Abstract
Immunologic tolerance is the ultimate goal of organ transplantation yet, is rarely attainable and an infrequent event in humans. Even with the use of conventional immunosuppression, which has successfully improved short-term allograft survival, long-term allograft survival has remained static and is complicated by serious side effects secondary to the long-term use of immunosuppressive agents. Accordingly, over the past several decades, there has been a push to fully understand both the cellular and molecular mechanisms that play a role in the induction and maintenance of tolerance, with recent data implicating B cells and donor specific alloantibody as a barrier to and potential mediator of allograft tolerance. The study of B cells and alloantibody in transplant tolerance has evolved over recent years from using rodent models to large animals to non-human primate models. This review will discuss the role of B cells and alloantibody as antagonists and facilitators of transplantation tolerance, and highlight the experimental models developed for elucidating the mechanisms of B cell tolerance to alloantigen.
1. Introduction
Since the first report of allograft tolerance in a murine model by Medawar and colleagues, a major goal in the field of transplantation has been to achieve immunologic tolerance and prevent allograft rejection (1). Clinically defined as long-term graft function in the absence of immunosuppression, tolerance is difficult to achieve in humans and the most successful reports of clinical ‘operational’ tolerance are in liver transplantation where elective weaning of immunosuppression is successful in 20% of adult liver transplant patients (2, 3). This may be due to the immune privileged status of a liver allograft, which is exemplified by the irrelevance of a positive cross-match, spontaneous recovery following severe rejection and an immunomodulating effect of the liver in cases of combined hepatic and renal allograft transplantation (2, 4–8). When compared to less ‘tolerogenic’ organs such as in kidney transplantation, the development of clinical operational tolerance is less frequent thus, these allografts typically require continuous, albeit in many cases reduced dose, immunosuppression therapy to prevent rejection (9, 10). Despite our advances in immunosuppression regimens that have significantly prolonged short-term allograft survival, long-term graft survival has not changed (9, 11). This is mainly due to chronic allograft nephropathy and renal toxicity associated with immunosuppression (12, 13). The sporadic occurrence of acquired immune tolerance in human transplant recipients, and the profoundly improved quality and length of life that accompanies it, continues to motivate investigation into this complex area of human biology.
Historically, the role of B cells as a barrier to graft acceptance has been limited to antibody production and hyperacute rejection. Advances in human leukocyte antigen (HLA) screening and desensitization therapies have all but eliminated the incidence of hyperacute rejection, however, the presence of donor specific alloantibody (DSA) remains a concern as it denies patients in renal failure an opportunity for a transplant and places patients at a higher risk of both acute and chronic antibody mediated rejection and subsequent graft loss (14–17). These effects of antibodies are most critical in presensitized recipients, as depletion of antibodies by plasmapheresis is usually short-lived, and their reappearance after the transplant correlates with a high incidence of acute humoral rejection (AHR). A recent paper by Burns et al. examined the time-course and pattern of donor specific anti-HLA antibody (DSA) levels post-kidney transplant in patients with low and high baseline (pre-transplant) DSA (18). Overall, they report a modestly higher incidence of AHR in the high baseline DSA group (40%) compared to the low DSA group (31%). By post-operative day 4, approximately 71% of all patients demonstrated a significant decrease in DSA suggesting absorption of the DSA by the allograft. The DSA levels remained low in patients who did not develop AHR, while in patients who went on to develop AHR, DSA levels increased by post-operative day 10 and the level of de novo DSA was directly correlated with the severity of AHR. These data confirm the long recognized role of post-transplant DSA as a barrier to early graft acceptance.
The role of DSA in transplant glomerulopathy and chronic antibody-mediated allograft injury is becoming clearer. We know from recent data that antibodies developed early after transplantation are more damaging to the allograft than antibodies developed after one year post-transplant (19), but that DSA can be detected years before any sign of humoral rejection (20). Consistent with this is the recent report that the incidence of transplant glomerulopathy, the chronic histologic lesion associated with antibody, occurs in 80% of positive crossmatch recipients 5 years after transplant compared to less than 5% in patients without DSA (15).
Beyond their role in antibody production, the presence of B cells themselves has been implicated in poor graft survival. In a seminal study by Sarwal et al., the presence of dense CD20+ B cell clusters in acutely rejecting renal allografts was observed to correlate with resistance to glucocorticoid therapy and accelerated graft failure (21). Because simultaneous immunohistochemical staining for CD20+ did not correlate with C4d deposition, it was suggested that B cells within the graft might have an antibody-independent role in acute rejection. Subsequent studies suggest that the presence of CD20+ B cells as well as CD20−CD38+ dense plasmablast infiltrates correlate with worse graft function and survival (22–24).
The preponderance of evidence from the clinic points to antibodies and B cells as critical barriers to graft acceptance. The explanation for their pathogenicity has focused on the ability of antibodies to bind to the graft endothelium and induce inflammation through the activation of complement, although their ability to directly activate endothelial cells independent of complement has also been described (25). In contrast to the clear pathogenic role of B cells and antibodies, a number of groups studying clinical tolerance in humans have observed a unexpected correlation between tolerance and an enriched B cell signature (26–28). These data, while aimed at identifying biomarkers of tolerance, raise the possibility that B cells may also play a paradoxical role in the maintenance of tolerance in humans. This review will provide an overview of the experimental models that have investigated the role of B cells and antibodies as barriers to tolerance induction, followed the fate of alloreactive B cells during successful tolerance induction and explored the emerging role of B cells as facilitators of transplantation tolerance.
2. B cells and Antibodies as Barriers to Tolerance in Experimental Models
One of the experimental models that has been extensively used to study tolerance in animals involves the approach of mixed chimerism whereby after receiving either total or non-myeloablative therapy plus engraftment of donor bone marrow, hematopoietic cells of both donor and recipient origin coexist to induce tolerance. In rodent models of mixed bone marrow chimerism, tolerance was shown to be dependent on central deletional mechanisms following the migration of donor cells to the recipient thymus (29–31). This experimental approach has been extended to large animals (pig) as well as non-human primates (monkey) with similar success including a recent report stating that some of the original monkeys have now maintained allograft tolerance for 10 years without immunosuppression or evidence of rejection (32–34). However, unlike the long-term chimerism observed in mice, mixed chimerism in monkeys is transient and undetectable after several months yet, even in the absence of immunosuppression there is continued survival of the kidney allograft, suggesting a role for peripheral tolerance or regulation by CD4+CD25+ T cells (34, 35). Furthermore, the absence of late deterioration in renal allograft function, lack of chronic vasculopathy and differential absence of allo-antibody in splenectomy compared to no splenectomy transplants suggest alloreactive B cells and alloantibodies as barriers to tolerance. Notably, these findings are consistent with recent studies by Porcheray et al., who analyzed five patients that received combined renal-bone marrow transplants from the same haploidentical donor (36). Even in the presence of T cell unresponsiveness, 3 of them developed de novo donor specific antibodies. Early alloantibody production in one of five patients resulted in acute antibody-mediated rejection while late alloantibody production observed in two patients has resulted in chronic rejection in one patient. The exact mechanism responsible for the production of antibody by B cells in the presence of T cell tolerance is still unclear however, one might speculate on post-transplant antibody production that is independent of T cell help or an inadequate control of T cell help in these models. These observations underscore the necessity of controlling alloantibody responses, and the ability of alloantibodies to antagonize stable transplantation tolerance.
An alternative model of studying transplantation tolerance uses the approach of targeting T cell co-stimulation, which avoids the potentially harmful effects of irradiation and conditioning regiments used in mixed chimerism models. The co-stimulation pathways of CD28–CD80/CD86 and CD154–CD40 have been extensively studied in these animal models. In particular targeting CD154 (CD40L), which is expressed on activated T cells and binds CD40 leading to APC activation and upregulation of B cell function (37), is effective in preventing allograft rejection and inducing long-term graft acceptance in animal models of transplantation, especially when combined with donor specific transfusion (DST) (38–41). Long-term survival of the allograft is associated with sustained inhibition of alloantibody responses (39). Indeed, in non-human primates treated with anti-CD154, late graft rejection was, in the majority of cases, coincident with the development of DSA, whereas rejection-free survival was characterized by the absence of DSA (42). As in the case of the bone marrow chimeras, it is unclear what triggers the late production of DSA production and graft rejection.
