Abstract
Socioeconomic position (SEP) across life is found to be related to adult physical performance, but the underlying pathways are not well characterized. Using a British birth cohort (N = 2956), the associations of SEP from childhood into midlife with objective physical performance measures in midlife were examined, adjusting for possible confounders or mediators, including indicators of muscle development and central nervous system function. Childhood and adulthood SEP were positively related to standing balance and chair rise performance, but not to grip strength after basic adjustments. When both father’s occupation and mother’s education were included in the same model, having a mother with low education was associated with 0.6 standard deviations (SD) (95% confidence interval (CI: 0.3, 0.8)) poorer standing balance time compared with having a mother with the highest educational level, and having a father in the lowest occupational group was associated with a 0.3 SD (95% CI: 0.1, 0.6) lower chair rise score compared with having a father in the highest occupational group. These associations were maintained, albeit attenuated, after adjustment. In contrast, the associations of own education and adult occupation with physical performance were generally not maintained after adjustment. SEP across life impacts on midlife physical performance, and thereby the ageing process.
Keywords: Physical performance, Ageing, Childhood, Lifetime socioeconomic position, Life course
Introduction
Socioeconomic gradients in disability are striking. For example, in the UK people in the lowest socioeconomic group, as indicated by their neighbourhood, have a disability free life expectancy which is 17 years shorter than that of people in the highest socioeconomic group [1]. As the difference in total life expectancy is less than this, people in lower socioeconomic groups spend more of their shorter lives with a disability [1]. Such evidence highlights the need to establish when in life socioeconomic gradients in disability and its precursors, such as lower physical performance levels, develop and to identify the pathways which underlie these associations so that appropriate strategies to reduce socioeconomic inequalities in the disability experience of subsequent generations of older people can be devised.
Poor adult socioeconomic position (SEP) has been shown to be associated with lower physical performance levels [2–9], which in turn are strong predictors of future disability [10, 11], morbidity [12] and survival [13]. Recent evidence from a systematic review and meta-analysis has also shown that there are modest associations between childhood SEP and physical performance, and that not all associations are fully explained by the continuity of SEP from childhood to adulthood [14].
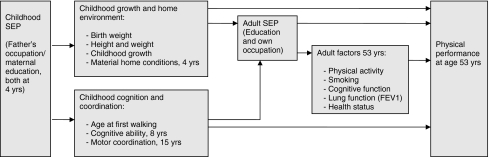
Using data from a British birth cohort study, we build on this body of research to examine the associations of indicators of SEP from across life with three objective measures of physical performance in midlife and to investigate pre-specified pathways which may underlie these associations. Two pathways starting in childhood were tested (Fig. 1). The first pathway included factors related to childhood growth and home environment, which have been shown to be associated with midlife physical performance possibly because of their impact on the development of muscle fibers in early life [8]. The second pathway included childhood cognition and coordination, which have also been shown to be related to midlife physical performance [8]. We hypothesized that father’s occupational class, a measure of material circumstances in childhood, would be more likely to be mediated by the former pathway, while maternal education, which is more a measure of childhood knowledge transfer and cognition, would be more strongly related to the latter. We also tested whether childhood SEP was related to midlife physical performance due to the continuity of SEP across life and, the pathways on which education and occupation in adulthood may be operating.
Fig. 1.
Pathway illustration
Materials and methods
The Medical Research Council (MRC) National Survey of Health and Development (NSHD) is a socially stratified sample of all births that took place in England, Scotland and Wales during 1 week in 1946 and consists of 2547 women and 2815 men [15]. In 1999, at age 53 years, 3035 were successfully contacted, and 2956 successfully completed at least one of the physical performance tests. Of the remaining 2327 no attempt was made to contact 40.7% as they had previously refused to participate, 24.9% were living abroad, 14.2% were untraced and 20.2% had died [16].
Physical performance at age 53 years
Physical performance was assessed by trained nurses during home visits at age 53 years using three tests (chair rising, standing balance and grip strength), following standardised protocols [9]. Chair rise time was measured as the time taken to rise from a sitting to a standing position and then sit down again ten times as fast as possible. Standing balance was measured as the time participants could maintain a one-legged stance, up to a maximum of 30 s. This test was performed twice, first with eyes open and then with eyes closed, with all participants asked to perform both tests. The time recorded during the second measurement, i.e. with eyes closed, was used in these analyses. Grip strength (kg) was measured isometrically using an electronic handgrip dynamometer, with two values recorded for each hand and the highest used in analyses. In order for high scores to indicate good chair rise performance as for the other two tests, the reciprocal of the time taken (multiplied by 100) was used. The distribution of standing balance time was skewed and so this was normalised using a logarithm transformation. For the purposes of the main analyses all three performance measures were standardised (mean = 0, SD = 1). This standardisation was done separately for men and women.
Socioeconomic position
Two indicators of childhood SEP recorded at age 4 years were selected based on previous findings [17]; mother’s education (secondary and higher; secondary only or primary and further education or higher; primary and further education (no qualifications attained); primary only), and father’s occupational class (categorised based on the UK Registrar General’s classification: I and II (advantaged), III non-manual, III manual, IV and V (least advantaged)).
