced in CXCR2−/− mice or neutralizing CXCR2 and its ligands (14,32,33). Neutrophils, however, seem to modulate neither axis, as CXCR4 and CXCR2 expression on EOCs and CXCL12 and CXCL1 serum levels were unaltered by LL-37 or neutropenia, respectively. In addition, LL-37 acts through FPR2 rather than CXCR4 in EOC-related monocytes (15,21), whereas some functional ligand activity for CXCR2 has been observed in neutrophils (23). The importance of LL-37-FPR interactions in mediating neutrophil-dependent EOC recruitment was unveiled in assays, where neutrophil- and LL-37-mediated EOC adhesion was abrogated by antagonizing FPRs. Notably, adherent neutrophils can seed LL-37 at sites of injury. Based on its cationic nature, LL-37 interacts with negatively charged proteins of the basement membrane and the EC glycocalyx. In a similar sequence of events, azurocidin deposited by emigrating neutrophils can induce monocyte adhesion (34), and activated platelets can deposit chemokines from intracellular stores to trigger monocyte arrest (35). The FPR2-LL-37 interaction is important during EC activation and proliferation leading to angiogenesis (8). Activation of embryonic endothelial progenitor cells with LL-37 ex vivo has been found to enhance NF-κB activity and to promote adhesion of these cells at sites of ischemia (36). Moreover, LL-37 has been implicated in regulating cell survival, with high concentrations being cytotoxic, whereas lower concentrations may prevent apoptosis (37,38). Here we show that LL-37 protects EOCs against apoptosis in a FPR2-dependent manner, providing an adjuvant mechanism for EOC accumulation under inflammatory conditions. However, unlike in ECs (8), LL-37 did not stimulate EOC proliferation.
Consequently, we identified effectors, by which LL-37-triggered EOC function may contribute to re-endothelialization. Namely, LL-37 stimulates release of the pro-angiogenic factors VEGF and EGF. A similar effect was identified for porcine cathelicidin PR-39, which inhibits ubiquitin-proteasome-dependent degradation of HIF-1α to increase VEGF expression (39). In our experiments, LL-37-treated EOC secretion exhibited a potent paracrine activity on ECs, resulting in enhanced migration and proliferation but reduced apoptosis. Considering the limited number of EOCs directly incorporating into the endothelial lining, this activity may stand out as a major mechanism underlying neutrophil-dependent re-endothelialization. Hence, LL-37 promotes re-endothelialization by a variety of mechanisms including EOC recruitment and survival, and endothelial recovery by paracrine effects.
Recent meta-analyses confirmed drug-eluting stents as a preferred option for coronary revascularization (1,40). Nevertheless, concerns regarding delayed re-endothelialization and in-stent thrombosis associated with drug-eluting stents have been raised (41,42). As a result, current treatment regimens after stent implantation have adopted a dual antiplatelet therapy for at least one year. We have therefore designed a LL-37-coated stent, which - likely through instructing the cathelicidin-EOC axis - limits in-stent stenosis in mice. LL-37 is known to harbour both pro- and anti-inflammatory properties, e.g. monocyte recruitment and activation (5,16), stimulation of wound healing and angiogenesis but also deactivation of macrophages (8,43,44). Given the limited in-stent neointima in LL-37-coated stents, we conclude that anti-inflammatory effects of LL-37 prevail in this model. Furthermore, LL-37-coated surfaces can exert potent antimicrobial effects (45) but little pro-inflammatory activity in terms of platelet or complement activation (46). Although our study was limited to 4 weeks after implantation, no thrombus formation was observed in these stents. Thus, stents exploiting pro-endothelial properties of LL-37 may also be suitable to circumvent problems of late thrombosis in drug-eluting stents after longer periods of clinical use. The maximum degree of neointima formation in mice is commonly obtained within 4 weeks after arterial injury. It is well established that covalent coatings with antimicrobial polypeptides are highly stable withstanding extreme environmental conditions e.g. severe changes in pH or temperature (47). Therefore, we have no reason to believe that the long-term efficiency and patency would be dramatically different beyond 4 weeks in our model. Previously, novel stent developments have been primarily tested and validated in large animal models (14,48). Our approach of implanting miniaturized and biofunctionalized stents in mouse carotid arteries allows for versatile analysis of novel stent coatings in a disease-relevant context of genetically altered mice.