The induction of tolerance with therapies directed at blocking co-stimulation on T cells has been shown to fail in the presence of memory alloreactive T cells (43, 44). These observations raise the question of whether recipients with a presensitized B cell compartment would also be resistant to tolerance induction. Using the 3–83 BCR-knockin (3–83KI) mouse in which the majority of B cells express the 3–83 BCR that recognizes the H-2Kk and H-2Kb alleles, Burns et al. (45) developed a murine heart transplant model that isolated the contributions of memory B cells from memory T cells. By transplanting C3H (H-2k) hearts into 3–83KI mice in the absence of immunosuppression, memory 3–83 B cells with dual specificity for H-2Kk and H-2Kb and memory T cells specific for H-2k but not H-2b were generated. When the C3H-primed 3–83KI recipients were transplanted with C57BL/6 hearts (H-2b), anti-CD154 treatment was no longer able to induce tolerance. Resistance to tolerance induction was transferrable by B cells but not T cells from presensitized 3–83KI mice, and not by B cells from naïve 3–83KI mice. Finally, they reported that the optimal resistance to tolerance induction was observed with the combination of memory B cells and alloantibodies, raising the possibility that memory B cells were rapidly differentiating into antibody-secreting cells upon alloantigen re-encounter, and that alloantibodies are the critical mediator of resistance to tolerance induction.
Insights into how antibodies prevent allograft acceptance were revealed in studies by Bickerstaff et al. (46) using a spontaneously accepting model of A/J (H-2a) renal allograft transplanted into C57BL/6 recipients (H-2b). When CCR5-deficient C57BL/6 recipients were used, the A/J renal allografts were acutely rejected. Histology revealed marked C3d deposition, neutrophil and macrophage margination, interstitial hemorrhage and edema and glomerular fibrin deposition, consistent with AHR. Further, rejection was associated with high serum donor-reactive antibody titers and was independent of CD8+ T cells. While the reason for the enhanced antibody production in CCR5-deficient mice requires further elucidation, these observations illustrate the ability of antibodies to prevent graft acceptance by binding directly to the allograft and inducing classic AHR as a mechanism by which graft acceptance is antagonized.
Burns et al. (47) tested whether alloantibodies could prevent tolerance via mechanisms independent of binding to the graft vascular endothelium to mediate AHR. To this end they used a skin allograft model, which is more resistant to AHR, and where long-term graft acceptance was induced by treatment with anti-CD154 in combination with donor spleen cells (DST). When anti-Kd mAbs were administered on the day of skin transplantation, anti-CD154/DST administration was no longer able to induce long term graft survival and a T cell-dependent acute rejection was precipitated. By using an approach of adoptive transfer of T cells from TCR75, OT-II and OT-I T cell receptor transgenic (TCR-Tg) mice into C57Bl/6 recipients of membrane ovalbumin (mOVA)-expressing BALB/c skins, they demonstrated that the ability of anti-Kd to enhance the priming of anti-Kd-specific T cells via the indirect pathway, as well as of non-Kd reactive OVA-specific CD4+ and CD8+ T cells. Further, the prevention of tolerance was mediated by anti-Kd binding to and converting DST from tolerogenic to immunogenic. Collectively their observations suggested that donor cells coated with alloantibodies were delivered to APC’s in a manner that enhanced their uptake, which then allowed the presentation of donor antigens recognized by the circulating alloantibody as well as other linked alloantigens. The uptake of these opsonins also facilitated the maturation of the APC’s so that they were able to stimulate alloreactive T cells in a CD40-CD154 independent manner.
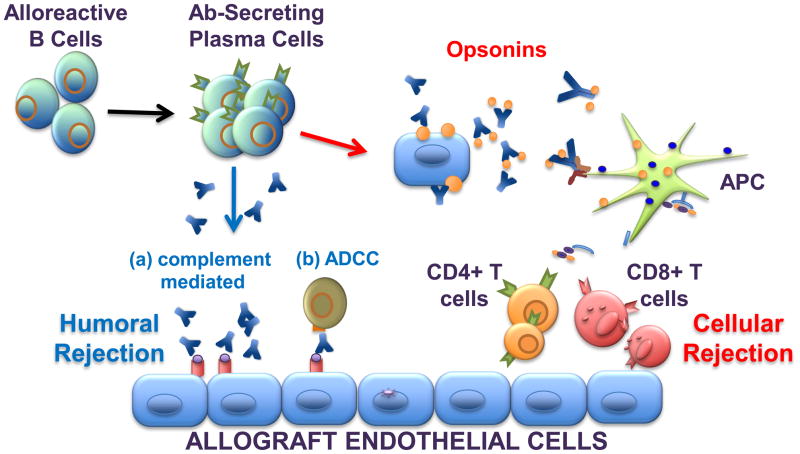
Studies focused on models of tolerance have revealed that antibodies can function within the graft to induce AHR or as opsonins to enhance T cell activation, thereby preventing the induction of tolerance (Figure 1). These two mechanisms do not provide a complete understanding of how antibodies prevent long-term graft acceptance and it is likely that other functions of B cells and antibodies may also contribute (Figure 1). Indeed, recent studies demonstrating the ability of alloantibodies to mediate chronic allograft rejection in a complement-independent manner, and where a role for antibodies and NK cells as well as other FcγRII+ cells was evoked, are likely to be relevant to explaining how alloantibodies prevent allograft acceptance even in the face of T cell tolerance.
Figure 1.
Antibodies play diverse roles in allograft rejection. Following allograft transplantation, alloreactive B cells are activated and differentiate into antibody secreting plasma cells. The secreted antibodies bind to alloantigen expressed on the graft endothelial cells to mediate humoral rejection via complement-dependent and antibody-dependent cellular cytotoxicity (ADCC) mechanisms, the latter involving FcγR-bearing natural killer cells. Alternatively, donor cells or cell fragments can be bound by antibodies to form opsonins, which are then engulfed by recipient FcγR-bearing macrophages and dendritic cells. Within these antigen presenting cells (APC), donor antigen is processed and presented in the context of MHC class I and II to recipient CD8+ or CD4+ T cells, respectively. These indirectly primed alloreactive effector T cells then mediate cellular rejection of the allograft. Both humoral and cellular processes can occur concurrently to induce mixed antibody and cell-mediated rejection.
Both the clinical and experimental data point to alloantibodies as barriers to the induction tolerance and long-term allograft acceptance as well as the importance of controlling alloantibody production long term. Insights into the fate of alloreactive B cells during the successful induction and maintenance of tolerance,, as well as when B cell tolerance is lost and alloantibody production is restored, are critical to achieving this goal.
3. Tracking the Fate of Alloreactive B cells During Transplantation Tolerance
Clear demonstration of B cell tolerance in the clinic comes from the studies of Fan et al. (48) in which ABO-incompatible heart transplantation during infancy resulted in the acquisition of B cell tolerance to donor blood group A and B antigens. Tolerance in that setting was associated with an absence of donor-reactive B cells, which could reflect functional inactivation or deletion. These findings of B cell tolerance to transplant antigens appear to recapitulate aspects of neonatal tolerance, as the development of antibodies against the non-self blood group antigens is relatively delayed and remain low during the first months of life in humans. More recently, Urschel et al. (49) reported a similar absence of donor specific anti-HLA antibodies when heart transplantation was performed in patients within the first 24 months of life. These observations raise the intriguing possibility of clinical induction of B cell tolerance to a broader range of transplant antigens. Further insights into the mechanisms of B cell tolerance in these transplant recipients will require an understanding of the fate of the B cells producing donor-reactive antibodies during tolerance, as well as a basic understanding of how self-reactive B cells are normally censored. Tolerance mechanisms associated with low-avidity soluble auto-antigens have been discussed in a number of recent excellent reviews (50–53), and as membrane bound MHC molecules are major targets of the allogeneic immune response, mechanisms gained from models of tolerance to membrane-bound high-avidity self-antigen are more relevant to transplantation and will be discussed here.