Educational level at 26 years was used as an indicator of SEP in young adulthood (university degree or higher, advanced secondary qualifications, ordinary secondary level, no formal qualifications). Head of household’s occupation at 53 years was used as a measure of adult SEP and was categorised in the same way as father’s occupation. If head of household occupation was missing at 53 years, information from age 43 (n = 50) or 36 years (n = 19) was used.
Covariates
Factors defined as being part of the “childhood growth and environment pathway” were: material home conditions at 4 years based on a construct score of several variables (including health visitor’s assessments of housing, clothing and mother’s management of child development) with a summary score ranging from 0 (poorest) to 8 (best conditions); birth weight (g); height (cm) and weight (kg) at 4 years measured using standardised protocols [18]; and height and weight velocities between 4 and 7 years.
Factors defined as being part of the “childhood cognition and coordination pathway” were: cognitive ability at 8 years based on a summary score of reading comprehension, pronunciation, vocabulary and nonverbal reasoning standardised (mean = 0, SD = 1); motor coordination, which included number of times they could tap the ground with their left foot in 15 s at 15 years; and age at first walking (months).
Covariates from adulthood (53 years), which could mediate or confound the main associations of SEP with physical performance, were body size, physical activity, lung function, cognitive performance and disabling medical conditions. Height (cm) and weight (kg) were measured by nurses according to standard protocols. Physical activity was based on self-reports of participation in sports and recreational activities during the previous 4 weeks (none, 1–4 times, 5+ times). Lung function, denoted by forced expiratory volume (FEV1), was measured using the Micromedical turbine electronic spirometer. Cognitive performance was assessed using the National Adult Reading Test [19]. Presence of one or more potentially disabling medical conditions, including diabetes, cancer, epilepsy, or cardiovascular disease, was included as a binary variable [9].
Statistical methods
To investigate the relationships between each of the SEP indicators and physical performance a sequential set of analyses was performed. First, crude summary statistics for each of the untransformed physical performance measures by the SEP categories were calculated. Geometric means were calculated for standing balance and chair rise times due to the skewed distributions of these measures prior to transformation. A Wald test was used to asses the overall association of SEP with each physical performance measure. Then, the Slope Index of Inequality (SII) [20] was used to test the associations of SEP with each of the three transformed physical performance measures. This involved modelling the SEP measures as ridit scores (proportion of population with higher SEP than the midpoint of the category) whereby the regression coefficients can be interpreted as the absolute difference in physical performance levels between the hypothetical individual at the top of the SEP hierarchy with the one at the bottom.
At each stage of the analyses a gender by SEP term was included to test for gender interactions. When using the SII, none of these interactions were significant and so in all SII analyses the genders were combined. Deviation from linearity was tested by adding a squared ridit score to the models, but no evidence of this was found. The pathways between SEP and physical performance were tested by sequentially adding groups of variables to the models. Each childhood SEP measure was first adjusted for gender, then for current height and weight, and then both SEP measures were included in the same model. A series of models were then fitted: (1) “childhood growth and home environment pathway”, (2) “childhood cognition and coordination pathway”, (3) education and adult SEP, and (4) adult covariates. For the adult SEP measures each variable was adjusted for gender, then current height and weight, and then both adult SEP measures were included in the same model. A further model was fitted, adding all childhood variables. Finally, a model adjusted for all variables was fitted. All analyses were adjusted for the initial stratified sampling design (non-manual households were oversampled with a ratio 4:1 compared to manual households [15]), and conducted in Stata 10.0.
Results
Table 1 shows the distribution of the physical performance measures by each of the SEP measures. In these crude analyses both balance and chair rise performance declined with decreases in all four indicators of SEP, with the exception of adult occupation and chair rise performance where no association was found. There was little evidence of an association between SEP and grip strength in either gender with the exception of a positive association between educational levels and grip strength among women.
Table 1.