Whereas the overall rate of stent thrombosis may be equivalent between drug-eluting and bare-metal stents at 5 years, its temporal incidence may differ, with higher rates of very late-stent thrombosis in drug-eluting stents (49). The underlying mechanisms have been differentially related to hypersensitivity or excessive fibrin in first-generation drug-eluting stents (50). Clinical follow-up at 3 years, however, indicates that an everolimus-eluting stent may have more favorable rates of stent thrombosis, possibly extending to very late-stent thrombosis (51). Although a link of re-endothelialization to late-stent thrombosis is not firmly established but based on differences in endothelialization of drug-eluting and bare-metal stents in autopsy and angioscopy studies, and comparison of drug-eluting stents in rabbit model showed a lesser reduction in neointima size despite better healing and re-endothelialization in zotarolimus-eluting stents (52), this may not be generalized to our purely pro-endothelial polymer stent concept without confounding effects of other drug eluents. Its beneficial effects may provide evidence for a link between endothelial recovery and reduced in-stent stenosis.
In conclusion, neutrophils play an important role during the early healing process after arterial injury by depositing cathelicidins as regulators of endothelial recovery. The FPR2-cathelicidin axis promotes adhesion and function of EOCs, improving re-endothelialization and limiting neointima formation. Accordingly, biofunctionalized LL37-coated stents showed effectiveness in a mouse model. These findings clearly warrant future studies to investigate the applicability of this strategy in large animal models and its translation into clinical use.
Materials and Methods
Arterial wire-injury
All animal studies were approved by local authorities. Apoe−/− mice were transplanted with BM from Cramp−/− mice (19), CAGgfp/gfp mice (The Jackson Laboratory), or wild-type controls by i.v. injection 24h after ablative whole-body irradiation. Mice were fed an atherogenic diet containing 21% fat for 1 week before and up to 4 weeks after injury. Wire-injury was performed and analyzed as described (22). Neutrophils were depleted by i.p. injection of mAb 1A8 (20). Platelets were depleted by i.p. injection of anti-platelet serum (20). Control animals were treated with IgG2a isotype control antibody or control serum. The braiding, coating, implantation, and processing of biofunctionalized stents is detailed in the supplemental material.
Cell culture
Isolation and culture of EOCs was as described (22). Briefly, peripheral blood mononuclear cells were separated by density gradient centrifugation and seeded on fibronectin-coated plates in MV2 endothelial growth medium (PromoCell) for 7 days.
Statistics
Data are expressed as mean±SD. Statistical calculations were performed using GraphPad Prism 5. Statistical tests are specified in figure legends. P-values < 0.05 were considered significant.
Supplementary Material
Figure S1: Neointimal cell composition.
Figure S2: LL-37 does not affect migration, apoptosis, and proliferation of smooth muscle cells (SMCs).
Figure S3: Neutropenia impairs endothelial recovery.
Figure S4: Neutropenia reduces incorporation of bone marrow-derived cells into the endothelial lining.
Figure S5: Platelet depletion reduces adhesion of EOCs.
Figure S6: Adhesion of adoptively transferred monocytes and EOCs partially depends on CXCR2.
Figure S7: Role of P2 receptors and TLR4 in LL-37-mediated EOC activation.
Figure S8: LL-37 does not induce endothelial cell (EC) proliferation.
Figure S9: Development of a LL-37-coated Nitinol stent.
Figure S10: Neointima sizes are reduced in mice receiving LL-37-coated stents. Figure S11: Re-endothelialization is improved in arteries with LL-37-coated stents. Figure S12: CRAMP prevents in-stent stenosis.