The importance of clonal deletion in B cell tolerance to auto-antigens was first revealed by the seminal studies of Goodnow and colleagues using the Hen Egg Lysozyme (HEL) system (54). The heavy and light chain cDNAs from the HyHEL10 anti-HEL B cell hybridoma were identified and used to generated HEL-BCR-Tg mice. In the absence of the HEL antigen, approximately 70–90% of mature B cells in the HEL-BCR-Tg mice were capable of binding HEL but when HEL was ubiquitously expressed as a membrane antigen, the mature B cell compartment was significantly reduced and the residual allotype-positive B cells were incapable of binding HEL. These observations suggest clonal deletion as the primary mechanism for the elimination of self-reactive B cells encountering antigen during development in the bone marrow.
In a second model for B cell tolerance to membrane bound autoantigen studied by Nemazee and colleagues, a BCR transgenic mouse was created from the heavy and light chains of the 3–83 hybridoma that recognizes several alleles of H-2K (k, b, bm3) with differing affinities (55). As in the HyHEL10 mouse model, the 3–83 BCR transgenic B cells were deleted in bone marrow upon encounter of cognate antigen. However a prescient observation was made that some of the 3–83 B cells modified their transgenic BCR to avoid recognizing self antigen, a process that is now termed receptor editing (56). Mechanistically, anti-self pre-B cells reactivate the RAG genes and the recombination machinery to reinitiate rearrangement at the light chain loci (kappa and lambda); thereby simultaneously deleting the self-reactive light chain and assembling a new BCR composed of the original heavy chain paired with a new light chain. Using mice in which the 3–83 heavy and light chains were ‘knocked-into’ the IgH and Igkappa loci, Pelanda R et al. (57) demonstrated that receptor editing is, in fact, the main mechanism of censoring B cell autoreactivity in the bone marrow. Further, by comparing receptor editing to clonal deletion of self-reactive B cells, it was found that deletion contributed little to the overall negative selection process and is likely to occur only after the editing process is exhausted, and that receptor editing is the dominant mechanism of censoring autoreactive B cells in the bone marrow (58).
In further studies using the 3–83 BCR transgenic mouse, Russell et al. reported that when H-2Kb autoantigen expression was restricted to the liver, significant deletion of 3.83 B cells was also observed suggesting that elimination of autoreactive B cells can occur at sites other than the bone marrow (59), an observation that may have important implications for transplantation. Additionally, an acute challenge with high-affinity cognate antigen introduced intra-peritoneally resulted in a significant decrease in the percentage of antigen-specific B cells in the peritoneal cavity. However only the highest affinity antigens were able to induce deletion whereas immature B cells underwent receptor editing. While differences in time of antigen exposure and B cell lineages in the peritoneal cavity suggest other explanations, these observations are nonetheless consistent with the notion that developing B cells are more sensitive to tolerance induction than mature B cells.
Overall, the mechanisms of mature B cell inactivation in the periphery remains poorly understood. The importance of controlling mature and post-mutational B cells that are autoreactive are underscored by the recent observations that the process of somatic hypermutation to generate high-affinity antibodies can result in the inadvertent generation of autoreactive B cells from non-autoreactive B cells (60). In those studies, it was observed that the majority of autoreactive B cells in a mouse model of systemic lupus erythematosus (SLE) arose from non-autoreactive B cells that diversified their immunoglobulin genes via somatic hypermutation. Thus, understanding how B cells that are either reactive to self-antigens not expressed in the bone marrow or reactive to antigens expressed only after full B maturation, develop is critical for the development of strategies that control of autoimmunity and also to understanding of alloantigen-specific B cell inactivation during transplantation tolerance.
One of the first studies tracking the fate of B cells reactive to transplantation antigens come from the studies by Sykes and colleagues investigating the fate of Mac-1-negative B-1b B cells secreting antibodies specific for the carbohydrate antigen, Gal alpha1,3Gal beta 1,4GlcNac (Gal) (61). In Gal-deficient mice, anti-Gal antibodies are produced by B-1b cells in the absence of overt sensitization, but this anti-Gal B cell response was tolerized in stable mixed bone marrow chimeras (62, 63). In subsequent analysis to determine the mechanism of Gal-specific B cell tolerance, Kawahara et al (64) reported that early B cell hypo-responsiveness was dependent on the persistence of antigen and B cell anergy, while the late hypo-responsiveness was independent of antigen and was due to clonal deletion and/or editing. While Gal-specific B cells were detectable by flow cytometry using fluorochrome-coupled Gal-conjugates, their low frequencies hampered further analysis of their fate during tolerance.
The opportunity to track the fate of alloreactive B cell during tolerance using the 3–83KI mouse system was first recognized by Chong and colleagues. B cells expressing the 3–83 BCR that recognize H-2Kb are present at high frequencies on the BALB/c background, and when these 3–83KI mice were used as recipients of H-2Kb expressing C57BL/6 allografts, they behaved comparably to wild-type (WT) BALB/c recipients. The 3–83KI mice acutely rejected the cardiac allografts with normal kinetics and long-term graft acceptance was successfully induced with anti-CD154 and DST (65, 66). This long-term graft acceptance was associated with profound clonal deletion of 3–83 B cells in the blood and in the lymph nodes, with a partial deletion in the spleen. Phenotypic analysis of remaining 3–83 B cells in the spleen indicated that the residual cells were enriched for immature IgMhiIgDlow B cells. The deletion was the result of a lack of CD4+ T cell help and required the presence of the allograft. Thus, similar to the situation of 3–83 cells recognizing liver-expressed self-antigen, clonal deletion was the major consequence of allograft tolerance however the B cell subset most susceptible to deletion was the mature B cell. It is unclear from those studies why the immature B cells, which are highly susceptible to elimination in models of B cell tolerance to self-antigen, are not eliminated. Also, whether the residual 3–83 B cells are controlled by cell intrinsic anergy mechanisms or have regulatory properties that facilitate the maintenance of tolerance are important issues that remained unaddressed.
Noorchashm and colleagues also used the 3–83 BCR-Tg mouse model to determine the fate of alloreactive B cells when they encountered alloantigen during the immature/transitional stage (67). To this end, they generated a bone marrow reconstitution model in BALB/c scid mice where bone marrow-derived B cells were allowed to reconstitute for two weeks. At this time, when the majority (65%) of the repopulating immature B cells were in the transitional IgMhiCD21lo stage, allogeneic bone marrow cells from T-cell-deficient BALB/c mice were infused. Following establishment of a steady-state B cell compartment (74 days post-bone marrow transplantation), the bone marrow chimeras were transplanted with a BALB/c cardiac allograft. To precipitate rejection, a normal population of T cells was adoptively transferred 14 days later. Allografts were acutely rejected, however this occurred without the development of BALB/c-specific alloantibody responses. To track the fate of alloreactive B cells in this model, T-cell-depleted mixed bone marrow chimeras comprising of bone marrow cells from 3.83-Tg and BALB/c mice (1:1) were generated and treated as above. Significant 3–83 B cell deletion was observed suggesting that the infusion of alloantigen bearing bone marrow during their development results in the elimination of antigen-reactive cells. In contrast, in chimeric mice that did not receive a bone marrow transfusion, the 3–83 B cells matured normally. However, when these mice were transplanted with a BALB/c heart, the 3–83 cells were not deleted but were arrested in an IgMloIgDloCD21lo phenotype with a high turnover rate, reminiscent of an anergic peripheral B cell phenotype. One explanation for the lack of 3–83 B cell elimination in the heart transplant model compared to B cell transfusion is inadequate alloantigen from the heart allograft accessing the bone marrow, compared to the transfusion of B cells. These observations with the heart allograft also contrast with the scenario where 3–83 transgenic mice were crossed to liver-H-2Kb transgenic mice and where 3–83 cell deletion was observed as well. It is possible that the environment where immature B cells encounter antigen may also affect they way they are censored.