Distribution of physical performance measures by indicators of socioeconomic position in the NSHD
| Balance time (seconds) | Chair rises time (seconds) | Grip strength (kg), men | Grip strength (kg), women | |||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (SD)c | N | Mean (SD)c | N | Mean (SD)a | N | Mean (SD)a | |
| Men | 1374 | 5.6 (4.7) | 1357 | 19.6 (9.3) | 1406 | 47.9 (12.1) | ||
| Women | 1410 | 4.5 (3.6) | 1400 | 21.2 (11.5) | 1444 | 27.4 (7.7) | ||
| Socioeconomic position | ||||||||
| Father’s occupation | ||||||||
| I and II | 603 | 5.8 (4.8) | 612 | 19.7 (7.8) | 306 | 48.6 (12.5) | 310 | 27.9 (8.0) |
| III Non manual | 490 | 5.5 (4.6) | 491 | 20.3 (8.7) | 240 | 47.2 (11.6) | 256 | 28.3 (7.7) |
| III Manual | 768 | 4.8 (3.9) | 755 | 20.4 (10.4) | 394 | 48.5 (12.4) | 407 | 26.9 (7.6) |
| IV and V | 689 | 4.5 (3.5) | 670 | 21.2 (11.7) | 355 | 46.7 (11.7) | 345 | 27.7 (7.6) |
| Overall test for associationb | P < 0.001 | P = 0.009 | P = 0.237 | P = 0.257 | ||||
| Mother’s education | ||||||||
| Secondary and further education or higher | 296 | 6.3 (5.3) | 300 | 19.5 (7.8) | 149 | 48.0 (12.9) | 153 | 27.4 (8.7) |
| Secondary only or primary and further education or higher | 290 | 6.5 (5.6) | 288 | 19.3 (9.9) | 157 | 47.7 (12.0) | 140 | 27.8 (6.9) |
| Primary and further education (no qualifications attained) | 366 | 5.3 (4.5) | 363 | 19.9 (9.8) | 193 | 48.7 (13.6) | 181 | 27.4 (7.2) |
| Primary only | 1521 | 4.6 (3.5) | 1501 | 21.0 (10.9) | 754 | 47.9 (11.7) | 814 | 27.3 (7.8) |
| Overall test for associationb | P < 0.001 | P = 0.005 | P = 0.746 | P = 0.883 | ||||
| Education at 26 years | ||||||||
| University degree and eq. | 268 | 6.9 (6.3) | 268 | 18.7 (6.7) | 200 | 47.0 (13.8) | 70 | 27.6 (8.0) |
| Adv. Secondary | 687 | 5.9 (4.8) | 696 | 19.7 (8.4) | 374 | 49.3 (12.0) | 317 | 29.0 (7.4) |
| Ordinary secondary | 658 | 4.9 (3.8) | 651 | 21.0 (11.5) | 257 | 47.8 (11.5) | 412 | 27.7 (7.8) |
| No qualifications | 1022 | 4.2 (3.1) | 992 | 21.3 (11.0) | 499 | 47.4 (11.8) | 563 | 26.7 (7.6) |
| Overall test for associationb | P < 0.001 | P < 0.001 | P = 0.267 | P = 0.032 | ||||
| Adult occupation | ||||||||
| I and II | 1207 | 5.6 (4.6) | 1195 | 20.0 (9.4) | 531 | 48.7 (12.2) | 692 | 28.4 (7.7) |
| III Non manual | 638 | 5.1 (4.1) | 635 | 20.1 (9.2) | 436 | 48.5 (11.9) | 212 | 27.1 (6.6) |
| III Manual | 483 | 4.4 (3.3) | 478 | 20.3 (11.0) | 183 | 48.6 (11.5) | 320 | 26.4 (7.9) |
| IV and V | 443 | 4.3 (3.5) | 432 | 21.8 (11.9) | 245 | 46.0 (11.8) | 212 | 27.1 (7.8) |
| Overall test for associationb | P < 0.001 | P = 0.687 | P = 0.092 | P = 0.167 | ||||
a Mean and SD is weighted for study design. b Wald test adjusted for height and gender, and study design. c Geometric mean
Childhood SEP (Table 2)
Table 2.
Childhood SEP and physical performance: Differences in standardised physical performance at age 53 years by father’s occupation and maternal education (the unit difference in physical performance is 1 standard deviation, and numbers in table are difference in outcome comparing the lowest (0th percentile) with the highest (100th percentile) SEP (Slope Index of Inequality, SII)
| Standing balance (std. devs.) (N = 1353) | Chair rises (std. devs.) (N = 1343) | Grip strength (std. devs.) (N = 1390) | ||||
|---|---|---|---|---|---|---|
| Difference in balance (per 1 SD) comparing low with high SEP | P-value | Difference in chair rises (per 1 SD) comparing low with high SEP | P-value | Difference in grip strength (per 1 SD) comparing low with high SEP | P-value | |
| Maternal education | ||||||
| Model 0: Adjusted for gender | −0.67 (−0.92, −0.41) | <0.001 | −0.23 (−0.48, 0.02) | 0.070 | −0.07 (−0.32, 0.17) | 0.556 |
| Model 1: Adjusted for gender, height and weight | −0.62 (−0.87, −0.36) | <0.001 | −0.23 (−0.48, 0.02) | 0.067 | −0.01 (−0.25, 0.22) | 0.931 |
| Model 2: Model 1 + Father’s occupation | −0.55 (−0.83, −0.28) | <0.001 | −0.12 (−0.39, 0.15) | 0.374 | −0.01 (−0.27, 0.24) | 0.912 |
| Model 3: Model 2 + Childhood growth and home environment | −0.48 (−0.75, −0.20) | 0.001 | −0.12 (−0.39, 0.16) | 0.404 | −0.03 (−0.28, 0.23) | 0.847 |
| Model 4: Model 2 + Childhood cognition and coordination | −0.44 (−0.72, −0.15) | 0.002 | −0.13 (−0.40, 0.15) | 0.377 | −0.02 (−0.29, 0.24) | 0.861 |
| Model 5: Model 2 + Education and adult SEP | −0.41 (−0.69, −0.13) | 0.005 | −0.11 (−0.39, 0.17) | 0.451 | 0.02 (−0.25, 0.28) | 0.900 |