Table S1: Differential leukocyte counts in mice with intact WBC and in neutropenic mice.
Table S2: Neointimal cell composition of neointima in mice with intact WBC or neutropenia.
Table S3: Immunohistochemical analysis of SMC homeostasis one week after wire-injury.
Table S4: Differential leukocyte counts in mice depleted of monocytes.
Table S5: mCXCL1 and CXCL12 serum levels in mice with intact WBC or neutropenia.
Acknowledgments
We thank Yvonne Jansen and Silvia Roubrocks for technical assistance.
Funding: This study was supported by Deutsche Forschungsgemeinschaft, German Heart Foundation, and IZKF, RWTH Aachen.
Footnotes
Competing interests: The authors do not declare any competing financial interests.
Author contributions: O.S. designed the study, performed intravital microscopy, analyzed data, wrote the manuscript and provided funding, S.W. performed in vitro assays, S.S. acquired stent data, Y.D. performed in vitro experiments, R.T.M. performed 2-photon microscopy, S.F.M. performed ELISA measurements, M.D. performed wire-injuries, R.S. and S.W. contributed to acquisition of stent data, F.S. and T.G. braided stents, S.V. contributed in-vitro data, M.v.Z. supervised 2-photon microscopy, B.A. provided reagents, C.T.P. and R.L.G. provided mouse strains, T.M.H. synthesized fluorescent LL-37, E.A.L. performed wire-injuries, acquired and analyzed stent data, A.Z. performed preliminary experiments and provided funding, D.K. supervised stent development, C.W. designed the study, wrote the manuscript and provided funding.
References and Notes
- 1.King SB, 3rd, Marshall JJ, Tummala PE. Revascularization for coronary artery disease: stents versus bypass surgery. Annu Rev Med. 2010;61:199–213. doi: 10.1146/annurev.med.032309.063039. [DOI] [PubMed] [Google Scholar]
- 2.Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Jüni P, Sianos G, Hellige G, van Domburg RT, Hess OM, Boersma E, Meier B, Windecker S, Serruys PW. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. The Lancet. 2007;369:667–678. doi: 10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 3.Hanawa T. Materials for metallic stents. J Artif Organs. 2009;12:73–9. doi: 10.1007/s10047-008-0456-x. [DOI] [PubMed] [Google Scholar]
- 4.Baetta R, Corsini A. Role of polymorphonuclear neutrophils in atherosclerosis: current state and future perspectives. Atherosclerosis. 2010;210:1–13. doi: 10.1016/j.atherosclerosis.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 5.Soehnlein O, Weber C, Lindbom L. Neutrophil granule proteins tune monocytic cell function. Trends Immunol. 2009;30:538–46. doi: 10.1016/j.it.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–39. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 7.Gautam N, Olofsson AM, Herwald H, Iversen LF, Lundgren-Akerlund E, Hedqvist P, Arfors KE, Flodgaard H, Lindbom L. Heparin-binding protein (HBP/CAP37): a missing link in neutrophil-evoked alteration of vascular permeability. Nat Med. 2001;7:1123–7. doi: 10.1038/nm1001-1123. [DOI] [PubMed] [Google Scholar]
- 8.Koczulla R, von Degenfeld G, Kupatt C, Krötz F, Zahler S, Gloe T, Issbrücker K, Unterberger P, Zaiou M, Lebherz C, Karl A, Raake P, Pfosser A, Boekstegers P, Welsch U, Hiemstra PS, Vogelmeier C, Gallo RL, Clauss M, Bals R. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–72. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 10.Hirschi KK. Vascular precursors: origin, regulation and function. Arterioscler Thromb Vasc Biol. 2010;30:1078–9. doi: 10.1161/ATVBAHA.110.208041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93:e17–24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 12.Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108:2710–2715. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 13.Blindt R, Vogt F, Astafieva I, Fach C, Hristov M, Krott N, Seitz B, Kapurniotu A, Kwok C, Dewor M, Bosserhoff AK, Bernhagen J, Hanrath P, Hoffmann R, Weber C. A novel drug-eluting stent coated with an integrin-binding cyclic Arg-Gly-Asp peptide inhibits neointimal hyperplasia by recruiting endothelial progenitor cells. J Am Coll Cardiol. 2006;47:1786–95. doi: 10.1016/j.jacc.2005.11.081. [DOI] [PubMed] [Google Scholar]
- 14.Hristov M, Zernecke A, Bidzhekov K, Liehn EA, Shagdarsuren E, Ludwig A, Weber C. Importance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circ Res. 2007;100:590–7. doi: 10.1161/01.RES.0000259043.42571.68. [DOI] [PubMed] [Google Scholar]
- 15.Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, Bidzhekov K, Rottenberg ME, Weber C, Lindbom L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–71. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–23. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 17.Kjeldsen L, Calafat J, Borregaard N. Giant granules of neutrophils in Chediak-Higashi syndrome are derived from azurophil granules but not from specific and gelatinase granules. J Leukoc Biol. 1998;64:72–7. doi: 10.1002/jlb.64.1.72. [DOI] [PubMed] [Google Scholar]
- 18.Adkison AM, Raptis SZ, Kelley DG, Pham CT. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest. 2002;109:363–71. doi: 10.1172/JCI13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 20.Drechsler M, Megens RTA, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–45. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 21.De Yang, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mause SF, Ritzel E, Liehn EA, Hristov M, Bidzhekov K, Müller-Newen G, Soehnlein O, Weber C. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation. 2010;122:495–506. doi: 10.1161/CIRCULATIONAHA.109.909473. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Cherryholmes G, Chang F, Rose DM, Schraufstatter I, Shively JE. Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils. Eur J Immunol. 2009;39:3181–94. doi: 10.1002/eji.200939496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–42. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Simon DI, Dhen Z, Seifert P, Edelman ER, Ballantyne CM, Rogers C. Decreased neointimal formation in Mac-1(−/−) mice reveals a role for inflammation in vascular repair after angioplasty. J Clin Invest. 2000;105:293–300. doi: 10.1172/JCI7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers C, Edelman ER, Simon DI. A mAb to the beta2-leukocyte integrin Mac-1 (CD11b/CD18) reduces intimal thickening after angioplasty or stent implantation in rabbits. Proc Natl Acad Sci U S A. 1998;95:10134–9. doi: 10.1073/pnas.95.17.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi S, Watanabe N, Nakazawa K, Suzuki J, Tsushima K, Tamatani T, Sakamoto S, Isobe M. Roles of P-selectin in inflammation, neointimal formation, and vascular remodeling in balloon-injured rat carotid arteries. Circulation. 2000;102:1710–7. doi: 10.1161/01.cir.102.14.1710. [DOI] [PubMed] [Google Scholar]
- 28.Wang K, Zhou X, Zhou Z, Mal N, Fan L, Zhang M, Lincoff AM, Plow EF, Topol EJ, Penn MS. Platelet, not endothelial, P-selectin is required for neointimal formation after vascular injury. Arterioscler Thromb Vasc Biol. 2005;25:1584–9. doi: 10.1161/01.ATV.0000172687.01179.d4. [DOI] [PubMed] [Google Scholar]