The 3–83 mouse system has proven to be a useful way to track the fate of both autoreactive as well as alloreactive B cells and has revealed that, depending on the stage of B cell development that the alloantigen encounter takes place or the approach to induce tolerance, the outcome of developmental arrest may be different. These observations underscore the multiple checkpoints in place that regulate B cell differentiation and raise the related question of how these checkpoints may be breached, especially as it pertains to graft rejection seen in the clinic and non-human primate scenarios (36, 42). Finally, despite its utility, several caveats of applying the 3–83 system to the study of B cell tolerance in transplantation exist: first, the precursor frequency of the 3–83 alloreactive B cells approaches 90% of B cells; second, the 3–83 mice cannot be on a C57BL/6 background, where most immunological genetic modifications exist, if 3–83 B cells are to develop into the periphery, and finally, there is a very high level of circulating 3–83 IgG in naïve 3–83 mice making it impossible to quantify the 3–83 IgG response post-transplantation. A system to track alloreactive B cells in wild-type mice would provide the most physiological answers, but the low frequency of these cells would limit the scope of mechanistic investigations. Thus, this approach together with a complementary system of an alloreactive BCR-KI mouse model on a C57BL/6 background will provide the ideal setting for investigations of alloreactive B cell fate in tolerance and rejection.
4. B cells as Mediators of Tolerance
Recent studies aimed at developing a gene signature to accurately diagnose the tolerant state reveal an enriched B-cell signature in operationally tolerant recipients relative to stable immunosuppressed recipients (27, 42, 68). These observations raise the possibility of using the B cell signature as a biomarker of tolerance with the hypothesis that B cells may play a role in the maintenance of transplantation tolerance. The latter notion is supported by the recent emerging literature of a rare subset of B cells with regulatory function.
B cells with the capacity to regulate T cell function were originally described by Shimamura et al. (69–71) and independently by Zubler et al. (72, 73) over 30 years ago. In a system of high dose (4 × 10^9) SRBC into C57BL/6 mice, Shimamura et al. (71) reported that splenic B cells were able to induce antigen-specific suppressor T cells that subsequently inhibited B cells responses. In contrast, Zubler et al. identified T cell-dependent B cells that caused potent specific feedback suppression of humoral responses via IgVH-restricted antibodies. A decade later Wolf et al. (74) reported that mice deficient in B cells developed experimentally-induced encephalomyelitis (EAE) comparably as mice sufficient in B cells, however their recovery was impaired suggesting that B cells may contribute to the immunomodulation of acute EAE. More recently, B regulatory cells (Bregs) have been reported to exert immunosuppressive function in murine models of colitis, arthritis, autoimmune diabetes (NOD mice), SLE, contact hypersensitivity and EAE (75, 76) (77). Studies aimed at identifying the B cells that have suppressive activities have not yielded a consensus phenotype but two phenotypically distinct subsets of Bregs have been extensively characterized. They are the immature/transitional 2-marginal zone precursors (T2-MZP) described by Mauri and colleagues (76, 78) and CD5+CD1dhi B10 cells by Tedder and colleagues (75, 79) (Table 1). Both these Breg subsets express high levels of CD1d, CD21, CD24, IgM and moderate levels of CD19. Additionally, T2-MZP Bregs express CD93 and CD23, while B10 cells express CD5. The relationship between these Bregs is not known, but functionally both subsets exert their immunomodulatory activity by their production of IL-10. Indeed, IL-10 producing B cells have frequently become used as a functional marker of regulatory B cells. Other mechanisms of immunomodulation include the secretion of TGF-beta by activated B cells that down-regulate Th1 immunity (80) or induce CD8+ anergy (81), and the induction of regulatory T cells.
Table 1.
| Mouse Spleen | Peritoneum | |||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 (Anergic) | B-1b | MZ | Follicular | Breg T2/MZP | Breg B10 | B-1a |
| B220+ | B220++ | B220+ | B220++ | B220+++ | B220+++ | B220+ | ||
| IgMhi | IgMlow/hi | IgMlow | IgMhi | IgMhi | IgMlow | IgM+ | IgMhi | IgMhi |
| IgDlow | IgDhi | IgDhi | IgDlow | IgDlow | IgDhi | IgDhi | IgDlo | IgDlow |
| CD23low | CD23+ | CD23+ | CD23+/− | CD23+/− | CD23++ | CD23+ | CD23+/− | CD23+/− |
| CD24hi | CD24dim | CD24low | CD24dim | CD24dim | CD24low | CD24hi | CD24hi | CD24dim |
| CD93+ | CD93+ | CD93+ | CD93− | CD93− | CD93− | CD93+ | CD93− | CD93− |
| CD43low | CD43− | CD43− | CD43− | CD43− | CD43+ | |||
| CD21low | CD21low | CD21+/− | CD21hi | CD21+ | CD21low | CD21hi | CD21+/− | |
| CD5− | CD5− | CD5+ | CD5+ | |||||
| CD1dhi | CD1dhi | |||||||
| CD11b+ | ||||||||
Yellow highlight: Cell surface markers in common between T2/MZP and T2 cells.
Blue highlight: Cell surface markers in common between B10 Bregs and B-1a cells.
Early studies by Reichardt P, Dombach B et al. (82), revealed that naïve B cells form very stable interactions with T cells while contacts between DC’s and T cells were brief and sequential. Additionally, DC interactions resulted in the generation of classical effector T cells, while those with naïve B cells generated activated T cells with regulatory capacity, also referred to as bTregs. These observations were confirmed and extended by a series of co-culture experiments performed by Tu and colleagues (83–86). In those studies, activated B cells (CD154-expressing cells plus IL-4 and Cyclosporine A) were mixed with purified naïve CD4+CD45RA+CD45RO−CD25− T cells in vitro. This resulted in a potent induction and expansion of regulatory T cells with the phenotype of CD4hiCD25+FoxP3+ (85). Similarly, these activated B cells were also able to induce CD8+CD45RA+CD45RO−CD25− T cells to become regulatory CD8hiCD25hiFoxP3+ T cells (84). The phenotype of the activated B cells were IgMlowIgDlow, expressed high levels of MHC Class I/II, CD80, and CD86, and interestingly, secreted IL-2. When compared to immature DC, the activated B cells were functionally superior at inducing regulatory T cells and this was proposed to be due to their ability to produce IL-2 (83). That activated B cells may be important for the expansion of regulatory T cells in vivo was reported recently also by Carter et al. (87) in a mouse model of autoimmune arthritis. They reported that in the absence of IL-10 producing T2-MZP B cells, disease exacerbation was associated with reduced induction of regulatory FoxP3+ T cells and the expansion of Th1/Th17 cells. These observations suggest that a close relationship can exist between the regulatory T and B cell subsets, and that this may explain how B cells may play an important role modulating autoreactivity.
There is accumulating evidence for a critical role for B cells in the induction and/or maintenance of allograft tolerance. Yan and colleagues (88) initially reported that the acceptance of PVG rat kidneys in fully-mismatched DA recipients was induced by the intravenous injection of donor B cells (MST >300 days) but not T cells (MST 17 days). Graft acceptance was associated with a rapid increase in IL-2, IFNgamma and IL-10 within the graft, to levels that far exceeded the rejecting grafts. However, the phenotype of the B cells and the mechanisms by which they induce graft acceptance was not elucidated.