| Model 6: Model 2 + adult life style, health and cognition | −0.44 (−0.72, −0.16) | 0.002 | −0.11 (−0.38, 0.16) | 0.412 | −0.00 (−0.26, 0.25) | 0.987 |
| Model 7: Fully adjusted | −0.32 (−0.61, −0.04) | 0.027 | −0.13 (−0.42, 0.15) | 0.349 | −0.00 (−0.26, 0.26) | 0.995 |
| Father’s occupation | ||||||
| Model 0: Adjusted for gender | −0.41 (−0.64, −0.19) | <0.001 | −0.37 (−0.61, −0.12) | 0.004 | −0.08 (−0.30, 0.14) | 0.471 |
| Model 1: Adjusted for gender, height and weight | −0.35 (−0.58, −0.12) | 0.003 | −0.37 (−0.61, −0.13) | 0.003 | 0.00 (−0.20, 0.22) | 0.944 |
| Model 2: Model 1 + Maternal education | −0.19 (−0.44, 0.06) | 0.130 | −0.33 (−0.60, −0.07) | 0.013 | 0.01 (−0.21, 0.23) | 0.919 |
| Model 3: Model 2 + Childhood growth and home environment | −0.12 (−0.36, 0.13) | 0.342 | −0.33 (−0.61, −0.05) | 0.019 | 0.02 (−0.21, 0.25) | 0.845 |
| Model 4: Model 2 + Childhood cognition and coordination | −0.09 (−0.34, 0.16) | 0.479 | −0.33 (−0.61,−0.04) | 0.024 | 0.02 (−0.21, 0.25) | 0.863 |
| Model 5: Model 2 + Education and adult SEP | −0.06 (−0.31, 0.20) | 0.655 | −0.31 (−0.59,−0.03) | 0.030 | 0.04 (−0.18, 0.27) | 0.724 |
| Model 6: Model 2 + adult life style, health and cognition | −0.07 (−0.32, 0.18) | 0.603 | −0.28 (−0.54, −0.13) | 0.040 | 0.02 (−0.20, 0.25) | 0.829 |
| Model 7: Fully adjusted | 0.05 (−0.20, 0.29) | 0.711 | −0.28 (−0.57, 0.01) | 0.059 | 0.04 (−0.19, 0.27) | 0.714 |
Model 0: Adjusted for gender
Model 1: Adjusted for gender, height and weight at 53 years
Model 2: Model 1 + adjusted for maternal education/father’s occupation
Model 3: Model 2 + adjusted for birth weight, height and weight at 4 years, and height and weight change 4–7 years, home environment at age 4 years
Model 4: Model 2 + adjusted for age of first walking, cognition at 8 years, coordination at 15 years
Model 5: Model 2 + adjusted for education and adult SEP
Model 6: Model 2 + adjusted for adulthood (53 years) physical activity, smoking, health conditions, cognitive function, and lung function
Model 7: Fully adjusted
Both indicators of childhood SEP were related to standing balance and chair rise performance, but not to grip strength. Using the SII, the difference in standing balance between those with mothers with the lowest educational level compared to those with the highest was −0.7 standard deviations (SD) (95% confidence interval (CI: −0.9, −0.4)), and for chair rise performance was −0.2 SD (−0.5, 0.0) in sex-adjusted models. A similar pattern of association was observed for father’s occupation; comparing the lowest with highest class was associated with −0.4 SD (−0.6, −0.2) poorer standing balance and chair rise performance.
Maternal education’s association with standing balance was only modestly attenuated by father’s occupation, but father’s occupation was no longer related to standing balance after adjustment for maternal education (model 2). Thus, the results suggest a stronger association of maternal education, than of father’s occupation, with standing balance. Adjustment for the “growth/environment pathway” (model 3) somewhat attenuated the association of maternal education with standing balance, and separate adjustment for the “cognition/coordination pathway” attenuated the association to a slightly greater extent (model 4). The association with maternal education was also slightly attenuated by education and adult SEP (model 5) and also for adult lifestyle, health and cognition (model 6). Despite an attenuation in effect size of approximately 50% in the fully adjusted model, an association of maternal education with standing balance remained with a difference of −0.3 SD between those with the lowest and highest maternal education (P = 0.03) (model 7).
For chair rise performance, the difference when comparing lowest with highest father’s occupational class was −0.3 SD (P = 0.01) in a model including both childhood SEP indicators, while the initially weaker association with maternal education was almost halved in size (model 2). The association with father’s occupation was robust to further adjustment for factors on both childhood pathways (models 3 and 4), and the indicators of adult SEP and other factors in adulthood had only modest impacts on the relationship (models 5 and 6). In the fully adjusted model the difference in chair rise performance comparing lowest with highest father’s occupational class was −0.3 SD (P = 0.06) (model 7).