- 29.Liehn EA, Schober A, Weber C. Blockade of keratinocyte-derived chemokine inhibits endothelial recovery and enhances plaque formation after arterial injury in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1891–6. doi: 10.1161/01.ATV.0000143135.71440.75. [DOI] [PubMed] [Google Scholar]
- 30.Yoshioka T, Takahashi M, Shiba Y, Suzuki C, Morimoto H, Izawa A, Ise H, Ikeda U. Granulocyte colony-stimulating factor (G-CSF) accelerates reendothelialization and reduces neointimal formation after vascular injury in mice. Cardiovasc Res. 2006;70:61–9. doi: 10.1016/j.cardiores.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Pearson JD. Endothelial progenitor cells - hype or hope? J Thromb Haemost. 2009;7:255–62. doi: 10.1111/j.1538-7836.2008.03214.x. [DOI] [PubMed] [Google Scholar]
- 32.Jones CP, Pitchford SC, Lloyd CM, Rankin SM. CXCR2 mediates the recruitment of endothelial progenitor cells during allergic airways remodeling. Stem Cells. 2009;27:3074–81. doi: 10.1002/stem.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keane MP, Belperio JA, Xue YY, Burdick MD, Strieter RM. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J Immunol. 2004;172:2853–60. doi: 10.4049/jimmunol.172.5.2853. [DOI] [PubMed] [Google Scholar]
- 34.Soehnlein O, Xie X, Ulbrich H, Kenne E, Rotzius P, Flodgaard H, Eriksson EE, Lindbom L. Neutrophil-derived heparin-binding protein (HBP/CAP37) deposited on endothelium enhances monocyte arrest under flow conditions. J Immunol. 2005;174:6399–405. doi: 10.4049/jimmunol.174.10.6399. [DOI] [PubMed] [Google Scholar]
- 35.Koenen RR, von Hundelshausen P, Nesmelova IV, Zernecke A, Liehn EA, Sarabi A, Kramp BK, Piccinini AM, Paludan SR, Kowalska MA, Kungl AJ, Hackeng TM, Mayo KH, Weber C. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat Med. 2009;15:97–103. doi: 10.1038/nm.1898. [DOI] [PubMed] [Google Scholar]
- 36.Pfosser A, El-Aouni C, Pfisterer I, Dietz M, Globisch F, Stachel G, Trenkwalder T, Pinkenburg O, Horstkotte J, Hinkel R, Sperandio M, Hatzopoulos AK, Boekstegers P, Bals R, Kupatt C. NF kappaB activation in embryonic endothelial progenitor cells enhances neovascularization via PSGL-1 mediated recruitment: novel role for LL37. Stem Cells. 2010;28:376–85. doi: 10.1002/stem.280. [DOI] [PubMed] [Google Scholar]
- 37.Nagaoka I, Tamura H, Hirata M. An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J Immunol. 2006;176:3044–52. doi: 10.4049/jimmunol.176.5.3044. [DOI] [PubMed] [Google Scholar]
- 38.Barlow PG, Li Y, Wilkinson TS, Bowdish DM, Lau YE, Cosseau C, Haslett C, Simpson AJ, Hancock RE, Davidson DJ. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J Leukoc Biol. 2006;80:509–20. doi: 10.1189/jlb.1005560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Post M, Volk R, Gao Y, Li M, Metais C, Sato K, Tsai J, Aird W, Rosenberg RD, Hampton TG, Sellke F, Carmeliet P, Simons M. PR39, a peptide regulator of angiogenesis. Nat Med. 2000;6:49–55. doi: 10.1038/71527. [DOI] [PubMed] [Google Scholar]
- 40.Spaulding C, Daemen J, Boersma E, Cutlip DE, Serruys PW. A pooled analysis of data comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356:989–97. doi: 10.1056/NEJMoa066633. [DOI] [PubMed] [Google Scholar]
- 41.Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–9. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 42.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 43.Carretero M, Escámez MJ, García M, Duarte B, Holguín A, Retamosa L, Jorcano JL, Río MD, Larcher F. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Invest Dermatol. 2008;128:223–36. doi: 10.1038/sj.jid.5701043. [DOI] [PubMed] [Google Scholar]