Le Texier et al. (89) more recently demonstrated in a rat model of long-term cardiac allograft tolerance induced by a deoxyspergualin analog (treatment from day 0–20 post-transplantation) that tolerant allografts contain B cells organized into germinal centers with minimal IgG production. Furthermore, B cells blocked in class switch to IgG progressively accumulated in the graft and blood after cessation of immunosuppression. Phenotypic analysis of the B cells in the blood of tolerant allograft recipients compared to those receiving syngeneic grafts revealed increased mRNA expression of BANK-1, a B-cell scaffold protein that is tyrosine phosphorylated upon BCR stimulation and may play a dominant role in attenuating CD40-mediated AKT activation, thereby preventing hyperactive B cell responses (90). In addition, the ratio of the transcripts for the inhibitory receptor, FcγR2b, relative to the activating receptor, FcγR2a, was upregulated in the blood and grafts of tolerant recipients compared to those undergoing chronic rejection. Taken together, these observations led the authors to conclude that tolerant recipients were characterized by the accumulation of B cells with an inhibitory profile. Indeed, either B cells or CD4+ T cells alone, from the spleens of tolerant recipients were able to transfer tolerance, leading the authors to conclude that B cells and T cells play important roles in the maintenance of transplantation tolerance (89). It remains to be tested as to whether the inhibitory phenotype on B cells is essential for the lack of differentiation into antibody-secreting plasma cells and/or to the regulatory properties of the transferred B cells.
In a modified model of mixed bone marrow chimerism to induce transplantation tolerance using a conditioning regimen of 3 Gy total body irradiation and anti-CD154 mAb followed by allogeneic bone marrow transplantation, Sykes and colleagues reported that both donor and recipient B cells as well as recipient CD4+ T cells were necessary for the rapid deletion of recipient CD8+ T cell and the induction of stable mixed bone marrow chimerism (91–93). Donor B cells were sufficient and the expression of MHC Class II was essential for recipient CD4+ T cells to mediate the deletion of recipient alloreactive CD8+ T cells (92). Likewise recipient Class II, B cells and dendritic cells were required for alloreactive CD8+ T cell tolerance (91). However, it was subsequently reported that MHC Class I and/or II, CD80 and CD86 on recipient DC’s but not B cells was necessary to promote recipient alloreactive CD8+ T cell tolerance (93). IL-10 production was not necessary for both donor and recipient B cells. Thus, in this model of mixed bone marrow chimerism as a means of achieving tolerance to solid organ allografts, donor and recipient B cells are both essential however, the mechanisms by which recipient B cells contribute to recipient CD8+ tolerance remains to be defined.
In another experimental model in which long-term acceptance of cardiac allografts in mice was induced with anti-CD45RB, Deng et al. (94) reported on a critical role for B cells, as anti-CD45RB treatment induced tolerance to cardiac allografts in wildtype but not in μMT−/− or Jh−/− recipients, distinct mouse models that lack B cells and antibodies. Tolerance was restored by the transfer of B cells as well as by B cells incapable of secreting antibodies, but not by B cells lacking CD45, CD40 and CD80/86. Subsequently, Huang et al. (95) reported that the expression of CD54 (ICAM-1) on B cells was necessary, suggesting that interactions between recipient T cells with recipient B cells is necessary for the induction of tolerance with anti-CD45RB. Zhao et al. (96) investigated the role of IL-10 in this model, and unexpectedly observed that IL-10 played a counter-regulatory role. Antibody-mediated neutralization of IL-10 enhanced graft survival and tolerance, an observation that was replicated using IL-10-deficient recipients. Further, this model of graft acceptance, induced with anti-CD45RB, resulted in DSA and chronic allograft vasculopathy, both of which were significantly reduced in the absence of IL-10. These observations collectively point to an unexpected counter-regulatory role for IL-10, however whether the source of IL-10 is from B cells and the mechanism by which B cells facilitate tolerance in this model are currently unclear but were speculated by the authors to be consistent with B cells facilitating the generation of induced Tregs.
An extensive investigation into the role of B cells in facilitating long-term islet graft survival in BALB/c recipients treated with the low-affinity antagonistic anti-T cell Ig mucin-1 (TIM-1) antibody (RMT-1) has been recently been reported (97). This anti-TIM-1 mAb was originally reported to inhibit autopathogenic Th1 and Th17 responses and to induce a strong Th2 response that protected from EAE (98). Additionally anti-Tim-1 is effective in prolonging the survival of allogeneic heart grafts in a STAT-6-dependent manner that was associated with a Th1 to Th2 cytokine switch (99). Subsequently Yuan et al (100) reported that anti-TIM-1 was able to overcome resistance to tolerance mediated by CD8+ Tc17 cells. These observations suggested that Tim-1 antibody treatment prevented alloreactive Th1 and CD8 Tc17 cell expansion while preserving Tregs and the induction of transplantation tolerance.
In opposition to the T cell-centric view of TIM-1 function and transplantation tolerance, Ding et al. (97) recently reported that the predominant lymphocyte expressing TIM-1 was B cells (5–8% of splenic B cells from naïve and 10–15% from anti-TIM-1 treated mice). Furthermore, the majority of B cells producing IL-4 and IL-10 were within the TIM-1 expressing population. Indeed, careful analysis of the B cells indicate that TIM-1 identified most of the IL-10-producing B cells, and that TIM-1 expression was enriched across a wide spectrum of B cell subsets, including the immature transitional 1 (IgM+IgD−CD21−CD23−), T2-MZP (IgM+IgD+CD21+CD23hi), marginal zone (IgM+IgD−CD21hiCD23−), follicular (IgM−IgD+CD21+CD23+), CD1dhiCD5+ and B1a (CD5+) B cells. Prolongation of graft acceptance with anti-TIM-1 was dependent on B cells while anti-TIM-1 treatment significantly accelerated rejection in the absence of B cells. Further, sort-purified TIM-1+ B cells from anti-TIM-1 treated islet allograft recipients transferred donor-specific tolerance and TIM-1+ B cell production of IL-4 and IL-10 was necessary. IL-10-deficiency had no effect on TIM-1 expression while IL-4- and IL-4R-deficiency significantly reduced TIM-1 expression and IL-10 production on B cells suggesting that IL-4 signaling was crucial for the differentiation into TIM-1+ IL-10+ regulatory B cells. These observations were confirmed in vitro, where TIM-1- B cells acquired TIM-1 expression and IL-10 production following BCR and IL-4 signaling. Collectively the studies by Ding et al. (97) delineate a phenotypically distinct population of TIM-1+ B cells that mediate islet allograft acceptance in BALB/c recipients, and whose regulatory properties were IL-4 and IL-10 dependent.
In summary, accumulating in vivo data demonstrate that B cells can acquire regulatory properties under different conditions of graft acceptance, but the mechanisms by which they do so are unexpectedly distinct, suggesting considerable heterogeneity in the regulatory B cell repertoire. Thus, there is a pressing need to understand when and how B cells play critical roles in mediating allograft tolerance and whether they can be harnessed for therapeutic purposes.
5. Concluding Remarks
Advances and insights gained from experimental models of tolerance have significantly increased our understanding of B cells and their role in transplantation tolerance. Indeed, over the last few years, results realized in the laboratory have made their way to the clinic and have been applied, albeit with varying success, to treatment modalities in human allograft transplantation. However, there remains many hurdles to achieving tolerance in the clinic; these include an incomplete understanding of the mechanisms involved in T and B cell tolerance to allogeneic solid organs, a lack of a reliable in vitro assay or predictor of tolerance, as well as an ethical concern and clinical risk of immunosuppression withdrawal in transplant recipients. In particular, there remains much to be understood regarding the role of B cells as mediators and antagonists of transplant tolerance, such that their protective activities can be preserved while their pathogenic activities curtailed. Another critical area of investigation is understanding how the humoral and T cell arm of the adaptive immune response is coordinately and independently suppressed over the life of the allograft. Rapid advances made over the last several years, combined with the fervent pursuit of translating laboratory results into the clinical realm, offer the possibility that we are getting closer to the day when immunologic tolerance is a reality.
Highlights.