Neither father’s occupation nor maternal education was related to grip strength before or after adjustment.
Adult SEP (Table 3)
Table 3.
Adult SEP and physical performance: Differences in standardised physical performance at age 53 years by educational level and adult occupation (the unit difference in physical performance is 1 standard deviation, and numbers in table are difference in outcome comparing the lowest (0th percentile) with the highest (100th percentile) SEP (Slope Index of Inequality, SII)
| Standing balance (std. devs.) (N = 1353) | Chair rises (std. devs.) (N = 1343) | Grip strength (std. devs.) (N = 1390) | ||||
|---|---|---|---|---|---|---|
| Difference in balance (per 1 SD) comparing low with high SEP | P-value | Difference in chair rises (per 1 SD) comparing low with high SEP | P-value | Difference in grip strength (per 1 SD) comparing low with high SEP | P-value | |
| Own education | ||||||
| Model 0: Adjusted for gender | −0.69 (−0.92, −0.46) | <0.001 | −0.18 (−0.42, 0.05) | 0.131 | −0.15 (−0.37, 0.08) | 0.207 |
| Model 1: Adjusted for gender, height and weight | −0.63 (−0.86, −0.40) | <0.001 | −0.17 (−0.41, −0.07) | 0.162 | −0.08 (−0.30, 0.14) | 0.484 |
| Model 2: Model 1 + Adult occupation | −0.52 (−0.76, −0.27) | <0.001 | −0.10 (−0.35, 0.15) | 0.429 | −0.04 (−0.28, 0.20) | 0.752 |
| Model 3: Model 2 + Childhood factors | −0.23 (−0.51, 0.05) | 0.111 | 0.01 (−0.28, 0.30) | 0.940 | −0.07 (−0.35, 0.20) | 0.606 |
| Model 4: Model 3 + Adult life style, health and cognition | −0.14 (−0.43, 0.16) | 0.370 | 0.08 (−0.21, 0.37) | 0.594 | −0.07 (−0.35, 0.22) | 0.643 |
| Adult occupation | ||||||
| Model 0: Adjusted for gender | −0.50 (−0.72, −0.28) | <0.001 | −0.20 (−0.42, 0.03) | 0.090 | −0.25 (−0.47, −0.03) | 0.024 |
| Model 1: Adjusted for gender, height and weight | −0.49 (−0.71, −0.26) | <0.001 | −0.23 (−0.46, −0.01) | 0.045 | −0.14 (−0.36, 0.08) | 0.212 |
| Model 2: Model 1 + Own education | −0.33 (−0.57, −0.10) | 0.005 | −0.20 (−0.44, 0.04) | 0.102 | −0.13 (−0.36, 0.10) | 0.282 |
| Model 3: Model 2 + Childhood factors | −0.30 (−0.53, −0.07) | 0.012 | −0.18 (−0.43, 0.06) | 0.141 | −0.13 (−0.37, 0.10) | 0.262 |
| Model 4: Model 3 + Adult life style, health and cognition | −0.23 (−0.46, 0.00) | 0.054 | −0.12 (−0.37, 0.12) | 0.328 | −0.10 (−0.34, 0.13) | 0.399 |
Model 0: Adjusted for gender
Model 1: Adjusted for gender, height and weight at 53 years
Model 2: + adult occupation/education
Model 3: + adjusted for father’s occupation and maternal education, birth weight, height and weight at 4 years, and height and weight change 4–7 years, age of first walking, cognition at 8 years, coordination at 15 years, home environment at age 4 years
Model 4: + adjusted for adulthood (53 years) physical activity, smoking, health conditions, cognitive function, and lung function
Both indicators of adult SEP were associated with standing balance. For example, when comparing lowest with highest educational level, the difference in standing balance was −0.7 SD (95% CI: (−0.9, −0.5)). Chair rise performance was only weakly associated with both indicators of adult SEP. Grip strength showed no associations with education or adult occupation after basic adjustments (model 1).
In a model including both adult SEP indicators, the association of education with standing balance was only slightly attenuated (P < 0.001) (model 2). Although the association of adult occupation with standing balance was maintained, approximately one-third of the initial effect was explained by education (model 0 and model 2). The association of education with standing balance was attenuated and no longer significant after further adjustment for childhood SEP and other childhood variables. In a fully adjusted model, with additional adjustment for adult factors, approximately one-fifth of the initial association remained (model 4). A similar pattern of change in association applied to adult occupation, but some 50% of the association was still evident in the fully adjusted model (model 4, P = 0.05). Both indicators of adult SEP were similarly related to chair rise performance both before and after mutual adjustment (model 2). These relatively weak relationships were largely attenuated after adjustment for factors in childhood and adulthood.
Education was positively associated with grip strength in women in unadjusted analyses (P = 0.03) (Table 1), but in SII analyses genders were combined as there was no evidence of gender interactions when these were formally tested. Education was not related to grip strength before or after adjustment (Table 3). Adult occupation was associated with grip strength in a model with adjustment only for gender (P = 0.02) but this association was fully explained by height and weight.