- 44.Brown KL, Poon GF, Birkenhead D, Pena OM, Falsafi R, Dahlgren C, Karlsson A, Bylund J, Hancock RE, Johnson P. Host Defense Peptide LL-37 Selectively Reduces Proinflammatory Macrophage Responses. J Immunol. 2011 doi: 10.4049/jimmunol.1002508. [DOI] [PubMed] [Google Scholar]
- 45.Gabriel M, Nazmi K, Veerman EC, Nieuw Amerongen AV, Zentner A. Preparation of LL-37-grafted titanium surfaces with bactericidal activity. Bioconjug Chem. 2006;17:548–50. doi: 10.1021/bc050091v. [DOI] [PubMed] [Google Scholar]
- 46.Gao G, Lange D, Hilpert K, Kindrachuk J, Zou Y, Cheng JT, Kazemzadeh-Narbat M, Yu K, Wang R, Straus SK, Brooks DE, Chew BH, Hancock RE, Kizhakkedathu JN. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials. 2011;32:3899–909. doi: 10.1016/j.biomaterials.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Costa F, Carvalho IF, Montelaro RC, Gomes P, Martins MC. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011;7:1431–40. doi: 10.1016/j.actbio.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Ma X, Hibbert B, Dhaliwal B, Seibert T, Chen YX, Zhao X, O’Brien ER. Delayed re-endothelialization with rapamycin-coated stents is rescued by the addition of a glycogen synthase kinase-3beta inhibitor. Cardiovasc Res. 2010;86:338–45. doi: 10.1093/cvr/cvq047. [DOI] [PubMed] [Google Scholar]
- 49.Garg S, Serruys P. Benefits of and safety concerns associated with drug-eluting coronary stents. Expert Rev Cardiovasc Ther. 2010;8:449–70. doi: 10.1586/erc.09.138. [DOI] [PubMed] [Google Scholar]
- 50.Nakazawa G, Finn AV, Vorpahl M, Ladich ER, Kolodgie FD, Virmani R. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. J Am Coll Cardiol. 2011;57:390–8. doi: 10.1016/j.jacc.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 51.Caixeta A, Lansky AJ, Serruys PW, Hermiller JB, Ruygrok P, Onuma Y, Gordon P, Yaqub M, Miquel-Hebert K, Veldhof S, Sood P, Su X, Jonnavithula L, Sudhir K, Stone GW SPIRIT II and III Investigators. Clinical follow-up 3 years after everolimus- and paclitaxel-eluting stents: a pooled analysis from the SPIRIT II and SPIRIT III randomized trials. JACC Cardiovasc Interv. 2010;3:1220–8. doi: 10.1016/j.jcin.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 52.Ertaş G, van Beusekom HM, van der Giessen WJ. Late stent thrombosis, endothelialisation and drug-eluting stents. Neth Heart J. 2009;17:177–80. doi: 10.1007/BF03086242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Neointimal cell composition.
Figure S2: LL-37 does not affect migration, apoptosis, and proliferation of smooth muscle cells (SMCs).
Figure S3: Neutropenia impairs endothelial recovery.
Figure S4: Neutropenia reduces incorporation of bone marrow-derived cells into the endothelial lining.
Figure S5: Platelet depletion reduces adhesion of EOCs.
Figure S6: Adhesion of adoptively transferred monocytes and EOCs partially depends on CXCR2.
Figure S7: Role of P2 receptors and TLR4 in LL-37-mediated EOC activation.
Figure S8: LL-37 does not induce endothelial cell (EC) proliferation.
Figure S9: Development of a LL-37-coated Nitinol stent.
Figure S10: Neointima sizes are reduced in mice receiving LL-37-coated stents. Figure S11: Re-endothelialization is improved in arteries with LL-37-coated stents. Figure S12: CRAMP prevents in-stent stenosis.
Table S1: Differential leukocyte counts in mice with intact WBC and in neutropenic mice.
Table S2: Neointimal cell composition of neointima in mice with intact WBC or neutropenia.
Table S3: Immunohistochemical analysis of SMC homeostasis one week after wire-injury.
Table S4: Differential leukocyte counts in mice depleted of monocytes.
Table S5: mCXCL1 and CXCL12 serum levels in mice with intact WBC or neutropenia.