Alloantibodies bind to graft endothelium to induce humoral rejection
Antibodies generate opsonins that enhance T cell-mediated rejection
Enrichment of B cells as a potential biomarker for tolerance to renal allografts
Evidence for a regulatory role by B cells in transplantation tolerance
Visualizing alloreactive B cells during tolerance will provide mechanistic insights
Acknowledgments
We thank Dr. David Rothstein for sharing his pre-publication manuscript (Ding et al.) and Dr. James Williams for his editorial comments. We are grateful for grant support R56AI043631, R01AI083452 and R01 AI072630 to ASC.
Abbreviations
- 3–83KI mice
3–83-knockin mouse
- ADCC
antibody-dependent cellular cytotoxicity
- AHR
acute humoral rejection
- APC
antigen presenting cells
- B10 cells
IL-10-producing regulatory B cells
- BCR
B cell receptor
- DSA
donor specific antibody
- EAE
experimentally-induced encephalomyelitis
- FcγR
Fc gamma receptors
- mOVA
membrane ovalbumin
- Gal
Galalpha1,3Gal beta 1,4GlcNac
- HEL
hen egg lysozyme
- ICAM-1
inter-cellular adhesion molecule-1
- MHC
major histocompatibility complex
- SLE
systemic lupus erythematosus
- T2-MZP
P immature/transitional 2-marginal zone precursors
- TIM-1
T cell Ig mucin-1
- TCR-Tg
T cell receptor transgenic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 2.Lerut J, Sanchez-Fueyo A. An appraisal of tolerance in liver transplantation. Am J Transplant. 2006;6(8):1774–1780. doi: 10.1111/j.1600-6143.2006.01396.x. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Fueyo A. Identification of tolerant recipients following liver transplantation. Int Immunopharmacol. 2010;10(12):1501–1504. doi: 10.1016/j.intimp.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Fong TL, Bunnapradist S, Jordan SC, Selby RR, Cho YW. Analysis of the United Network for Organ Sharing database comparing renal allografts and patient survival in combined liver-kidney transplantation with the contralateral allografts in kidney alone or kidney-pancreas transplantation. Transplantation. 2003;76(2):348–353. doi: 10.1097/01.TP.0000071204.03720.BB. [DOI] [PubMed] [Google Scholar]
- 5.Gordon RD, Fung JJ, Markus B, Fox I, Iwatsuki S, Esquivel CO, et al. The antibody crossmatch in liver transplantation. Surgery. 1986;100(4):705–715. [PMC free article] [PubMed] [Google Scholar]
- 6.Orlando G, Soker S, Wood K. Operational tolerance after liver transplantation. Journal of hepatology. 2009;50(6):1247–1257. doi: 10.1016/j.jhep.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen A, Davies HF, Jamieson NV, Evans DB, Calne RY. Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. Transplantation. 1995;59(6):919–921. [PubMed] [Google Scholar]
- 8.Ruiz R, Kunitake H, Wilkinson AH, Danovitch GM, Farmer DG, Ghobrial RM, et al. Long-term analysis of combined liver and kidney transplantation at a single center. Arch Surg. 2006;141(8):735–741. doi: 10.1001/archsurg.141.8.735. discussion 741–732. [DOI] [PubMed] [Google Scholar]
- 9.Orlando G, Hematti P, Stratta RJ, Burke GW, 3rd, Di Cocco P, Pisani F, et al. Clinical operational tolerance after renal transplantation: current status and future challenges. Annals of surgery. 2010;252(6):915–928. doi: 10.1097/SLA.0b013e3181f3efb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roussey-Kesler G, Giral M, Moreau A, Subra JF, Legendre C, Noel C, et al. Clinical operational tolerance after kidney transplantation. Am J Transplant. 2006;6(4):736–746. doi: 10.1111/j.1600-6143.2006.01280.x. [DOI] [PubMed] [Google Scholar]
- 11.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11(3):450–462. doi: 10.1111/j.1600-6143.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- 12.Halloran PF. Immunosuppressive drugs for kidney transplantation. The New England journal of medicine. 2004;351(26):2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 13.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. The New England journal of medicine. 2003;349(24):2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 14.Castillo-Rama M, Castro MJ, Bernardo I, Meneu-Diaz JC, Elola-Olaso AM, Calleja-Antolin SM, et al. Preformed antibodies detected by cytotoxic assay or multibead array decrease liver allograft survival: role of human leukocyte antigen compatibility. Liver Transpl. 2008;14(4):554–562. doi: 10.1002/lt.21408. [DOI] [PubMed] [Google Scholar]
- 15.Gloor JM, Winters JL, Cornell LD, Fix LA, DeGoey SR, Knauer RM, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10(3):582–589. doi: 10.1111/j.1600-6143.2009.02985.x. [DOI] [PubMed] [Google Scholar]
- 16.Ogura K, Terasaki PI, Koyama H, Chia J, Imagawa DK, Busuttil RW. High one-month liver graft failure rates in flow cytometry crossmatch-positive recipients. Clinical transplantation. 1994;8(2 Pt 1):111–115. [PubMed] [Google Scholar]
- 17.O’Leary JG, Kaneku H, Susskind BM, Jennings LW, Davis GL, Klintmalm GB, et al. High Mean Fluorescence Intensity Donor-Specific Anti-HLA Antibodies Associated With Chronic Rejection Postliver Transplant. Am J Transplant. 2011 doi: 10.1111/j.1600-6143.2011.03593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns JM, Cornell LD, Perry DK, Pollinger HS, Gloor JM, Kremers WK, et al. Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. Am J Transplant. 2008;8(12):2684–2694. doi: 10.1111/j.1600-6143.2008.02441.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee PC, Zhu L, Terasaki PI, Everly MJ. HLA-specific antibodies developed in the first year posttransplant are predictive of chronic rejection and renal graft loss. Transplantation. 2009;88(4):568–574. doi: 10.1097/TP.0b013e3181b11b72. [DOI] [PubMed] [Google Scholar]
- 20.Lee PC, Terasaki PI, Takemoto SK, Lee PH, Hung CJ, Chen YL, et al. All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation. 2002;74(8):1192–1194. doi: 10.1097/00007890-200210270-00025. [DOI] [PubMed] [Google Scholar]
- 21.Sarwal M, Chua MS, Kambham N, Hsieh SC, Satterwhite T, Masek M, et al. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. The New England journal of medicine. 2003;349(2):125–138. doi: 10.1056/NEJMoa035588. [DOI] [PubMed] [Google Scholar]
- 22.Hippen BE, DeMattos A, Cook WJ, Kew CE, 2nd, Gaston RS. Association of CD20+ infiltrates with poorer clinical outcomes in acute cellular rejection of renal allografts. Am J Transplant. 2005;5(9):2248–2252. doi: 10.1111/j.1600-6143.2005.01009.x. [DOI] [PubMed] [Google Scholar]
- 23.Tsai EW, Rianthavorn P, Gjertson DW, Wallace WD, Reed EF, Ettenger RB. CD20+ lymphocytes in renal allografts are associated with poor graft survival in pediatric patients. Transplantation. 2006;82(12):1769–1773. doi: 10.1097/01.tp.0000250572.46679.45. [DOI] [PubMed] [Google Scholar]
- 24.Zarkhin V, Kambham N, Li L, Kwok S, Hsieh SC, Salvatierra O, et al. Characterization of intra-graft B cells during renal allograft rejection. Kidney Int. 2008;74(5):664–673. doi: 10.1038/ki.2008.249. [DOI] [PubMed] [Google Scholar]
- 25.Bian H, Reed EF. Alloantibody-mediated class I signal transduction in endothelial cells and smooth muscle cells: enhancement by IFN-gamma and TNF-alpha. J Immunol. 1999;163(2):1010–1018. [PubMed] [Google Scholar]
- 26.Brouard S, Mansfield E, Braud C, Li L, Giral M, Hsieh SC, et al. Identification of a peripheral blood transcriptional biomarker panel associated with operational renal allograft tolerance. Proc Natl Acad Sci U S A. 2007;104(39):15448–15453. doi: 10.1073/pnas.0705834104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1848–1861. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307(5947):168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 30.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169(2):493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. J Immunol. 1994;153(3):1087–1098. [PubMed] [Google Scholar]