Discussion
Indicators of SEP in childhood and adulthood were positively related to midlife standing balance and chair rise performance, but not to grip strength. The associations of childhood SEP were maintained, albeit attenuated, after adjustment for possible mediating factors across the life course. In contrast, the associations of own education and adult occupation with these performance measures were found to be explained by these factors, with the exception of adult occupation which showed an association with standing balance even after adjustment.
Grip strength has previously been shown to be strongly related to height [8, 16], possibly due to the fact that taller individuals have more muscle mass. The observed relationship between adult occupation and grip strength in models with adjustment only for sex was due to the fact that those of higher occupational classes were taller. Our findings of an association of adult SEP with standing balance and chair rise performance and no evidence of an association with grip strength (after adjustment for height), were consistent with previous analyses from the NSHD using a binary categorization of occupational social class (i.e. manual vs non-manual) [9]. However, in another paper which examined unadjusted associations between adult occupational class in 6 categories and grip strength, grip strength was stronger in the higher adult occupational classes [16]. This discrepancy in findings is due to the fact that our analyses included adjustment for current height and the initial sampling design, unlike the previous analyses.
Results from several other studies are in line with our findings, showing poorer physical performance in those with lower adult SEP [2–7]. Some studies have also reported socioeconomic gradients in grip strength in older populations [2, 4, 5, 7]. This discrepancy is possibly because the relationship has not yet emerged in the younger population we examined. Most of these previous studies did not have a life course design, and did not consider early life factors, and some did not adjust for height [5]. Further, many studies do not include objective measures of physical performance and instead rely on self-reported measures of functioning, which may be more subject to bias [21–26].
A recent systematic review found, similarly to us, that childhood SEP was related to chair rise performance independently of adult SEP and body size. However, the review found that the relationships with grip strength and standing balance were greatly attenuated and no longer significant after adjustment [14]. Substantial heterogeneity in effect sizes were observed between studies, which may be due to the variation in study characteristics including methods of measuring childhood SEP. Standing balance was analysed as a binary variable using a measure with eyes open, and hence the review results are not fully comparable with our own.
Previous investigations of the associations of childhood SEP with individual measures of physical performance in the NSHD have considered only father’s occupation. Differences in results regarding father’s occupation and chair rising between these previous analyses and our own are probably due to the use of SII as opposed to a binary stratification thus increasing the power to detect a trend across all categories. Associations with father’s occupational class and mother’s education (after mutual adjustment) with a summary physical performance score was previously found in the NSHD [17]. However, this study did not investigate as wide a range of covariates as we have examined and by grouping the results from different tests of performance into a summary score was unable to detect differences in the associations by type of performance measure which may be important when considering the most appropriate ways to intervene and the most likely pathways of association.
That the associations of maternal education with standing balance, and of father’s occupation with chair rises were maintained after adjustments for a wide range of covariates, indicates that midlife physical performance levels have roots in childhood. It may be that an accumulation of negative events in the lower socioeconomic groups during childhood, a developmentally sensitive period, might influence midlife physical performance by affecting the peak level of performance achieved.
The maintenance of an association of maternal education with standing balance, and of father’s occupation with chair rises, despite adjustments could be due to unmeasured factors associated with childhood SEP. Standing balance has previously been shown to be strongly associated with factors in childhood and to be less influenced by factors later in life in the NSHD [8, 19]. A substantial part of the maternal education-balance link was mediated by the “cognition/coordination” pathway, and it is possible that if we had been able to include other variables which are on this pathway the association may have been fully explained. For example, higher maternal education has previously been found to be related to healthier eating habits and more exercise in childhood [27, 28]. It has also been shown to be related to maternal smoking habits, but this information was not available in this study.
We found no clear evidence to suggest that the “cognition/coordination” and the “growth/home environment” pathways differed in their level of influence on the associations of maternal education and father’s occupation with midlife physical performance. Both cognitive, growth and home environment factors were important mediators for the relationships between childhood SEP and midlife physical performance. This may be because, although we defined factors as being on one or other pathway there is overlap between them.
The fact that most of the associations of education and adult occupation with the performance measures were mediated by adult health and health-related factors suggests that these factors are on the chain of risk between adult SEP and physical performance.
The strength of this study is being able to relate socioeconomic position at different stages of life with objectively assessed physical performance in middle age, to adjust for a wide range of prospectively measured covariates and to investigate specific pathways which may underlie the associations.
A possible limitation of all historic birth cohorts is their generalizability. As this cohort, born in 1946, experienced a different childhood environment with more absolute deprivation, especially in the lower socioeconomic groups, than more recent cohorts, one could argue that the associations found might not be applicable to younger cohorts. However, a recent review concluded that the adult health effects of poor childhood SEP persist in later birth cohorts despite the fact that these cohorts have not generally experienced the same absolute levels of deprivation as our cohort [29], which suggests that our findings may apply to younger cohorts.
We also need to consider the representativeness of our sample. Comparisons with UK Census data of people of a similar age show that NSHD participants followed up to age 53 years were similar in many respects to the national population but did have higher adult occupational class and levels of education [30]. In comparisons of characteristics within the NSHD, it was found that the 2956 participants with physical performance data at age 53 years were more likely to be women (P<0.01) had higher maternal education (P = 0.03) and paternal occupation levels (P = 0.01) than those 2406 participants without these measures. Further, those participants with complete data on physical performance, mediators and confounders (n = 1438) who were included in the main analyses did not differ by gender, current height or weight, maternal education, father’s occupation, adult occupation, grip strength, chair rise time, or balance performance from participants at 53 years who were not included in the main analyses due to missing data on covariates (n = 1518). However, those included in analyses did have higher educational levels (P < 0.01) than those excluded.
It is unlikely that the SEP-physical performance relationships in the subsample with lower education levels that was excluded from the analyses would have been different enough from the SEP-physical performance relationships in those with similar education levels who participated to substantially influence the findings. If anything the observed relationships are likely to be weaker as it was more likely to exclude lower SEP and those with poor performance (i.e. because of poor health/unable to do tests or participate in study). Also, in sensitivity analyses using maximum available samples the relationships between SEP and physical performance were very similar to the relationships found in the restricted sample with complete data on all covariates (results not shown).
Study participants unable to perform the physical performance tests were excluded from our analyses, which might also introduce bias. Sensitivity analyses were performed in which those unable to perform the tests for health reasons were included by assigning them values corresponding to the 99th percentile for chair rises (30 s, n = 154) and the 1st percentile for balance time (1 s, n = 113) and grip strength (11.2 kg, n = 69). In these analyses a somewhat stronger association of SEP with physical performance was found, indicating that estimates from the main analyses presented might be conservative. This was especially true for chair rising where in sensitivity analyses stronger evidence of relationships with maternal and own education were found (results not shown). Those unable to perform the tests generally had lower educational levels and a greater probability of dying before age 60 (15% of those unable to do the balance test died compared to only 2% among those able to do the test) which may explain these differences.
The slope index of inequality (SII) has some attractive properties: it gives an inequality measure across the full range of SEP; not just comparing the two extreme groups, it accounts for differing group sizes, and allows a more fair comparison across different measures of SEP. The use of SII assumes a linear association between the ranked SEP score and the physical performance outcomes and this assumption was justified by our tests for deviation from linearity.
Conclusions
Socioeconomic position across life impacts on midlife physical performance, and thereby the ageing process. Policies aiming to reduce socioeconomic differences in childhood will probably have long term health gains including a beneficial impact on physical performance in later life.
Funders
The manuscript is funded by MRC Unit for Lifelong Health and Ageing in UK and supported in part by the Intramural Research Program, National Institute on Aging, NIH in USA, and the Research Council of Norway. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
References
- 1.Marmot M. The marmot review fair society, healthy lives. In: Marmot M, editor. London: UCL, 2010.
- 2.Mohd Hairi F, Mackenbach JP, Andersen-Ranberg K, et al. Does socio-economic status predict grip strength in older Europeans? Results from the SHARE study in non-institutionalised men and women aged 50+ J Epidemiol Community Health. 2010;64:829–837. doi: 10.1136/jech.2009.088476. [DOI] [PubMed] [Google Scholar]
- 3.Brunner E, Shipley M, Spencer V, et al. Social inequality in walking speed in early old age in the Whitehall II study. J Gerontol A Biol Sci Med Sci. 2009;64:1082–1089. doi: 10.1093/gerona/glp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syddall H, Evandrou M, Cooper C, et al. Social inequalities in grip strength, physical function, and falls among community dwelling older men and women: findings from the Hertfordshire Cohort Study. J Aging Health. 2009;21:913–939. doi: 10.1177/0898264309340793. [DOI] [PubMed] [Google Scholar]
- 5.Russo A, Onder G, Cesari M, et al. Lifetime occupation and physical function: a prospective cohort study on persons aged 80 years and older living in a community. Occup Environ Med. 2006;63:438–442. doi: 10.1136/oem.2005.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coppin AK, Ferrucci L, Lauretani F, et al. Low socioeconomic status and disability in old age: evidence from the InChianti study for the mediating role of physiological impairments. J Gerontol A Biol Sci Med Sci. 2006;61:86–91. doi: 10.1093/gerona/61.1.86. [DOI] [PubMed] [Google Scholar]