- 32.Cosimi AB, Sachs DH. Mixed chimerism and transplantation tolerance. Transplantation. 2004;77(6):943–946. doi: 10.1097/01.tp.0000117779.23431.3f. [DOI] [PubMed] [Google Scholar]
- 33.Huang CA, Fuchimoto Y, Scheier-Dolberg R, Murphy MC, Neville DM, Jr, Sachs DH. Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J Clin Invest. 2000;105(2):173–181. doi: 10.1172/JCI7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59(2):256–262. [PubMed] [Google Scholar]
- 35.Andreola G, Chittenden M, Shaffer J, Cosimi AB, Kawai T, Cotter P, et al. Mechanisms of Donor-Specific Tolerance in Recipients of Haploidentical Combined Bone Marrow/Kidney Transplantation. Am J Transplant. 2011;11(6):1236–1247. doi: 10.1111/j.1600-6143.2011.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porcheray F, Wong W, Saidman SL, De Vito J, Girouard TC, Chittenden M, et al. B-cell immunity in the context of T-cell tolerance after combined kidney and bone marrow transplantation in humans. Am J Transplant. 2009;9(9):2126–2135. doi: 10.1111/j.1600-6143.2009.02738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229(1):152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5(6):686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 39.Larsen CP, Alexander DZ, Hollenbaugh D, Elwood ET, Ritchie SC, Aruffo A, et al. CD40-gp39 interactions play a critical role during allograft rejection. Suppression of allograft rejection by blockade of the CD40-gp39 pathway. Transplantation. 1996;61(1):4–9. doi: 10.1097/00007890-199601150-00002. [DOI] [PubMed] [Google Scholar]
- 40.Parker DC, Greiner DL, Phillips NE, Appel MC, Steele AW, Durie FH, et al. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc Natl Acad Sci U S A. 1995;92(21):9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000;6(4):464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 42.Preston EH, Xu H, Dhanireddy KK, Pearl JP, Leopardi FV, Starost MF, et al. IDEC-131 (anti-CD154), sirolimus and donor-specific transfusion facilitate operational tolerance in non-human primates. Am J Transplant. 2005;5(5):1032–1041. doi: 10.1111/j.1600-6143.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- 43.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111(12):1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2(6):501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 45.Burns AM, Ma L, Li Y, Yin D, Shen J, Xu J, et al. Memory alloreactive B cells and alloantibodies prevent anti-CD154-mediated allograft acceptance. J Immunol. 2009;182(3):1314–1324. doi: 10.4049/jimmunol.182.3.1314. [DOI] [PubMed] [Google Scholar]
- 46.Bickerstaff A, Nozaki T, Wang JJ, Pelletier R, Hadley G, Nadasdy G, et al. Acute humoral rejection of renal allografts in CCR5(−/−) recipients. Am J Transplant. 2008;8(3):557–566. doi: 10.1111/j.1600-6143.2007.02125.x. [DOI] [PubMed] [Google Scholar]
- 47.Burns AM, Chong AS. Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming. J Immunol. 2011;186(1):214–221. doi: 10.4049/jimmunol.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan X, Ang A, Pollock-Barziv SM, Dipchand AI, Ruiz P, Wilson G, et al. Donor-specific B-cell tolerance after ABO-incompatible infant heart transplantation. Nat Med. 2004;10(11):1227–1233. doi: 10.1038/nm1126. [DOI] [PubMed] [Google Scholar]
- 49.Urschel S, Campbell PM, Meyer SR, Larsen IM, Nuebel J, Birnbaum J, et al. Absence of donor-specific anti-HLA antibodies after ABO-incompatible heart transplantation in infancy: altered immunity or age? Am J Transplant. 2010;10(1):149–156. doi: 10.1111/j.1600-6143.2009.02877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodnow CC. Transgenic mice and analysis of B-cell tolerance. Annu Rev Immunol. 1992;10:489–518. doi: 10.1146/annurev.iy.10.040192.002421. [DOI] [PubMed] [Google Scholar]
- 51.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol. 2007;7(8):633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nemazee D, Weigert M. Revising B cell receptors. J Exp Med. 2000;191(11):1813–1817. doi: 10.1084/jem.191.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, Clarke SH. Regulation of B-cell development by antibody specificity. Curr Opin Immunol. 2004;16(2):246–250. doi: 10.1016/j.coi.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353(6346):765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 55.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337(6207):562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 56.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177(4):1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pelanda R, Schwers S, Sonoda E, Torres RM, Nemazee D, Rajewsky K. Receptor editing in a transgenic mouse model: site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997;7(6):765–775. doi: 10.1016/s1074-7613(00)80395-7. [DOI] [PubMed] [Google Scholar]
- 58.Melamed D, Nemazee D. Self-antigen does not accelerate immature B cell apoptosis, but stimulates receptor editing as a consequence of developmental arrest. Proc Natl Acad Sci U S A. 1997;94(17):9267–9272. doi: 10.1073/pnas.94.17.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russell DM, Dembic Z, Morahan G, Miller JF, Burki K, Nemazee D. Peripheral deletion of self-reactive B cells. Nature. 1991;354(6351):308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo W, Smith D, Aviszus K, Detanico T, Heiser RA, Wysocki LJ. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. J Exp Med. 2010;207(10):2225–2237. doi: 10.1084/jem.20092712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohdan H, Swenson KG, Kruger Gray HS, Yang YG, Xu Y, Thall AD, et al. Mac-1-negative B-1b phenotype of natural antibody-producing cells, including those responding to Gal alpha 1,3Gal epitopes in alpha 1,3-galactosyltransferase-deficient mice. J Immunol. 2000;165(10):5518–5529. doi: 10.4049/jimmunol.165.10.5518. [DOI] [PubMed] [Google Scholar]
- 62.Ohdan H, Yang YG, Shimizu A, Swenson KG, Sykes M. Mixed chimerism induced without lethal conditioning prevents T cell- and anti-Gal alpha 1,3Gal-mediated graft rejection. J Clin Invest. 1999;104(3):281–290. doi: 10.1172/JCI6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang YG, deGoma E, Ohdan H, Bracy JL, Xu Y, Iacomini J, et al. Tolerization of anti-Galalpha1-3Gal natural antibody-forming B cells by induction of mixed chimerism. J Exp Med. 1998;187(8):1335–1342. doi: 10.1084/jem.187.8.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawahara T, Shimizu I, Ohdan H, Zhao G, Sykes M. Differing mechanisms of early and late B cell hyporesponsiveness induced by mixed chimerism. Am J Transplant. 2005;5(12):2821–2829. doi: 10.1111/j.1600-6143.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 65.Li Y, Ma L, Shen J, Chong AS. Peripheral deletion of mature alloreactive B cells induced by costimulation blockade. Proc Natl Acad Sci U S A. 2007;104(29):12093–12098. doi: 10.1073/pnas.0705240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y, Ma L, Yin D, Shen J, Chong AS. Long-term control of alloreactive B cell responses by the suppression of T cell help. J Immunol. 2008;180(9):6077–6084. doi: 10.4049/jimmunol.180.9.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parsons RF, Vivek K, Rostami SY, Zekavat G, Ziaie SM, Luo Y, et al. Acquisition of humoral transplantation tolerance upon de novo emergence of B lymphocytes. J Immunol. 2010;186(1):614–620. doi: 10.4049/jimmunol.1002873. [DOI] [PubMed] [Google Scholar]
- 68.Pallier A, Hillion S, Danger R, Giral M, Racape M, Degauque N, et al. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 2010;78(5):503–513. doi: 10.1038/ki.2010.162. [DOI] [PubMed] [Google Scholar]