- 7.Rautio N, Heikkinen E, Ebrahim S. Socio-economic position and its relationship to physical capacity among elderly people living in Jyvaskyla, Finland: five- and ten-year follow-up studies. Soc Sci Med. 2005;60:2405–2416. doi: 10.1016/j.socscimed.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 8.Kuh D, Hardy R, Butterworth S, et al. Developmental origins of midlife physical performance: evidence from a British birth cohort. Am J Epidemiol. 2006;164:110–121. doi: 10.1093/aje/kwj193. [DOI] [PubMed] [Google Scholar]
- 9.Kuh D, Bassey EJ, Butterworth S, et al. Grip strength, postural control, and functional leg power in a representative cohort of British men and women: associations with physical activity, health status, and socioeconomic conditions. J Gerontol A Biol Sci Med Sci. 2005;60:224–231. doi: 10.1093/gerona/60.2.224. [DOI] [PubMed] [Google Scholar]
- 10.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 12.Cooper R, Kuh D, Cooper C, et al. Objective measures of physical capability and subsequent health: a systematic review. Age and Ageing. 2010 (in press). [DOI] [PMC free article] [PubMed]
- 13.Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birnie K, Cooper R, Martin RM, et al. Childhood socioeconomic position and objectively measured physical capability levels in adulthood: a systematic review and meta-analysis. PLoS ONE. 2011;6(1):e15564. doi: 10.1371/journal.pone.0015564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadsworth M, Kuh D, Richards M, et al. Cohort profile: the 1946 national birth cohort (MRC national survey of health and development) Int J Epidemiol. 2006;35:49–54. doi: 10.1093/ije/dyi201. [DOI] [PubMed] [Google Scholar]
- 16.Kuh D, Bassey J, Hardy R, et al. Birth weight, childhood size, and muscle strength in adult life: evidence from a birth cohort study. Am J Epidemiol. 2002;156:627–633. doi: 10.1093/aje/kwf099. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, Butterworth S, Wadsworth ME, et al. Childhood socioeconomic status predicts physical functioning a half century later. J Gerontol A Biol Sci Med Sci. 2006;61:694–701. doi: 10.1093/gerona/61.7.694. [DOI] [PubMed] [Google Scholar]
- 18.Braddon FEM, Rodgers B, Wadsworth MEJ, et al. Onset of obesity in a 36 year birth cohort study. Brit Med J (Clin Res Ed) 1986;293:299–303. doi: 10.1136/bmj.293.6542.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuh D, Cooper R, Hardy R, et al. Lifetime cognitive performance is associated with midlife physical performance in a prospective national birth cohort study. Psychosom Med. 2009;71:38–48. doi: 10.1097/PSY.0b013e31818a1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackenbach JP, Kunst AE. Measuring the magnitude of socio-economic inequalities in health: an overview of available measures illustrated with two examples from Europe. Soc Sci Med. 1997;44:757–771. doi: 10.1016/S0277-9536(96)00073-1. [DOI] [PubMed] [Google Scholar]
- 21.Sekine M, Chandola T, Martikainen P, et al. Socioeconomic inequalities in physical and mental functioning of British, Finnish, and Japanese civil servants: role of job demand, control, and work hours. Soc Sci Med. 2009;69:1417–1425. doi: 10.1016/j.socscimed.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gjonca E, Tabassum F, Breeze E. Socioeconomic differences in physical disability at older age. J Epidemiol Community Health. 2009;63:928–935. doi: 10.1136/jech.2008.082776. [DOI] [PubMed] [Google Scholar]
- 23.Jang SN, Choi YJ, Kim DH. Association of socioeconomic status with successful ageing: differences in the components of successful ageing. J Biosoc Sci. 2009;41:207–219. doi: 10.1017/S0021932008003052. [DOI] [PubMed] [Google Scholar]
- 24.Stansfeld SA, Head J, Fuhrer R, et al. Social inequalities in depressive symptoms and physical functioning in the Whitehall II study: exploring a common cause explanation. J Epidemiol Community Health. 2003;57:361–367. doi: 10.1136/jech.57.5.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laaksonen E, Martikainen P, Head J, et al. Associations of multiple socio-economic circumstances with physical functioning among Finnish and British employees. Eur J Public Health. 2009;19:38–45. doi: 10.1093/eurpub/ckn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osler M, Madsen M, Nybo Andersen AM, et al. Do childhood and adult socioeconomic circumstances influence health and physical function in middle-age? Soc Sci Med. 2009;68:1425–1431. doi: 10.1016/j.socscimed.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giskes K, Lenthe Fv F, Brug HJ, et al. Dietary intakes of adults in the Netherlands by childhood and adulthood socioeconomic position. Eur J Clin Nutr. 2004;58:871–880. doi: 10.1038/sj.ejcn.1601889. [DOI] [PubMed] [Google Scholar]
- 28.McVeigh JA, Norris SA, Wet T. The relationship between socio-economic status and physical activity patterns in South African children. Acta Paediatr. 2004;93:982–988. doi: 10.1111/j.1651-2227.2004.tb02699.x. [DOI] [PubMed] [Google Scholar]
- 29.Galobardes B, Lynch JW, Davey Smith G. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. J EpidemiolCommunity Health. 2008;62:387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- 30.Wadsworth ME, Butterworth SL, Hardy RJ, et al. The life course prospective design: an example of benefits and problems associated with study longevity. Soc Sci Med. 2003;57:2193–2205. doi: 10.1016/S0277-9536(03)00083-2. [DOI] [PubMed] [Google Scholar]