- 69.Shimamura T, Hashimoto K, Sasaki S. Feedback suppression of the immune response in vivo. II. Involvement of prostaglandins in the generation of suppressor-inducer B lymphocytes. Cell Immunol. 1982;69(1):192–195. doi: 10.1016/0008-8749(82)90063-6. [DOI] [PubMed] [Google Scholar]
- 70.Shimamura T, Hashimoto K, Sasaki S. Feedback suppression of the immune response in vivo. I. Immune B cells induce antigen-specific suppressor T cells. Cell Immunol. 1982;68(1):104–113. doi: 10.1016/0008-8749(82)90093-4. [DOI] [PubMed] [Google Scholar]
- 71.Shimamura T, Habu S, Hashimoto K, Sasaki S. Feedback suppression of the immune response in vivo. III. Lyt-1+ B cells are suppressor-inducer cells. Cell Immunol. 1984;83(1):221–224. doi: 10.1016/0008-8749(84)90242-9. [DOI] [PubMed] [Google Scholar]
- 72.Zubler RH, Benacerraf B, Germain RN. Feedback suppression of the immune response in vitro. II. IgVH-restricted antibody-dependent suppression. J Exp Med. 1980;151(3):681–694. doi: 10.1084/jem.151.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zubler RH, Cantor H, Benacerraf B, Germain RN. Feedback suppression of the immune response in vitro. I. Activity of antigen-stimulated B cells. J Exp Med. 1980;151(3):667–680. doi: 10.1084/jem.151.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184(6):2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 76.Mauri C, Blair PA. Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheumatol. 2010;6(11):636–643. doi: 10.1038/nrrheum.2010.140. [DOI] [PubMed] [Google Scholar]
- 77.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197(4):489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178(12):7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 79.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 80.Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167(2):1081–1089. doi: 10.4049/jimmunol.167.2.1081. [DOI] [PubMed] [Google Scholar]
- 81.Parekh VV, Prasad DV, Banerjee PP, Joshi BN, Kumar A, Mishra GC. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-beta 1. J Immunol. 2003;170(12):5897–5911. doi: 10.4049/jimmunol.170.12.5897. [DOI] [PubMed] [Google Scholar]
- 82.Reichardt P, Dornbach B, Rong S, Beissert S, Gueler F, Loser K, et al. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood. 2007;110(5):1519–1529. doi: 10.1182/blood-2006-10-053793. [DOI] [PubMed] [Google Scholar]
- 83.Zheng J, Liu Y, Lau YL, Tu W. CD40-activated B cells are more potent than immature dendritic cells to induce and expand CD4(+) regulatory T cells. Cell Mol Immunol. 2010;7(1):44–50. doi: 10.1038/cmi.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng J, Liu Y, Qin G, Chan PL, Mao H, Lam KT, et al. Efficient induction and expansion of human alloantigen-specific CD8 regulatory T cells from naive precursors by CD40-activated B cells. J Immunol. 2009;183(6):3742–3750. doi: 10.4049/jimmunol.0901329. [DOI] [PubMed] [Google Scholar]
- 85.Tu W, Lau YL, Zheng J, Liu Y, Chan PL, Mao H, et al. Efficient generation of human alloantigen-specific CD4+ regulatory T cells from naive precursors by CD40-activated B cells. Blood. 2008;112(6):2554–2562. doi: 10.1182/blood-2008-04-152041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zheng J, Liu Y, Qin G, Lam KT, Guan J, Xiang Z, et al. Generation of human Th1-like regulatory CD4+ T cells by an intrinsic IFN-gamma- and T-bet-dependent pathway. Eur J Immunol. 2010;41(1):128–139. doi: 10.1002/eji.201040724. [DOI] [PubMed] [Google Scholar]
- 87.Carter NA, Vasconcellos R, Rosser EC, Tulone C, Munoz-Suano A, Kamanaka M, et al. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186(10):5569–5579. doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]
- 88.Yan Y, van der Putten K, Bowen DG, Painter DM, Kohar J, Sharland AF, et al. Postoperative administration of donor B cells induces rat kidney allograft acceptance: lack of association with Th2 cytokine expression in long-term accepted grafts. Transplantation. 2002;73(7):1123–1130. doi: 10.1097/00007890-200204150-00020. [DOI] [PubMed] [Google Scholar]
- 89.Le Texier L, Thebault P, Lavault A, Usal C, Merieau E, Quillard T, et al. Long-term allograft tolerance is characterized by the accumulation of B cells exhibiting an inhibited profile. Am J Transplant. 2011;11(3):429–438. doi: 10.1111/j.1600-6143.2010.03336.x. [DOI] [PubMed] [Google Scholar]
- 90.Aiba Y, Yamazaki T, Okada T, Gotoh K, Sanjo H, Ogata M, et al. BANK negatively regulates Akt activation and subsequent B cell responses. Immunity. 2006;24(3):259–268. doi: 10.1016/j.immuni.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 91.Fehr T, Haspot F, Mollov J, Chittenden M, Hogan T, Sykes M. Alloreactive CD8 T cell tolerance requires recipient B cells, dendritic cells, and MHC class II. J Immunol. 2008;181(1):165–173. doi: 10.4049/jimmunol.181.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fehr T, Wang S, Haspot F, Kurtz J, Blaha P, Hogan T, et al. Rapid deletional peripheral CD8 T cell tolerance induced by allogeneic bone marrow: role of donor class II MHC and B cells. J Immunol. 2008;181(6):4371–4380. doi: 10.4049/jimmunol.181.6.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mollov JL, Lucas CL, Haspot F, Kurtz J, Gaspar C, Guzman A, et al. Recipient dendritic cells, but not B cells, are required antigen-presenting cells for peripheral alloreactive CD8+ T-cell tolerance. Am J Transplant. 2010;10(3):518–526. doi: 10.1111/j.1600-6143.2009.02967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Deng S, Moore DJ, Huang X, Lian MM, Mohiuddin M, Velededeoglu E, et al. Cutting edge: transplant tolerance induced by anti-CD45RB requires B lymphocytes. J Immunol. 2007;178(10):6028–6032. doi: 10.4049/jimmunol.178.10.6028. [DOI] [PubMed] [Google Scholar]
- 95.Huang X, Moore DJ, Mohiuddin M, Lian MM, Kim JI, Sonawane S, et al. Inhibition of ICAM-1/LFA-1 interactions prevents B-cell-dependent anti-CD45RB-induced transplantation tolerance. Transplantation. 2008;85(5):675–680. doi: 10.1097/TP.0b013e3181663422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao G, Moore DJ, Lee KM, Kim JI, Duff PE, O’Connor MR, et al. An unexpected counter-regulatory role of IL-10 in B-lymphocyte-mediated transplantation tolerance. Am J Transplant. 2010;10(4):796–801. doi: 10.1111/j.1600-6143.2010.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ding Q, Yeung M, Camirand G, Qiang Z, Akiba H, Yagita H, et al. Regulatory B cells are indentified by TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011 doi: 10.1172/JCI46274. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiao S, Najafian N, Reddy J, Albin M, Zhu C, Jensen E, et al. Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J Exp Med. 2007;204(7):1691–1702. doi: 10.1084/jem.20062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ueno T, Habicht A, Clarkson MR, Albin MJ, Yamaura K, Boenisch O, et al. The emerging role of T cell Ig mucin 1 in alloimmune responses in an experimental mouse transplant model. J Clin Invest. 2008;118(2):742–751. doi: 10.1172/JCI32451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yuan X, Ansari MJ, D’Addio F, Paez-Cortez J, Schmitt I, Donnarumma M, et al. Targeting Tim-1 to overcome resistance to transplantation tolerance mediated by CD8 T17 cells. Proc Natl Acad Sci U S A. 2009;106(26):10734–10739. doi: 10.1073/pnas.0812538106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duan B, Morel L. Role of B-1a cells in autoimmunity. Autoimmun Rev. 2006;5(6):403–408. doi: 10.1016/j.autrev.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 102.Samitas K, Lotvall J, Bossios A. B cells: from early development to regulating allergic diseases. Archivum immunologiae et therapiae experimentalis. 2010;58(3):209–225. doi: 10.1007/s00005-010-0073-2. [DOI] [PubMed] [Google Scholar]